Abstract
Duck blood is rich in protein. It is one of the main by-products in the slaughter industry. The objective of this research was to optimize and establish a method for producing duck plasma antioxidant peptides. The composition of duck plasma powder was analyzed. Protease selection experiment (Alcalase, Protamex, and Flavourzyme) and single-factor experiment were performed, and response surface methodology was used to determine the optimal hydrolysis conditions for duck plasma. Among the proteases, Alcalase hydrolysate exhibited the strongest 1,1-diphenyl-2-picrylhydrazyl scavenging rate. The optimum enzymatic hydrolysis conditions were hydrolysis time of 6 h, temperature of 65.5°C, pH 10.0, and enzyme-to-substrate ratio of 0.3%. The 1,1-diphenyl-2-picrylhydrazyl scavenging rate reached 64.84%, and the ratio of essential amino acids was 38.76%. Briefly, the duck plasma hydrolysate exhibited strong antioxidant properties and reasonable composition of amino acids. Thus, it may be used as a nutritional or functional ingredient in foods or medicines. This research provides a theoretical basis for comprehensive processing and high value utilization of duck plasma.
Key words: duck blood plasma, hydrolysis, response surface methodology, antioxidant activity
Introduction
Duck blood is a major edible by-product in the slaughter industry. It is also used in fertilizers, binders, and feedstuffs. Blood is a good source of nutrients, and it is considered a nonallergenic protein compared with soy and dairy proteins (Sorapukdee and Narunatsopanon, 2017). However, most of the duck blood is discarded as waste. Because of the unpalatability and dark color of the blood cells and whole blood, the application of duck blood plasma (DBP) in food is considered more acceptable. Duck blood plasma can be separated using a high-speed centrifuge or separator. Blood plasma is rich in protein. It accounts for more than 60% of the blood by weight (Ockerman and Hansen, 2000). It is a potential resource for the development of bioactive peptides.
Hydrolysis is an effective way to produce bioactive peptides from proteins in food resources (Korhonen and Pihlanto, 2006). Recently, peptides from food sources are gaining attention (Nedjar-Arroume et al., 2008; Miyake et al., 2011; Samaranayaka and Li-Chan, 2011; Bah et al., 2016; Cheng et al., 2016; Wu et al., 2017), and these natural peptides are considered relatively safe. Excessive free radicals could cause or accelerate chronic diseases (Wu et al., 2017). The intake of antioxidants could protect the body from free radicals and block the progression of these diseases (Miyake et al., 2011; Bo et al., 2016). The food-derived antioxidative peptides showed activities in vivo conditions affecting the body's antioxidant system (Korhonen, 2009; Sarmadi and Ismail, 2010; Samaranayaka and Li-Chan, 2011). Thus, producing antioxidant peptides by hydrolysis may be a promising method to use the DBP. It has been reported that the hydrolysates of porcine plasma (Liu et al., 2010) and bovine plasma (Seo et al., 2015) have antioxidant activity. Peptides are consecutively formed and degraded during hydrolysis. However, to our knowledge, there has not been any research on the preparation of antioxidant peptides by hydrolysis of DBP. Thus, the hydrolysis parameters should be carefully selected and optimized to obtain the strongest antioxidant peptides from DBP hydrolysates (DBPH).
Response surface methodology (RSM) is a common and effective method to optimize hydrolysis. Response surface methodology has been widely applied in food research for process optimization (Paseephol et al., 2007; Ren et al., 2008; Ye et al., 2018; Zheng et al., 2018b). Therefore, the objective of this study was to establish optimal conditions for the hydrolysis of DBP to produce antioxidant peptides based on the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity. This could be an effective method to improve the utilization of DBP and reduce environment pollution. These peptides may be used in human diet in the future.
Materials and methods
Chemicals and Reagents
Duck plasma powder was provided from Purun Biotechnology Co. (Shangdong, China). Alcalase 2.4 L, Protamex, and Flavourzyme 500 mg were obtained from Novozymes (Denmark). Glutathione and DPPH were purchased from Sigma Chemical Co. (Missouri). All other chemicals and reagents were of analytical grade and were obtained from Jiancheng Chemical Regent Co., Ltd. (Nanjing, China).
Proximate Compositions
The proximate compositions of the plasma sample, including moisture, CP, lipid, and ash, were assayed as per the method of Association of Official Analytical Chemists (2012). Each sample was determined in triplicate.
Amino Acid Composition
The amino acids of the plasma sample were analyzed as per the method described by Zhu et al. (2014). Samples were hydrolyzed with 6 mol HCl at 110°C for 24 h. Then, the solution was filtered through a 0.45-μm membrane filter (Millipore, Bedford, MA). The amino acids were analyzed using a Hitachi L-8900A Auto Amino Acid Analyzer (Hitachi Ltd., Japan). Tryptophan was not analyzed.
Preparation of Protein Hydrolysate
Three proteases (Alcalase, Protamex, and Flavourzyme) were evaluated to determine the optimum enzyme for hydrolyzing DBP. Briefly, the substrate (DBP) was dissolved in distilled water, homogenized at 5,000 rpm, and preheated in a water bath to attain optimum conditions (Alcalase pH = 9.0, 60°C; Protamex pH = 6.0, 50°C; and Flavourzyme pH = 6.0, 50°C), and then, the enzymes were added at the same enzyme-to-substrate (E/S) ratio (0.4 g enzyme/100 g protein). The solution was incubated and stirred for 4 h. During the reaction, the pH was adjusted using 1 mol HCl or 1 mol NaOH. Enzymatic hydrolysis was terminated by heating at 100°C for 10 min. Then, the mixture was rapidly cooled to room temperature in an ice bath and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was collected to measure the antioxidant activity and the degree of hydrolysis (DH).
