Abstract
The objective of this study was to compare 2 laying hen strains in 5 production periods regarding phytase activity, phytate (InsP6) degradation, and myo-inositol (MI) release in the digestive tract and phosphorus (P) and calcium (Ca) utilization. One offspring of 10 nonrelated roosters per strain (Lohmann Brown-classic (LB) or Lohmann LSL-classic (LSL)) was placed in one of 20 metabolic units in a completely randomized block design in week 8, 14, 22, 28, and 58 of life. All hens were fed the same corn and soybean meal–based diet at one time, but the diet composition was adjusted to the requirements in the respective period. For 4 consecutive days, excreta were collected quantitatively at 24-hour intervals. In week 10, 16, 24, 30, and 60, the blood plasma, digesta of crop, gizzard, jejunum, ileum, and ceca, and mucosa of the jejunum was collected. The concentration of inorganic P in the blood plasma was higher in LB than in LSL hens (P = 0.026). Plasma Ca concentrations increased with each period (P < 0.001) in both strains. In jejunum digesta, the MI concentration did not differ between strains, but InsP6 concentration was higher in LB than in LSL hens (P = 0.002) and the highest in week 30 and 60. Total phosphatase and phytase activities were higher in LB than in LSL hens (P ≤ 0.009). Period effects were also significant for these enzymes. Concentrations of some constituents of the cecal content were different between the strains. The MI concentration in the egg albumen and yolk was higher in LB than in LSL hens. Differences in InsP6- and MI-related metabolism of the 2 hen strains existed. These differences were partly dependent of the period. Especially, week 24 was a period of remarkable change of metabolism. Great differences also existed among individuals, making it worth to have a closer look at the metabolism of individuals in addition to evaluating treatment means. Further studies on metabolic, genetic, and microbiome level may help explain these differences.
Key words: laying hen, mucosal phytase, myo-inositol, phosphorus, phytate degradation
Introduction
The functioning of all living organisms is dependent on the continuous supply of phosphates for the formation of structural body compartments, such as the bones and other physiological mechanisms. Fertilizer and feed phosphate supply is largely based on finite rock phosphate stores, which makes efficient utilization necessary along the food chain. To the farm animal sector, exploring possibilities for a reduction in feed phosphate application, improving the efficacy of phytase supplements in nonruminant feeding (Rodehutscord, 2008), and breeding for improved phosphorus (P) utilization (Beck et al., 2016) are the main approaches to address these challenges. Phytic acid (myo-inositol 1,2,3,4,5,6-hexakis (dihydrogen phosphate); InsP6) including its salts (phytate) is the primary storage form of P in plant seeds and feedstuffs obtained from plant seeds. The utilization of phosphate bound as InsP6 by the animal requires stepwise cleavage of P from the inositol ring, which is catalyzed by phytases and other phosphatases. The cleavage yields isomers of lower inositol phosphates with different degrees of phosphorylation as intermediate products and myo-inositol (MI) as end product. Although it has often been assumed that poultry is unable for endogenous InsP6 degradation, recent studies showed substantial InsP6 degradation in the digestive tract of broiler chickens. Between 62 and 74% of InsP6 contained in the diet disappeared until the terminal ileum of broiler chickens in the absence of exogenous and intrinsic plant phytase and when mineral P was not provided and calcium (Ca) supply was low (Tamim et al., 2004; Shastak et al., 2014; Zeller et al., 2015a, Zeller et al., 2015b). These results revealed the high potential of poultry for gastrointestinal InsP6 hydrolysis by enzymes of the mucosa or gut bacteria. Because remarkable InsP6 disappearance was also found in the digestive tract of gnotobiotic broiler chickens, mucosal enzymes likely contribute to InsP6 hydrolysis (Sommerfeld et al., 2019). This is consistent with studies that found some phytase activity in purified brush-border membrane (BBM) vesicles from different sections of the small intestine (Maenz and Classen, 1998; Onyango et al., 2006; Huber et al., 2015).
Unlike broiler chickens, laying hens were hardly studied in regard to InsP6 breakdown and MI release in the digestive tract. Maenz and Classen (1998) found a higher total endogenous phytase activity in the duodenum and the proximal jejunum in the BBM vesicles of laying hens than those of broilers. Another study found endogenous phytase activity in the small intestinal mucosa and in the content of some digestive tract segments and fecal disappearance to be significantly higher in 47-week-old than in 20-week-old hens (53 vs. 25%) (Marounek et al., 2008). Significant differences between 2 laying hen strains in total tract InsP6 degradation were reported in a study by Abudabos (2012). However, the release of MI in the digestive tract of laying hens has not been studied yet, and knowledge about the function and potential effects of MI in poultry is lacking (Gonzalez-Uarquin et al., 2020), including transfer of MI into the egg.
Phytate degradation by laying hens is of particular interest for 2 major reasons. First, hens require less P in their diet than growing birds (Ahmadi and Rodehutscord, 2012) but need substantially more Ca because of eggshell formation. This causes amounts of Ca and P passing through the digestive tract being more extreme than in broiler chickens and likely causing more distinct interactions with InsP6 degradation and MI release than in broiler chickens. Second, the laying hen's productive live span usually is 12 mo or longer. It follows a rearing period in that body growth instead of egg production is targeted, causing changes of requirements for Ca and P. Therefore, conditions for InsP6 degradation change markedly during the life span of a hen. The consequence of these changes for InsP6 degradation by hens has not been investigated to date. Therefore, the objective of this study was to characterize endogenous phosphatase activity and InsP6 degradation products and utilization of P and Ca by 2 laying hen strains in different stages of production.
The hypotheses of this study were as follows:
-
1.
The genetic background of the hens affects the mucosal phytase activity and therefore InsP6 disappearance, inositol phosphate pattern, and MI in different gut sections and MI in the blood of laying hens and their eggs.
-
2.
Owing to adaption processes over time, results are affected by the stage of production and may interact with the genetic background of the hens.
Materials and methods
Birds and Housing
The study was conducted at the Agricultural Experiment Station of the University of Hohenheim, Germany. It was approved by the Regierungspräsidium Tübingen, Germany (Project no. HOH50/17 TE) in accordance with the German Animal Welfare Legislation.
A total of 140 Lohmann Brown-classic (LB) and 140 Lohmann LSL-classic (LSL) newly hatched female chickens were obtained from a breeding company (Lohmann Tierzucht GmbH, Cuxhaven, Germany), representing 2 distinct genetic backgrounds. For each strain, 14 nonrelated roosters had been used for egg production. Ten offspring per rooster were placed at the beginning.
Birds were raised in floor pens on deep litter bedding according to the standardized routine procedure of the Experiment Station before being moved to metabolic units in week 8, 14, 22, 28, and 58 at the beginning of one of five measurement periods representing the different stages of production. During rearing, all hens were weighed at intervals to monitor BW development. Before the beginning of the first period, offspring of 10 roosters per strain were chosen based on the average BW of the female offspring. At the onset of each of the five periods, one randomly chosen offspring of each of the 10 roosters per strain was placed in one metabolism unit (1 m × 1 m × 1 m), resulting in 10 replicates per strain, in a completely randomized block design 10 d before slaughter. Metabolism units were equipped with a wooden perch, a nest, a feeding trough, water cups, and a wire mesh floor. For total excreta collection, metal trays were installed under the units. Nine hours of light and 15 h of darkness were provided during the first 2 periods, and 16 h of light and 8 h of darkness were provided during the last 3 periods. Temperature in the barn was set to 18°C to 22°C during all sampling periods.
Diets
All hens were fed the same diet at one time, but the diet composition was adjusted to the requirements and level of feed intake in the respective period (Table 1). Diets were based on corn and soybean meal to minimize plant intrinsic phytase activity and meeting or exceeding the breeding company supply recommendations. Diets provided in the metabolism units contained TiO2 as the indigestible marker and were in mash form. Diets were mixed in the certified feed mill of the Agricultural Experiment Station of the University of Hohenheim. Feed and water were provided for ad libitum consumption throughout the experiment. Calculated nutrient concentrations were confirmed by analyses (Table 1).
