Abstract
Heparanase, the sole heparan sulfate degrading endoglycosidase, regulates multiple biological activities that enhance tumor growth, angiogenesis and metastasis. Much of the impact of heparanase on tumor progression is related to its function in mediating tumor-host crosstalk, priming the tumor microenvironment to better support tumor growth and metastasis. We have utilized mice over-expressing (Hpa-tg) heparanase to reveal the role of host heparanase in tumor initiation, growth and metastasis. While in wild type mice tumor development in response to DMBA carcinogenesis was restricted to the mammary gland, Hpa-tg mice developed tumors also in their lungs and liver, associating with reduced survival of the tumor-bearing mice. Consistently, xenograft tumors (lymphoma, melanoma, lung carcinoma, pancreatic carcinoma) transplanted in Hpa-tg mice exhibited accelerated tumor growth and shorter survival of the tumor-bearing mice compared with wild type mice. Hpa-tg mice were also more prone to the development of metastases following intravenous or subcutaneous injection of tumor cells. In some models, the growth advantage was associated with infiltration of heparanase-high host cells into the tumors. However, in other models, heparanase-high host cells were not detected in the primary tumor, implying that the growth advantage in Hpa-tg mice is due to systemic factors. Indeed, we found that plasma from Hpa-tg mice enhanced tumor cell migration and invasion attributed to increased levels of pro-tumorigenic factors (i.e., RANKL, SPARC, MIP-2) in the plasma of Hpa-Tg vs. wild type mice. Furthermore, tumor aggressiveness and short survival time were demonstrated in wild type mice transplanted with bone marrow derived from Hpa-tg but not wild type mice. These results were attributed, among other factors, to upregulation of pro-tumorigenic (i.e., IL35+) and downregulation of anti-tumorigenic (i.e., IFN-γ+) T-cell subpopulations in the spleen, lymph nodes and blood of Hpa-tg vs. wild type mice and their increased infiltration into the primary tumor. Collectively, our results emphasize the significance of host heparanase in mediating the pro-tumorigenic and pro-metastatic interactions between the tumor cells and the host tumor microenvironment, immune cells and systemic factors.
Keywords: Tumor microenvironment, host factors, immune cells, metastasis, heparanase
Introduction
Heparanase is an endoglucuronidase that cleaves heparan sulfate (HS), thereby altering the structure and function of HS proteoglycans (HSPG) and contributing to tumor-mediated remodeling of both cell surfaces and the extracellular matrix (ECM) [1–7]. These actions dynamically impact multiple regulatory pathways, most notably by augmenting the bioavailability of growth factors and cytokines bound to HS [2, 8–10]. Besides, heparanase upregulates the expression of multiple genes (i.e., VEGF, HGF, RANKL and MMP-9, IL-2, IFN-γ) that promote aggressive tumor progression and inflammation [9, 11]. Intense research effort in the last two decades revealed that heparanase expression is up-regulated in various human carcinomas, sarcomas, and hematological malignancies [10, 12, 13]. In many cases, heparanase induction correlates with increased tumor metastasis, vascular density, and shorter postoperative survival of cancer patients [10, 12, 13], thus providing strong clinical support for the pro-tumorigenic function of the enzyme and encouraging the development of heparanase inhibitors as anti-cancer drugs [8, 14–16]. Compelling evidence tie heparanase to all steps of tumor formation, including tumor initiation, growth, metastasis, and chemoresistance [17–21], further supporting the notion that heparanase is a valid drug target. Less attention was directed toward deciphering the role of heparanase in host cells that constitute the tumor microenvironment and play instrumental roles in tumorigenesis [5, 22, 23]. Heparanase is expressed by cells of the immune system (e.g., neutrophils, T-cells, NK cells, dendritic cells, macrophages) [13, 24–27] playing essential roles in immune cell activation, inflammation and autoimmunity [26, 28, 29]. Heparanase is also intimately involved in cytokine gene expression, bioavailability and signal transduction [24–26, 30, 31]. For example, TNFα expression is markedly induced in macrophages following heparanase overexpression or its exogenous addition [30]. In contrast, much lower expression of TNFα and other cytokines was quantified in macrophages isolated from Hpa-knockout (Hpa-KO) vs. wild type (WT) mice [25].
