Abstract
Nuclear factor erythroid-2-related factor 2 (Nrf2), is an inducible transcription factor that improves redox balance through stimulating antioxidant gene expression. In older humans the Nrf2 response to a single bout of acute exercise is blunted compared to young indicating impaired redox signaling. The purpose of this randomized controlled trial was to investigate if the signaling impairment could be reversed with exercise training in older men and women, while also comparing to young. Young (18–28y, n=21) and older (≥60y, n=19) men and women were randomized to 8-week aerobic exercise training (ET; 3d/wk, 45min/d) or a non-exercise control group (CON). Nrf2 nuclear localization, gene expression for NQO1, HO1, and GCLC, and GCLC protein were measured in PBMCs in response to acute exercise trial (AET; 30-min cycling at 70% VO2 peak pre- and post-intervention at 7 timepoints (Pre, +10m, +30m, +1h, +4h, +8h, +24h). Young had greater Nrf2 signaling response compared to older at pre-intervention (p=0.05), whereas the older had significantly higher basal Nrf2 levels (p=0.004). ET decreased basal Nrf2 expression compared to CON (p=0.032) and improved the Nrf2 signaling response in both young and older (p<0.05). The degree of restoration in Nrf2 signaling response was related to the degree of change in basal Nrf2 (p=0.039), which was driven by older adults (p=0.014). Lower basal nuclear Nrf2 levels were associated with changes seen in AET responses for Nrf2 and GCLC protein, as well as NQO1 and GCLC mRNA. Together these data demonstrate that exercise training improves Nrf2 signaling and downstream gene expression and that lower basal Nrf2 levels are associated with a more dynamic acute response. Our results provide evidence that the impaired Nrf2 signaling in sedentary older adults can be restored to a degree with moderate exercise training, albeit not to the level seen in young.
Keywords: Redox Homeostasis, Exercise, Aging, Nrf2 Signaling, NQO1, GCLC
Graphical Abstract
1. Introduction
Aging is associated with reductions in physiological function and overall decreased stress resilience [1–3]. This contributes to an increased risk for developing non-communicable diseases including cardiovascular disease, diabetes, cancer and dementia [4]. One major thread connecting these diseases is dysregulated redox homeostasis due to oxidative stress imbalances [5–8]. The redox stress hypothesis of aging highlights the increasingly oxidative milieu in older organisms contributing to the lack of ability to respond to a redox stressor [9]. One of the predictions that can be made from this hypothesis is the declining ability of the cell or tissue to respond in a dynamic manner to redox stressors such as acute exercise or ischemia/reperfusion [10–12]. Cells accomplish appropriate responses to redox stressors primarily through redox stress response proteins like the inducible transcription factor nuclear factor erythroid-2-related factor 2 (Nrf2). Nrf2 is responsible for activating a host of gene targets including antioxidant, metabolic, and xenobiotic gene products. Under unstimulated conditions, Nrf2 is bound to its negative regulator and redox sensor, Keap1, in the cytosol. Oxidative or electrophilic stress activates Nrf2 by modifying Keap1 cysteine residues preventing Nrf2 degradation, increasing Nrf2 nuclear accumulation, and leading to increased mRNA transcripts of downstream targets [13–16]. The ability of a cell or organism to mount a successful response to redox stressors is dependent on an intact Nrf2 system to provide dynamic adjustments to the redox environment [17–19].
Loss of responsiveness in the Keap1-Nrf2 signaling system is a characteristic of older organisms and cells in response to various redox stressors [20]. Bronchial epithelial cells derived from older humans showed impaired Nrf2 responses when treated with the phytonutrient sulforaphane, compared to cells from young donors [21]. Similarly, impairments were found in redox sensitive cell signaling pathways, chaperone protein responses, and ability to degrade oxidatively damaged proteins via the proteasome in human fibroblasts and C. elegans when challenged with several different oxidative stress treatments [22]. These data indicate that the redox stress response is impaired in aging organisms independent of the type of redox stress.
Regular exercise is one of the most successful interventions to prevent or delay age-associated diseases. Aerobic (non-damaging) exercise induces a series of biochemical signaling cascades that result in changes in cell physiology, many of which are mediated by redox mechanisms [23–26]. Transient redox stress elicited by acute exercise increases Nrf2 activation in young animals and humans, thus improving cellular resistance to subsequent redox stressors [10, 27–29]. However, animal studies demonstrate that Nrf2 activation in response to acute exercise is impaired with aging [30], and we have previously shown that older men have an impaired Nrf2 signaling response to an acute exercise bout [10]. Taken together, these data highlight an important problem that has yet to be addressed in aging humans: the delineation between acute responses and adaptations to regular exercise in redox stress response systems. The purpose of this randomized controlled trial was to investigate whether an eight-week exercise training intervention could improve or reverse the age-related decline in redox stress response signaling in older men and women, with young adults serving as additional controls. This study tested the hypothesis that the divergent pattern of responses to acute exercise seen between age groups would be attenuated after exercise training, due to an improved response in older individuals.
2. Methods
2.1. Subjects
Twenty-six young (18–28y) and 29 older (60–77y) men and women from the surrounding community and campus completed screening for study participation. Subjects were eligible to participate if they were currently inactive as defined by no regular exercise for at least 6 months prior to study admission, as per self-report. Subjects were generally healthy, not overly obese (BMI ≤ 33.0 kg/m2), non-smokers, and not taking antioxidant supplements in excess of a multi-vitamin. Multivitamins were not excluded because the dosages of antioxidants are within the range of normal dietary intake, and we are unaware of any confounding effects in redox measures at moderate doses. Any condition that would contraindicate maximal exercise testing or participation in exercise training including clinically significant electrocardiogram (EKG) abnormalities at rest or during the maximal exercise test, elevated blood pressure at rest or musculoskeletal problems excluded subjects from participating in the study. Women who were taking estrogen replacement therapy or birth control were excluded from participation as research has previously shown that estrogen has a significant effect on markers of oxidative stress [12]. All participants signed a written informed consent approved by the Northern Arizona University Institutional Review Board.
2.2. Study Design
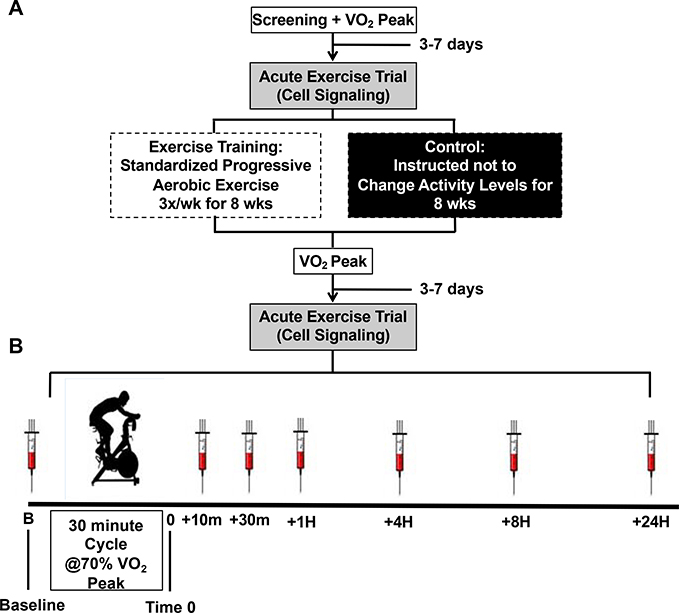
The study was an 8-week randomized controlled trial where young and older individuals were randomly assigned to either an exercise training intervention (ET) or a non-exercise control group (CON). Subjects completed pre-screening over the phone to determine eligibility for study participation. If there were no obvious exclusions, the subject was scheduled for a screening visit in the lab followed by a maximal graded exercise test on a cycle ergometer to determine baseline aerobic capacity (VO2 peak). In a separate visit subjects completed an acute exercise trial (30 minutes of cycling at 70% VO2 peak) to measure redox signaling capacity responses from blood samples taken at baseline and six additional time points as described below (see figure 1). After baseline testing was complete, subjects were randomized to ET or CON groups. Subjects randomized to CON were asked not to change their activity levels during the study and were offered the opportunity to receive the supervised exercise training program after completing the control arm of the study as a way to minimize attrition. Subjects randomized to ET performed supervised aerobic exercise training three times per week for eight weeks (24 sessions). After the 8 weeks of either ET or CON, subjects returned to the lab and completed the same testing as performed before the intervention. To control for any potential confounding effects of diet on the acute exercise trial, subjects completed a 2-day food log prior to the baseline test and then were asked to consume the same diet for the two days prior to the post-intervention trial. Study design is shown in Figure 1.
Figure 1. Study design.
(A) Subjects were randomized to the eight-week exercise training intervention (ET) or non-exercise control (CON). VO2 peak testing and acute exercise trial were completed pre- and post-intervention. (B) Acute Exercise Trial: After a baseline blood draw, subjects cycled on a stationary cycle ergometer for 30 minutes at 70% VO2 peak. Following the acute exercise trial (Time 0), blood was drawn at 10-min, 30-min, 1-hr, 4-hrs, 8-hrs and 24-hrs post-exercise. For time-course comparisons all time points were normalized to the individuals’ baseline blood draw. Acute exercise trial time course responses were tested before and after the intervention.
2.3. Screening visit
After participants signed the informed consent they completed a health history questionnaire and the modified Historical Leisure Physical Activity questionnaire for assessment of lifetime physical activity [31, 32]. Height, weight, waist circumference and resting blood pressure were measured and a 12-lead supine resting EKG was obtained to rule out any cardiac abnormalities that would preclude participants from performing a maximal aerobic capacity test. Body composition was measured using seven-site skinfold using a Lange skinfold caliper. Body density was calculated using normalized equations for men and women specified by age. The body fat percent equation by Siri et al. was used for final body fat calculations [33]. All skinfold measures were done by the same researcher to prevent intertester variability.
2.4. Peak Oxygen Consumption Test
VO2 peak was measured with a graded exercise test (GXT) performed on a cycle ergometer as previously described [34]. The starting workload was selected based on the predicted maximal workload for each individual and was increased every minute until volitional exhaustion. Participants were instructed to maintain a pedaling rate of 60–70 rpm throughout the test. Oxygen uptake was measured via indirect calorimetry using a metabolic measurement cart (CareFusion, Yorba Linda, CA). Heart function was monitored with continuous 12-lead EKG. VO2 peak was considered achieved if two of the following three criteria were met: 1) a plateau in VO2 with an increase in workload, 2) a respiratory exchange ratio (RER) ≥ 1.10, or 3) heart rate within 10 beats of the age-predicted maximal heart rate [35]. Standard contraindications to exercise testing, as well as termination criteria, outlined by the American College of Sports Medicine were followed at all times [36].
