Abstract
This study aimed to improve the nutritional quality of Harbin dry sausages using natural plant-based Jerusalem artichoke powder (JAP) and olive oil as animal fat replacers. Low-fat Harbin dry sausages were manufactured with 2 different formulations containing JAP and olive oil as pork fat replacers. The texture, rheological properties, microstructure, water holding capacity, muscle protein structure, physicochemical indices, microbiological characteristics, and sensory evaluation of the sausages were analyzed. The result showed that Harbin dry sausages with JAP and olive oil were healthier than control sausages based on the lower fat content and improved fatty acid composition. Scanning electron microscopy showed gel network formation in sausages with a high JAP content. Low-field nuclear magnetic resonance illustrated that the water-holding capacity of the modified sausages was improved, suggesting that the replacers enhanced protein gel formation by changes in C–H stretching and bending vibrations, a reduction in α-helixes, and increases in β-sheets and random coils accompanying the exposure of reactive groups and microenvironment of the tertiary structure. Dynamic rheological and texture tests indicated that the replacers improved the elasticity of sausages. The reduction of fat and addition of replacers significantly enhanced lipid oxidative resistance. Overall, JAP and olive oil improved the fatty acid composition, gel structure, lipid oxidative resistance, and sensory quality of the sausages. These results may contribute to the development of healthy meat products to further reduce animal fat.
Key words: Jerusalem artichoke powder, olive oil, fat replacer, LF-NMR, Raman spectroscopy
Introduction
As one of traditional meat products, Harbin dry sausage is widely consumed in China. However, excessive intake of red meat and processed meat products has potential health risks and might cause some diseases, including cardiovascular disease, coronary heart disease (CHD), high blood pressure, and obesity, because of their high fat and fatty acid composition (Wang and Hu, 2017). Animal fat reduction is a key strategy for improving the health and nutrition of fatty foods. However, animal fat is one of the important components to ensure the quality of sausages, which affects various properties, including water holding capacity, protein binding characteristics, texture, and taste (Yang et al., 2015). Various strategies have been developed to resolve this issue, including addition of starch ingredients, such as rice and pea flour, as animal fat replacers to stimulate protein gel formation during the cooking process (Pereira et al., 2020), or addition of gels, such as guar gum and carrageenan, as fat analogs (Marchetti et al., 2014; Rather et al., 2017). However, better approach was to develop natural plant-based fat analogs or plant-based ingredients as fat replacers, which have also been developed as sources of healthy fatty acids, fiber, minerals, antioxidants, and vitamins (Pintado et al., 2018).
Jerusalem artichoke (JA) is a natural perennial root plant and contains high amounts of inulin, phenolic compounds, mineral elements, and dietary fiber (Afoakwah et al., 2015). Jerusalem artichoke powder (JAP) is usually used in dairy products. It contains high amounts of inulin, which is a low-calorie ingredient composed of linear polyfructose chains connected by β(1-2) linkages (Tárrega et al., 2011). Inulin-type polyfructose could reduce postprandial blood glucose (Lightowler et al., 2018) and could promote the growth of intestinal probiotics and improve gastrointestinal functions (Wilson and Whelan, 2017). More importantly, when it is mixed with warm water at high concentrations, inulin can form gels that are analogous to fats (Keenan et al., 2014), which indicates its potential characteristic as a fat substitute. In addition, abundant natural antioxidants of phenolic compounds in JAP has high antioxidant activity (Gibson et al., 2017), which further showed its potential characteristic as a fat substitute. Thus, the inulin and phenolic compounds in JAP could provide analogous properties to those of fats and antioxidants. In addition, JAP has high nutritional value because it is rich in mineral elements and dietary fiber. Therefore, JAP is a promising natural replacer of animal fat in sausages.
Besides fat reduction, the development of healthy lipid formulations is a major research goal to improve the health and nutrition of fatty foods. Those alterations in fatty acid profiles of fatty foods using vegetable or fish oils with positive effects on human health have attracted substantial interest. Olive oil contains high levels of unsaturated fatty acids (UFAs) (Triki et al., 2013), which is consistent with health recommendations (van Hees et al., 2010). Unsaturated fatty acids include polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs). Polyunsaturated fatty acids contain essential fatty acids and are beneficial to human health. For example, a study has shown that replacing 5% of energy derived from saturated fat with equivalent energy from PUFAs can reduce the risk of CHD by 25% (Li et al., 2015). Monounsaturated fatty acids are not essential fatty acids in the diet, but replacing saturated fat acids with MUFAs from plant sources can decrease the risk of CHD (Kris-Etherton et al., 2001). Olive oil has also been shown the beneficial effects on reducing the risk of cardiovascular diseases and cancer, as well as reducing the concentration of blood lipids (Nocella et al., 2018). Before being used as a fat replacer, the oil was usually pre-emulsified with emulsifier including soy protein isolate (SPI) or fish protein isolate through high-speed stirring (Kang et al., 2016; Cheetangdee, 2017).
Considering both animal fat reduction and lipid profile improvement, in this study, the effects of JAP (natural plant-based-gels) combined with olive oil (natural plant-based oil) used as partial animal fat replacers were evaluated on the quality of Harbin dry sausages, which have not been explored previously.
Materials and methods
Preparation of Pre-emulsified Olive Oil and JAP Gel
Pre-emulsified olive oil was prepared following previously described methods for pre-emulsified soy oils (Kang et al., 2016), with minor modifications. The ratio of SPI: olive oil: water was set to 1:10:10. Soy protein isolate and water were homogenized at 4°C, 2,000 rpm for 60 s. Next, olive oil was added gradually with homogenization for 60 s. The mixture was homogenized continuously for 120 s and packed in polythene bags to acquire the final pre-emulsified olive oil, then the oil was maintained at 4°C before use. For JAP gel preparation, JAP and water were mixed at a ratio of 2:1. Next, 0.25 mg/mL Ca2+ was added and the mixture was homogenized at 80°C, 2,000 rpm for 30 min to form fat analogs of JAP gels, then the gels were maintained at 4°C before use.
Sausage Preparation
Sausages were manufactured at the Food Pilot Plant of the Shandong Meat Quality Control Centre (Shandong, China). Three group sausages with 50 kg per batch were prepared. Control sausages contained 90% lean meat and 10% back fat. Animal fat reduction group A contained 90% lean meat, 4% back fat, 2% JAP, and 4% pre-emulsified olive oil. Animal fat-reduction group B contained 90% lean meat, 2% back fat, 4% JAP, and 4% pre-emulsified olive oil. The three formulations also contained other necessary additives. The JAP gels and pre-emulsified olive oil were prepared and other additives were dissolved in water in advance, and then chilled at 4°C. Then the minced lean meat was chopped with a chopper (BZBJ-15; Expro Stainless Steel Mechanical & Engineering Co., Ltd., Hangzhou, China) for 1 min. The meat batters were followed by addition of back fat, the chilled JAP gels, pre-emulsified olive oil and other additives, and then chopped for 4 min for pre-emulsification. At last, the meat batters were cured at 4°C for 48 h and then stuffed into natural casings. The sausages were dried at 65°C for 2 h, smoked at 55°C for 6 h, and cooked with steam for about 20 min, then the products were stored at 4°C after being cooled to room temperature.
Sampling Procedure
Changes in the water content, thiobarbituric acid reactive substances (TBARS) values, and total aerobic microorganisms plate counts were assessed every sixth day during the storage period according to Kim et al. (2011) with minor modifications. For other indices, samples were selected from each group at the same time at the initial phase of ripening to obtain initial values. For all measured properties, 3 replicates were obtained from each group on the same day.
Determination of the pH and Water Content
The pH measurement was carried out in accordance with the previous report (Ruiz-Capillas et al., 2012) with some modifications. Samples (5 g each) were dissected and homogenized in 90 mL of 0.85% saline using a BagMixer (400CC; Interscience, Saint-Nom la Bretèche, France) for 90 s. The pH of the filtrate was measured using a pH meter (Testo 206-PH1; Testo AG, Lenzkirch, Germany). The water content was estimated in accordance with previous analytical methods (Adam et al., 2009) with minor modifications, and samples (20 g each) were placed in a dry evaporation pan and dried to a constant weight at 106°C for about 5 h. The water content was calculated based on the difference between the weights of the initial sample and dried sample.
