Abstract
Crossbreeding advantage in hybrids compared with their parents, termed heterosis, has been exhaustively exploited in chicken breeding over the last century. Reports for crossbreeding of elite laying chickens covering rearing and laying period remain infrequent. In this study, resource populations of Rhode Island Red (RIR) and White Leghorn (WL) pure-bred chickens were reciprocally crossed to generate 4 distinct groups that were evaluated for prelaying growth, egg production, and egg quality. Birds monitored for prelaying growth consists of 105 (RIR), 131 (WL), 207 (RIR × WL) and 229 (WL × RIR), and 30 pullets from each group were evaluated. Egg laying records were collected from 102, 89, 147, and 191 hens in the 4 populations, respectively. In addition, expression of 5 candidate genes for egg production in the ovarian follicles was measured by RT-qPCR. Results showed that BW of hatched chicks in the WL line was higher than the other populations. However, the 2 crossbreds grew faster than WL purebred throughout the prelaying period. Low to medium heterosis was observed for BW and body length before the onset of lay. White Leghorn and the hybrids commenced laying earlier than RIR pullets and egg production traits were favorable in the crossbreds compared with purebreds. Heterosis for egg number and clutch size was moderate in WL × RIR but low in RIR × WL hens. Expression of antimullerian hormone gene was high in WL and RIR × WL hybrids, suggesting WL parent-specific enhancing dominant expression. Shell weight was higher in the crossbreds than purebreds at 52 wk of age, but RIR hens laid eggs with higher shell ratio than the other populations (P < 0.05). Conversely, WL and the hybrids had higher eggshell strength than RIR birds (P < 0.05). Eggshell strength was the only egg quality trait that showed heterosis above 10% in WL × RIR hybrids at 32 and 52 wk of age. White Leghorn × RIR hens demonstrated higher percent heterosis for economic traits than birds of the reciprocal hybrid. This means that RIR breed is a better dam than a sire line for growth, egg laying, and egg quality traits.
Key words: heterosis, chickens, egg production, clutch size, egg quality
Introduction
Hybrid vigor has been of primary interest to breeders because of its extensive application in the improvement of agricultural species (Li et al., 2016). In poultry, heterosis has been exploited since 19th century and remains one of the powerful tools of poultry improvement in the modern times. Production of hybrid birds will therefore continue to be indispensable for higher productivity of modern egg laying chickens (Hristakieva et al., 2014)
Heterosis for important traits in laying chickens has been studied extensively within the last century. Various crossbreeding schemes involving several elite and local breeds report heterosis in growth and development pattern (Williams et al., 2002; Sutherland et al., 2018a), egg production (Wang et al., 2005; Schreiweis et al., 2006; Silversides, 2010; Hristakieva et al., 2014; Amuzu-Aweh et al., 2015; Amin et al., 2017), and egg quality traits (Tuiskula-Haavisto et al., 2002; Schreiweis et al., 2006; Bekele et al., 2009; Liu et al., 2011, 2018). Heterosis for these traits is however poorly correlated between studies and stages of life and is therefore breeds combination, developmental phase, and trait specific. There is therefore a need for more studies on heterosis covering the entire lifespan and multiple traits in chickens.
Highly organized follicular development and hierarchy distinguishes efficient layer hens with nonefficient ones. In the latter, excessive follicle development lowers efficiency in reproduction (Johnson et al., 2008). A catalog of candidate genes with potential roles on egg laying has been documented (Li et al., 2011; Ngu et al., 2015; Zhang et al., 2019). Excessive expression of antimullerian hormone (AMH) in the granulosa cells of avian ovary was linked to lower efficiency in egg laying (Johnson et al., 2008; Zhang et al., 2019). Zona pellucida member 2 (ZP2) was another candidate for egg laying and was speculated to facilitate the binding and penetration of spermatozoa to the germinal disc (Nishio et al., 2014). Insulin-like growth factor I (IGF-I) and ZP2 have shown low expression in the ovary of less efficient hens as compared with more efficient ones (Zhang et al., 2019).
Rhode Island Red (RIR) and White Leghorn (WL) are lines commercially used in layer production worldwide. Crosses between RIR and WL produce tint eggs, which constitute higher and higher market shares in parts of Asia, and especially China. According to the statistics of China Animal Agriculture Association, tint eggs constitutes averagely 61% of the eggs from domestic breeds and 24% from the imported breeds in recent 4 yr. This study evaluated heterosis for prelaying growth, egg production, and egg quality traits in chicken genotypes generated from reciprocal crossing involving RIR and WL pure-bred chickens. Expression of some candidate genes for egg production and clutch traits was also measured.
Materials and methods
Statement of Ethics
The study was approved by the Animal Care and Use Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, No. IAS2020-12), where the experiments were conducted. All experiments were performed in accordance with the relevant guidelines and regulations set by Ministry of Agriculture of the People's Republic of China.
Management of Breeder Birds
Two resource populations of RIR and WL chickens that were not selected for any economic trait were used for this study. They were randomly mating populations without pedigree information. Birds were kept in individual cages in the same laying pen and fed with breeder diet (19% CP; 2,840 kcal/kg ME; 3.5% Ca and 0.32% nonphytate P) ad libitum. Two randomly mating parental lines were used to generate 4 populations by reciprocal crossing using artificial insemination with a mating ration of 1:4 or 1:5. Briefly, a total of 14 RIR roosters was mated with 70 WL hens (RIR × WL crossbred), 11 RIR roosters with 44 RIR hens (RIR purebred), 14 WL roosters with 48 RIR hens (WL × RIR crossbred), and 14 WL roosters with 70 WL hens (WL purebred). Individual fresh and undiluted semen was used for the artificial insemination at a 2-day interval. Eggs were collected from the second day after the first insemination for 14 d. The number of eggs set for incubation in a single hatch was 422, 635, 665, and 597 for RIR, WL, RIR × WL, and WL × RIR, respectively.
