Abstract
The present experiment aimed to compare toxic effects of dietary organic or inorganic selenium (Se) and to predict of Se intake and tissue Se concentrations in broiler chickens based on feather Se concentrations. A total of four hundred twenty 7-day-old Ross 308 male broiler chicks were allotted to 1 of 7 dietary treatments in a completely randomized design. Each treatment had 6 replicates with 10 birds per replicate. Organic Se (selenium yeast) or inorganic Se (sodium selenite) was added to the basal diet at the levels of 5, 10, or 15 mg/kg Se. All experimental diets were fed to birds on an ad libitum basis for 28 d. Results indicated that a significant interaction (P < 0.01) was observed between Se sources and inclusion levels for the BW gain and feed intake of broiler chickens with inorganic Se at 15 mg/kg in diets showing a greatest negative effect. Increasing inclusion levels of Se in diets increased (linear, P < 0.01) plasma concentrations of uric acid. Increasing inclusion levels of Se in diets increased (linear, P < 0.01) relative liver weight. No significant interactions were observed between Se sources and inclusion levels in diets on hepatic antioxidant capacity. Increasing inclusion levels of Se in diets increased (linear, P < 0.01) Se concentrations in the liver, breast, and feather. The concentrations of Se in the breast, liver, and feather were greater (P < 0.05) for organic Se than for inorganic Se in diets. The toxic levels of organic or inorganic Se in broiler diets were near 7 or 9 mg/kg based on the BW gain, respectively. The prediction equations indicate that feather Se concentrations in broiler chickens can be used to predict both daily Se intake and Se concentrations in the liver and breast.
Key words: broiler chicken, selenium, selenium toxicity, selenium source, tissue selenium concentration
Introduction
Selenium (Se) is an essential trace element that has many vital functions in animals, such as antioxidant defense, immune system, and reproduction (NRC, 1994; Surai, 2002; Kumar and Priyadarsini, 2014). Especially, Se is the essential component of antioxidant enzymes such as glutathione peroxidase, which protects the cell from damages caused by free radicals (Rotruck et al., 1973). Therefore, the symptoms of Se deficiency include the gastrointestinal and thyroid problems, impaired immune response, reduced performance, and increased lipid peroxidation (Surai, 2002; Kumar and Priyadarsini, 2014; Kieliszek and Blazejak, 2016). On the other hand, Se toxicity is characterized by various symptoms such as respiratory disorders, anemia, ataxia, diarrhea, liver cirrhosis, and possibly even death (Mahan and Moxon, 1984; Surai, 2002; Suchy et al., 2014). Therefore, Se concentrations in the diet should be precisely managed to prevent Se deficiency or toxicity in animals.
Dietary Se sources commonly used in the animal industry are categorized into organic and inorganic Se. In the past, inorganic Se such as sodium selenite (Na2SeO3) was the most widely used in animal diets. In 2000, organic Se such as Se-enriched yeast and selenomethionine was approved by US Food and Drug Administration for feed supplements (Federal Register, 2000). Since its legal approval, many animal experiments have been conducted to compare the relative efficacy of organic Se with inorganic Se in animal diets (Payne and Southern, 2005; Yoon et al., 2007; Lu et al., 2018). It has been appreciated that dietary organic Se has greater bioavailability and retention than inorganic Se in animals (Payne and Southern, 2005; Yoon et al., 2007; Li et al., 2018), which has been attributed to the faster and higher absorption of organic Se in the intestine (Robinson and Thomson, 1983; Foster and Sumar, 1995). It is hypothesized, therefore, that a greater efficiency and accumulation rate of organic Se than inorganic Se may result in organic Se exerting more toxic effects on animals. However, it was reported that organic Se was less toxic to pigs because a greater retention of organic Se in the tissues may decrease the amounts of free Se in the plasma, which damages the cell membrane integrity of tissues that are highly sensitive to free Se (Kim and Mahan, 2001). However, although some previous studies have investigated the toxic levels of dietary Se in poultry (Echevarria et al., 1988; Payne et al., 2005; Lu et al., 2019), there is a lack of data regarding comparison of the toxic effects of organic and inorganic Se in broiler diets.
The feather can be a good indicator of heavy metal and trace element concentrations in the body (Couloigner et al., 2015; Kim et al., 2019, 2020). Moreover, the feather of broiler chickens is continuously and simply collected in an animal-friendly method without the requirement for euthanizing birds. Although there are potential benefits, however, experiments predicting Se concentrations in poultry tissues using feather Se concentrations are currently limited.
Therefore, the objectives of the present experiment were to compare toxic effects of organic or inorganic Se in broiler diets and to predict Se intake and tissue Se concentrations in broiler chickens using feather Se concentrations.
Materials and methods
Diets, Animals, and Experimental Design
All experimental procedures were reviewed and approved by the Institutional Animal Care and the Use Committee of Chung-Ang University. A total of six hundred 1-day-old Ross 308 male broiler chicks were obtained from a local commercial hatchery (Dongsan Hatchery, Cheonan, Republic of Korea) and transferred in an environmentally controlled room. Birds were distributed randomly in each cage and were provided with corn–soybean meal–based diets and fresh water for 7 d. After all broiler chickens were weighed again at 7 d of age, 180 birds with extremely high and low BW were removed from the experiment. The remaining 420 birds were allotted to each battery cage (76 cm × 78 cm × 45 cm = width × length × height for each cage) with a similar average BW (initial BW ± SD = 165.3 ± 1.23 g) among cages. All birds were allotted to 1 of 7 dietary treatments with 6 replicates in a completely randomized design. Each replicate consisted of 10 birds. Two galvanized iron troughs (76 cm long × 12 cm wide × 8 cm depth) that hung outside of the battery cage were installed for a feeder and a drinker. A 2-phase feeding program with a grower diet from 7 to 21 d and a finisher diet from 22 to 35 d was used. The experiment was established with 2 different Se sources, including the Se-enriched yeast (1,000 mg/kg Se, Sel-Plex 1000; Alltech, Inc., Nicholasville, KY) or sodium selenite (≥45.6% purity; Sigma-Aldrich Chemical Company, St. Louis, MO). A basal diet (CON) was formulated to meet or exceed the nutritional recommendation of Ross 308 (Aviagen, 2017; Table 1). The analyzed concentrations of Se in the basal diets for growing and finishing broiler chickens were 0.56 and 0.54 mg/kg, respectively (Table 2), which exceeded the current requirements of dietary Se (0.3 mg/kg) in broiler chickens (Aviagen, 2017). Additional 6 diets were prepared by supplementing the basal diet with either organic (selenium yeast) or inorganic Se (sodium selenite) at the inclusion levels of 5, 10, or 15 mg/kg Se in replace of the celite. The inclusion levels of Se were decided based on the previous animal experiments reporting that toxic levels of dietary Se ranged from 5 to 15 mg/kg (Ort and Latshaw, 1978; Kim and Mahan, 2001; Ryu et al., 2005), and therefore, those inclusion levels were used to compare the toxic effects of different sources of dietary Se in the current experiment. The analyzed Se concentrations in the grower and finisher diets were close to the expected levels in diets (Table 2). All diets were in a mash form and were fed to the birds on an ad libitum basis for 28 d. The temperature of the room was maintained at 30°C during the first wk and then gradually decreased to 24°C at the conclusion of the experiment. A 24-h lighting schedule was adopted throughout the entire experiment. The BW gain (BWG) and feed intake (FI) were recorded at the end of the experiment. Mortality was recorded daily. The feed efficiency (FE, g/kg) was calculated by dividing BWG with FI after mortality correction for FI (Kim et al., 2017).