1,1-Diphenyl-2-Picrylhydrazyl Scavenging Activity
1,1-Diphenyl-2-picrylhydrazyl radical is a stable free radical with the maximum absorption at 517 nm. In the presence of antioxidants, free radicals are cleared, resulting in the decrease of absorbance at 517 nm. Thus, the antioxidant activity can be evaluated as per the change of absorbance value. The DPPH assay has been widely used to evaluate the antioxidant capacities of natural compounds (Kaur and Geetha, 2006). The assay was performed as per the method described by Liu et al. (2017). Briefly, DPPH was dissolved in 95% alcohol at a concentration of 0.2 mmol/L. The sample group consisted of 1 mL sample solution and 1 mL DPPH (in 95% ethanol). The blank was made up of 1 mL of 95% ethanol and 1 mL sample solution. The control group contained 1 mL DPPH mixed with 1 mL of 95% ethanol. All the mixtures were incubated at 25°C for 30 min in the dark. Subsequently, absorbance was measured at 517 nm. The DPPH radical scavenging activity was calculated as per the following formula:
| (1) |
Degree of hydrolysis
The DH of plasma hydrolysate was calculated by measuring the nitrogen content soluble in 10% trichloroacetic acid as per the method of Fonkwe and Singh (1996) with slight modification. Briefly, the hydrolysate (20 mL) was mixed with an equal volume of 20% trichloroacetic acid. The mixture (10% trichloroacetic acid) was stirred at room temperature and centrifuged at 15,000 × g for 10 min at 4°C. The supernatant was collected and lyophilized to calculate the content of soluble protein by the Kjeldahl method (Amiza et al., 2011). The percent DH was expressed as follows:
| (2) |
Single-Factor Experiment
Alcalase hydrolysis was studied as per the method described in the section of “Preparation of Protein Hydrolysisates.” Enzyme concentration, temperature, time, and pH could affect the antioxidant ability of the hydrolysate. Single-factor experiments were conducted as follows:
Effect of hydrolysis time: Duck blood plasma was hydrolyzed for different durations (0.5, 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, 7.5, and 8.5 h), and the E/S ratio, pH value, and temperature were set at 0.3%, 9.0, and 60°C, respectively.
Effect of E/S ratio: The E/S ratios in the experiment were set as 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6%, whereas the hydrolysis time, pH value, and temperature were set at 5.5 h, 9.0, and 60°C, respectively.
Effect of pH values: Duck blood plasma was hydrolyzed at different pH values (8.0, 8.5, 9.0, 9.5, 10.0, 10.5, 11.0), whereas the E/S ratio was 0.3% and the incubation was performed at a constant temperature of 60°C for 5.5 h.
Effect of incubation temperature: Duck blood plasma was hydrolyzed at various incubation temperatures (50, 55, 60, 65, 70, 75, and 80°C), whereas the E/S ratio, pH value, and hydrolysis time were fixed at 0.3%, 9.5, and 5.5 h, respectively.
Optimization of Hydrolysis Conditions
Based on the single-factor experiment, RSM was used to study the influence of different hydrolysis conditions on DPPH scavenging activity. A central composite design (CCD) was used to optimize hydrolysis conditions using 4 independent variables, which were reaction temperature (X1), pH (X2), time (X3), and the ratio of E/S (X4). Design–Expert software, version 8.6 (Stat-Ease, Inc., Minneapolis), package was used to establish the RSM–CCD model. The factors and design levels for the CCD independent variables are shown inTables 1 and 2. The experiments were performed in a randomized form and repeated thrice under identical conditions (Hamid et al., 2014). Central composite design was used to fit the second-order response surface model, which required 30 experiments, including 6 center points, 16 factorial points, and 8 axial points (Table 2). The second-order polynomial regression equation is as follows:
| (3) |
where Y is the predicted response representing DPPH scavenging rate; β0 is constant, and βi, βii, βij, are the regression coefficients for linear, quadratic, and interaction terms, respectively. The experiments were run randomly. The DBPH was lyophilized and stored at −20°C for further analysis.
Table 1.
Factors and design levels for the RSM-CCD independent variables.
| Factor | Code | Level |
||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Reaction temperature (°C) | X1 | 55 | 60 | 65 | 70 | 75 |
| pH | X2 | 7.5 | 8.5 | 9.5 | 10.5 | 11.5 |
| Reaction time (h) | X3 | 4.5 | 5 | 5.5 | 6 | 6.5 |
| Ratio of E/S (%, w/w) | X4 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
Abbreviations: CCD, central composite design; E/S, enzyme to substrate; RSM, response surface methodology.
Table 2.
The CCD-RSM design for optimizing hydrolysis conditions.
