Abstract
Multiplexed immunohistochemical techniques give insight into contextual cellular relationships by offering the ability to collect cell-specific data with spatial information from formalin-fixed, paraffin embedded tissue sections. We established an automated sequential elution-stripping multiplex immunohistochemical assay to address two controversial scientific questions in the field of hepatopathology: 1) whether epithelial-to-mesenchymal transition or mesenchymal-to-epithelial transition occurs during liver injury and repair of a chronic liver disease and 2) if there is a stromal:epithelial relationship along the canals of Hering that would support the concept of this biliary structure being a stem/progenitor cell niche. Our 4-plex assay includes both epithelial and mesenchymal clinical immunohistochemical markers and was performed on clinical human liver specimens in patients with primary biliary cholangitis. The assay demonstrated that in each specimen, co-expression of epithelial and mesenchymal markers was observed in extraportal cholangiocytes. In regard to possible mesenchymal components in a stem cell niche, 82.3% ± 5.5% of extraportal cholangiocytes were intimately associated with a vimentin-positive cell. Co-expression of epithelial and mesenchymal markers by extraportal cholangiocytes is evidence for epithelial to mesenchymal transition in primary biliary cholangitis. Vimentin-positive stromal cells are frequently juxtaposed to extraportal cholangiocytes, supporting an epithelial:mesenchymal relationship within the hepatobiliary stem cell niche. Our automated sequential elution-stripping multiplex immunohistochemical assay is a cost-effective multiplexing technique that can be readily applied to a small series of clinical pathology samples in order to answer scientific questions involving cell:cell relationships and cellular antibody expression.
Keywords: multiplex immunohistochemistry, colocalization, epithelial to mesenchymal transition, stem cell niche, primary biliary cholangitis
Introduction
In order to elucidate contextual relationships at the cellular level, immunohistochemistry (IHC) is moving away from traditional single-marker approaches and going towards multiplexed immunohistochemical (mIHC) techniques. mIHC offers the ability to collect cell-specific information with spatial context from formalin-fixed, paraffin embedded (FFPE) tissue sections. This data has the potential to provide a wealth of information that can lead to greater diagnostic, prognostic and treatment accuracy and can also be used to address basic scientific questions.
Conventional chromogenic and fluorescent IHC methods are inadequate to detect multiple targets within one section primarily due to the nature and limited number of long-established fluorophores and chromogens (1). Nevertheless, there are a number of complex mIHC modalities available using a variety of approaches to collect spatially resolved multi-parametric data. Recently, the development of stable covalently deposited chromogens has increased the applicability of chromogenic multiplexing (2). Next generation techniques such as mass cytometry (CyTOF) and Digital Slide Profiling (DSP) use histological methodology as the foundation for their multiplexing approach, however these systems are not easily accessible (3, 4). Therefore, there is a need for simpler cost effective multiplexing techniques that are more readily applicable to the pathology community (5).
In order to overcome the difficulty of determining colocalization of markers within individual cells, we established an automated sequential elution-stripping multiplex immunohistochemical (smIHC) assay. The assay consists of repeated cycles of antibody labeling, detection, whole-slide digital image acquisition, chromogen elution and antibody stripping (6–8). The smIHC method is a straightforward approach to observe colocalization and an effective alternative to more complex, costly mIHC methods. We used this approach to evaluate clinical liver biopsy specimens in order to address two scientific questions: 1) to determine if epithelial-to-mesenchymal transitions (EMT) or mesenchymal-to-epithelial transitions (MET) occur during injury and repair in chronic liver disease and 2) to demonstrate that a stromal:epithelial relationship exists along the canals of Hering (CoH), substantiating the notion that this biliary structure is a stem/progenitor cell niche. We approached the first question by using smIHC to assess the presence of EMT/MET in primary biliary cholangitis (PBC), a disease in which EMT has previously been described (9, 10), by evaluating simultaneous cell expression of epithelial and mesenchymal markers. Also, given the use of vimentin as one such mesenchymal marker, we could explore the question of CoH-associated stromal cells in normal livers. Such cells would help to define a quiescent version of the cell:cell relationships already demonstrated in the ductular reactions (DR) of PBC, which are understood to be the activated state of the niche.
Materials & Methods
Case selection
The study protocol was approved by the New York University (NYU) Langone Health Institutional Review Board. A retrospective review of the pathology database (Powerpath; Sunquest) was performed between January 1, 2013 and December 31, 2018, to identify: 1) liver core biopsies and liver explant specimens with a diagnosis of PBC and 2) liver core biopsies and wedge resections without pathological alteration, to serve as case controls. For each PBC case, hematoxylin-eosin (H&E) slides derived from FFPE tissue were reviewed by three board-certified pathologists (J.D.P., K.S. and N.D.T.). The review entailed both confirmation of the diagnosis of PBC and histological staging (stage 1, 2, 3 or 4) according to the Scheuer staging system. Any cases with histological disparities among reviewers were re-reviewed for consensus assessment. The cases were then grouped as early (stage 1), intermediate (stages 2–3) or late (stage 4) stage of disease. Clinical information was gathered from the electronic medical record, including patient age, gender and serologic test results (serum autoantibodies).