Table 1.
Ingredient, calculated, and analyzed composition of the experimental diets.
| Ingredient, g/kg | Week 10 | Week 16 | Week 24 | Week 30 | Week 60 |
|---|---|---|---|---|---|
| Corn | 667.2 | 667.2 | 574.0 | 606.5 | 599.0 |
| Soybean meal | 177.8 | 177.8 | 283.6 | 263.7 | 252.6 |
| Alfalfa meal | 105.5 | 105.5 | - | - | - |
| Soybean oil | 10.0 | 10.0 | 19.9 | 11.9 | 17.7 |
| DL-Methionine | 2.5 | 2.5 | 3.5 | 3.5 | 3.4 |
| Monocalcium phosphate | 12.2 | 12.2 | 10.5 | 10.4 | 9.3 |
| Limestone, fine1 | 11.4 | 11.4 | 42.6 | 26.9 | 28.6 |
| Limestone, coarse2 | - | - | 52.1 | 63.7 | 76.1 |
| Sodium chloride | 3.0 | 3.0 | 3.3 | 3.0 | 2.9 |
| Choline chloride | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Sodium bicarbonate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Vitamin mix3 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mineral mix4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| TiO2 | 5.0 | 5.0 | 5.0 | 5.0 | 4.9 |
| Calculated concentration | |||||
| Crude protein, g/kg DM | 167 | 167 | 195 | 188 | 183 |
| Total P, g/kg DM | 6.4 | 6.4 | 6.3 | 6.1 | 5.9 |
| Nonphytate P, g/kg DM | 4.5 | 4.5 | 4.0 | 3.9 | 3.7 |
| Ca, g/kg DM | 10.0 | 10.0 | 43.1 | 41.3 | 45.0 |
| Analyzed concentration5 | |||||
| Total P, g/kg DM | 6.5 | 6.6 | 6.2 | 6.2 | 6.0 |
| InsP6-P, g/kg DM | 2.0 | 2.1 | 2.3 | 2.1 | 2.4 |
| Ca, g/kg DM | 10.0 | 10.0 | 43.2 | 46.2 | 47.6 |
| Myo-inositol, μmol/g DM | 2.2 | 2.2 | 1.7 | 1.1 | 1.1 |
| Ins(1,2,3,4,5)P5, μmol/g DM | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 |
| Ins(1,2,4,5,6)P5, μmol/g DM | 0.7 | 0.7 | 1.0 | 0.8 | 0.8 |
| InsP6, μmol/g DM | 10.9 | 11.1 | 12.1 | 11.4 | 13.2 |
Particle size: > 0.25 mm—0.9%, 0.125-0.25 mm—3.3%, 0.063-0.125 mm—11.0%, <0.063 mm—84.8%.
Particle size: > 1.18 mm—12.3%, 1-1.18 mm—34.8%, 0.5-1 mm—48.8%, 0.063-0.5 mm—0.6%, <0.063 mm—3.6%.
Vitamin premix (Miavit GmbH, Essen, Germany), provided per kg of the complete diet: 10,000 IU vitamin A, 3,000 IU vitamin D3, 30 mg vitamin E, 2.4 mg vitamin K3, 100 mcg biotin, 1 mg folic acid, 3 mg vitamin B1, 6 mg vitamin B2, 6 mg vitamin B6, 30 mcg vitamin B12, 50 mg nicotinamide, 14 mg calcium-D-pantothenate.
Trace element premix (Gelamin Gesellschaft für Tierernährung mbH, Memmingen, Germany), provided per kg of complete diet: 80 mg manganese from manganese-(II)-oxide, 60 mg zinc from zinc oxide, 25 mg iron from ferrous-(II)-sulfate monohydrate, 7.5 mg copper from cupric-(II)-sulfate pentahydrate, 0.6 mg iodine from calcium iodate, 0.2 mg selenium from sodium selenite.
All other InsP isomers below the limit of quantification or limit of detection.
Experimental Procedures, Samplings, and Measurements
Before the beginning of each period, hens were distributed to the metabolism units. Samples of each diet were obtained before and after the excreta collection phases in week 9, 15, 23, 29, and 59 to determine the DM content of the respective diet. The animals were inspected at least twice daily. During excreta collection phases in week 23, 29, and 59 the eggs of all hens were collected and weighed. Eggs from the second last collection day were kept at room temperature until further processing for MI analysis. Feed was weighed at the beginning and feed and birds at the end of each period. Excreta were collected from the trays quantitatively at 24-hour intervals for 4 consecutive days. Feathers, skin scales, and spilled feed were carefully removed before each excreta collection. Feed residues from the trays were weighed, and feed intake was corrected for these residues. Excreta were immediately frozen after each collection at −20°C.
Before slaughtering in week 10, 16, 24, 30, and 60, feed was deprived 2 h followed by 1 h ad libitum access to feed to standardize gut fill. The hens were individually stunned with a gas mixture of 35% CO2, 35% N2, and 30% O2 and killed by decapitation. The trunk blood was collected in tubes containing sodium fluoride for MI analysis or lithium heparin for P and Ca analysis. Blood samples were then centrifuged for 10 min at 2,500 × g to obtain plasma. Digesta from the crop, gizzard, jejunum, the terminal part of the ileum, defined as the last two-thirds of the section between Meckel's diverticulum and 2 cm prior the ileo-ceco-colonic junction, and both ceca were collected. The crop and gizzard were clamped with an arterial clamp to prevent emptying, then opened and upended. The crop and gizzard digesta were gently removed with a spatula without scraping the mucosa. The jejunum, terminal ileum, and ceca were cut lengthwise, and the digesta were gently removed with a spatula without scraping the mucosa. About 15 cm of the proximal jejunum was taken and freed of any fat, and jejunal mucosa was stripped off carefully using microscopic slides and shock-frozen into liquid nitrogen. Immediately after finishing the collection, mucosa samples were transported on dry ice and subsequently stored at −80°C until analysis.
Digesta samples were immediately frozen at −20°C, freeze dried, and pulverized (PULVERISETTE 9, Fritsch GmbH, Idar-Oberstein, Germany). Pulverized samples were stored in airtight containers until further analysis.
The yolk and albumen from one egg per hen were separated and weighed, respectively. Then, they were freeze-dried, weighed again, and pulverized with a pestle and mortar and stored until further analysis.
Sample Preparation and Analyses
Excreta samples were thawed at +3°C, weighed, pooled for each individual hen for each period, and homogenized. The DM of the excreta was analyzed in triplicate. A subsample of the excreta was freeze-dried and pulverized. Pulverized samples were stored in airtight containers until further analysis. Feed were ground to pass through a 0.5-mm sieve (Ultra Centrifugal Mill ZM 200, Retsch GmbH, Haan, Germany) and excreta samples were analyzed for the DM according to the official method in Germany (method no. 3.1) (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten (VDLUFA) 2007).
Pulverized feed, digesta, and excreta samples were analyzed for P, Ca, and titanium by using inductively coupled plasma-optical emission spectrometry after wet digestion using a modified method of Boguhn et al. (2009), described in detail by Zeller et al. (2015a). Calcium and P utilization was calculated as the proportion of intake which was not recovered in excreta (based on quantitative data).
Extraction and measurement of InsP3-6 isomers in feed and digesta were carried out using the method of Zeller et al. (2015a) with modifications by Sommerfeld et al. (2018) and high-performance ion chromatography detection (ICS-3000 system, Dionex, Idstein, Germany). By using this methodology, separation of enantiomers is not possible and, therefore, we were unable to distinguish between the D- and L-forms. Some InsP3 isomers could not be identified because standards were unavailable. A clear discrimination of the isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because of coelution; therefore, in this study, we used the term InsP3x for these InsP3 isomers of unknown proportion.
Myo-inositol in feed, digesta, yolk, albumen, and plasma samples was analyzed according to Sommerfeld et al. (2018) using a gas-chromatograph/mass spectrometer after derivatization of the samples. The total MI content in the albumen and yolk was calculated based on the concentration of MI in the freeze-dried material and its mass. The content of MI in the egg (without shell) was calculated as the sum of MI content in egg yolk and albumen.