| Run number | Code level of variables |
Response value Y (%) | Type of point | |||
|---|---|---|---|---|---|---|
| x1 (X1) | x2 (X2) | x3 (X3) | x4 (X4) | |||
| 1 | 60 (−1) | 8.5 (−1) | 5 (−1) | 0.2 (−1) | 37.36 | Factorial |
| 2 | 70 (+1) | 8.5 (−1) | 5 (−1) | 0.2 (−1) | 47.63 | Factorial |
| 3 | 60 (−1) | 10.5 (+1) | 5 (−1) | 0.2 (−1) | 50.8 | Factorial |
| 4 | 70 (+1) | 10.5 (+1) | 5 (−1) | 0.2 (−1) | 45.02 | Factorial |
| 5 | 60 (−1) | 8.5 (−1) | 6 (+1) | 0.2 (−1) | 39.69 | Factorial |
| 6 | 70 (+1) | 8.5 (−1) | 6 (+1) | 0.2 (−1) | 52.62 | Factorial |
| 7 | 60 (−1) | 10.5 (+1) | 6 (+1) | 0.2 (−1) | 59.69 | Factorial |
| 8 | 70 (+1) | 10.5 (+1) | 6 (+1) | 0.2 (−1) | 56.66 | Factorial |
| 9 | 60 (−1) | 8.5 (−1) | 5 (−1) | 0.4 (+1) | 38.53 | Factorial |
| 10 | 70 (+1) | 8.5 (−1) | 5 (−1) | 0.4 (+1) | 49.46 | Factorial |
| 11 | 60 (−1) | 10.5 (+1) | 5 (−1) | 0.4 (+1) | 56.26 | Factorial |
| 12 | 70 (+1) | 10.5 (+1) | 5 (−1) | 0.4 (+1) | 49.67 | Factorial |
| 13 | 60 (−1) | 8.5 (−1) | 6 (+1) | 0.4 (+1) | 38.46 | Factorial |
| 14 | 70 (+1) | 8.5 (−1) | 6 (+1) | 0.4 (+1) | 53.75 | Factorial |
| 15 | 60 (−1) | 10.5 (+1) | 6 (+1) | 0.4 (+1) | 59.28 | Factorial |
| 16 | 70 (+1) | 10.5 (+1) | 6 (+1) | 0.4 (+1) | 57.08 | Factorial |
| 17 | 55 (−α) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 44.29 | Axial |
| 18 | 75 (+α) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 52.25 | Axial |
| 19 | 65 (0) | 7.5 (−α) | 5.5 (0) | 0.3 (0) | 31.84 | Axial |
| 20 | 65 (0) | 11.5 (+α) | 5.5 (0) | 0.3 (0) | 51.58 | Axial |
| 21 | 65 (0) | 9.5 (0) | 4.5 (−α) | 0.3 (0) | 50.13 | Axial |
| 22 | 65 (0) | 9.5 (0) | 6.5 (+α) | 0.3 (0) | 62.15 | Axial |
| 23 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.1 (−α) | 50.77 | Axial |
| 24 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.5 (+α) | 53.68 | Axial |
| 25 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 62.05 | Center |
| 26 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 62.65 | Center |
| 27 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 63.33 | Center |
| 28 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 61.65 | Center |
| 29 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 63.78 | Center |
| 30 | 65 (0) | 9.5 (0) | 5.5 (0) | 0.3 (0) | 62.65 | Center |
Abbreviations: CCD, central composite design; RSM, response surface methodology; x, the actual level of variables; X, the code level of variables.
Statistical Analysis
The assays of DH and DPPH were conducted in triplicate, and data analysis was performed by the Duncan's multiple range test using SAS 9.0 software (P < 0.05). The response values of the RMS model were analyzed using ANOVA.
Results and discussion
Duck Blood Plasma Compositions
The proximate chemical compositions of spray-dried DBP are summarized in Table 3. Spray-dried DBP had high protein content (72.38%). The lipid, moisture, and ash content were 1.8, 7.5, and 10.2%, respectively. The protein content was lower than that (89.7%) of whole duck blood powder (Sorapukdee and Narunatsopanon, 2017). On the other hand, the protein content of DBP was higher than that of duck blood corpuscle powder (38.74%) (Zheng et al., 2014). Thus, DBP with high protein content and low lipid is suitable for preparing bioactive peptides.
Table 3.
Proximate composition of spray-dried duck blood plasma.1
| Item | Duck blood plasma |
|---|---|
| Protein (%) | 72.38 ± 4.43 |
| Lipid (%) | 1.8 ± 0.09 |
| Moisture (%) | 7.5 ± 0.52 |
| Ash (%) | 10.2 ± 0.84 |
Values represent means ± SD from triplicate measurement.
Amino Acid Composition
The amino acid composition of DBP is shown in Table 4. Duck blood plasma was rich in Glu, Asp, Leu, and Lys, which is consistent with previous reports. Researchers (Sorapukdee and Narunatsopanon, 2017, Zheng et al., 2018b) showed that these were the main amino acids in duck blood and blood cells. The amino acid composition was reasonable, and the ratio of essential amino acids (38.92%) was higher than the ideal protein value recommended by the Food and Agriculture Organization of the United Nations (WHO, 2007). In addition, duck plasma is rich in antioxidant amino acids and hydrophobic amino acids (Table 4). The presence of hydrophobic amino acids plays a significant role in the elimination of free radicals (Chen et al., 1996; Samaranayaka and Li-Chan, 2011). It is reported that antioxidant amino acids contribute to the antioxidant activity of peptides. Therefore, DBP is a high-quality raw material and can be considered a food source to produce antioxidant peptides.
Table 4.
Amino acid composition of duck blood plasma (DBP) and their hydrolysates (DBPH).
| Amino acid | DBP (g/100 g) | DBPH (g/100 g) | Amino acid | DBP (g/100 g) | DBPH (g/100 g) |
|---|---|---|---|---|---|
| Asp | 6.23 ± 0.16 | 6.84 ± 0.21 | Ile1,2 | 3.08 ± 0.07 | 3.51 ± 0.14 |
| Thr1 | 3.67 ± 0.09 | 4.24 ± 0.14 | Leu1,3 | 6.18 ± 0.22 | 6.93 ± 0.26 |
| Ser | 3.50 ± 0.05 | 3.87 ± 0.09 | Tyr2 | 3.36 ± 0.15 | 3.80 ± 0.13 |
| Glu | 10.61 ± 0.26 | 11.53 ± 0.28 | Phe1,3 | 3.54 ± 0.06 | 3.79 ± 0.15 |
| Gly | 2.76 ± 0.14 | 3.11 ± 0.07 | Lys1,3 | 6.06 ± 0.23 | 6.57 ± 0.27 |
| Ala3 | 3.49 ± 0.13 | 4.21 ± 0.16 | His2 | 1.99 ± 0.15 | 3.04 ± 0.20 |
| Cys2 | 2.44 ± 0.08 | 2.68 ± 0.15 | Arg | 5.38 ± 0.21 | 6.72 ± 0.22 |
| Val1,3 | 4.32 ± 0.18 | 4.56 ± 0.19 | Pro3 | 4.76 ± 0.20 | 3.59 ± 0.21 |
| Met1,2 | 1.51 ± 0.12 | 1.66 ± 0.08 |
Essential amino acids.
Antioxidant amino acids.
Hydrophobic amino acids.
