Abstract
Tibial dyschondroplasia (TD) is a metabolic tibiotarsal bone disease in rapidly growing birds throughout the world, which is characterized by gait disorders, reduced growth, and in an unrecoverable lameness in many cases. The short production cycle in chickens, long metabolism cycle in most of the drugs with the severe drug residue, and high treatment cost severely restrict the enthusiasm for the treatment of TD. Traditional Chinese medicine (TCM) has been used for the prevention, treatment, and cure of avian bone diseases. Previously, a couple of traditional Chinese medicines has been reported being useful in treating TD. This review will discuss the TCM used in TD and the alternative TCM to treat TD. Selecting a TCM approach and its pharmacologic effects on TD chickens mainly focused on the differentiation, proliferation, and apoptosis of chondrocytes, angiogenesis, matrix metabolism, oxidative damage, cytokines, and calcification of cartilage in tibia.
Key words: chicken, pharmacologic effect, tibial dyschondroplasia, traditional Chinese medicine
Tibial dyschondroplasia
Tibial dyschondroplasia (TD) is the most critical tibiotarsal bone disease in fast-growing poultry that disturbs the healthy development of the tibial growth plate (GP) (Nabi et al., 2016). Tibial dyschondroplasia is characterized by an avascular and nonmineralized GP, gait disorders, reduced growth, and unrecoverable lameness (Figure 1) (Mehmood et al., 2017). Previous research has indicated that almost 30% of bone diseases in poultry are due to TD and that this disease leads to greater than 10% morbidity in China, creating significant economic losses in the poultry industry (Li et al., 2008; Dan et al., 2009; Zhang et al., 2018b). Affected chickens are less disease resistant and show reductions in production performance and osteomyelitis (Shahzad et al., 2014, 2015).
Figure 1.
The different changes of tibial metaphysis in tibial dyschondroplasia chickens.
Healthy GP development requires cartilage vascularization and mineralization with a well-structured morphology, whereas in TD, the differentiation of chondrocytes appears to be abnormal. The avian GP has random columns of chondrocytes along with deeply penetrating blood vessels (Pines et al., 2005). It has stated that the examination of a histologic section of GP illustrated that a large number of chondrocytes were in the resting zone of the normal broiler, and the chondrocytes regularly proliferated and differentiated from top to bottom (Piróg et al., 2010). Angiogenesis is increased in the cartilage profoundly in the hypertrophic zone to prepare the osteoblasts for calcification in normal bone ossification. The blood vessels are thick and rich in blood in normal bone ossification, whereas chondrocytes are small in size with large capsules, and nucleus present in the center.
In TD GP, the chondrocytes are unorganized and round in shape, having fewer blood vessels, and there is no demarcation between proliferative zone and hypertrophic zone (Mehmood et al., 2019a). The chondrocytes are immature and larger than normal chondrocytes. Tibial dyschondroplasia lesions are present in the proximal GP of the tibia bone, which includes avascular, nonmineralized (noncalcified) tissue, and dull cartilage. Histologically, cartilage does not show any blood vessels and vascularization because the prehypertrophic (avascular) zone enlarges and combines with avascular cartilage zones (Leach and Monsonego-Ornan, 2007; Nabi et al., 2016). The sketch of healthy and TD GP is shown in Figure 2.
Figure 2.
Proximal end of tibial growth plate diagram of normal and TD chickens (modified from Leach and Monsonego-Ornan, 2007). Abbreviation: TD, tibial dyschondroplasia.
Pathogenesis of TD
Studies have shown that with TD, angiogenesis, and vascular development are inhibited in osteoblasts, osteoclasts, and mesenchymal stem cells, vascular infiltration in the hypertrophic zone of cartilage is reduced, and osteoclasts, osteoblasts, and mesenchymal stem cells lacked sufficient nutritional inputs. Consequently, calcified cartilage cannot complete the bone sedimentary process, which leads to white cartilage deposition (Rath et al., 2007; Borjesson et al., 2013). It is clear that the occurrence of TD in broilers is mainly associated with pathologic changes of tibial because of the following: 1) the apoptosis of chondrocytes, especially the nuclear dissolution, preventing the further development of a large number of chondrocytes; 2) chondrocytes in the resting and proliferative zone cannot further differentiate within the hypertrophic zone, and a large number of cells gather near the GP; 3) vascular endothelial cell impairment and reduced angiogenesis within the tibia GP. The accompanying deterioration of blood flow impairs or kills off chondrocytes within the zone of tibia GP osteogenesis; 4) the resulting failure of osteogenesis causes an accumulation of white cartilaginous tissue in place of healthy bone (Leach and Monsonego-Ornan, 2007; Nabi et al., 2016; Mehmood et al., 2018a, 2019a, 2019b; Yao et al., 2018; Zhang et al., 2018a, 2018b). Currently, research focuses on increasing the growth rate and feed conversion ratio of broilers; consequently, poultry bone disease incidence is also increasing in the broiler industry. It is reported that the chicken muscle tissue and bone growth and development destroy the original balance and are also a reason for the leg deformities in chicken. Previous studies indicated that GP were resistant to angiogenesis in TD chickens, and the chondrocytes around TD lesions failed to provide appropriate angiogenesis signals to stimulate normal GP vascularization. A reduction of blood vessels at the site of osteogenesis induces deterioration and necrosis of chondrocytes (Figure 1) (Zhang et al., 2018a, 2018b). Rath et al. (2007) have reported apoptosis of capillary endothelial cells in GP in thiram-induced TD chickens, and the mortality of cells increased with the duration of the thiram dosing period, accompanied by chondrocyte cell death. Previously, studies have found that the gene expression of tibial GP chondrocytes significantly changes during the occurrence of TD in chickens, and the cartilage matrix protein composition changes follow. Tibial dyschondroplasia triggers abnormal chondrocyte protein secretion in TD, including Col II, Col X, Aggrecan, and fibroblast growth factor, bone adhesion protein (osteonectin), osteopontin, conversion, transforming growth factor-β, insulin-like growth factor 1, epidermal growth factor, and tumor necrosis factor, and so on. (Tian et al., 2013). Meanwhile, changes of cartilage extracellular matrix (ECM) composition accompany abnormal chondrocyte protein secretion in TD lesion areas, including heat shock family proteins (HSP), extracellular matrix metalloproteinase 9, aggrecan, Col II, Runx2, P2RX7, caspases, BECN1, Sox9, Hif-1 alpha/vascular endothelial growth factor (VEGF), Cox-2, Wnt4, BMP2, MMP-13, and extracellular matrix metalloproteinase inducers (Tian et al., 2013; Shahzad et al., 2014, 2015; Iqbal et al., 2016; Nabi et al., 2016; Zhang et al., 2018a, 2018b; Mehmood et al., 2018b, 2019a, 2019b; Yao et al., 2018).
Causes of TD
Since TD was reported in 1965, the factors related to TD occurrence have been discovered, including heredity (variety breeding), environment (temperature, light, feeding density), nutrient elements (electrolyte, calcium and phosphorus ratio), vitamin D3, and poisons (thiram in particular). (Zhang et al., 2018a, 2018b; Mehmood et al., 2019a, 2019b). Tibial dyschondroplasia appears to be induced by multiple factors, and causes are diverse. For example, soybean meal in feed has been linked to changes in TD incidence, along with other factors such as vitamin D deficiency, hyperthyroidism, and abnormal levels of biochemical markers such as IL-1and nitric oxide. Rath et al. (2007) and Li et al. (2008) have demonstrated that thiram is highly effective in inducing TD and that the symptoms are nearly identical to naturally occurring TD signs (Zhang et al., 2019a). Our previous studies have also found that thiram can be used to induce TD in poultry efficiently, and thiram has been widely used to model TD in many controlled induction experiments (Zhang et al., 2018a). Our previous studies have indicated that thiram promotes apoptosis of chondrocytes, inducing nuclear dissolution, which serves to greatly the number of functioning chondrocytes within osteogenesis zones (Figure 3). In addition, thiram disrupts angiogenesis within the tibial GP, further impairing chondrocyte activity and undermining osteogenesis at that location (Figure 3) (Mehmood et al., 2018a, Mehmood et al., 2018b; Zhang et al., 2018a, 2018b; Mehmood et al., 2019a).
Figure 3.
Model diagram of the influence of thiram on the development of tibia growth plate in broiler chickens.