Management of Experimental Birds
Healthy female chicks from the 4 genetic groups (RIR = 105, WL = 131, RIR × WL = 207, and WL × RIR = 229) were hatched and given unique identification by wing-banding. They were vaccinated and transferred to brooding pen. The birds were reared under the same standard brooding procedures. At 8 wk of age, the chicks were transferred to rearing cages (187 × 36 × 34 cm) in a different pen with 5 birds per cage. Birds from all the genetic groups shared the same pens throughout the period of the experiment. At the end of 18 wk of age, birds from the 4 genetic groups (RIR = 89, WL = 102, RIR × WL = 191 and WL × RIR = 147) were transferred to the laying hens' house for observation of egg production. Rearing and laying pens had 3 rows of cages, and each cage had 3 tiers. Birds in the 4 groups were randomized within tiers in group cages. The pullets were fed the same diet containing 16% CP, 2,800 kcal/kg ME, and 2% Ca and 0.32% nonphytate P from 8 wk of age to 5% egg production. During the laying period, diet contained 16.5% CP, 2,700 kcal/kg ME, and 3.5% Ca and 0.32% nonphytate P was offered ad libitum. The lighting program consisted of a systematic reduction of light from 24 h at day-old to 10 h at 8 wk of age. Light was supplied for 9 h throughout the growing period up to 20 wk of age. Thereafter, lighting period was increased with 1 h every wk up to 27 wk of age. From 28 to 43 wk, constant lighting of 16 h was maintained, and lighting of 16.5 h was supplied afterward.
Body Weight and Body Size
Body weight and body length were measured at the age of 6 and 18 wk. During each round of measurement, a sample of 30 birds each from the 4 genotypes was used. BW was measured using a digital scale and body length was measured as the distance from the shoulder joint to ischial tuberosity using a measuring tape.
Egg Production
Egg collection and recording were carried out for individual hens once daily from the age at first egg (AFE; the age of pullet in days when it lay its first egg) until 52 wk of age. Total egg number up to 32 (EN32) and 52 (EN52) week of age was computed for individual hens, and an average for each genetic group was calculated. Clutch size (CS), the number of eggs laid on successive days by a hen, was calculated as the total number of eggs laid within a period divided by the number of sequences recorded (Blake and Ringer 1987). The length of pause (LP) was determined following Reddy et al. (2005) and was calculated by dividing total number of pause days with number of pauses recorded within a period for each hen.
Egg Quality Evaluation
Eggs were collected from 15 hens each for the 4 genetic groups at 32 and 52 wk of age for evaluation of external and internal quality traits. Three consecutively laid eggs were collected from each selected hen for egg quality evaluation. Egg weight, yolk weight, and shell weight were measured using a digital scale with a sensitivity of 0.01 g (HC-UTP-313; Haihua Chao Electrical Appliances, Shanghai, China). Egg length and egg width represent the longest and shortest dimensions observed on the external surface of an egg respectively were measured using FHK egg dimension meter (Fujihira Ind. Co. Ltd., Tokyo, Japan). Eggshell thickness, the measure of deposition, and uniformity of calcification on the external surface of an egg was measured at the acute, middle, and obtuse poles of each egg using Eggshell Thickness Gauge (Orka Food Technology Ltd., Herzliya, Israel) with sensitivity of 0.01 μm. Eggshell strength representing the force required to break the shell of an intact egg was measured at the obtuse pole of the egg using Egg Force Reader (Orka Food Technology Ltd.). Albumen height and Haugh unit were measured using Egg Analyzer (Orka Food Technology Ltd.). Albumen height represents the elevation of egg white when egg content is poured on a flat surface and Haugh unit measures the freshness of an egg. Egg shape index was computed as the ratio of egg width to egg length (Duman et al., 2016), whereas shell ratio was calculated as the percentage of shell weight from egg weight (Rath et al., 2015).
Expression of Candidate Genes for Laying Traits in the Ovary Pure-Bred and Hybrid Chickens
Based on previous reports linking egg production traits with some candidate genes, relative expression of 5 such genes including AMH, progesterone receptor, 3-hydroxybutyrate dehydrogenase, type 1 A, IGF-,I and ZP2 was measured using RT-qPCR. Four birds each from the 4 genotypes with egg production and CS corresponding to their group average were slaughtered at 52 wk of age. Total RNA was purified from 40 to 50 mg of ovarian tissues mainly prehierarchical follicles using Trizol reagent (Invitrogen). Genomic DNA was eliminated using gDNA Eraser (TaKaRa, Shiga, Japan). cDNA was reverse transcribed from 1 μg of total RNA using PrimeScript RT reagent (TaKaRa) following the manufacturer's instructions. Quantitative PCR was performed in QuantStudio7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA) using SYBR FAST qPCR kit (Kapa Biosystems, Wilmington, MA) in a 10 μL reaction volume. Each 10 μL of PCR reaction mixture contained 5 μL of KAPA SYBR FAST qPCR master mix (2 × ), 0.4 μL each of forward and reverse primers (10 μmol), 1.5 μL of template cDNA, 0.2 μL of ROX Reference Dye II (50 × ), and 2.5 μL of ddH2O. Thermocycling protocol consisted of initial denaturation at 95°C for 3 min, 40 cycles of amplification were performed (95°C for 30 s and 60°C for 34 s) followed by thermal denaturation (95°C for 15 s, 60°C for 1 min, and 95°C for 15 s) to generate melting curves to verify amplification specificity. The candidate genes were amplified in the same plate (384 wells) with β-actin gene as an endogenous control. Primers for the genes as shown in Table 1 were designed using NCBI primer tool (https://www.ncbi.nlm.nih.gov/) and synthesized by BGI (Beijing, China). Samples were run in 3 technical replicates. Melting curves showed a single peak, implying that amplification was specific. The relative abundance of transcripts was calculated using the 2−ΔΔCT method.
Table 1.
Primers of the candidate genes used in qRT-PCR.
| Gene | Primer sequence (5’→3′) | Product length (bp) | Accession number |
|---|---|---|---|
| AMH | F: CCTGGAGGAAGTGAAGTGGG R: CGGGTAGAAGAGCAGCAGAG |
115 | NM_205030.1 |
| PGR | F: AGGCTTCTGGTTGCCACTAC R: GTTGTGCTGCCCTTCCATTG |
83 | NM_205262.1 |
| BDH1A | F: GATCTCCACGTTTGGGGAGG R: TCACTACACGACCCTTTGACC |
137 | NM_001006547.2 |
| IGF-I | F: CCAGAAACACTGTGTGGTGC R: TCCCTTGTGGTGTAAGCGTC |
123 | NM_001004384.2 |
| ZP2 | F: TGCATCAGACGCTGCACTTA R: CCCTTGGGATTGTCCTCCCT |
73 | NM_001039098.1 |
| β-actin | F: CTCTGACTGACCGCGTTACT R: TACCAACCATCACACCCTGAT |
172 | NM_205518.1 |
Abbreviations: AMH, antimullerian hormone; BDH1A, 3-hydroxybutyrate dehydrogenase, type 1 A; IGF-I, insulin-like growth factor I; PGR, progesterone receptor; ZP2, zona pellucida member 2.