Table 1.
Composition and nutrient content of experimental diets.
| Items | Grower diets (7–21 d) | Finisher diets (22–35 d) |
|---|---|---|
| Ingredients (%) | ||
| Corn | 54.56 | 59.36 |
| Soybean meal (45% CP) | 23.66 | 18.99 |
| Corn gluten meal | 10.40 | 10.00 |
| Tallow | 4.06 | 4.73 |
| Salt | 0.30 | 0.30 |
| Monodicalcium phosphate | 1.71 | 1.50 |
| Limestone | 1.69 | 1.55 |
| Threonine (98.5%) | 0.14 | 0.13 |
| DL-Methionine (88%) | 0.32 | 0.29 |
| Lysine H2SO4 (54%) | 0.76 | 0.75 |
| Choline (50%) | 0.10 | 0.10 |
| Mineral premix1 | 0.10 | 0.10 |
| Vitamin premix2 | 0.10 | 0.10 |
| Sodium bicarbonate | 0.10 | 0.10 |
| Celite3 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Energy and nutrient contents4 | ||
| AMEn (kcal/kg) | 3,113 | 3,206 |
| CP (%) | 21.57 | 19.51 |
| Lysine (%) | 1.29 | 1.16 |
| Methionine + Cysteine (%) | 1.01 | 0.93 |
| Calcium (%) | 1.00 | 0.90 |
| Available phosphorus (%) | 0.45 | 0.40 |
Provided per kilogram of the complete diet: Zn (as ZnO), 100 mg; Mn (as MnSO2·H2O), 120 mg; Fe (as FeSO4·7H2O), 60 mg; Cu (as CuSO4·5H2O), 16 mg; Co (as CoCO3), 1,000 μg; I (as Ca(IO3)2·H2O), 1.25 mg; Se (as Na2SeO3), 300 μg.
Provided per kilogram of the complete diet: vitamin A (from vitamin A acetate), 13,000 IU; vitamin D3, 5,000 IU; vitamin E (from DL-α-tocopheryl acetate), 80 IU; vitamin K3, 4 mg; vitamin B1, 4 mg; vitamin B2, 10 mg; vitamin B6, 6 mg; vitamin B12, 20 μg; calcium pantothenate, 20 mg; folic acid, 2 mg; biotin, 200 μg; niacin, 60 mg.
Additional organic (selenium yeast) or inorganic Se (sodium selenite) was supplemented to the basal diets at the inclusion levels of 5, 10, or 15 mg/kg Se in replace of the celite.
Calculated values from Aviagen (2017).
Table 2.
Analyzed selenium (Se) concentrations in experimental diets.
| Treatments | Added Se (mg/kg) | Analyzed Se concentrations (mg/kg) |
|
|---|---|---|---|
| Grower diets | Finisher diets | ||
| CON1 | 0 | 0.56 | 0.54 |
| Organic Se | 5 | 5.10 | 5.42 |
| 10 | 10.35 | 9.65 | |
| 15 | 15.63 | 14.44 | |
| Inorganic Se | 5 | 4.87 | 5.63 |
| 10 | 10.97 | 10.04 | |
| 15 | 15.47 | 14.89 | |
CON: control diet (basal diet).
Sample Collection and Chemical Analysis
At the conclusion of the experiment, 1 bird per replicate with a BW near the replicate mean BW (i.e., 6 birds per treatment) was selected and weighed from each cage. The selected birds were euthanized by CO2 inhalation and then immediately dissected to collect samples. Blood samples were immediately extracted from each bird via heart punctures into a 10-mL sodium heparin tube (Becton Dickinson and Co, Franklin Lakes, NJ) and then immediately centrifuged at 3,000 × g at 4°C for 20 min to collect the plasma. The supernatant was obtained in a 1.7-mL microtube (Axygen, Union City, CA) and then stored in the refrigerator at −20°C before analysis. The concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and uric acid in the plasma were analyzed using a Hitachi Automatic Analyzer 7020 (Hitachi Ltd., Tokyo, Japan). After blood collection, the organ samples such as the liver, spleen, kidney, bursa of Fabricius, and thymus were individually weighed. Relative organ weight was expressed as a percentage of BW.
The liver samples were collected in a 1.7-mL microtube (Axygen, Union City, CA), immediately frozen in liquid nitrogen, and then stored at −80°C before the analysis for the glutathione peroxidase (GSH-Px), catalase, superoxide dismutase, malondialdehyde, and reactive oxygen species (ROS) concentrations. The protein concentrations in the liver, which were required for calculating antioxidant capacity, were determined using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL) as per the instructions. Absorbance at 562 nm was detected using a microplate reader (SpectraMax 190; Molecular Devices Corp., Sunnyvale, CA). The concentrations of GSH-Px in the liver were measured to confirm antioxidant capacity. Briefly, the liver samples were weighed as approximately 0.1 g in a 5-mL microcentrifuge tube (Chem Bio, Seoul, Republic of Korea) and added 200 μL of cold GSH-Px assay buffer. The samples were homogenized in ice using an electric homogenizer (Tissue tearor; Biospec Products, Inc., Bartlesville, OK). Homogenized liver samples were centrifuged at 13,000 × g and 4°C for 15 min. The supernatants were collected into a 1.7-mL microtube and used to measure the concentrations of GSH-Px in the liver using a GSH-Px assay kit (Abcam, Cambridge, UK) as per the manufacturer's instructions. Absorbance was read on a microplate reader at 340 nm. The concentrations of catalase in the liver were measured to determine the antioxidant capacity. In short, the liver samples were weighed as 0.5 g in a 5-mL microcentrifuge tube and added 2.5 mL cold PBS containing 1 mmol EDTA, and homogenized in ice. The homogenates were centrifuged at 13,000 × g and 4°C for 15 min. The supernatants were collected and tested for the concentrations of catalase in the liver using an OxiSelect Catalase Activity Assay Kit (Cell Biolabs, San Diego, CA) as per the manufactures' instructions. The absorbance was read using a microplate reader at 520 nm. The concentrations of superoxide dismutase in the liver were measured to determine the antioxidant capacity. Namely, 0.5 g of liver samples were weighed and placed into a 5-mL microcentrifuge tube and homogenized in ice with 2.5 mL of cold 1X lysis buffer (10 mmol Tris, pH 7.5; 50 mmol NaCl, 0.1 mmol EDTA). Tris and sodium chloride were purchased from Sigma-Aldrich (St. Louis, MO). Afterward, the homogenate was transferred in a 1.7-mL microtube and centrifuged at 13,000 × g and 4°C for 15 min. The supernatants were collected in a 1.7-mL microtube and used to determine the concentrations of superoxide dismutase in the liver using an OxiSelect superoxide dismutase activity assay kit (Cell Biolabs, San Diego, CA) as per the manufacturer's instructions. The absorbance of supernatant was measured at 490 nm with a microplate reader. The concentrations of malondialdehyde in the liver were measured as an index of lipid peroxidation. Briefly, the liver samples were weighed approximately 0.1 g in a 5-mL microcentrifuge tube and suspended in 2.0 mL ice-cold PBS containing 0.05% butylated hydroxytoluene. The liver samples were then homogenized in ice at the low speed. Homogenized liver samples were centrifuged at 13,000 × g and 4°C for 15 min. The supernatants were collected into a 1.7-mL microtube and used to determine the concentrations of malondialdehyde in the liver using an OxiSelect TBARS Assay Kit (Cell Biolabs, San Diego, CA) as per the manufacturer's instructions. Absorbance was measured using a microplate reader at 532 nm. The concentrations of ROS in the liver were measured as an index of oxidative stress. Namely, the liver samples were weighed as approximately 0.5 g in a 5-mL microcentrifuge tube and suspended in 2.5 mL PBS and homogenized in ice. The homogenates were centrifuged at 13,000 × g and 4°C for 15 min, and then, the supernatants were collected. The concentrations of ROS in the liver were determined using an OxiSelect In Vitro ROS/RNS Assay kit (Cell Biolabs, San Diego, CA) as per the manufacturer's instructions. Fluorescence was measured using a fluorescence microplate reader (Nikon TS-1000; Nikon, Tokyo, Japan) at 480 nm (excitation wavelengths) and 530 nm (emission wavelengths). The results were expressed relative to the fluorescence intensity of the CON group.