Determination of the Crude Fat Content and Fatty Acid Composition
Fat contents were determined with Soxhlet extraction method in accordance with the previous report (Shin and Park, 2015) with some modifications; the homogenized test portion (1 g) was put into in a beaker and dried in 105°C oven for 4 h. After being cooled to a temperature of 25°C, the test portion was packed with the defatted filter bag and weighed accurately, then put into the extraction tube of Soxhlet extractor and extracted at 55°C for about 12 h with diethyl ether. After extraction, the filter bag was removed and dried in 105°C oven for 4 h, then cooled and weighed accurately. Fat content was calculated as follows:
Fat content, % = (B−C)/A × 100, where A = test portion weight, B = weight of extraction bag after drying, C = weight of extraction bag before extraction.
The fatty acid composition was measured by gas chromatography in accordance with previously described methods (Magrinyà et al., 2012) with minor modifications. Fatty acid was extracted from 1.5 g of chopped sausages using 20 mL of chloroform and methanol (2:1, v/v). And the extraction was subsequently re-extracted twice with 10 mL of the same solvent. Then, fatty acid methyl esters were acquired through derivative using sodium methoxide and boron trifluoride. Subsequently fatty acid composition of each sausage sample was measured in terms of a percentage of peak area normalization.
Analyses of Nitrosylmyoglobin (Mb-NO) and Lipid Oxidation
Mb-NO analysis was carried out in accordance with the previous method (Gao et al., 2014) with minor modifications. The minced sample (10 g) was homogenized in 90 mL of phosphate buffer (pH 6.0, 0.01 mol/L) using a BagMixer (400CC; Interscience, Saint-Nom la Bretèche, France) for 90 s. Then the mixture was placed in a dark room at 4°C for 1 h and filtered with a nitrocellulose membrane (0.22 mm). The absorbance spectra of pigment extracts from the sausages were evaluated from 350 nm to 700 nm using a UV–Vis spectrophotometer (TU-1810; Beijing Purkinje General Instrument Co., Ltd., Beijing, China). For lipid oxidation, the minced sample (10 g) was homogenized in 50 mL of trichloroacetic acid solution (7.5% of trichloroacetic acid, 0.1% of EDTA, w/v) using a BagMixer (400CC; Interscience, Saint-Nom la Bretèche, France) for 90 s. Then the lipid oxidation was measured by the thiobarbituric acid (TBA) method accordingly as described previously by measuring the absorbance of the reaction products from the oxidation products of unsaturated fatty acids and TBA (Racanicci et al., 2008).
Dynamic Rheological Characteristics
Dynamic rheological characteristics were evaluated using a rotational rheometer (MCR102; Anton Paar, Graz, Austria). Each sample was placed between 2 plates separated by 1 mm and silicon oil was applied to prevent moisture loss (Bolger et al., 2018). Next, a dynamic rheological characteristics test was conducted by frequency sweep measurements from 0.1 to 100 Hz at a strain of 0.5%. The storage modulus (G′) and the loss modulus (G″) were recorded during sweep measurements.
Scanning Electron Microscopy and Low-field Nuclear Magnetic Resonance
For scanning microstructure of the sausage samples, sausage slices were fixed in 2.5% glutaraldehyde at 4°C for 12 h. Then the samples were reinforced in 1% osmium tetroxide for 5 h. Finally, they were washed 3 times, dehydrated in increasing concentrations of ethanol, and evaluated by scanning electron microscopy (JSM-7500F; JEOL, Tokyo, Japan) after being coated with gold.
Low-field nuclear magnetic resonance (LF-NMR) relaxation surveys were performed using an NMR analyzer (NM120-040V-1; Niumag Analytical Instrument Corporation, Suzhou, China). Before measurements, all samples were thermostated to temperature of 25°C. Samples (15 g each) were placed in a 40-mm glass tube and the NMR probe was inserted. Proton transverse relaxation time (T2) was detected with a Carr–Purcell–Meiboom–Gill sequence. The analysis conditions were as follows: a resonance frequency was set as 25 MHz and the test temperature was 32°C, a series of 10,000 echoes were used for 30 scan repetitions. Three relaxation times (T2b, T21, and T22) and water populations (P2b, P21, and P22) were acquired as the amplitude of every second echo.
Texture Profile Analysis
Texture profile was analyzed in accordance with the method of Zhu et al. After removing the casing, the sausage sample was cut into a cylinder with 2-cm thickness and 1.5 cm in diameter. Then the texture profile analysis was performed using a texture analyzer (TA-XT Plus; Probe P/0.5; Stable Micro Systems Godalming, UK). Texture parameters consisting of hardness, springiness, cohesiveness, chewiness, and resilience were calculated using Texture Expert version 1.22.
Microbiological Analysis
Microbiological analysis was carried out to detect the total aerobic microorganism counts with plate count method in accordance with the standard procedures of AOAC 966.23 (AOAC, 2016). Samples (10 g each) were chopped and homogenized in sterilized saline (0.85%, w/v) for 90 s in a BagMixer (400CC; Interscience) and then a series of decimal dilutions were prepared. Three consecutive appropriate decimal dilutions (1 mL) were removed in the plate and then 20 mL of plate count agar was poured and incubated at 35°C for 48 h to determine the aerobic plate counts.
Raman Spectroscopic Analysis
Raman spectra were measured using a DXR2xi Raman Imaging Microscope spectrometer (DXR2xi, Thermo Fisher Scientific, Waltham, MA) as described by Liu et al. (2011). An Nd:YAG laser with a power of about 21.5 mW was used. Raman spectra in the range of 300 to 3,500 cm−1 were recorded under the following conditions: exposure time, 0.20 s (5 Hz); scan times, 100 and the spectra were smoothed using OMNICxi 8.0 (DXR2xi; Thermo Fisher Scientific). Baseline correction and normalization against the phenylalanine band near 1,003 cm−1 were performed. The secondary structures of proteins were determined as percentages of α-helixes, β-sheets, β-turns, and random coils (Tadpitchayangkoon et al., 2010; Nawrocka et al., 2015).
Sensory Evaluation
Sensory evaluation of the samples was performed based on a nine-point scale from one to nine (Magrinyà et al., 2012). The evaluation was carried out by a panel of 10 peoples who possessed experiences in sensory evaluation. Sausages are cut into cylindrical slices with 3-cm thickness and placed in a clean plate. The panelists were requested to rank sensory indicators and overall acceptability of the sample on a 9-point scale (1 = very bad; 9 = very good) under artificial light. Water and salt-free crackers were provided to the panel to purify their taste buds between tasting different samples.
Statistical Analysis
Data were analyzed using SPSS Version 18.0 (IBM, New York, NY) with single-factor experiments. A completely randomized design of 3 replications was used. The differences among groups were evaluated by one-way analysis of variance and Duncan multiple range tests with P < 0.05 accepted as the level of significance. The differences were represented with different lowercase or uppercase letters.
Results and discussion
Fat Content and Fatty Acid Profile
The fat content and lipid sources in the sausage formulation have a great influence on the fatty acid composition (Pintado et al., 2018). The initial fat contents for the 2 sample groups were both significantly lower (P < 0.05) than that of the control (Table 1), which indicating a noticeable fat reduction in the 2 sample groups. On the other hand, when pork fat was replaced with JAP and olive oil, the total SFA was lower than that of the control (P < 0.05). The decrease in total SFA was mainly attributed to reductions in the percentages of myristic acid, stearic acid, and palmitic acid. In addition, when the pork fat was replaced as shown in Table 1, total UFA was significantly higher (P < 0.05) than that in the control. The increase was mainly explained by increases in the percentages of octadecenoic acid, oleic acid, and linolenic acid, particularly oleic acid. This result can be explained by the high oleic acid content (55–83%) in olive oil (Hernández et al., 2018; Bontempo et al., 2019). Previous research has shown that oleic acid in olive oil was associated with the decrease in blood pressure (Terés et al., 2008) and could reduce the incidence of stroke (Samieri et al., 2011). Moreover, the ratio of total UFA to total SFA was higher in fat-reduction groups than in the control (P < 0.05). Considering that SFAs are associated with several major illnesses and UFAs have beneficial health effects, particularly oleic acid, the fatty acid profiles of the 2 fat-reduction groups were superior to that of the control from a health perspective.