Sequential elution-stripping multiplex immunohistochemical (smIHC) assay
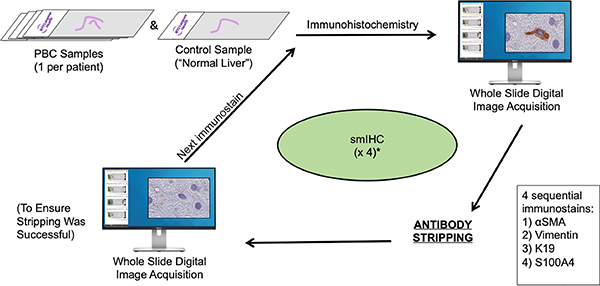
For each case yielded by a pathology database query, our customized smIHC assay was performed on one representative FFPE tissue block chosen by J.D.P, K.S. and N.D.T (Figure 1). The smIHC assay is a 4-plex chromogenic assay based on the elution stripping methodology of Glass et al (7) modified for use on the Ventana Discovery Ultra platform (Ventana Medical Systems, USA). It consists of four rounds of sequential chromogenic IHC detected by horseradish peroxidase (HRP) enzyme deposition of 3-amino-9-ethylcarbozole (AEC). Upon completion of immunolabeling, slides were counterstained, wet-mounted and imaged on a Leica SCN 400 whole slide scanner (Leica Biosystems, USA). Subsequently, coverslips were removed, the chromogen was eluted through graded alcohols, and the antibody-HRP immune complex was “stripped” allowing the application of the next antibody in the sequence (7, 11, 12).
Figure 1.
Customized 4-plex smIHC assay consists of repeated cycles of antibody labeling and detection (immunohistochemistry, whole-slide digital image acquisition, chromogen elution/ antibody stripping, whole slide digital imaging to ensure successful stripping) that allows for four sequential IHC assays to be formed on each FFPE tissue-derived section. Box insert: four sequential antibodies in the smIHC assay..
To establish the working protocol, each antibody was optimized as a monoplex on the same FFPE liver tissue sample using all assay reagents, including the elution and stripping components. Based on signal-to-noise performance and antigen retrieval requirements, an initial multiplex marker order was determined. The multiplex sequence was iteratively tested with crossover controls in order to verify adequate elution and stripping between each sequential antibody application. Details for the four antibodies and their final order in the smIHC assay are shown in Table 1 (11, 13).
Table 1.
Multiplex sequence order and control series
| Primary Secondary Antibodies | Negative Control | Cross Over Control 1 | Cross Over Control 2 | Cross Over Control 3 | Positive Control | |
|---|---|---|---|---|---|---|
| 1 | anti-aSMA | No (A) | Yes | Yes | Yes | Yes |
| anti-Mouse | Yes | No | Yes | Yes | Yes (H) | |
| 2 | anti-Vimentin | No (B) | No | Yes | Yes | Yes |
| anti-Mouse | Yes | Yes (E) | No | Yes | Yes (I) | |
| 3 | anti-K19 | No (C) | N/A | No | Yes | Yes |
| anti-Mouse | Yes | N/A | Yes (F) | No | Yes (J) | |
| 4 | anti-s100A4 | No (D) | N/A | N/A | No | Yes |
| anti-Rabbit | Yes | N/A | N/A | Yes (G) | Yes (K) | |
The final multiplex sequence is numbered and shows the application of primary and secondary antibodies for negative, crossover, and positive controls. Primary and secondary antibody pairs are highlighted. Each column corresponds to tissue section run through the entire multiplex labeling sequence (detection, scanning, elution and stripping performed between each antibody pair in sequence) unless otherwise specified (N/A). Yes: indicates reagent was applied. No: indicates antibody diluent substituted for primary antibodies. No: no secondary antibody reagent applied. Letters correspond to images shown in supplemental Figure 1.
Crossover controls consisted of an additional positive control sample for each marker in the multiplex. The control sample was treated identically in the protocol sequence, except it did not receive the corresponding secondary antibody in the sequence. In the next round, the crossover control did not receive primary antibody, but instead received the secondary antibody in the sequence to determine if elution and stripping prevented cross-labeling between antibody sequences as shown in Table 1. Based on this iterative testing process, adjustments to antibody sequencing order, concentration, time and temperature for protocol parameters were tested until stable reproducible labeling were achieved for the 4-plex assay (instrument sequence protocols are shown in Supplemental Table 1) (13). The antibodies used in the multiplex assay include both mesenchymal and epithelial markers and are presented in their final sequence order. (Table 2)
Table 2.