Selection of Protease Enzymes
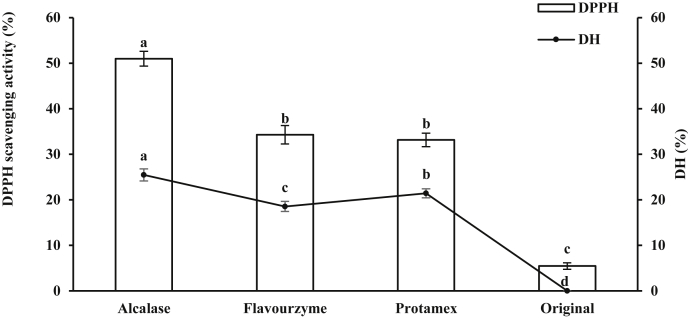
Protease was one of the important factors affecting the antioxidant activity of peptides. Different proteases could affect the antioxidant property of protein hydrolysates (Dong et al., 2008; Yang et al., 2013). Therefore, it is important to select an appropriate protease. Three kinds of proteases were used to hydrolyze DBP. The DPPH scavenging activity was used to evaluate the antioxidant property of the hydrolysates. As shown in Figure 1, all the hydrolysates exhibited higher DPPH scavenging rate than DBP (P < 0.05). The order of the antioxidant activity was as follows: Alcalase hydrolysate > Flavourzyme hydrolysate > Protamex hydrolysate. The DPPH scavenging rate of Alcalase hydrolysate was significantly higher than that of others (P < 0.05). The difference between Flavourzyme hydrolysate and Protamex hydrolysate was not significant (P > 0.05). The data indicated that antioxidant activity of the protein hydrolysates was significantly influenced by the type of protease. Different hydrolysates had different antioxidant activities, perhaps because of the different amino acid compositions (Zhang et al., 2011). Alcalase hydrolysate exhibited the highest DPPH scavenging rate (50.96%), which was consistent with previous reports. Similar to porcine blood plasma (Liu et al., 2010) and bovine blood plasma (Seo et al., 2015) hydrolyzed by proteases, Alcalase hydrolysate showed significant antioxidant activity. The results showed that Alcalase is an effective enzyme to produce antioxidant peptides.
Figure 1.
Effects of protease types on the degree of hydrolysis (DH) and the DPPH scavenging abilities of duck blood plasma (DBP) hydrolysis.
Degree of hydrolysis is an important parameter in the hydrolysis process. Degree of hydrolysis affects the size and the amino acid composition of peptides, which could modulate their biological activity (Sila and Bougatef, 2016). The hydrolysate of Alcalase exhibited the highest DH (25.47%), followed by that of Flavourzyme and then Protamex (Figure 1). The molecular weight of the hydrolysates with high DH is lower than the hydrolysates with low DH (Li et al., 2007). In the study, Alcalase hydrolysates, which had the highest DH, exhibited the highest DPPH scavenging activity, which is consistent with the results of the studies by Li et al. (2007) and Ren et al. (2008). Thus, Alcalase was selected to prepare antioxidant peptides for further studies.
Single-Factor Experiment
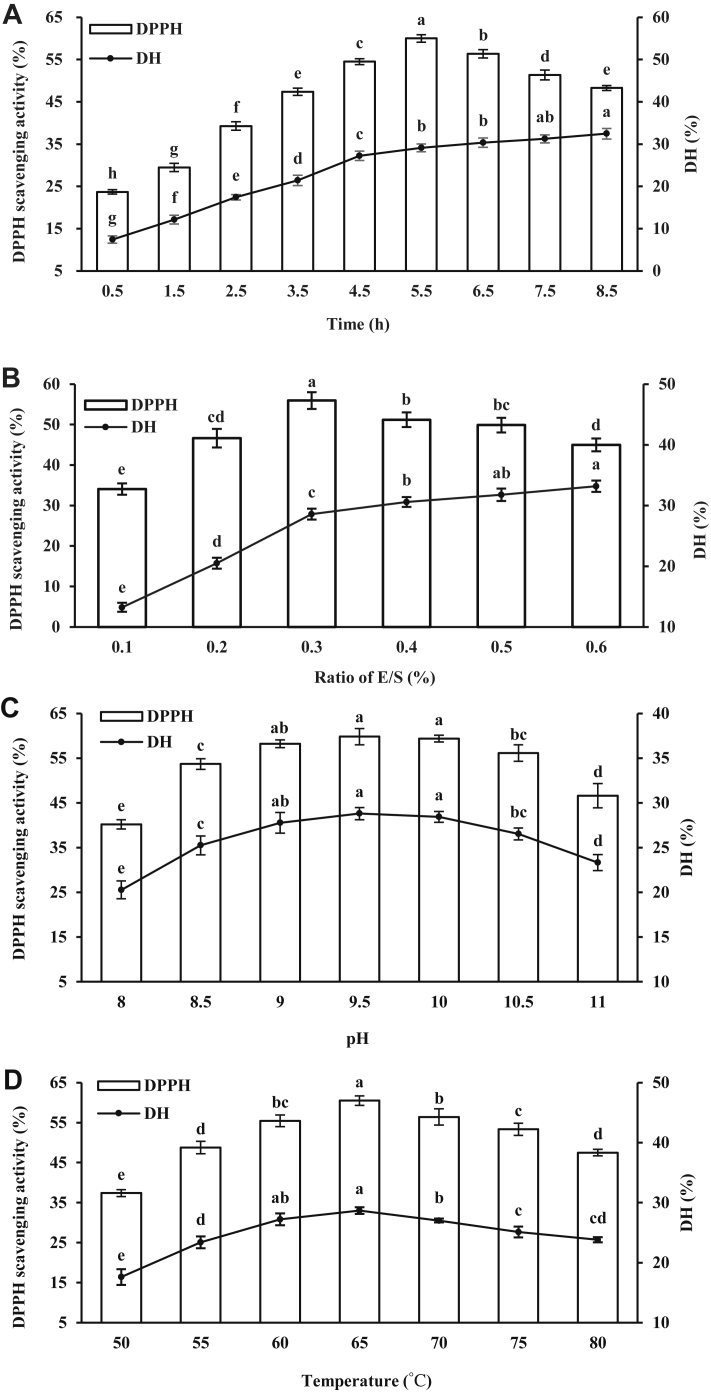
To obtain the desired hydrolysate, optimization of process parameters for enzymatic hydrolysis is essential. As shown in Figure 2A, DH value increased with the prolongation of enzymatic hydrolysis time; however, the DH increased slightly beyond 5.5 h. Liu et al. (2010) and Seo et al. (2015) showed that the DH of the hydrolysates increased with an increase in hydrolysis time. The DPPH scavenging rate increased first and then decreased with the prolongation of enzymatic hydrolysis time. At the hydrolysis time of 5.5 h, the scavenging rate was the highest (60.01%). It may be because that the peptides with antioxidant activity are enzymolyzed again with the extension of reaction time, which results in the decrease of its antioxidant activity. Thus, the antioxidant activity of the hydrolysates increased and then reduced. Hence, the optimal reaction time was 5.5 h.