2.5. Exercise Intervention
Subjects randomized to the exercise intervention performed supervised aerobic exercise training three times per week for eight weeks (24 sessions in total) at the NAU Student Recreation Center. The intervention was progressive in duration and intensity and included a mix of moderate intensity continuous training and high intensity interval training. Every session, however, was standardized so that each subject performed the same duration and relative intensity at a given session. Intensity was monitored by heart rate monitors (Polar, Inc., Finland) and Rate of Perceived Exertion [37]. Relative intensity was normalized to the individual’s maximal heart rate measured during the maximal exercise test. The intervention was progressive in duration and intensity and included a mix of moderate intensity continuous training and high intensity interval training. Durations started with 30-minute constant workload sessions, and progressed to 50-minute constant workload by the end of the intervention, while also progressing the intensity through increasing steady state heart rate range each week. High intensity interval sessions started modestly and progressed in intensity and duration each week. A mix of work:rest ratios were used to provide variation for subjects engagement as well as ensuring adequate range of intensity / duration combinations that meet ACSM weekly exercise prescription recommendations [38]. Target RPE and HR range were provided for each individual every exercise session.
2.6. Acute Exercise Trial
Subjects reported to the laboratory pre- and post-intervention to complete the acute exercise trial. The trial started between 6:00 and 9:00 am and subjects were instructed to refrain from any exercise or physical exertion for 48-hours prior. An intravenous (IV) catheter was inserted into the antecubital vein and a baseline blood sample was collected. The subject then performed the acute exercise trial consisting of 30-minutes of cycling at 70% VO2 peak, maintaining a pedaling rate of 60–70 rpm throughout. We have previously shown that this protocol elicits Nrf2 activation, as well as significant increase in antioxidant enzyme activity [10, 29]. Oxygen consumption and heart rate were measured throughout the trial using the same system as described above. After completion of the exercise, blood was drawn again at the following time points: 10-min, 30-min, 1-hr, 4-hrs, 8-hrs, and 24-hrs. The blood was drawn via the IV catheter through the 1-hr time point and thereafter with venipuncture using a 21-G butterfly needle (Becton Dickinson, Franklin Lakes, NJ). Subjects were allowed to leave the lab after the +1-hour blood draw and return for the last three draws. The acute exercise session was designed as a physiological stimulus to induce signaling cascades in the redox stress response system that are associated with physiological adaptations. We have previously shown that Nrf2 content does not change over similar sampling time points without exercise [11]. The intensity of the acute exercise session was normalized to the individuals VO2 peak to standardize the stimulus regardless of differences in maximal aerobic capacity in order to make valid comparisons in molecular measurements across age groups.
2.7. PBMC Isolation and Cell Counting
Whole blood was collected into EDTA vacutainers to prevent coagulation, diluted 1:1 with phosphate buffered saline (PBS) and layered onto Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden). Samples were spun at 900xg for 30 minutes at room temperature. Following centrifugation, the lymphocyte rich layer was collected and washed twice in PBS with phosphatase inhibitors (Halt Phosphatase Cocktail, ThermoFisher, Waltham, MA) at 400xg for 10 mins at 4°C. The supernatant was discarded, and the cell pellet was re-suspended in 1.5 mL second wash buffer. Cells were counted using Countess Automated Cell Counter (Invitrogen, Carlsbad, CA). Aliquots of 2×106 PBMC cell lysate were suspended with 100 μL of RNAlater (Ambion Biotechnology, Austin, TX) and stored at −80°C to preserve RNA stability until gene expression analyses. Additional volume of 1×106 and 3×106 cells per mL sample were aliquoted for whole cell and nuclear fractionation, respectively, for western blot measures.
2.8. Cell Fractionation and Preparation for Western Blotting
Cells were fractionated into cytosolic and nuclear fractions using a modified version of the REAP protocol [39]. Briefly, 3×106 cells were lysed in the presence of phosphatase and protease inhibitors in 0.1% Triton PBS solution by pipet mixing five times followed by a 10 second pop-spin in a microcentrifuge. Supernatant was aliquoted as the cytosolic fraction and mixed with 4x Laemmli sample buffer in a 3:1 ratio. The pellet was resuspended in 1000μL 0.1% Triton PBS solution followed by a 10 second pop-spin. Following centrifugation, the supernatant was discarded, and the nuclear fraction was resuspended in 1x Laemmli sample buffer. For whole cell lysates, 1×106 cells were loaded into microcentrifuge tubes and lysed using 0.1% Triton PBS and protease-phosphatase inhibitor cocktails, followed by mixing with 4X Laemmli sample buffer. Nuclear fractions and whole cell lysates were sonicated by microprobe (Sonifier 150, Branson Ultrasonics, Danby, Connecticut) on ice at level 2, three times for 5 seconds, interspersed by two minutes of cooling on ice. All samples were heated at 100°C for 5 minutes followed immediately by freezing at −80°C for storage until analysis.
2.9. Western Blot Analyses
Samples were separated by SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. Nuclear fractions were incubated with rabbit polyclonal primary antibody for Nrf2 (H-300, Santa Cruz Biotechnology, Dallas TX, 1:500), while the whole cell fraction was incubated with GCLC primary antibody (EP13475, Abcam, Cambridge MA, 1:1000), followed by incubation with their respective anti-rabbit and anti-mouse secondary antibodies (925–32211, and 926–68070, Li-Cor Biosciences, Lincoln NE,1:20,000). Mouse monoclonal antibodies were used for internal control proteins β-actin and Lamin-B1 (sc-4778, and sc-365214, Santa Cruz Biotechnology, Dallas TX, 1:1000). β-actin and Lamin-B1 served as loading controls in whole cell and nuclear fractions, respectively. All samples were multiplexed by co-incubation with rabbit and mouse monoclonal primary antibodies respectively, followed by co-incubation with anti-rabbit and anti-mouse secondary antibodies designed to appear in the 800 and 700 channels respectively. Membranes were imaged using Li-Cor Odyssey Fc Infrared Imaging System (Li-Cor Biosciences, Lincoln NE). Band intensity was measured by densitometry using Image Studio Lite (Li-Cor Biosciences, Lincoln NE) and changes in protein levels were determined by comparing band intensity from post-intervention samples to levels from pre-intervention samples. For all acute exercise trial analyses, proteins of interest were normalized to 1 at baseline within each individual to analyze patterns and magnitudes of responses by age and training group. Basal protein expression was compared across age and training group by normalizing to internal control proteins and expressed as a ratio in arbitrary units.
2.10. RT-qPCR – Reverse transcriptase quantitative polymerase chain reaction
RNA was extracted using the Qiagen RNeasy Plus Mini Kit (Qiagen, Gaithersberg, MD) following the manufacturers’ instructions. Briefly, Qiagen RNA extraction kits were used to isolate purified RNA from participants at baseline, 1-hr, 4-hrs, 8-hrs, and 24-hrs after completion of the acute exercise trial. RNA quality was assessed with a NanoDrop (ThermoFisher), using RNA amount (ng/μL) and purity (A260/280). Once an acceptable RNA quantity was measured the RNA was converted into cDNA via iScript cDNA conversion kit (BioRad, Hercules, CA). RT-qPCR utilized paired primers for NADPH Quinone Oxidoreductase 1 (NQO1), Heme Oxygenase 1 (HO1), and Glutamate Cysteine Ligase Catalytic subunit (GCLC) with Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) as an internal reference gene for normalization. The assays were run in triplicate on a CFX96-Touch (Bio-Rad, Hercules, CA) and each run included negative controls, and non-template controls. Differences in quantification cycles (Cq) were recorded after adjusting for the amount of mRNA in a sample and normalized to the internal reference gene (GAPDH). GAPDH was selected as the housekeeping gene as previous studies have shown it to be a reliable, stably expressed gene in response to aerobic exercise in humans [40, 41]. Experimental design, quality control, and minimum information for reporting qPCR steps from the MIQE guidelines were followed [42]. The gene-specific primer pairs are outlined in Table 1.
Table 1.
Forward and reverse primers for RT – qPCR
| Primers | Forward 5’ to 3’ | Reverse 5’ to 3’ |
|---|---|---|
| NQO1 | GGATTGGACCGAGCTGGAA | AATTGCAGTGAAGATGAAGGCAAC |
| HO-1 | AAGAGGCCAAGACTGCGTTC | GGTGTCATGGGTCAGCAGC |
| GCLC | GCTGTCTTGCAGGGAATGTT | ACACACCTTCCTTCCCATTG |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
2.11. Statistical Analyses
Subject characteristics and baseline data were analyzed by 2-way ANOVA (Age x Intervention Group). Significant differences in subject characteristics and baseline values were added as covariates to the statistical models to control for any confounding variables. Each marker measured for gene expression, and protein content was analyzed across time points (repeated measures), as well as between age groups, and by sex. Baseline acute exercise responses for gene expression data were analyzed using (2 × 5) repeated measures ANOVA (Age by Time) with significant differences in baseline anthropometric data placed in the model as covariates (sex, body mass, BMI, waist circumference, body fat percent). Similar analyses were run for protein expression responses but using 2 × 7 repeated measures ANOVA (Age x Time). All analyses on the effects of training or control intervention were 2 × 2 × 5 (training group x intervention time x sampling time point for gene expression) or 2 × 2 × 7 (training group x intervention time x sampling time point for protein expression) repeated measures ANOVA with covariates age, sex, and any other significantly different physical or physiological measurements entered into the model. Statistical significance was set at p < 0.05 and the analyses were performed using statistical programs SPSS and R. Data are reported as mean ± SEM unless otherwise noted.
