Abstract
System xc- is a heterodimeric amino acid antiporter that, in the central nervous system, is best known for linking the import of L-cystine (CySS) with the export of L-glutamate for the production and maintenance of cellular glutathione (GSH) and extracellular glutamate levels, respectively. Yet, mice that are null for system xc- are healthy, fertile, and, morphologically, their brains are grossly normal. This suggests other glutamate and/or cyst(e)ine transport mechanisms may be upregulated in compensation. To test this, we measured the plasma membrane expression of Excitatory Amino Acid Transporters (EAATs) 1–3, the Alanine-Serine-Cysteine-Transporter (ASCT) 1, the sodium-coupled neutral amino acid transporter (SNAT) 3 and the L Amino Acid Transporter (LAT) 2 in striatum, hippocampus and cortex of male and female mice using Western Blot analysis. Present results demonstrate brain region and transporter-specific changes occurs in female system xc- null mice with increased expression of EAAT1 and ASCT1 occurring in the striatum and cortex, respectively, and decreased SNAT 3 expression in cortex. In male system xc- null brain, only SNAT3 was altered significantly - increasing in the cortex, but decreasing in the striatum. Total levels of GSH and CyS were similar to that found in age and sex-matched littermate control mice, however, reductions in the ratio of reduced to oxidized GSH (GSH/GSSG) — a hallmark of oxidative stress — were found in all three brain regions in female system xc- null mice, whereas this occurred exclusively in the striatum of males. Protein levels of Superoxide dismutase (SOD) 1 were reduced, whereas SOD2 was enhanced in the hippocampus of male xc- null mice only. Finally, striatal vulnerability to 3-nitropropionic acid (3-NP)-mediated oxidative stress in either sex showed no genotype difference, although 3-NP was more toxic to female mice of either genotype, as evidenced by an increase in moribundity as compared to males.
Keywords: System xc-, Compensation, Cysteine, Oxidative Stress, Glutathione
1. Introduction
System xc- — an obligate exchanger best known for linking the import of L-cystine [but see (Kobayashi et al., 2015)] to the export of L-glutamate in a 1:1 ratio (Bassi et al., 2001) — is expressed highly in a subset of astrocytes throughout the brain including in the striatum, hippocampus, cortex and cerebellum (Ottestad-Hansen et al., 2018; Shih et al., 2006; Zhang et al., 2014).The glutamate exported from system xc- is an important contributor to the ambient extracellular glutamate levels that bathe the central nervous system in vivo (Baker et al., 2002a; Baker et al., 2002b; De Bundel et al., 2011; Massie et al., 2011; McCullagh and Featherstone, 2014). Rapid reduction of intracellular cystine to cysteine allows for its incorporation into proteins as well as into GSH (Bassi et al., 2001; Lewerenz et al., 2013; Sato et al., 1999). GSH is one of a number of endogenous antioxidant molecules employed to protect against oxidative stress/damage [for review see (Gilgun-Sherki et al., 2001; Halliwell, 2006). One of the major determinates of the rate of GSH synthesis is the availability of its substrate cyst(e)ine (Deneke and Fanburg, 1989; Kranich et al., 1996; Sagara et al., 1993). It is important to note that neurons are only able to import a small amount of cystine and therefore must be supplied with cysteine (Kranich et al., 1996; Shanker et al., 2001), primarily through release and catabolism of GSH (Hanigan and Ricketts, 1993) from astrocytes (Dringen et al., 1997). Remarkably, it has been estimated that ≈10% of astrocytic GSH is released per hour (Dringen et al., 1997), indicating that synthesis must match release in order to maintain a constant concentration of GSH.
Both GSH and cysteine are common sources of reducing equivalents for combating oxidative stress, which is the imbalance between the formation and removal of reactive oxygen species (ROS), that can damage DNA, proteins and lipids (Bains and Shaw, 1997). Apart from their antioxidant effects, GSH and cysteine participate in signal transduction, and regulate gene expression, cellular metabolism and cell proliferation (Lu, 2009; Nkabyo et al., 2005; Noda et al., 2002; Ramirez et al., 2007; Wu et al., 2004).
Cells that lack system xc- are unable to grow in vitro without addition of a reducing agent such as 2-Mercaptoethanol (2-ME), which maintains viability and supports growth (Chintala et al., 2005; Jackman et al., 2010; Shih et al., 2006), either via direct reduction of extracellular cystine to cysteine or by forming a mixed disulfide of 2-ME/cysteine for uptake by non-xCT (e.g. system L) transport systems (Ishii et al., 1981). Despite this, system xc- appears to be dispensable in mammalian development and health as mice null for the antiporter, resulting from either transgenic knockout of, or a natural null mutation in the gene Slc7a11, which encodes its substrate specific light chain, xCT (xCT−/− and Slc7a11sut/sut mice, respectively) (Chintala et al., 2005; Sato et al., 2005), are healthy and fertile. Of importance, Slc7a11sut/sut male mice, bred homozygously, have been reported to show enlarged lateral ventricles and striatal and cortical “thinning” at 15 weeks of age (Shih et al., 2006), an effect unobserved in our SLC7A11sut/sut mouse colony bred heterozygously (Sears and Hewett; unpublished observations) or in xCT−/− null mice (De Bundel et al., 2011; Sato et al., 2005). Given these findings, we hypothesized that other transport systems may compensate for the loss of system xc- to regulate amino acid levels in vivo. Toward this end, we investigated whether plasma membrane protein expression of prominent glutamate and cyst(e)ine CNS transport systems, many of which are expressed in astrocytes —namely Excitatory Amino Acid Transporters 1–3 (EAAT1, Slc1a3; EAAT2, Slc1a2; EAAT3, Slc1a1), the Alanine-Serine-Cysteine-Transporter 1 (ASCT1; Slc1a4), System N transporter 3 (SNAT3, Slc38a8) and/or L Amino Acid Transporter 2 (LAT2; Slc7a8) —are upregulated in cortex, striatum and hippocampus of male and female Slc7a11sut/sut system xc- null mice. Herein, we report that changes in expression of glutamate and cysteine transporters occurs in brains of system xc- null mice in both a transporter-specific, region-specific and sexually dimorphic manner.
2. Methods
2.1. Animals:
This study was conducted in accordance with the National Institute of Health guidelines for the use of experimental animals and has been approved by the Institutional Animal Care and Use Committee of Syracuse University. Mice were maintained on a 12-hour light/dark schedule and provided mouse chow and water ad libitum. Littermate mice wild-type (Slc7a11+/+) or lacking system xc- (Slc7a11sut/sut) for this study (Holmdahl and Malissen, 2012) were derived from F1 heterozygous breeding units (C3H/HeSnJ-Slc7a11sut/J; JAX # 001310). Slc7a11sut/sut mice harbor a natural null mutation in exon 12 of Slc7a11, which encodes for xCT, the substrate specific light chain of system xc- (Chintala et al., 2005). Slc7a11sut/sut mice have no xCT mRNA (Chintala et al., 2005) or protein (McCullagh and Featherstone, 2014) in brain. We have additionally validated lack of system xc- activity in astrocytes derived from said mice (Jackman et al., 2010).
F2 and F3 generations only are used for experimentation at 17–20 weeks of age (Wolfer et al., 2002; Wolfer and Lipp, 2000). Male and female mice are used to address sex as a biological variable. Mice are separated by sex at weaning, and following initial genotyping, are placed 4–5/cage ensuring that members of each genotype are represented to control for any environmental effects (Pick and Little, 1965; Wolfer and Lipp, 2000). Investigators blind to genotype performed experimental procedures and subsequent analyses. Mice were regenotyped upon the conclusion of each study and results crosschecked with the original. Genotyping was performed via PCR analysis of tail genomic DNA samples: WT primers (230 bp) 5’- GAA GTG CTC CGT GAA GG −3’ (forward), 5’- ATC TCA ATC CTG GGC AGA TG - 3’ (reverse); mutant primers (2280 bp) 5’- CCA CTG TTG TAG GTC AGC TTA GG −3’ (forward), 5’- CAG GAC CTG TGA ATA TGA TAG GG −3’ (reverse).
2.2. Immunoblots:
Mice were perfused transcardially with ice-cold 1× phosphate buffered saline (PBS) under full anesthesia. Bilateral striata, hippocampi and cortices were dissected, snap frozen with liquid nitrogen, and stored separately at −80°C prior to use. Plasma membrane and total proteins were extracted as described in each subsection and the resulting protein concentrations were quantified using the BCA assay kit (Pierce, Rockford, IL). Following SDS-PAGE and transfer, total protein levels were quantified using Revert™ Total Protein Stain (Lincoln, NE). Following reversal of Revert™, membranes were blocked in Odyssey® blocking buffer at 25°C for 1 hr, after which primary antibodies to the proteins of interest were added in in Odyssey® blocking buffer containing 0.2% Tween-20. All antibodies were added overnight at 4°C. Following thorough removal of antibody [3× 5ml 1XPBS with 0.1% Tween-20 (PBS-T) at 10 min intervals], species specific IR-Dyes [680LT or 800CW conjugated goat polyclonal anti-rabbit or 800CW conjugated goat polyclonal anti-mouse secondary antibody (LI-COR Biosciences; Lincoln, NE)] were added for 1 hr at 25°C at 1:10,000 dilution. Results were recorded on LI-COR ODYSSEY® Fc Imaging system (LI-COR Biosciences) and protein levels quantified using Image Studio 3.1 (LI-COR Biosciences; Lincoln, NE). To ensure that we were working within the linear range, the optimum concentration for each antibody and antigen were determined via serial antibody and protein titration (Supplemental Figure 1).