Table 1.
Fatty acid and fat contents.
| Physicochemical indexes | Treatments |
||
|---|---|---|---|
| Control | Group A | Group B | |
| Fat content (%) | 9.32 ± 1.11a | 7.76 ± 0.58b | 6.17 ± 0.39c |
| Fatty acids (%) | |||
| Tricosane acid C 23:0 | 0.51 ± 0.00c | 0.74 ± 0.00a | 0.68 ± 0.01b |
| Octadecenoic acid C18:1 | 0.51 ± 0.01c | 0.74 ± 0.00a | 0.68 ± 0.01b |
| Margaric acid C17:0 | 0.51 ± 0.02 | ud | ud |
| Miristic acid C14:0 | 1.04 ± 0.02a | 0.74 ± 0.01b | 0.68 ± 0.00c |
| Eicosadienoic acid C20:2 | 0.51 ± 0.00a | 0.74 ± 0.00a | 0.68 ± 0.01b |
| Eicosenoic acid C20:1 | 0.51 ± 0.01c | 0.74 ± 0.01a | 0.68 ± 0.02b |
| Oleic acid C18:1 | 40.51 ± 2.30b | 44.85 ± 1.55a | 46.62 ± 1.32a |
| Linolenic acid C18:3 | 0.51 ± 0.00c | 0.74 ± 0.02a | 0.68 ± 0.001b |
| Linoleic acid C18:2 | 15.39 ± 0.16a | 15.44 ± 1.32a | 14.86 ± 0.95a |
| Stearic acid C18:0 | 12.82 ± 0.13a | 11.03 ± 0.24b | 10.14 ± 0.06c |
| Palmitic acid C16:0 | 25.13 ± 0.14a | 22.79 ± 0.77b | 22.30 ± 0.94b |
| Palmitoleic acid C16:1 | 2.00 ± 0.10a | 1.78 ± 0.19a | 1.87 ± 0.25a |
| Total SFA | 40.02 ± 0.26a | 35.37 ± 0.77b | 33.80 ± 1.16b |
| Total UFA | 59.48 ± 2.37b | 63.99 ± 0.29a | 65.54 ± 0.37a |
| UFA/SFA | 1.49 ± 0.06c | 1.81 ± 0.05b | 1.94 ± 0.07a |
Control represents sausages contained 90% lean meat and 10% back fat. Group A represents fat-reduction group A contained 90% lean meat, 4% back fat, 2% JAP, and 4% pre-emulsified olive oil. Group B represents fat-reduction group B contained 90% lean meat, 2% back fat, 4% JAP, and 4% pre-emulsified olive oil. The meaning of the control, group A, and group B were consistent with those of other figures and tables in this article. Values are expressed as means ± SD; a, b, and c indicate that values in the same row are significantly different (P < 0.05). In addition, ud indicates undetected.
Abbreviations: JAP, Jerusalem artichoke powder; SFA, saturated fatty acid; UFA, unsaturated fatty acid.
Dynamic Rheological Characteristics
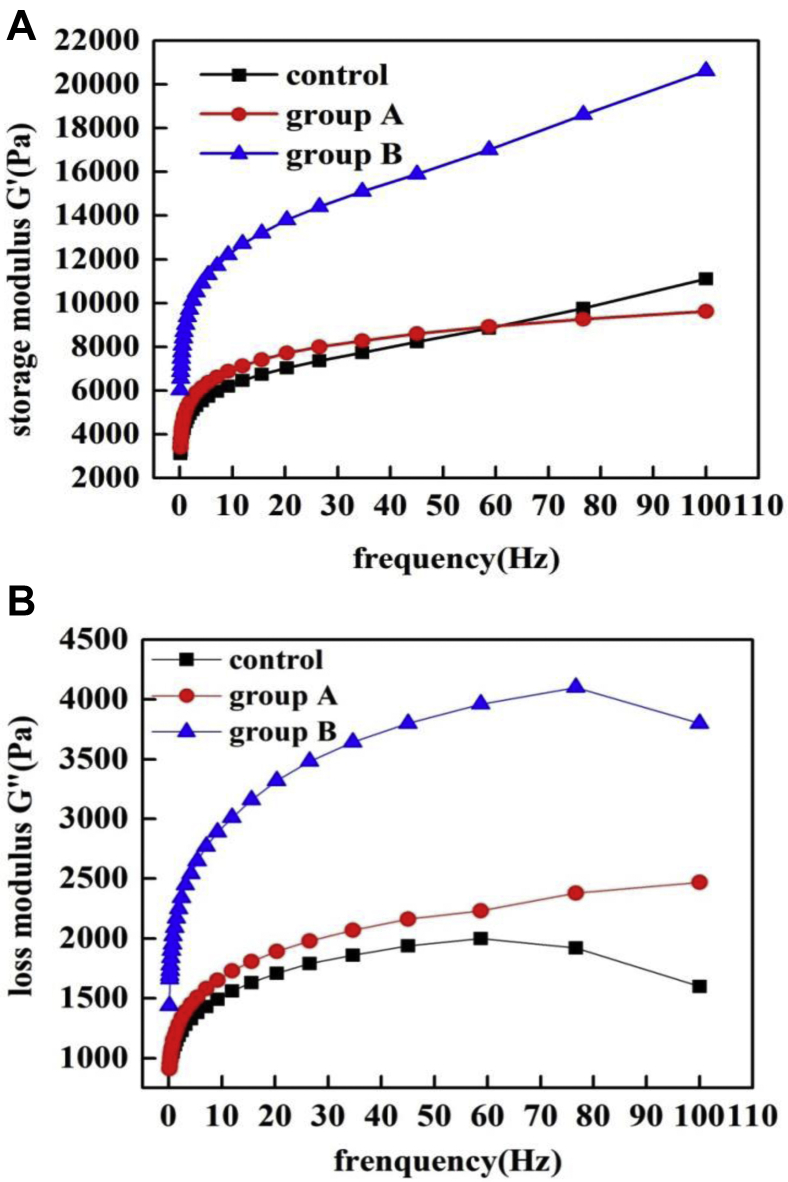
For measuring mechanical behavior of viscoelasticity of the sample, a dynamic rheological characteristics test was conducted by frequency sweep measurements. The effects of oscillatory shear frequency on viscoelastic properties of the sausages are summarized in Figure 1. When the frequency is less than 10, the G′ and G″ of the 3 group sausages all increased rapidly with the increase of the frequency. When the frequency increased over 10, the G′ and G″ increase slowly. It showed that the viscoelasticity of sausages increased with the increase of frequency, and the frequency influence was greatest in the frequency range from 0 to 10. In the frequency range from 0 to 100, the G′ of group A with a low proportion of JAP was similar to that of the control (Figure 1A) and G″ of group A was slightly higher than that of the control (Figure1B). The G′ and G″ of group B (with a relatively higher proportion of JAP) were much higher than those of the control and group A and exhibited high-frequency sensitivity. That illustrated that the addition of JAP could act as a rheological modifier to improve viscoelasticity. Previous studies have shown that the addition of starch (Bolger et al., 2018) and xanthan gum (Rather et al., 2015) could increase G′ and G″ of sausages and resulted in increases in the viscoelasticity characteristics of sausages. The higher viscoelasticity observed in group B might therefore be explained by the increase in JAP owing to its starch properties and colloidal substance properties (Afoakwah et al., 2015).
Figure 1.