Calcium and inorganic P (Pi) in blood plasma were analyzed at the IDEXX BioResearch Vet Med Labor GmbH (Ludwigsburg, Germany). Calcium was measured photometrically by the Arsenazo method in a Beckman Olympus AU480 (Beckman Coulter, Krefeld, Germany). Inorganic P was measured photometrically as a phosphomolybdate complex in Beckman Olympus AU480 (Beckman Coulter).
The particle size of the limestone samples was measured as described by Grubješić et al. (2019) by wet-sieving analysis using a sieve shaker (AS200, Retsch GmbH, Haan, Germany) with sieve sizes of 2.000, 1.180, 1.000, 0.500, 0.250, 0.125, and 0.063 mm.
Brush-Border Membrane Enrichment
Brush-border membrane enrichment of enterocytes was performed according to Huber et al. (2015). In short, mucosa samples of the jejunum were ground under liquid nitrogen and mixed with 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid/mannitol buffer (HEPES 2 mmol, mannitol 50 mmol, PMSF 25 mmol). After homogenization and chilling on ice, mucosa was mixed with 1 M MgCl2 and shaken to precipitate basolateral membranes. Precipitates containing enriched BBM after sequential centrifugation were resuspended in HEPES/mannitol buffer with protease inhibitors. 50 μL aliquots of BBM homogenates were shock-frozen in liquid nitrogen and stored at −80°C until analysis.
Protein Quantification for Brush-Border Membranes
Protein concentrations of the BBM were determined using a protein assay according to Bradford (Bradford Reagent, 5 ×, SERVA, Heidelberg, Germany). Analyses were performed in triplicate according to the manufacturer's protocol.
Endogenous Phosphatase Activities
The activity of phosphatases associated with the BBM was measured by using the phytic acid (phytate)/total phosphorus kit (K-PHYT 05/17 assay; Megazyme International, Ireland) adapted to be applied for the BBM as described by Gonzalez-Uarquin et al. (2020). In short, the kit-own enzymes were replaced by BBM preparations (160 μg protein), phytate was added as substrate for the BBM-associated phosphatases at pH 5.5 (optimal for phytase), and released Pi was quantified. In a second BBM aliquot obtained from the same animal, measurements were made using a pH of 10.4 (optimal for alkaline phosphatase [ALP]). In a third BBM aliquot, measurements were made at both pH values, to assess total phosphatases activity. The amount of Pi/mg BBM protein per min released at the respective pH indicated the activity of endogenous total phosphatases, phytase, and ALP, respectively. Enzyme activities could not be determined in week 10 and 16 because of shortage in tissue material from the smaller individuals in these periods.
Statistical Analysis
The comparisons were performed using the MIXED procedure and pairwise t-tests using the software package SAS (version 9.3; SAS Institute Inc., Cary, NC). Data without normal distribution were either log or square root transformed. Results are presented as the LSmeans and the pooled SEM of the untransformed data. The individual hen was considered as the experimental unit. The following model was used:
where Yijklm = response variable, μ = overall mean, αi = effect of strain (fixed), βj = effect of period (fixed), the interaction between strain and period (fixed), γk = block (random), the interaction between block and period (random), δl = metabolism unit (random), ϕm = father/rooster (random), and εijklm = residual error. Statistical significance was declared at P < 0.05.
Results
Performance Traits
The BW of the hens increased with each period (P < 0.001 for the period) and was consistently higher for LB than for LSL hens (P < 0.001 for strain; Table 2). The ADFI was affected by the interaction strain × period as it was not different between strains in week 10, 16, and 24 but higher for LSL than for LB hens in week 30 and 60. The average egg weight in the excreta collection period did not differ between strains but increased with each period (P < 0.001 for period).
Table 2.
BW at the end, ADFI, and egg weight of laying hens in the excreta-collection periods in different weeks of life.
| Period | Strain | BW |
ADFI |
Average egg weight |
|---|---|---|---|---|
| g | g/day | g | ||
| Week 10 | LB1 | 754z | 48.5f,g | - |
| LSL2 | 676z | 45.3g | - | |
| Week 16 | LB | 1,231y | 59.0e | - |
| LSL | 1,119y | 55.3e,f | - | |
| Week 24 | LB | 1,673x | 101.7d | 52.3x |
| LSL | 1,555x | 102.1d | 52.3x | |
| Week 30 | LB | 1,763w | 113.9b,c | 57.1w |
| LSL | 1,682w | 124.6a | 58.8w | |
| Week 60 | LB | 1,963v | 108.5c,d | 61.1v |
| LSL | 1,751v | 120.3a,b | 63.9v | |
| Pooled SEM | 34.7 | 3.20 | 1.21 | |
| P values | Strain | <0.001 | 0.293 | 0.189 |
| Periodv-z | <0.001 | <0.001 | <0.001 | |
| Strain × perioda-g | 0.080 | 0.025 | 0.510 |
a-gDifferent superscript lowercase letters within a column indicate significant interaction effects between strains and periods (P < 0.05).
v-zDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strain.
When the main effect of the strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 9 to 10 hens.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Blood Traits
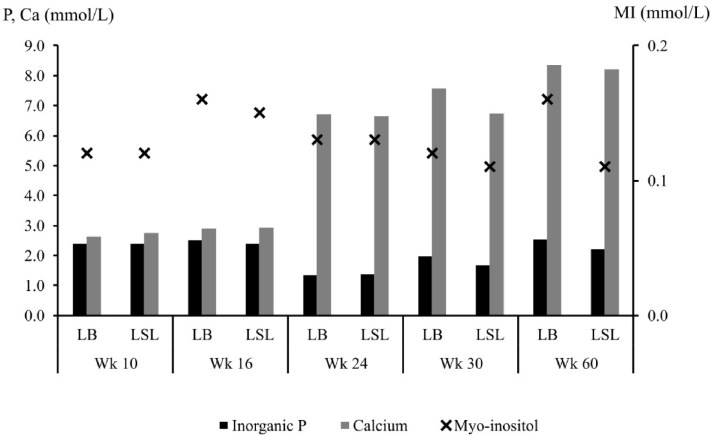
The concentration of Pi in blood plasma was higher in LB than in LSL hens (P = 0.026 for strain; Figure 1). The lowest concentration was measured in week 24, a significantly higher concentration in week 30 and the significantly highest in week 10, 16, and 60 (P < 0.001 for period). Plasma Ca concentrations increased with each period (P < 0.001 for period) in both strains. The plasma MI concentration ranged between 0.11 and 0.16 mmol/L. There was no difference between the strains in week 10, 16, 24, and 30 but a higher concentration in LB than in LSL hens in week 60, causing an interaction between the main effects (P = 0.004).
Figure 1.
Concentrations of inorganic P (SEM = 0.09), calcium (SEM = 0.27), and myo-inositol (SEM = 0.01) in the blood plasma of 2 laying hen strains (LB and LSL) in different weeks of life (n = 9 to 10 hens). The concentration of inorganic P was higher in LB than in LSL hens (P = 0.026 for strain) and higher in weeks 10, 16, and 60 than in week 30, which was higher than week 24 (P < 0.001 for the period). The concentration of Ca was increased with each week (P < 0.001 for period). The concentration of myo-inositol did only differ between strains in week 60, causing a strain × period interaction (P = 0.004). Abbreviations: Ca, calcium; LB, Lohmann Brown-classic; LSL, Lohmann LSL-classic; P, phosphorus.
Myo-Inositol, InsP6, Ca, and P Concentrations and Enzyme Activity in Gut Sections
Crop
In the crop digesta, the MI concentration was affected by the interaction strain × period (P = 0.008; Table 3). The LSL hens had higher MI concentrations than LB hens in week 10 but lower concentrations in week 24. In all other periods, there was no difference between the strains. Higher InsP6 concentrations in the crop digesta were measured in LSL than in LB hens (P = 0.044 for strain), and the concentration was higher in week 16, 30, and 60 than in week 10 and 24 (P < 0.001 for period). The Ca concentration in the crop was markedly higher in week 24 than in week 10 and 16 and further increased in week 30, followed by a decrease in week 60 (P < 0.001 for period). The P concentration in the crop was higher in LSL than in LB hens (P = 0.032 for strain) and higher in week 10, 16, and 30 than in week 24 and 60.