2.2.1. Glutamate and Cyst(e)ine Transporter Expression:
Plasma membrane protein from five striata pooled from three mice, five hippocampi pooled from three mice, or two cortices taken from one mouse were isolated using a Plasma Membrane Protein Extraction Kit [Abcam, Cambridge, U.K. (ab65400)] from system xc- null (Slc7a11sut/sut) and wild-type (Slc7a11+/+) mice in parallel to allow for side-by-side comparisons that controlled for procedural variabilities in the amount of protein extracted. Pooled tissue was disrupted by 75 strokes in a dounce homogenizer containing 1mL homogenize buffer plus protease inhibitors. Plasma membrane proteins were isolated from total membranous proteins via aqueous polymer two-phase (PEG/Dextran) separation as described by manufacturer. Plasma membrane fraction were resuspended in PBS containing 0.001% Triton X-100 (40 μL) and stored at −80°C until used. Proteins (3 μg for EAAT1, 1 μg for EAAT2, 20 μg for EAAT3, 6μg for ASCT1, 6–8 μg for SNAT3 and 35μg for LAT2) were separated by 8% SDS-PAGE under severe reducing conditions (0.1M DTT and 8M Urea) —which effectively prevented formation of multimers (Supplemental Figure 1) — and electrophoretically transferred to a PVDF membrane (Bio-Rad; Hercules, CA). Rabbit polyclonal antibodies (Table 1) were used to detect EAAT1 (1.3 μg/mL), EAAT2 (1.3 μg/mL), EAAT3 (1.2 μg/mL), ASCT1 (2.3μg/mL) and SNAT3 (0.3μg/mL). A mouse monoclonal antibody was used to detect LAT2 (3.3μg/mL). Transporter protein levels were quantified by normalizing values to their respective total protein signals, followed by baseline correction to their wild-type levels from the same protein extraction (set to 1).
Table 1.
| Protein | Host Species | Company | Catalog Number | RRID | Epitope |
|---|---|---|---|---|---|
| EAAT1 | Rabbit | Abcam | Ab416 | AB_304334 | C-terminus of rat EAAT1 |
| EAAT2 | Rabbit | Abcam | Ab41621 | AB_941789 | C-terminus of rat EAAT2 |
| EAAT3 | Rabbit | Abcam | Ab124802 | AB_10974334 | Human EAAT3 residues 150-250 |
| ASCT1 | Rabbit | Alomone Labs | ANT-081 | AB_2756719 | Mouse Slc1a4 residues 500-512 |
| LAT2 | Mouse | OriGene | TA500514 | AB_11124222 | Full length Slc7a8 from HEK293T cells |
| SNAT3 | Rabbit | Proteintech | 14315-1-AP | AB_941782 | Residues 1-191 of Slc38a3 fusion protein |
| SOD1 | Rabbit | Millipore | 07-043 | AB_310587 | C-terminus of human Cu/Zn SOD |
| SOD2 | Rabbit | Sigma Aldrich | HPA001814 | AB_1080134 | Residues 2-146 of human SOD2 |
| Catalase | Mouse | Sigma Aldrich | C0979 | AB_258720 | Human erythrocyte catalase |
2.2.2. Antioxidant Expression:
Total protein from two striata, two hippocampi, or one cortex was extracted using RIPA Buffer (50mM Tris, 0.5% Deoxycholate, 0.1% SDS). Proteins (16μg for SOD1, 8μg for SOD2 and 25μg for Catalase) were separated by 8% (Catalase), 11% (SOD2), or 15% (SOD1) SDS-PAGE under reducing conditions and electrophoretically transferred to a PVDF membrane (Bio-Rad; Hercules, CA). Rabbit polyclonal antibodies (Table 1) were used to detect SOD1 (1.3 μg/mL), SOD2 (0.3 μg/mL) and a mouse monoclonal antibody was used to detect Catalase (0.7μg/mL). Antioxidant levels were quantified by normalizing values to their respective total protein signals, followed by baseline correction to the wild-type levels (set to 1).
2.3. Glutathione and Cysteine measurements:
Fully anesthetized naïve mice were perfused transcardially with ice-cold PBS. Striata, hippocampi, and cortices were rapidly dissected and snap-frozen separately in liquid nitrogen. The concentrations of reduced and oxidized glutathione (GSH and GSSG) and cysteine (CyS and CySS) were determined via high-performance liquid chromatography by the Emory-Children’s Pediatric Biomarkers Core facility (Atlanta, GA). Total GSH = GSH + 2(GSSG); Total CyS = CyS + 2(CySS).
2.4 3. -Nitropropionic Acid (3-NP) Dosing Protocol:
Mice were acclimated to handling by performing mock daily i.p. injections 5 days prior to each study. 3-NP (Sigma Aldrich Chemicals, St. Louis, MO) was dissolved in normal saline at 25mg/ml and adjusted to pH 7.4 using 5M NaOH. The solution was filter sterilized and stored at 4°C for no more than one week. Mice were weighed daily just prior to the first dose of 3-NP and injected twice daily at 8–12 h intervals by an experimenter blinded to the genotype. Mice completing the study received a total dose of 920 mg/kg 3-NP (i.p.) over twelve days using an escalating dosing protocol as follows (b.i.d) : 20 mg/kg X 2 days, 30 mg/kg X 3 days, 40 mg/kg X 3 days, 50 mg/kg X 3 days, 60 mg/kg X 1 day and were sacrificed ~12 hr after the final injection. Four separate experiments were performed over eight months.
2.5. Behavioral Scoring:
The severity scale for 3-NP-induced motor disorders was performed as described (Fernagut et al., 2002). General locomotor activity, hindlimb clasping, hindlimb dystonia and/or truncal dystonia and postural adjustment reflexes were assessed and recorded just prior to each injection by an observer blinded to genotype. Three scales were assigned corresponding to no abnormality (0), moderate (1,3) or severe deficits (2,5). Any mouse attaining a cumulative behavioral score ≥ 9 or sustaining a weight loss ≥ 20% was immediately sacrificed.
2.6. Histological Analysis:
Brain sections (40μm) cut serially through the rostro-caudal extent of each brain (+1.54 to −0.46 relative to bregma) (Microm HM-550 cryostat, ThermoScientific) were stained with 0.5% thionin as described in detail (Claycomb et al., 2011). Images (2400 pixels) were captured by scanning (Epson Perfection 3170). The lesion (denoted by pale thionin staining) and total striatal area of slices +1.42, +0.98, +0.50, +0.02 and −0.46 from bregma was measured using NIH ImageJ by three individuals blinded to the animal’s genotype. Striatal and lesion volumes were calculated using the Cavalieri principle (volume = (s1d1) + (s2d2) + (s3d3) + (s4d4) + (s5d5)) where s is the area and d is the distance between slices as described (Shih et al., 2005). The percent of the striatum that had a lesion was calculated bilaterally as follows: (L/T) x 100, where L and T represent the lesion volume and total striatal volume, respectively. Lesion incidence is expressed as the percent of the total mice subjected to the systemic injection paradigm with quantifiable lesions.
2.7. Succinate Dehydrogenase Activity
Succinate dehydrogenase (SDH) activity in crude brain mitochondrial preparations from the striatum were quantified two hours following injection with either saline or 200 mg/kg 3-NP as described (Shih et al., 2005).
2.8. Statistical Analyses:
All statistical analyses were performed using GraphPad Prism (Version 6.0.3 or higher, Graphpad Software, Inc., La Jolla, CA) as described in the individual figure legends.
3. Results
3.1. Comparison of Cysteine and Glutamate Transporter Expression between age- and sex-matched Slc7a11+/+ and Slc7a11sut/sut mice.