Pharmacological Mechanism of TCM for the Treatment of TD
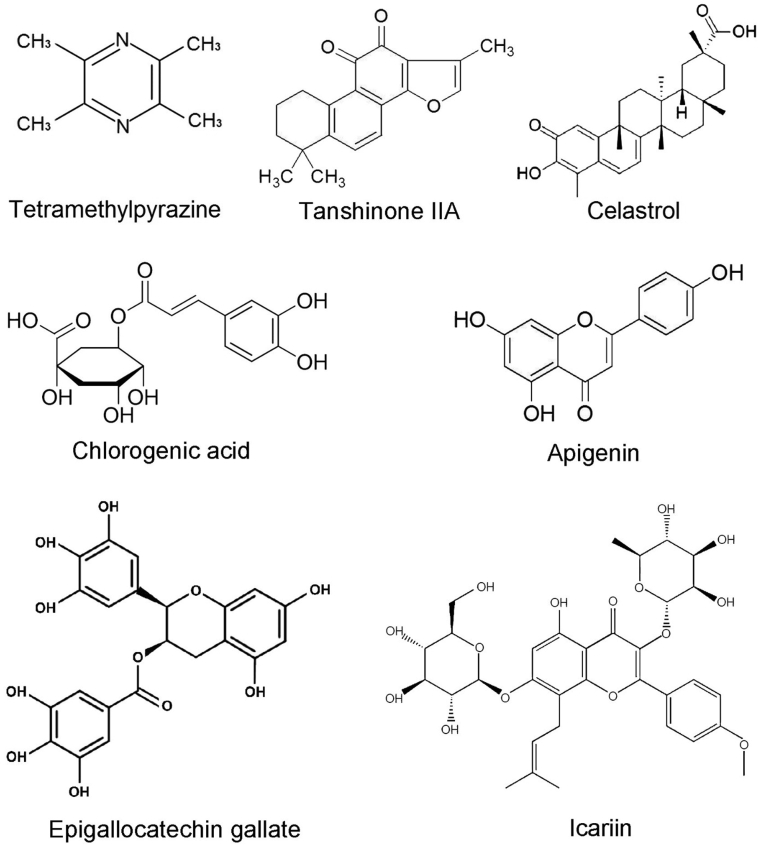
For the treatment of TD in chickens, there is still no specific drug widely available. Previous studies showed that administering vitamin C and vitamin D3 and changing the proportion of calcium and phosphorus in the diet reduces the incidence of TD in chickens (Leach and Monsonego-Ornan, 2007; Landy and Toghyani, 2018). Owing to the short production cycle in chickens, the long metabolism cycle of most drugs, high treatment costs, and issues regarding the accumulation of drug residues in commercial chickens serve as deterrents for the broad-scale application of drugs to combat TD. The application of traditional Chinese medicines (TCM) has a long history and has been used for the prevention, clinical treatment, and cure of disorders or diseases (Hao et al., 2015). Recently, some key TCM have come under scrutiny as potential tools for combating TD, application of which is not accompanied by the issues presented with the use of synthetic drugs. In particular, the application of a single herb or single TCM herb extract has generated significant interest (Nabi et al., 2016; Zhang et al., 2018a; Mehmood et al., 2019b). At present, the pharmacologic effects of TCM on TD chickens are mainly focused on the differentiation, proliferation, and apoptosis of chondrocytes, angiogenesis, matrix metabolism, oxidative damage, cytokine stimulation, and calcification of cartilage in the tibia (Leach and Monsonego-Ornan, 2007; Nabi et al., 2016; Zhang et al., 2018b; Yao et al., 2018; Mehmood et al., 2019a). Currently, there are several kinds of TCM reported to treat TD (Figure 4), which are as follows.
Figure 4.
Chemical structure of some traditional Chinese medicines used for TD treatment. Abbreviation: TD, tibial dyschondroplasia.
Tetramethylpyrazine
Tetramethylpyrazine (TMP) is one of the most important bioactive component extracted from the TCM herb Chuanxiong, has been found to function as a vasodilator, improving minicirculation, eliminating free radicals, and is antiapoptotic and anti-inflammatory (Liang et al., 2005; Mehmood et al., 2018a; Zhang et al., 2018c). Tetramethylpyrazine was reported to play an essential role in angiogenesis during the impairment and recovery of GP in TD chickens via regulating the expression of the relevant gene of the hypoxia inducible factor-1α (HIF-1α)/VEGF pathway (Mehmood et al., 2018a). Mehmood et al. (2019b) have reported that TMP treatment upregulates the expression of ITGB3 in TD chickens. Thus, TMP could be considered as an essential agent to avoid the losses and costs associated with TD.
Tanshinone IIA
Tanshinone IIA is a fat-soluble bioactive component of Salvia miltiorrhiza, which has anti-inflammatory property, can scavenge oxygen free radicals, and possesses antioxidant effects (La-Zhi et al., 2008). Tanshinone IIA can promote the increase of bone marrow mesenchymal differentiation, bone mineral density, bone strength, and fracture healing while preventing bone loss. In previous studies, we found that Tanshinone IIA can reduce the incidence of thiram-induced TD in chickens, significantly improve the development of tibial cartilage, and downregulate Hsp90 and VEGF in TD chickens (Mehmood et al., 2017). Studies have shown that Tanshintone IIA can significantly downregulate β-catenin, block Wnt/β-catenin signal pathway, as well as change the expression of downstream target genes, such as Hsp90 and VEGF, so it plays an essential role in protecting relevant tissues and organs (Liu et al., 2013; Mehmood et al., 2017). Recently, our study found that TD chickens treated by Tanshinone IIA can restore gene (WNT5α, β-catenin, and BMP-2) expression in Wnt/β-catenin pathway and improve GP development patterns in TD broilers (Yang et al., 2019).
Celastrol
Celastrol has been commonly used as an anti-inflammatory agent and immune regulator, including dermatitis, anticancer, Alzheimer disease, systemic lupus erythematosus, cartilage-protective, rheumatoid arthritis, and dermatomyositis in China (Nabi et al., 2016; Zhang et al., 2018d; Li and Hao, 2019). Nabi et al. (2016) reported that treatment with celastrol significantly inhibited the expression of Hsp90 and increased the expression of receptors Flk-1 in the GP in thiram-induced TD chickens. At the same time, celastrol could decrease the level of aspartic acid transaminase, alanine aminotransferase, and malondialdehyde by reducing liver stress. Celastrol promotes broiler liver detoxification, restores antioxidative activity, reduces liver damage, and elevates the production of bone metabolism–related enzymes (Nabi et al., 2016). Meanwhile, administration of celastrol to TD chickens can promote the GP vascularization and restore the angiogenesis (Nabi et al., 2016).
Chlorogenic Acid
Chlorogenic acid (CGA), is known as one of the most common polyphenolic compounds, mainly in Eucommia, honeysuckle, and green tea. Pharmacologic studies have found that CGA plays an important and therapeutic role in antioxidation, anti-inflammatory, antiviral, antitumor, cardioprotection, and free radical scavenging activities. (Kwak et al., 2013; Han et al., 2017; Nabavi et al., 2017). Of note, CGA inhibits the expression of Jun-D, c-Jun, c-Fos, Fra-1, Fra-2, ALP, Runx2, and Osterix genes involved in the differentiation of preosteoblasts into osteoblasts (Yi, 2013). Zhang and Hu (2016) found that CGA can enhance the proliferation of osteoblasts and accelerate the transition process S phase. Chlorogenic acid may increase the expression of Bcl-2 and decrease the Bax expression during apoptosis, thereby inhibiting osteoblast apoptosis (Zhang and Hu, 2016). Zhang et al. (2019b) reported that CGA possesses a positive therapeutic effect on TD chickens via regulating caspase-3, caspase-9, MMP-9, MMP-10, MMP-13, and BECN1 expression.
Apigenin
Apigenin is one of the most common flavonoids compounds, mainly in Daphne, Verbenaceae, and Papyridae, and is widely distributed in warm tropical vegetables and fruits. Pharmacologic studies have found that apigenin plays several therapeutic roles in antitumor, cardiovascular and cerebrovascular protection, antiapoptosis, anti-inflammatory, and antioxidant functions. (Salehi et al., 2019). Our previous research found that administering the apigenin to TD chickens restored chondrocyte columnar organization with vascularization, which ultimately abrogated the lameness (Iqbal et al., 2016). Meanwhile, the expression levels of Hsp90 and VEGF were increased in thiram-treated chondrocytes culture medium, whereas apigenin therapy to chondrocytes reduced the Hsp90 and VEGF expression levels. Apigenin therapy is considered as a promising approach to control and treat TD in chickens (Mehmood et al., 2017).