Table 3.
Concentrations of myo-inositol, InsP6, Ca, and P in the dried crop and gizzard content of laying hens in different weeks of life.
| Period | Strain | Crop |
Gizzard |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Myo-inositol |
InsP6 |
Ca |
P |
Myo-inositol |
InsP6 |
Ca |
P |
||
| μmol/g | μmol/g | g/kg | g/kg | μmol/g | μmol/g | g/kg | g/kg | ||
| Week 10 | LB1 | 1.1b | 8.0w | 7.6x | 4.5v | 0.9v | 4.1b-d | 3.1c | 2.0b |
| LSL2 | 1.4a | 9.8w | 8.3x | 5.1v | 0.9v | 4.5b,c | 2.9c | 2.0b | |
| Week 16 | LB | 1.6a | 10.8v | 6.4x | 4.8v | 1.0v | 4.4b,c | 3.5c | 1.8b |
| LSL | 1.5a | 10.9v | 7.0x | 5.0v | 1.0v | 4.8b,c | 2.7c | 1.9b | |
| Week 24 | LB | 0.8b-d | 9.0w | 30.1w | 3.7w | 0.3x | 4.6b-d | 35.5b | 1.4c,d |
| LSL | 0.6e | 9.2w | 42.1w | 4.1w | 0.2x | 4.2c,d | 70.2a | 1.3c,d | |
| Week 30 | LB | 0.8c-e | 10.4v | 61.6v | 5.2v | 0.3x | 4.8b,c | 79.4a | 1.7b,c |
| LSL | 0.9b,c | 11.0v | 50.8v | 4.9v | 0.2x | 3.3d | 66.6a | 1.2d | |
| Week 60 | LB | 0.7d,e | 10.4v | 38.3w | 3.8w | 0.6w | 10.0a | 74.7a | 2.9a |
| LSL | 0.7c-e | 11.8v | 39.7w | 4.6w | 0.4w | 5.8b | 67.9a | 1.6b,c | |
| Pooled SEM | 0.08 | 0.55 | 6.19 | 0.21 | 0.07 | 0.59 | 6.29 | 0.17 | |
| P values | Strain | 0.343 | 0.044 | 0.572 | 0.032 | 0.014 | 0.042 | 0.649 | 0.019 |
| Periodv-x | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Strain × perioda-e | 0.008 | 0.418 | 0.405 | 0.053 | 0.609 | 0.008 | <0.001 | <0.001 | |
a-eDifferent superscript lowercase letters within a column indicate significant interaction effects between strains and periods (P < 0.05).
v-xDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
When the main effect of the strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 9 to 10 hens.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Gizzard
The MI concentration in the gizzard digesta was higher in LB than in LSL hens (P = 0.014 for strain) and lower in week 24 and 30 than in the other periods (Table 3). The InsP6 concentration did not differ between strains in week 10, 16, and 24 but was significantly higher in LB than in LSL hens in week 30 and 60 causing an interaction (P = 0.008). The Ca concentration was significantly lower in week 10 and 16 than in all other weeks but with a lower concentration in LB than in LSL hens in week 24 resulting in an interaction (P < 0.001). The P concentration did not differ between strains in week 10 and 16, decreased in week 24 in both strains, and was higher in LB than in LSL hens in week 30 and 60 (P < 0.001 for interaction).
Jejunum
In the jejunum digesta, the MI concentration was higher in week 60 and lower in week 24 than in the other weeks, but did not differ between strains (Table 4). The InsP6 concentration was higher in LB than in LSL hens (P = 0.002 for strain) and highest in week 30 and 60, followed by week 16, 10, and 24 (P < 0.001 for period). The Ca concentration did not differ between strains in week 10, 30, and 60. It was higher in LB than in LSL hens in week 16 and higher in LSL than in LB hens in week 24 resulting in an interaction (P = 0.001). The P concentration was lower in LSL than in LB hens (P = 0.020 for strain) and increased from week 10 to 16 and from 24 to 30.
Table 4.
Concentrations of myo-inositol, InsP6, Ca, and P in the dry jejunum and ileum content of laying hens in different weeks of life.
| Period | Strain | Jejunum |
Ileum |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Myo-inositol |
InsP6 |
Ca |
P |
Myo-inositol |
InsP6 |
Ca |
P |
||
| μmol/g | μmol/g | g/kg | g/kg | μmol/g | μmol/g | g/kg | g/kg | ||
| Week 10 | LB1 | 5.3w | 16.9x | 11.2e | 7.1x | 3.0a | 33.2b-e | 24.0x | 13.5v |
| LSL2 | 5.5w | 15.7x | 10.6e | 6.8x | 0.8d,e | 31.5c-e | 30.7x | 16.1v | |
| Week 16 | LB | 5.3w | 23.4w | 16.3d | 8.8w | 1.1c,d | 24.4f | 20.5x | 10.1w |
| LSL | 5.2w | 17.2w | 12.7e | 7.5w | 0.8d,e | 28.2d-f | 28.4x | 12.7w | |
| Week 24 | LB | 4.5x | 15.2y | 33.8c | 6.4x | 1.8a,b | 34.9b-d | 67.2v | 12.1w |
| LSL | 4.5x | 13.4y | 46.4a | 6.3x | 1.3b,c | 27.9e,f | 86.5v | 10.2w | |
| Week 30 | LB | 5.4w | 26.7v | 51.2a | 10.8v | 2.1a,b | 39.4a,b | 71.7v | 13.7v,w |
| LSL | 5.2w | 22.3v | 43.9a,b | 8.9v | 0.8e | 38.4a-c | 72.1v | 12.9v,w | |
| Week 60 | LB | 7.2v | 26.4v | 35.1b,c | 9.8v | 2.5a | 35.0b-d | 58.8w | 10.9w |
| LSL | 6.5v | 21.0v | 42.8a-c | 9.2v | 1.3b,c | 44.4a | 55.7w | 13.3w | |
| Pooled SEM | 0.29 | 1.17 | 3.11 | 0.47 | 0.37 | 2.74 | 7.19 | 1.13 | |
| P values | Strain | 0.374 | 0.002 | 0.947 | 0.020 | <0.001 | 0.674 | 0.107 | 0.123 |
| Periodv-y | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.034 | |
| Strain × perioda-e | 0.621 | 0.130 | 0.001 | 0.065 | 0.021 | 0.025 | 0.761 | 0.056 | |
a-eDifferent superscript lowercase letters within a column indicate significant interaction effects between strains and periods (P < 0.05).
v-yDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
When the main effect of the strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 8 to 10 hens.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Ileum
In the digesta of the terminal ileum, the MI concentration was higher in LB than in LSL hens in week 10, 30, and 60 but not in week 16 and 24, resulting in an interaction (P = 0.021; Table 4). The InsP6 concentration was higher in LB than in LSL hens in week 24 but lower in week 60. The concentration of Ca was the highest in week 24 and 30, followed by that in week 60. The P concentration was significantly higher in week 10 than in week 16, 24, and 60. The P concentration in week 30 did not differ from all other periods.
Enzyme Activity in the Jejunum
The enzyme activities in the jejunum were measured in week 24, 30, and 60 (Table 5). Total phosphatase and phytase activities were both higher in LB than in LSL hens (P ≤ 0.009 for strain). Total phosphatase activity was higher in week 60 than in week 24 and 30, whereas the ALP and phytase activity was lower in week 30 than in week 24 and 60.
Table 5.