The breast, liver, and feather samples were collected to analyze Se concentrations in the tissues and stored in the refrigerator at −20°C for further analysis. The tissue samples were weighed and digested to analyze Se concentrations in the tissues as per the method described by Son et al. (2018) with slight modifications (Kim et al., 2016). In short, approximately 1 g of each liver, breast, and feather sample was weighed and then was added along with 10 mL of HNO3 and 5 mL of HClO4 into a 500-mL Kjeldahl flask to digest the liver, breast, and feather samples. The machine was switched off when the samples became colorless. After the digestion was finished, the Kjeldahl flasks were maintained to cool for a few min at the room temperature. The digested solutions were filtered using a Whatman filter paper (Whatman Grade 42; Whatman International Ltd., Maidstone, England). The filtrates were used to determine Se concentrations using an inductively coupled plasma spectrometer (Optima 5300 DV; Perkin Elmer Inc., Shelton, CT).
Statistical Analysis
Statistical analysis was performed using the PROC MIXED procedure of SAS (SAS Institute Inc., Cary, NC). All data were analyzed using a completely randomized design. Each replicate was considered as the experimental unit for all analyses. Outlier data were checked using the UNIVARIATE procedure of SAS (Steel et al., 1997). The LSMEANS procedure was used to calculate treatment means, and the PDIFF option of SAS was used to separate the means if the difference was significant. The statistical model included Se sources, inclusion levels in diets, and their interactions. Orthogonal polynomial contrast tests were also performed to verify the linear and quadratic effect of increasing inclusion levels of organic and inorganic Se in diets (Seo et al., 2018). The one-slope broken-line (Robbins et al., 2006; Alhotan et al., 2017) was performed to predict the toxic level of Se in diets based on BWG using the nonlinear regression (NLIN) procedure of SAS (SAS Institute Inc., Cary, NC). The one-slope broken-line model was used as Y = L + U × (X–R), where L is the asymptote, U is the slope, X is organic and inorganic Se concentrations in diets, and R is the breakpoint × value. In addition, a regression analysis was performed to predict daily Se intake and Se concentrations in the liver and breast from Se concentrations in the feather. The results of daily Se intake and Se tissues concentrations between Se sources were combined because there were no significant interactions between Se sources and inclusion levels in diets on daily Se intake and Se concentrations in the tissues. Significance for statistical tests was set at P < 0.05.
Results
Growth Performance
There was a significant interaction (P < 0.01) between Se sources and inclusion levels in diets for the BWG and FI of broiler chickens (Table 3). A greater reduction in BWG and FI for birds fed diets containing 15 mg/kg inorganic Se than for those fed diets containing 15 mg/kg organic Se led to the significant interaction. Different Se sources did not affect BWG, FI, and FE, whereas increasing inclusion level of Se in diets, regardless of Se sources, decreased BWG, FI (linear and quadratic, P < 0.01), and FE (linear, P < 0.01). Birds fed diets containing 15 mg/kg Se had less (P < 0.05) FI than those fed diets containing 5 or 10 mg/kg Se. The BWG and FE for birds fed diets containing 15 mg/kg Se were less (P < 0.05) than for those fed diets containing 10 mg/kg Se, which were less (P < 0.05) than those fed diets containing 5 mg/kg Se. In comparison of 7 dietary treatments, birds fed diets containing 10 or 15 mg/kg organic Se had less (P < 0.05) BWG and FI than birds fed the CON diet. However, this reduction in BWG and FI was only observed for inorganic Se at the level of 15 mg/kg in diets. However, birds fed diets containing 15 mg/kg inorganic Se had less (P < 0.05) BWG and FI than those fed diets containing 15 mg/kg organic Se. Birds fed diets containing 15 mg/kg organic or inorganic Se had less (P < 0.05) FE than birds fed the CON diet.
Table 3.
Effect of selenium (Se) sources and inclusion levels in diets on growth performance of broiler chickens.
| Items | Added Se (mg/kg) | Growth performance |
||
|---|---|---|---|---|
| BWG1 (g) | FI1 (g) | FE1 (g/kg) | ||
| CON1 | 0 | 1,746a | 2,656a | 658a,b |
| Organic Se | 5 | 1,673a,b | 2,505a,b | 670a |
| 10 | 1,538b | 2,442b | 628b | |
| 15 | 1,280c | 2,182c | 587c | |
| Inorganic Se | 5 | 1,715a | 2,639a | 650a,b |
| 10 | 1,625a,b | 2,553a,b | 635b | |
| 15 | 1,032d | 1,859d | 555c | |
| SEM (n = 6) | 52.7 | 65.6 | 11.1 | |
| Main effect | ||||
| Se source | ||||
| Organic Se | 1,497 | 2,376 | 628 | |
| Inorganic Se | 1,457 | 2,350 | 613 | |
| SEM (n = 18) | 31.3 | 38.7 | 6.3 | |
| Se level | ||||
| 5 | 1,694a | 2,572a | 660a | |
| 10 | 1,581b | 2,497a | 632b | |
| 15 | 1,156c | 2,020b | 571c | |
| SEM (n = 12) | 38.3 | 47.4 | 7.7 | |
| P-values | ||||
| 1-way ANOVA | <0.01 | <0.01 | <0.01 | |
| 2-way ANOVA | ||||
| Se source | 0.38 | 0.63 | 0.10 | |
| Se level | <0.01 | <0.01 | <0.01 | |
| Source × level | <0.01 | <0.01 | 0.21 | |
| Contrast | ||||
| Se level (linear) | <0.01 | <0.01 | <0.01 | |
| Se level (quadratic) | <0.01 | <0.01 | 0.10 | |
a–dMeans with different superscripts within a column differ (P < 0.05).