The current research was undertaken to elucidate the impact of host heparanase on tumor initiation and progression, emphasizing its role in mediating the crosstalk between the tumor and immune cells of the tumor microenvironment. Applying heparanase overexpressing mice (Hpa-tg), we have first examined the susceptibility of WT and Hpa-tg mice to the chemical carcinogen DMBA [18]. We found that Hpa-tg mice developed an aggressive disease endowed with reduced survival of the mice, indicating that high levels of host heparanase facilitate tumor initiation and progression. In subsequent experiments, we investigated transplantable tumors (lymphoma, melanoma, lung and pancreatic carcinoma) and demonstrated accelerated tumor growth and metastasis, associated with shorter survival time of the tumor-bearing Hpa-tg vs. wild type (WT) mice. This result was attributed to elevated levels of pro-tumorigenic immune cell populations and systemic factors in Hpa-tg vs. WT mice. The nature of the immune cell subpopulations was identified by FACS analysis. Tumor aggressiveness and short survival time were also noted in WT mice transplanted with bone marrow (BM) derived from Hpa-tg but not WT mice, suggesting a pro-tumorigenic effect of BM-derived circulating cells and systemic factors.
Results
Hpa-tg mice develop extensive disease endowed with reduced survival in response to DMBA carcinogenesis
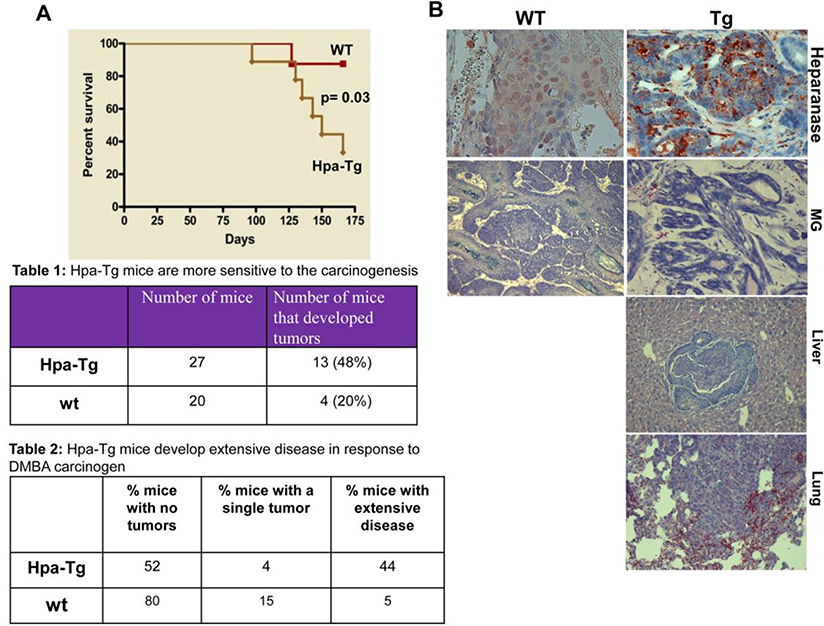
In order to explore the significance of heparanase contributed by the host and by cells of the tumor microenvironment, we first employed transgenic mice (Hpa-tg), which, under the actin promoter, over-express heparanase in essentially all cell types and tissues [32]. For this purpose, we exposed WT and Hpa-tg mice to DMBA, a carcinogen that has been shown to predominantly affect the mammary gland. This carcinogen was preferred because the mammary tissue appeared more developed in the Hpa-tg mice [32] and heparanase was implicated in breast cancer progression [33, 34]. Mice were administrated (p.o) with four consecutive applications of DMBA (1.5 mg/mouse) and the survival rates were inspected. Hpa-tg mice were more sensitive to the carcinogen and exhibited a markedly reduced survival rate compared with WT (Cont.) mice following DMBA treatment (Fig. 1A). In a subsequent experiment, mice were treated with a lower dose of DMBA (1 mg/mouse) and sacrificed once tumors were observed, or when morbidity became apparent. Forty-eight percent of the Hpa-tg mice developed tumors as compared to only 20% of the WT mice (Table 1 in Fig. 1). Moreover, 44% of the Hpa-tg mice revealed extensive disease vs. only 5% of the wild type mice (Table 2 in Fig. 1). Notably, while tumor development was most often confined to the mammary gland (MG) of WT mice (Fig. 1B, second panels), Hpa-tg mice developed tumors also in the liver (Fig. 1B, third panel), lung (Fig. 1B, fourth panel) and abdominal cavity, often with no precise recognition of the tissue of origin (extensive disease; Table 2 in Fig. 1). These results clearly imply that host heparanase facilitates tumor initiation and progression once expressed at high levels.
Figure 1. Hpa-tg mice are more susceptible to DMBA carcinogenesis.