Icariin
Icariin (ICA), extracted from Herba epimedii, has been shown to be effective for the treatment of various bone regeneration and repair (Zhang et al., 2018a). In recent years, studies have found that ICA has the following effects for treating bone diseases: 1) It can significantly improve bone density and bone formation; 2) Icariin has the function of promoting the metabolic activity of chondrocytes and the synthesis of cartilage matrix, promoting the proliferation of chondrocytes for the growth of cartilage, which can be used for the repair of cartilage tissue; 3) Promoting osteoblast differentiation; 4) Effective anti-inflammatory activity that can be used to treat osteoarthritis; 5) It can effectively inhibit the absorbance of mature osteoclasts and the formation of osteoclast-like cells (Xu et al., 2016; Wang et al., 2018). In our previous studies, we have found that ICA upregulated WNT4 and P2RX7 mRNA expressions and downregulated VEGF expression, as well as restored the GP width, reduced chondrocyte damage and “white cartilage mass," promoted the development of blood vessels in GPs, increased growth performance, and reduced lameness in TD chickens. Meanwhile, ICA administration recovered GP lesion, improved the performance, and prevented lameness (Zhang et al., 2018a).
Epigallocatechin Gallate
Epigallocatechin gallate is the most effective active catechin in green tea. Epigallocatechin gallate as a potent antibacterial, antiviral, antiarteriosclerosis, anti-inflammatory, antioxidant, and antitumor agent has been reported (Chen et al., 2014a; Granja et al., 2017). Epigallocatechin gallate has a vigorous antioxidant activity and can protect cells and DNA from damage owing to its oxygen free radical scavenging ability (antioxidant) (Chen et al., 2014a). The occurrence of TD is also closely related to the expression of Hsps. The upregulated expression of Hsp90 affects the expression of VEGF and its receptor, resulting in obstructed vascular formation in the proliferation zone of tibial GP cells in broilers, insufficient oxygen supply of cells, and hypoxia (Iqbal et al., 2016). However, epigallocatechin gallate can inhibit aryl hydrocarbon receptor activity of Hsp90 client protein by binding to Hsp90c terminal. Epigallocatechin gallate can significantly increase the transcription level of VEGF in TD broilers and considerably reduce the transcription levels of Hsp90 and Flk-1. Therefore, the prevention and recovery of broiler TD can be achieved through epigallocatechin gallate (Iqbal et al., 2016).
Biological activities for the Selection of TCM Treating TD
Research efforts of TCM use for treating TD have been made significant progress. Meanwhile, TCM application has little side effects, low price, low drug residue, and high safety margins, and TCM substances are easy to obtain. The use of TCM not only avoids the gastrointestinal reactions caused by oral drugs but also avoids the first-pass effect of liver metabolism (An et al., 2019; Zhang et al., 2019c). What is more? Chinese herbal medicine contains rich active ingredients, such as polysaccharides, alkaloids, volatile oils, and organic acids (Yu et al., 2019). These active ingredients are conducive for regulating immune function and improving the production performance of chickens. Keeping in view the characteristics of TCM, we have identified some protocols and features that should be kept in mind while selecting TCM (Table 1; Figure 5).
Table 1.
Alternative traditional Chinese medicines for treating TD.
| Name | Active components | Biological activity | Mechanism of action | References |
|---|---|---|---|---|
| Morinda officinalis | Iridoids glycoside | Antiapoptotic and anticatabolic, anti-inflammatory | ↓ Proinflammatory cytokines ↓ MMP-3 and MMP-13 |
(Wang et al., 2014) |
| Resveratrol | Phytoalexin, polyphenolic | Regulates apoptosis, degrades extracellular matrix and protects chondrogenesis | ↑ Sirt1 ↓ MMP1, MMP3 and MMP13, NF-κB and p38MAPK pathways. |
(Liu et al., 2017b; Jin et al., 2018; Wang and Bai, 2019) |
| Rhizoma atractylodis macrocephalae | Sesquiterpene, atractylenolide | Promotes chondrogenic differentiation | ↓Osteoclast differentiation | (Li et al., 2012) |
| Fructus psoraleae | Volatile oil, coumarin, flavones, lipids, resins | Promotes viability and cartilaginous formation | ↑ Type II collagen ↑ Aggrecan, and Sox-9 |
(Pan et al., 2016) |
| Semen plantaginis | Flavonoids, triterpenoids, iridoid glycosides | Antioxidant | ↓VEGF, HIF-1α | (Tzeng et al., 2016) |
| Hesperetin | Flavonoids | Antioxidant and antiapoptotic | TLR4/NF-κB, Nrf2 pathways | (Chen et al., 2019; Muhammad et al., 2019) |
| Paeoniflorin | Paeoniflorin | Angiogenesis | ↓VEGF/VEGFR2 ↓Jagged1/Notch1 |
(Yuan et al., 2018) |
| Daidzein | Flavonoids, isoflavones | Antioxidant | (Yi et al., 2019) | |
| Curculigo orchioides | Phenols and phenolic glycosides | Antioxidant | ↑Caspase-3 and caspase-8, ROS-mediated, ↓Bcl-2 | (Hejazi et al., 2018) |
| Paeonol | Paeonol | Antiapoptotic and degrades extracellular matrix | ↑ IL-1β, ↓ ROS, apoptosis | (Liu et al., 2017a) |
| Angelica | Ferulic acid, butylidenephthalide, and polysaccharides | Anti-apoptotic | ↑ mTOR, p70S6K, Notch1, ↓BNIP3, hypoxia | (Xue et al., 2019) |
| Eucommia ulmoides | Lignans, iridoids, phenolics, steroids, flavonoids | Antiapoptotic and extracellular matrix biosynthesis | ↑Cartilage metabolism ↓Apotosis ↓MMP-1, -3 and -13 |
(Lu et al., 2013; Li et al., 2014) |
| Tetrandrine | Alkaloids | Anti-inflammatory, antiapoptosis and antioxidant | Apoptosis, ↓iNOS, COX-2, TNF-α | (Xie et al., 2002; Ng et al., 2006; Shine et al., 2018) |
| Puerarin | Isoflavone | Antioxidant, anti-inflammatory, anti-apoptotic and bone formation | ↓Oxidative stress, ↓nuclear factor-κB protein ↑VEGFA |
(Zhao et al., 2016; Guo et al., 2018) |
| Naringin | Flavanone glycoside | Angiogenesis, antioxidant, and protects chondrocytes | ↓Caveolin-1, p-p38, and p-ATF-2 TNF-α and p38MAPK pathways |
(Su et al., 2014; Song et al., 2017) |
| Polygonum multiflorum | Polyphenol, tetrahydroxystilbene, glucoside | Angiogenesis | ↑Vascular endothelial growth factor, angiopoietin 1, and angiopoietin receptor-2 | (Mu et al., 2017) |
| Magnolia officinalis | Neolignans, lignans, sesquiterpenes, alkaloids, and phenylethanoid | Antioxidant, extracellular matrix biosynthesis, and protects chondrocytes | ↓NF-κB | (Chen et al., 2014b; Amorati et al., 2015) |
| Berberine II | Alkaloids (Isoquinoline) | Antiapoptotic, extracellular matrix biosynthesis, and protects chondrocytes | ↓NF-κB | (Zhou et al., 2015a; Lu et al., 2019) |
| Quercetin | Flavonoid glycosides | Antioxidant, angiogenesis, and bone repair | ↓ IL-6, IL-1α, IL-3, ↑ IL-4, NF-κB | (Zhou et al., 2015b; Forte et al., 2016) |
| Betulinic acid | Triterpene | Antioxidant and extracellular matrix biosynthesis | ↓ Extracellular matrix (ECM) ↓ Transforming growth factor-β1/Smad signaling pathway |
(Yi et al., 2014; Jiang et al., 2017) |
| Sophoridine | Matrine | Antiapoptosis and antioxidant | ↓Caspase-3 and Bax, ↑ Bcl-2 |
(Zhao et al., 2015) |
| Baicalin | Baicalin | Protects chondrocytes | ↓ H2O2 ↓ ECM-genes |
(Cao et al., 2018) |
| Iso quercitrin | Flavonoids | Bone formation | (Li et al., 2019) | |
| Genistein | Isoflavone | Anti-inflammatory, angiogenesis, enhancing bone formation, and inhibiting bone resorption | ↑ RUNX2 ↑BMP and angiogenesis pathways |
(Cheng et al., 2014) |
| Bauhinia championii flavone | Flavonoids | Antioxidant, anti-inflammatory, and antiapoptotic | ↓ Apoptosis ↓caspase-3 and TLR4, ↑Bcl-2 |
(Jian et al., 2016) |
| Velvet antler | Amino acids, polypeptides and proteins | Angiogenesis, proliferation, and differentiation of chondrocytes | ↑ CEPCs and VEGF ↑ Jagged-1, Notch1, NICD, HES1, Hes1 and Hey2 |
(Li et al., 2018; Ma et al., 2019) |
| Achyranthes bidentata | Phytosterone, phytoecdysteroids, saccharides and saponins | Promotes chondrocyte proliferation, anti-inflammatory, and antiapoptotic | ↑ Wnt/β-catenin pathway ↑Frizzled-2, β-catenin and cyclin D1 ↓ glycogen synthase kinase 3β (GSK-3β) |
(Weng et al., 2014; Zhang et al., 2014) |
| Sinomenine | Alkaloids | Anti-apoptotic of chondrocytes | ↓ MMP-13 ↑ TIMP-1 ↓ Caspase-3 activity and apoptosis ↑ Cell viability |
(Ju et al., 2010) |
| Ginsenosides | Saponins, ginsenoside | Angiogenesis, antioxidative possesses osteoblast differentiation and osteogenic stimulatory | ↑Cell growth, ALP, Coll-I synthesis ↑ BMP-2 and Runx2 ↓ ROS production |
(Siddiqi et al., 2014; Kang et al., 2019) |
| Gambogic acid | Gamboges, guttic acid | Angiogenesis, antioxidative possesses | ↓Hsp90 inhibitions | (Nabi et al., 2016) |
| White mulberry | Gallic acid, chlorogenic acid, protocatechuic acid, rutin, caffeic acid | Immunomodulation, anti-inflammation, antioxidation, and relieves cartilage degeneration | ↓ Proteoglycans ↑ Mineral density ↓ Bone damage |
(Yimam et al., 2015; He et al., 2018) |
Abbreviations: HIF-1α, hypoxia inducible factor-1α; TD, tibial dyschondroplasia; TNF, tumor necrosis factor.