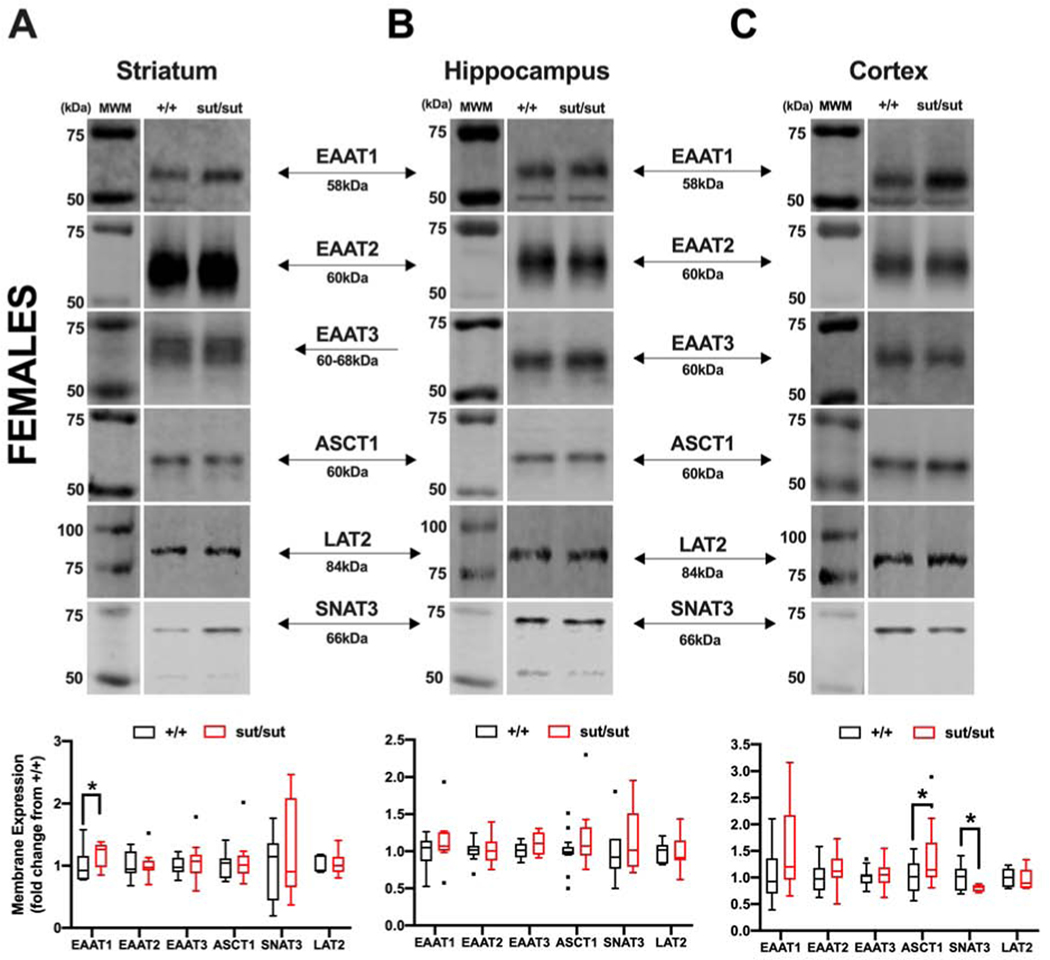
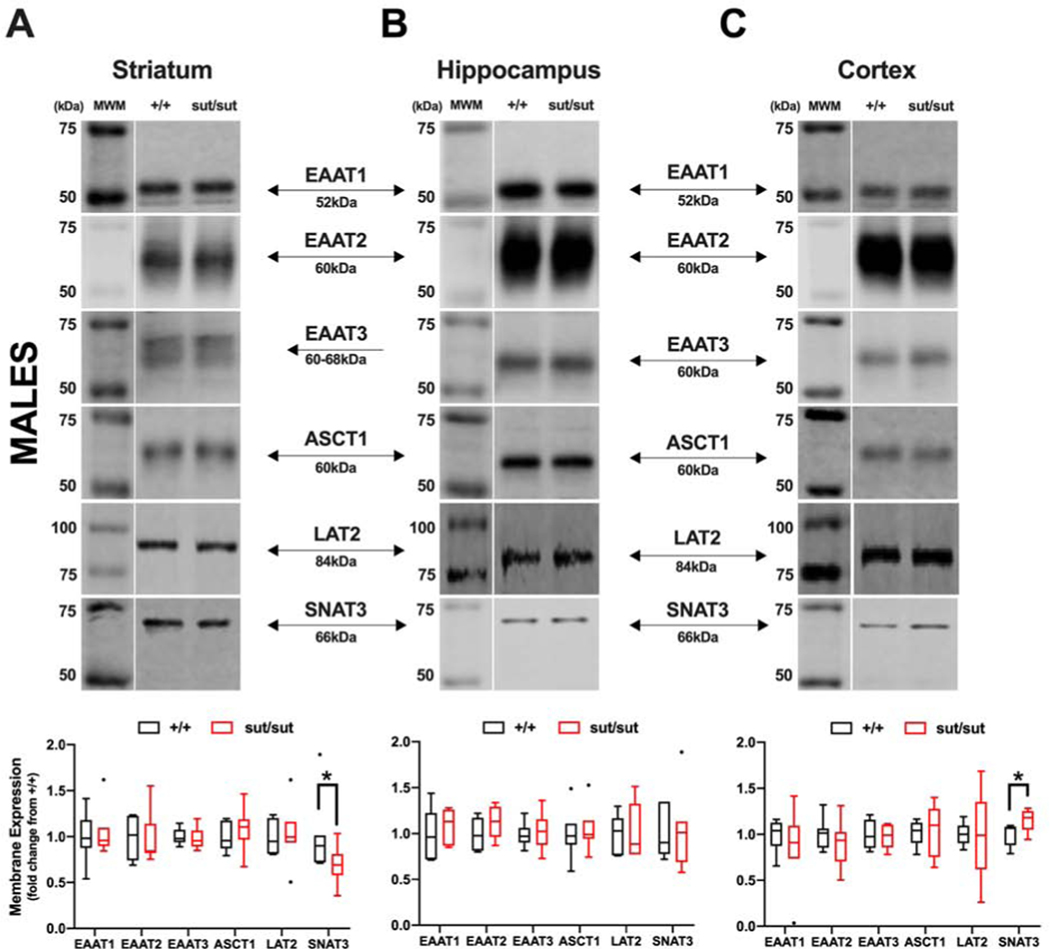
Given the fact that mice null for system xc- thrive and survive despite the inability of their cells to proliferate and persist in culture without addition of a reducing agent, we set out to determine whether the surface expression of other glutamate or cyst(e)ine transport systems — namely EAATs 1 and 2, EAAT3, ASCT1, SNAT3 and LAT2 — that with exception of EAAT3 are, like system xc-, predominately expressed on the plasma membrane of astrocytes— was altered in the areas of the striatum, hippocampus, and cortex of 17–20 week old female and male Slc7a11sut/sut mice as compared to Slc7a11+/+ age and sex-matched littermate controls. Overall, we find significant region- and transporter-specific changes in both sexes. In the striatum of female Slc7a11sut/sut mice, the only transporter that was altered was EAAT1 and its levels increased (p = 0.044) (Figure 1A). No change in the level of any transporter tested was found in the hippocampus. (Figure 1B). In the cortex, ASCT1 increased (p = 0.026) whereas SNAT3 decreased (p < 0.001) (Figure 1C). In male Slc7a11sut/sut mice, the only change in the plasma membrane expression of any transporter examined was that of SNAT3, which significantly decreased in striatum (p = 0.027) and increased in the cortex (p = 0.026) (Figure 2).
Figure 1: Comparison of glutamate and cysteine transporter levels between wild-type (+/+) and system xc- null (sut/sut) female mice in striatum, hippocampus, and cortex.
Plasma membrane striatal, hippocampal, and cortical protein extracted in pairs from +/+ and sut/sut female mice [5 striata or 5 hippocampi (5 mice per 2 samples), or 2 cortices (1 mouse per sample)] were separated under reducing conditions via 8% SDS-PAGE. Representative blots and protein quantification for EAAT1, 2, 3, ASCT1, SNAT3 and LAT2 protein in striatum (A), hippocampus (B) and cortex (C). Protein quantification was performed by normalizing to each samples’ representative total protein to correct for differences in loading followed by baseline correction to the paired +/+ levels. Values are graphed as Tukey’s box and whisker plots with the box representing the 2nd and 3rd quartiles, and whiskers representing points within 1.5 times the interquartile distance. An asterisk (*) represents a significant between-group difference calculated using a ratio-paired t-test on transformed data. Between-group differences for each brain areas are as follows: (A) Striatum: EAAT1 (p = 0.044; n = 6), EAAT2 (p = 0.477; n = 11), EAAT3 (p = 0.234; n = 10), ASCT1 (p = 0.343; n = 14), SNAT3 (p = 0.279; n = 6) and LAT2 (p = 0.376; n = 6). (B) Hippocampus: EAAT1 (p = 0.085; n = 11), EAAT2 (p = 0.169; n = 11 ), EAAT3 (p = 0.086; n = 7), ASCT1 (p = 0.144; n = 14), SNAT3 (p = 0.213; n = 7) and LAT2 (p = 0.436; n = 7). (C) Cortex: EAAT1 (p = 0.092; n = 14), EAAT2 (p = 0.2391; n = 14), EAAT3 (p = 0.426; n = 14), ASCT1 (p = 0.0267; n = 13), SNAT3 (p = 0.0001; n = 6), and LAT2 (F1,12 = 8.313 p = 0.260; n = 6).
Figure 2: Comparison of glutamate and cysteine transporter levels between wild-type (+/+) and system xc- null (sut/sut) male mice in striatum, hippocampus, and cortex.
Plasma membrane striatal, hippocampal, and cortical protein extracted in pairs from +/+ and sut/sut male mice [5 striata or 5 hippocampi (5 mice per 2 samples), or 2 cortices (1 mouse per sample)] were separated under reducing conditions via 8% SDS-PAGE. Representative blot for detection of EAAT1, 2, 3, ASCT1, SNAT3 and LAT2 protein in striatum (A), hippocampus (B), and cortex (C). Protein quantification was performed by normalizing to each samples’ representative total protein levels to correct for differences in loading followed by baseline correction to the paired +/+ levels. Values are graphed as Tukey’s box and whisker plots with the box representing the 2nd and 3rd quartiles, and whiskers representing points within 1.5 times the interquartile distance. An asterisk (*) represents a significant between-group difference calculated using a ratio-paired t-test on transformed data. (A) Striatum: EAAT1 (p = 0.3866; n = 7), EAAT2 (p = 0.468; n = 7), EAAT3 (p = 0.351; n = 6), ASCT1 (p = 0.493; n = 7), SNAT3 (p = 0.0267; n = 7) and LAT2 (p = 0.465; n = 7). (B) Hippocampus: EAAT1 (p = 0.186; n = 7), EAAT2 (p = 0.079; n = 7), EAAT3 (p = 0.487; n = 6), ASCT1 (p = 0.469; n = 7), SNAT3 (p = 0.438; n = 7) and LAT2 (p = 0.386; n = 6). (C) Cortex: EAAT1 (p = 0.149; n = 7), EAAT2 (p = 0.161; n = 7), EAAT3 (p = 0.325; n = 7), ASCT1 (p = 0.429; n = 7), SNAT3 (p = 0.026; n = 5) and LAT2 (p = 0.254; n = 5).