A. Control (Con) and Hpa-tg mice were administrated 4 times with DMBA (p.o; 1.5 mg/mouse) and mouse survival rates were recorded. B,C. DMBA (p.o; 1 mg/mouse) was similarly administrated, and mice were sacrificed once tumors became apparent or mice became morbid. At termination, tumor development was inspected in all internal organs and categorized as no, single, or extensive disease (C) according to visual inspection and histology (B). Mammary gland (MG) tumors in WT and Hpa-tg mice and tumors developed in the liver and lungs of Hpa-tg mice were subjected to immunostaining applying anti-heparanase antibody (B). Table 1 denotes the percentage of tumor-bearing Hpa-tg vs. WT mice. Table 2 denotes the percentage of Hpa-tg vs. WT mice with no tumors, single tumor or extensive disease.
Impact of host heparanase on tumor xenograft growth
Primary tumor growth
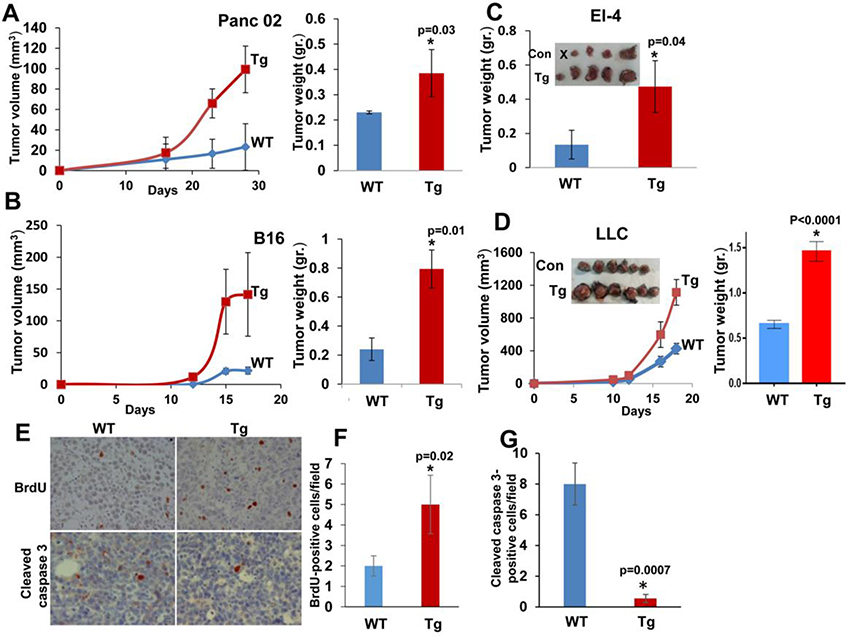
We implanted tumor-derived cells sub-cutaneous (s.c) in WT and Hpa-tg syngeneic C57BL mice and tumor growth was inspected over time. We found that Panc02 (Fig. 2A), B16 melanoma (Fig. 2B), El-4 lymphoma (Fig. 2C) and Lewis lung carcinoma (LLC; Fig. 2D) cells developed 3–4 folds bigger tumors (in terms of size and weight) once implanted in Hpa-tg as compared to WT mice, clearly demonstrating the impact of host heparanase. Immunostaining of the EL4 tumors ascribed the accelerated tumor growth observed in Hpa-tg mice to increased cell proliferation, evidenced by BrdU incorporation (Fig. 2E upper panel, F), and decreased apoptotic cell death, evidenced by decreased staining for cleaved caspase 3 (Fig. 2E lower panel, G).
Figure 2. Tumor growth prevails in Hpa-tg mice.
A-D. Tumor growth. The indicated cell line was inoculated subcutaneously (s.c) in WT (Con) and Hpa-tg mice and tumor growth was inspected over time (A, B, D, left panels). At termination, tumors were excised, weighed (A-D right panels) and fixed in formalin for histological evaluation. E-G. Immunostaining. Tumor xenografts produced by El-4 mouse lymphoma cells were subjected to immunostaining applying anti-BrdU (E, upper panels) and anti-cleaved caspase-3 (E, lower panels) antibodies. Quantification of the staining is shown graphically in F and G, respectively. Original magnification: x40.