Activity of total phosphatase, alkaline phosphatase, and phytase in the jejunum of laying hens in different weeks of life.
| Period | Strain | Total phosphatase activity |
ALP activity |
Phytase activity |
|---|---|---|---|---|
| μg Pi/mg BBM protein/min | μg Pi/mg BBM protein/min | μg Pi/mg BBM protein/min | ||
| Week 24 | LB1 | 1.05w | 1.42v | 3.11v |
| LSL2 | 1.00w | 1.34v | 2.81v | |
| Week 30 | LB | 1.12w | 1.10w | 2.59w |
| LSL | 0.97w | 1.13w | 2.28w | |
| Week 60 | LB | 1.35v | 1.48v | 3.56v |
| LSL | 1.13v | 1.32v | 2.78v | |
| Pooled SEM | 0.064 | 0.098 | 0.183 | |
| P values | Strain | 0.009 | 0.378 | 0.003 |
| Periodv,w | 0.003 | 0.010 | 0.001 | |
| Strain × period | 0.386 | 0.645 | 0.305 |
v,wDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
When the main effect of strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 8 to 10 hens.
Abbreviation: ALP, alkaline phosphatase.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Ceca
In the ceca digesta, the MI concentration was higher in LSL than in LB hens (P < 0.001 for strain; Table 6) and higher in week 30 than in all other weeks (P = 0.006 for period). The concentration of Ins(1,2,3,4)P4 was higher in LSL than in LB hens (P = 0.007 for strain) but was not detectable in week 24 and 30 and in LSL in week 60. Ins(1,2,3,4,5)P5 concentration differed between strains only in week 24 where it was higher for LB than for LSL hens. The highest InsP6 concentration was measured in week 60 with no difference between the strains. The concentrations were significantly lower in week 10, 24, and 30 and in between in week 16. The Ca concentration in the ceca was the highest in week 24, followed by week 10, 30, and 60 and the lowest in week 16. The P concentration was higher in LB than in LSL hens, increased from week 16 to week 24 and decreased in week 30. Titanium concentration was significantly higher in LB than in LSL hens and higher in week 60 than in all other periods.
Table 6.
Concentrations of myo-inositol, InsP6, Ca, and P in the dry ceca content of laying hens in different weeks of life.
| Period | Strain |
Myo-inositol |
Ins(1,2,3,4)P4 |
Ins(1,2,3,4,5)P5 |
Ins(1,2,4,5,6)P5 |
InsP6 |
Ca |
P |
Ti |
|---|---|---|---|---|---|---|---|---|---|
| μmol/g | μmol/g | μmol/g | μmol/g | μmol/g | g/kg | g/kg | g/kg | ||
| Week 10 | LB1 | 0.7w | 0.4 | 1.4b,c | n.d. | 5.4w | 20.9w | 12.2x | 10.4x |
| LSL2 | 0.9w | 1.1 | 1.9a,b | n.d. | 1.6w | 15.7w | 10.3x | 6.0x | |
| Week 16 | LB | 0.7w | 0.6 | 1.7a-c | 0.4 | 6.8v,w | 13.1x | 12.8x | 17.4w |
| LSL | 1.1w | 1.0 | 2.0a | n.d. | 2.5v,w | 12.8x | 11.5x | 7.0w | |
| Week 24 | LB | 0.3w | n.d. | 1.3b,c | n.d. | 2.6w | 41.3v | 36.1v | 12.8w,x |
| LSL | 0.9w | n.d. | 0.6d | 0.3 | 3.2w | 35.8v | 26.9v | 8.5w,x | |
| Week 30 | LB | 1.0v | n.d. | 1.5a-c | n.d. | 3.0w | 17.1w | 14.2w,x | 11.5w,x |
| LSL | 1.5v | n.d. | 1.1c,d | 0.3 | 3.0w | 20.0w | 12.4w,x | 7.8w,x | |
| Week 60 | LB | 0.5w | 0.3 | 1.8a,b | 0.9 | 16.0v | 16.0w | 18.6w | 30.4v |
| LSL | 0.9w | <LOQ | 2.0a,b | 0.4 | 6.0v | 20.1w | 13.9w | 14.6v | |
| Pooled SEM | 0.18 | 0.15 | 0.24 | 0.16 | 1.93 | 2.98 | 1.52 | 1.99 | |
| P values | Strain | <0.001 | 0.007 | 0.892 | 0.025 | 0.479 | 0.899 | 0.001 | <0.001 |
| Periodv-x | 0.006 | 0.200 | 0.001 | 0.045 | 0.035 | <0.001 | <0.001 | <0.001 | |
| Strain × perioda-d | 0.696 | 0.074 | 0.020 | . | 0.094 | 0.096 | 0.526 | 0.507 |
a-dDifferent superscript lowercase letters within a column indicate significant interaction effects between the strains and periods (P < 0.05).
v-xDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
When the main effect of the strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 6 to 10 hens.
Abbreviations: Ca, calcium; P, phosphorus.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Calcium and P Utilization
Most traits were significantly affected by the period (P < 0.001). The Ca intake increased with each period, independent of the strain (Table 7). The Ca excretion increased up to week 30. Week 60 did not differ from week 24 and 30. Calcium utilization decreased from week 10 to week 16 and increased to week 24 and to week 60. Phosphorus excretion increased up to week 24 and decreased from week 30 to week 60. Phosphorus utilization was highest in week 10 and 60, lower in week 16 and week 30, and the lowest in week 24. Phosphorus intake differed between the strains only in week 30 and week 60 with LSL hens showing a higher intake (P = 0.021 for interaction).
Table 7.
Ca and P utilization of laying hens in different weeks of life.
| Period | Strain | Ca intake |
Ca excretion |
Ca utilization |
P Intake |
P Excretion |
P Utilization |
|---|---|---|---|---|---|---|---|
| g/day | g/day | % | g/day | g/day | % | ||
| Week 10 | LB1 | 0.44z | 0.26y | 39.6x | 0.29e,f | 0.18y | 38.0v |
| LSL2 | 0.42z | 0.26y | 39.2x | 0.27f | 0.17y | 37.2v | |
| Week 16 | LB | 0.51y | 0.33x | 36.7y | 0.34d | 0.22x | 34.9w |
| LSL | 0.49y | 0.34x | 30.6y | 0.32d,e | 0.24x | 27.7w | |
| Week 24 | LB | 4.08x | 2.01w | 50.9w | 0.58c | 0.47v | 20.0y |
| LSL | 4.05x | 2.05w | 49.4w | 0.58c | 0.48v | 16.9y | |
| Week 30 | LB | 4.87w | 2.35v | 53.2w | 0.65b | 0.49v | 25.4x |
| LSL | 5.28w | 2.78v | 47.8w | 0.71a | 0.51v | 27.6x | |
| Week 60 | LB | 5.25v | 2.29v,w | 57.3v | 0.66b | 0.43w | 34.7v |
| LSL | 5.79v | 2.57v,w | 55.6v | 0.72a | 0.45w | 38.2v | |
| Pooled SEM | 0.12 | 0.16 | 2.86 | 0.02 | 0.02 | 2.31 | |
| P values | Strain | 0.380 | 0.300 | 0.261 | 0.206 | 0.438 | 0.472 |
| Periodv-z | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Strain × Perioda-f | 0.067 | 0.368 | 0.683 | 0.021 | 0.926 | 0.152 |
a-fDifferent superscript lowercase letters within a column indicate significant interaction effects between the strains and periods (P < 0.05).
v-zDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
Data are given as LSmeans; n = 9 to 10 hens.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Myo-Inositol in Eggs
Myo-inositol concentration in the albumen and yolk and the MI content in the albumen was higher in LB than in LSL hens (Table 8). The MI content in the yolk, however, was significantly higher in week 60 than in week 24. Myo-inositol in the egg (albumen + yolk) was additionally affected by the period and was lower in week 24 than in the other periods. Overall, about 58% of the total MI in the egg was found in the albumen and 42% in the yolk.
Table 8.