3.2. Comparison of reducing equivalents between age- and sex-matched Slc7a11+/+ and Slc7a11sut/sut mice.
We previously determined that male Slc7a11sut/sut mice had little to no alteration in their levels of reduced and oxidized cysteine (CyS and CySS) or glutathione (GSH and GSSG) in hippocampus and cortex (Sears et al., 2019). Herein, we report no change in the total CyS, CyS and CySS concentrations nor in the CyS/CySS ratio is present in the striatum of male Slc7a11sut/sut mice (Supplemental Figure 2A). However, despite the maintenance of total GSH, the GSH/GSSG ratio is significantly decreased (p = 0.02). Although we find no statistically significant change in either GSH or GSSH concentrations themselves, this ratio reduction appears to be driven mainly by the increase in GSSG (Supplemental Figure 2B). In females, total CyS levels in Slc7a11sut/sut mice show no statistically significant alteration nor do the concentrations of CyS or CySS in any brain region tested as compared to Slc7a11+/+ females (Figure 3A, 3C, 3E). Yet, the ratio of CyS/CySS is decreased in the cortex (p = 0.049), owing to a slight but non-significant increase in CySS (p = 0.378); no change in ratio in the striatum or hippocampus is found (Figure 3A–E). In contrast to what we found in males (Sears et al., 2019), the concentration of GSH is significantly decreased in the female Slc7a11sut/sut hippocampus (p = 0.007) (Figure 3D) with non-significant reductions noted in the striatum (p = 0.459) (Figure 3B) and cortex (p = 0.196) (Figure 3F). A slight but non-statistically significant increase in GSSG concentration is evident in all brain regions tested (Figure 3B, 3D, 3F). In keeping with this, the ratio of reduced to oxidized glutathione (GSH/GSSG) is lower in all three brain regions (Figure 3B, 3D, 3F), although this change is statistically significant only in the hippocampus (p = 0.006) (Figure 3D). These shifts to oxidized states suggest that certain brain regions in both female and male Slc7a11sut/sut mice are under oxidative stress.
Figure 3: Comparison of CyS and GSH redox in wild-type (+/+) and system xc- null (sut/sut) female mice in striatum, hippocampus, and cortex.
, CyS, CySS, GSH and GSSG levels were measured in +/+ and sut/sut females using HPLC. CyS, CySS, and the ratio of CyS:CySS of striatum (A), hippocampus (C) and cortex (E) are graphed as the mean + SEM. Total GSH (GSH + 2GSSG), GSH, GSSG, and the ratio of GSH:GSSG of striatum (B), hippocampus (D), and cortex (F) are graphed as the mean + SEM. An asterisk (*) represents between-group differences calculated using a Mann-Whitney test. Significance was set at P < 0.05.
3.3. Comparison of antioxidant protein expression levels between age- and sex-matched Slc7a11+/+ and Slc7a11sut/sut mice.
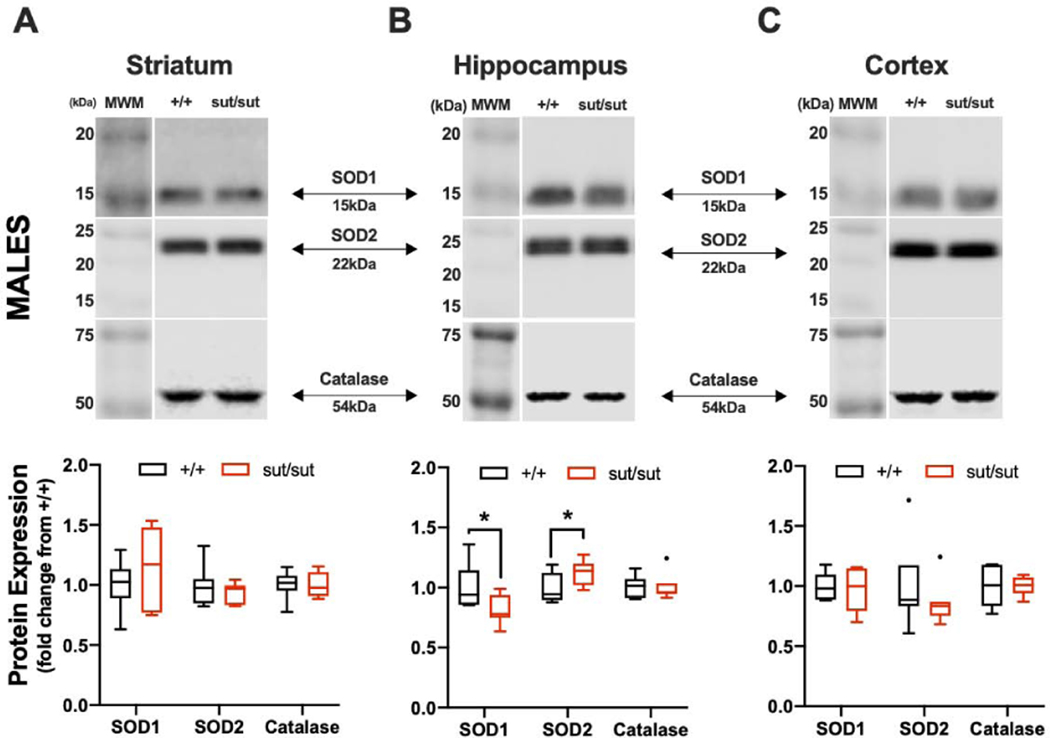
We next explored whether expression levels of other antioxidants might be upregulated, in a region specific manner, in efforts to protect the brain from oxidative stress. No statistically significant changes in expression of SOD1, 2 or catalase was found in any region of female Slc7a11sut/sut mouse brain, although the levels of SOD1 (p = 0.066) and catalase (p = 0.087) in the striatum tended to be smaller (Figure 4A). In Slc7a11sut/sut males, changes were restricted to the hippocampus, with total SOD1 protein levels being smaller (p = 0.050) and SOD2 levels larger (p = 0.033), than that isolated from male Slc7a11+/+ hippocampi (Figure 5B).
Figure 4: Comparison of antioxidant levels between wild-type (+/+) and system xc- null (sut/sut) female mice in striatum, hippocampus, and cortex.
Bilateral striatal, hippocampal, or cortical tissue was pooled from one mouse per sample and the protein extracted was separated under reducing conditions via 8% (catalase), 11% (SOD2), or 15% (SOD1) SDS-PAGE. Representative blot for detection of SOD1, SOD2, and catalase protein in striatum (A), hippocampus (B), and cortex (C). Protein quantification was performed by normalizing to each samples’ representative total protein to correct for differences in loading followed by baseline correction to the protein +/+ levels. Values are graphed as Tukey’s box and whisker plots with the box representing the 2nd and 3rd quartiles and whiskers representing points within 1.5 times the interquartile distance. There are no between-group differences calculated using a one-sided t-test with Welch’s correction. (A) Striatum: SOD1 (F1,6 = 36.86; p = 0.066), SOD2 (F1,6 = 1.022; p = 0.190) and catalase (F1,6 = 178.9; p = 0.087). (B) Hippocampus: SOD1 (F1,5 = 2.468; p = 0.383), SOD2 (F1,5 = 1.318; p = 0.364) and catalase (F1,6 = 1.922; p = 0.230). (C) Cortex: SOD1 (F1,6 = 4.702; p = 0.231), SOD2 (F1,6 = 2.655; p = 0.116) and catalase (F1,6 = 2.045; p = 0.448).
Figure 5: Comparison of antioxidant levels between wild-type (+/+) and system xc- null (sut/sut) male mice in striatum, hippocampus, and cortex.
Bilateral striatal, hippocampal, or cortical tissue was pooled from one mouse per sample and the protein extracted separated under reducing conditions via 8% (catalase), 11% (SOD2), or 15% (SOD1) SDS-PAGE. Representative blot for detection of SOD1, SOD2, and catalase protein in striatum (A), hippocampus (B), and cortex (C). Protein quantification was performed by normalizing to each samples’ representative total protein to correct for differences in loading followed by baseline correction to the protein +/+ levels. Values are graphed as Tukey’s box and whisker plots with the box representing the 2nd and 3rd quartiles, and whiskers representing points within 1.5 times the interquartile distance. An asterisk (*) represents a significant between-group difference calculated using a one-sided t-test with Welch’s correction. Between-group differences for each brain area are as follows: (A) Striatum: SOD1 (F1,6 = 4.193; p = 0.108), SOD2 (F1,6 = 7.71; p = 0.195) and catalase (F1,6 = 1.115; p = 0.442). (B) Hippocampus: SOD1 (F1,6 = 8.003; p = 0.050), SOD2 (F1,6 = 1.044; p = 0.033) and catalase (F1,6 = 1.801; p = 0.432). (C) Cortex: SOD1 (F1,6 = 1.574; p = 0.443), SOD2 (F1,6 = 9.502; p = 0.181) and catalase (F1,6 = 4.313; p = 0.422).
3.4. Comparison of the toxic effect of 3-nitropropionic acid (3-NP) of age- and sex-matched Slc7a11+/+ and Slc7a11sut/sut mice.
Finally, we compared the neurobehavioral and neurotoxicological responses between age and sex-matched Slc7a11+/+ and Slc7a11sut/sut mice to oxidative damage in vivo facilitated by repeated systemic administration of 3-nitropropionic acid (3-NP) (Beal, 1995; Reynolds et al., 1998; Schulz et al., 1996). We first confirmed that 3-NP significantly reduced SDH activities in striatum of both Slc7a11+/+and Slc7a11sut/sut mice to a similar extent, and that this occurs independent of sex (Supplemental Figure 3).