Data Analysis
Phenotype and the expression data were subjected to linear orthogonal contrast procedure in Genstat (20th Edition, VSN International, Hemel Hempstead, UK). The 3 orthogonal contrasts consist (purebreds vs. crossbreds), (within purebreds), and (within crossbreds). The following model was used:
The following model was used:
Where Yij is the phenotype, μis the population average, Giis the fixed effect of genetic group, and εijis the random error.
Heterosis was calculated as per Fairfull et al. (1987) using the model below;
Where F1 is the performance value of the hybrid, P1 and P2 are the performance values of the 2 parental lines.
Results
Body Weight and Body Size
Chicks weight at 6 and 18 wk of age differ along purebred and crossbred line, within the purebred but not within the crossbreds. Within the purebreds, RIR chicks were heavier than WL chicks (P < 0.05). Except within purebreds at 6 wk of age, body length differs in all the 3 orthogonal contrasts at both ages (P < 0.05). White Leghorn pullets were at least 200 g lighter than other populations (Table 2).
Table 2.
Body weight and body length of Rhode Island Red, White Leghorn, and their reciprocal hybrids at 6 and 18 wk of age.
| Trait1 | Population2 |
SEM |
P-value3 |
|||||
|---|---|---|---|---|---|---|---|---|
| RIR | WL | RIR × WL | WL × RIR | |||||
| BW6 (g) | 421.87 | 363.97 | 423.17 | 417.90 | 6.56 | <0.0001 | <0.0001 | 0.166 |
| BL6 (cm) | 12.51 | 12.57 | 12.24 | 13.39 | 0.14 | <0.0001 | 0.722 | <0.0001 |
| BW18 (g) | 1,266.95 | 1,042.65 | 1,277.10 | 1,213.3 | 35.83 | 0.019 | 0.001 | 0.141 |
| BL18 (cm) | 20.34 | 17.60 | 19.81 | 21.28 | 0.49 | <0.0001 | <0.0001 | <0.0001 |
BW6 = body weight at 6 wk, BL6 = body length at 6 wk, BW18 = body weight at 18 wk, BL18 = body length at 18 wk.
RIR × WL = offspring of Rhode Island Red sires crossed to White Leghorn dams, and WL × RIR = offspring of White Leghorn sires crossed to Rhode Island Red dams.
(purebreds vs. crossbreds), (within purebreds), and (within crossbreds).
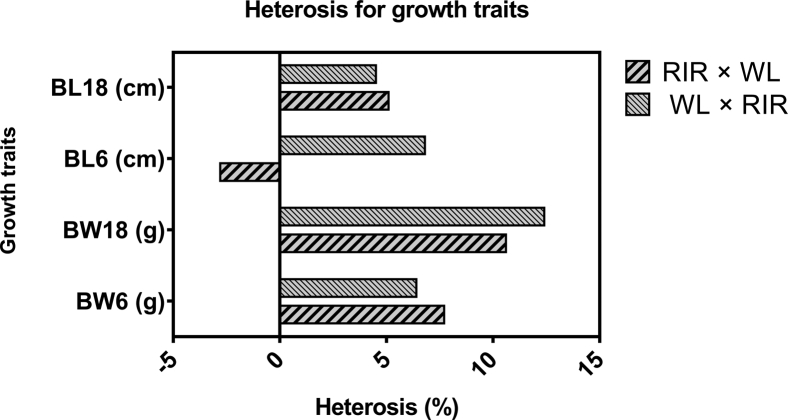
As shown in Figure 1, heterosis for prelaying growth traits was low (1% < heterosis <10%) except for BW at 18 wk, where WL × RIR hybrid had heterosis of 12%.
Figure 1.
Heterosis for growth traits at 6 and 18 wk of age. Abbreviations: BL, body length; RIR × WL, offspring of Rhode Island Red sires crossed to White Leghorn dams; WL × RIR, offspring of White Leghorn sires crossed to Rhode Island Red dams.
Egg Production
The performance of parental lines and their reciprocal hybrids for egg production is presented in Table 3. Significant differences were observed between purebred and crossbreds in AFE, EN32, clutch size up to 32 wk (CS32), EN52, clutch size up to 52 wk (CS52), and length of pause up to 52 wk (LP52) (P < 0.05). Similarly, AFE and EN32 differ between RIR and WL but not within the crossbreds. White Leghorn and the 2 reciprocal hybrids matured earlier than RIR pullets. Hybrids laid more eggs than parental breeds throughout the data collection period. Additionally, WL hens laid more eggs than RIR hens at early phase of the laying cycle (EN32). There was a general reduction in CS with increasing age of the birds in all populations.
Table 3.
Least square means and SEM for egg-laying traits of Rhode Island Red, White Leghorn, and their reciprocal hybrids.
| Trait1 | Population2 |
SEM |
P-value3 |
|||||
|---|---|---|---|---|---|---|---|---|
| RIR | WL | RIR × WL | WL × RIR | |||||
| AFE (day) | 154.76 | 140.30 | 140.35 | 138.35 | 1.76 | <0.0001 | <0.0001 | 0.069 |
| EN32 | 55.58 | 66.36 | 71.51 | 74.12 | 2.22 | <0.0001 | <0.0001 | 0.058 |
| CS32 (day) | 6.97 | 7.42 | 10.94 | 10.65 | 0.99 | <0.0001 | 0.810 | 0.769 |
| LP32 (day) | 1.50 | 1.66 | 1.49 | 1.53 | 0.17 | 0.225 | 0.646 | 0.766 |
| EN52 | 148.79 | 146.83 | 168.09 | 173.54 | 3.10 | <0.0001 | 0.713 | 0.126 |
| CS52 (day) | 5.52 | 5.57 | 7.11 | 7.82 | 0.59 | <0.0001 | 0.914 | 0.113 |
| LP52 (day) | 2.10 | 2.25 | 1.86 | 1.84 | 0.38 | 0.008 | 0.490 | 0.920 |
AFE = age at first egg, EN = egg number, CS = clutch size, LP = length of a pause.
RIR × WL = offspring of Rhode Island Red sires crossed to White Leghorn dams, and WL × RIR = offspring of White Leghorn sires crossed to Rhode Island Red dams.
(purebreds vs. crossbreds), (within purebreds), and (within crossbreds).