Source, species and clonality for antibodies used in the EMT/MET smIHC assay
| Antibody | Marker Type | Source | RRID* | Lot # | Species | Clonality |
|---|---|---|---|---|---|---|
| αSMA | Mesenchymal | Dako (M0851) | AB_2223500 | 00048599 | Mouse | 1A4 |
| Vimentin | Mesenchymal | Ventana (790–2917) | AB_2335925 | E04396 | Mouse | V9 |
| K19 | Epithelial | Cell Marque (319M) | AB_1158234 | 1634004C | Mouse | A53_B/A2.26 |
| S100A4 | Mesenchymal | Dako (A5114) | AB_2335679 | 00013143 | Rabbit | Poly |
RRID Research Resource Identifiers www.antibodyregistry.org. EMT/MET: epithelial-to-mesenchymal transition/mesenchymal-to-epithelial transition, respectively. smIHC: sequential elution-stripping multiplex immunohistochemical assay:
Four-micrometer tissue sections were collected onto Inkjet Plus slides (Cat# 22–042-924, Fisher Scientific, USA ), air-dried and stored at room temperature (RT) prior to use. Prior to staining, sections were incubated for 1 h at 60°C followed by instrument deparaffinization. The first marker in the sequence, alpha-SMA, was retrieved for 40 min at 95°C using Cell Conditioner 1 (Tris-Borate-EDTA pH 8.5, Cat# 950–224, Ventana Medical Systems). Endogenous peroxidase activity was blocked with 10% hydrogen peroxide (Cat# R3821310, Ricca Chemical, USA) for 12 min. Alpha-SMA was diluted 1:100 in diluent (Cat# 251–018, Ventana Medical Systems) and incubated for 60 min at RT. Alpha-SMA was detected with Biocare Mouse-on-Mouse Horseradish Peroxidase (HRP) polymer Cat# MM620, Lot#120216, Biocare Medical, USA) for 30 min at 37°C and detected with Ventana Discovery 3-Amino-9-ethylcarbazol (AEC) incubated for 8 min. Sections were counterstained with hematoxylin (Cat# 7211 Thermo Scientific, USA), and wet mounted in Glycerol:PBS 1:1 (Thermo Fisher Scientific, USA). Slides were then scanned. Coverslips were removed in running deionized water. AEC was eluted by treating the sections with graded series of ethanol (75%, 95%, 100%) each for 5 min. The slides were returned to deionized water (DIH2O) by the reverse process (100%, 95%, 75%) also for 5 min each. The immune complex was then denatured (stripped) on the Discovery Ultra with 95°C reaction buffer for 32 min. Upon completion, labeling of the next marker in the multiplex sequence would commence. No additional antigen retrieval treatments were performed for the remainder of the multiplex. Following blocking of endogenous peroxidase with 10% H2O2 (same time as above), the second marker in sequence, vimentin, was applied neat for 60 min at RT. This was detected with Ventana goat anti-mouse, HRP-conjugated multimer (Cat# 760–4310, Ventana Medical Systems) incubated for 8 min and detected with AEC. Sections were counterstained, mounted, imaged and subsequently eluted and stripped. Sections were blocked and the third marker in the multiplex sequence, keratin 19, was applied after diluting 1:100 in Ventana diluent and incubating for 60 min at 37°C. Keratin 19 is a standard marker for hepatobiliary cells of the ductular reaction and for normal cholangiocytes of the canals of Hering and ductules (14). This was detected with Ventana goat anti-mouse, horseradish peroxidase conjugated multimer incubated for 8 min and detected with AEC. Sections were counterstained, mounted, imaged and subsequently eluted and stripped. Following blocking of endogenous peroxidase, the fourth marker in sequence, S100A4, was applied after diluting 1:500 in Ventana diluent and incubating for 60 min at 37°C. This was detected with Ventana goat anti-rabbit, HRP-conjugated multimer (Cat# 760–4311, Ventana Medical Systems) incubated for 8 min and detected with AEC. Sections were counterstained, mounted and imaged. Positive and negative (no primary, full multiplex) as well as crossover (between every marker) controls were run with the study sample group and subsequently imaged.
Antibody visualization and evaluation
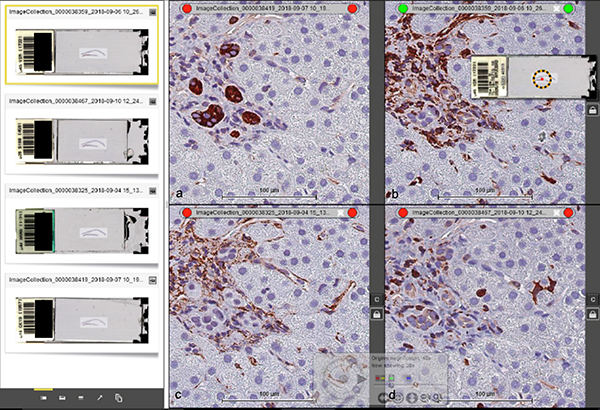
Using SlidePath Digital Image Hub software (Leica Microsystems, USA), four side-by-side viewing panes (one for each antibody) were used to concurrently evaluate expression patterns in extraportal cholangiocytes i.e., those comprising CoH and ductular reactions, and in nearby cells (Figure 2). The leftmost window displays four whole-slide scans of a single slide, each consisting of one of four antibodies from our smIHC assay. The four panes to the right are magnifications of each stain (Figures 2a, b, c, d). The four panes have been manually aligned and then “locked” using the padlock icon such that location and magnification changes made to one pane are automatically synchronized to the other three panes. This allows for cells in the single FFPE tissue section to be evaluated for expression of all four antibodies simultaneously.
Figure 2.
Side-by-side (descending order) viewing of four different antibodies from smIHC using Leica SlidePath Digital Image Hub software. (a) K19, (b) vimentin. (c) αSMA and (d) S100A4. The four images can be synchronized in the same view pane by “locking” them together with the padlock icon. As a result, location and magnification changes made to one pane automatically apply to the other three panes. Scale bar = 100 μm.
IHC expression-patterns of all extraportal cholangiocytes and adjacent cells were evaluated for each core biopsy specimen. For explant specimens, a near-equivalent portion of tissue (30 mm x 3 mm) was selected at random for evaluation.
Data analysis
All data were placed in a database spreadsheet with identifying information removed for confidentiality. Statistical analysis was performed using Student t test and Mann-Whitney U test.
Results
Clinical data
Fourteen PBC liver specimens and three control specimens consisting of histologically normal liver parenchyma were retrospectively identified in the pathology database. Baseline characteristics and clinical data for each case are presented in Table 3. Clinical data for the PBC cases show a female:male gender distribution of 6:1. The PBC cases show an age distribution of 48 ± 11 (± standard deviation) years, while the control cases show an age distribution of 49 ± 8 years. These age differences are not statistically significant.