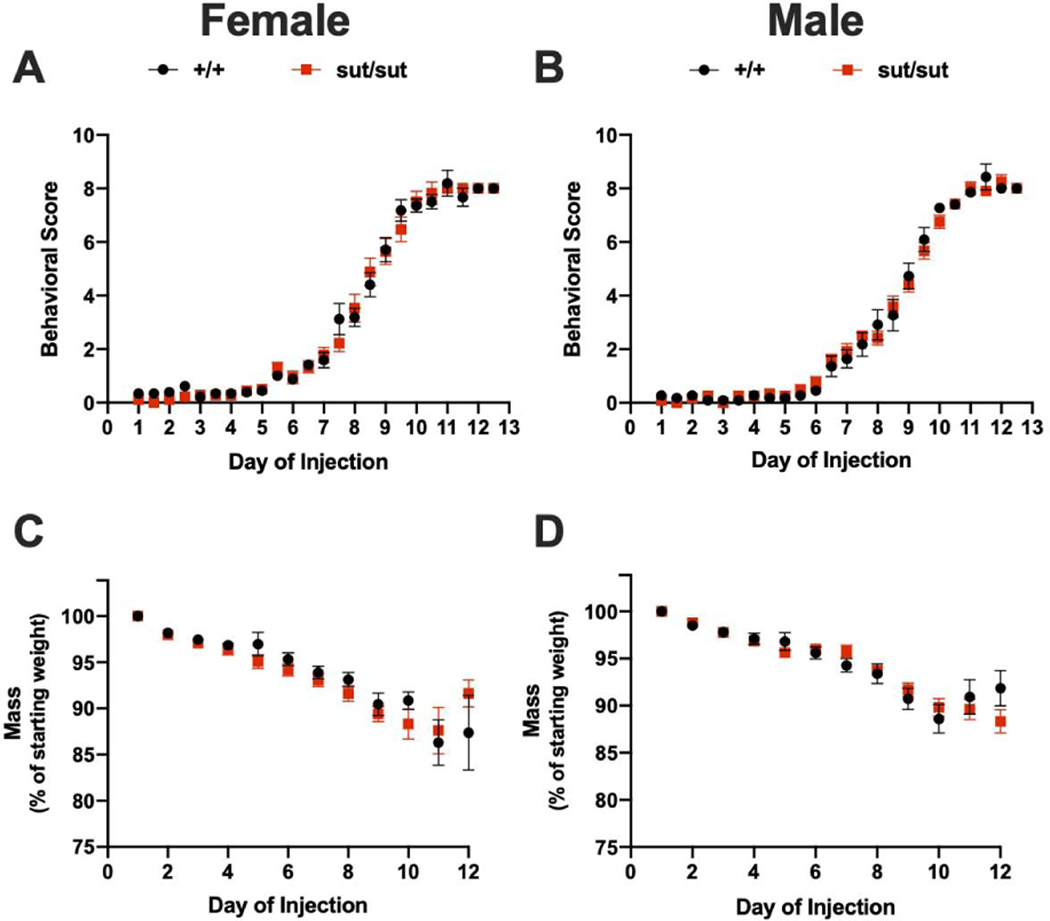
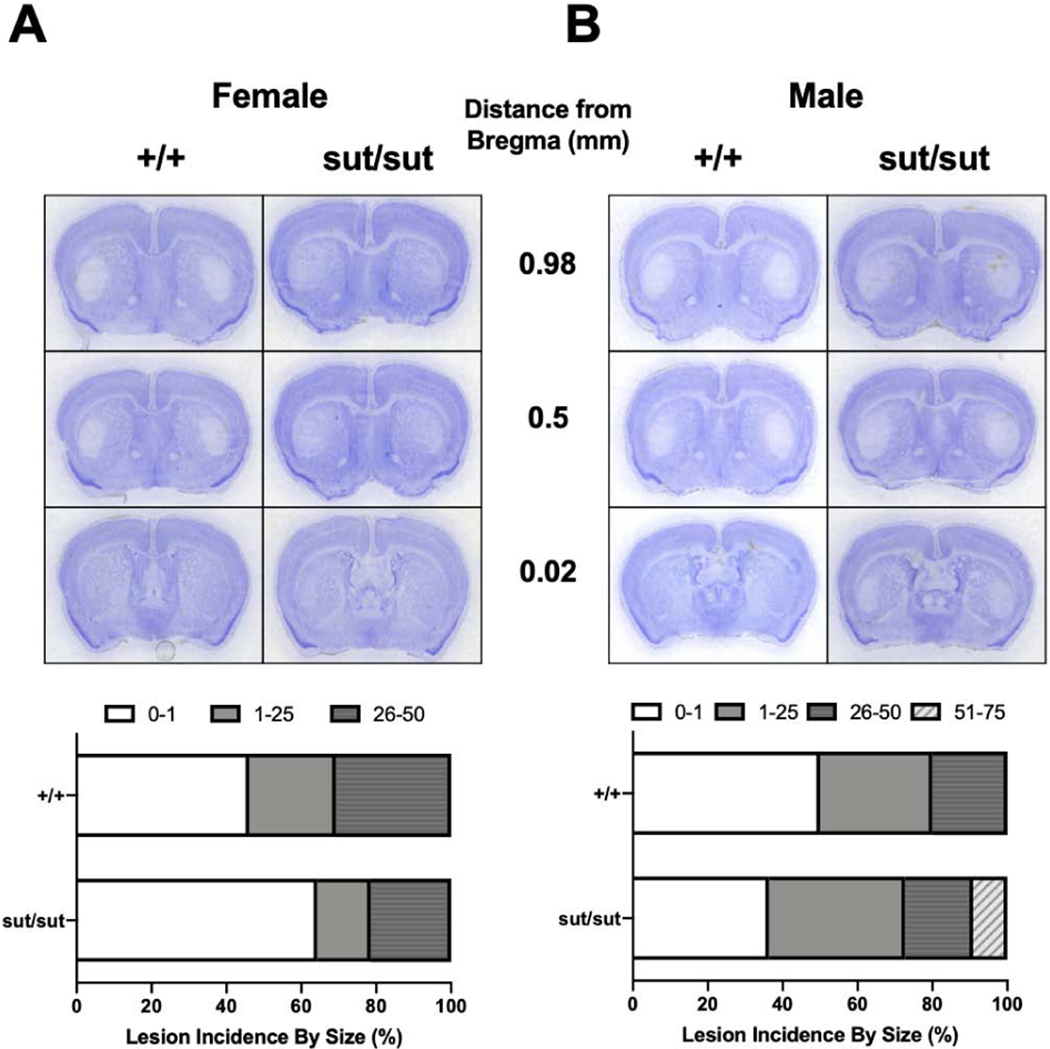
Motor symptoms are nonexistent until after 3-NP injection day 5 with cumulative behavioral scores increasing at the same rate irrespective of genotype in both females (day effect: p < 0.001, genotype effect: p = 0.73) (Figure 6A) and males (day effect: p < 0.001, genotype effect: p = 0.911) (Figure 6B). Over the 12 day paradigm, mice of both sexes progressively lose weight, as expected, with no differences in the percentage of weight lost occurring between genotypes (female: p = 0.666, male: p = 0.972). Consistent with weight loss, we find no genotype difference in moribundity (Fisher’s exact test; p > 0.05) for either sex. However, we do find that 3-NP is more toxic to female mice regardless of genotype with 94% (Slc7a11+/+) and 83% (Slc7a11sut/sut) of females becoming moribund before the end of the study compared to just 46% (Slc7a11+/+) and 42% (Slc7a11sut/sut) of males. This notwithstanding, the rate at which the mice in each genotypic group were removed — graphed as % survival — did not differ for either sex (Figure 7A,B). Despite the high moribundity in females, only 54% of Slc7a11+/+ and 36% of Slc7a11sut/sut mice show neurodegeneration (p = 0.288) (Figure 8A). The lesion incidence for Slc7a11+/+ and Slc7a11sut/sut males is 50% and 64%, respectively (p = 0.425) (Figure 8B). Finally, the lesion sizes for each sex and genotype do not differ (Figure 8A, B), with similar percentages of Slc7a11+/+ and Slc7a11sut/sut mice having small (females: 23% vs. 14%, p = 0.462; males: 30% vs 36%, p = 0.562) and medium sized lesions (females 30% vs 21%; p = 0.454; males: 20% vs. 18%, p = 0.669). No Slc7a11+/+ mice from either sex nor any Slc7a11sut/sut female mice have lesions that cover greater than 50% of the striatum, whereas 9% of Slc7a11sut/sut males do (p = 0.524). Overall, we find that female mice, regardless of genotype, show more systemic toxicity to 3-NP than males. Despite this, there is no genotype difference in either sex in striatal lesion incidence or size.
Figure 6: Comparison of weight loss and behavioral scores between wild-type (+/+) and system xc- null (sut/sut) mice exposed to 3-nitrpropionic acid (3NP).
A, B) Behavioral assessments were scored and recorded prior to each injection. Any mouse receiving a score of 9 or greater was removed from the study. Both scores for each day are graphed (mean ± SEM) with a.m. scores on integers, and p.m. scores on half- integers. Any symbol graphed without error bars means the error falls within the confines of the symbol. There was no statistically significant between-group differences in either male or female mice as determined by mixed-effects analysis with Sidak’s multiple comparisons test. C, D) Female (17 +/+, 18 sut/sut) and male (11 +/+, 12 sut/sut) mice were injected with 3NP twice per day for 12 days as described in methods. Mice were weighed once each day prior to the first injection. To facilitate comparison of weight loss, mass is expressed as the mean weight normalized to the mean starting weight ± SEM. For some points the error bars are shorter than the height of the symbol. Mean starting weights were as follows: Female: +/+ = 28.06g ± 0.81, sut/sut = 26.19g ± 0.45 and Male: +/+ = 34.36g ± 0.96, sut/sut = 33.21g ± 0.70. There was a significant between-group difference in female (p = 0.030) but not male (p = 0.2989) weight. There was a significant day-effect in weight loss for both female (p < 0.0001) and male (p < 0.0001) mice, but no genotype-effect in female (p = 0.666) or male (p = 0.971) mice.