3. Results
Fifty-five subjects were screened and of those 9 were disqualified or lost prior to pre-testing (VO2 max too high n=1, declined to participate n=5, unable to contact n=3). A total of 46 individuals completed pre-testing and started the intervention. Two young individuals dropped out citing time commitment, two subjects were withdrawn due to issues with venipuncture (one young, one older), and two older individuals were withdrawn by study personnel due to medical issues (blood pressure dysregulation, arrhythmia at post-testing). All six subjects that were withdrawn during testing or intervention were in the ET group, there were no dropouts in the CON group. The data presented are from 40 individuals who completed the intervention; 21 young and 19 older, with equal representation between sexes. Baseline subject characteristics are shown in Table 2. As expected, there were age-related differences at baseline where the young individuals had significantly lower body mass, body mass index (BMI), and body fat percentage (BF%), as well as smaller waist circumference. Similarly, age-related differences in the response to maximal exercise testing showed expected results where young individuals had significantly higher VO2 peak values compared to older adults (38.5 vs. 25.8 mL/kg/min, respectively, p<0.001), higher maximal heartrate (189 vs 148 bpm, respectively, p<0.001), and higher maximal workload (210 vs. 155 watts, respectively, p<0.001). Importantly, none of the baseline characteristics differed between those randomized to the ET intervention versus those randomized to the control group (see Table 2).
Table 2.
Baseline physical characteristics
| Physical Characteristics & Exercise Testing | Young | Older | p Value | ||||
|---|---|---|---|---|---|---|---|
| CON (n=10) | ET (n=11) | CON (n=9) | ET (n=10) | Age | Training Grp | Age x Training Grp | |
| Men / Women Ratio (n) | 5 / 5 | 5 / 6 | 5 / 4 | 5 / 5 | - | - | - |
| Age | 21.9 (± 3) | 21.6 (± 3) | 65.1 (± 3) | 68.3 (± 5) | <0.001 | NS | NS |
| Height(cm) | 177 (± 11) | 171 (± 10) | 172 (± 13) | 170 (± 8.9) | NS | NS | NS |
| Mass(Kg) | 68.3 (± 12.3) | 66.0 (± 8.4) | 87.3 (± 14.8) | 76.8 (± 16.5) | 0.002 | NS | NS |
| BMI (Kg/m^2) | 21.6 (± 2.5) | 22.5 (± 3.9) | 29.3 (± 2.3) | 26.3 (± 3.8) | <0.001 | NS | NS |
| Body Fat % | 15 (± 7.5) | 17 (± 10.3) | 29.9 (± 3.9) | 25.2 (± 4.2) | <0.001 | NS | NS |
| Waist Circ. (cm) | 76.7 (± 6.7) | 76.6 (± 6.4) | 102 (± 10.2) | 93.5 (± 11) | <0.001 | NS | NS |
| HLPA | 2.83 (± 0.3) | 2.95 (± 0.6) | 2.86 (± 0.9) | 2.36 (± 0.47) | NS | NS | NS |
|
VO2 Peak | |||||||
| Absolute VO2 (mL/min) | 2621 (± 724) | 2563 (± 554) | 2161 (± 570) | 2067 (± 510) | 0.01 | NS | NS |
| Relative VO2 (mL/Kg/min) | 38.0 (± 6.6) | 38.9 (± 7.4) | 24.6 (± 3.5) | 26.9 (± 3.1) | <0.001 | NS | NS |
| Max Heart Rate (bpm) | 189 (± 9) | 188 (± 8) | 145 (± 21) | 150 (± 18) | <0.001 | NS | NS |
| RER | 1.21 (± 0.06) | 1.28 (± 0.1) | 1.31 (± 0.05) | 1.28 (± 0.08) | 0.04 | NS | NS |
| Workload (Watts) | 212 (± 62) | 208 (± 42) | 158 (± 39) | 153 (± 41) | 0.001 | NS | NS |
|
Acute Exercise Trial | |||||||
| % of VO2 Peak | 69.0 (± 2.4) | 70.5 (± 2.4) | 71.4 (± 3.9) | 71 (± 2.7) | NS | NS | NS |
| % of HR Max | 81 (± 6) | 81 (± 6) | 79 (± 8) | 82 (± 7) | NS | NS | NS |
BMI – Body Mass Index, HLPA – Historical Leisure Physical Activity Questionnaire, RER – Respiratory Exchange Ratio. Data are shown as mean (± SD)
The goal for the acute exercise trial was an average intensity of 70% VO2 peak. The attained mean VO2 peak for the acute exercise trial across the whole cohort was 70.5% of VO2 peak. Young individuals averaged 69.8% of VO2 peak while older individuals averaged 71.2% of VO2 peak (Table 2). The exercise intensity did not differ statistically between young and older individuals or between ET and CON.
3.1. Effects of the exercise intervention on cardiorespiratory fitness
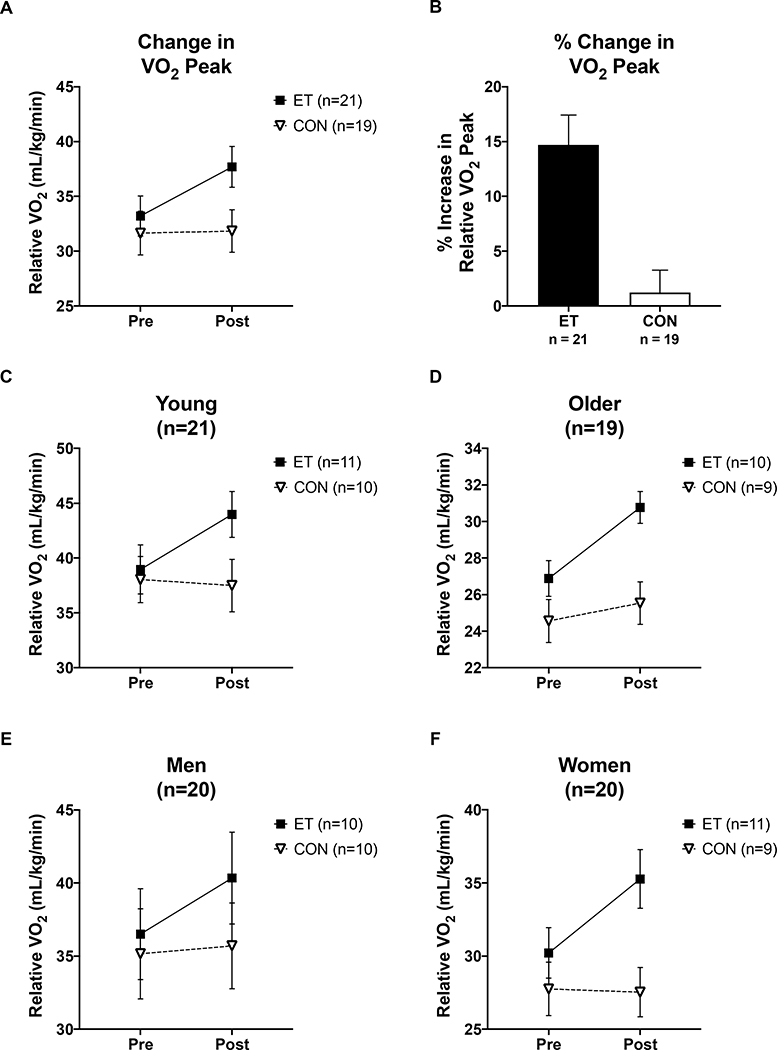
Figure 2 shows the changes in peak oxygen consumption in response to the ET or CON intervention. Aerobic capacity increased significantly in ET (mean change: 4.5 mL/kg/min, +14.7%) and did not change in CON (mean change: 0.2 mL/kg/min, +1%), indicated by main effect of group, p = 0.004, and intervention by group interaction, p = 0.013. When including covariates in the model, main effects of training group (ET vs CON), age, sex, and change in BMI (ΔBMI) were also associated with VO2 peak measurements (RM ANCOVA, ET vs CON p < 0.001, Age p < 0.001, Sex p < 0.001, ΔBMI p = 0.022). Within the ET group, there were no differences in the magnitude of improvement across age or sex (Repeated Measures ANCOVA ΔVO2 x Age p =0.405 and ΔVO2 x Sex, p = 0.4, Figure 2C-F) indicating that the individuals who underwent the ET intervention responded to the same degree regardless of age or sex.
Figure 2. VO2 peak changes in response to the intervention.
(A&B) Relative VO2 peak (ml/kg/min) improved in trained subjects but not control subjects, regardless of age or sex (Intervention x Training group interaction p = 0.013). (C-F) Changes in VO2 peak by age and sex. Within the exercise trained group, there were no differences in the degree of fitness improvement by age or sex indicating the exercise training intervention had the same effect across age and sex (Compare all ET group changes in panels C-F). (Δ indicates the difference between Pre and Post Intervention for a given measurement). Black squares indicate ET group, open triangles indicate CON group. Data shown as Mean ± SEM.
There were no significant changes in resting blood pressure, maximal heart rate, and RER in either ET or CON. There were no significant changes in overall body mass in ET or CON, but there was a significant decrease in body fat mass, and waist circumference in ET, with no change in CON (one-way ANOVA ΔBody Fat %, p = 0.023, Δwaist circumference, p = 0.001).