Table 3.
Baseline characteristics and clinical data of each PBC and control patient
| Case # | Gender | Age | Clinical presentation | AutoAbs |
|---|---|---|---|---|
| PBC 1 | F | 58 | Fatty liver | AMA+/ANA+/ASMA− |
| PBC 2 | M | 55 | Elevated LFTs | AMA+/ANA+/ASMA+ |
| PBC 3 | F | 41 | Elevated LFTs | AMA+/ANA+/ASMA− |
| PBC 4 | M | 54 | Elevated LFTs | AMA+/ANA−/ASMA− |
| PBC 5 | F | 38 | Elevated LFTs | AMA+/ANA−/ASMA− |
| PBC 6 | F | 52 | History of PBC | AMA+/ANA+/ASMA− |
| PBC 7 | F | 52 | History of PBC | AMA+/ANA−/ASMA− |
| PBC 8 | F | 26 | Elevated LFTs | AMA+/ANA+/ASMA− |
| PBC 9 | F | 64 | Elevated LFTs | AMA+/ANA+/ASMA− |
| PBC 10 | F | 51 | Fatty liver | AMA+/ANA+/ASMA− |
| PBC 11 | F | 29 | History of PBC with cirrhosis | AMA+/ANA+/ASMA− |
| PBC 12 | F | 39 | History of PBC with cirrhosis | AMA+/ANA−/ASMA− |
| PBC 13 | F | 61 | History of PBC with cirrhosis | AMA+/ANA+/ASMA− |
| PBC 14 | F | 53 | History of PBC with cirrhosis | AMA+/ANA−/ASMA− |
| Control 1 | F | 57 | Liver lesion, FNH | None evaluated |
| Control 2 | M | 37 | Elevated LFTs | AMA−/ANA−/ASMA− |
| Control 3 | F | 52 | Liver lesion, melanoma | None evaluated |
AutoAbs, autoantibodies; PBC, primary biliary cholangitis; F, female; M, male; LFTs, liver function tests; AMA, anti-mitochondrial antibodies; ANA, anti-nuclear antibodies; ASMA, anti-smooth muscle antibodies.
Pathological data
Specimen characteristics and pathological data obtained by histologic review of each case by J.D.P., K.S. and N.D.T. are presented in Table 4. All liver needle biopsy specimens were deemed adequate by the number of portal tracts sampled and core length. Explant specimens consisted of recipient livers (total hepatectomies) in cases of liver transplantation.
Table 4.
Specimen and pathological characteristics of each PBC and control specimen
| Case # | Type of Specimen | Pathological Findings | Consensus Scheuer Stage | Stage Grouping |
|---|---|---|---|---|
| PBC 1 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 2 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 3 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 4 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 5 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 6 | Needle biopsy | Portal hepatitis, FDLs | 1 of 4 | Early |
| PBC 7 | Needle biopsy | Periportal hepatitis, DR | 2 of 4 | Intermediate |
| PBC 8 | Needle biopsy | Periportal hepatitis, FDLs, DR | 2 of 4 | Intermediate |
| PBC 9 | Needle biopsy | Periportal hepatitis, DR, BD loss | 3 of 4 | Intermediate |
| PBC 10 | Needle biopsy | Periportal hepatitis, DR, BD loss | 3 of 4 | Intermediate |
| PBC 11 | Explant | Cirrhosis | 4 of 4 | Late |
| PBC 12 | Explant | Cirrhosis | 4 of 4 | Late |
| PBC 13 | Explant | Cirrhosis | 4 of 4 | Late |
| PBC 14 | Explant | Cirrhosis | 4 of 4 | Late |
| Control 1 | Wedge resection | Normal liver parenchyma | N/A | N/A |
| Control 2 | Needle biopsy | Normal liver parenchyma | N/A | N/A |
| Control 3 | Needle biopsy | Normal liver parenchyma | N/A | N/A |
PBC, primary biliary cholangitis; FDLs, florid duct lesions; DR, ductular reaction; BD, bile duct. N/A, not applicable
Immunohistochemical expression patterns of extraportal cholangiocytes
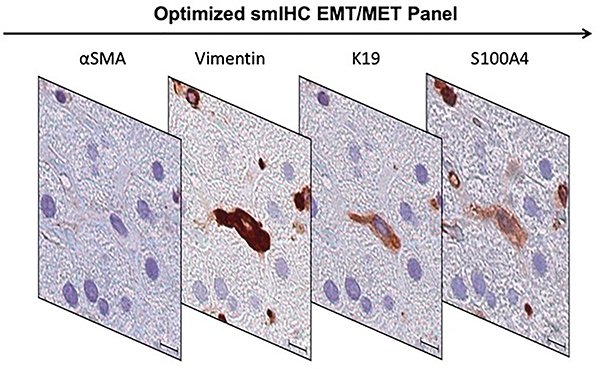
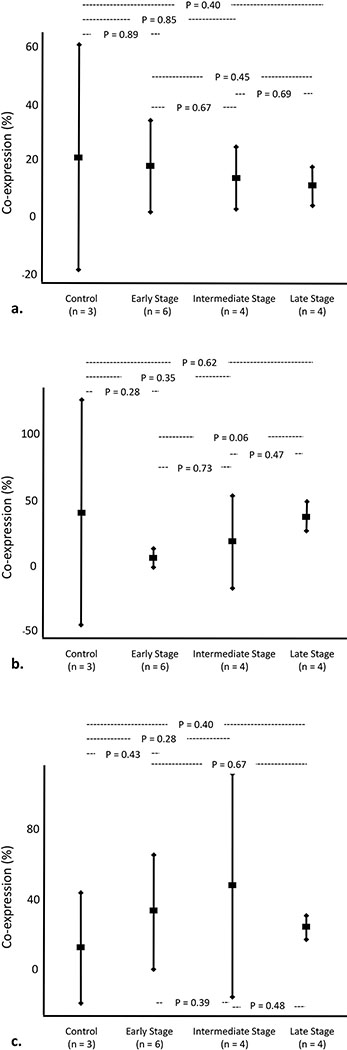
In each specimen, our optimized smIHC EMT/MET assay demonstrated expression of mesenchymal markers (vimentin and S100A4) in some K19-positive extraportal cholangiocytes (Figure 3). K19-positive extraportal cholangiocytes did not demonstrate co-expression of αSMA in any specimen. The percent of K19-positive extraportal cholangiocytes that co-express vimentin and/or S100A4 are presented in Table 5. Co-expression did not differ significantly among PBC stage groups or between PBC stage groups and control cases (Figure 4).