Figure 7: Comparison of the toxicity of 3NP between wild-type (+/+) and system xc- null (sut/sut) age-and sex-matched mice.
Mice were injected twice daily with 3-NP for 12 days as described in methods. Kaplan-Meier survival curve depicting the rate in which (A) female ( n= 17 +/+; 18 sut/sut) or (B) male (n= 11 +/+; 12 sut/sut) were removed from the study because of moribundity, defined as any mouse sacrificed due to severe behavioral deficits (score ≥ 9) or excessive weight loss ( > 20%), including any mouse found dead (2F sut/sut mice). There were no genotypic differences in the rate of removal from the study in either sex as determined by the Mantel-COX log-rank test.
Figure 8: Comparison of striatal lesion size and incidence between wild-type (+/+) and system xc- null (sut/sut) mice exposed to 3-nitropropionic Acid (3NP).
Thionin stained representative images of 3NP exposed mice at 0.98mm, 0.5mm, and 0.02mm from bregma from A) Female (13 +/+, 14 sut/sut) and B) male (10 +/+, 11 sut/sut) mice. Graph depicts the percent of mice from each genotype in 4 defined groups: 0–1%, 1–25%, 26–50% and 51–75%. No lesion exceeded 75% of the striatum. There was no significant difference in any of the defined groups as determined by Fisher’s exact test. Sample sizes differ from those described in figures 6-8 as some slices were lost during procurement and/or staining. Brains from mice found dead were also excluded from analysis.
4. Discussion
Present results demonstrate brain region and transporter-specific transporter alterations occur in both female and male Slc7a11sut/sut mice. Despite maintaining the total CyS and GSH levels and steady redox states of cyst(e)ine, female and male mice demonstrate region specific reductions in the ratio of GSH/GSSG, a hallmark of oxidative stress. Region specific alterations in the protein levels of antioxidants superoxide dismutase 1, 2 and catalase were also shown to be sexually dimorphic. Yet interestingly, there was no genotype difference in striatal vulnerability to 3-nitropropionic acid-mediated oxidative stress in either sex, although 3-NP was more toxic to female mice of either genotype, as evidenced by an increase in moribundity as compared to males.
The import of cystine by system xc- has been shown to be vital to cell growth and proliferation, as melanocytes (Shih et al., 2006), fibroblasts (Sato et al., 2005), astrocytes (Jackman et al., 2010; Shih et al., 2006) and meningeal cells (Shih et al., 2006) lacking system xc- are unable to survive in vitro without the addition of a reducing agent. Yet mice lacking system xc- — including the Slc7a11sut/sut used herein —appear healthy and breed normally, perhaps due to the fact that plasma contains sufficient concentrations of cysteine (Bannai, 1984) for uptake by non-system xc- transport systems. Brain atrophy has been reported to occur in Slc7a11sut/sut mice at ≈13–15 weeks of age (Shih et al., 2006), though we find no genotypic differences in the same morphological measures in our colony (Sears and Hewett, in preparation). We attribute these differential findings to breeding strategies: independent homozygous breeder lines generated experimental animals in (Shih et al., 2006) whereas we breed heterozygotes and restrict our use of mice to F2 and F3 generations, thereby preventing genetic drift (Wolfer et al., 2002; Wolfer and Lipp, 2000). Interestingly, nominal changes in cellular proliferation in both the subventricular zone and dentate gyrus, measured at 3 and 11 months of age, have been reported in Slc7a11sut/sut mice (Liu et al., 2007).
We and others find that once astrocytes and meningeal cells (Jackman et al., 2010; Shih et al., 2006) are grown to confluence in vitro, system xc- null cells can survive sans reducing agents, suggesting the existence of some compensatory mechanisms. Whether more global compensatory mechanisms occur in brains of Slc7a11sut/sut mice was the focus of this investigation. Toward this end, we chose to determine whether plasma membrane expression levels of transporters known to be expressed by astrocytes that flux cyst(e)ine and/or glutamate —(ASCT1, LAT2, and SNAT3) (Arriza et al., 1993; Hayes et al., 2005; Watts et al., 2014), and, EAAT1 and 2 (Lehre et al., 1995), respectfully —were altered in naïve Slc7a11sut/sut mouse brain striatum, cortex and hippocampus. The expression levels of EAAT3, which fluxes cysteine but is located predominately on neurons (Chen and Swanson, 2003; Holmseth et al., 2012; Rothstein et al., 1994) was also ascertained.
Via extrusion, system xc- contributes to the ambient extracellular glutamate levels that bathe synapses (Baker et al., 2002a; Baker et al., 2002b; De Bundel et al., 2011; Massie et al., 2011; McCullagh and Featherstone, 2014). Optimal basal extracellular glutamate concentrations in brain are also maintained by glutamate removal facilitated by EAAT1 and EAAT2 expressed mainly on astrocytes (Anderson and Swanson, 2000; Danbolt, 2001; O’Donovan et al., 2017). Enhancements in extracellular glutamate concentrations are known to rapidly upregulate glutamate transporter expression and hence transport capacity (Duan et al., 1999; Gegelashvili et al., 1996; O’Donovan et al., 2017), which led us to hypothesize that expression of EAAT 1 and/or 2 might be downregulated in Slc7a11sut/sut brain to balance for the reduction in extracellular glutamate concentrations reported to occur in system xc- null mice (De Bundel et al., 2011; Massie et al., 2011; McCullagh and Featherstone, 2014). However, this was not the case. No changes in EAAT2 were evident in any brain region assessed in either female or male mice. Interestingly, EAAT1 expression in female Slc7a11sut/sut striatum was significantly increased as compared to wild-type female mice. The biological significance of this finding is uncertain given that EAAT2 is quantitatively the most important transporter with respect to glutamate clearance in brain [for review see (Robinson, 1998)] and glutamate levels were reported to be decreased (not increased) in striatum of Slc7a11sut/sut mice of both sexes (McCullagh and Featherstone, 2014). These findings are consistent, however, with other studies that showed no change in the total expression of EAAT 1 or 2 in the hippocampus of male xCT−/− mice (De Bundel et al., 2011); the effects in female mice were not explored. While no change in total EAAT1 expression in either male or female Slc7a11sut/sut mice was found previously (McCullagh and Featherstone, 2014), it is possible the effects were masked as measurements were made using whole brain protein extracts, which would reflect total protein levels, whereas we assessed only plasma membrane expression.
Like system xc- (Ottestad-Hansen et al., 2018; Zhang et al., 2014), the alanine, serine cysteine preferring, ASCT1 (Foster et al., 2016; Sakai et al., 2003), the System N transporter, SNAT3 (Rubio-Aliaga and Wagner, 2016; Todd et al., 2017), and the L-type neutral amino acid transporter, LAT2, (Jackman et al., 2010; Kim et al., 2004) are expressed prominently on astrocytes, with the latter heterodimerizing with the glycoprotein CD98hc (aka 4F2hc, Slc3a2 ) for plasma membrane expression (Pineda et al., 1999). EAAT3 is located predominately on neurons (Holmseth et al., 2012; Rothstein et al., 1994). Consistent with the idea that compensation can occur, we found increased expression of ASCT1 in the cortex of Slc7a11sut/sut female mice, though interestingly SNAT3 was decreased. No change in LAT-2 or EAAT3 was evident in any brain region tested (Figure 1). With respect to redox status, we find normal total CyS levels and CyS/CySS redox ratios in hippocampus and striatum, but not cortex —where the ratio is more oxidized — as compared to female Slc7a11+/+ mice (Figure 3). Despite maintenance of total GSH levels in naïve female Slc7a11sut/sut mice, the GSH/GSSG ratio was reduced in all brain regions examined, providing evidence of basal oxidative stress (Figure 3). Interestingly, reductions (not enhancements) of striatal levels of the cellular antioxidant enzymes SOD1 and catalase were also found in female Slc7a11sut/sut mice (Figure 4). Despite this, no enhancement in striatal lesion size or incidence engendered by repeated 3-NP injection in female SLC7A11sut/sut mice as compared to female Slc7a11+/+ mice was found. Thus, we posit that maintenance of the CyS/CySS redox potential in striatum, is an important adaptive response that protects against insults resulting from altered bioenergetics or primary mitochondrial dysfunction, such as occurs following systemic 3-NP exposure (Beal, 1995; Reynolds et al., 1998; Schulz et al., 1996). This idea is supported by evidence demonstrating that the cellular Cys/CySS redox couple represents a distinct node in the circuitry for thiol/disulfide redox signaling, independent from GSH/GSSG (Jones et al., 2004; Paul et al., 2018), and that CyS itself plays a protective role in the suppression of certain forms of cell death, including ferroptosis, itself mediated by CyS deprivation and involving mitochondria (Gao et al., 2019; Sbodio et al., 2018; Yu and Long, 2016).