3.2. Exercise-induced Nrf2 signaling: Pre-Intervention
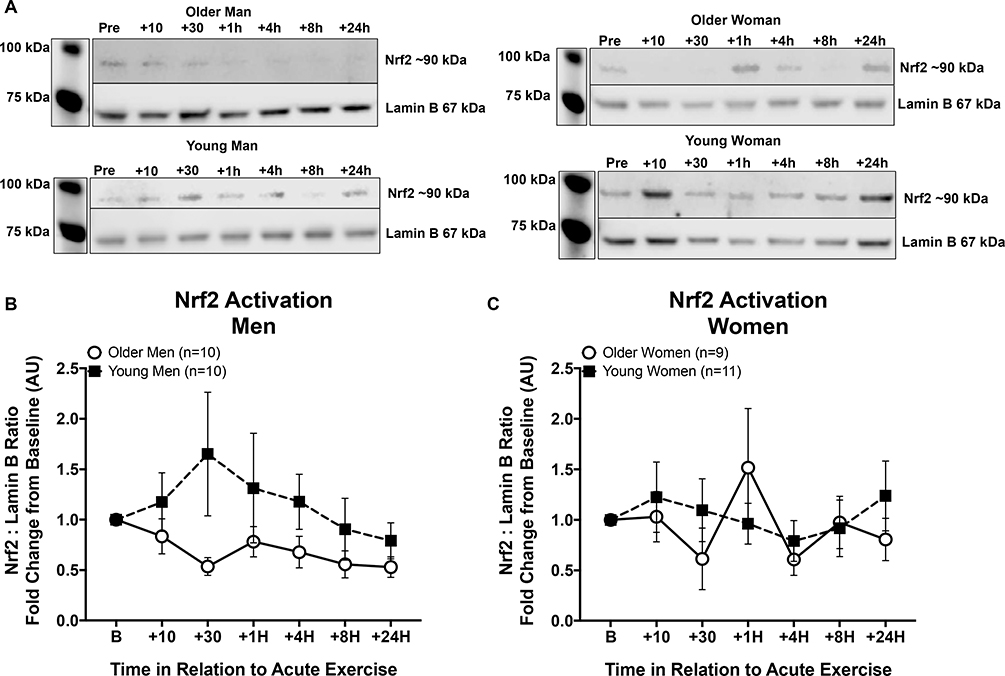
Age-group differences in Nrf2 nuclear localization in response to the acute exercise trial were not significant when analyzed without covariates (RM ANOVA, main effect of age, p = 0.118) but approached significance when entering measures of fitness and body composition into the model (RM ANCOVA, main effect of age, p = 0.05). When the data were examined further, unexpected sex differences emerged. Figure 3 shows the pre-intervention responses in men (A) and women (B). Young men showed an increase in Nrf2 nuclear localization with a peak at thirty minutes after a single acute exercise session, while older men did not respond (within sex main effect of age, p < 0.07, with or without covariates), in agreement with our earlier findings in men [10]. In contrast, there were no significant differences between young and older women in the responses (within sex main effect of age, p = 0.714, with covariates p = 0.279).
Figure 3. Nrf2 signaling responses to a single acute exercise session by age and sex.
All responses are normalized to the individual’s baseline draw (B) and set equal to 1 in order to control for any differences in absolute expression levels across individuals. (A) Representative Nrf2 western blots with Lamin B used as internal control, for an older man, older woman, young man, and young woman pre-Intervention. (B) Nrf2 signaling responses in young and older men. Black squares indicate response in young men and open circles indicate response in older men across time. (C) Young women (black squares) and older women (open circles) showed minimal Nrf2 signaling responsiveness to an acute exercise bout and were not significantly different from each other. Analyzed together with fitness and body composition covariates in the model, there was a significant main effect of age with young individuals showing a higher response in Nrf2 activation than older individuals (RM ANCOVA Age p = 0.05). Data shown as Mean ± SEM.
3.3. Molecular Signaling Responses to Acute Exercise: Effect of Exercise Training
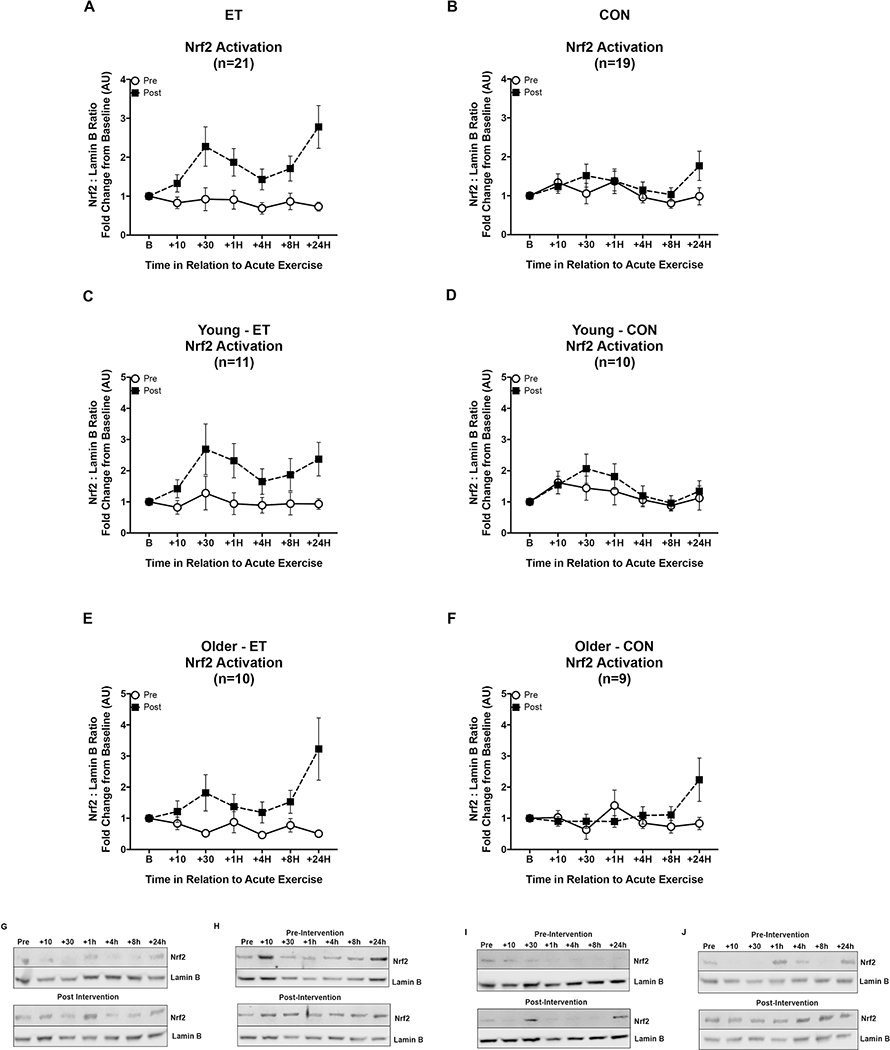
Nrf2 signaling responses after exercise training were significantly higher in ET whereas the response did not change in CON (intervention by group interaction p = 0.012, effect of age p = 0.014, and effect of ET versus CON, p = 0.034, Figure 4A & B). This response was partially explained by the degree of improvement of VO2 peak in the training group, (p = 0.019), the change in nuclear baseline levels of Nrf2 (p = 0.039), and the interaction of time by age (p = 0.007). Both young and older subjects significantly improved their Nrf2 signaling responses post-ET, however, when comparing only the ET group pre-post intervention the main effect of age was greater than the age differences at baseline (p = 0.04, Figure 4 C versus E), while the inactive controls did not show any significant change from pre-intervention (Figure 4 D&F).
Figure 4. Nrf2 signaling responses to acute exercise before and after exercise training or inactive control intervention by age.
Open circles indicate Nrf2 activation pre-intervention, and black squares indicate Nrf2 activation post-intervention. Panels A, C, & E are ET subjects while panels B, D, & F are CON subjects. (A&B) Eight weeks of aerobic exercise training increased the Nrf2 signaling response to an acute exercise bout in the whole cohort, while the Nrf2 signaling in the control group did not change. (C&E) The increase in Nrf2 signaling after training was similar in pattern for young and older adults, although young improved their responses to a greater degree than older ET subjects (main effect of age, p = 0.014). (D&F) Nrf2 signaling did not change from pre-intervention in older or young control groups. Representative western blots for (G) Young-ET, (H) Young-CON, (I) Older-ET, and (J) Older-CON. Data shown as Mean ± SEM.
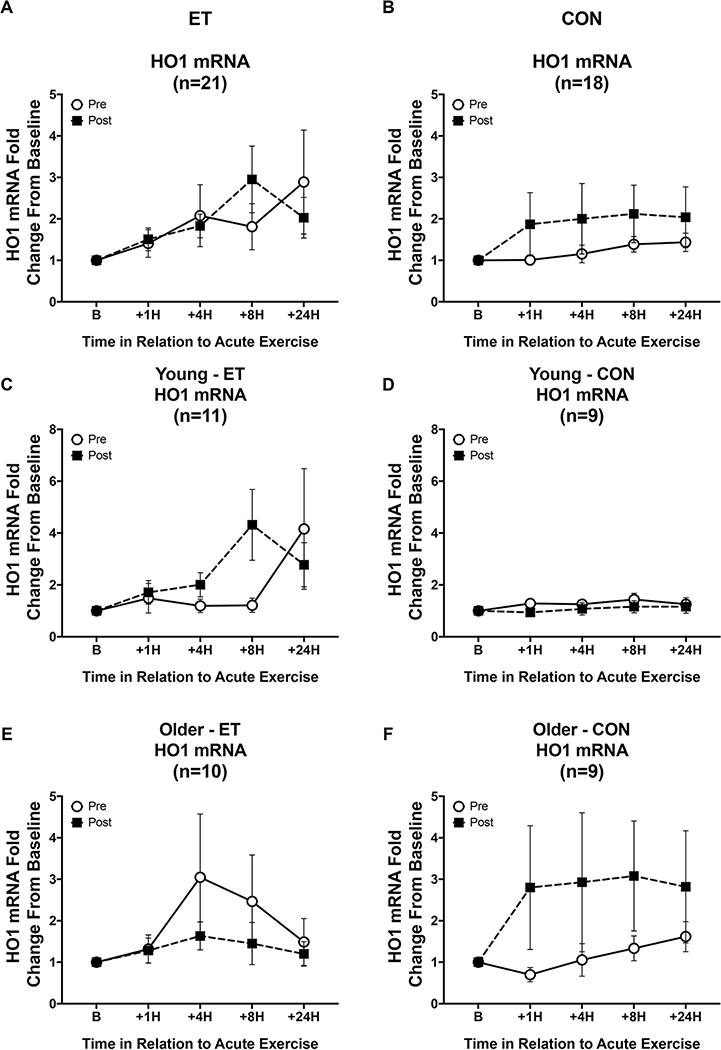
The acute exercise trial induced a significant increase in HO1 mRNA in the cohort as a whole (Figure 5, main effect of time, p = 0.039). However, there were no significant differences between the groups or in response to the intervention. Additionally, there were no effects of age, sex, or any other covariate on HO1 mRNA response.
Figure 5. HO1 mRNA signaling responses to an acute exercise bout before and after the intervention.
Open circles indicate HO1 mRNA responses pre-intervention, while black squares indicate responses post-intervention. (A&B) There was a significant response to an acute exercise bout across the whole cohort (p = 0.039), but there were no differences in HO1 mRNA responses after training or control intervention. (C-F) Additionally, there were no significant differences across age or sex. The pattern of responses differs across ET and CON within age groups, but these were not statistically significant. Data shown as Mean ± SEM.