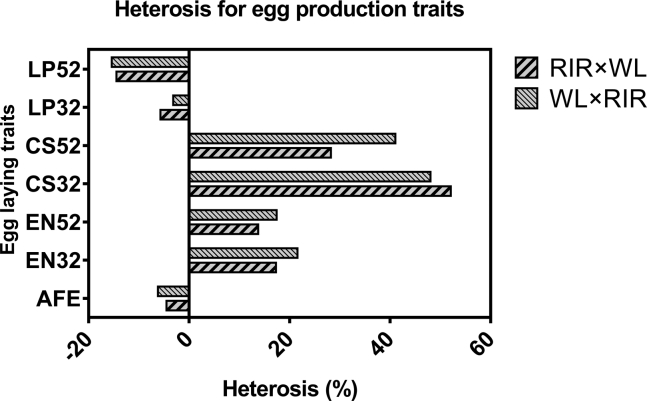
Heterosis for egg laying traits is presented in Figure 2. Hybrids demonstrated low and negative heterosis for AFE but positive heterosis for EN and CS. Moderate heterosis for EN at early (17.3–21.6%) and late periods (13.8–17.4%) of lay was observed in the hybrids. At both periods, WL × RIR hybrids had the upper limit of heterosis, whereas the reciprocal hybrids had the lower limit. The degree of heterosis for CS between periods follows the pattern of EN. Contrastingly, RIR × WL hybrids had the upper limit (52.1%) at 32 wk of age, whereas WL × RIR had the upper limit (41.0%) at 1 yr of age. LP showed low to moderate but negative heterosis (−3.2–−15.4%) among hybrids. The trend in LP was contrasting to CS, with RIR × WL hybrids at the lower limit at 32 wk while WL × RIR at the lower limit at 1 yr of age.
Figure 2.
Heterosis for egg production traits. Abbreviations: LP, length of pause; CS, clutch size; EN, egg number; AFE, age at first egg; RIR × WL, offspring of Rhode Island Red sires crossed to White Leghorn dams; WL × RIR, offspring of White Leghorn sires crossed to Rhode Island Red dams.
Expression of Candidate Genes for Egg Production
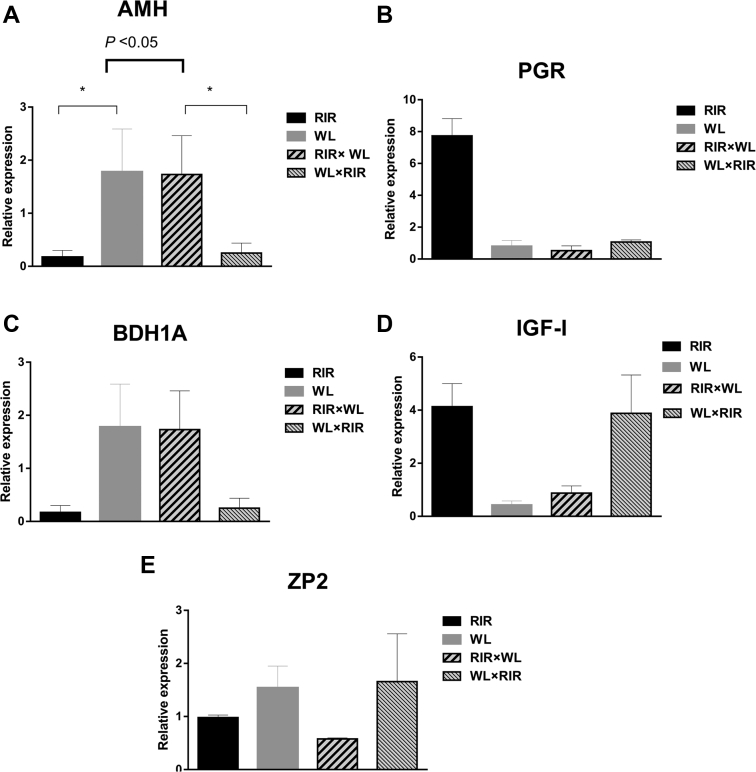
The results of the RT-qPCR analysis showed that AMH was highly expressed in follicles of WL than in RIR and in RIR × WL hybrids than in WL × RIR (P < 0.05). However, no significant difference between purebreds and crossbreds was observed (Figure 3). In addition, the expression of the other 4 genes was similar under the 3 modes of orthogonal contrasts.
Figure 3.
Relative expression of candidate genes in the ovary tissues of purebred and their reciprocal hybrids at 52 wk of age. (A) AMH; (B) PGR; (C) BDH1A; (D) IGF-I; and (E) ZP2. Abbreviations: AMH, antimullerian hormone; PGR, progesterone receptor; BDH1A, 3-hydroxybutyrate dehydrogenase, type 1 A; IGF-I, insulin-like growth factor I; ZP2, zona pellucida member 2, RIR × WL , offspring of Rhode Island Red sires crossed to White Leghorn dams, and WL × RIR, offspring of White Leghorn sires crossed to Rhode Island Red dams.
Egg Quality Traits
External and internal egg quality traits in the 4 populations at 32 and 52 wk of age are presented in Table 4. All external egg traits were similar between purebreds and crossbreds except egg weight and shell weight at 52 wk of age. Traits including egg length, egg weight, and eggshell index at 32 wk and eggshell thickness, eggshell strength, shell weight, and shell ratio at 52 wk differ within the purebreds, while eggshell index at 32 wk and shell ratio at 52 wk differ within the crossbreds (P < 0.05).
Table 4.
Least square means and SEM for egg weight and eggshell traits of Rhode Island Red, White Leghorn, and their reciprocal hybrids.