Figure 2.
Effect of reaction time (A), ratio of E/S (B), pH (C), and temperature (D) on the DH and DPPH scavenging activity of DBPH. Abbreviations: DBPH, duck blood plasma hydrolysate; DH, degree of hydrolysis; DPPH, 1,1-diphenyl-2-picrylhydrazyl; E/S, enzyme to substrate.
As shown in Figure 2B, DH increased with the increase in the E/S ratio. The scavenging capacity of DPPH increased first and then decreased with the increase in the E/S ratio. When the E/S ratio was 0.3%, the DPPH scavenging capacity was the highest. As we all know, when the substrate protein is enough, more antioxidant peptides can be got by the increasing with the E/S ratio. However, when the ratio of E/S is too high, the antioxidant activity of the hydrolysates will be reduced with the increasing of the ratio of E/S because it might be attributed to the recleavage of antioxidative fractions or enzyme inhibition (Zheng et al., 2018b). Therefore, the optimum E/S ratio was 0.3%.
The DPPH scavenging rate and DH of DBPH were effected by pH significantly. Duck blood plasma hydrolysates showed the highest DPPH scavenging rate (59.82%) at pH = 9.5 and DH (28.81%) compared with other pH values (Figure 2C). The optimum pH for alkaline protease was 9.5, perhaps because very high or very low pH reduces the catalytic activity of the enzyme. Therefore, the optimum hydrolysis pH was determined to be 9.5.
The DPPH scavenging rate and DH of DBPH were effected by reaction temperature significantly. As shown in Figure 2D, with the increase in reaction temperature, the DH and DPPH scavenging rate increased first and then decreased. Both of them reached their maximum value at 65°C, 60.50% and 28.67%, respectively. The enzyme activity is strongest at the optimum temperature. Excessive temperature can cause denaturation and inactivation of the enzyme. Thus, the optimum temperature was 65°C.
Optimization of Hydrolysis Parameters by RSM
Based on the single-factor experiment, RSM was used to study the influence of different hydrolysis conditions on DPPH scavenging activity. The results of 30 treatment conditions designed by CCD method are presented in Table 2. The DPPH scavenging rate ranged from 31.84% to 63.78% under different conditions. By applying multiple regression analysis, the relationship between the response variables and the 4 independent variables can be calculated by Equation (4).
| (4) |
where X1, X2, X3, and X4 refer to the coded values of reaction temperature, pH, time, and ratio of E/S, respectively.
The ANOVA was performed to determine the fitness and statistical significance of the quadratic model for hydrolysis process. ANOVA and the effect of each model terms are listed in Table 5. As shown in Table 5, the model F value (292.84) and P value (P < 0.0001) showed that the model provides statistically significant results. The P value of lack of fit (0.6259) was used to measure the fitness of the model, and it did not show significance (P > 0.05). Thus, the model is suitable. Furthermore, in this case, R2 = 0.9964, R2Adj = 0.9930, R2Pred = 0.9849, indicating that the model is reliable for the prediction of DPPH scavenging activity and was fitted to the second-order equation.
Table 5.
ANOVA for the quadratic polynomial mode.
| Source | Sum of square | df | Mean square | F-value | P-value Prob > F |
|---|---|---|---|---|---|
| Model | 2,249.11 | 14 | 160.65 | 292.84 | <0.00011 |
| X1 | 94.96 | 1 | 94.96 | 173.10 | <0.00011 |
| X2 | 564.93 | 1 | 564.93 | 1,029.76 | 0.00151 |
| X3 | 184.48 | 1 | 184.48 | 336.28 | <0.00011 |
| X4 | 14.79 | 1 | 14.79 | 26.96 | 0.00011 |
| X1X2 | 280.83 | 1 | 280.83 | 511.72 | <0.00011 |
| X1X3 | 12.53 | 1 | 12.53 | 22.84 | 0.00251 |
| X1X4 | 0.22 | 1 | 0.22 | 0.55 | 0.3211 |
| X2X3 | 23.57 | 1 | 23.57 | 42.97 | <0.00011 |
| X2X4 | 3.26 | 1 | 3.26 | 5.94 | 0.02772 |
| X3X4 | 10.89 | 1 | 10.89 | 19.85 | 0.01532 |
| X1X1 | 359.15 | 1 | 359.15 | 654.66 | <0.00011 |
| X2X2 | 758.46 | 1 | 758.46 | 1,382.54 | 0.00031 |
| X3X3 | 74.77 | 1 | 74.77 | 136.29 | <0.00011 |
| X4X4 | 189.69 | 1 | 189.69 | 345.77 | <0.00011 |
| Residual | 8.23 | 15 | 0.55 | ||
| Lack of fit | 6.14 | 10 | 0.51 | 0.83 | 0.6259 |
| Pure error | 3.09 | 5 | 0.62 | ||
| Cor total | 2,257.34 | 29 | |||
| R-Squared | 0.9964 | ||||
| Adj R-Squared | 0.9930 | ||||
| Pred R-Squared |
0.9849 | ||||
| Adeq Precision | 58.689 |
Significant within a 99.9% confidence interval.