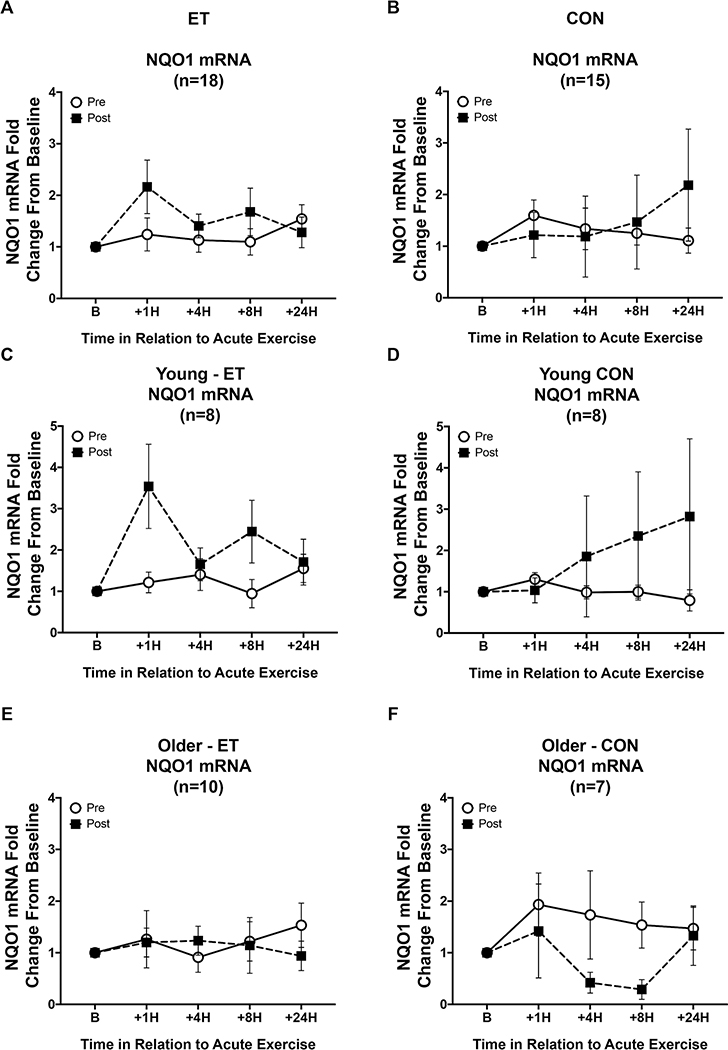
NQO1 mRNA response showed a significant interaction between intervention and age (p = 0.045, Figure 6), however when including the change in baseline Nrf2 expression (Δ Baseline Nrf2) the interaction was no longer significant. There was a significant interaction between the NQO1 mRNA response and measures of body composition and improvements in fitness (Time x ΔWaist circumference, p = 0.004, Time x Δ Body Fat %, p = 0.008, Time x ΔVO2 peak p = 0.017). There was a significant three-way interaction between intervention, time, and training group (Figure 6, p = 0.028). The large standard error in the young control group was due to the response of one individual post-intervention. Analyzing the data without this outlier did not change the results. Additionally, there were six individuals who did not show any NQO1 amplification, hence the lower number of subjects for this gene set than the others. Interestingly, each of the individuals who did not show NQO1 amplification were of Native American, Asian, or Middle Eastern ethnicity.
Figure 6. NQO1 mRNA signaling responses to an acute exercise bout before and after the intervention.
Open circles indicate NQO1 mRNA responses to the acute exercise trial pre-intervention, while black squares indicate responses post-intervention. (A&B) In the whole cohort, there was a slight increase in NQO1 mRNA response after training, which was dependent on baseline Nrf2 expression changes (Δ Baseline Nrf2), but no difference in response after control intervention. (C&E) This increase in NQO1 mRNA response after ET appears to be driven by younger individuals, not older. (D&F) Post-control intervention older individuals show a decreased response while younger controls show a delayed increase in NQO1 mRNA driven by one outlier in the post-intervention CON response. Panels A, C, & E are ET subjects, while B, D, & F are CON subjects. Data shown as Mean ± SEM.
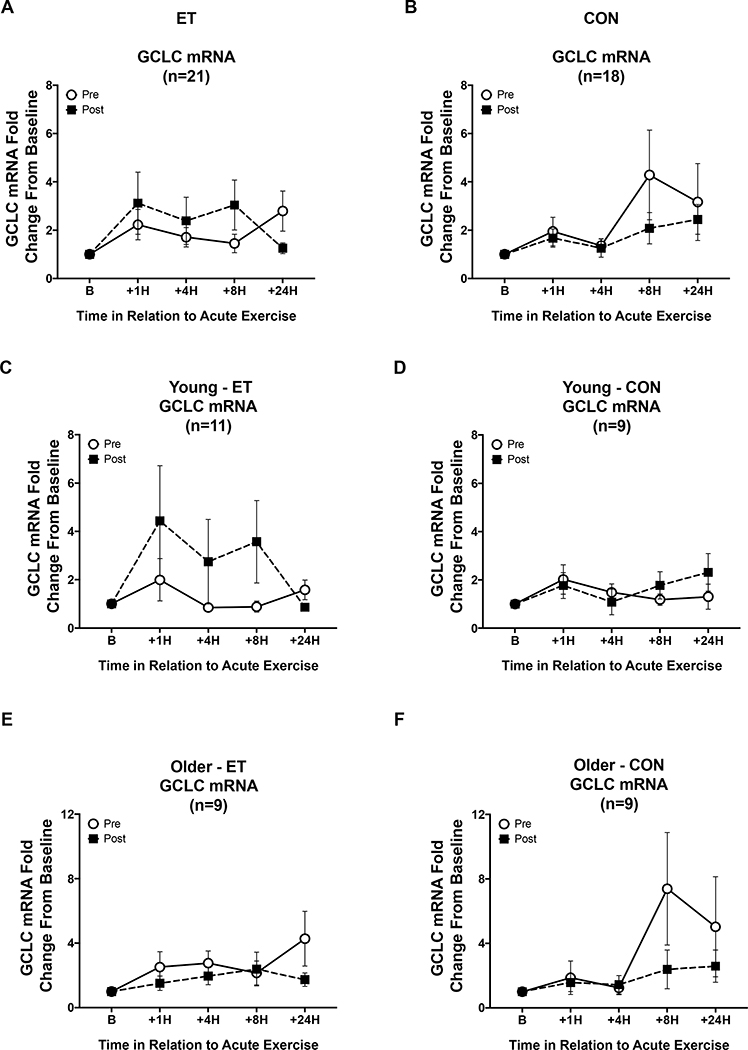
For changes in GCLC mRNA responses to acute exercise, there was a significant main effect of time (p = 0.005, Figure 7) There was also a three-way interaction between intervention by time by training group (p = 0.05, Figure 7).There was a significant interaction between the intervention and change in baseline nuclear Nrf2 protein content (Intervention x Δ Baseline Nrf2 p = 0.006). There was a significant interaction between the change in baseline Nrf2 levels and GCLC mRNA response to acute exercise (Time x Δ Baseline Nrf2 p < 0.001). There was also a significant three-way interaction between the intervention, GCLC response, and the change in baseline Nrf2 protein expression (Intervention x Time x Δ Baseline Nrf2 p < 0.001).
Figure 7. GCLC mRNA signaling responses to an acute exercise bout before and after the intervention.
Open circles are pre-intervention GCLC mRNA responses and black squares are post-intervention responses. Panels A, C, & E are ET subjects and B, D, & F are CON subjects. (A&B) Overall comparison of intervention, time, and training group responses to the acute exercise trial. There were significant interactions between intervention, time, and training group in GCLC mRNA response (p = 0.05) as well as an intervention by time by ΔBaseline GCLC protein interaction (p = 0.001). (C-F) Show acute exercise trial responses by age and training group. Data shown as Mean ± SEM.
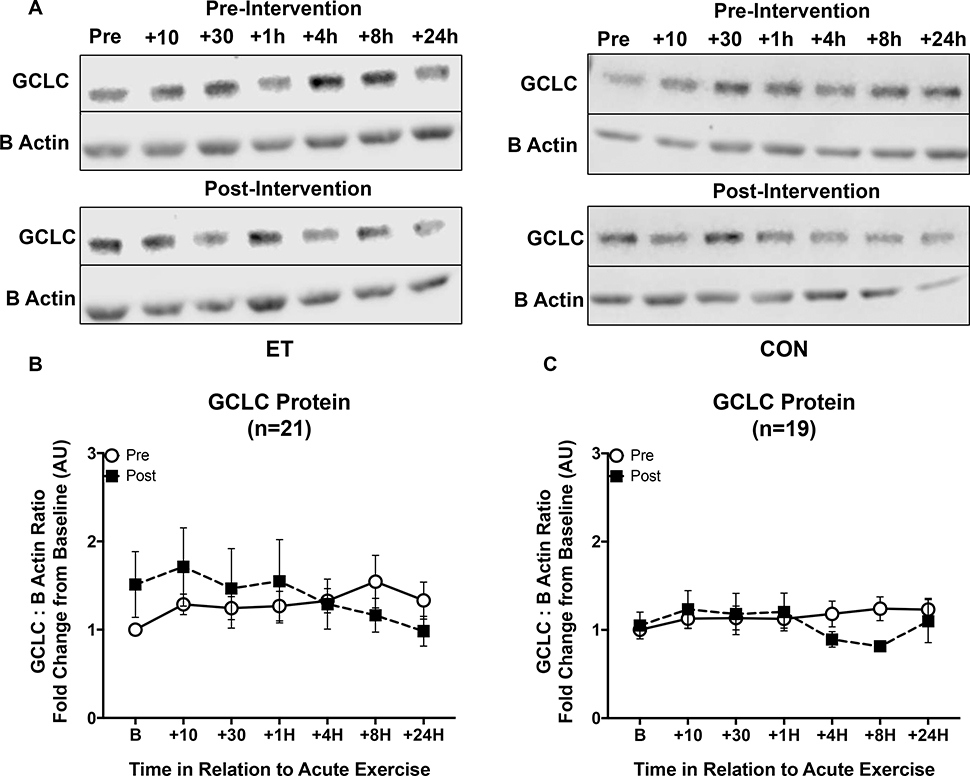
For GCLC protein responses there was a main effect of the change in peak VO2 (ΔVO2 peak) on GCLC protein responses to ET (p = 0.044). There was also an intervention by time interaction (p = 0.024), and a three-way interaction intervention by time by ΔVO2 (p = 0.031, Figure 8). There was a significant interaction between GCLC protein response and the change in baseline GCLC protein content (Time x Δ Baseline GCLC protein, p = 0.05). The new steady state protein concentrations after training were primarily being driven by men, although there were no significant differences in GCLC protein response by age or sex.