Concentrations and amount of myo-inositol in the albumen, yolk, and egg (without the shell) of laying hens in different weeks of life.
| Period | Strain | Albumen |
Albumen |
Yolk |
Yolk |
Egg |
|---|---|---|---|---|---|---|
| μmol | μmol/g | μmol | μmol/g | μmol | ||
| Week 24 | LB1 | 15.0 | 0.45 | 10.9w | 0.91 | 25.9w |
| LSL2 | 12.1 | 0.37 | 10.4w | 0.79 | 22.5w | |
| Week 30 | LB | 17.2 | 0.48 | 12.0v,w | 0.86 | 28.3v |
| LSL | 15.1 | 0.42 | 10.9v,w | 0.71 | 26.8v | |
| Week 60 | LB | 16.8 | 0.46 | 13.8v | 0.83 | 31.2v |
| LSL | 12.2 | 0.35 | 12.3v | 0.70 | 24.1v | |
| Pooled SEM | 1.15 | 0.030 | 0.89 | 0.052 | 1.70 | |
| P values | Strain | 0.009 | 0.010 | 0.056 | 0.007 | 0.016 |
| Periodv,w | 0.072 | 0.262 | 0.012 | 0.112 | 0.046 | |
| Strain × period | 0.449 | 0.619 | 0.771 | 0.718 | 0.204 |
v,wDifferent superscript lowercase letters within a column indicate significant main effects of the periods (P < 0.05), irrespective of the strains.
When the main effect of the strain was significant, LB hens differed significantly from LSL hens (P < 0.05).
Data are given as LSmeans; n = 9 to 10 hens.
LB = Lohmann Brown-classic.
LSL = Lohmann LSL-classic.
Inositol Phosphate Isomers in Gut Sections
Analyzed concentrations of InsP isomers (InsP3-5) in the crop, gizzard, jejunum, and terminal ileum are given in Supplementary Tables 1-3. Some strain differences were detected for InsP5 isomers. Overall, concentrations of Ins(1,2,4,5,6)P5 and Ins(1,2,3,4,5)P5 were higher in LSL than in LB chickens in the crop but lower in LSL than in LB hens in the jejunum. In the ileum, strain effects on the concentrations of Ins(1,2,4,5,6)P5 were different between periods while those of Ins(1,2,3,4,5)P5 were not affected by strain. Concentrations of InsP3 and InsP4 were below the limit of quantification or limit of detection in most cases in all sections.
Discussion
Unlike broiler chickens, stepwise degradation of phytate and appearance of the end product MI has not been well studied in laying hens before. Differences between broiler chickens and hens are likely to exist because of the long life span of hens and the different demands for P and Ca, especially in the laying period. A genetic effect on different traits of P utilization was reported for Japanese quail (Beck et al., 2016) and broiler chickens (Zhang et al., 2003; de Verdal et al., 2011). Thus, the 2 laying hen strains used in this study might differ in traits related to P utilization as well. Consequently, the first objective herein was to compare InsP degradation in these 2 strains. The second objective was to measure the traits during five different periods, starting in the rearing phase. The BW development of hens in the rearing phase overall was consistent with the values reported by the breeding company to be typical for these strains.
To the best of our knowledge, here, for the first time, concentrations of InsP isomers and minerals in the digestive tract are presented based on individual bird measurements. We assessed individuals because we were interested in interindividual variation within a strain and linkages with metabolic, physiologic, and genetic traits of individuals that will be reported elsewhere and may provide new insights into metabolic interrelations. In standard prececal digestibility experiments, digesta obtained from several birds are pooled into one sample that reduces the variation among replicates but masks individual digestive performance. On the one hand, working with individuals thus may have effects on statistical data evaluation. Scheideler (1986) faced similar issues in experiments with laying hens. On the other hand, the present data showed differences between strains and periods and interactions although variation was high, indicating individual metabolic and digestive conditions. The present study used 10 individuals per treatment, a number which is in the range of numbers often used in metabolomic studies. However, owing to lack of information on variance among individual hens, we were unable to use power analysis as a tool to decide on the number of replicates. Furthermore, the sample size of individuals might be limiting for conducting repeated chemical analyses of one sample.
InsP Degradation
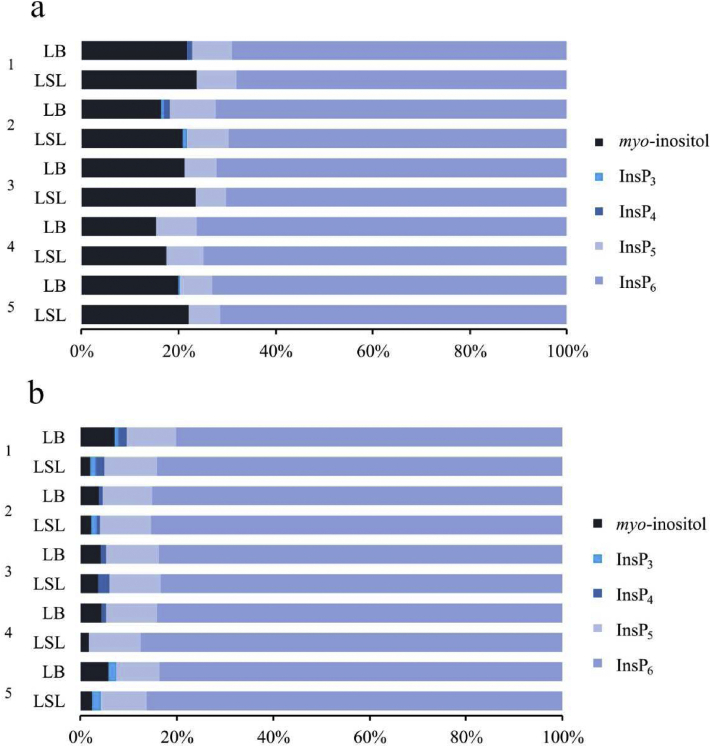
The concentrations of InsP isomers in the crop and gizzard were similar to those analyzed in the feed, suggesting that InsP6 degradation in the upper part of the digestive tract of laying hens was low when phytase was not contained in the feed. The appearance of Ins(1,2,4,5,6)P5 and Ins(1,2,3,4,5)P5 in the jejunum and a positive relationship between concentrations of these isomers and InsP6 (r = 0.962; P < 0.001 for Ins(1,2,4,5,6)P5 and r = 0.882; P < 0.001 for Ins(1,2,3,4,5)P5) indicate the activity of a mucosal 3-phytase and a mucosal 6-phytase. In addition, the presence of Ins(1,2,3,4,6)P5 in the jejunum of hens in week 10 and 16 was an indication for the activity of a 5-phytase. This InsP5 isomer was not detected in week 24 to 60. This confirmed the hypothesis that the period had an effect on some of the traits. For these traits, period effects were likely caused by the high Ca level in the diets. Supplementation of Ca has previously been shown to inhibit InsP degradation by broilers (Zeller et al., 2015b; Sommerfeld et al., 2018). The presence of a 5-phytase is consistent with the appearance of Ins(1,2,3,4,6)P5 in the ileum in all periods. InsP isomers less phosphorylated than InsP5 did not appear in the jejunum, which indicates an initial slow degradation of InsP6 and InsP5 and a fast degradation of lower InsP by phosphatases. There was a higher presence of lower InsP in the ileum than in the jejunum in some treatments, confirming the finding by Maenz and Classen (1998) that mucosal phytase activity decreased along the digestive tract. Supporting the hypothesis of differences between the 2 hen strains, LB hens had higher concentrations of InsP6, Ins(1,2,3,4,5)P5, and Ins(1,2,4,5,6)P5 in the jejunum than LSL hens. However, these differences were not observed in the ileum. Differences in jejunum concentrations of InsP6 and InsP5 isomers were not reflected in measured mucosa enzyme activities. This was unexpected, but measured concentrations alone may not reflect the quantities of the hydrolyzed substrate.
Myo-Inositol
The higher MI concentration in the crop in week 10 and 16 than in the other weeks most likely mirrored the MI concentration in the diets that were also higher in those weeks. Except for week 24, the relative difference between feed MI and crop MI was similar in all periods. Hence, MI release in the crop is supposed to be low.