Figure 5.
Mechanism of traditional Chinese medicines for improving bone remodeling in TD chickens. Abbreviation: TD, tibial dyschondroplasia.
Promote the Proliferation of Chondrocytes and Inhibit the Apoptosis of Chondrocytes
Previous results showed that Eucommia ulmoides could promote or inhibit the proliferation of chondrocytes; E. ulmoides increased the bone growth rate by promoting chondrogenesis or inhibiting the proliferation of chondrocytes, as well as increasing the expression levels of BMP-2 and insulin-like growth factor-1 (Kim et al., 2015). Puerarin increased the proliferation of chondrocytes in osteoarthritis (Peng et al., 2019b). Antler extracts promoted chondrocyte proliferation and differentiation and prevented chondrocyte apoptosis (Yao et al., 2019). Emodin can promote the proliferation of chondrocytes by inhibiting the expression of extracellular signal-regulated kinase and Wnt/beta-catenin pathways in chondrocytes and downregulate the expression of a series of inflammatory mediators (Liu et al., 2018). Psoralen, achyransaceae polysaccharide, and soybean isoflavone promote osteoblast differentiation and proliferation by activating the Wnt/β-catenin signaling pathway (Weng et al., 2014; Yu et al., 2015; Zheng et al., 2017). Traditional Chinese medicine can be used for the prevention and treatment of TD by regulating the chondrocyte cycle, promoting chondrocyte proliferation.
Degradation and Synthesis of Extracellular Matrix
Extracellular matrix is a noncellular 3-dimensional macromolecular network composed of collagen, proteoglycan/glycosaminoglycan, elastin, fibronectin, laminin, and several other glycoproteins (Theocharis et al., 2016). Studies have shown that TCM can promote the synthesis of collagen and proteoglycan in cartilage matrix and inhibit its degradation, which may be one of the protective mechanisms of cartilage. Results showed that ICA promotes cartilage repair via regulating chondrocyte proliferation and differentiation, as well as ECM synthesis (Wang et al., 2016). Curcumin inhibits the production of proinflammatory cytokines and prostanoids and the degradation of matrix-metalloproteases (Henrotin et al., 2010). Psoralen can promote the synthesis of ECM and increase the expression of cartilage genes, which may be a useful bioactive component to activate the function of chondrocytes (Xu et al., 2015). Astragaloside IV significantly induced osteogenesis-related gene expression, such as ALP, Col1a2, osteocalcin, and Runx2 (Bian et al., 2011). Chlorogenic acid has a positive therapeutic effect on TD by regulating the caspase and BECN1 expression,and regulating the degradation of ECM.
Angiogenesis
Blood plays a role of nutrient transport in growth and development, and blood vessel degeneration often leads to severe damage to tissues and organs, as well as bone development. The growth and development of bones cannot be initiated and maintained without angiogenesis (Mehmood et al., 2018a, Mehmood et al., 2018b; Zhang et al., 2018b). Capillary invasion mediated by VEGF is the key mechanism linking chondrogenesis and osteogenesis, which determines the development and growth rate of bone (Mehmood et al., 2018a, Mehmood et al., 2018b). Thiram can change the differentiation of abnormal chondrocytes by altering the expression of the HIF-1α/VEGF pathway of chondrocytes. Previously, Drynaria fortunei promoted angiogenesis associated with modified MMP-2/TIMP-2 balance (Mehmood et al., 2018a; Huang et al., 2018). Icariin can significantly improve abnormal angiogenesis in the GP of TD and promote vascular recovery (Zhang et al., 2018c). Mehmood et al., 2018a, Mehmood et al., 2018b demonstrated that the TMP enhances angiogenesis in TD chickens via regulation of the HIF-1α/VEGF signaling pathway. Administering celastrol to TD chickens prevented unvascularized GP and reinstated angiogenesis (Nabi et al., 2016). Screening the TCM that promotes tibia angiogenesis is considered as another important target in treating broilers TD.
Scavenging Oxygen Free Radicals
Free radicals can inhibit the synthesis of chondrocyte DNA, matrix proteoglycan, and collagen and cause severe damage to the membrane structure of cartilage. In addition, free radicals can induce apoptosis in chondrocytes, resulting in high levels of cell count reductions within critical zones of bone GP (Qin et al., 2019). Administration of Dendrobium officinale polysaccharides to aged mice significantly decreased oxidative stress of bone marrow mesenchymal stem cell (Peng et al., 2019a). Scutellarin reduced the levels of oxidative stress in collagen-induced arthritis mice (Zhang et al., 2017). Usually, chondrocyte proliferation and apoptosis are in a dynamically balanced state; however, excessive apoptosis of chondrocytes in TD chickens reduces cell density and numbers within bone GP resulting in impairment of cartilage formation, as well as subsequent ossification processes.
Meanwhile, oxygen-free radicals can also accelerate the apoptosis of chondrocytes, reducing the content of proteoglycan in the cartilage matrix. Some key selected therapeutic TCM agents help prevent the occurrence of TD in chickens via scavenging radical oxygen species, thereby reducing oxidative damage to cartilaginous chondrocytes. Li et al. (2007) reported that thiram destroyed the oxidative balance via decreasing superoxide dismutase and GSH-Px content. Our previous study found that the antioxidant index of the liver had significant changes in TD chickens, the levels of superoxide dismutase, glutathione peroxidase, and total antioxidant capability were significantly reduced, and the content of malondialdehyde was increased considerably. Icariin, anacardic acid, and tetramethylpyrazine can restore the serum biochemical indexes and antioxidant imbalance of TD broilers.
Conclusion
Currently, research on the mechanisms of TCM-derived products for the treatment of TD is now providing a strong foundation in efforts to uncover potential treatments for TD. However, there are still many problems in the clinical application of TCM. For example, the composition of TCM and specific mechanisms of action are not clear; the chemical structure of polysaccharides, flavonoids, and glycoside monomers is complex, and large-scale (industrial) production is difficult; moreover, dosage of naturally occurring substances is not easily standardized, and lack of quantitative indicators and unified standards is difficult to implement on a large scale. Future research on the mechanism of TCM treatment should be combined with the latest scientific achievements to deepen further the understanding TCM derivative treatment and to lay the foundation.