Figure 8. GCLC protein responses to an acute exercise bout before and after eight weeks of ET or CON.
Open circles indicate pre-intervention GCLC protein responses to acute exercise, while black squares indicate post-intervention responses. (A) Representative blots of GCLC with β-Actin used as an internal control for ET and CON groups pre- and post-intervention respectively. (B) GCLC shows an increase in the steady state concentrations after exercise training compared to pre-intervention in trained subjects. (C) GCLC protein responses were not different after the inactive control intervention. There was a significant interaction between the GCLC protein response and the change in baseline GCLC protein content (p = 0.016). There were no significant differences between young and older individuals or by sex in response to the training. Data shown as Mean ± SEM.
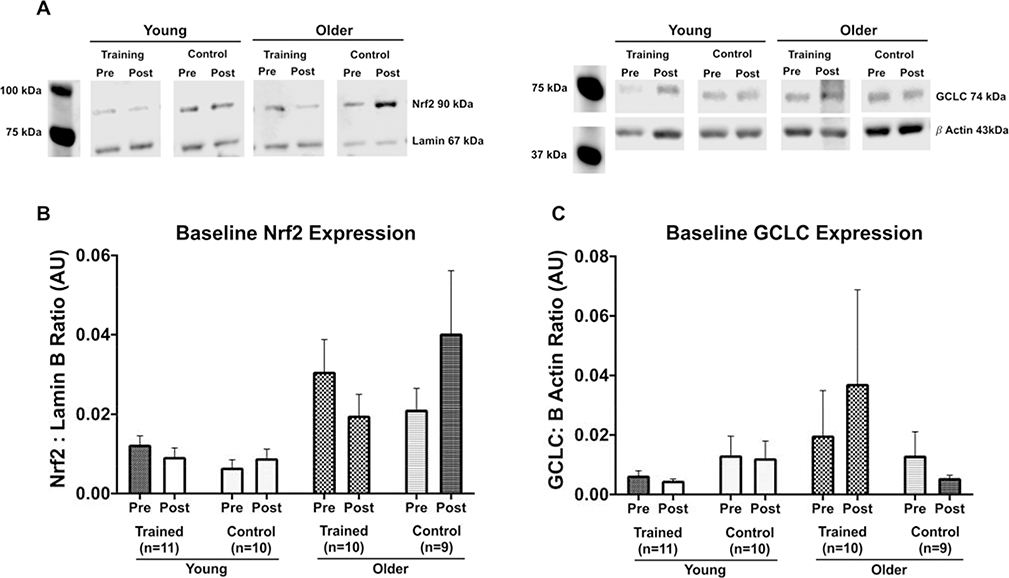
3.4. Aerobic Exercise Training Changes Basal Steady State Levels of Nrf2 but not GCLC
The Nrf2 and GCLC protein analysis for acute exercise responses was normalized to 1 for each of the individual’s pre-intervention baseline values in order to make comparisons across individuals. This does not allow for analysis of basal expression levels for each protein across age and sex. Therefore, to test whether there were basal expression differences in Nrf2 or GCLC across age, sex, or in response to the intervention, we analyzed each individual’s baseline samples pre and post intervention. There was a main effect of age on basal expression levels of Nrf2 before the intervention where older adults had significantly higher levels of Nrf2 at rest compared to young (independent sample t test, p = 0.004, Figure 9A). Basal Nrf2 levels decreased in older adults after the ET but increased in CON (Intervention by Training group interaction p = 0.034). Young individuals did not change significantly from pre to post intervention in either ET or CON groups. There was no statistically significant difference in in GCLC protein content across age or sex at pre-intervention (independent sample t test p = 0.428, Figure 9B). After exercise training GCLC protein was elevated at baseline driven by the increases shown in older adults, although this did not reach statistical significance (Figure 9B). There were no sex differences in response to exercise training or control intervention for basal GCLC protein expression.
Figure 9. Baseline expression of Nrf2 and GCLC before and after ET or CON.
(A) Representative blot images for young and older ET and CON individuals for Nrf2 and GCLC protein expression respectively. (B) Baseline Nrf2 protein expression was higher in older adults before the exercise intervention compared to young (independent sample t test, p = 0.004) but decreased with ET closer to young Nrf2 expression levels. Older controls showed the opposite pattern, increasing baseline Nrf2 expression after eight weeks of CON. Repeated measures ANCOVA shows a significant interaction between training group and pre-post intervention (p = 0.032). The majority of these differences are explained by age differences (p < 0.001) (C) There was no change in GCLC protein in young trained or control subjects. In older subjects there was no significant difference in basal GCLC protein after training or control interventions. Data shown as Mean ± SEM.
4. Discussion
Exercise is one of the most powerful tools available for improving health span [43–45]. Many of the beneficial effects rely on redox-dependent cell signaling mechanisms to induce exercise adaptations [46]. ROS and other electrophiles generated by exercise are critical signaling molecules that lead to improved physiological function and healthy adaptations [47–53]. However, we and others have shown that redox dependent activation of Nrf2 through acute exercise is impaired in aging populations [10, 27].
This investigation tested the hypothesis that exercise training (repeated stimulus of acute exercise) would restore Nrf2 signaling effects in older adults, with an additional comparison to the responses of young. To our knowledge, this is the first randomized control trial to measure responses to acute exercise as well as effects of exercise training on Nrf2 time course responses in young and older men and women. Notably, the pre-intervention results replicate previous findings from our lab which was the first study to show that Nrf2 signaling in response to acute exercise was impaired with age in humans [10]. The current data, which in contrast to our earlier study included both sexes, demonstrate an effect of age on Nrf2 activation in response to the acute exercise trial when accounting for baseline levels of body composition and fitness. However, this effect of age was no longer significantly different when including baseline levels of Nrf2 expression suggesting that the variation in Nrf2 responses to exercise are dependent on basal Nrf2 expression levels. Additionally, there were clearly divergent responses between men and women. These sex differences were not anticipated, and by design the study was not powered to detect sex differences once the data were parsed by age and training group.
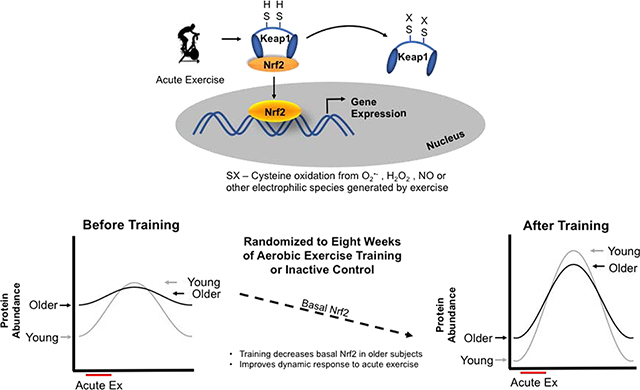
It is well established that exercise training changes the molecular signaling patterns that manifest from a single acute exercise bout, however this has never been tested in the Nrf2 signaling pathway and aging human populations. The decrease in basal Nrf2 expression observed in the older group after exercise training may seem counterintuitive. However, since Nrf2 is a stress response transcription factor, it would be expected to be lowly expressed under resting unstressed conditions. Elevated Nrf2 levels at rest in older adults suggests that there are higher levels of redox stress compared to young and is in line with findings in skeletal muscle in mice where older mice have higher activation of redox-sensitive transcription factors at rest but fail to increase the activation in response to an acute stressor [54]. Lowering the basal redox stress levels would therefore be advantageous. The decrease in baseline Nrf2 levels in older adults after training is an indicator that training reduces redox stress under resting conditions. This subsequently improved the signaling capacity to respond to stressful events like exercise with the appropriate magnitude and timing necessary to adapt to the stimulus similar to that observed in young. A recent investigation that compared individuals that were stratified based on their acute exercise oxidative stress response, measured as urinary F2-isoprostanes, demonstrated that higher oxidative stress responses to acute exercise resulted in greater performance adaptations to endurance training than individuals with lower oxidative stress response to acute exercise [55]. The study also found that the oxidative stress response was correlated to resting levels of oxidative stress. The same group more recently demonstrated that targeted antioxidant supplementation to restore a deficiency was beneficial for exercise performance, whereas supplementation in non-deficient individuals had no effect on exercise performance [56]. In agreement with our data, young and older mice subjected to exercise training showed decreases in Nrf2 content after training [57]. This general pattern of response in redox signaling systems has been hypothesized and predicted by others in the field [58], where accumulating oxidative stress in aging cells leads to an inability to respond further to a redox stressor. As far as we are aware this pattern of response has not been demonstrated in humans until now.
Nrf2 activation response to acute exercise improved significantly in response to the exercise intervention (Figure 4). The older individuals in the ET group showed significant improvement compared to pre intervention levels. Studies in mouse myocardium show similar improvements in Nrf2 signaling and redox status after exercise training [27]. Contrary to our hypothesis, there was greater improvement in Nrf2 signaling in young individuals compared to older in response to the training. Comparing the magnitude of peak improvement (+30-min time point) in young (+1.41-fold) versus older individuals (+1.30-fold), the relative improvements in Nrf2 signaling after ET were greater in young individuals than in older individuals (p = 0.04) suggesting that the improvements elicited from exercise training do not completely reverse biological aging. Similar differences have been found in lifelong trained versus untrained older adults [59]. Their data showed that redox signaling changes that occur with aging are not completely reversed even with lifelong training, again pointing to an underlying biological aging process independent of training status [59, 60]. Our results are in agreement with these findings, and show that redox signaling decline in older adults is an inherent biological aging process independent of training status. Whether the response can be further augmented with greater exercise volume (intensity, duration) or through synergistic effects between exercise and phytonutrient Nrf2 activators remains to be elucidated.