Abbreviations: BWG, BW gain; CON, control diet (basal diet); FI, feed intake; FE, feed efficiency (BWG:FI, g/kg).
The toxic levels of each Se source in broiler diets were calculated using the one-slope broken-line model based on their BWG (Figure 1 and Figure 2). The equations of one-slope broken-line analysis of BWG at different inclusion levels of organic or inorganic Se in diets were Y = 1,709–51.57 × (X–6.68) with a R2 of 0.979 or Y = 1,730–118.60 × (X–9.11) with a R2 of 0.999, respectively. Result indicated that the toxic levels of organic and inorganic Se in diets were approximately 7 and 9 mg/kg, respectively.
Figure 1.
The one-slope broken-line analysis of BW gain (BWG) at different dietary organic selenium (Se) levels. The toxic level of organic Se in broiler diets was predicted to be 7 mg/kg.
Figure 2.
The one-slope broken-line analysis of BW gain (BWG) at different dietary inorganic selenium (Se) levels. The toxic level of inorganic Se in broiler diets was predicted to be 9 mg/kg.
Plasma Measurements
No significant interactions were observed between Se sources and inclusion levels in diets on plasma measurements (Table 4). The Se sources did not affect plasma measurements. However, increasing inclusion levels of Se in diets increased (linear, P < 0.01) plasma concentrations of uric acid. There was a quadratic association (P < 0.01) between inclusion levels of Se in diets and plasma concentrations of AST. Birds fed diets containing 10 mg/kg Se had less (P < 0.05) plasma concentrations of AST than those fed other diets. The plasma concentrations of uric acid for birds fed diets containing 15 mg/kg Se were greater (P < 0.05) than for those fed other diets. In comparison of 7 dietary treatments, plasma concentrations of ALT were the least (P < 0.05) for birds fed the CON diet, whereas those of ALT were greatest (P < 0.05) for birds fed 15 mg/kg organic Se. The plasma concentrations of uric acid were greater (P < 0.05) for birds fed diets containing 15 mg/kg inorganic Se than those fed the CON diet.
Table 4.
Effect of selenium (Se) sources and inclusion levels in diets on plasma measurements of broiler chickens.
| Items | Added Se (mg/kg) | Plasma measurements |
|||
|---|---|---|---|---|---|
| ALT1 (U/L) | AST1 (U/L) | Creatinine (mg/dL) | Uric acid (mg/dL) | ||
| CON1 | 0 | 2.9c | 270 | 0.14 | 6.4b |
| Organic Se | 5 | 4.1a,b | 261 | 0.16 | 7.4b |
| 10 | 3.5b,c | 225 | 0.17 | 8.0b | |
| 15 | 4.7a | 272 | 0.17 | 9.7a,b | |
| Inorganic Se | 5 | 3.3b,c | 261 | 0.18 | 7.7b |
| 10 | 4.0a,b | 218 | 0.16 | 7.1b | |
| 15 | 3.5b,c | 281 | 0.17 | 12.7a | |
| SEM (n = 6) | 0.35 | 21.0 | 0.013 | 1.37 | |
| Main effect | |||||
| Se source | |||||
| Organic Se | 4.1 | 253 | 0.16 | 8.3 | |
| Inorganic Se | 3.6 | 254 | 0.17 | 9.2 | |
| SEM (n = 18) | 0.21 | 11.0 | 0.008 | 0.72 | |
| Se level | |||||
| 5 | 3.7 | 261a | 0.17 | 7.5b | |
| 10 | 3.7 | 222b | 0.16 | 7.6b | |
| 15 | 4.1 | 276a | 0.17 | 11.2a | |
| SEM (n = 12) | 0.26 | 13.7 | 0.010 | 0.89 | |
| P-values | |||||
| 1-way ANOVA | 0.03 | 0.19 | 0.51 | 0.02 | |
| 2-way ANOVA | |||||
| Se source | 0.11 | 0.94 | 0.77 | 0.41 | |
| Se level | 0.48 | 0.02 | 0.76 | <0.01 | |
| Source × level | 0.08 | 0.91 | 0.51 | 0.26 | |
| Contrast | |||||
| Se level (linear) | 0.30 | 0.41 | 0.75 | <0.01 | |
| Se level (quadratic) | 0.67 | <0.01 | 0.49 | 0.10 | |
a–cMeans within a variable with no common superscript differ significantly (P < 0.05).
Abbreviations: CON, control diet (basal diet); ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Relative Organ Weight
No significant interactions were observed between Se sources and inclusion levels in diets for relative organ weight (Table 5). Different Se sources in diets did not influence relative organ weight. However, increasing inclusion levels of Se in diets decreased (linear, P < 0.05) relative bursa of Fabricius and thymus weights. Increasing inclusion levels of Se in diets increased (linear, P < 0.01) relative liver weight. The birds fed diets containing 15 mg/kg Se had less (P < 0.05) relative thymus weight than those fed diets containing other diets. The relative liver weight for birds fed diets containing 15 mg/kg Se were greater (P < 0.05) than for those fed diets containing 10 mg/kg Se, which were greater (P < 0.05) than those fed diets containing 5 mg/kg Se. In comparison of 7 dietary treatments, birds fed diets containing 15 mg/kg organic or inorganic Se had greater (P < 0.05) relative liver weight than birds fed the CON diet. Birds fed diets containing 15 mg/kg organic Se had greater (P < 0.05) relative liver weight than those fed diets containing 15 mg/kg inorganic Se.
Table 5.
Effect of selenium (Se) sources and inclusion levels in diets on relative organ weight of broiler chickens.
| Items | Added Se (mg/kg) | Relative organ weight (%) |
||||
|---|---|---|---|---|---|---|
| Liver1 | Spleen1 | Kidney1 | BF1,2 | Thymus1 | ||
| CON2 | 0 | 2.7c,d,e | 0.14 | 0.23 | 0.22 | 0.19 |
| Organic Se | 5 | 2.4e | 0.15 | 0.19 | 0.23 | 0.18 |
| 10 | 2.9c,d | 0.13 | 0.19 | 0.20 | 0.21 | |
| 15 | 3.7a | 0.13 | 0.21 | 0.18 | 0.16 | |
| Inorganic Se | 5 | 2.5d,e | 0.13 | 0.20 | 0.24 | 0.18 |
| 10 | 3.1b,c | 0.14 | 0.18 | 0.22 | 0.18 | |
| 15 | 3.3b | 0.10 | 0.19 | 0.19 | 0.10 | |
| SEM (n = 6) | 0.14 | 0.014 | 0.022 | 0.023 | 0.030 | |
| Main effect | ||||||
| Se source | ||||||
| Organic Se | 3.0 | 0.14 | 0.19 | 0.20 | 0.18 | |
| Inorganic Se | 3.0 | 0.12 | 0.19 | 0.22 | 0.16 | |
| SEM (n = 18) | 0.08 | 0.008 | 0.012 | 0.013 | 0.015 | |
| Se level | ||||||
| 5 | 2.5c | 0.14 | 0.19 | 0.24 | 0.18a | |
| 10 | 3.0b | 0.13 | 0.18 | 0.21 | 0.19a | |
| 15 | 3.5a | 0.12 | 0.20 | 0.18 | 0.13b | |
| SEM (n = 12) | 0.10 | 0.010 | 0.015 | 0.016 | 0.018 | |
| P-values | ||||||
| 1-way ANOVA | <0.01 | 0.44 | 0.70 | 0.38 | 0.24 | |
| 2-way ANOVA | ||||||
| Se source | 0.64 | 0.19 | 0.91 | 0.48 | 0.18 | |
| Se level | <0.01 | 0.30 | 0.66 | 0.08 | 0.03 | |
| Source × level | 0.09 | 0.42 | 0.74 | 0.98 | 0.48 | |
| Contrast | ||||||
| Se level (linear) | <0.01 | 0.16 | 0.63 | 0.02 | 0.04 | |
| Se level (quadratic) | 0.92 | 0.70 | 0.42 | 0.97 | 0.10 | |
a–eMeans with different superscripts within a column differ (P < 0.05).