Significant within a 95% confidence interval.
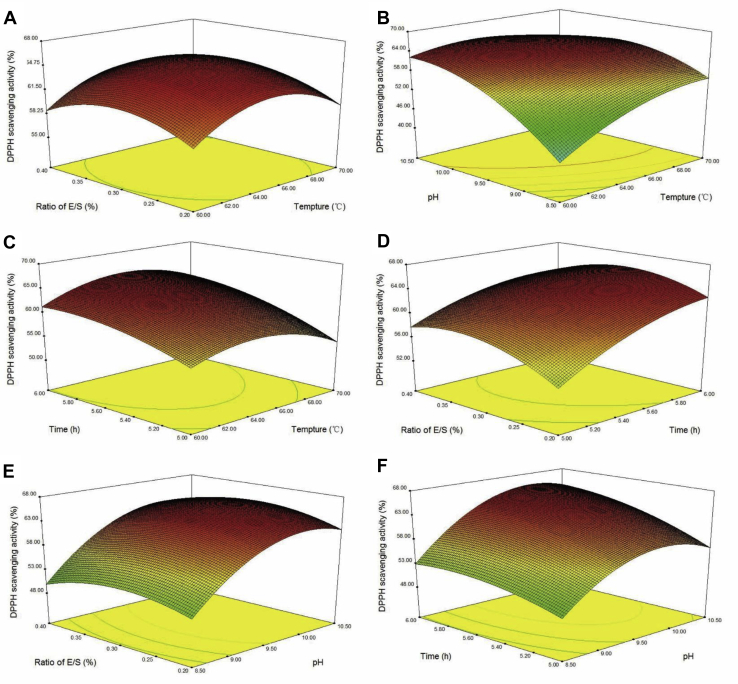
Table 5 shows that the DPPH scavenging rate was affected by the 4 terms significantly (P < 0.01). Among the 4 variables, the ratio of E/S had the largest effect on the DPPH scavenging activity, followed by pH, and the effects of time and temperature were the smallest. Similarly, the quadratic terms and interaction terms without X1X4 show significance (P < 0.01) on the DPPH scavenging activity. This indicated that the effect of each variable on the response value is not a simple linear relationship but a quadratic relationship, and the interaction exists between the variables. The interactions between the 2 factors are shown in Figure 3. As shown in the Figures 3A–C, with the increase in enzymatic hydrolysis temperature, DPPH scavenging rate increased first and then decreased. As shown in Figures 3A, 3D, 3E, the DPPH scavenging rate increased first and then decreased with the increase in the E/S ratio. As for Figures 3C, 3D, 3F, the antioxidant activity increased with the prolongation of hydrolysis time. In Figures 3B, 3E, 3F, with the increase in pH value, the antioxidant activity increased first and then decreased. When the pH was about 9.5, the DPPH scavenging rate reached its maximum. We can predict that the interaction between pH and temperature/time is significant based on the shape of contour, which is consistent with the variance analysis.
Figure 3.
Response surface plots showing correlation of (A) ratio of E/S and hydrolysis temperature; (B) hydrolysis temperature and hydrolysis pH; (C) hydrolysis time and hydrolysis temperature; (D) ratio of E/S and hydrolysis time; (E) ratio of E/S and hydrolysis pH, and (F) hydrolysis time and hydrolysis pH on the DPPH scavenging activity. Abbreviations: DPPH, 1,1-diphenyl-2-picrylhydrazyl; E/S, enzyme to substrate.
Verification of the Predictive Model
In accordance with the established model for optimization analysis, the highest DPPH scavenging activity of 65.58% occurred at the hydrolysis temperature (X1) of 65.41°C, pH (X2) of 10.05, time (X3) of 6 h, and ratio of E/S (X4) of 0.3%. To further optimize the hydrolysis, the test parameters were revised to hydrolysis temperature (X1) of 65.5°C, pH (X2) of 10, time (X3) of 6 h, and ratio of E/S (X4) of 0.3%. Verification tests (n = 3) were carried out, and the DPPH scavenging rate was found to be 64.84% ± 1.24%. There is no significant difference between the measured value and the estimated value by the analysis using SAS software. Therefore, the model was reliable and can be used for optimizing hydrolysis. In addition, RSM was used to optimize the process conditions of grass carp protein to prepare antioxidant peptides (Ren et al., 2008). Chicken blood corpuscle was hydrolyzed and optimized by RSM, and then, AEDKKLIQ was identified with antioxidant activity as well as that of glutathione (Zheng et al., 2018a).
In the experiment, the DPPH scavenging rate of DBPH was 64.84% at the concentration of 1.0 mg/mL, which was lower than those in the RSM model of duck protein (84.36%) (Wang et al., 2018). Porcine hemoglobin hydrolysate exhibited similar DPPH scavenging activity (67%) at the concentration of 1.5 mg/mL (Sun et al., 2012). Thus, DBPH showed antioxidant activity.