Both NQO1 and GCLC mRNA showed significant responses to the acute exercise bouts as well as age related differences in signaling post intervention. The three-way interaction of intervention, time, and training group for NQO1 mRNA response indicates that only the ET group improved signaling responses after training. The change in basal Nrf2 levels explains the age-related differences in signaling post-ET. GCLC mRNA responded to the acute exercise bout and the interaction of intervention by time by training group indicates ET improved GCLC mRNA responses after training. Again, the responses to acute exercise after training were explained by the changes in baseline Nrf2 levels. Taken together these gene expression data support the conclusions drawn by the Nrf2 western blotting data.
While HO1 mRNA did respond to a single acute bout across the entire cohort, there were no age or sex differences (Figure 5). Furthermore, there were no significant effects of changing Nrf2 levels on HO1 mRNA responses to acute exercise before or after exercise training. Other literature has shown that HO1 is transcriptionally regulated by a number of other transcription factors [61, 62], therefore we speculate that Nrf2 has less of a contribution to HO1 responses to acute exercise in human PBMCs. The divergent responses pre-post intervention for older adults by training group (Figure 5, E & F) are puzzling. One possible explanation is that redox stress decreased with training, and the increase in Nrf2 signaling is maintaining higher steady state levels of mRNA, so it appears that the response to acute exercise did not change. Conversely, the older controls had lower amounts after the intervention and needed to respond to a greater degree in order to maintain redox homeostasis. However, these responses were not significantly different.
It is expected that time course changes in steady state proteins such as GCLC will likely take longer duration to show any significant accumulation. This was the rationale for performing the 4-, 8-, and 24-hour blood draws and the notion that any increase in protein content would be seen after peaks in gene expression occurred. Changes in steady state protein concentration is dependent on multiple inputs including protein turnover, localization, and post-translational modifications, providing a healthy debate over just how important mRNA accretion is for protein accumulation in response to exercise [63]. However, the peak of GCLC protein accumulation occurred at 8 hours after the acute exercise bout at pre-intervention, which is in line with the time course of expected changes where Nrf2 peaked at 30 minutes, and GCLC mRNA peaked at 1 hour for young and 8 hours in older subjects. Together these time course data provide rationale for using mRNA as an outcome measure, particularly when the primary outcome is transcription factor activity. The GCLC protein changes post-ET were directly related to the degree of improvement in VO2 peak. Additionally, the degree of responsiveness to an acute exercise bout after ET was dependent on the degree of change in baseline GCLC protein.
Our overall findings suggest that exercise training reduces baseline levels of Nrf2 in order to allow the system to respond more robustly to subsequent redox stressors. The lack of responsiveness to stressors is a general phenomenon in aging populations that leads to overall impaired stress resilience. While older adults did improve Nrf2 nuclear localization in response to acute exercise after ET, this did not necessarily translate to improved mRNA accretion. The increases in mRNA levels in GCLC were modest in older adults, and the changes in baseline GCLC protein seem to be driven by older adults, but they were not significant. These non-significant changes could be due to sex differences confounding the results because the changes in GCLC levels after ET seemed to be primarily driven by older men. Transcriptomic approach may be better able to detect changes across the host of genes regulated by Nrf2.
The free radical theory of aging described by Denham Harman in the late 1950’s was and still is an influential theoretical framework in redox biology and aging fields [64, 65]. Although the macromolecular damage hypothesis has been discarded, it has been replaced with more refined and nuanced versions of the hypothesis [9, 26]. The redox stress hypothesis of aging and the cell signaling disruption theory of aging both posit that the observed increases in oxidative stress in aging do not necessarily lead to damaged macromolecules but impair redox homeostasis and the ability to elicit a molecular signaling response to restore redox homeostasis. Based on these hypotheses, one would predict that older cells would show attenuated redox signaling responses, such as the Keap1-Nrf2 signaling axis, to stimuli compared to younger cells. This was previously shown in an eloquent series of experiments by Meng et al. [22] who tested the redox stress response capacity hypothesis using five different redox stressors in a cell culture model and in C. Elegans. Older cells and organisms showed lower ROS production, impaired redox cell signaling, and impaired redox homeostasis in response to redox stressors compared to young [22]. Similarly to our human data, their results show that redox stress associated with aging leads to further impaired redox signaling responses resulting in a decreased ability to restore redox homeostasis.
Experimental manipulation of baseline oxidative stress has been shown to alter the response to acute exercise in young men [66]. The subjects underwent a 12-day intervention of either a pro-oxidant stimulus of passive smoking (elevated baseline) or an antioxidant stimulus of vitamin C supplementation (lowered baseline). The subsequent oxidative stress response to acute exercise was lower in the passive smoking group than in the group that had been treated with an antioxidant. It is now widely accepted that exercise-induced ROS signaling is key to long-term adaptations and therefore the impaired response in the passive smoking group would be considered mal-adaptive. Our results show the older individuals who underwent exercise training demonstrated lower baseline Nrf2 levels and augmented responses to acute exercise, which is consistent with the findings of Theodorou et al. [66].
Strengths and Limitations
The RCT design of this study along with the extremely tight data in the control groups strengthens the results and data interpretation. The adherence to the exercise intervention and low drop out (none in the control groups) is both a testament to the dedication of the participants and the study personnel. The main limitation of the study was that it was underpowered to adequately detect sex differences. Rather than limiting the study cohort to older individuals, we thought it was important to be able to compare the response to the exercise intervention to those seen in young and there were no available data indicating sex differences in the response. Recently published data from Drosophila show sexually dimorphic responses to oxidative stress [67]. Future studies should include an adequate subject number to analyze sex differences in redox signaling responses in humans. We make the assumption that higher baseline levels of Nrf2 pre-intervention were due to elevated or aberrant production of ROS or other electrophilic species, and that exercise training helped reduce baseline production of those reactive species. However, this is somewhat speculative since ROS or any reactive species adducts were not measured.
5. Conclusions
The major finding of the current investigation was that the observed age-related impairment in stimulated Nrf2 activation was improved through exercise training, demonstrating that the pathway retains plasticity to respond. The second novel finding was that basal nuclear Nrf2 levels were elevated in older adults, which may explain the inability to respond to an acute stimulus. The exercise intervention lowered the basal nuclear Nrf2 content and the pre-post change in the levels was a significant predictor of the improvement in the stimulated response. The effect of the Nrf2 activation on the downstream response was less robust in the older individuals and may have been affected by the unexpected sex differences. Future studies should add a transcriptomic approach because of the number of gene targets regulated by Nrf2. Furthermore, the age-related differences in signaling responses for both Nrf2 and downstream target genes NQO1 and GCLC after ET prevailed after the intervention, which suggests that despite the improvement in older adults, there remains an element of biological aging not affected by exercise training or that a greater stimulus is needed. Our findings support the redox stress hypothesis of aging and the decay of redox stress response capacity, and add additional evidence for exercise hormesis theoretical frameworks [9, 26, 50]. Future work should investigate the degree to which these signaling responses are apparent in other tissues in aging models, and model systems of disease where oxidative stress is a key clinical manifestation of the particular disease.
Highlights.
Older individuals show attenuated nuclear accumulation of Nrf2 and downstream gene expression following acute exercise compared to young
Exercise Training (ET) can reverse some of the age-related impairment
The degree of improvement in signaling is evident to a greater degree in young than older individuals after ET suggesting ET does not completely reverse age-related biological declines in Nrf2-dependent signaling systems
The major improvements in signaling effects driven by ET in older individuals comes from decreasing baseline expression levels of Nrf2.
Acknowledgements
We would like to thank all undergraduates and graduate students in the Traustadóttir Lab that helped with testing and training subjects. We would also like to thank our participants for their hard work and adherence.