The higher MI concentration in the ileum of LB than LSL hens in some periods can be related to differences between the strains in InsP degradation or MI uptake through the intestinal wall, or both. The hypothesis of strain differences in MI release is supported by the significantly higher total phosphatase and phytase activities in the LB hens. Total phosphatase activity in the jejunum was positively related to the MI concentration in the jejunum (r = 0.854; P = 0.030) and the ileum (r = 0.828; P = 0.042). Alkaline phosphatase activity and MI concentration in both segments were unrelated (r ≤ 0.432; P ≥ 0.392) and the relationship between phytase activity and MI concentration was smaller in the jejunum (r = 0.450; P = 0.371) than ileum (r = 0.770; P = 0.073). These observations point to a phosphatase, but not the ALP, being the relevant enzyme for MI release. Enzymes produced by the gut microbiota may also provide enzymes involved in InsP6 degradation (Witzig et al., 2015). However, the study with gnotobiotic broilers by Sommerfeld et al. (2019) assumed that microbial enzymes were absent in the digestive tract and a prececal InsP6 disappearance of 42% was found in that study. This value suggested that mucosal phosphatases had a remarkable impact on InsP6 degradation. Nevertheless, the quantitative contribution of mucosal and microbial enzyme sources is not known to date.
The MI amount in the yolk was positively related to the MI concentration in the jejunum (r = 0.944; P = 0.005) and positively, although weaker, related to the MI concentration in the ileum (r = 0.708; P = 0.116). This was supported by a relationship between the MI amount in the yolk and the total phosphatase activity in the jejunum (r = 0.965; P = 0.002). These relationships suggest that a major proportion of MI absorption occurs in the jejunum and is transferred into the egg, mainly into the yolk. In the jejunum, the proportion of MI in the sum of all InsP and MI was higher than in the ileum, whereas that of InsP6 in the sum of all InsP and MI was lower in the jejunum than in the ileum (Supplementary Figure 1). This indicates a faster MI absorption than MI release in the jejunum than in the ileum. Myo-inositol released from phytate seems to be preferentially absorbed through the MI transporter SMIT 1 in the jejunum of laying hens (Herwig et al., 2019). In that study, more MI was found in the egg yolk when phytase or MI were added to the feed, indicating that at least a part of the intestinal MI is transferred into the egg. It is not known to which extent the MI in the egg derives from absorbed MI and thus from the feed or from metabolic synthesis. However, with the results obtained in the present study, it can be estimated to what extent the feed may have contributed to MI found in the egg. Based on the results of week 30, the estimate assumes the following:
-
-
30 μmol MI contained in daily egg mass
-
-
106 g DM intake/day
-
-
1 μmol MI/g DM of feed and 11 μmol InsP6/g DM of feed
-
-
10% prececal InsP6 disappearance and 10% of disappeared InsP6 totally dephosphorylated.
Based on these data and assumptions, a total of 118 μmol MI is provided for potential prececal absorption (106 contained in the feed and 12 from InsP6 degradation). An unknown variable is the absorption rate of MI from the small intestine. Should it be 100%, then prececal the MI absorption exceeds the amount of MI in the egg by 4-fold. However, 100% absorption is not realistic. Therefore, an attempt has been made to estimate the absorbed amount of MI. Based on prececal DM digestibility and the measured MI concentration in ileum digesta, the MI flow at the terminal ileum was calculated and values ranged between 15 and 87 μmol MI per day (depending on the treatment). Taken the aforementioned 118 μmol MI reaching the prececal digestive tract per day into account, an absorption of between 31 and 103 μmol MI per day (depending on the treatment) is calculated as the difference. The lower level of this range corresponds to the amount of MI contained in the daily egg mass (30 μmol).
Strain Effects Exemplified by the Ceca
Concentrations of several constituents of the cecal content were different between the strains (MI, Ins(1,2,3,4)P4, Ins(1,2,4,5,6)P5, P, Ti). It has been estimated that only about 20% of the digesta and mainly small particles enter the ceca (Son et al., 2002; Svihus et al., 2013). A muscular ring of tissue at the cecal openings that is narrow and filled with villi might be involved in filtering material during the filling of the ceca (Clarke, 1978). Furthermore, there are a lot of cecal types among avian species (Clench, 1999), with morphological differences between species. Differences in marker retention times between 2 genetic broiler lines selected for divergent digestion efficiency were found in the proventriculus and gizzard, ceca, and total digestive tract (Rougière and Carré, 2010). Differences in the morphology and function of the ceca of broilers from 2 different breeding origins were also observed by Maisonnier et al. (2001). In further experiments comparing different strains, possible morphological differences should be investigated in addition to physiological differences.
As reviewed by Svihus et al. (2013), some publications showed reflux from the lower digestive tract into the ileum does not exist, whereas the study by Sacranie et al. (2012) gave some indications for reflux of material from the colon and ceca to the upper segments of the intestine. Thus, the relevance of cecal material for the animal remains unanswered and may have contributed, at least in part, to the strain effects in the ileum.
As indicated by the high SE, differences existed not only between the strains but also among individuals. Morphological differences among individuals as mentioned before might have been causative for that variation. Another possibility for the variation among individuals could be the time point of sampling. The ceca empty only a few times a day, leading to a longer retention time than in the other parts of the digestive tract that are characterized by a more continuous flow of digesta with a more frequent emptying (Svihus et al., 2013). Depending on the time that has passed since last emptying, the InsP degradation might have differed at the time of sampling.
Period Effects Exemplified by Week 24
The results of the present study indicate that week 24 was a period of remarkable change of metabolism most likely related to the onset of the laying period. An increased Ca concentration in the layer feed, which is needed for eggshell formation, might have led to a reduced P absorption, as it is known that high dietary Ca levels can reduce P absorption (Hurwitz and Bar, 1965; van der Klis et al., 1997). A lower P absorption might have led to the reduced plasma Pi level observed in week 24. A lower absorption of Ca and P during the small intestinal passage might possibly explain the higher concentrations of these minerals in the ceca. However, P supply exceeded the requirement according to more recent estimates of the P requirement of laying hens (Ahmadi and Rodehutscord, 2012; Jing et al., 2018). Thus, studies using a low P supply are needed to provoke adaptive metabolic responses of birds from different strains.
Conclusion
Some traits were affected by the hen strain, and effects were partly dependent on the study period. A lower mucosal phosphatase activity, lower concentrations of InsP isomers and MI in the digestive tract, and lower MI concentrations in the plasma and egg of LSL hens than those of LB hens consistently suggest strain differences in digestive InsP degradation and MI uptake through the intestinal wall, or both. Different concentrations of several constituents of the cecal content suggest differences in the morphology and function of the ceca between strains need more studies. The 2 strains studied herein likely had a different metabolism that deserves further research. Further studies on a metabolic, genetic, and microbiome level may help explain the large differences that existed among individual hens.
Acknowledgments
This study was funded by the Deutsche Forschungsgemeinschaft, Germany (DFG, German Research Foundation) – Project number RO 1217/10-1 and was part of the Research Unit 2601: Inositol phosphates and myo-inositol in the domestic fowl: Exploring the interface of genetics, physiology, microbiome, and nutrition. The authors appreciate the work done in the animal house by Fernando Gonzalez Uarquin, Clara Heumann-Kiesler, Tanja Hofmann, Michael Oster, Daniel Rissi, Katrin Röhm, Sonja Schmucker, Nares Trakooljul, and Solveig Vollmar and the staff of the experimental station and the institute's laboratories, especially Melanie Liebscher, Helga Ott, Margit Schollenberger, and Helga Terry. Hans-Peter Piepho gave advice in developing the statistical model.
Hatchlings for this study including pedigree information were provided by Lohmann Tierzucht GmbH, Cuxhaven, Germany, which is gratefully acknowledged.
Conflict of Interest Statement: The authors declare they have no conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.08.064.
Supplementary data
Supplementary Figure 1.
Relative proportions of InsP3-6 and myo-inositol in the digesta of the jejunum (a) and the terminal ileum (b) of 2 laying hen strains (LB and LSL) in different weeks of life (n = 8-10 hens). The sum of the concentrations of InsP3-6 and myo-inositol on a molar basis is defined as 100%.