The bioactivities of a peptide are mostly dependent on its amino acid composition (Ponall et al., 2010; Chalamaiah et al., 2018). The antioxidant activities of peptides show a close relationship with the amino acid sequence, composition, structure, and hydrophobicity (Zou et al., 2016; Wang et al., 2019). The amino acid composition of DBPH is shown in Table 4. Duck blood plasma hydrolysate was found to be rich in Glu, Leu, Asp, Arg, and Lys, which was consistent with the main amino acids of duck blood of Sorapukdee and Narunatsopanon (2017) and Zheng et al. (2018b). Proper positioning of antioxidant amino acids (Cys, Tyr, His, and Met) has been reported to contribute greatly to the potency of antioxidant peptides (Bellia et al., 2008; Udenigwe and Aluko, 2011). The antioxidant amino acid amounts to 14.69% in DBPH, which is higher than that in rapeseed peptides (He et al., 2012). Hydrophobic amino acids play a significant role in the elimination of free radicals (Chen et al., 1996; Samaranayaka and Li-Chan, 2011). Hydrophobic amino acid content (Phe, Ala, Val, Pro, Leu, and Lys) was 29.65% in DBPH. Sila and Bougatef (2016) reported that peptides containing more hydrophobic amino acids showed stronger antioxidant activity. The results revealed a high content of antioxidant amino acids and hydrophobic amino acids in DBPH. Thus, the antioxidant ability may be because of these amino acids. In addition, as shown in Table 4, the amino acid composition is reasonable and the ratio of essential amino acids (38.76%) was high. Thus, DBPH can be a potential nutritional ingredient in function foods.
Conclusion
An efficient and feasible technology for producing antioxidant peptides from DBP was developed and optimized by RSM. The optimum protease was found to be Alcalase. The optimum conditions to obtain the highest DPPH scavenging rate were as follows: hydrolysis temperature, 65.5°C; E/S ratio, 0.3%; pH, 10.0; and hydrolysis time, 6.0 h. Under the given conditions, the DPPH scavenging rate was 64.84%. The experimental value agreed with the predicted value within a 95% confidence interval, suggesting a good fit between the models and the experimental data. Therefore, this method can be used to produce duck plasma antioxidant peptides.
This study indicated that DBPH shows antioxidant activity. Based on the amino acid composition, DBPH possesses a high nutritional value. Enzymatic hydrolysis is a feasible and manageable technique to industrially produce protein hydrolysate. Although further isolation and characterization of antioxidant peptides is required, the results indicate that the duck plasma could be used as a high quality source of protein or as an additive for function foods, as well as a research and development base for animal blood. It is not a waste product but a valuable protein resource with antioxidant activity.
Acknowledgments
This research was financially supported by the Natural Science Foundation of China (Grant No. 31671872).
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
References
- Amiza M.A., Nurul Ashikin S., Faazaz A.L. Optimization of enzymatic protein hydrolysis from silver catfish (Pangasius sp.) frame. Int. Food Res. J. 2011;18:775–781. [Google Scholar]
- AOAC . 19th ed. Association of Official Analytical Chemists; Washington, DC: 2012. The Official Methods of Analysis. [Google Scholar]
- Bah C.S.F., Carne A., McConnell M.A., Mros S., Bekhit Ael D.A. Production of bioactive peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions using plant and fungal protease preparations. Food Chem. 2016;202:458–466. doi: 10.1016/j.foodchem.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Bellia F., Amorini A.M., LaMendola D., Vecchio G., Tavazzi B., Giardina B., Di Pietro V., Lazzarino G., Rizzarelli E. Newglycosidic derivatives of histidine-containing dipeptides with antioxidant properties and resistant to carnosinase activity. Eur. J. Med. Chem. 2008;43:373–380. doi: 10.1016/j.ejmech.2007.03.038. [DOI] [PubMed] [Google Scholar]
- Bo Y.C., Lu Y., Zhao Y., Zhao E.J., Yuan L., Lu W.Q., Cui L.L., Lu Q.J. Association between dietary vitamin C intake and risk of esophageal cancer: a dose-response meta-analysis. Int. J. Cancer. 2016;138:1843–1850. doi: 10.1002/ijc.29838. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M., Yu W., Wu J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem. 2018;245:205–222. doi: 10.1016/j.foodchem.2017.10.087. [DOI] [PubMed] [Google Scholar]
- Chen H.M., Muramoto K., Yamauchi F., Nokihara K. Antioxidant activity of design peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996;44:2619–2623. [Google Scholar]
- Cheng F.Y., Lai I.C., Lin L.C., Sakata R. The in vitro antioxidant properties of alcalase hydrolysate prepared from silkie fowl (Gallus gallus) blood protein. Anim. Sci. J. 2016;87:921–928. doi: 10.1111/asj.12509. [DOI] [PubMed] [Google Scholar]
- Dong S., Zeng M., Wang D., Liu Z., Zhao Y., Yang H. Antioxidant and biochemical properties of protein hydrolysates prepared from silver carp (Hypophthalmichthys molitrix) Food Chem. 2008;107:1485–1493. [Google Scholar]
- Fonkwe L.G., Singh R.K. Protein recovery from mechanically deboned turkey residue by enzymic hydrolysis. Process Biochem. 1996;31:605–616. [Google Scholar]
- Hamid S.B.A., Chowdhury Z.Z., Karim M.Z. Catalytic extraction of microcrystalline cellulose (MCC) from elaeis guineensis using central composite design (CCD) Bioresources. 2014;9:7403–7426. [Google Scholar]
- He R., Ju X., Yuan J., Wang L., Girgih A.T., Aluko R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012;49:432–438. [Google Scholar]
- Kaur I.P., Geetha T. Screening methods for antioxidants - a review. Mini. Rev. Med. Chem. 2006;6:305–312. doi: 10.2174/138955706776073448. [DOI] [PubMed] [Google Scholar]
- Korhonen H. Milk-derived bioactive peptides: from science to applications. J. Funct. Foods. 2009;1:177–187. [Google Scholar]
- Korhonen H., Pihlanto A. Bioactive peptides: production and functionality. Int. Dairy J. 2006;16:945–960. [Google Scholar]