Storage modulus G′ (A) and loss modulus G″ (B) curves for sausages during the dynamic frequency test.
pH and Water Content
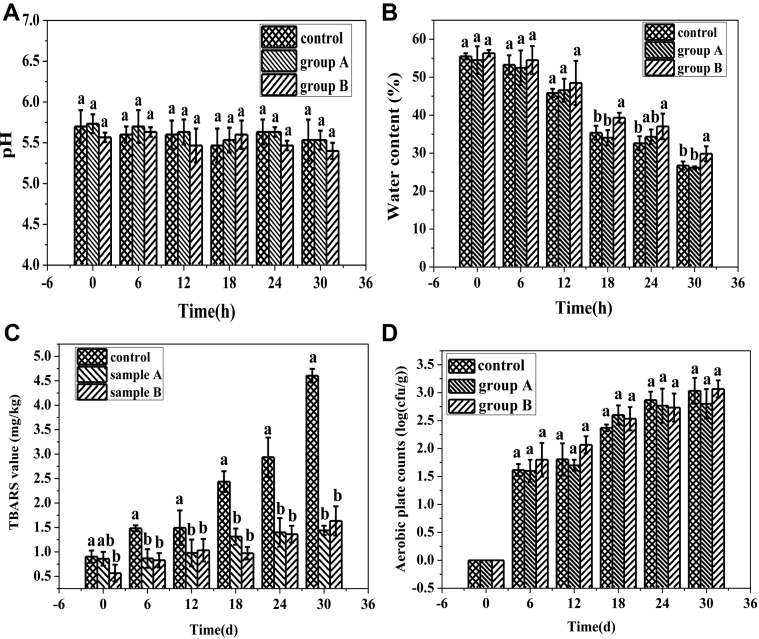
The initial pH and water content have crucial effects on the shelf life of sausages. As shown in Figure 2A and Figure 2B, there were no significant differences (P > 0.05) between the control and the 2 sample groups in the initial water content and pH value, which suggested that animal fat reduction had little impact on the quality and shelf life of the sausages. The pH was stable during storage, further guaranteeing the stability of product quality. Initial water contents in the 3 groups of sausages ranged from 54.5 to 56.3% and were similar to those of commercially available of Harbin sausages (water content, ≤ 60%). The water content in the control and sample group were all decreased with the increase of storage time. The moisture in group B was higher (P < 0.05) than those in the control and group A at a later storage period, illustrating that high JAP content could improve the water holding capacity of the sausages, which might be explained by the high water absorption capacity of JAP (Afoakwah et al., 2015).
Figure 2.
Summary of changes in the pH (A), water content (B), TBARS (C), and aerobic plate counts (D) in the sausages during storage at 4°C. Values are expressed as means ± SD; a, b, c indicate significant differences between different sausages at the same time (P < 0.05). Abbreviation: TBARS, thiobarbituric acid reactive substances.
Lipid Oxidation
As shown in Figure 2C, the TBARS values in the 3 groups all increased as the storage duration increased. However, the values in the control were significantly higher (P < 0.05) and increased more substantially than those in the 2 fat-reduction groups throughout the storage period. The higher antioxidant ability in the fat-reduction groups might attribute to the reduction of animal fat and addition of JAP. A previous study has demonstrated that JAP has high antioxidant ability due to its intramolecular hydrogen bonding, which could prevent free radical-mediated oxidation, and the high phenolic compound content, which could scavenge free radicals (Gibson et al., 2017). Samples with reduced animal fat contents had high oxidation resistance and could maintain TBARS levels at less than 2 mg/kg to ensure quality stability of the sausages.
Microbiological Analysis
The total aerobic microorganism counts indicate the level of microorganism in a product and represent the hygienic condition of the food according to AOAC 966.23 (AOAC, 2016). And the total aerobic plate counts are regularly used to predict and determine the shelf life of food products (Giménez et al., 2017). As shown in Figure 2D, changes in aerobic plate counts were similar in the 3 groups; all counts increased slowly to about 103 cfu/g and there were no significant differences among groups (P > 0.05), indicating that the fat replacers had little influence on bacterial growth. Counts (about 103 cfu/g) in all 3 groups on d 30 were all below the established limit (104 cfu/g) in accordance with Chinese national standard “National food safety standards of cooked meat products” (National Health Commission of the Peoples Republic of China, 2016).
Scanning Electron Microscopy
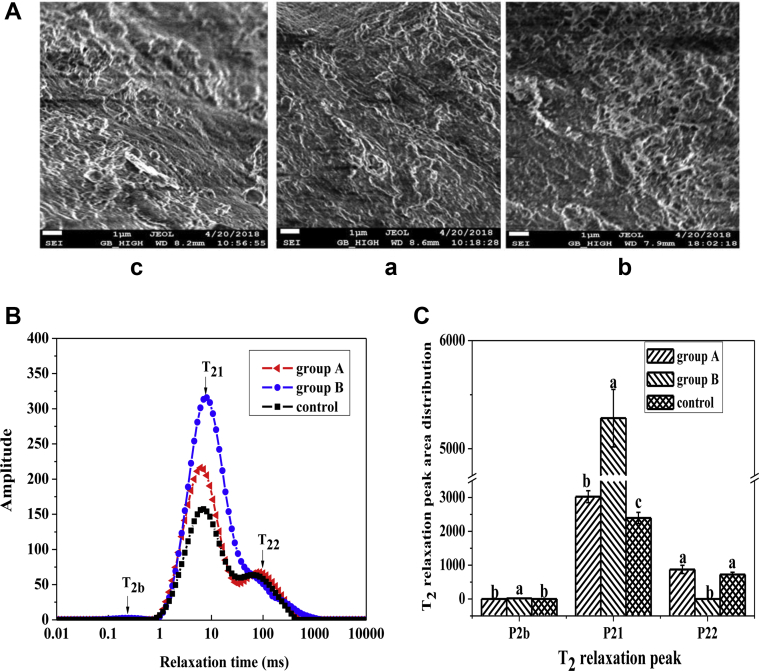
As shown in Figure 3Ac, the myofibrillar and fat gel exhibited an irregular structure, including many fat globules on the surface of the sausage slice, probably owing to the inability to emulsify fully and to form colloids with a certain structure (Chin et al., 2000). Figure 3Aa showed the relatively compact and dense texture and the appearance of fewer fat globules. The increase in JAP gel content (Figure 3Ab) produced a more regular spongy gelatin network structure compared with that of group A, which might result in the formation of polymers due to the crosslinking reaction among JAP polysaccharides, SPI, myofibrillar protein, and water (Kumar et al., 2016). This structure could substantially increase the elastic characteristics of the sausages, consistent with the results for the dynamic rheological characteristics summarized in Figure 1A and Figure 1B. Therefore, JAP is a potential animal fat replacer based on its hydrophilic and gel properties.
Figure 3.
Microstructure at 5,000 × magnification in the 3 groups of sausages (A). Note that a, b, and c denote group A, group B, and the control, respectively. The distribution of T2 (B) and water populations of 3 components (C) in the 3 groups of sausages. P2b, P21, and P22 represent proportions of water closely associated with macromolecules, water located in the myofibrillar network, and free water outside of the myofibrillar network, respectively.
Low-field Nuclear Magnetic Resonance
The distribution of T2 relaxation times and proportions of 3 water components in Harbin dry sausages was determined by LF-NMR T2 measurements and presented in Figures 3B, 3C. The three T2 peaks are associated with the 3 water components in sausages. T2b represents hydration water, which is tightly combined with macromolecular structures, T21 is assigned to immobilized water in the myofibrillar network, and T22 represents free water, corresponding to water outside of the myofibrillar lattice (Duflot et al., 2019). The relaxation time results showed that T2b, T21, and T22 of the 3 groups were lower than reported values, indicating that water hydration was tighter and the mobility of immobilized water was weaker (Shao et al., 2016). P2b and P21, especially P21, were significantly higher in group B than in group A and the control (P < 0.05), indicating that group B had relatively more immobilized water and more intramyofibrillar network space that was ascribed to polymer gel formation among JAP polysaccharides, SPI, myofibrillar proteins, and water. Water-holding capacity during aging can be ascribed to the amount of water located in the myofibrillar network (Shao et al., 2016), so group B had higher water-holding capacity than the control and group A. P22 of group B was lower than those in group A and the control (P < 0.05), indicating that group B had less free water outside of the myofibrillar lattice. A low free water content can prevent biochemical reactions (Quek et al., 2007) and the growth of microorganisms (Andrews and Harris, 2000).