Tibial dyschondroplasia is the most important tibiotarsal bone disease in fast-growing poultry that disturbs normal development of the tibial GP. The long metabolic cycles of most drugs combined with residue buildup and high treatment cost seriously restrict the utility of synthetic therapeutics for the treatment of TD. The use of TCM not only avoids the gastrointestinal reactions caused by oral drugs but also avoids the first-pass effects on liver metabolism. The principle of selecting TCM and its pharmacologic effects on TD chickens is primarily focused on the differentiation, proliferation, and apoptosis of chondrocytes, angiogenesis, matrix metabolism, oxidative damage, cytokines, and calcification of cartilage in tibia.
Acknowledgments
The study was supported by the the National Key Research and Development Program of China (Project No. 2016YFD0501200 and 2017YFD0502200).
Conflict of Interest Statement: None of the authors have any conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.08.055.
Contributor Information
Zhaoxin Tang, Email: tangzx@scau.edu.cn.
Ying Li, Email: lying@scau.edu.cn.
Supplementary data
References
- Amorati R., Zotova J., Baschieri A., Valgimigli L. Antioxidant activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their reaction with Peroxyl radicals. J. Org. Chem. 2015;80:10651–10659. doi: 10.1021/acs.joc.5b01772. [DOI] [PubMed] [Google Scholar]
- An W., Lai H., Zhang Y., Liu M., Lin X., Cao S. Apoptotic pathway as the therapeutic target for Anticancer traditional Chinese medicines. Front Pharmacol. 2019;10:758. doi: 10.3389/fphar.2019.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Q., Huang J.H., Liang Q.Q., Shu B., Hou W., Xu H., Zhao Y.J., Lu S., Shi Q., Wang Y.J. The osteogenetic effect of astragaloside IV with centrifugating pressure on the OCT-1 cells. Pharmazie. 2011;66:63–68. [PubMed] [Google Scholar]
- Borjesson A.E., Lagerquist M.K., Windahl S.H., Ohlsson C. The role of estrogen receptor alpha in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 2013;70:4023–4037. doi: 10.1007/s00018-013-1317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Zhang Y., Wang T., Li B. Endoplasmic Reticulum stress is involved in Baicalin protection on chondrocytes from Patients with osteoarthritis. Dose Response. 2018;16 doi: 10.1177/1559325818810636. 712707828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.H., Tsai C.F., Hsu Y.W., Lu F.J. Epigallocatechin gallate eye drops protect against ultraviolet B-induced corneal oxidative damage in mice. Mol. Vis. 2014;20:153–162. [PMC free article] [PubMed] [Google Scholar]
- Chen Y.J., Tsai K.S., Chan D.C., Lan K.C., Chen C.F., Yang R.S., Liu S.H. Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes. J. Orthop. Res. 2014;32(4):573–580. doi: 10.1002/jor.22577. [DOI] [PubMed] [Google Scholar]
- Chen X., Wei W., Li Y., Huang J., Ci X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2019;308:269–278. doi: 10.1016/j.cbi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- Cheng K., Chen K.M., Ge B.F., Zhen P., Gao Y.H., Ma H.P. [Comparison research with icariin and genistein by anti-inflammatory reaction and angiogenesis pathway to inhibit bone loss on ovariectomized rats] Zhong Yao Cai. 2014;37(4):627–631. (In Chinese) [PubMed] [Google Scholar]
- Dan H., Simsa-Maziel S., Hisdai A., Sela-Donenfeld D., Monsonego O.E. Expression of matrix metalloproteinases during impairment and recovery of the avian growth plate. J. Anim. Sci. 2009;87:3544–3555. doi: 10.2527/jas.2009-2068. [DOI] [PubMed] [Google Scholar]
- Forte L., Torricelli P., Boanini E., Gazzano M., Rubini K., Fini M., Bigi A. Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: an in vitro osteoblast–osteoclast–endothelial cell co-culture study. Acta Biomater. 2016;32:298–308. doi: 10.1016/j.actbio.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Granja A., Frias I., Neves A.R., Pinheiro M., Reis S. Therapeutic potential of Epigallocatechin gallate Nanodelivery Systems. Biomed. Res. Int. 2017;2017:5813793. doi: 10.1155/2017/5813793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B.Q., Xu J.B., Xiao M., Ding M., Duan L.J. Puerarin reduces ischemia/reperfusion-induced myocardial injury in diabetic rats via upregulation of vascular endothelial growth factor A/angiotensin-1 and suppression of apoptosis. Mol. Med. Rep. 2018;17:7421–7427. doi: 10.3892/mmr.2018.8754. [DOI] [PubMed] [Google Scholar]
- Han D., Chen W., Gu X., Shan R., Zou J., Liu G., Shahid M., Gao J., Han B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget. 2017;8:14680–14692. doi: 10.18632/oncotarget.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao P.P., Jiang F., Chen Y.G., Yang J., Zhang K., Zhang M.X., Zhang C., Zhao Y.X., Zhang Y. Traditional Chinese medication for cardiovascular disease. Nat. Rev. Cardiol. 2015;12:115–122. doi: 10.1038/nrcardio.2014.177. [DOI] [PubMed] [Google Scholar]
- He X., Fang J., Ruan Y., Wang X., Sun Y., Wu N., Zhao Z., Chang Y., Ning N., Guo H., Huang L. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): a review. Food Chem. 2018;245:899–910. doi: 10.1016/j.foodchem.2017.11.084. [DOI] [PubMed] [Google Scholar]
- Hejazi I.I., Khanam R., Mehdi S.H., Bhat A.R., Rizvi M., Thakur S.C., Athar F. Antioxidative and anti-proliferative potential of Curculigo orchioides Gaertn in oxidative stress induced cytotoxicity: in vitro, ex vivo and in silico studies. Food Chem. Toxicol. 2018;115:244–259. doi: 10.1016/j.fct.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Clutterbuck A.L., Allaway D., Lodwig E.M., Harris P., Mathy-Hartert M., Shakibaei M., Mobasheri A. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141–149. doi: 10.1016/j.joca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Huang S.T., Chang C.C., Pang J.S., Huang H.S., Chou S.C., Kao M.C., You H.L. Drynaria fortunei promoted angiogenesis associated with modified MMP-2/TIMP-2 balance and activation of VEGF ligand/receptors expression. Front Pharmacol. 2018;9:979. doi: 10.3389/fphar.2018.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M.K., Liu J., Nabi F., Rehman M.U., Zhang H., Tahir A.H., Li J. Recovery of chicken growth plate by heat-shock protein 90 Inhibitors Epigallocatechin-3-gallate and Apigenin in thiram-induced tibial dyschondroplasia. Avian Dis. 2016;60:773–778. doi: 10.1637/11425-041816-Reg. [DOI] [PubMed] [Google Scholar]
- Jian J., Xuan F., Qin F., Huang R. The antioxidant, anti-inflammatory and anti-apoptotic Activities of the Bauhinia Championii Flavone are Connected with protection against myocardial ischemia/reperfusion injury. Cell Physiol Biochem. 2016;38:1365–1375. doi: 10.1159/000443080. [DOI] [PubMed] [Google Scholar]
- Jiang L., Chen F.X., Zang S.T., Yang Q.F. Betulinic acid prevents high glucoseinduced expression of extracellular matrix protein in cardiac fibroblasts by inhibiting the TGFbeta1/Smad signaling pathway. Mol. Med. Rep. 2017;16:6320–6325. doi: 10.3892/mmr.2017.7323. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhang H., Ma T., Lan H., Feng S., Zhu H., Ji Y. Resveratrol protects Murine chondrogenic ATDC5 cells against LPS-induced inflammatory injury through up-regulating MiR-146b. Cell Physiol Biochem. 2018;47:972–980. doi: 10.1159/000490141. [DOI] [PubMed] [Google Scholar]
- Ju X.D., Deng M., Ao Y.F., Yu C.L., Wang J.Q., Yu J.K., Cui G.Q., Hu Y.L. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi. 2010;130:1053–1060. doi: 10.1248/yakushi.130.1053. [DOI] [PubMed] [Google Scholar]