Relative organ weight was expressed as a percentage of BW.
Abbreviations: CON, control diet (basal diet); BF, bursa of Fabricius.
Antioxidant Capacity in the Liver
No significant interactions were observed between Se sources and inclusion levels in diets on antioxidant capacity (Table 6). Different Se sources and inclusion levels in diets did not influence antioxidant capacity in the liver. There were no linear and quadratic effects of increasing inclusion levels of Se in diets on antioxidant capacity in the liver of broiler chickens. There were no significant differences in antioxidant capacity in the liver among Se treatment groups with the CON group.
Table 6.
Effect of selenium (Se) sources and inclusion levels in diets on antioxidant capacity in the liver of broiler chickens.
| Items | Added Se (mg/kg) | Antioxidant capacity in the liver |
||||
|---|---|---|---|---|---|---|
| GSH-Px1 (nmol/mg protein) | CAT1 (U/mg protein) | SOD1 (U/mg protein) | MDA1 (μm/mg protein) | ROS1 (% of CON) | ||
| CON1 | 0 | 20.3 | 5.46 | 31.9 | 1.04 | 1.00 |
| Organic Se | 5 | 21.4 | 5.57 | 30.2 | 1.22 | 1.62 |
| 10 | 21.7 | 5.52 | 39.0 | 1.28 | 1.87 | |
| 15 | 21.8 | 5.41 | 37.5 | 1.30 | 1.91 | |
| Inorganic Se | 5 | 19.8 | 5.53 | 31.3 | 1.08 | 1.09 |
| 10 | 19.7 | 5.38 | 37.3 | 1.18 | 1.67 | |
| 15 | 20.3 | 5.59 | 35.9 | 1.19 | 1.62 | |
| SEM (n = 6) | 2.63 | 0.079 | 4.61 | 0.107 | 0.314 | |
| Main effect | ||||||
| Se source | ||||||
| Organic Se | 21.6 | 5.50 | 35.6 | 1.27 | 1.80 | |
| Inorganic Se | 19.9 | 5.50 | 34.8 | 1.15 | 1.46 | |
| SEM (n = 18) | 1.42 | 0.041 | 2.14 | 0.064 | 0.172 | |
| Se level | ||||||
| 5 | 20.6 | 5.55 | 30.7 | 1.15 | 1.35 | |
| 10 | 20.7 | 5.45 | 38.2 | 1.23 | 1.77 | |
| 15 | 21.1 | 5.50 | 36.7 | 1.25 | 1.77 | |
| SEM (n = 12) | 1.74 | 0.052 | 2.82 | 0.079 | 0.219 | |
| P-values | ||||||
| 1-way ANOVA | 0.99 | 0.33 | 0.60 | 0.57 | 0.12 | |
| 2-way ANOVA | ||||||
| Se source | 0.40 | 0.97 | 0.81 | 0.22 | 0.16 | |
| Se level | 0.98 | 0.42 | 0.12 | 0.66 | 0.25 | |
| Source × level | 0.99 | 0.09 | 0.91 | 0.99 | 0.83 | |
| Contrast | ||||||
| Se level (linear) | 0.84 | 0.51 | 0.08 | 0.39 | 0.19 | |
| Se level (quadratic) | 0.95 | 0.23 | 0.18 | 0.75 | 0.35 | |
Abbreviations: CAT, catalase; CON, control diet (basal diet); GSH-Px, glutathione peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species; SOD, superoxide dismutase.
Selenium Concentrations in the Tissue
There were no significant interactions between Se sources and inclusion levels in diets on Se concentrations in the breast, liver, and feather (Table 7). The Se concentrations in the tissues for birds fed diets containing organic Se were greater (P < 0.05) than for those fed diets containing inorganic Se. Increasing inclusion levels of Se in diets increased (linear, P < 0.01) Se concentrations in the breast, liver, and feather. The Se concentrations in the liver and feather for birds fed diets containing 15 mg/kg Se were greater (P < 0.05) than for those fed diets containing 10 mg/kg Se, which were greater (P < 0.05) than those fed diets containing 5 mg/kg Se. In addition, birds fed diets containing 15 mg/kg Se had greater (P < 0.05) Se concentrations in the breast than those fed diets containing 5 mg/kg Se. In comparison of 7 dietary treatments, birds fed diets containing more than 10 mg/kg organic or inorganic Se had greater (P < 0.05) Se concentrations in the breast than those fed the CON diet. However, birds fed diets containing more than 5 mg/kg organic or inorganic Se had greater (P < 0.05) Se concentrations in the liver and feather than those fed the CON diet.
Table 7.
Effect of selenium (Se) sources and inclusion levels in diets on Se concentrations in the tissues of broiler chickens.
| Items | Added Se (mg/kg) | Se concentrations in the tissues (mg/kg) |
||
|---|---|---|---|---|
| Breast | Liver | Feather | ||
| CON1 | 0 | 0.25d | 0.32d | 0.67d |
| Organic Se | 5 | 0.51b,c,d | 0.63c | 1.81c |
| 10 | 0.67a,b | 0.85b | 2.68b | |
| 15 | 0.91a | 1.18a | 4.32a | |
| Inorganic Se | 5 | 0.32c,d | 0.57c | 1.57c |
| 10 | 0.58b,c | 0.81b | 2.32b,c | |
| 15 | 0.65a,b | 0.93b | 3.09b | |
| SEM (n = 6) | 0.010 | 0.054 | 0.296 | |
| Main effect | ||||
| Se source | ||||
| Organic Se | 0.70 | 0.89 | 2.94 | |
| Inorganic Se | 0.52 | 0.77 | 2.32 | |
| SEM (n = 18) | 0.061 | 0.034 | 0.183 | |
| Se level | ||||
| 5 | 0.42b | 0.60c | 1.69c | |
| 10 | 0.63a,b | 0.83b | 2.50b | |
| 15 | 0.78a | 1.06a | 3.70a | |
| SEM (n = 12) | 0.075 | 0.041 | 0.225 | |
| P-values | ||||
| 1-way ANOVA | <0.01 | <0.01 | <0.01 | |
| 2-way ANOVA | ||||
| Se source | <0.05 | 0.02 | 0.02 | |
| Se level | <0.01 | <0.01 | <0.01 | |
| Source × level | 0.73 | 0.17 | 0.25 | |
| Contrast | ||||
| Se level (linear) | <0.01 | <0.01 | <0.01 | |
| Se level (quadratic) | 0.78 | 0.94 | 0.50 | |
a–eMeans with different superscripts within a column differ (P < 0.05).