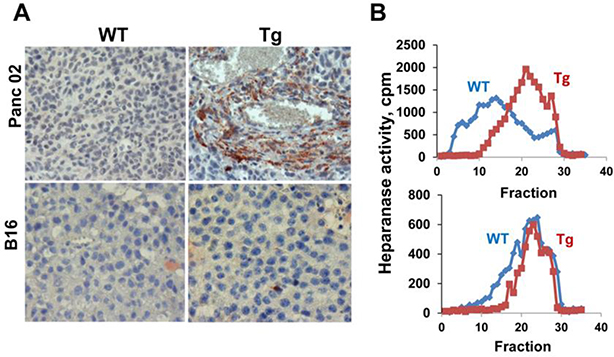
The finding that EL4 lymphoma cells grew aggressively in Hpa-tg mice is particularly instructive because EL4 cells lack heparanase expression [25], leaving only host cells to contribute heparanase and thereby support tumor growth. The contribution of host heparanase was further exemplified by demonstrating a marked 8.5-fold and 2.5-fold decrease in tumor growth following inoculation of EL4 or LLC cells into heparanase-KO mice, respectively (Supp Fig. 1), strongly indicating that host heparanase plays a decisive role in tumor progression. Subjecting tumors produced by Panc02 cells to immunostaining for heparanase revealed very weak staining in tumors developed in WT mice (Fig. 3A, upper left panel). In contrast, tumors developed in Hpa-tg mice were decorated with cells that exhibited intense staining for heparanase (Fig. 3B, upper right panel, Tg), most likely representing heparanase-high host cells that populate the tumor, resulting in increased heparanase content (Fig. 3A, upper panel) and activity (Fig. 3B, upper panel). This suggests that stromal cells are highly abundant in Panc02 tumors and likely promote their growth. In subsequent studies, we have identified the recruited host immune cells by dissociation of the primary tumors into single cells, followed by flow cytometry analysis (Figs. 7,8).
Figure 3.
Heparanase is a convenient marker of the tumor microenvironment. A. Immunostaining. Five-micron sections of tumor xenografts produced by PANC02 (upper panels) and B16 melanoma (lower panels) cells in WT and Hpa-tg (Tg) mice were subjected to immunostaining employing anti-heparanase antibody. Note, abundant heparanase-positive stromal cells in Hpa-tg tumors produced by Panc02 but not B16 melanoma cells. B. Heparanase activity. Heparanase enzymatic activity was evaluated in corresponding tumor extracts (Panc02, upper panel; B16 melanoma, lower panel). Note that the recruitment of stromal cells correlates with increased heparanase activity in tumors produced by Panc02 cells.
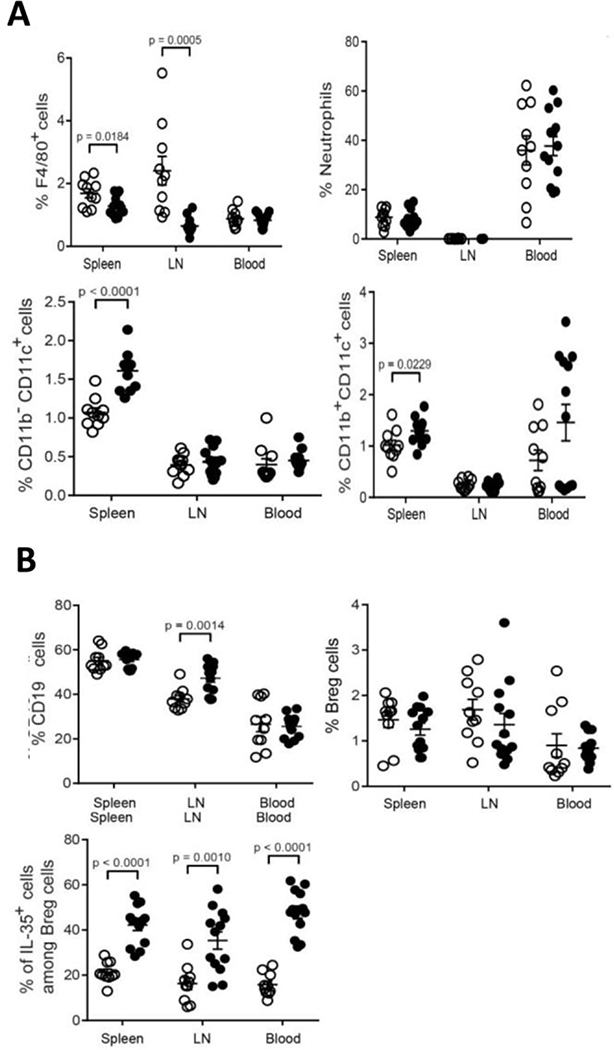
Figure 7.