Funding: This project was funded by the NIH R15AG055077 to TT
Footnotes
Declaration of competing interest: The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Miller BF, Seals DR, Hamilton KL, A viewpoint on considering physiological principles to study stress resistance and resilience with aging, Ageing Res Rev 38 (2017) 1–5. [DOI] [PubMed] [Google Scholar]
- [2].Pomatto LCD, Davies KJA, The role of declining adaptive homeostasis in ageing, J Physiol 595(24) (2017) 7275–7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pomatto LCD, Sun PY, Davies KJA, To Adapt or not to Adapt: Consequences of Declining Adaptive Homeostasis and Proteostasis with Age, Mech Ageing Dev (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, Geroscience: linking aging to chronic disease, Cell 159(4) (2014) 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK, Oxidative stress in cardiovascular disease, Indian journal of biochemistry & biophysics 46(6) (2009) 421–40. [PubMed] [Google Scholar]
- [6].Kaneto H, Katakami N, Kawamori D, Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M, Yamasaki Y, Involvement of oxidative stress in the pathogenesis of diabetes, Antioxid Redox Signal 9(3) (2007) 355–66. [DOI] [PubMed] [Google Scholar]
- [7].Markesbery WR, Oxidative stress hypothesis in Alzheimer’s disease, Free radical biology & medicine 23(1) (1997) 134–47. [DOI] [PubMed] [Google Scholar]
- [8].Panieri E, Santoro MM, ROS homeostasis and metabolism: a dangerous liason in cancer cells, Cell death & disease 7(6) (2016) e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sohal RS, Orr WC, The redox stress hypothesis of aging, Free radical biology & medicine 52(3) (2012) 539–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Done AJ, Gage MJ, Nieto NC, Traustadóttir T, Exercise-induced Nrf2-signaling is impaired in aging, Free Radic Biol Med 96 (2016) 130–8. [DOI] [PubMed] [Google Scholar]
- [11].Done AJ, Newell MJ, Traustadóttir T, Effect of exercise intensity on Nrf2 signalling in young men, Free Radic Res 51(6) (2017) 646–655. [DOI] [PubMed] [Google Scholar]
- [12].Davies SS, Traustadóttir T, Stock AA, Ye F, Shyr Y, Harman SM, Roberts, LJ 2nd, Ischemia/reperfusion unveils impaired capacity of older adults to restrain oxidative insult, Free Radic Biol Med 47(7) (2009) 1014–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M, Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain, Genes Dev 13(1) (1999) 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M, Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages, The Journal of biological chemistry 275(21) (2000) 16023–9. [DOI] [PubMed] [Google Scholar]
- [15].Tong KI, Padmanabhan B, Kobayashi A, Shang C, Hirotsu Y, Yokoyama S, Yamamoto M, Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response, Molecular and cellular biology 27(21) (2007) 7511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL, The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer, Redox Biol 1 (2013) 45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, Symons JD, Paine R 3rd, Hoidal JR, Rajasekaran NS, Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle, Biochimica et biophysica acta 1822(6) (2012) 1038–50. [DOI] [PubMed] [Google Scholar]
- [18].Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, Hoidal JR, Wang L, Rajasekaran NS, Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium, Free Radic Biol Med 52(2) (2012) 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Narasimhan M, Hong J, Atieno N, Muthusamy VR, Davidson CJ, Abu-Rmaileh N, Richardson RS, Gomes AV, Hoidal JR, Rajasekaran NS, Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells, Free Radic Biol Med 71 (2014) 402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang H, Davies KJA, Forman HJ, Oxidative stress response and Nrf2 signaling in aging, Free Radic Biol Med 88(Pt B) (2015) 314–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhou L, Zhang H, Davies KJA, Forman HJ, Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells, Redox Biology 14 (2018) 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meng J, Lv Z, Qiao X, Li X, Li Y, Zhang Y, Chen C, The decay of Redox-stress Response Capacity is a substantive characteristic of aging: Revising the redox theory of aging, Redox Biol 11 (2017) 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Henriquez-Olguin C, Diaz-Vegas A, Utreras-Mendoza Y, Campos C, Arias-Calderon M, Llanos P, Contreras-Ferrat A, Espinosa A, Altamirano F, Jaimovich E, Valladares DM, NOX2 Inhibition Impairs Early Muscle Gene Expression Induced by a Single Exercise Bout, Frontiers in physiology 7 (2016) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Henriquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, Sylow L, Holmdahl R, Richter EA, Jaimovich E, Jensen TE, Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise, Nat Commun 10(1) (2019) 4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Henriquez-Olguin C, Renani LB, Arab-Ceschia L, Raun SH, Bhatia A, Li Z, Knudsen JR, Holmdahl R, Jensen TE, Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2, Redox Biol 24 (2019) 101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Viña J, Borras C, Abdelaziz KM, Garcia-Valles R, Gomez-Cabrera MC, The Free Radical Theory of Aging Revisited: The Cell Signaling Disruption Theory of Aging, Antioxidants & Redox Signaling 19(8) (2013) 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KJ, Odelberg SJ, Firpo MA, Paine R 3rd, Hoidal JR, Abel ED, Rajasekaran NS, Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training, PloS one 7(9) (2012) e45697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Done AJ, Traustadóttir T, Nrf2 mediates redox adaptations to exercise, Redox Biol 10 (2016) 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nordin TC, Done AJ, Traustadottir T, Acute exercise increases resistance to oxidative stress in young but not older adults, Age (Dordr) 36(6) (2014) 9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KS, Odelberg SJ, Firpo MA, Paine R 3rd, Hoidal JR, Abel ED, Rajasekaran NS, Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training, PloS one 7(9) (2012) e45697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cottreau CM, Ness RB, Kriska AM, Physical activity and reduced risk of ovarian cancer, Obstet Gynecol 96(4) (2000) 609–14. [DOI] [PubMed] [Google Scholar]
- [32].Winters-Hart CS, Brach JS, Storti KL, Trauth JM, Kriska AM, Validity of a questionnaire to assess historical physical activity in older women, Med Sci Sports Exerc 36(12) (2004) 2082–7. [DOI] [PubMed] [Google Scholar]
- [33].Siri WE, Body composition from fluid spaces and density: analysis of methods. 1961, Nutrition 9(5) (1993) 480–91; discussion 480, 492. [PubMed] [Google Scholar]
- [34].Traustadóttir T, Stock AA, Harman SM, High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults, Age (Dordr) 30(4) (2008) 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH 3rd, Holloszy JO, Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds, Journal of applied physiology (Bethesda, Md. : 1985) 71(5) (1991) 2004–11. [DOI] [PubMed] [Google Scholar]
- [36].M. American College of Sports, ACSM's guidelines for exercise testing and prescription, Sixth edition. Philadelphia: : Lippincott Williams & Wilkins, [2000] ©20002000. [Google Scholar]
- [37].Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T, Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study, Circulation 115(24) (2007) 3086–94. [DOI] [PubMed] [Google Scholar]
- [38].Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP,M. American College of Sports, American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise, Med Sci Sports Exerc 43(7) (2011) 1334–59. [DOI] [PubMed] [Google Scholar]
- [39].Suzuki K, Bose P, Leong-Quong RY, Fujita DJ, Riabowol K, REAP: A two minute cell fractionation method, BMC research notes 3 (2010) 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA, Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise, Physiological genomics 18(2) (2004) 226–31. [DOI] [PubMed] [Google Scholar]
- [41].Catoire M, Mensink M, Boekschoten MV, Hangelbroek R, Muller M, Schrauwen P, Kersten S, Pronounced effects of acute endurance exercise on gene expression in resting and exercising human skeletal muscle, PloS one 7(11) (2012) e51066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT, The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin Chem 55(4) (2009) 611–22. [DOI] [PubMed] [Google Scholar]
- [43].Pareja-Galeano H, Garatachea N, Lucia A, Exercise as a Polypill for Chronic Diseases, Prog Mol Biol Transl Sci 135 (2015) 497–526. [DOI] [PubMed] [Google Scholar]
- [44].Garatachea N, Pareja-Galeano H, Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Moran M, Emanuele E, Joyner MJ, Lucia A, Exercise attenuates the major hallmarks of aging, Rejuvenation Res 18(1) (2015) 57–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Garatachea N, Santos-Lozano A, Hughes DC, mez-Cabello A, Ara I, Physical Exercise as an Effective Antiaging Intervention, BioMed Research International 2017 (2017) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Merry TL, Ristow M, Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice, J Physiol 594(18) (2016) 5195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Goto S, Naito H, Kaneko T, Chung HY, Radak Z, Hormetic effects of regular exercise in aging: correlation with oxidative stress, Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 32(5) (2007) 948–53. [DOI] [PubMed] [Google Scholar]
- [48].Radak Z, Chung HY, Goto S, Exercise and hormesis: oxidative stress-related adaptation for successful aging, Biogerontology 6(1) (2005) 71–5. [DOI] [PubMed] [Google Scholar]
- [49].Radak Z, Chung HY, Koltai E, Taylor AW, Goto S, Exercise, oxidative stress and hormesis, Ageing research reviews 7(1) (2008) 34–42. [DOI] [PubMed] [Google Scholar]
- [50].Radak Z, Ishihara K, Tekus E, Varga C, Posa A, Balogh L, Boldogh I, Koltai E, Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve, Redox Biol 12 (2017) 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M, Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling, Antioxid Redox Signal 18(10) (2013) 1208–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J, Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats, J Physiol 567(Pt 1) (2005) 113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J, Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance, The American journal of clinical nutrition 87(1) (2008) 142–9. [DOI] [PubMed] [Google Scholar]
- [54].Vasilaki A, McArdle F, Iwanejko LM, McArdle A, Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age, Mech Ageing Dev 127(11) (2006) 830–9. [DOI] [PubMed] [Google Scholar]
- [55].Margaritelis NV, Theodorou AA, Paschalis V, Veskoukis AS, Dipla K, Zafeiridis A, Panayiotou G, Vrabas IS, Kyparos A, Nikolaidis MG, Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability, Acta Physiol (Oxf) 222(2) (2018). [DOI] [PubMed] [Google Scholar]
- [56].Margaritelis NV, Paschalis V, Theodorou AA, Kyparos A, Nikolaidis MG, Antioxidant supplementation, redox deficiencies and exercise performance: A falsification design, Free Radic Biol Med 158 (2020) 44–52. [DOI] [PubMed] [Google Scholar]
- [57].Crilly MJ, Tryon LD, Erlich AT, Hood DA, The role of Nrf2 in skeletal muscle contractile and mitochondrial function, Journal of applied physiology 121(3) (2016) 730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Musci RV, Hamilton KL, Linden MA, Exercise-Induced Mitohormesis for the Maintenance of Skeletal Muscle and Healthspan Extension, Sports (Basel, Switzerland) 7(7) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cobley JN, Sakellariou GK, Owens DJ, Murray S, Waldron S, Gregson W, Fraser WD, Burniston JG, Iwanejko LA, McArdle A, Morton JP, Jackson MJ, Close GL, Lifelong training preserves some redox-regulated adaptive responses after an acute exercise stimulus in aged human skeletal muscle, Free Radic Biol Med 70 (2014) 23–32. [DOI] [PubMed] [Google Scholar]
- [60].Cobley JN, Moult PR, Burniston JG, Morton JP, Close GL, Exercise improves mitochondrial and redox-regulated stress responses in the elderly: better late than never!, Biogerontology 16(2) (2015) 249–64. [DOI] [PubMed] [Google Scholar]
- [61].Alam J, Cook JL, How many transcription factors does it take to turn on the heme oxygenase-1 gene?, Am J Respir Cell Mol Biol 36(2) (2007) 166–74. [DOI] [PubMed] [Google Scholar]
- [62].Inouye S, Hatori Y, Kubo T, Saito S, Kitamura H, Akagi R, NRF2 and HSF1 coordinately regulate heme oxygenase-1 expression, Biochem Biophys Res Commun 506(1) (2018) 7–11. [DOI] [PubMed] [Google Scholar]
- [63].Miller BF, Konopka AR, Hamilton KL, Last Word on Viewpont: On the rigorous study of exercise adaptations: why mRNA might not be enough?, Journal of Applied Physiology 121(2) (2016) 601–601. [DOI] [PubMed] [Google Scholar]
- [64].Harman D, Aging: A Theory Based on Free Radical and Radiation Chemistry, Journal of Gerontology 11(3) (1956) 298–300. [DOI] [PubMed] [Google Scholar]
- [65].Harman D, The Biologic Clock: The Mitochondria?, Journal of the American Geriatrics Society 20(4) (1972) 145–147. [DOI] [PubMed] [Google Scholar]
- [66].Theodorou AA, Paschalis V, Kyparos A, Panayiotou G, Nikolaidis MG, Passive smoking reduces and vitamin C increases exercise-induced oxidative stress: does this make passive smoking an anti-oxidant and vitamin C a pro-oxidant stimulus?, Biochem Biophys Res Commun 454(1) (2014) 131–6. [DOI] [PubMed] [Google Scholar]
- [67].Pomatto LCD, Tower J, Davies KJA, Sexual Dimorphism and Aging Differentially Regulate Adaptive Homeostasis, The journals of gerontology. Series A, Biological sciences and medical sciences 73(2) (2018) 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]