CON: control diet (basal diet).
Prediction for Daily Se Intake and Tissue Se Concentrations From Feather Se Concentrations
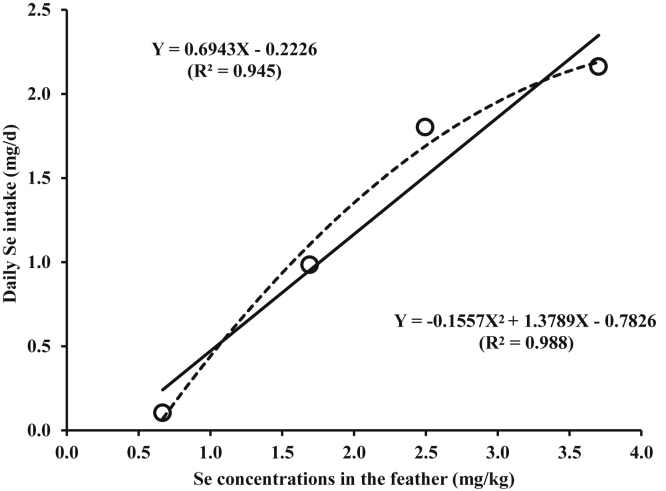
A regression analysis was performed to confirm prediction equations for daily Se intake and Se concentrations in the liver and breast from Se concentrations in the feather. However, there were no significant interactions between Se sources and inclusion levels in diets on daily Se intake and tissue Se concentrations. Thus, the regression analysis was performed with combining data from both organic Se and inorganic Se. The regression analysis was carried out with Se concentrations in the feather ranging from 0.67 to 3.70 mg/kg as x variables, whereas daily Se intake ranging from 0.10 to 2.16 mg/day (Figure 3), Se concentrations in the liver from 0.32 to 1.06 mg/kg (Figure 4), and Se concentrations in the breast from 0.24 to 0.78 mg/kg (Figure 5) as y variables. The resulting equations were Y = 0.6943X–0.2226 (R2 = 0.945) and Y = −0.1557X2 + 1.3789X–0.7826 (R2 = 0.998) for daily Se intake (Figure 3), Y = 0.2439X + 0.1808 (R2 = 0.989) and Y = −0.0244X2 + 0.3513X + 0.093 (R2 = 0.998) for Se concentrations in the liver (Figure 4), and Y = 0.1828X + 0.1263 (R2 = 0.984) and Y = −0.0123X2 + 0.2371X + 0.082 (R2 = 0.988) for Se concentrations in the breast (Figure 5).
Figure 3.
Linear and quadratic regression analyses for estimating selenium (Se) intake (mg/day) from Se concentrations in the feather (mg/kg).
Figure 4.
Linear and quadratic regression analyses for estimating selenium (Se) concentrations in the liver (mg/kg) from Se concentrations in the feather (mg/kg).
Figure 5.
Linear and quadratic regression analyses for estimating selenium (Se) concentrations in the breast (mg/kg) from Se concentrations in the feather (mg/kg).
Discussion
The toxic effects of Se were observed for 10 mg/kg organic Se but for 15 mg/kg inorganic Se based on BWG and FI, which may indicate that toxic level of Se in diets may be less for inorganic Se than for organic Se. However, a greater reduction in BWG and FI was observed for birds fed diets containing 15 mg/kg inorganic Se than for those fed diets containing 15 mg/kg organic Se. This result may indicate that the severity for toxic effects of Se (i.e., toxicity) at the high inclusion level in diets may be observed more prominently for inorganic Se than for organic Se. Accordingly, it is likely suggested that the toxic level and toxicity of Se depend on Se sources in diets. However, most of the previous studies have compared Se sources with low inclusion levels in broiler diets (e.g., 0.15 to 0.50 mg/kg), which are close or less than the recommended levels of the FDA (2017), European Commission (2019), and NRC (1994). Therefore, the direct comparison of our data with those from the previous experiments is difficult. Although there is a lack of poultry experiments regarding high inclusion levels of Se in diets, some pig experiments have been conducted with the similar objective of the present experiment. Kim and Mahan (2001) reported that increasing inclusion levels of organic or inorganic Se in diets from 0 to 20 mg/kg decreased the BW of growing–finishing pigs and suggested that inorganic Se would be more toxic to pigs than organic Se when diets contained more than 15 mg/kg Se. This finding is in accordance with our observation regarding toxic effects of diets containing 15 mg/kg inorganic Se on broiler chickens.
It should be noted, however, that the statistical analysis used in the previous experiments has a limitation with respect to establishing a toxic level of Se between Se sources because the analysis was based primarily on multiple comparisons among established Se levels within each Se source in diets. For this reason, the toxic level of Se in broiler diets may vary depending on the experimental design (i.e., Se sources and dietary Se levels). To overcome this limitation, therefore, the one-slope broken-line analysis was adopted in the current experiment to predict the approximate toxic levels of Se sources in broiler diets (Alhotan et al., 2017). We determined the toxic levels of organic or inorganic Se as being approximately 7 or 9 mg/kg Se in broiler diets, respectively, when the BWG of broiler chickens was the response variable. This result may indicate that the toxic level of Se in broiler diets is close between organic and inorganic Se.
The plasma concentrations of ALT are commonly used as an indicator of liver functions (Lu et al., 2014). In the current experiment, birds fed diets containing supplemental Se exhibited greater plasma concentrations of ALT than those fed the CON diet. This observation may indicate decreased liver functions when broiler chickens were fed diets containing more than 5 mg/kg Se, regardless of Se sources (Grunkemeyer, 2010). In addition, the current experiment also revealed that increasing inclusion levels of Se in diets linearly increased the relative liver weight of broiler chickens, which may indicate toxic effects of Se on the liver of broiler chickens. Similarly, broiler chickens fed diets containing 15 mg/kg Se, regardless of Se sources had increased plasma concentrations of uric acid than those fed diets containing 5 or 10 mg/kg Se. The plasma concentrations of uric acid have been widely applied as an indicator for kidney functions (Kanbay et al., 2010), indicating impaired renal function when broiler chickens were fed diets containing more than 15 mg/kg Se.
Increasing inclusion levels of Se in diets linearly decreased the relative weights of the bursa of Fabricius in broiler chickens. The bursa of Fabricius is a lymphoid organ that plays an important role in avian immune systems (Schat et al., 2014). The relative weights of bursa of Fabricius are often used as an indicator of the immune response and functionality in broiler chickens (Al-Khalifa et al., 2012). In the agreement with our finding, Peng et al. (2009) reported that 35-day-old broiler chickens fed diets containing 15 mg/kg Se had less relative weights of the bursa of Fabricius than those fed the CON diet. In addition, the relative weights of the thymus, which is another important lymphoid organs, were also decreased with increasing inclusion levels of Se in diets with broiler chickens fed diets containing 15 mg/kg Se showing the least relative weights of the thymus. Therefore, our results may indicate that extremely high inclusion levels of Se in diets, regardless of Se sources, may impair immune systems in broiler chickens.