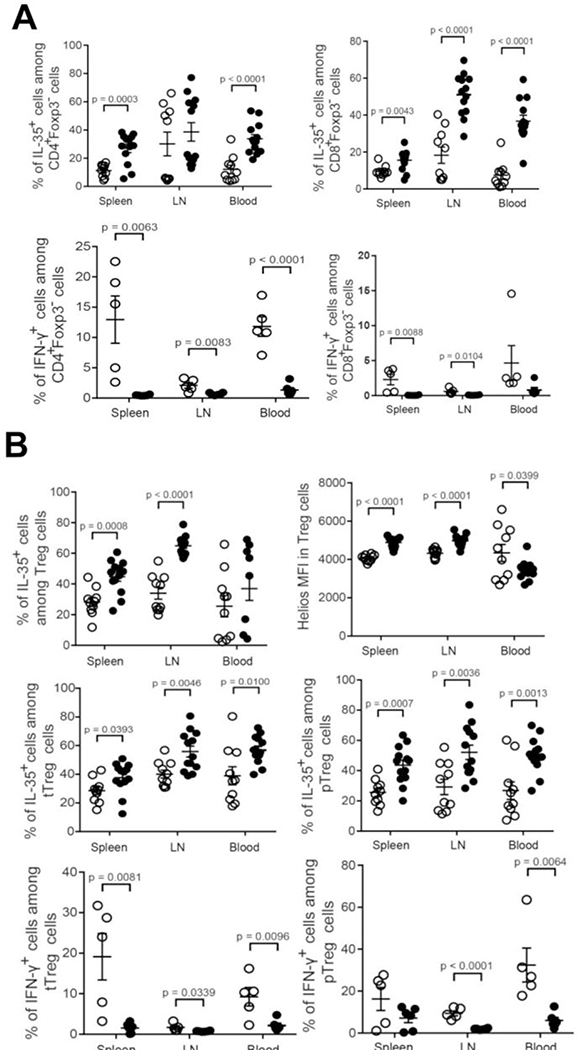
Percentage of immune cell populations in the spleen, inguinal lymph nodes and blood of LLC tumor bearing WT (open dots) vs. Hpa-tg (closed dots) mice. A. Percent macrophages (top, left panel), neutrophils (Ly6G) (top, right panel), CD11b−CD11c+ dendritic cells (bottom, left panel), and CD11b+CD11c+ dendritic cells (bottom, right panel). Note, decreased percentage of macrophages and increased percentage of dendritic cells in Hpa-tg (closed dots) vs. WT (open dots) mice. B. Percent CD19+ (top, left panel), CD19+CD5+CD1d+ Breg cells (top, right panel), and IL-35+ cells among Breg cells (bottom, left panel). Note increased levels of IL-35+ Breg cells in Hpa-tg mice. Unpaired t-tests were performed for comparison between WT and Hpa-tg mice for the different innate cell types. The actual percentage of immune cells ± SEM is presented in Supp. Table 1.
Figure 8.
Percentage of immune cell sub-populations in the spleen, inguinal lymph nodes and blood of LLC tumor-bearing WT (open dots) vs. Hpa-tg (closed dots) mice. A. Percent IL-35+ cells among CD4+Foxp3− cells (top, left panel), IL-35+ cells among CD8+Foxp3− cells (top, right panel), IFN-γ+ cells among CD4+Foxp3− (bottom, left panel), and IFN-γ+ cells among IFN-γ+ CD4+Foxp3− T-cells (bottom, right panel). Note, increased levels of IL-35+CD4+ and IL-35+CD8+ T cells and decreased levels of IFN-γ+CD4+ and IFN-γ+CD8+ T in Hpa-tg vs. WT mice. B. Percent IL-35+ cells among tTreg cells (top, left panel), Helios MFI cells in Treg cells (top, right panel), IL-35+ cells among tTreg cells (middle, left panel), IL-35+ cells among pTreg cells (middle, right panel), IFN-γ+ cells among tTreg cells (bottom, right panel), and IFN-γ+ cells among pTreg cells (bottom, left panel). Spleen, inguinal lymph nodes (LN) and blood were collected from LLC tumor-bearing WT (open dots) and Hpa-tg (black dots) mice. Single-cell suspensions were prepared and subjected to flow cytometry analysis as described in ‘Methods’. Unpaired t-tests were performed for comparison between WT and Hpa-tg mice for the different innate cell types. The actual percentage of immune cells ± SEM is presented in Supp. Table 1.