Figure 3.
An optimized smIHC EMT/MET panel consists of sequential αSMA, vimentin, K19 and S100A4 antibody labeling performed on Case 6 shows a spindled extraportal cholangiocyte co-expressing vimentin, K19 and S100A4. Scale bar = 10 μm.
Table 5.
Percentage of K19-positive extraportal cholangiocytes that co-express mesenchymal markers
| Case # | % K19+ Extraportal Cholangiocytes with Vimentin Co-expression Only | % K19+ Extraportal Cholangiocytes with S100A4 Co-expression Only | % K19+ Extraportal Cholangiocytes with Vimentin and S100A4 Co-expression |
|---|---|---|---|
| PBC 1 | 20.0% (2/10) | 0.0% (0/10) | 20.0% (2/10) |
| PBC 2 | 0.0% (0/5) | 40% (2/5) | 0.0% (0/5) |
| PBC 3 | 27.3% (3/11) | 18.2% (2/11) | 18.2% (2/11) |
| PBC 4 | 19.0 (4/21) | 0.0% (0/21) | 76.6% (16/21) |
| PBC 5 | 38.4% (5/13) | 0.0% (0/13) | 15.4% (2/13) |
| PBC 6 | 0.0% (0/15) | 6.7% (1/15) | 66.7% (10/15) |
| PBC 7 | 18.1% (2/11) | 0.0% (0/11) | 81.9% (8/11) |
| PBC 8 | 5.6% (1/18) | 27.8% (5/18) | 27.8% (5/18) |
| PBC 9 | 20.0% (2/10) | 0.0% (0/10) | 80.0% (8/10) |
| PBC 10 | 9.1% (1/11) | 45.5% (5/11) | 0.0% (0/11) |
| PBC 11 | 12.0% (9/75) | 37.3% (28/75) | 20.0% (15/75) |
| PBC 12 | 10.7% (7/65) | 27.6% (18/65) | 29.2% (19/65) |
| PBC 13 | 15.1% (8/53) | 39.6% (21/53) | 24.5% (13/53) |
| PBC 14 | 4.8% (3/62) | 45.2% (28/62) | 19.4% (12/62) |
| Control 1 | 6.3% (1/16) | 0.0% (0/16) | 0.0% (0/16) |
| Control 2 | 16.7% (2/12) | 58.3% (7/12) | 8.3% (1/12) |
| Control 3 | 37.5% (6/16) | 62.5% (10/16) | 25.0% (4/16) |
Figure 4.
Quantification of K19-positive extraportal cholangiocytes that co-express (a) vimentin, (b) S100A4 and (c) both vimentin and S100A4. Vertical bars represent 95% confidence intervals.