As expected, increasing inclusion levels of Se in diets increased Se concentrations in the breast, liver, and feather. In addition, feeding diets containing organic Se to broiler chickens had greater Se concentrations in the breast, liver, and feather than feeding diets containing inorganic Se to broiler chickens in the current experiment. However, the interactions were not significant between Se sources and inclusion levels in diets. Kim and Mahan (2001) demonstrated that increasing inclusion levels of Se in diets increased Se concentrations in the loin muscles and liver of growing–finishing pigs. Moreover, pigs fed diets containing organic Se had greater Se concentrations in the loin muscle and liver compared with those fed diets containing inorganic Se. Payne and Southern (2005) also reported that organic Se was deposited more effectively in breast muscles of broiler chickens compared with inorganic Se. It is suggested that the organic Se enters in the specific Se pathway during turnover of the methionine pool and is consistently reabsorbed to the metabolic Se pool (Foster and Sumar, 1997; Burk and Hill, 2015). In contrast, inorganic Se directly enters and is used in the synthesis of selenoproteins (Foster and Sumar, 1997). Therefore, it is appreciated that organic Se can retain in the tissue more efficiently than inorganic Se, which was confirmed by our findings. Our results further suggest greater retention efficiency for organic Se than for inorganic Se even at very high inclusion level of Se in diets.
Among collected tissues, Se concentrations in the feather were greater than those in the breast and liver, regardless of Se sources and inclusion levels. The reason for this observation may be owing to the relatively lower tissue turnover rate of the feather than the breast and liver. Our previous experiments also reported the greater responsiveness of the feather to other dietary trace minerals such as Pb and Hg than the breast and liver (Kim et al., 2019, 2020), which suggests that the feather can serve as the potential tissue of broiler chickens for accessing the effects of varying levels of dietary heavy metals with respect to their body retention (Kim et al., 2019, 2020). Moreover, the feather can be easily and continuously sampled without the sacrifice of chickens, and thus, the concentrations of heavy metals in the feather may be used to investigate the status of heavy metals in other tissues of broiler chickens.
As a consequence, linear and quadratic regression analyses were performed to generate prediction equations for daily Se intake and Se concentrations in the liver and breast from Se concentrations in the feather. Both linear and quadratic regressions for predicting Se concentrations in the liver (R2 = 0.989 and 0.998) from Se concentrations in the feather were significant with very high R2 values. Furthermore, it was difficult to determine better fit the data for either linear or quadratic regressions when 2 regressions were significant with high R2 values and had similar visual closeness. Based on simplicity and adoptability, however, it may be inferred that the linear equation is more suitable compared with the quadratic equation (Dilger and Adeola, 2006; Kim et al., 2019). On the other hand, only linear regressions for predicting daily Se intake and Se concentrations in the breast from Se concentrations in the feather were significant with high R2 values (R2 = 0.945 and 0.984, respectively) although quadratic regressions for daily Se intake and Se concentrations in the breast showed higher R2 values than linear regressions. Therefore, it can be concluded that Se concentrations in the feather can be used to predict daily Se intake and Se concentrations in the breast and liver using a linear regression equation. However, it should be noted that the current prediction equations were generated from very high and wide inclusion levels of Se in diets, which greatly exceeded the practical concentrations of Se in broiler diets (e.g., less than 1 mg/kg). Therefore, our equations may have the limitation to predict daily Se intake and Se concentrations in the liver and breast from Se concentrations in the feather when broiler chickens were fed diets containing the commercial level of Se. As a consequence, to improve the predictability for a commercial feeding situation, further researches conducting with varying inclusion levels near to practical concentrations of Se in broiler diets are required.
Conclusion
The toxic levels of Se in broiler diets are similar for organic and inorganic Se based on the one-slope broken-line analysis. Organic Se can be retained more efficiently in the poultry body than inorganic Se. The feather is the most responsive tissue to dietary Se concentrations and is preferable for identifying the Se status in broiler chickens. The daily Se intake and Se concentrations in the liver and breast of broiler chickens can be properly predicted based on Se concentrations in the feather.
Acknowledgments
This research was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01093204), Rural Development Administration, Republic of Korea.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Alhotan R.A., Vedenov D.V., Pesti G.M. Estimation of the maximum safe level of feed ingredients by spline or broken-line nonlinear regression models. Poult. Sci. 2017;96:904–913. doi: 10.3382/ps/pew317. [DOI] [PubMed] [Google Scholar]
- Al-Khalifa H., Givens D.I., Rymer C., Yaqoob P. Effect of n-3 fatty acids on immune function in broiler chickens. Poult. Sci. 2012;91:74–88. doi: 10.3382/ps.2011-01693. [DOI] [PubMed] [Google Scholar]
- Aviagen . Ross Breeders Limited; Newbridge, Midlothian, Scotland, UK: 2017. Ross 308 AP Broiler: Nutrition Specifications. [Google Scholar]
- Burk R.F., Hill K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015;35:109–134. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- Couloigner F., Jlali M., Briens M., Rouffineau F., Geraert P., Mercier Y. Selenium deposition kinetics of different selenium sources in muscle and feathers of broilers. Poult. Sci. 2015;94:2708–2714. doi: 10.3382/ps/pev282. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Adeola O. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing pigs fed conventional and low-phytate soybean meals. J. Anim. Sci. 2006;84:627–634. doi: 10.2527/2006.843627x. [DOI] [PubMed] [Google Scholar]
- Echevarria M.G., Henry P.R., Ammerman C.B., Rao P.V., Miles R.D. Estimation of the relative bioavailability of inorganic selenium sources for poultry. 1. Effect of time and high dietary selenium on tissue selenium uptake. Poult. Sci. 1988;67:1295–1301. doi: 10.3382/ps.0671295. [DOI] [PubMed] [Google Scholar]
- European Commission . 2019. Register of feed additives. Pursuant to regulation (EC) No 1831/2003. Annex I: List of additives (Edition 5/2019)https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed-eu-reg-comm_register_feed_additives_1831-03.pdf [Google Scholar]