With respect to male Slc7a11sut/sut mice, adaptations were found only in SNAT3, where it is increased in cortex but decreased in striatum (Figure 2). While no statistically significant alterations in CyS, CySS, GSH and GSSG levels are found in Slc7a11sut/sut male cortex (Sears et al., 2019) or striatum (Supplemental Figure 2A), the striatal GSH/GSSG ratio is more oxidized, owing to an increase in GSSG (Supplemental Figure 2B). This evidence of oxidative stress was not reflected in any change in the striatal expression of the antioxidant enzymes SOD1,2 or catalase, although alterations in SOD1 and 2 expression are evident in the hippocampus (Figure 5). xCT−/− mice (sex not specified) too show no difference in GSH levels in striatum; GSSG levels were not ascertained (Massie et al., 2011). Like in females, we find no statistical difference in 3-NP mediated striatal lesion incidence or size between genotypes, though there was a trend for an increase in both parameters in male Slc7a11sut/sut mice. These results differ from those of Massie and colleagues who demonstrated protection against striatal dopamine loss in xCT-/mice (sex non-specified) following 6-hydroxydopamine lesion, an effect attributed to the reduction in striatal glutamate levels found (Massie et al., 2011). It should be noted that striatal reductions in glutamate levels of Slc7a11sut/sut mice of both sexes has also been reported (McCullagh and Featherstone, 2014).
It is important to note that we did not do an exhaustive analysis of all cyst(e)ine transporting systems, thus, it is possible that the expression and function of others [e.g., ASCT2 (Slc1a5)] (Broer et al., 1999; Fotiadis et al., 2013) could change in response to loss of system xc-. In fact, our attempts to quantify ASCT2 were stymied by lack of a suitable antibody. Additionally, compensatory adaptation could occur through an increase in the rate of influx via transporters already present on the membrane. Finally, an increase in the transsulfuration pathway, which has been shown to be upregulated when system xc- is inhibited, could be responsible for producing/maintaining cysteine concentrations (Kandil et al., 2010).
Finally, we find 3NP to be more toxic to female than male mice, as seen by increased moribundity: only 10–20% of female mice completed the 12 day dosing paradigm as compared to 40–50% of males, of either genotype. How and why this occurs is not known, but a sex difference in basal striatal SDH activity, the ability of 3-NP to inhibit said activity, and ultimately the striatal vulnerability to such inhibition can be ruled out (Figure 8 and Supplemental Figure 3). Although systemic 3-NP circulates throughout the body and should lead to SDH inhibition in all tissues and cells, surprisingly very little can be found in the literature related to toxicity to tissues besides brain. Hence, that idea that peripheral toxic responses to 3-NP are sexually dimorphic requires further investigation.
In sum, we report that adaptive changes in expression of transporters capable of fluxing cyst(e)ine occurs in naïve brains of system xc- mice in a transporter- and region-specific, sexually dimorphic manner. Associated with these changes is the maintenance of total GSH and CyS, normal CyS/CySS but not GSH/GSSG ratios in both male and female striatum. Despite this, 3-NP mediated striatal injury is not enhanced, suggesting a potentially important role for CyS in neuroprotection. Still unknown are the mechanisms underlying the differences between male and female mice or how the plasma membrane expression of transporters and/or cellular antioxidants change in brain of Slc7a11sut/sut and Slc7a11+/+ mice of either sex change during 3-NP exposure. It is possible that during this oxidative challenge a larger pool of these or other transporters further compensate for the loss of system xc- via trafficking to the plasma membrane, as has been demonstrated for system xc- itself (Chase et al., 2020).
Supplementary Material
Protein dilution series of striatal, hippocampal, and cortical plasma membrane proteins (EAAT 1 and 2: 1, 2, 4, 8μg, EAAT3: 4, 8, 16, 32μg, ASCT1: 2, 4, 8, 16μg, SNAT3: 2, 4, 8, 16μg and LAT2: 6, 12, 24, 48μg) were separated under reducing conditions via 8% SDS-PAGE. Accuracy of the serial dilution was confirmed using total protein levels visualized using Revert™ Total Protein Stain (Lincoln, NE). EAATs 1, 2, 3, ASCT1, SNAT3, and LAT2 monomers were detected with two dilutions of antibody (EAATs 1 and 2: 2μg/mL and 1μg/mL, EAAT3: 1.756μg/mL and 0.878μg/mL ASCT1: 4μg/mL and 1.6μg/mL, SNAT3: 0.534μg/mL and 0.267μg/mL, LAT2: 1μg/mL and 2μg/mL) via Western Blot Analysis. Representative blots and protein quantification for EAATs 1 (A), 2 (B), 3 (C), ASCT1 (D), SNAT 3 (E) and LAT2 (F) protein. Data curves were fit via linear regression: (A) EAAT1 R2 = 0.99, (B) EAAT2 R2 = 0.98, (C) EAAT3 R2 = 0.97, (D) ASCT1 R2 = 0.99, (E) SNAT3 R2 = 0.99, (F) LAT2 R2 = 0.99. Optimal protein amounts [3μg for EAAT1, 1μg for EAAT2, 20μg for EAAT3, 6μg for ASCT1, 8μg (striatum and cortex) and 6μg (hippocampus) for SNAT3 and 35μg for LAT2] and antibody concentrations (1.33μg/mL for EAATs 1 and 2, 1.17μg/mL for EAAT3, 2.28μg/mL for ASCT1, 0.267μg/mL for SNAT3 and 3.33μg/mL for LAT2) were selected from the middle of the linear range.
, CyS, CySS GSH and GSSG levels were measured in the striatum of +/+ and sut/sut males using HPLC. A) CyS, CySS, and the ratio of CyS:CySS are graphed as the mean + SEM. B) GSH, GSSG, and the ratio of GSH:GSSG are graphed as the mean + SEM. An asterisk (*) represents between-group differences calculated using a Mann-Whitney test. Significance was set at P < 0.05.
+/+ and sut/sut female (A) and male (B) littermates were injected with 200 mg/kg 3-NP or saline (n = 3–4 each for both sexes). Two hours later, striatal SDH activity was measured as described in methods. Data are normalized to +/+ control group ([- 3-NP], set to 100%) and expressed as mean + SEM. An asterisk denotes significant between-group differences (+/− 3-NP treatment) as determined by two-way ANOVA followed by Bonferroni’s multiple comparison test on transformed data. There was a significant treatment effect in both females (p = 0.0007) and males (p = 0.0038), but no difference between genotypes in females (p = 0.929) or males (0.5268).
Highlights –
Cysteine transporter modifications occur in brains of system xc- null mice
Transporter adaptations are sexually dimorphic and brain region specific
CyS/CySS but not GSH/GSSG ratios are maintained in a sexually dimorphic and brain region specific manner.
System xc- null mice of either sex are no more susceptible to in vivo oxidative stress than wild-type littermate controls.
Acknowledgements:
We thank Megan LeBlanc and Myles M. Morgan for their help with optimizing antibody concentrations, as well as Shannon Pitt and Nathena Murray for their assistance with measurement of lesions. We would also like to acknowledge support for this project from both the Undergraduate Research and Creative Works and WiSE programs at Syracuse University.
Funding: This work was supported by NINDS 2R01NS051445 and 1R01NS105767.
Abbreviations:
- 3-NP
3-Nitropropionic Acid
- EAAT
Excitatory Amino Acid Transporter
- ASCT
Alanine Serine Cysteine Transporter
- LAT
L Amino Acid Transporter
- GSH
Glutathione
- GSSG
Glutathione Disulfide
- CyS
Cysteine
- CySS
Cystine
- SOD
Superoxide Dismutase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Swanson RA, 2000. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32, 1–14. [PubMed] [Google Scholar]
- Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG, 1993. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem 268, 15329–15332. [PubMed] [Google Scholar]
- Bains JS, Shaw CA, 1997. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Brain Res Rev 25, 335–358. [DOI] [PubMed] [Google Scholar]
- Baker DA, Shen H, Kalivas PW, 2002a. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids 23, 161–162. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW, 2002b. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 22, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S, 1984. Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779, 289–306. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Gasol E, Manzoni M, Pineda M, Riboni M, Martin R, Zorzano A, Borsani G, Palacin M, 2001. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc. Pflugers Arch 442, 286–296. [DOI] [PubMed] [Google Scholar]
- Beal MF, 1995. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol 38, 357–366. [DOI] [PubMed] [Google Scholar]
- Broer A, Brookes N, Ganapathy V, Dimmer KS, Wagner CA, Lang F, Broer S, 1999. The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. J Neurochem 73, 2184–2194. [PubMed] [Google Scholar]
- Chase LA, VerHeulen Kleyn M, Schiller N, King AG, Flores G, Engelsman SB, Bowles C, Smith SL, Robinson AE, Rothstein J, 2020. Hydrogen peroxide triggers an increase in cell surface expression of system xc(−) in cultured human glioma cells. Neurochem Int 134, 104648. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA, 2003. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem 84, 1332–1339. [DOI] [PubMed] [Google Scholar]
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT, 2005. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 102, 10964–10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb RJ, Hewett SJ, Hewett JA, 2011. Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition. Epilepsia 52, 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC, 2001. Glutamate uptake. Prog Neurobiol 65, 1–105. [DOI] [PubMed] [Google Scholar]
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, Vermoesen K, Bannai S, Sato H, Michotte Y, Smolders I, Massie A, 2011. Loss of System x(c)(−) Does Not Induce Oxidative Stress But Decreases Extracellular Glutamate in Hippocampus and Influences Spatial Working Memory and Limbic Seizure Susceptibility. Journal of Neuroscience 31, 5792–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke SM, Fanburg BL, 1989. Regulation of cellular glutathione. Am J Physiol 257, L163–173. [DOI] [PubMed] [Google Scholar]
- Dringen R, Kranich O, Hamprecht B, 1997. The gamma-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res 22, 727–733. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA, 1999. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19, 10193–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F, 2002. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience 114, 1005–1017. [DOI] [PubMed] [Google Scholar]