| Trait1 | Population2 |
SEM |
P-value3 |
|||||
|---|---|---|---|---|---|---|---|---|
| RIR | WL | RIR × WL | WL × RIR | |||||
| EW32 (g) | 54.0 | 55.5 | 54.7 | 54.9 | 2.36 | 0.625 | 0.416 | 0.234 |
| EW52 (g) | 58.50 | 58.01 | 60.13 | 60.91 | 1.81 | 0.030 | 0.780 | 0.541 |
| EL32 (cm) | 5.60 | 5.73 | 5.52 | 5.57 | 0.12 | 0.608 | <0.001 | 0.329 |
| EL52 (cm) | 5.62 | 5.67 | 5.69 | 5.72 | 0.22 | 0.250 | 0.518 | 0.656 |
| Ewt32 (cm) | 4.16 | 4.24 | 4.19 | 4.24 | 0.15 | 0.578 | 0.028 | 0.127 |
| Ewt52 (cm) | 4.17 | 4.18 | 4.21 | 4.27 | 0.64 | 0.051 | 0.905 | 0.160 |
| ESI32 | 72.6 | 74.4 | 74.8 | 76.1 | 1.62 | 0.399 | <0.001 | 0.039 |
| ESI52 | 74.1 | 73.59 | 73.94 | 74.81 | 1.26 | 0.413 | 0.598 | 0.320 |
| EST32 (mm) | 0.39 | 0.39 | 0.40 | 0.40 | 0.03 | 0.294 | 0.786 | 0.470 |
| EST52 (mm) | 0.35 | 0.41 | 0.38 | 0.37 | 0.02 | 0.442 | 0.007 | 0.577 |
| ESS32 (kg/cm2) | 3.28 | 3.52 | 3.69 | 3.74 | 0.43 | 0.869 | 0.818 | 0.884 |
| ESS52 (kg/cm2) | 3.15 | 3.79 | 3.54 | 3.82 | 0.32 | 0.238 | 0.016 | 0.244 |
| SW32 (g) | 5.14 | 5.14 | 5.24 | 5.27 | 0.27 | 0.742 | 0.136 | 0.487 |
| SW52 (g) | 5.31 | 5.85 | 5.83 | 6.03 | 0.26 | 0.014 | 0.009 | 0.281 |
| SR32 (%) | 9.40 | 9.50 | 9.58 | 9.58 | 0.47 | 0.944 | 0.254 | 0.112 |
| SR52 (%) | 9.07 | 10.15 | 9.51 | 10.07 | 0.39 | 0.350 | <0.001 | 0.039 |
| Alh32 (mm) | 5.30 | 4.70 | 5.70 | 4.80 | 0.39 | 0.819 | 0.177 | 0.160 |
| Alh52 (mm) | 5.90 | 5.50 | 5.90 | 5.00 | 0.36 | 0.162 | 0.127 | 0.001 |
| HU32 | 73.60 | 68.10 | 75.90 | 68.90 | 2.28 | 0.763 | 0.846 | 0.378 |
| HU52 | 76.60 | 73.10 | 75.30 | 68.40 | 2.24 | 0.039 | 0.105 | 0.001 |
| YW32 (g) | 15.24 | 15.32 | 16.21 | 15.80 | 0.60 | 0.009 | 0.106 | 0.507 |
| YW52 (g) | 16.61 | 17.20 | 18.35 | 18.14 | 0.56 | <0.001 | 0.171 | 0.588 |
EW = egg weight, EL = egg length, Ewt = egg width, ESI = egg shape index, ST = shell thickness, ESS = eggshell strength, SW = shell weight, SR = shell ratio, Alh = albumen height, HU = Haugh unit; YW = yolk weight.
RIR × WL = offspring of Rhode Island Red sires crossed to White Leghorn dams, and WL × RIR = offspring of White Leghorn sires crossed to Rhode Island Red dams.
(purebreds vs. crossbreds), (within purebreds), and (within crossbreds).
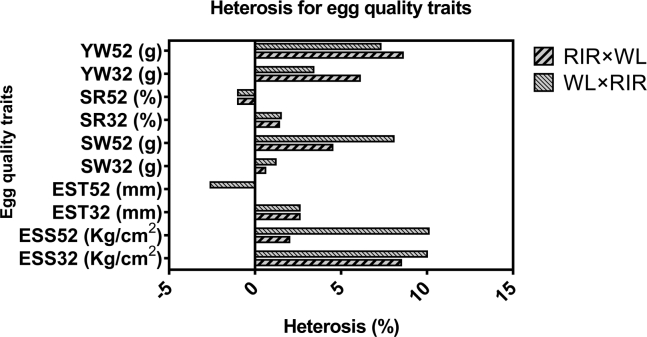
Heterosis observed for eggshell thickness, shell weight, and shell ration of eggs for the hybrids was less than 5% at 32 wk of age although it increased up to 10% for shell weight at 52 wk of age (Figure 4). Similarly, low heterosis was observed for eggshell strength in the hybrids at 32 and 52 wk of age. At both periods, WL × RIR showed higher heterosis than its reciprocal cross. However, 2.0% heterosis for eggshell strength observed in eggs laid by RIR × WL hybrid was lower than what was observed in the same population at 32 wk of age.
Figure 4.
Heterosis for egg quality traits in hybrids. Abbreviations: YW, yolk weight; SR, shell ratio; SW, shell weight; ST, shell thickness; ESS, egg shell strength; RIR × WL, offspring of Rhode Island Red sires crossed to White Leghorn dams; WL × RIR, offspring of White Leghorn sires crossed to Rhode Island Red dams.
As shown in Table 4, internal egg quality traits that differ between purebreds and crossbreds include yolk weight at both ages and Haugh unit at 52 wk (P < 0.05), but all internal egg traits were similar between WL and RIR purebreds. The internal traits that differ within the crossbreds were albumen height and Haugh unit at 52 wk (P < 0.05). Heterosis for yolk weight at 32 and 52 wk of age were generally low but higher in RIR × WL (6.1–8.6%) than its reciprocal hybrid (3.4–7.3%; Figure 4).
Discussion
Heterosis is still an important genetic phenomenon extensively utilized by poultry breeders in the development of commercial birds. We monitored heterosis for hatching traits, prelaying growth traits, egg production as well as egg quality traits in purebred WL, RIR, and their reciprocal hybrids. We also measured the expression of candidate genes reported to have a significant influence on EN and CS in the 4 genotypes.
Results showed prelaying BW and body length of the WL pullets lagging behind the other populations. This corroborates the slow posthatching growth of WL chickens. Within breed, body size at sexual maturity has been reported to influence laying performance and egg quality in hens (Lacin et al., 2008).
Observed marginal heterosis for growth traits in the crossbreds follows the pattern of previous studies (Siwendu et al., 2013; Lalev et al., 2014). Negative heterosis range of −4 to −22% was however reported for BW at 6 wk of age and at the onset of lay in hybrids of White Plymouth Rock lines (Williams et al., 2002). Similarly, negative heterosis for growth rate was documented in F1 and F2 hybrids of Red Jungle Fowl and White Plymouth Rock (WPR) lines (Sutherland et al., 2018a).