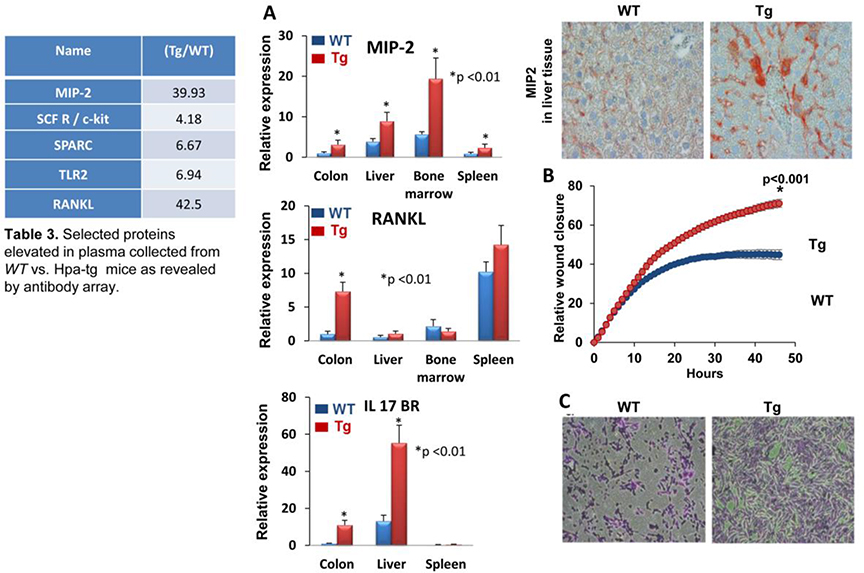
Unlike the Panc02 model, heparanase staining was similar in B16 melanoma tumors grown in WT or Hpa-tg mice (Fig. 3A, lower panels). There was also no difference in heparanase enzymatic activity determined in extracts derived from these tumors (Fig. 3B, lower panel). We, therefore, assumed that increased growth of B16 melanoma tumors in Hpa-tg mice is not due primarily to the recruitment of host cells but rather to systemic factors that are more abundant in the plasma of Hpa-tg mice. To examine this possibility, plasma was collected from WT and Hpa-tg mice and applied onto Biotin Label-based Antibody Array (RayBio®). We found that indeed the levels of several proteins were elevated in the plasma of Hpa-tg mice (Table 3 in Fig. 4). Increased expression of the biological mediators that were revealed in the array was further examined by real-time PCR analyses, employing RNA that was extracted from different mouse tissues. We could not confirm the elevation of TLR2 in the colon, liver, or spleen of Hpa-tg mice (not shown). However, the expression of MIP-2, RANKL, and IL17BR was increased significantly in Hpa-tg tissue(s) (Fig. 4A); increased MIP-2 levels in the liver of Hpa-tg mice was further confirmed by immunostaining (Fig. 4A upper right panel). These results not only add the above mediators to the growing list of genes regulated by heparanase [9, 35–37] but also provide, possibly, a mechanism by which an abundance of systemic mediators in the plasma of Hpa-tg mice supports tumor growth.
Figure 4. Plasma proteins and cell motility.
A. Increased expression of selected proteins and genes in Hpa-tg vs. WT tissues. Plasma collected from tumor-free WT and Hpa-tg mice was subjected to antibody array. Several proteins that were up-regulated in the Hpa-tg mice (fold increase in hpa-tg vs. WT mice is shown in table 3 of figure 4) were selected for validation by qPCR. Briefly, total RNA was extracted from the indicated tissue of WT and Hpa-tg mice and subjected to real-time PCR analyses applying primers sets specific for MIP-2 (A, upper panel), RANKL (A, middle panel), IL17BR (A, lower panel), Sections of liver tissue were subjected to immunostaining applying anti-MIP-2 antibody (A, top right panel). B. Plasma of Hpa-tg mice enhances cell motility. Upper panel: Wound closure. 3×105 Panc02 cells were seeded into 96 wells plate in triplicates. Scratch was conducted using Wound Maker (Essen); plasma (1%) of WT (blue) and Hpa-tg (Tg, red) mice, diluted in DMEM medium, was added and pictures were taken by Essen Incucyte Zoom every 2 h, monitoring the closure of the scratch wound. Lower panel: Invasion assay. LLC cells (2×105) were plated on Matrigel and chemo-attraction was initiated by adding medium supplemented with 10% of WT plasma (left) vs. Hpa-tg (Tg) plasma (right) to the lower compartment for 6 h. Shown are representative images of cells invading through Matrigel. Note, that Hpa-tg plasma is far more effective than WT plasma in chemo-attraction of cancer cells.