- Federal Register . 2000. Food additives permitted in feed and drinking water of animals; Selenium yeast.https://www.federalregister.gov/documents/2000/06/06/00-14214/food-additives-permitted-in-feed-and-drinking-water-of-animals-selenium-yeast [Google Scholar]
- Foster L.H., Sumar S. Selenium in the environment, food and health. Nutr. Food Sci. 1995;5:17–23. [Google Scholar]
- Foster L.H., Sumar S. Selenium in health and disease: a review. Crit. Rev. Food Sci. Nutr. 1997;37:211–228. doi: 10.1080/10408399709527773. [DOI] [PubMed] [Google Scholar]
- Grunkemeyer V.L. Advanced diagnostic approaches and current management of avian hepatic disorders. Vet. Clin. North Am. Exot. Anim. Pract. 2010;13:413–427. doi: 10.1016/j.cvex.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kanbay M., Solak Y., Dogan E., Lanaspa M.A., Covic A. Uric acid in hypertension and renal disease: the chicken or the egg? Blood Purif. 2010;30:288–295. doi: 10.1159/000321074. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Blazejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609. doi: 10.3390/molecules21050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Jung H., Pitargue F.M., Han G.P., Choi H.S., Kil D.Y. Effect of dietary calcium concentrations in low non-phytate phosphorus diets containing phytase on growth performance, bone mineralization, litter quality, and footpad dermatitis incidence in growing broiler chickens. Asian-Australas. J. Anim. Sci. 2017;30:979–983. doi: 10.5713/ajas.17.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.W., Kim J.H., Shin J.E., Kil D.Y. Relative bioavailability of copper in tribasic copper chloride to copper in copper sulfate for laying hens based on egg yolk and feather copper concentrations. Poult. Sci. 2016;95:1591–1597. doi: 10.3382/ps/pew049. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee H.K., Park G.H., Choi H.S., Ji S.Y., Kil D.Y. Determination of the toxic level of dietary mercury and prediction of mercury intake and tissue mercury concentrations in broiler chickens using feather mercury concentrations. J. Appl. Poult. Res. 2019;28:1240–1247. [Google Scholar]
- Kim J.H., Park G.H., Han G.P., Choi H.S., Ji S.Y., Kil D.Y. Prediction of lead intake and tissue lead concentrations in broiler chickens using feather lead concentrations. Biol. Trace Elem. Res. 2020;193:517–523. doi: 10.1007/s12011-019-01726-2. [DOI] [PubMed] [Google Scholar]
- Kim Y.Y., Mahan D.C. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 2001;79:942–948. doi: 10.2527/2001.794942x. [DOI] [PubMed] [Google Scholar]
- Kumar B.S., Priyadarsini K.I. Selenium nutrition: How important is it? Biomed. Prev. Nutr. 2014;4:333–341. [Google Scholar]
- Li K.X., Wang J.S., Yuan D., Zhao R.X., Wang Y.X., Zhan X.A. Effects of different selenium sources and levels on antioxidant status in broiler breeders. Asian-Australas. J. Anim. Sci. 2018;31:1939–1945. doi: 10.5713/ajas.18.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Harper A.F., Zhao J., Corl B.A., LeRoith T., Dalloul R.A. Effects of a dietary antioxidant blend and vitamin E on fatty acid profile, liver function, and inflammatory response in broiler chickens fed a diet high in oxidants. Poult. Sci. 2014;93:1658–1666. doi: 10.3382/ps.2013-03827. [DOI] [PubMed] [Google Scholar]
- Lu J., Qu L., Shen M.M., Hu Y.P., Guo J., Dou T.C., Wang K.H. Comparison of dynamic change of egg selenium deposition after feeding sodium selenite or selenium-enriched yeast. Poult. Sci. 2018;97:3102–3108. doi: 10.3382/ps/pey161. [DOI] [PubMed] [Google Scholar]
- Lu L., Qu L., Shen M.M., Wang X.G., Guo J., Hu Y.P., Dou T.C., Wang K.H. Effects of high-dose selenium-enriched yeast on laying performance, egg quality, clinical blood parameters, organ development, and selenium deposition in laying hens. Poult. Sci. 2019;98:2522–2530. doi: 10.3382/ps/pey597. [DOI] [PubMed] [Google Scholar]
- Mahan D.C., Moxon A.L. Effect of inorganic selenium supplementation on selenosis in postweaning swine. J. Anim. Sci. 1984;58:1216–1221. doi: 10.2527/jas1984.5851216x. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ort J.F., Latshaw J.D. The toxic level of sodium selenite in the diet of laying chickens. J. Nutr. 1978;108:1114–1120. doi: 10.1093/jn/108.7.1114. [DOI] [PubMed] [Google Scholar]
- Payne R.L., Lavergne T.K., Southern L.L. Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poult. Sci. 2005;84:232–237. doi: 10.1093/ps/84.2.232. [DOI] [PubMed] [Google Scholar]
- Payne R.L., Southern L.L. Changes in glutathione peroxidase and tissue selenium concentrations of broilers after consuming a diet adequate in selenium. Poult. Sci. 2005;84:1268–1276. doi: 10.1093/ps/84.8.1268. [DOI] [PubMed] [Google Scholar]
- Peng X., Cui Y., Cui W., Deng J., Cui H. The decrease of relative weight, lesions, and apoptosis of bursa of Fabricius induced by excess dietary selenium in chickens. Biol. Trace Elem. Res. 2009;131:33–42. doi: 10.1007/s12011-009-8345-6. [DOI] [PubMed] [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci. 2006;84(E. Suppl.):E155–E165. doi: 10.2527/2006.8413_supple155x. [DOI] [PubMed] [Google Scholar]
- Robinson M.F., Thomson C.D. The role of selenium in the diet. Nutr. Abstr. Rev. 1983;53:3–26. [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Ryu Y.-C., Rhee M.-S., Lee K.-M., Kim B.-C. Effects of different levels of dietary supplemental selenium on performance, lipid oxidation, and color stability of broiler chickens. Poult. Sci. 2005;84:809–815. doi: 10.1093/ps/84.5.809. [DOI] [PubMed] [Google Scholar]
- Schat K.A., Kaspers B., Kaiser P. Acad. Press; Cabridge, MA: 2014. Avian Immunology. [Google Scholar]
- Seo S., Jeon S., Ha J.K. Guidelines for experimental design and statistical analyses in animal studies submitted for publication in the Asian-Australasian Journal of Animal Sciences. Asian-Australas. J. Anim. Sci. 2018;31:1381–1386. doi: 10.5713/ajas.18.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son A.R., Jeong J., Park K.R., Kim M., Lee S.D., Yoo J., Do Y., Reddy K.E., Lee H. Effects of graded concentrations of supplemental selenium on selenium concentrations in tissues and prediction equations for estimating dietary selenium intake in pigs. PeerJ. 2018;6:e5791. doi: 10.7717/peerj.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R.G.D., Torrie J.H., Dickey D.A. 3rd ed. McGraw Hill Book Co.; New York, NY: 1997. Principles and Procedures of Statistics: A Biometrical Approach. [Google Scholar]
- Suchy P., Strakova E., Herzig I. Selenium in poultry nutrition: a review. Czech J. Anim. Sci. 2014;59:495–503. [Google Scholar]
- Surai P.F. Selenium in poultry nutrition. 1. Antioxidant properties, deficiency and toxicity. Worlds Poult. Sci. J. 2002;58:333–347. [Google Scholar]
- U.S. Food & Drug Administration (FDA) 2017. Code of federal regulations. Title 21 CFR Part 537.920 Selenium.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=573.920 [Google Scholar]
- Yoon I., Werner T.M., Butler J.M. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poult. Sci. 2007;86:727–730. doi: 10.1093/ps/86.4.727. [DOI] [PubMed] [Google Scholar]