Egg production, the singular most important trait in laying chickens (Wolc et al., 2014), is actually a composite trait determined by AFE, CS, and LP. Results here showed that hybrids and WL started laying eggs around 2 wk earlier than RIR thereby laying more eggs than RIR at the early phase of lay. Attaining sexual maturity at an early age was shown to increase total egg production as long as the development of body and reproductive organs is not compromised. Similar AFE was reported for WL chickens (Rosa et al., 2018). However, late AFE (149–155 d) was also previously reported (Szwaczkowski et al., 2003; Goraga et al., 2010; Liu et al., 2011; Tomar et al., 2015) for the same breed. Similarly, RIR populations reared in India and Ethiopia were reported to have started laying at comparable age with birds in the current study (Khawaja et al., 2013a; Kumar et al., 2014). In several crossbreeding studies, F1 and higher-order hybrids attained sexual maturity earlier than their parental breeds (Tuiskula-Haavisto et al., 2002; Khawaja et al., 2013b; Tomar et al. 2015; Das et al., 2016; Sutherland et al., 2018a). In addition to AFE, 2 other crucial traits that determine egg production are CS and LP. At the early phase of lay, RIR and WL lines lay about 7 eggs consecutively, separated by short pause of one and half a day. There was however a reduction in CS and corresponding increase in LP at older age consistent with reports of previous studies in WL and RIR breeds (Wolc et al., 2019). However, CS for early and late periods of lay larger than observed in our study was documented (Roy et al., 2014; Wolc et al., 2019). Egg number reported for WL pure lines in other studies is comparable to those observed in the current study (Liu et al., 2011; Khawaja et al., 2013a). The superior performance in egg number exhibited by WL compared with RIR at 32 wk was neutralized when the birds reached 1 yr. This compensation in egg production by RIR showed that the delay in AFE was perhaps to promote body growth. Cumulative egg production up to 32 and 52 wk in the hybrids was higher than those reported for RIR × WL hybrids in a similar crossbreeding study (Das et al., 2016). However, higher egg number at a comparable age in the early phase of laying was documented in RIR × WL and reciprocal hybrids of RIR and Fayoumi chickens (Tuiskula-Haavisto et al., 2002; Khawaja et al., 2013a).
Hybrid vigor for AFE in the crossbred was negative and moderate, and WL × RIR population commenced egg laying 2 d earlier than its reciprocal hybrid. Comparable heterosis (−8.32%) was reported in F1 hybrid generated from 2 lines of WPR chickens (Lalev et al. 2014), but a lower heterosis (−22to−29%) was reported for hybrids of WPR lines divergently selected for BW (Sutherland et al. 2018b; Williams et al., 2002). Negative heterosis for AFE in our study and previous ones allows the hybrids to reach sexual maturity earlier than their parents. Heterosis for cumulative egg number after 1 yr of age for the 2 hybrids reduced compared with the early phase of lay. The percent heterosis range of 13.8 to 21% observed in our study is lower to 31 to 36% reported in WPR hybrids (Williams et al., 2002). However, marginal heterosis (8.25%) was reported for the same trait (Lalev et al., 2014). The highest heterosis observed in this study was for CS (41–52%) and suggested that the trait can be effectively improved through crossbreeding. Taken together, results from the present and previous studies corroborate the assertion that reproductive traits exhibit higher heterosis than growth traits in chickens (Sutherland et al., 2018a).
Pattern of differential expression of genes are potential mechanisms underlying heterosis (Mai et al., 2019; Zhou et al., 2019). Antimullerian hormone primarily regresses the right oviduct of hens during development in addition to completely inhibiting its development in roosters (Johnson et al., 2008). Antimullerian hormone expression was reported to be high in the prehierarchal follicles than preovulatory follicles and was suggested to control the rate of follicular growth and maintenance of follicular hierarchies (Johnson et al., 2009). This finely organized follicular development might be achieved by decreasing the sensitivity of small prehierarchal follicles to FSH via its receptor (Johnson et al., 2009). Antimullerian hormone was also suggested to play a role in protecting ovarian reserve (Lemcke et al., 2018). However, excessive AMH expression may impair optimal follicle selection (Johnson et al., 2009) and thereby reduce the number of potential oviposition. This might be achieved by disrupting the finely organized recruitment of prehierarchal follicles resulting in irregular laying patterns. The result of this study shows that AMH exhibits WL specific enhancing dominance expression and may not play a major role in egg laying and clutch traits determination.
External eggshell qualities protect internal egg content from mechanical damage and microbial invasion thereby reducing economic losses because of eggshell breakage (Solomon, 2010; Zhang et al., 2015; Arango et al., 2016). Lower eggshell thickness of eggs were reported for WL (Liu et al., 2011), RIR (Khawaja et al., 2013a), and Rhode Island White (Zhang et al., 2015) than in the present study. There was a marginal increase in eggshell thickness with increasing age in the WL population. Rhode Island Red birds had a lower eggshell thickness than the other populations at 52 wk of age. Tuiskula-Haavisto et al. (2002) reported a comparable eggshell strength in RIR × WL hybrids, whereas the eggshell strength observed in the present study were higher than reported for WL and RIR pure-bred lines (Liu et al., 2011, 2018; Rao et al., 2015).
Conclusions
The 2 reciprocal crosses exhibited similar laying performance which is superior to their parental purebred lines. Within the crossbreds, hens of WL × RIR demonstrated higher favorable heterosis for prelaying body growth, on-set of sexual maturity, egg number, clutch size, eggshell weight, and eggshell strength up to 1 yr of age. By this result, RIR breed has lent itself the advantage of being a good sire but also a better dam line for growth, egg laying, and egg quality traits. Candidate gene expression analysis suggests WL dominant allele-specific expression for AMH which may not play a crucial role in egg production or clutch size determination. There is a need, however, to study the complete transcriptome in the ovary of purebred and their hybrids to grasp the possible dynamics of expression of all genes that influence heterosis for egg number and clutch size.
Acknowledgments
The authors thank Yong Zhao and Chunjie Niu (Beijing Bainianliyuan Ecological Agriculture Co., LTD, Beijing, China) for their assistance in data recording. Financial support of this study was provided by the Chinese Agricultural Research System (grant number CARS-40), Beijing Municipal Science and Technology Project (grant number D171100007817005), Central Public-interest Scientific Institution Basal Research Fund (grant number 2018-YWF-YB-20), and the Agricultural Science and Technology Innovation Program (grant number ASTIP-IAS04). GSCAAS fellowship awarded to Adamu Mani Isa for the Ph.D. study is hereby acknowledged.
Conflict of Interest Statement: There are no conflicts of interest.