Raman Spectrum Analysis
C–H Stretching and Bending Vibrations
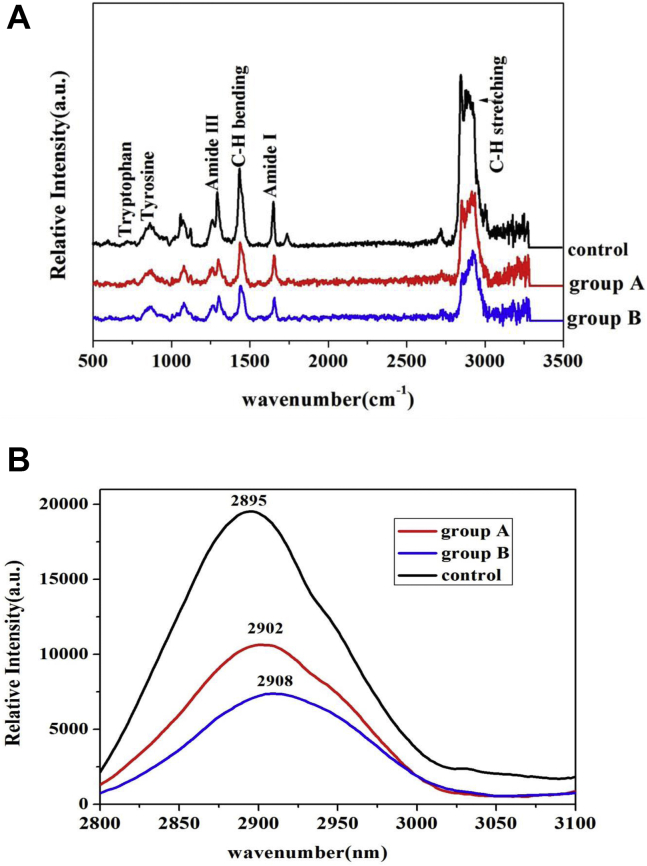
Raman spectra are shown in Figure 4. The high peaks centered at 2,800–3,000 cm−1 and 1,440–1,465 cm−1 (Figure 4A) corresponded to C–H stretching and C–H bending bands (Chen et al., 2016). The C–H stretching band position (Figure 4B) shifted from 2,895 cm−1 in the control to 2,902 cm−1 in group A and 2,908 cm−1 in group B. Spectral changes to higher wavenumbers might be related to protein unfolding and the exposure of aliphatic residues (Leelapongwattana et al., 2008). In addition, the Raman intensities of C–H stretching and C–H bending vibrations were decreased in the 2 fat-reduction groups (Figure 4A). Herrero attributed the decreased intensity to the strengthening of hydrophobic interactions around aliphatic residues, which might promote gel formation (Herrero et al., 2008). Increases in the intensities of the C–H stretching band and C–H bending band could increase protein denaturation, and excessive protein denaturation might negatively affect gel properties (Liu et al., 2011). Accordingly, in group B, the high JAP content promoted protein unfolding and strengthened hydrophobic interactions, which might be conducive to the formation of an orderly gel network (Sun et al., 2011).
Figure 4.
Raman spectra for the 3 groups in the region 500–3,500 cm−1 (A) and 2,800–3,100 cm−1 (B).
Protein Secondary Structure
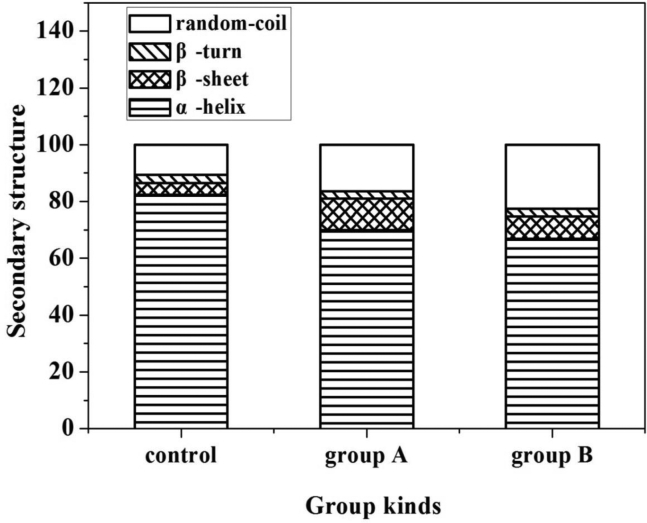
The Raman band centered near 1,655 cm−1 has been attributed to the amide I vibrational mode (Chen and Han, 2011). The amide bond of proteins has a few vibrational modes and amide I is the most useful for analyses of secondary structure. This band comprises overlapping components in the 1,645–1,658 cm−1, 1,665–1,680 cm−1, 1,680–1,690 cm−1, and 1,660–1,665 cm−1, assigned to α-helices, β-sheets, β-turns, and random coils, respectively (Sheng et al., 2016). These components constitute the protein secondary structure skeleton. Figure 5 shows that the secondary structure of the control contained 82.46% α-helices, 4.04% β-sheets, 2.85% β-turns, and 10.65% random coils. Compared with the control group, the β-sheet and random coil contents of the 2 fat-reduction groups increased, and the α-helix content decreased, particularly in group B. This was in agreement with the previous report indicating that an increase in the β-sheet content was usually accompanied by a decrease in the α-helix content (Tadpitchayangkoon et al., 2010). It has been reported that β-sheets and other extended structures are relevant to the increase in hydration, in which more protein-water bonds form and aggregate as a result of the partial ordered gel network formation (Herrero et al., 2008; Nawrocka et al., 2017). In addition, β-sheets could increase the interaction between water and adjacent proteins, resulting in a reduction in interactions between proteins and nearby matrix and an increase in the juiciness of the meat (Beattie et al., 2004). These results indicated that group B has better gel network because of the increase in extended structures (β-sheets and random coils).
Figure 5.
Secondary structure components of the tree group sausages calculated from the amide I band.
Protein Tertiary Structure
Raman spectra could reflect the tertiary structure of proteins by monitoring local environments (Kang et al., 2016). The Raman bands of aromatic amino acids, including tryptophan and tyrosine, have strong hydrophobicity and can provide information of local environments based on hydrophobic interactions of proteins (Guo et al., 2019).
The Raman band near 760 cm−1 reflects the stretching vibration of tryptophan residues, representing the H-bonding strength and hydrophobicity (Nawrocka et al., 2017). Table 2 showed that the normalized intensity of the tryptophan band (I760 cm/I1003) decreased in the 2 fat-reduction groups. The decrease in the tryptophan band illustrated that the tryptophan residues were exposed to a hydrophilic environment from a hydrophobic environment because of the addition of the fat replacers (Kang et al., 2016). The exposure of tryptophan residues might result in H-bond formation due to the presence and exposure of the indole ring in Trp residues (Nawrocka et al., 2017).
Table 2.
Normalized intensities of tryptophan and tyrosine residues in sausages.
| Treatment | Trp band (I760/I1003 cm−1) | Tyr doublet (I850/I830 cm−1) |
|---|---|---|
| Control | 0.836 ± 0.021a | 1.651 ± 0.121a |
| Group A | 0.667 ± 0.043b | 1.013 ± 0.053c |
| Group B | 0.668 ± 0.13b | 1.201 ± 0.049b |
Values are expressed as means ± SD; a, b, and c indicate that values in the same column are significantly different (P < 0.05).
The tyrosine doublet intensity ratio (I850/I830) provides information about hydrogen bonding of the phenolic hydroxyl group. A high I850/I830 ratio (0.9–2.5) reveals that the tyrosine residue is exposed to a polar environment, whereas a low ratio indicates that the tyrosine residue is buried in a hydrophobic environment (Kang et al., 2016). The I850/830 ratios of the 3 groups were all in the range of 0.9–2.5, indicating that the tyrosine residues were all exposed and could participate in moderate hydrogen bonding (Zhang et al., 2015). On the other hand, the I850/830 ratios were lower in the 2 fat-reduction groups than in the control group, illustrating that buried tyrosine residues increased and tyrosyl hydroxyl groups might participate in the formation of strong hydrogen bonds as hydrogen bond donors (Liu et al., 2011). In addition, decreases in the I850/830 ratios reflect the partial unfolding and aggregation of protein molecules (Liu et al., 2011), which might result in changes in the protein tertiary structure. Nawrocka pointed out that decreases in I850/830 ratios and subsequent changes in the protein tertiary structure increase the water absorption capacity of the gel (Nawrocka et al., 2017). Accordingly, our results showed that the addition of replacers was beneficial for the formation of hydrogen bonds and the water retention capacity.