Evaluation of cells adjacent to extraportal cholangiocytes
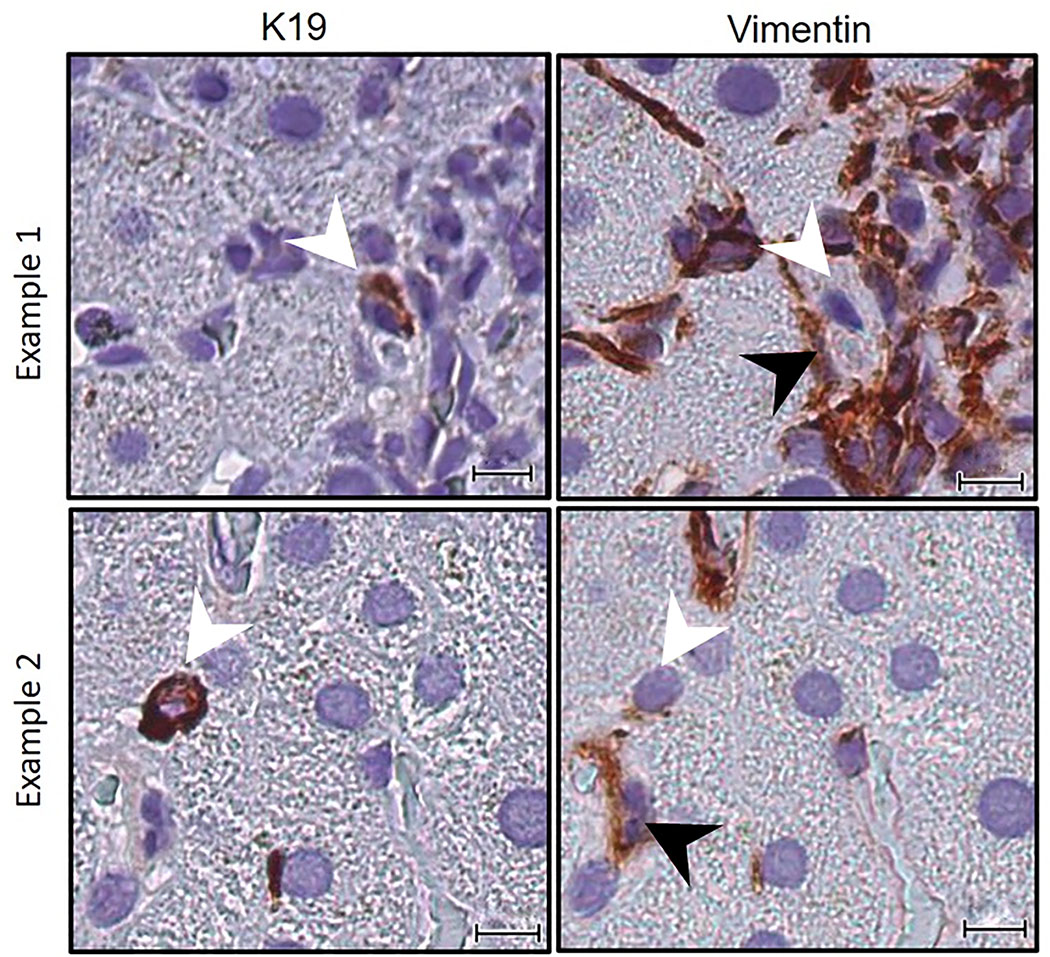
An optimized smIHC EMT/MET assay demonstrated that at least some (82.3 ± 5.5%) of extraportal cholangiocytes from each specimen were closely associated with a vimentin-positive stromal cell (Figure 5, Table 6). The percentage of extraportal cholangiocytes associated with a vimentin-positive stromal cell are presented in Table 6. Similar numbers were seen in both normal controls (76.4% ± 6.9%) and PBC ( 83.6% ± 11.9%) p = 0.21).
Figure 5.
An optimized smIHC EMT/MET panel performed on Case 2 shows two examples of K19-positive extraportal cholangiocytes (white arrowheads) and their intimate association with a vimentin-positive mesenchymal cell (black arrowheads). Scale bar = 10 μm.
Table 6.
Percentage of K19-positive extraportal cholangiocytes associated with a vimentin-positive stromal cell
| Case # | % K19+ Extraportal Cholangiocytes Associated with Vimentin+ Stromal Cell |
|---|---|
| PBC 1 | 90.0% (9/10) |
| PBC 2 | 100.0% (5/5) |
| PBC 3 | 90.1% (10/11) |
| PBC 4 | 85.7% (18/21) |
| PBC 5 | 92.3% (12/13) |
| PBC 6 | 66.7% (10/15) |
| PBC 7 | 100% (11/11) |
| PBC 8 | 83.3% (15/18) |
| PBC 9 | 90.0% (9/10) |
| PBC 10 | 90.9% (10/11) |
| PBC 11 | 69.3% (52/75) |
| PBC 12 | 81.5% (53/65) |
| PBC 13 | 64.2% (34/53) |
| PBC 14 | 66.1% (41/62) |
| Control 1 | 81.3% (13/16) |
| Control 2 | 66.7% (8/12) |
| Control 3 | 81.3% (13/16) |
Discussion
In this study, we developed an automated smIHC assay to evaluate for EMT/MET and stem cell niche components in clinical human liver specimens with PBC pathology. The assay is customizable and runs on a Ventana Discovery Ultra platform using standard procedures and reagents. Routine, primary antibodies or more exotic, exploratory markers can be used with this method. Our smIHC assay was developed with three historical limitations in mind. Firstly, we selected a technique with IHC methodology as the foundation, rather than immunofluorescence-based, because immunofluorescence studies in human liver tissue are difficult to interpret due to interfering autofluorescence (AF) (15). Secondly, in the EMT/MET hepatobiliary literature, there is acknowledged difficulty in determining colocalization of epithelial and mesenchymal IHC markers within individual cells, either by double IHC in one slide or by IHC on sequential sections of a single specimen (16). Lastly, we required an assay that was relatively simple, readily applicable, reproducible and cost-effective. There are insuperable financial and practical barriers to applying next generation techniques such as CyTOF and DSP to our study of seventeen samples. For these techniques, substantial investment is required to acquire instrumentation and develop the technical resources to operate systems not designed for high throughput. In contrast, our smIHC technique can be performed readily in any immunohistochemistry laboratory with clinical IHC antibodies and preexisting paraffin tissue blocks.
In regard to EMT/MET, some animal models have shown supportive evidence for these processes occurring in the liver, while others have refuted this concept (16). Nevertheless, the number of studies evaluating for EMT/MET in human liver tissue is limited (17, 18). EMT/MET is defined as the process by which an epithelial cell acquires mesenchymal characteristics, and the reverse of that process. In order to identify cells that are undergoing EMT/MET in paraffin-embedded tissues, some investigators have used immunohistochemical co-expression of epithelial and mesenchymal markers as a surrogate, although there is an acknowledged difficulty determining colocalization of antibodies within individual cells and a need for multiplexing approaches (9, 16, 19). Characteristic immunohistochemical markers for epithelial cells include keratins, while mesenchymal markers include α-smooth muscle actin (αSMA) and vimentin. One mesenchymal marker, S100A4 (fibroblast-specific protein 1) is thought to play a mediating role in EMT/MET and is a proposed marker of cells undergoing EMT/MET (9, 19–21).