- Kang J.I., Choi Y., Cui C.H., Lee D., Kim S.C., Kim H.M. Pro-angiogenic Ginsenosides F1 and Rh1 inhibit vascular Leakage by modulating NR4A1. Sci. Rep. 2019;9:4502. doi: 10.1038/s41598-019-41115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Lee J.I., Song M., Lee D., Song J., Kim S.Y., Park J., Choi H.Y., Kim H. Effects of Eucommia ulmoides extract on longitudinal bone growth rate in adolescent female rats. Phytother Res. 2015;29:148–153. doi: 10.1002/ptr.5195. [DOI] [PubMed] [Google Scholar]
- Kwak S.C., Lee C., Kim J.Y., Oh H.M., So H.S., Lee M.S., Rho M.C., Oh J. Chlorogenic acid inhibits osteoclast differentiation and bone resorption by down-regulation of receptor activator of nuclear factor kappa-B ligand-induced nuclear factor of activated T cells c1 expression. Biol. Pharm. Bull. 2013;36:1779–1786. doi: 10.1248/bpb.b13-00430. [DOI] [PubMed] [Google Scholar]
- Landy N., Toghyani M. Evaluation of one-alpha-hydroxy-cholecalciferol alone or in combination with cholecalciferol in Ca-P deficiency diets on development of tibial dyschondroplasia in broiler chickens. Anim. Nutr. 2018;4:109–112. doi: 10.1016/j.aninu.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La-zhi Y., Qin Z., Hong-xing Z. Effect of Salviol on proliferation and Antioxidation of chondrocytes. Chinese Journal Rehabilitation. 2008;23:83–84. [Google Scholar]
- Leach R.J., Monsonego-Ornan E. Tibial dyschondroplasia 40 years later. Poult. Sci. 2007;86:2053–2058. doi: 10.1093/ps/86.10.2053. [DOI] [PubMed] [Google Scholar]
- Li J., Bi D., Pan S., Zhang Y. Effect of diet with thiram on liver antioxidant capacity and tibial dyschondroplasia in broilers. Br. Poult. Sci. 2007;48:724–728. doi: 10.1080/00071660701665858. [DOI] [PubMed] [Google Scholar]
- Li J., Bi D., Pan S., Zhang Y., Zhou D. Effects of high dietary vitamin A supplementation on tibial dyschondroplasia, skin pigmentation and growth performance in avian broilers. Res. Vet. Sci. 2008;84:409–412. doi: 10.1016/j.rvsc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Li Z.Y., Deng X.L., Huang W.H., Li L., Li H., Jing X., Tian Y.Y., Lv P.Y., Yang T.L., Zhou H.H., Ouyang D.S. Lignans from the bark of Eucommia ulmoides inhibited Ang II-stimulated extracellular matrix biosynthesis in mesangial cells. Chin Med. 2014;9:8. doi: 10.1186/1749-8546-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang Z., Mao M., Zhao M., Xiao X., Sun W., Guo J., Liu C., Yang D., Qiao J., Huang L., Li L. Velvet antler Mobilizes endothelial Progenitor cells to promote angiogenesis and repair vascular endothelial injury in rats following myocardial Infarction. Front Physiol. 2018;9:1940. doi: 10.3389/fphys.2018.01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X., Wang Y., Lu C., Zheng D., Zhang J. Isoquercitrin, a flavonoid glucoside, exerts a positive effect on osteogenesis in vitro and in vivo. Chem. Biol. Interact. 2019;297:85–94. doi: 10.1016/j.cbi.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Li X., Wei G., Wang X., Liu D.H., Deng R.D., Li H., Zhou J.H., Li Y.W., Zeng H.P., Chen D.F. Targeting of the Sonic Hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012;35:1328–1335. doi: 10.1248/bpb.b12-00265. [DOI] [PubMed] [Google Scholar]
- Li J., Hao J. Treatment of Neurodegenerative diseases with bioactive components of Tripterygium wilfordii. Am. J. Chin Med. 2019;47:769–785. doi: 10.1142/S0192415X1950040X. [DOI] [PubMed] [Google Scholar]
- Liang M.J., He L.C., Yang G.D. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum Chuanxiong. Life Sci. 2005;78:128–133. doi: 10.1016/j.lfs.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Liu Z., Lang Y., Li L., Liang Z., Deng Y., Fang R., Meng Q. Effect of emodin on chondrocyte viability in an in vitro model of osteoarthritis. Exp. Ther. Med. 2018;16:5384–5389. doi: 10.3892/etm.2018.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang Y., Li D., Zhou L., Yin P. Effect of Tanshinone Ⅱ A on COX-2-β-catenin signal transduction pathway-mediated VEGF expression in human colorectal cancer cells. China J. Traditional Chin. Med. Pharm. 2013;28:108–112. [Google Scholar]
- Liu S., Yang H., Hu B., Zhang M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/beta-catenin signaling pathways. Exp. Ther. Med. 2017;14:5057–5062. doi: 10.3892/etm.2017.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhong S., Kong R., Shao H., Wang C., Piao H., Lv W., Chu X., Zhao Y. Paeonol alleviates interleukin-1beta-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed. Pharmacother. 2017;95:914–921. doi: 10.1016/j.biopha.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Lu L., Hu J., Wu Q., An Y., Cui W., Wang J., Ye Z. Berberine prevents human nucleus pulposus cells from IL1betainduced extracellular matrix degradation and apoptosis by inhibiting the NFkappaB pathway. Int. J. Mol. Med. 2019;43:1679–1686. doi: 10.3892/ijmm.2019.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Jiang J., Xie G., Liu W., Yan G. Effects of an aqueous extract of Eucommia on articular cartilage in a rat model of osteoarthritis of the knee. Exp. Ther. Med. 2013;6:684–688. doi: 10.3892/etm.2013.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Duan C.C., Yang Z.Q., Ding J.L., Liu S., Yue Z.P., Guo B. Crosstalk between Activin A and Shh signaling contributes to the proliferation and differentiation of antler chondrocytes. Bone. 2019;123:176–188. doi: 10.1016/j.bone.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Iqbal M.K., Rehman M.U., Shahzad M., Li K., Huang S., Nabi F., Zhang L., Li J. In vitro effect of Apigenin and Danshen in tibial dyschondroplasia through inhibition of heat-shock protein 90 and vascular endothelial growth factor expressions in avian growth plate cells. Avian Dis. 2017;61:372–377. doi: 10.1637/11641-032817-RegR. [DOI] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Li K., Wang L., Rehman M.U., Nabi F., Iqbal M.K., Luo H., Shahzad M., Li J. Effect of tetramethylpyrazine on tibial dyschondroplasia incidence, tibial angiogenesis, performance and characteristics via HIF-1alpha/VEGF signaling pathway in chickens. Sci. Rep. 2018;8:2495. doi: 10.1038/s41598-018-20562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Iqbal M.K., Rehman M.U., Li K., Huang S., Shahzad M., Nabi F., Mujahid I., Li J. Tetramethylpyrazine mitigates toxicity and liver oxidative stress in tibial dyschondroplasia chickens. Pak Vet. J. 2018;38:76–80. [Google Scholar]
- Mehmood K., Zhang H., Yao W., Jiang X., Waqas M., Li A., Wang Y., Lei L., Zhang L., Qamar H., Li J. Protective effect of Astragaloside IV to inhibit thiram-induced tibial dyschondroplasia. Environ. Sci. Pollut. Res. Int. 2019;26:16210–16219. doi: 10.1007/s11356-019-05032-1. [DOI] [PubMed] [Google Scholar]
- Mehmood K., Zhang H., Jiang X., Yao W., Tong X., Iqbal M.K., Rehman M.U., Iqbal M., Waqas M., Qamar H., Zhang J., Li J. Ligustrazine recovers thiram-induced tibial dyschondroplasia in chickens: Involvement of new molecules modulating integrin beta 3. Ecotoxicol Environ. Saf. 2019;168:205–211. doi: 10.1016/j.ecoenv.2018.10.080. [DOI] [PubMed] [Google Scholar]
- Mu Y., Xu Z., Zhou X., Zhang H., Yang Q., Zhang Y., Xie Y., Kang J., Li F., Wang S. 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-D-Glucoside attenuates ischemia/reperfusion-induced Brain injury in rats by promoting angiogenesis. Planta Med. 2017;83:676–683. doi: 10.1055/s-0042-120544. [DOI] [PubMed] [Google Scholar]