References
- Abudabos A.M. Intestinal phytase activity in chickens (Gallus domesticus) Afr. J. Microbiol. Res. 2012;6:4932–4938. [Google Scholar]
- Ahmadi H., Rodehutscord M. A meta-analysis of responses to dietary nonphytate phosphorus and phytase in laying hens. Poult. Sci. 2012;91:2072–2078. doi: 10.3382/ps.2012-02193. [DOI] [PubMed] [Google Scholar]
- Beck P., Piepho H.-P., Rodehutscord M., Bennewitz J. Inferring relationships between phosphorus utilization, feed per gain, and bodyweight gain in an F2 cross of Japanese quail using recursive models. Poult. Sci. 2016;95:764–773. doi: 10.3382/ps/pev376. [DOI] [PubMed] [Google Scholar]
- Boguhn J., Baumgärtel T., Dieckmann A., Rodehutscord M. Determination of titanium dioxide supplements in different matrices using two methods involving photometer and inductively coupled plasma optical emission spectrometer measurements. Arch. Anim. Nutr. 2009;63:337–342. doi: 10.1080/17450390903052623. [DOI] [PubMed] [Google Scholar]
- Clarke P.L. The structure of the ileo-caeco-colic junction of the domestic fowl (Gallus gallus L.) Br. Poult. Sci. 1978;19:595–600. doi: 10.1080/00071667808416519. [DOI] [PubMed] [Google Scholar]
- Clench M.H. The avian cecum: Update and motility review. J. Exp. Zool. 1999;283:441–447. [Google Scholar]
- de Verdal H., Narcy A., Bastianelli D., Chapuis H., Même N., Urvoix S., Le Bihan-Duval E., Mignon-Grasteau S. Improving the efficiency of feed utilization in poultry by selection. 2. Genetic parameters of excretion traits and correlations with anatomy of the gastro-intestinal tract and digestive efficiency. BMC Genet. 2011;12:71. doi: 10.1186/1471-2156-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Uarquin F., Molano E., Heinrich F., Sommerfeld V., Rodehutscord M., Huber K. Research note: jejunum phosphatases and systemic myo-inositol in broiler chickens fed without or with supplemented phytase. Poult. Sci. 2020;99:5972–5976. doi: 10.1016/j.psj.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Uarquin F., Rodehutscord M., Huber K. Myo-inositol: its metabolism and potential implications for poultry nutrition-a review. Poult. Sci. 2020;99:893–905. doi: 10.1016/j.psj.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubješić G., Titze N., Krieg J., Rodehutscord M. Determination of in situ ruminal crude protein and starch degradation values of compound feeds from single feeds. Arch. Anim. Nutr. 2019;73:414–429. doi: 10.1080/1745039X.2019.1641377. [DOI] [PubMed] [Google Scholar]
- Herwig E., Schwean-Lardner K.V., Walk C., van Kessel A.G., Bedford M.R., Classen H.L. Effect of phytase and myo-inositol supplementation on the expression of inositol transporters in the small intestine of laying hens, and blood and yolk inositol concentrations. Poult. Sci. 2019;98(E-Suppl. 1):194. (Abstr.) [Google Scholar]
- Huber K., Zeller E., Rodehutscord M. Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult. Sci. 2015;94:1009–1017. doi: 10.3382/ps/pev065. [DOI] [PubMed] [Google Scholar]
- Hurwitz S., Bar A. Absorption of calcium and phosphorus along the gastrointestinal tract of the laying fowl as influenced by dietary Ca and egg shell formation. J. Nutr. 1965;86:433–438. doi: 10.1093/jn/86.4.433. [DOI] [PubMed] [Google Scholar]
- Jing M., Zhao S., Rogiewicz A., Slominski B.A., House J.D. Assessment of the minimal available phosphorus needs of laying hens: implications for phosphorus management strategies. Poult. Sci. 2018;97:557–567. doi: 10.3382/ps/pey057. [DOI] [PubMed] [Google Scholar]
- Maenz D.D., Classen H.L. Phytase activity in the small intestinal brush border membrane of the chicken. Poult. Sci. 1998;77:557–563. doi: 10.1093/ps/77.4.557. [DOI] [PubMed] [Google Scholar]
- Maisonnier S., Gomez J., Chagneau A.M., Carré B. Analysis of variability in nutrient digestibilities in broiler chickens. Br. Poult. Sci. 2001;42:70–76. doi: 10.1080/00071660020035082. [DOI] [PubMed] [Google Scholar]
- Marounek M., Skřivan M., Dlouhá G., Břeňová N. Availability of phytate phosphorus and endogenous phytase activity in the digestive tract of laying hens 20 and 47 weeks old. Anim. Feed Sci. Technol. 2008;146:353–359. [Google Scholar]
- Onyango E.M., Asem E.K., Adeola O. Dietary cholecalciferol and phosphorus influence intestinal mucosa phytase activity in broiler chicks. Br. Poult. Sci. 2006;47:632–639. doi: 10.1080/00071660600963651. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M. Approaches for saving limited phosphate resources. Arch. Tierz. 2008;51:39–48. [Google Scholar]
- Rougière N., Carré B. Comparison of gastrointestinal transit times between chickens from D+ and D- genetic lines selected for divergent digestion efficiency. Animal. 2010;4:1861–1872. doi: 10.1017/S1751731110001266. [DOI] [PubMed] [Google Scholar]
- Sacranie A., Svihus B., Denstadli V., Moen B., Iji P.A., Choct M. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poult. Sci. 2012;91:693–700. doi: 10.3382/ps.2011-01790. [DOI] [PubMed] [Google Scholar]
- Scheideler S.E. Iowa State Univ.; Ames: 1986. Utilization of Phosphorus in Poultry as Influenced by Dietary Calcium and Phosphorus Source. PhD Diss. [Google Scholar]
- Shastak Y., Zeller E., Witzig M., Schollenberger M., Rodehutscord M. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 2014;93:2548–2559. doi: 10.3382/ps.2014-03961. [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., van Kessel A.G., Classen H.L., Schollenberger M., Kühn I., Rodehutscord M. Phytate degradation in gnotobiotic broiler chickens and effects of dietary supplements of phosphorus, calcium, and phytase. Poult. Sci. 2019;98:5562–5570. doi: 10.3382/ps/pez309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J.H., Ragland D., Adeola O. Quantification of digesta flow into the caeca. Br. Poult. Sci. 2002;43:322–324. doi: 10.1080/00071660120121562. [DOI] [PubMed] [Google Scholar]
- Svihus B., Choct M., Classen H.L. Function and nutritional roles of the avian caeca: a review. Worlds Poult. Sci. J. 2013;69:249–264. [Google Scholar]
- Tamim N.M., Angel R., Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- van der Klis J.D., Versteegh H.A.J., Simons P.C.M., Kies A.K. The efficacy of phytase in corn-soybean meal-based diets for laying hens. Poult. Sci. 1997;76:1535–1542. doi: 10.1093/ps/76.11.1535. [DOI] [PubMed] [Google Scholar]
- Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA) 1st ed. VDLUFA; Darmstadt, Germany: 2007. Handbuch der landwirtschaftlichen Versuchs und Untersuchungsmethodik (VDLUFA–Methodenbuch), vol. III: Die Chemische Untersuchung von Futtermitteln. [Google Scholar]
- Witzig M., Camarinha-Silva A., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L.E., Rodehutscord M. Spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One. 2015;10:e0143442. doi: 10.1371/journal.pone.0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015;4:e1. doi: 10.1017/jns.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L.E., Rodehutscord M. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 2015;94:1018–1029. doi: 10.3382/ps/pev087. [DOI] [PubMed] [Google Scholar]
- Zhang W., Aggrey S.E., Pesti G.M., Edwards H.M., Jr., Bakalli R.I. Genetics of phytate phosphorus bioavailability: Heritability and genetic correlations with growth and feed utilization traits in a randombred chicken population. Poult. Sci. 2003;82:1075–1079. doi: 10.1093/ps/82.7.1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.