- Foster AC, Farnsworth J, Lind GE, Li YX, Yang JY, Dang V, Penjwini M, Viswanath V, Staubli U, Kavanaugh MP, 2016. D-Serine Is a Substrate for Neutral Amino Acid Transporters ASCT1/SLC1A4 and ASCT2/SLC1A5, and Is Transported by Both Subtypes in Rat Hippocampal Astrocyte Cultures. PLoS One 11, e0156551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Kanai Y, Palacin M, 2013. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34, 139–158. [DOI] [PubMed] [Google Scholar]
- Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, Jiang X, 2019. Role of Mitochondria in Ferroptosis. Mol Cell 73, 354–363 e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Civenni G, Racagni G, Danbolt NC, Schousboe I, Schousboe A, 1996. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. Neuroreport 8, 261–265. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D, 2001. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharm 40, 959–975. [DOI] [PubMed] [Google Scholar]
- Halliwell B, 2006. Oxidative stress and neurodegeneration: where are we now? J Neurochem 97, 1634–1658. [DOI] [PubMed] [Google Scholar]
- Hanigan MH, Ricketts WA, 1993. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 32, 6302–6306. [DOI] [PubMed] [Google Scholar]
- Hayes D, Wiessner M, Rauen T, McBean GJ, 2005. Transport of L-[14C]cystine and L-[14C]cysteine by subtypes of high affinity glutamate transporters over-expressed in HEK cells. Neurochem Int 46, 585–594. [DOI] [PubMed] [Google Scholar]
- Holmdahl R, Malissen B, 2012. The need for littermate controls. Eur J Immunol 42, 45–47. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC, 2012. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci 32, 6000–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Bannai S, Sugita Y, 1981. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J Biol Chem 256, 12387–12392. [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ, 2010. Regulation of system x(c)(−)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia 58, 1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM Jr., Kirlin WG, 2004. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18, 1246–1248. [DOI] [PubMed] [Google Scholar]
- Kandil S, Brennan L, McBean GJ, 2010. Glutathione depletion causes a JNK and p38MAPK-mediated increase in expression of cystathionine-gamma-lyase and upregulation of the transsulfuration pathway in C6 glioma cells. Neurochem Int 56, 611–619. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim IJ, Hwang S, Kook JH, Lee MC, Shin BA, Bae CS, Yoon JH, Ahn SG, Kim SA, Kanai Y, Endou H, Kim JK, 2004. System L-amino acid transporters are differently expressed in rat astrocyte and C6 glioma cells. Neurosci Res 50, 437–446. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Sato M, Kasakoshi T, Tsutsui T, Sugimoto M, Osaki M, Okada F, Igarashi K, Hiratake J, Homma T, Conrad M, Fujii J, Soga T, Bannai S, Sato H, 2015. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J Biol Chem 290, 8778–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranich O, Hamprecht B, Dringen R, 1996. Different preferences in the utilization of amino acids for glutathione synthesis in cultured neurons and astroglial cells derived from rat brain. Neurosci Let 219, 211–214. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC, 1995. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15, 1835–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P, 2013. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18, 522–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RR, Brown CE, Murphy TH, 2007. Differential regulation of cell proliferation in neurogenic zones in mice lacking cystine transport by xCT. Biochem Biophys Res Commun 364, 528–533. [DOI] [PubMed] [Google Scholar]
- Lu SC, 2009. Regulation of glutathione synthesis. Mol Aspects Med 30, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, Conrad M, Plesnila N, Sato H, Michotte Y, 2011. Dopaminergic neurons of system x(c)(−)-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J 25, 1359–1369. [DOI] [PubMed] [Google Scholar]
- McCullagh EA, Featherstone DE, 2014. Behavioral characterization of system xc- mutant mice. Behav Brain Res 265, 1–11. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Go YM, Ziegler TR, Jones DP, 2005. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol 289, G70–78. [DOI] [PubMed] [Google Scholar]
- Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY, 2002. Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif 35, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan SM, Sullivan CR, McCullumsmith RE, 2017. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottestad-Hansen S, Hu QX, Follin-Arbelet VV, Bentea E, Sato H, Massie A, Zhou Y, Danbolt NC, 2018. The cystine-glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia 66, 951–970. [DOI] [PubMed] [Google Scholar]
- Paul BD, Sbodio JI, Snyder SH, 2018. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol Sci 39, 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick JR, Little JM, 1965. Effect of Type of Bedding Material on Thresholds of Pentylenetetrazol Convulsions in Mice. Lab Anim Care 15, 29–33. [PubMed] [Google Scholar]
- Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M, 1999. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem 274, 19738–19744. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J, 2007. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol 293, L972–981. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Carter RJ, Morton AJ, 1998. Dopamine modulates the susceptibility of striatal neurons to 3-nitropropionic acid in the rat model of Huntington’s disease. J Neurosci 18, 10116–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, 1998. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int 33, 479–491. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW, 1994. Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Wagner CA, 2016. Regulation and function of the SLC38A3/SNAT3 glutamine transporter. Channels (Austin) 10, 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara JI, Miura K, Bannai S, 1993. Maintenance of neuronal glutathione by glial cells. J Neurochem 61, 1672–1676. [DOI] [PubMed] [Google Scholar]
- Sakai K, Shimizu H, Koike T, Furuya S, Watanabe M, 2003. Neutral amino acid transporter ASCT1 is preferentially expressed in L-Ser-synthetic/storing glial cells in the mouse brain with transient expression in developing capillaries. J Neurosci 23, 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S, 2005. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 280, 37423–37429. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S, 1999. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274, 11455–11458. [DOI] [PubMed] [Google Scholar]
- Sbodio JI, Snyder SH, Paul BD, 2018. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc Natl Acad Sci U S A 115, 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Henshaw DR, MacGarvey U, Beal MF, 1996. Involvement of oxidative stress in 3-nitropropionic acid neurotoxicity. Neurochem Int 29, 167–171. [DOI] [PubMed] [Google Scholar]
- Sears SMS, Hewett JA, Hewett SJ, 2019. Decreased epileptogenesis in mice lacking the System xc (−) transporter occurs in association with a reduction in AMPA receptor subunit GluA1. Epilepsia Open 4, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker G, Allen JW, Mutkus LA, Aschner M, 2001. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res 902, 156–163. [DOI] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH, 2006. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci 26, 10514–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH, 2005. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280, 22925–22936. [DOI] [PubMed] [Google Scholar]
- Todd AC, Marx MC, Hulme SR, Broer S, Billups B, 2017. SNAT3-mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia 65, 900–916. [DOI] [PubMed] [Google Scholar]
- Watts SD, Torres-Salazar D, Divito CB, Amara SG, 2014. Cysteine transport through excitatory amino acid transporter 3 (EAAT3). PLoS One 9, e109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP, 2002. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci 25, 336–340. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP, 2000. Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol 85, 627–634. [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND, 2004. Glutathione metabolism and its implications for health. J Nutr 134, 489–492. [DOI] [PubMed] [Google Scholar]
- Yu X, Long YC, 2016. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci Rep 6, 30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein dilution series of striatal, hippocampal, and cortical plasma membrane proteins (EAAT 1 and 2: 1, 2, 4, 8μg, EAAT3: 4, 8, 16, 32μg, ASCT1: 2, 4, 8, 16μg, SNAT3: 2, 4, 8, 16μg and LAT2: 6, 12, 24, 48μg) were separated under reducing conditions via 8% SDS-PAGE. Accuracy of the serial dilution was confirmed using total protein levels visualized using Revert™ Total Protein Stain (Lincoln, NE). EAATs 1, 2, 3, ASCT1, SNAT3, and LAT2 monomers were detected with two dilutions of antibody (EAATs 1 and 2: 2μg/mL and 1μg/mL, EAAT3: 1.756μg/mL and 0.878μg/mL ASCT1: 4μg/mL and 1.6μg/mL, SNAT3: 0.534μg/mL and 0.267μg/mL, LAT2: 1μg/mL and 2μg/mL) via Western Blot Analysis. Representative blots and protein quantification for EAATs 1 (A), 2 (B), 3 (C), ASCT1 (D), SNAT 3 (E) and LAT2 (F) protein. Data curves were fit via linear regression: (A) EAAT1 R2 = 0.99, (B) EAAT2 R2 = 0.98, (C) EAAT3 R2 = 0.97, (D) ASCT1 R2 = 0.99, (E) SNAT3 R2 = 0.99, (F) LAT2 R2 = 0.99. Optimal protein amounts [3μg for EAAT1, 1μg for EAAT2, 20μg for EAAT3, 6μg for ASCT1, 8μg (striatum and cortex) and 6μg (hippocampus) for SNAT3 and 35μg for LAT2] and antibody concentrations (1.33μg/mL for EAATs 1 and 2, 1.17μg/mL for EAAT3, 2.28μg/mL for ASCT1, 0.267μg/mL for SNAT3 and 3.33μg/mL for LAT2) were selected from the middle of the linear range.
, CyS, CySS GSH and GSSG levels were measured in the striatum of +/+ and sut/sut males using HPLC. A) CyS, CySS, and the ratio of CyS:CySS are graphed as the mean + SEM. B) GSH, GSSG, and the ratio of GSH:GSSG are graphed as the mean + SEM. An asterisk (*) represents between-group differences calculated using a Mann-Whitney test. Significance was set at P < 0.05.
+/+ and sut/sut female (A) and male (B) littermates were injected with 200 mg/kg 3-NP or saline (n = 3–4 each for both sexes). Two hours later, striatal SDH activity was measured as described in methods. Data are normalized to +/+ control group ([- 3-NP], set to 100%) and expressed as mean + SEM. An asterisk denotes significant between-group differences (+/− 3-NP treatment) as determined by two-way ANOVA followed by Bonferroni’s multiple comparison test on transformed data. There was a significant treatment effect in both females (p = 0.0007) and males (p = 0.0038), but no difference between genotypes in females (p = 0.929) or males (0.5268).