- Li B., Chen F., Wang X., Ji B.P., Wu Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionizationemass spectrometry. Food Chem. 2007;102:1135–1143. [Google Scholar]
- Liu D.M., Chen X., Huang J.C., Huang M., Zhou G.H. Generation of bioactive peptides from duck meat during post-mortem aging. Food Chem. 2017;237:408–415. doi: 10.1016/j.foodchem.2017.05.094. [DOI] [PubMed] [Google Scholar]
- Liu Q., Kong B., Xiong Y.L., Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. [Google Scholar]
- Miyake Y., Fukushima W., Tanaka K., Sasaki S., Kiyohara C., Tsuboi Y., Yamada T., Oeda T., Miki T., Kawamura N., Sakae N. Dietary intake of antioxidant vitamins and risk of Parkinson’s disease: a case–control study in Japan. Eur. J. Neurol. 2011;18:106–113. doi: 10.1111/j.1468-1331.2010.03088.x. [DOI] [PubMed] [Google Scholar]
- Nedjar-Arroume N., Dubois-Delval V., Adje E.Y., Traisnel J., Krier F., Mary P., Kouach M., Briand G., Guillochon D. Bovine hemoglobin: an attractive source of antibacterial peptides. Peptides. 2008;29:969–977. doi: 10.1016/j.peptides.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Ockerman H.W., Hansen C.L. Animal By-Product Processing and Utilization. Press, Technomic Publishing Co., Inc.; Pennsylvania: 2000. Blood utilization; pp. 325–354. [Google Scholar]
- Paseephol T., Small D., Sherkat F. Process optimization for fractionating Jerusalem artichoke fructans with ethanol using response surface methodology. Food Chem. 2007;104:73–80. [Google Scholar]
- Ponall T.L., Udenige C.C., Aluko R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010;58:4712–4718. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- Ren J.R., Zhao M.M., Shi J., Wang J.S., iang Y.M., Cui C., Kakuda Y., Sophia Jun X. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci. Technol. 2008;41:1624–1632. [Google Scholar]
- Samaranayaka A.G.P., Li-Chan E.C.Y. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J. Funct. Foods. 2011;3:229–254. [Google Scholar]
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Seo H.W., Jung E.Y., Go G.W., Kim G.D., Joo S.T., Yang H.S. Optimization of hydrolysis conditions for bovine plasma protein using response surface methodology. Food Chem. 2015;185:106–111. doi: 10.1016/j.foodchem.2015.03.133. [DOI] [PubMed] [Google Scholar]
- Sila A., Bougatef A. Antioxidant peptides from marine by-products: isolation, identification and application in food systems. A review. J. Funct. Foods. 2016;21:10–26. [Google Scholar]
- Sorapukdee S., Narunatsopanon S. Comparative study on compositions and functional properties of porcine, chicken and duck blood. Korean J. Food Sci. 2017;37:228–241. doi: 10.5851/kosfa.2017.37.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Luo Y.K., Shen H.X., Li X., Yao L. Purification and characterisation of a novel antioxidant peptide from porcine haemoglobin hydrolysate. Int. J. Food Sci. Technol. 2012;47:148–154. [Google Scholar]
- Udenigwe C.C., Aluko R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011;12:3148–3161. doi: 10.3390/ijms12053148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Xie N.N., Li B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: a review. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12571. [DOI] [PubMed] [Google Scholar]
- Wang D.Y., Zhang M.H., Zou Y., Sun Z.L., Xu W.M. Optimization of flavourzyme hydrolysis condition for the preparation of antioxidant peptides from duck meat using response surface methodology. J. Poult. Sci. 2018;55:217–223. doi: 10.2141/jpsa.0160155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Vol. 935. WHO; Geneva, Switzerland: 2007. (Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint WHO/FAO/UNU Expert Consultation Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation). [Google Scholar]
- Wu J., Huo J., Huang M., Zhao M., Luo X., Sun B. Structural characterization of a tetrapeptide from sesame flavor-type Baijiu and its preventive effects against AAPH-induced oxidative stress in HepG2 cells. J. Agric. Food Chem. 2017;65:10495–10504. doi: 10.1021/acs.jafc.7b04815. [DOI] [PubMed] [Google Scholar]
- Yang R.L., Zhao X.J., Kuang Z.S., Ye M.Q., Luo G.Q., Xiao G.S., Liao S.T., Li L., Xiong Z.Y. Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology. J. Food Agric. Environ. 2013;11 [Google Scholar]
- Ye N.H., Hu P., Xu S.L., Chen M.M., Wang S.Y., Hong J., Chen T.T., Cai T.T. Preparation and characterization of antioxidant peptides from carrot seed protein. J. Food Qual. 2018;2018:8579094. [Google Scholar]
- Zhang T., Li Y., Miao M., Jiang B. Purification and characterisation of a new antioxidant peptide from chickpea (Cicer arietium L.) protein hydrolysates. Food Chem. 2011;128:28–33. doi: 10.1016/j.foodchem.2011.02.072. [DOI] [PubMed] [Google Scholar]
- Zheng Z.J., Huang Y., Wu R.J., Zhao L.M., Wang C.F., Zhang R.J. Response surface optimization of enzymatic hydrolysis of duck blood corpuscle using commercial proteases. Poult. Sci. 2014;3:2641–2650. doi: 10.3382/ps.2014-03898. [DOI] [PubMed] [Google Scholar]
- Zheng Z.J., Si D.Y., Ahmad B., Li Z.X., Zhang R.J. A novel antioxidative peptide derived from chicken blood corpuscle hydrolysate. Food Res. Int. 2018;106:410–419. doi: 10.1016/j.foodres.2017.12.078. [DOI] [PubMed] [Google Scholar]
- Zheng Z.J., Wei X.B., Shang T.T., Huang Y., Hu C., Zhang R.J. Bioconversion of duck blood cell: process optimization of hydrolytic conditions and peptide hydrolysate characterization. BMC Biotechnol. 2018;18:67. doi: 10.1186/s12896-018-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.Z., Zhang W.G., Kang Z.L., Zhou G.H., Xu X.L. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014;96:783–789. doi: 10.1016/j.meatsci.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Zou T.B., He T.P., Li H.B., Tang H.W., Xia E.Q. The Structure activity relationship of the antioxidant peptides from natural proteins. Molecules. 2016;21 doi: 10.3390/molecules21010072. [DOI] [PMC free article] [PubMed] [Google Scholar]