Texture
As could be seen in Table 3, time increased (P < 0.05) hardness of the 3 groups during storage. However, there were no significant differences in hardness among the 3 groups (P > 0.05). Cohesiveness values increased in initial phase and declined thereafter, with no significant differences among the 3 groups (P > 0.05). Resilience also increased over time and then declined, with no significant differences among the 3 groups (P > 0.05). The reduction of fat content in sausages usually resulted in an increase in the hardness and cohesiveness, and excessively hardness and cohesiveness made it difficult to slice the sausages (Cegiełka and Tambor 2012). The aforementioned results indicated that the fat reduction in this article had no negative impact on hardness, cohesiveness, and resilience of the sausage. On the other hand, springiness values were higher in group B than in group A and the control (P < 0.05) during the storage period. Previous study indicated that the sausages showed softer character than the control group when the inulin was added in the meat batters as a gel (Cegiełka and Tambor 2012). So the springiness increase might attribute to the formation of inulin gel contained in JAP. And other study also proved that inulin could increase the softer character and springiness of the sausages (Mendoza et al., 2001). Chewiness in the 3 groups were also increased initially and declined thereafter; however, group A and group B were more stable than the control. At the end of the storage period, values for the 2 fat-reduction samples were better than those of the control (P < 0.05). Chewiness increase might attribute to the inulin in JAP, which could promote and strengthen connections between the various food matrix (Menegas et al., 2013). In summary, the addition of the fat replacer resulted in texture modification, especially enhanced the springiness and chewiness of the sausages.
Table 3.
Texture of sausages during storage at 4°C.
| Texture | Storage time (D) |
|||||
|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | |
| Hardness | ||||||
| Control | 1,400.01 ± 165.90a,F | 8,242.17 ± 501.78a,E | 26,380.04 ± 1,466.24a,D. | 36,603.67 ± 951.58a,C | 52,268.38 ± 2,806.25a,B | 56,521.74 ± 1,119.68a,A |
| Group A | 1,503.73 ± 155.58a,F | 8,466.07 ± 329.87a,E | 26,752.52 ± 1,002.86a,D | 36,840.17 ± 1,149.83a,C | 52,640.33 ± 4,332.78a,B | 56,805.27 ± 1,049.26a,A |
| Group B | 1,384.23 ± 144.07a,F | 8,080.11 ± 424.51a,E | 26,126.67 ± 997.31a,D | 35,275.08 ± 1,057.91a,C | 51,587.33 ± 3,505.65a,B | 56,281.33 ± 2,809.91a,A |
| Springiness | ||||||
| Control | 0.525 ± 0.05a,B | 0.795 ± 0.03b,A | 0.669 ± 0.07b,A | 0.776 ± 0.02b,A | 0.763 ± 0.07b,A | 0.737 ± 0.05b,A |
| Group A | 0.557 ± 0.07a,B | 0.809 ± 0.04b,A | 0.679 ± 0.08b,A,B | 0.793 ± 0.10b,A | 0.810 ± 0.07b,A | 0.777 ± 0.12b,A |
| Group B | 0.6167 ± 0.09a,B | 0.936 ± 0.04a,A | 0.854 ± 0.03a,A | 0.956 ± 0.05a,A | 0.947 ± 0.05a,A | 0.927 ± 0.08a,A |
| Cohesiveness | ||||||
| Control | 0.452 ± 0.03b,C | 0.608 ± 0.07a,B | 0.743 ± 0.10a,A | 0.630 ± 0.09a,A,B | 0.568 ± 0.01a,B,C | 0.474 ± 0.06a,C |
| Group A | 0.560 ± 0.05a,C | 0.653 ± 0.06a,A,B | 0.780 ± 0.135a,A | 0.673 ± 0.11a,A,B | 0.655 ± 0.08a,A,B | 0.509 ± 0.04a,C |
| Group B | 0.476 ± 0.05b,C | 0.630 ± 0.01a,B | 0.725 ± 0.09a,A | 0.625 ± 0.06a,B | 0.572 ± 0.04a,B | 0.464 ± 0.02a,C |
| Chewiness | ||||||
| Control | 6,152.73 ± 632.40a,E | 14,933.53 ± 1,174.65a,D | 22,532.50 ± 1,401.57a,B | 25,057.33 ± 1,111.94a,A | 26,264.57 ± 1,012.75b,A | 20,076.81 ± 2,216.89b,C |
| Group A | 5,061.83 ± 659.21b,E | 12,559.48 ± 687.27a,D | 21,645.52 ± 1,193.83a,C | 24,350.27 ± 906.22a,B | 28,122.78 ± 1,607.56a,b,A | 25,814.74 ± 1,065.73a,B |
| Group B | 5,435.87 ± 540.48a,b,E | 13,883.87 ± 1,342.03a,D | 20,950.95 ± 997.10a,C | 24,044.25667 ± 192.60a,B | 29,174.33 ± 1,065.69a,A | 24,142.51 ± 1,635.48a,B |
| Resilience | ||||||
| Control | 0.130 ± 0.02a,E | 0.277 ± 0.01b,A | 0.256 ± 0.03a,A,B | 0.222 ± 0.03b,B,C | 0.204 ± 0.03a,C,D | 0.173 ± 0.04a,D,E |
| Group A | 0.118 ± 0.01a,D | 0.292 ± 0.03a,b,A | 0.282 ± 0.02a,b,A,B | 0.244 ± 0.02a,b,A,B,C | 0.236 ± 0.02a,b,B,C | 0.215 ± 0.02a,C |
| Group B | 0.132 ± 0.01a,D | 0.334 ± 0.04a,A | 0.318 ± 0.02a,A | 0.278 ± 0.02a,B | 0.251 ± 0.01a,B,C | 0.232 ± 0.03a,C |
Values are expressed as means ± SD; a, b, and c indicate that values in the same column are significantly different (P < 0.05); A, B, and C indicate that values in the same row are significantly different (P < 0.05).
Sensory Evaluation
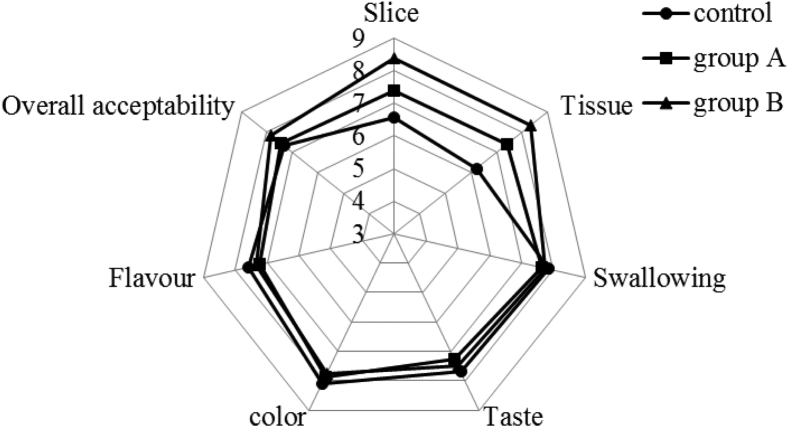
Figure 6 shows the sensory scores of the 3 group sausages on a 1–9 hedonic scale. It was observed that the 2 low-fat groups (group A and group B) added with JAP and pre-emulsified olive oil had no significant difference with the control group in flavor, color, taste, swallowing, and overall acceptability (P > 0.05). However, group A and group B both scored higher than the control in tissue and slice character (P < 0.05), and group B scored higher than group A in tissue and slice character (P < 0.05), which illustrated that the fat replacers addition improved slicing and tissue characteristics, especially JAP addition. So the fat-reduction samples improved sensory quality of the sausages. Previous report of partial replacement of animal fat by inulin has also obtained similar results that the sensory quality was improved (Mendoza et al., 2001).
Figure 6.