Tumor cell migration and invasion
We investigated the possible contribution of systemic factors to tumor cell migration and invasion. For this purpose, we applied the Wound Maker scratch assay to monitor the rate of cell migration (wound closer), and the Matrigel invasion assay to monitor cell invasion. As presented in Figure 4B, plasma from Hpa-tg mice induced an increased rate of wound closure (Essen Incucyte Zoom) by Panc02 cells as compared to plasma from WT mice. The Hpa-tg plasma also accelerated the invasion of LLC cells through Matrigel to a much higher extent than WT plasma (Fig. 4C). These results can be attributed to soluble factors revealed by the antibody array to be elevated in the plasma of Hpa-tg vs. WT mice (Table 3 in Fig. 4).
Impact of host heparanase on cancer metastasis
LLC cells
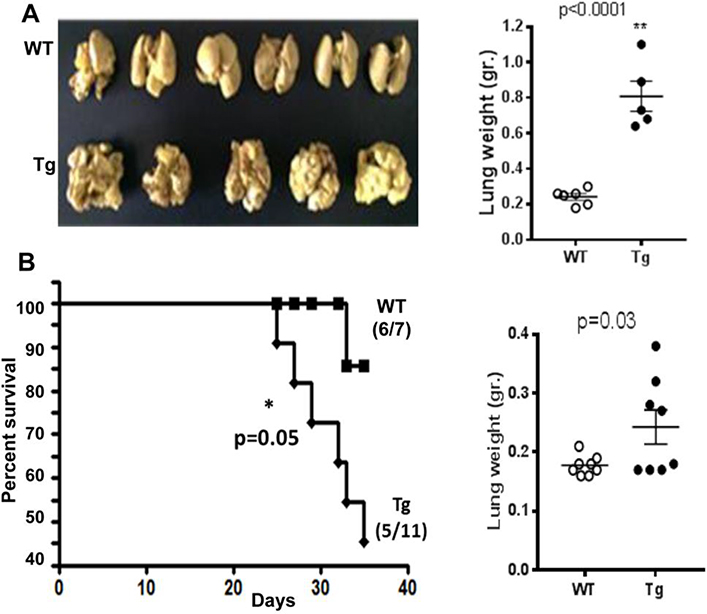
Apart from an effect on primary tumor growth, it is likely that host-derived heparanase will also modify and dictate the site/niche of secondary tumor development, thereby contributing to metastasis. To examine this, mouse Lewis Lung Carcinoma (LLC2) cells were injected into the tail vein of Hpa-tg and WT mice. As demonstrated in Figure 5A, inspection of the mouse lungs on day 17 after cell inoculation revealed massive colonization (~ 100 colonies/lung) of metastatic cells in the lungs of Hpa-tg mice as opposed to little or no colonization in the lungs of WT mice (Fig. 5A, left panel). Similarly, while the weight of lungs excised from WT mice was nearly the same as that of control mice (~200 mg) that were not injected with tumor cells, the weight of Hpa-tg lungs infiltrated with metastatic LLC cells was 3–4 folds higher than that of WT mice (Fig. 5A, right panel), attributed to metastatic tumor colonies developed in the lungs.
Figure 5. Overexpression of host heparanase accelerates tumor metastasis.
A. Experimental metastasis. LLC2 cells (1×106) suspended in 100 μl PBS were injected into the tail vein of Hpa-tg and WT C57BL mice and the lungs were dissected on day 17 post-injection. Lung colonization by tumor foci was visualized after fixation of the tissue in Bouin’s solution (left panel). Lungs were excised from Hpa-tg and WT mice and their weights measured (right panel). B. Spontaneous metastasis. LLC cells (3.5×105) were injected S.C to WT (cont.) and Hpa-tg (TG) mice; tumors were removed after 18 days when approaching a similar tumor volume (750 mm3), and survival of the mice was monitored (left panel). Lungs were excised from Hpa-tg and WT mice and their weights measured (right panel).
In a spontaneous tumor metastasis model (Fig. 5B), LLC cells were injected s.c to WT and Hpa-tg mice; tumors were removed after 18 days when approaching a similar tumor volume (~750 mm3), and survival of the mice was monitored. Survival of Hpa-tg mice was markedly reduced. Thus, while only one out of seven mice in the WT group died (14%), 55% of the Hpa-tg mice (6/11) died due to lung metastases (Fig. 5B, left panel). Macroscopically, lung metastases were noted in 8/8 Hpa-tg mice as opposed to 1/8 in WT mice, as also reflected by measurements of lungs weights (Fig. 5B, right panel), indicating that overexpression of heparanase in the lung tissue facilitates the homing and growth of metastatic cells. Collectively, these results indicate that host-derived heparanase is an important determinant in the formation of appropriate metastatic niche and dictating the extent of metastatic spread, colonization and growth.