References
- Amin E., El-Dlebshany A.E., Debes A. Using general and specific combining abilities to expected breeding values, genetic values and hybrid performance in chickens. Egypt. Poult. Sci. J. 2017;37:1289–1302. [Google Scholar]
- Amuzu-Aweh E.N., Bovenhuis H., De Koning D.-J., Bijma P. Predicting heterosis for egg production traits in crossbred offspring of individual White Leghorn sires using genome-wide SNP data. Genet. Select. Evol. 2015;47:27. doi: 10.1186/s12711-015-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango J., Wolc A., Settar P., O'Sullivan N.P. Model comparison to evaluate a shell quality bio-complex in layer hens. Poult. Sci. 2016;95:2520–2527. doi: 10.3382/ps/pew286. [DOI] [PubMed] [Google Scholar]
- Bekele F., Gjoen H.M., Kathle J., Adnoy T., Abebe G. Genotype X environment interaction in two breeds of chickens kept under two management systems in Southern Ethiopia. Trop. Anim. Health Prod. 2009;41:1101–1114. doi: 10.1007/s11250-008-9290-7. [DOI] [PubMed] [Google Scholar]
- Blake A.G., Ringer R.K. Changes in ring-necked pheasants’ (Phasianus colchicus) egg formation time, oviposition lag time, and egg sequence length due to ahemeral light-dark cycles. Poult. Sci. 1987;66:231–236. doi: 10.3382/ps.0660231. [DOI] [PubMed] [Google Scholar]
- Das A., Kumar S., Rahim A., Mishra A. Characterization of production and reproduction performances in Rhode Island Red-White Strain chicken. Iranian J. Appl. Anim. Sci. 2016;6:707–713. [Google Scholar]
- Duman M., Sekeroglu A., Yildirim A., Eleroglu H., Camci O. Relation between egg shape index and egg quality characteristics. Eur. Poult. Sci. 2016;80:1–9. [Google Scholar]
- Fairfull R., Gowe R., Nagai J. Dominance and epistasis in heterosis of White Leghorn strain crosses. Can. J. Anim. Sci. 1987;67:663–680. [Google Scholar]
- Goraga Z., Nassar M., Schramm G.-P., Brockmann G.A. Phenotypic characterization of chicken inbred lines that differ extremely in growth, body composition and egg production traits. Arch. Anim. Breed. 2010;53:337–349. [Google Scholar]
- Hristakieva P., Oblakova M., Lalev M., Mincheva N. Heterosis effect in hybrid laying hens. Biotech. Anim. Husb. 2014;30:303–311. [Google Scholar]
- Johnson P.A., Kent T.R., Urick M.E., Giles J.R. Expression and regulation of anti-mullerian hormone in an oviparous species, the hen. Biol. Reprod. 2008;78:13–19. doi: 10.1095/biolreprod.107.061879. [DOI] [PubMed] [Google Scholar]
- Johnson P.A., Kent T.R., Urick M.E., Trevino L.S., Giles J.R. Expression of anti-Mullerian hormone in hens selected for different ovulation rates. Reproduction. 2009;137:857–863. doi: 10.1530/REP-08-0406. [DOI] [PubMed] [Google Scholar]
- Khawaja T., Khan S.H., Mukhtar N., Parveen A., Fareed G. Production performance, egg quality and biochemical parameters of three way crossbred chickens with reciprocal F1 crossbred chickens in sub-tropical environment. Ital. J. Anim. Sci. 2013;12:e21. [Google Scholar]
- Khawaja T., Khan S.H., Mukhtar N., Ullah N., Parveen A. Production performance, egg quality and biochemical parameters of Fayoumi, Rhode Island Red and their reciprocal crossbred chickens. J. Appl. Anim. Res. 2013;41:208–217. [Google Scholar]
- Kumar N., Belay Z.N., Shenkutie A.M., Taddele H. Comparative study of performance of Rhode Island red and Bovans white under intensive management in Mekelle, Ethiopia. Int. J. Livest. Res. 2014;4:92–98. [Google Scholar]
- Lacin E., Yildiz A., Esenbuga N., Macit M. Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech. J. Anim. Sci. 2008;53:466–471. [Google Scholar]
- Lalev M., Mincheva N., Oblakova M., Hristakieva P., Ivanova I. Estimation of heterosis, direct and maternal additive effects from crossbreeding experiment involving two White Plymouth Rock lines of chickens. Biotech. Anim. Husb. 2014;30:103–114. [Google Scholar]
- Lemcke R., Stephens C., Hildebrandt K., Johnson P. Anti-Müllerian hormone type II receptor in avian follicle development. Biol. Reprod. 2018;99:1227–1234. doi: 10.1093/biolre/ioy140. [DOI] [PubMed] [Google Scholar]
- Li D., Huang Z., Song S., Xin Y., Mao D., Lv Q., Zhou M., Tian D., Tang T., Wu Q., Liu X., Chen T., Song X., Fu X., Zhao B., Liang C., Li A., Liu G., Li S., Hu S., Cao X., Yu J., Yuan L., Chen C., Zhu P. Integrated analysis of phenome, genome, and transcriptome of hybrid rice uncovered multiple heterosis-related loci for yield increase. Proc. Nat. Acad. Sci. 2016:E6026–E6035. doi: 10.1073/pnas.1610115113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Sun D.X., Yu Y., Liu W.J., Tang S.Q., Zhang Y., Wang Y.C., Zhang S.L., Zhang Y. Genetic effect of the follicle-stimulating hormone receptor gene on reproductive traits in Beijing You chickens. Poult. Sci. 2011;90:2487–2492. doi: 10.3382/ps.2010-01327. [DOI] [PubMed] [Google Scholar]
- Liu W., Li D., Liu J., Chen S., Qu L., Zheng J., Xu G., Yang N. A genome-wide SNP scan reveals novel loci for egg production and quality traits in white leghorn and brown-egg dwarf layers. PLoS One. 2011;6:e28600. doi: 10.1371/journal.pone.0028600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Sun C., Yan Y., Li G., Shi F., Wu G., Liu A., Yang N. Genetic variations for egg quality of chickens at late laying period revealed by genome-wide association study. Sci. Rep. 2018;8:10832. doi: 10.1038/s41598-018-29162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai C., Wen C., Sun C., Xu Z., Chen S., Yang N. Implications of gene inheritance patterns on the heterosis of abdominal fat deposition in chickens. Genes. 2019;10:842. doi: 10.3390/genes10100824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio S., Kohno Y., Iwata Y., Arai M., Okumura H., Oshima K., Nadano D., Matsuda T. Glycosylated chicken ZP2 accumulates in the egg coat of immature oocytes and remains localized to the germinal disc region of mature eggs. Biol. Reprod. 2014;91:107. doi: 10.1095/biolreprod.114.119826. [DOI] [PubMed] [Google Scholar]