The sensory properties of the 3 group sausages.
Conclusion
Overall, the replacement of pork fat with JAP and olive oil reducted the fat content, increased the ratio of total UFA to total SFA, and, in particular, increased the oleic acid content derived from the olive oil. These results indicated that sausages in the fat-reduction groups had better health-related characteristics than those of the control. The addition of fat replacers significantly enhanced the formation of muscle protein gels and the gel network by changes in C–H stretching and bending vibrations, reductions of α-helixes, and increases in β-sheets and random coils accompanying the exposure of reactive groups and the microenvironment of the tertiary structure. The structural characteristics could improve the elasticity, texture, and water holding capacity of the sausages. Furthermore, the reduction of fat and the addition of replacers significantly enhanced lipid oxidative resistance and sensory quality of the sausages. Thus, JAP and olive oil have the potential to serve as heathy substitutes for animal fat in meat products.
Acknowledgments
The research was financially supported by the National Natural Science Foundation of China (No. 31501512), Shandong Province Key Research and Development plan-Special Plan for Medical Food (2019YYSP023), and the regulation mechanism of quality deterioration of fresh produce through water activity and microorganism (No. 2016YFD0400105). The authors would like to thank Editage (www.editage.cn) for English language editing.
Conflict of Interest Statement: The authors declared that there had no conflict of interest.
References
- Adam M., Cervenka L., Rezkova S., Ventura K., Kalovsky J. Determination of water content in meat pâté by karl fischer titration and its moisture sorption characteristics. Ital. J. Food Sci. 2009;21:89–95. [Google Scholar]
- Afoakwah N.A., Dong Y., Zhao Y., Xiong Z., Owusu J., Wang Y., Zhang J. Characterization of Jerusalem artichoke (Helianthus tuberosus L.) powder and its application in emulsion-type sausage. LWT-Food Sci. Technol. 2015;64:74–81. [Google Scholar]
- Andrews J.H., Harris R.F. The ecology and biogeography of microorganisms on plant surfaces. Annu. Rev. Phytopatho. 2000;38:145–180. doi: 10.1146/annurev.phyto.38.1.145. [DOI] [PubMed] [Google Scholar]
- AOAC . AOAC International; Gaithersburg, MD: 2016. Official Methods of Analysis, Method 966. 23, Bacteriological Analytical Manual Aerobic Plate Count. 20th ed. [Google Scholar]
- Beattie R.J., Bell S.J., Farmer L.J., Moss B.W., Patterson D. Preliminary investigation of the application of Raman spectroscopy to the prediction of the sensory quality of beef silverside. Meat Sci. 2004;66:903–913. doi: 10.1016/j.meatsci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Bolger Z., Brunton N.P., Monahan F.J. Impact of inclusion of flaxseed oil (pre-emulsified or encapsulated) on the physical characteristics of chicken sausages. J. Food Eng. 2018;230:39–48. [Google Scholar]
- Bontempo L., Paolini M., Franceschi P., Ziller L., García-González D.L., Camin F. Characterisation and attempted differentiation of European and extra-European olive oils using stable isotope ratio analysis. Food Chem. 2019;276:782–789. doi: 10.1016/j.foodchem.2018.10.077. [DOI] [PubMed] [Google Scholar]
- Cegiełka A., Tambor K. Effect of inulin on the physical, chemical and sensory attributes of Polish chicken burgers. J. Food Res. 2012;1:169–178. [Google Scholar]
- Cheetangdee N. Characteristic of sausages as influenced by partial replacement of pork back-fat using pre-emulsified soybean oil stabilized by fish proteins isolate. Agric. Nat. Resour. 2017;51:310–318. doi: 10.1007/s13197-017-2623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Han M. Raman spectroscopic study of the effects of microbial transglutaminase on heat-induced gelation of pork myofibrillar proteins and its relationship with textural characteristics. Food Res. Int. 2011;44:1514–1520. doi: 10.1016/j.meatsci.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Chen X., Li J., Zhou T., Li J., Yang J., Chen W., Xiong Y.L. Two efficient nitrite-reducing Lactobacillus strains isolated from traditional fermented pork (NanxWudl) as competitive starter cultures for Chinese fermented dry sausage. Meat Sci. 2016;121:302–309. doi: 10.1016/j.meatsci.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Chin K.B., Keeton J.T., Miller R.K., Longnecker M.T., Lamkey J.W. Evaluation of konjac Blends and soy protein isolate as fat replacements in low-fat Bologna. J. Food Sci. 2000;65:756–763. [Google Scholar]
- National Health Commission of the People's Republic of China . China Standard Press; Bejing, China: 2016. Chinese national food safety standard GB2726, National Food Safety Standards of Cooked Meat Products. [Google Scholar]
- Duflot M., Sánchez-Alonso I., Duflos G., Careche M. LF 1H NMR T2 relaxation rate as affected by water addition, NaCl and pH in fresh, frozen and cooked minced hake. Food Chem. 2019;277:229–237. doi: 10.1016/j.foodchem.2018.10.106. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li D., Liu X. Bacteriocin-producing Lactobacillus sakei C2 as starter culture in fermented sausages. Food Control. 2014;35:1–6. [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Giménez B., Graiver N., Califano A., Zaritzky N. Quality attributes and shelf life of high-pressure preserved beef as affected by pre-treatment conditions. Food Bioproc. Tech. 2017;10:2013–2022. [Google Scholar]
- Guo L., Yu B., Wang S., Zhu Y., Li P., Wang B., Hung M., Sun J. Effect of ripening with Penicillium roqueforti on texture, microstructure, water distribution and volatiles of chicken breast meat. Int. J. Food Sci. Tech. 2019;54:1550–1557. [Google Scholar]
- Hernández M.L., Velázquez-Palmero D., Sicardo M.D., Fernández J.E., Diaz-Espejo A., Martínez-Rivas J.M. Effect of a regulated deficit irrigation strategy in a hedgerow ‘Arbequina’olive orchard on the mesocarp fatty acid composition and desaturase gene expression with respect to olive oil quality. Agr. Water Manag. 2018;204:100–106. [Google Scholar]
- Herrero A.M., Cambero M.I., Ordóñez J.A., De La Hoz L., Carmona P. Raman spectroscopy study of the structural effect of microbial transglutaminase on meat systems and its relationship with textural characteristics. Food Chem. 2008;109:25–32. doi: 10.1016/j.foodchem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Kang Z.L., Chen F.S., Ma H.J. Effect of pre-emulsified soy oil with soy protein isolate in frankfurters: a physical-chemical and Raman spectroscopy study. LWT-Food Sci. Technol. 2016;74:465–471. [Google Scholar]
- Keenan D.F., Resconi V.C., Kerry J.P., Hamill R.M. Modelling the influence of inulin as a fat substitute in comminuted meat products on their physico-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014;96:1384–1394. doi: 10.1016/j.meatsci.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Kim I.S., Jin S.K., Mandal P.K., Kang S.N. Quality of low-fat pork sausages with tomato powder as colour and functional additive during refrigerated storage. J. Food Sci. Tech. 2011;48:591–597. doi: 10.1007/s13197-010-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton P.M., Zhao G., Binkoski A.E., Coval S.M., Etherton T.D. The effects of nuts on coronary heart disease risk. Nutr. Rev. 2001;59:103–111. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Kumar Y., Kairam N., Ahmad T., Yadav D.N. Physico chemical, microstructural and sensory characteristics of low-fat meat emulsion containing aloe gel as potential fat replacer. Int. J. Food Sci. Tech. 2016;51:309–316. [Google Scholar]
- Leelapongwattana K., Benjakul S., Visessanguan W., Howell N.K. Raman spectroscopic analysis and rheological measurements on natural actomyosin from haddock (Melanogrammus aeglefinus) during refrigerated (4°C) and frozen (−10°C) storage in the presence of trimethylamine-N-oxide demethylase from kidney of lizardfish (Saurida tumbil) Food Chem. 2008;106:1253–1263. [Google Scholar]