In human liver tissue, ductular reaction occurs in many acute and chronic liver diseases as a response to injury (22). Cholangiocytes comprising ductular reaction can exhibit a spindled morphology. This mesenchymal appearance of epithelial cells raises the question of whether these cholangiocytes are undergoing EMT/MET. In the autoimmune liver disease primary biliary cholangitis (PBC), destruction of small bile ducts leads to ductular reaction formation, purportedly as a compensatory mechanism allowing the continuation of bile flow (23). This autoimmune destruction appears to involve the smallest branches of the biliary trees, including the CoH which link the hepatocellular bile canaliculi to interlobular bile ducts (24, 25). Intriguingly, we have noted that keratin 19 (K19) immunolabeling demonstrates occasional spindled-morphology in the epithelial cholangiocytes that comprise CoH, suggestive of EMT/MET.
In regard to the second question, the CoH are also thought to harbor hepatobiliary stem/progenitor cells (HSPCs) and thus function as a stem cell niche (25–27). Keratin 19 is one of the more common markers for demonstrating cells of this structure. As seen in other organs, one might expect a juxtaposition of HSPC and a mesenchymal cell (28). However, positioning of niche components has not yet, to our knowledge, been histologically visualized, in part due to the difficulty of double labeling for markers of either niche component that allows for close analysis of cell:cell relationships.
As presented in Table 1, our smIHC assay was optimized with a panel of both epithelial and mesenchymal antibodies, specifically tailored to address the question of whether EMT/MET occurs during liver injury and repair. In particular, the assay allows us to determine if a given epithelial cell with mesenchymal morphologic phenotype (i.e. a spindled K19-positive extraportal cholangiocyte) also expresses mesenchymal markers. Our study demonstrated that K19-positive extraportal cholangiocytes in normal livers and in PBC often co-express vimentin and/or S100-A4. This co-expression of epithelial and mesenchymal markers is evidence for EMT/MET though our data suggests that it is a baseline state in normal livers that persists when PBC is present. While vimentin positivity may occur in epithelial cells, co-expression with S100A4 more specifically suggests potential bidirectional transition occurring in extraportal cholangiocytes in normal and in PBC affected livers (29).
Of note, co-expression of K19 and αSMA was not observed in extraportal cholangiocytes. This result gives some insight into which cell type the extraportal cholangiocytes are differentiating towards or from. Activated HSCs are defined by αSMA expression, therefore the absence of αSMA co-expression in this study suggests that the transition occurring in extraportal cholangiocytes does not involve differentiation towards or from activated HSCs (30).
With regards to the hepatobiliary stem cell niche, we herein for the first time demonstrate that vimentin-positive stromal cells are frequently juxataposed to extraportal cholangiocytes in human liver. In bone marrow, it has been shown that hematopoietic stem cells reside in specialized microenvironments (niches) (28). The bone marrow stem cell niche includes mesenchymal stromal cells that are thought to support stem cell function (28). Within the liver, the CoH are thought to harbor HSPCs, the niche for which is also thought to comprise an HSPC juxtaposed to a mesenchymal cell (31). Our assay showed that 82.3% ± 5.5% of extra portal cholangiocytes are associated with a vimentin-positive cell, confirming that there is a stromal:epithelial relationship in the CoH that would support the concept of this biliary structure being a stem/progenitor cell niche. Different authors have suggested different identities for such stromal companion cells, namely hepatic stellate cells (32, 33), macrophages (33) and portal fibroblasts (34). However, these suggestions are generally based on study of DR, the activated form of the niche, rather than the quiescent structures in the normal controls seen here. These data showing frequent vimentin positive stromal cells adjacent to K19 positive, extraportal cholangiocytes in similar numbers would therefore support this concept of this HSPC niche (19, 28).
In general, our smIHC is an application of digital pathology and uses whole slide digital image acquisition and visualization with SlidePath Digital Image Hub software. The technique fosters ease of collaboration among coinvestigators, speeds up IHC expression analysis, allows for simple organization of case material and permits permanent archiving of antibody labeling. The pane “locking” mechanism within the SlidePath software permits ease of colocalization and streamlines data collection.
Conclusion
In summary, smIHC is a cost-effective multiplexing technique that can be readily applied to a small series of clinical pathology samples in order to answer scientific questions involving cell:cell relationships and cellular antibody expression. Application of our smIHC assay to a series of clinical primary biliary cholangitis cases revealed evidence of epithelial-to-mesenchymal transition/mesenchymal-to-epithelial transition occurring in human liver tissue as well as hitherto unreported histological visualization of potential human stem cell niche components. We anticipate that this smIHC assay will have multiple applications, from further exploring the findings of this paper, but more broadly to both basic science and clinical research.
Supplementary Material
Acknowledgements
This work was supported in part by the Pathology Department Translational Research Program (TRP) at NYU Langone Health (NYULH). The authors would like to acknowledge the contributions of Malcolm Shumel and George Boukas in the development of the assay.
The NYULH Center for Biospecimen Research and Development, Histology and Immunohistochemistry Laboratory (RRID:SCR_018304), is supported in part by the Laura and Isaac Perlmutter Cancer Center Support Grant; NIH/NCI P30CA016087 and the National Institutes of Health S10 Grants; NIH/ORIP S10OD01058 and S10OD018338.