- Muhammad T., Ikram M., Ullah R., Rehman S.U., Kim M.O. Hesperetin, a Citrus flavonoid, attenuates LPS-induced Neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-kappaB signaling. Nutrients. 2019;11 doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nabavi S.F., Tejada S., Setzer W.N., Gortzi O., Sureda A., Braidy N., Daglia M., Manayi A., Nabavi S.M. Chlorogenic acid and Mental diseases: from Chemistry to medicine. Curr. Neuropharmacol. 2017;15:471–479. doi: 10.2174/1570159X14666160325120625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi F., Shahzad M., Liu J., Li K., Han Z., Zhang D., Iqbal M.K., Li J. Hsp90 inhibitor celastrol reinstates growth plate angiogenesis in thiram-induced tibial dyschondroplasia. Avian Pathol. 2016;45:187–193. doi: 10.1080/03079457.2016.1141170. [DOI] [PubMed] [Google Scholar]
- Ng L.T., Chiang L.C., Lin Y.T., Lin C.C. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am. J. Chin Med. 2006;34:125–135. doi: 10.1142/S0192415X06003692. [DOI] [PubMed] [Google Scholar]
- Pan X., Xu K., Qiu X., Zhao W., Wang D., Yang L. The extract of Fructus Psoraleae promotes viability and cartilaginous formation of rat chondrocytes in vitro. Evid. Based Complement Alternat Med. 2016;2016:2057631. doi: 10.1155/2016/2057631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H., Yang M., Guo Q., Su T., Xiao Y., Xia Z.Y. Dendrobium officinale polysaccharides regulate age-related lineage commitment between osteogenic and adipogenic differentiation. Cell Prolif. 2019;52:e12624. doi: 10.1111/cpr.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Xie Z., Pei J., Wang B., Gao Y., Qu Y. Puerarin alters the function of monocytes/macrophages and exhibits chondroprotection in mice. Mol. Med. Rep. 2019;19:2876–2882. doi: 10.3892/mmr.2019.9936. [DOI] [PubMed] [Google Scholar]
- Pines M., Hasdai A., Monsonego-Ornan E. Tibial dyschondroplasia–tools, new insights and future prospects. World's Poult. Sci. J. 2005;61:285–297. [Google Scholar]
- Piróg K.A., Oihane J., Yoshihisa K., Meadows R.S., Kadler K.E., Boot-Handford R.P., Briggs M.D. A mouse model offers novel insights into the myopathy and tendinopathy often associated with pseudo achondroplasia and multiple epiphyseal dysplasia. Hum. Mol. Genet. 2010;19:52–64. doi: 10.1093/hmg/ddp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D., Zhang H., Zhang H., Sun T., Zhao H., Lee W.H. Anti-osteoporosis effects of osteoking via reducing reactive oxygen species. J. Ethnopharmacol. 2019;244:112045. doi: 10.1016/j.jep.2019.112045. [DOI] [PubMed] [Google Scholar]
- Rath N.C., Huff W.E., Huff G.R. Thiram-induced changes in the expression of genes relating to vascularization and tibial dyschondroplasia. Poult. Sci. 2007;86:2390–2395. doi: 10.3382/ps.2007-00219. [DOI] [PubMed] [Google Scholar]
- Salehi B., Venditti A., Sharifi-Rad M., Kregiel D., Sharifi-Rad J., Durazzo A., Lucarini M., Santini A., Souto E.B., Novellino E., Antolak H., Azzini E., Setzer W.N., Martins N. The therapeutic potential of Apigenin. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad M., Gao J., Qin P., Liu J., Wang Z., Zhang D., Li J. Expression of genes encoding matrilin-3 and Cyclin-I during the impairment and recovery of chicken growth plate in tibial dyschondroplasia. Avian Dis. 2014;58:468–473. doi: 10.1637/10781-012614-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Shahzad M., Liu J., Gao J., Wang Z., Zhang D., Nabi F., Li K., Li J. Differential expression of extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) in avian tibial dyschondroplasia. Avian Pathol. 2015;44:13–18. doi: 10.1080/03079457.2014.987210. [DOI] [PubMed] [Google Scholar]
- Shine V.J., Anuja G.I., Suja S.R., Raj G., Latha P.G. Bioassay guided fractionation of Cyclea peltata using in vitro RAW 264.7 cell culture, antioxidant assays and isolation of bioactive compound tetrandrine. J. Ayurveda Integr. Med. 2020;11:281–286. doi: 10.1016/j.jaim.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi M.H., Siddiqi M.Z., Ahn S., Kim Y.J., Yang D.C. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J. Pharm. Pharmacol. 2014;66:1763–1773. doi: 10.1111/jphp.12306. [DOI] [PubMed] [Google Scholar]
- Song N., Zhao Z., Ma X., Sun X., Ma J., Li F., Sun L., Lv J. Naringin promotes fracture healing through stimulation of angiogenesis by regulating the VEGF/VEGFR-2 signaling pathway in osteoporotic rats. Chem. Biol. Interact. 2017;261:11–17. doi: 10.1016/j.cbi.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Su Y.X., Yan H., Chen B.J., Zahn Q., Wang Y.R., Lu M.L., Wang W.T., He Z., Sheng L. [Effect of naringin of Drynaria Rhizome, a Chinese medical component of Zhuanggu Jianxi Recipe containing serum on caveolin-p38MAPK signal pathway in IL-1beta induced rabbit degenerated chondrocytes] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:1492–1498. [PubMed] [Google Scholar]
- Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Tian W.X., Li J.K., Qin P., Wang R., Ning G.B., Qiao J.G., Li H.Q., Bi D.R., Pan S.Y., Guo D.Z. Screening of differentially expressed genes in the growth plate of broiler chickens with tibial dyschondroplasia by microarray analysis. Bmc Genomics. 2013;14:276. doi: 10.1186/1471-2164-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng T., Liu W.Y., Liou S., Hong T., Liu I. Antioxidant-rich extract from Plantaginis Semen Ameliorates diabetic Retinal injury in a Streptozotocin-induced diabetic rat model. Nutrients. 2016;8:572. doi: 10.3390/nu8090572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang D., Yang D., Zhen W., Zhang J., Peng S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018;29:535–544. doi: 10.1007/s00198-017-4255-1. [DOI] [PubMed] [Google Scholar]
- Wang F., Wu L., Li L., Chen S. Monotropein exerts protective effects against IL-1beta-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int. Immunopharmacol. 2014;23:575–580. doi: 10.1016/j.intimp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Wang P., Zhang F., He Q., Wang J., Shiu H.T., Shu Y., Tsang W.P., Liang S., Zhao K., Wan C. Flavonoid compound icariin activates hypoxia inducible factor-1alpha in chondrocytes and promotes articular cartilage repair. PLoS One. 2016;11:e148372. doi: 10.1371/journal.pone.0148372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Bai L. Resveratrol inhibits apoptosis by increase in the proportion of chondrocytes in the S phase of cell cycle in articular cartilage of ACLT plus Mmx rats. Saudi J. Biol. Sci. 2019;26:839–844. doi: 10.1016/j.sjbs.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng X., Lin P., Liu F., Chen J., Li H., Huang L., Zhen C., Xu H., Liu X., Ye H., Li X. Achyranthes bidentata polysaccharides activate the Wnt/beta-catenin signaling pathway to promote chondrocyte proliferation. Int. J. Mol. Med. 2014;34:1045–1050. doi: 10.3892/ijmm.2014.1869. [DOI] [PubMed] [Google Scholar]
- Xie Q.M., Tang H.F., Chen J.Q., Bian R.L. Pharmacological actions of tetrandrine in inflammatory pulmonary diseases. Acta Pharmacol. Sin. 2002;23:1107–1113. [PubMed] [Google Scholar]
- Xu K., Pan X., Sun Y., Xu W., Njunge L., Yang L. Psoralen activates cartilaginous cellular functions of rat chondrocytes in vitro. Pharm. Biol. 2015;53:1010–1015. doi: 10.3109/13880209.2014.952835. [DOI] [PubMed] [Google Scholar]
- Xu J.H., Yao M., Ye J., Wang G.D., Wang J., Cui X.J., Mo W. Bone mass improved effect of icariin for postmenopausal osteoporosis in ovariectomy-induced rats: a meta-analysis and systematic review. Menopause. 2016;23:1152–1157. doi: 10.1097/GME.0000000000000673. [DOI] [PubMed] [Google Scholar]