- Ngu N.T., Xuan N.H., Vu C.T., An N.T., Dung T.N., Nhan N.T.H. Effects of genetic polymorphisms on egg production in indigenous Noi chicken. J. Exper. Biol. Agric. Sci. 2015;3:487–493. [Google Scholar]
- Rao S.V.R., Raju M., Prakash B., Reddy E.P.K., Panda A. Effect of dietary inclusion of toasted guar (Cyamopsis tetragonoloba) meal as a source of protein on performance of White Leghorn layers. Br. Poult. Sci. 2015;56:733–739. doi: 10.1080/00071668.2015.1113498. [DOI] [PubMed] [Google Scholar]
- Rath P.K., Mishra P.K., Mallick B.K., Behura N.C. Evaluation of different egg quality traits and interpretation of their mode of inheritance in White Leghorns. Vet. World. 2015;8:449. doi: 10.14202/vetworld.2015.449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy I., David C., Singh K. Relationship between Intersequence pauses, laying persistency and concentration of prolactin during the productive period in white Leghorn hens. Asian-austr. J. Anim. Sci. 2005;18:686–691. [Google Scholar]
- Rosa J.O., Venturini G.C., Chud S., Cristina T., Pires B.C., Buzanskas M.E., Stafuzza N.B., Furquim G.R., da Cruz R., Aparecida V. Bayesian inference of genetic parameters for reproductive and performance traits in White Leghorn hens. Czech J. Anim. Sci. 2018;63:230–236. [Google Scholar]
- Roy B.G., Kataria M.C., Roy U. Study of oviposition pattern and clutch traits in a White leghorn (WL) layer population. J. Agric. Vet. Sci. 2014;7:59–67. [Google Scholar]
- Schreiweis M.A., Hester P.Y., Settar P., Moody D.E. Identification of quantitative trait loci associated with egg quality, egg production, and body weight in an F2 resource population of chickens. Anim. Genet. 2006;37:106–112. doi: 10.1111/j.1365-2052.2005.01394.x. [DOI] [PubMed] [Google Scholar]
- Silversides F. Laying performance of six pure lines of chickens and four commercial hybrids at the Agassiz Research Centre. Can. J. Anim. Sci. 2010;90:341–347. [Google Scholar]
- Siwendu N.A., Norris D., Ngambi J.W., Shimelis H.A., Benyi K. Heterosis and combining ability for body weight in a diallel cross of three chicken genotypes. Trop. Anim. Health Prod. 2013;45:965–970. doi: 10.1007/s11250-012-0317-8. [DOI] [PubMed] [Google Scholar]
- Solomon S.E. The eggshell: strength, structure and function. Br. Poult. Sci. 2010;51(Suppl 1):52–59. doi: 10.1080/00071668.2010.497296. [DOI] [PubMed] [Google Scholar]
- Sutherland D.A.T., Honaker C.F., Dorshorst B., Andersson L., Brisbin I.L., Jr., Siegel P.B. Growth patterns for three generations of an intercross between red junglefowl and chickens selected for low body weight. J. Anim. Breed. Genet. 2018;135:300–310. doi: 10.1111/jbg.12336. [DOI] [PubMed] [Google Scholar]
- Sutherland D.A.T., Honaker C.F., Dorshorst B., Andersson L., Siegel P.B. Asymmetries, heterosis, and phenotypic profiles of red junglefowl, White Plymouth Rocks, and F 1 and F 2 reciprocal crosses. J. Appl. Genet. 2018;59:193–201. doi: 10.1007/s13353-018-0435-8. [DOI] [PubMed] [Google Scholar]
- Szwaczkowski T., Cywa-Benko K., Wezyk S. A note on inbreeding effect on productive and reproductive traits in laying hens. Anim. Sci. Pap. Rep. 2003;21:121–129. [Google Scholar]
- Tomar A., Poonia J., Chaudhari M., Kumar P. Evaluation of production performance of some economic traits in White Leghorn birds. Haryana Vet. 2015;54:19–21. [Google Scholar]
- Tuiskula-Haavisto M., Honkatukia M., Vilkki J., de Koning D.J., Schulman N.F., Maki-Tanila A. Mapping of quantitative trait loci affecting quality and production traits in egg layers. Poult. Sci. 2002;81:919–927. doi: 10.1093/ps/81.7.919. [DOI] [PubMed] [Google Scholar]
- Wang H., Sun D.-X., Yu Y., Wang D.Z., Yuan Relationship between differential gene expression in ovary and heterosis of egg number traits in a chicken diallel cross. Asian-austr. J. Anim. Sci. 2005;18:767–771. [Google Scholar]
- Williams S.M., Price S.E., Siegel P.B. Heterosis of growth and reproductive traits in fowl. Poult. Sci. 2002;81:1109–1112. doi: 10.1093/ps/81.8.1109. [DOI] [PubMed] [Google Scholar]
- Wolc A., Arango J., Jankowski T., Dunn I., Settar P., Fulton J.E., O'Sullivan N.P., Preisinger R., Fernando R.L., Garrick D.J., Dekkers J.C. Genome-wide association study for egg production and quality in layer chickens. J. Anim. Breed. Genet. 2014;131:173–182. doi: 10.1111/jbg.12086. [DOI] [PubMed] [Google Scholar]
- Wolc A., Jankowski T., Arango J., Settar P., Fulton J.E., O'Sullivan N.P., Dekkers J.C.M. Investigating the genetic determination of clutch traits in laying hens. Poult. Sci. 2019;98:39–45. doi: 10.3382/ps/pey354. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen L., Han K., Zhang X., Zhang G., Dai G., Wang J., Xie K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019;208:106–114. doi: 10.1016/j.anireprosci.2019.106114. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhu F., Liu L., Zheng C.W., Wang de H., Hou Z.C., Ning Z.H. Integrating transcriptome and genome re-sequencing data to identify key genes and mutations affecting chicken eggshell qualities. PLoS One. 2015;10:e0125890. doi: 10.1371/journal.pone.0125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Z., Lamont S.J., Abasht B. RNA-Seq analyses identify additivity as the predominant gene expression pattern in F1 chicken embryonic brain and Liver. Genes. 2019;10:27. doi: 10.3390/genes10010027. [DOI] [PMC free article] [PubMed] [Google Scholar]