- Li Y., Hruby A., Bernstein A.M., Ley S.H., Wang D.D., Chiuve S.E., Sampson L., Rexrode K.M., Rimm E.B., Willett W.C., Hu F.B. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective cohort study. J. Am. Coll. Cardiol. 2015;66:1538–1548. doi: 10.1016/j.jacc.2015.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowler H., Thondre S., Holz A., Theis S. Replacement of glycaemic carbohydrates by inulin-type fructans from chicory (oligofructose, inulin) reduces the postprandial blood glucose and insulin response to foods: report of two double-blind, randomized, controlled trials. Eur. J. Nutr. 2018;57:1259–1268. doi: 10.1007/s00394-017-1409-z. [DOI] [PubMed] [Google Scholar]
- Liu R., Zhao S.M., Xie B.J., Xiong S.B. Contribution of protein conformation and intermolecular bonds to fish and pork gelation properties. Food Hydrocoll. 2011;25:898–906. [Google Scholar]
- Magrinyà N., Bou R., Rius N., Codony R., Guardiola F. Effect of fermentation time and vegetable concentrate addition on quality parameters of organic botifarracatalana, a cured–cooked sausage. J. Agr. Food Chem. 2012;60:6882–6890. doi: 10.1021/jf301218k. [DOI] [PubMed] [Google Scholar]
- Marchetti L., Andres S.C., Califano A.N. Low-fat meat sausages with fish oil: Optimization of milk proteins and carrageenan contents using response surface methodology. Meat Sci. 2014;96:1297–1303. doi: 10.1016/j.meatsci.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Mendoza E., Garcia M.L., Casas C., Selgas M.D. Inulin as a fat substitute in low fat, dry fermented sausages. Meat Sci. 2001;57:387–393. doi: 10.1016/s0309-1740(00)00116-9. [DOI] [PubMed] [Google Scholar]
- Menegas L.Z., Pimentel T.C., Garcia S., Prudencio S.H. Dry-fermented chicken sausage produced with inulin and corn oil. Physicochemical, microbiological and textural characteristics and acceptability during storage. Meat Sci. 2013;93:501–506. doi: 10.1016/j.meatsci.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Nawrocka A., Szymańska-Chargot M., Miś A., Ptaszyńska A.A., Kowalski R., Waśko P., Gruszecki W.I. Influence of dietary fibre on gluten proteins structure-a study on model flour with application of FT-Raman spectroscopy. J. Raman Spectrosc. 2015;46:309–316. [Google Scholar]
- Nawrocka A., Szymańska-Chargot M., Miś A., Wilczewska A.Z., Markiewicz K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins–A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017;69:410–421. [Google Scholar]
- Nocella C., Cammisotto V., Fianchini L., D'Amico A., Novo M., Castellani V., Stefanini L., Violi F., Carnevale R. Extra virgin olive oil and cardiovascular diseases: Benefits for human health. Endocr. Metab. Immune Disord. Drug Targets. 2018;18:4–13. doi: 10.2174/1871530317666171114121533. [DOI] [PubMed] [Google Scholar]
- Pintado T., Herrero A.M., Jiménez-Colmenero F., Cavalheiro C.P., Ruiz-Capillas C. Chia and oat emulsion gels as new animal fat replacers and healthy bioactive sources in fresh sausage formulation. Meat Sci. 2018;135:6–13. doi: 10.1016/j.meatsci.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Pereira J., Hu H., Xing L., Zhang W., Zhou G. Influence of rice flour, Glutinous rice flour, and Tapioca starch on the functional properties and quality of an emulsion-type cooked sausage. Foods. 2020;9:9. doi: 10.3390/foods9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quek S.Y., Chok N.K., Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. 2007;46:386–392. [Google Scholar]
- Racanicci A.M., Danielsen B., Skibsted L.H. Mate (Ilex paraguariensis) as a source of water extractable antioxidant for use in chicken meat. Eur. Food Res. Technol. 2008;227:255–260. [Google Scholar]
- Rather S.A., Masoodi F.A., Akhter R., Gani A., Wani S.M., Malik A.H. Xanthan gum as a fat replacer in goshtaba-a traditional meat product of India: effects on quality and oxidative stability. J. Food Sci. Tech. 2015;52:8104–8112. doi: 10.1007/s13197-015-1960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather S.A., Masoodi F.A., Akhter R., Rather J.A., Amin F. Effects of guar gum as a fat substitute in low fat meat emulsions. J. Food Process. Preserv. 2017;41:1–9. [Google Scholar]
- Ruiz-Capillas C., Triki M., Herrero A.M., Rodriguez-Salas L., Jiménez-Colmenero F. Konjac gel as pork backfat replacer in dry fermented sausages: processing and quality characteristics. Meat Sci. 2012;92:144–150. doi: 10.1016/j.meatsci.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Samieri C., Féart C., Proust-Lima C., Peuchant E., Tzourio C., Stapf C., Berr P., Barberger-Gateau P. Olive oil consumption, plasma oleic acid, and stroke incidence: the Three-City Study. Neurology. 2011;77:418–425. doi: 10.1212/WNL.0b013e318220abeb. [DOI] [PubMed] [Google Scholar]
- Shao J.H., Deng Y.M., Jia N., Li R.R., Cao J.X., Liu D.Y., Li J.R. Low-field NMR determination of water distribution in meat batters with NaCl and polyphosphate addition. Food Chem. 2016;200:308–314. doi: 10.1016/j.foodchem.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Sheng L., Wang J., Huang M., Xu Q., Ma M. The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol. 2016;92:600–606. doi: 10.1016/j.ijbiomac.2016.07.068. [DOI] [PubMed] [Google Scholar]
- Shin J.M., Park S.K. Comparison of fat determination methods depending on fat definition in bakery products. LWT-Food Sci. Technol. 2015;63:972–977. [Google Scholar]
- Sun J., Li X., Xu X., Zhou G. Influence of various levels of flaxseed gum addition on the water-holding capacities of heat-induced porcine myofibrillar protein. J. Food Sci. 2011;76:C472–C478. doi: 10.1111/j.1750-3841.2011.02094.x. [DOI] [PubMed] [Google Scholar]
- Tadpitchayangkoon P., Park J.W., Mayer S.G., Yongsawatdigul J. Structural changes and dynamic rheological properties of sarcoplasmic proteins subjected to pH-shift method. J. Agr. Food Chem. 2010;58:4241–4249. doi: 10.1021/jf903219u. [DOI] [PubMed] [Google Scholar]
- Tárrega A., Torres J.D., Costell E. Influence of the chain-length distribution of inulin on the rheology and microstructure of prebiotic dairy desserts. J. Food Eng. 2011;104:356–363. [Google Scholar]
- Terés S., Barceló-Coblijn G., Benet M., Alvarez R., Bressani R., Halver J.E., Escribá P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA. 2008;105:13811–13816. doi: 10.1073/pnas.0807500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triki M., Herrero A.M., Rodríguez-Salas L., Jiménez-Colmenero F., Ruiz-Capillas C. Chilled storage characteristics of low-fat, n-3 PUFA-enriched dry fermented sausage reformulated with a healthy oil combination stabilized in a konjac matrix. Food Control. 2013;31:158–165. [Google Scholar]
- van Hees A.M., Saris W.H., Hul G.B., Schaper N.C., Timmerman B.E., Lovegrove J.A., Roche H.M., Blaak E.E. Effects of dietary fat modification on skeletal muscle fatty acid handling in the metabolic syndrome. Int. J. Obes. 2010;34:859–870. doi: 10.1038/ijo.2010.6. [DOI] [PubMed] [Google Scholar]
- Wang D.D., Hu F.B. Dietary fat and risk of cardiovascular disease: Recent controversies and advances. Annu. Rev. Nutri. 2017;37:423–446. doi: 10.1146/annurev-nutr-071816-064614. [DOI] [PubMed] [Google Scholar]
- Wilson B., Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroen. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- Yang H., Han M., Bai Y., Han Y., Xu X., Zhou G. High pressure processing alters water distribution enabling the production of reduced-fat and reduced-salt pork sausages. Meat Sci. 2015;102:69–78. doi: 10.1016/j.meatsci.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang Y., Tang X., Chen Y., You Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chem. 2015;188:111–118. doi: 10.1016/j.foodchem.2015.04.129. [DOI] [PubMed] [Google Scholar]