Footnotes
Disclosure statement
There are no conflicts of interest.
References
- 1.Dixon AR, Bathany C, Tsuei M, et al. Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev Mol Diagn. 2015;15(9):1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day WA, Lefever MR, Ochs RL, et al. Covalently deposited dyes: a new chromogen paradigm that facilitates analysis of multiple biomarkers in situ. Lab Invest. 2017;97(1):104–113. [DOI] [PubMed] [Google Scholar]
- 3.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24(11):1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11(4):417–422. [DOI] [PubMed] [Google Scholar]
- 5.Hofman P, Badoual C, Henderson F, et al. Multiplexed Immunohistochemistry for Molecular and Immune Profiling in Lung Cancer—Just About Ready for Prime-Time? Cancers. 2019;11(3):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolognesi MM, Manzoni M, Scalia CR, et al. Multiplex staining by sequential immunostaining and antibody removal on routine tissue sections. J Histochem Cytochem. 2017;65(8):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009;57(10):899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Brand M, Peters RP, Catsburg A, et al. Development of a multiplex real-time PCR assay for the rapid diagnosis of neonatal late onset sepsis. J Microbiol Methods. 2014;106:8–15. [DOI] [PubMed] [Google Scholar]
- 9.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Seminars in liver disease. 2011;31(1):11–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan J, Wang Q, Zhang Z, Sun L. Curcumin mitigates the epithelial‐to‐mesenchymal transition in biliary epithelial cells through upregulating CD109 expression. Drug Develop Res. 2019;80(7):992–999. [DOI] [PubMed] [Google Scholar]
- 11.Pirici D, Mogoanta L, Kumar-Singh S, et al. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Microbiol Methods. 2009;57(6):5675–5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujikawa T, Kumar S, Borkar RN, et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Reports. 2017;19(1):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Der Loos CM. Chromogens in multiple immunohistochemical staining used for visual assessment and spectral imaging: the colorful future. J Histotechnol. 2010;33(1):31–40. [Google Scholar]
- 14.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatol. 2004;39(6):1739–1745. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell AJ, Pradel LC, Chasson L, Van Rooijen N, Grau GE, Hunt NH, et al. Technical advance: autofluorescence as a tool for myeloid cell analysis. J Leukoc Biol. 2010;88(3):597–603. [DOI] [PubMed] [Google Scholar]
- 16.Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. American journal of physiology-gastrointestinal liver Physiol. 2013;305(12):G881–G90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Huang G, Li X, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer. 2013;13(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caja L, Bertran E, Campbell J, et al. The transforming growth factor‐beta (TGF‐β) mediates acquisition of a mesenchymal stem cell‐like phenotype in human liver cells. J Cell Physiol. 2011;226(5):1214–1223. [DOI] [PubMed] [Google Scholar]
- 19.Banales JM, Huebert RC, Karlsen T, et al. Cholangiocyte pathobiology. Nat Review Gastroenterol Hepatol. 2019;16(5):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial–mesenchymal transition, fibrosis and regeneration in diseases? J Mol Med. 2008;86(5):507–22. [DOI] [PubMed] [Google Scholar]
- 21.Xue C, Plieth D, Venkov C, Xu C, Neilson EG. The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 2003;63(12):3386–3394. [PubMed] [Google Scholar]
- 22.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatol. 2011;54(5):1853–1863. [DOI] [PubMed] [Google Scholar]
- 23.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatol. 2009;50(1):291–308. [DOI] [PubMed] [Google Scholar]
- 24.Saxena R, Hytiroglou P, Thung SN, Theise ND. Destruction of canals of Hering in primary biliary cirrhosis. Human Path. 2002;33(10):983–988. [DOI] [PubMed] [Google Scholar]
- 25.Khan FM, Komarla AR, Mendoza PG, et al. Keratin 19 demonstration of canal of Hering loss in primary biliary cirrhosis:“minimal change PBC”? Hepatol. 2013;57(2):700–707. [DOI] [PubMed] [Google Scholar]
- 26.Roskams T Relationships among stellate cell activation, progenitor cells, and hepatic regeneration. Clinics in liver disease. 2008;12(4):853–860. [DOI] [PubMed] [Google Scholar]
- 27.Theise ND, Saxena R, Portmann BC, et al. The canals of Hering and hepatic stem cells in humans. Hepatol. 1999;30(6):1425–1433. [DOI] [PubMed] [Google Scholar]
- 28.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms: a comprehensive immunohistochemical formalin-and alcohol-fixed tumors. Am J Clin Path. 1987;88(3):286–296. [DOI] [PubMed] [Google Scholar]
- 30.Moreira RK. Hepatic Stellate Cells and Liver Fibrosis. Arch Pathol Lab Med. 2007;131(11):1728–1734. [DOI] [PubMed] [Google Scholar]
- 31.Theise ND. Gastrointestinal stem cells. III. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G189–G93. [DOI] [PubMed] [Google Scholar]
- 32.Carpino G, Cardinale V, Folseraas T, et al. Hepatic stem/progenitor cell activation differs between primary sclerosing and primary biliary cholangitis. Am J Path. 2018;188(3):627–639. [DOI] [PubMed] [Google Scholar]
- 33.Lanzoni G, Cardinale V, Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatol. 2016;64(1):277–286. [DOI] [PubMed] [Google Scholar]
- 34.Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59(2):247–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.