- Xue Y., Dongmei L., Yige Z., Hang G., Li H. Angelica polysaccharide moderates hypoxia-evoked apoptosis and autophagy in rat neural stem cells by downregulation of BNIP3. Artif. Cells Nanomed Biotechnol. 2019;47:2492–2499. doi: 10.1080/21691401.2019.1623228. [DOI] [PubMed] [Google Scholar]
- Yang H., Zhang H., Tong X., Zhang J., Shen Y. Recovery of chicken growth plate by TanshinoneA through wnt/beta-catenin pathway in thiram-induced Tibial Dyschondroplasia. Ecotoxicol Environ. Saf. 2019;183:109575. doi: 10.1016/j.ecoenv.2019.109575. [DOI] [PubMed] [Google Scholar]
- Yao B., Zhang M., Leng X., Zhao D. Proteomic analysis of the effects of antler extract on chondrocyte proliferation, differentiation and apoptosis. Mol. Biol. Rep. 2019;46:1635–1648. doi: 10.1007/s11033-019-04612-1. [DOI] [PubMed] [Google Scholar]
- Yao W., Zhang H., Jiang X., Mehmood K., Iqbal M., Li A., Zhang J., Wang Y., Waqas M., Shen Y., Li J. Effect of Total flavonoids of Rhizoma drynariae on tibial dyschondroplasia by regulating BMP-2 and Runx2 expression in chickens. Front Pharmacol. 2018;9:1251. doi: 10.3389/fphar.2018.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. South-Central University for Nationalities; Wuhan, China: 2013. Preliminary Study of Chlorogenic Acid on Activity of Cultured Osteoblasts in Vitro. Master degree. (In Chinese) [Google Scholar]
- Yi Y., Adrjan B., Li J., Hu B., Roszak S. NMR studies of daidzein and puerarin: active anti-oxidants in traditional Chinese medicine. J. Mol. Model. 2019;25:202. doi: 10.1007/s00894-019-4090-8. [DOI] [PubMed] [Google Scholar]
- Yi J., Xia W., Wu J., Yuan L., Wu J., Tu D., Fang J., Tan Z. Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J. Vet. Sci. 2014;15:141–148. doi: 10.4142/jvs.2014.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimam M., Lee Y.C., Kim T.W., Moore B., Jiao P., Hong M., Kim H.J., Nam J.B., Kim M.R., Oh J.S., Cleveland S., Hyun E.J., Chu M., Jia Q. UP3005, a Botanical composition containing Two standardized extracts of Uncaria gambir and Morus alba, improves Pain Sensitivity and cartilage degradations in Monosodium Iodoacetate-induced rat OA disease model. Evid. Based Complement Alternat Med. 2015;2015:785638. doi: 10.1155/2015/785638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Liu Z., Tong Z., Zhao Z., Liang H. Soybean isoflavone treatment induces osteoblast differentiation and proliferation by regulating analysis of Wnt/beta-catenin pathway. Gene. 2015;573:273–277. doi: 10.1016/j.gene.2015.07.054. [DOI] [PubMed] [Google Scholar]
- Yu G., Luo Z., Wang W., Li Y., Zhou Y., Shi Y. Rubus chingii Hu: a review of the Phytochemistry and Pharmacology. Front Pharmacol. 2019;10:799. doi: 10.3389/fphar.2019.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R., Shi W., Xin Q., Yang B., Hoi M.P., Lee S.M., Cong W., Chen K. Tetramethylpyrazine and Paeoniflorin inhibit Oxidized LDL-induced angiogenesis in human Umbilical Vein endothelial cells via VEGF and Notch pathways. Evid. Based Complement Alternat Med. 2018;2018:3082507. doi: 10.1155/2018/3082507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Mehmood K., Jiang X., Yao W., Iqbal M., Li K., Tong X., Wang L., Wang M., Zhang L., Nabi F., Rehman M.U., Li J. Effect of icariin on tibial dyschondroplasia incidence and tibial characteristics by regulating P2RX7 in chickens. Biomed. Res. Int. 2018;2018:6796271. doi: 10.1155/2018/6796271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Mehmood K., Jiang X., Yao W., Iqbal M., Waqas M., Rehman M.U., Li A., Shen Y., Li J. Effect of tetramethyl thiuram disulfide (thiram) in relation to tibial dyschondroplasia in chickens. Environ. Sci. Pollut. Res. Int. 2018;25:28264–28274. doi: 10.1007/s11356-018-2824-2. [DOI] [PubMed] [Google Scholar]
- Zhang H., Mehmood K., Jiang X., Li Z., Yao W., Zhang J., Tong X., Wang Y., Li A., Waqas M., Iqbal M., Li J. Identification of differentially expressed MiRNAs profile in a thiram-induced tibial dyschondroplasia. Ecotoxicol Environ. Saf. 2019;175:83–89. doi: 10.1016/j.ecoenv.2019.03.043. [DOI] [PubMed] [Google Scholar]
- Zhang H., Chang Z., Mehmood K., Yang M.K., Liu Z., Duan Z., Yuan F., Ali M.M., Adnan M., Qasim M.U., Shaheen S., Abbas R.Z., Tian Y., Guo R. Tetramethylpyrazine inhibited hypoxia-induced expression of calcium-sensing receptors in pulmonary artery smooth muscle cells in chickens. J. Biol. Regul. Homeost Agents. 2018;32:489–495. [PubMed] [Google Scholar]
- Zhang J., Huang S., Tong X., Zhang L., Jiang X., Zhang H., Mehmood K., Li J. Chlorogenic acid alleviates thiram-induced tibial dyschondroplasia by modulating caspases, BECN1 expression and ECM degradation. Int. J. Mol. Sci. 2019;20:3160. doi: 10.3390/ijms20133160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Chang J., Zhao Y., Xu H., Wang T., Li Q., Xing L., Huang J., Wang Y., Liang Q. Fabrication of a triptolide-loaded and poly-gamma-glutamic acid-based amphiphilic nanoparticle for the treatment of rheumatoid arthritis. Int. J. Nanomedicine. 2018;13:2051–2064. doi: 10.2147/IJN.S151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sun S., Li W., Zhang W., Wang X., Yang S.Y. Effect of Scutellarin inhibits collageninduced arthritis through TLR4/NFkappaBmediated inflammation. Mol. Med. Rep. 2017;16:5555–5560. doi: 10.3892/mmr.2017.7292. [DOI] [PubMed] [Google Scholar]
- Zhang M., Hu X. Mechanism of chlorogenic acid treatment on femoral head necrosis and its protection of osteoblasts. Biomed. Rep. 2016;5:57–62. doi: 10.3892/br.2016.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Huai Y., Miao Z., Qian A., Wang Y. Systems Pharmacology for Investigation of the mechanisms of action of traditional Chinese medicine in drug Discovery. Front Pharmacol. 2019;10:743. doi: 10.3389/fphar.2019.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu X., Xu T., Qin S. beta-Ecdysterone suppresses interleukin-1beta-induced apoptosis and inflammation in rat chondrocytes via inhibition of NF-kappaB signaling pathway. Drug Dev. Res. 2014;75:195–201. doi: 10.1002/ddr.21170. [DOI] [PubMed] [Google Scholar]
- Zhao L., Wang Y., Liu J., Wang K., Guo X., Ji B., Wu W., Zhou F. Protective effects of genistein and puerarin against Chronic alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Agric. Food Chem. 2016;64:7291–7297. doi: 10.1021/acs.jafc.6b02907. [DOI] [PubMed] [Google Scholar]
- Zhao P., Zhou R., Zhu X.Y., Hao Y.J., Li N., Wang J., Niu Y., Sun T., Li Y.X., Yu J.Q. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int. J. Mol. Med. 2015;36:633–644. doi: 10.3892/ijmm.2015.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Lin P., Ma Y., Shao X., Chen H., Chen D., Liu X., Li X., Ye H. Psoralen promotes the expression of cyclin D1 in chondrocytes via the Wnt/beta-catenin signaling pathway. Int. J. Mol. Med. 2017;40:1377–1384. doi: 10.3892/ijmm.2017.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liu S., Yu L., He B., Wu S., Zhao Q., Xia S., Mei H. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis: An International Journal Programmed Cell Death. 2015;20:1187–1199. doi: 10.1007/s10495-015-1152-y. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wu Y., Jiang X., Zhang X., Xia L., Lin K., Xu Y. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS One. 2015;10:e129605. doi: 10.1371/journal.pone.0129605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.