Pancreatic carcinoma cells
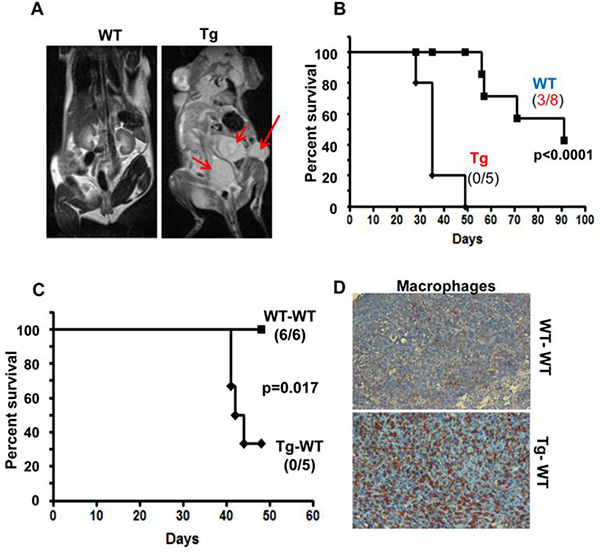
To further explore the effect of heparanase-high host on experimental metastasis, we employed mouse Panc02 cells, which normally do not metastasize when injected into the tail vein. MRI imaging performed on day 40 following Panc02 cell inoculation revealed substantial tumor development in Hpa-tg but not in WT mice (Fig. 6A, arrows). This was associated with a marked decrease in the survival rate of Hpa-tg mice. Thus, by 50 days following cell inoculation all Hpa-tg mice died while none of the WT mice were dead. Forty percent of the WT mice were still alive when the experiment was terminated on day 90 (Fig. 6B), differences that are statistically highly significant (p=0.0001). This suggests that heparanase not only facilitates cell invasion and metastasis (seed) but also converts target tissues to a congenial soil. To better elucidate the cellular mechanism(s) that underlines the pro-metastatic host effect, we employed bone marrow transplantation. WT mice were irradiated lethally and their bone marrow (BM) was substituted with BM cells collected from WT (WT-WT) or Hpa-tg (Tg-WT) mice. Following recovery, Panc02 cells were injected into the tail vein. As shown in figure 6C, the survival rate was reduced significantly in mice receiving Hpa-tg BM. Thus, upon termination of the experiment on day 50, all the Tg-WT mice were dead while all the WT-WT mice were still alive (Fig. 6C). Notably, macrophages were highly abundant in Tg-WT vs. WT-WT tumors (Fig. 6D), suggesting the contribution of macrophages to the pro-metastatic impact of heparanase. These and other results [24–26, 38] prompted us to investigate the relative levels of various immune cell populations in Hpa-tg vs. WT mice.
Figure 6.
Host heparanase facilitates pancreatic tumor metastasis associating with reduced survival. A,B. Inoculation (i.v) of Panc02 mouse pancreatic cancer cells to WT and Hpa-tg mice. A. Representative MRI images of WT and Hpa-tg mice on day 40 following Panc02 cell injection. Note, many tumor lesions in Hpa-tg mice (arrows) vs. none in WT mice. B. Survival of WT and Hpa-tg mice following Panc02 cell inoculation. Note, reduced survival of Hpa-tg mice. C,D. BM transplantation. WT mice were lethally irradiated and their BM was replaced with BM cells (5×106) collected from WT (WT-WT) or Hpa-tg (Tg-WT) tibias. Panc02 cells were injected i.v six weeks thereafter and the survival rate was followed (C). Note a significant decrease in the survival rate of mice that have high heparanase levels in BM-derived cells. D. Immunostaining. Upon termination, tumors were excised and subjected to immunostaining applying anti-F4/80 antibody, a marker for mouse macrophages. Note, high abundance of macrophages in tumors developed in Hpa-tg mice.
Immune cell populations in Hpa-tg vs. WT mice
Having demonstrated the significance of the host in tumor initiation, growth and metastasis, we have next attempted to identify the type of host immune cells that may support tumor progression in Hpa-tg vs. WT mice. For this purpose, we first examined (flow cytometry) the percentage of various immune cell populations in the spleen, inguinal lymph nodes (LN) and blood-derived from WT and Hpa-tg mice, 17 days after subcutaneous inoculation of LLC cells (Figs. 7, 8; Supp. Table 1). Likewise, LLC tumors grown in the flank of WT and Hpa-tg C57BL mice were excised, dissociated and subjected to FACS analysis to examine the type and level of immune cells that infiltrate the primary tumors (Fig. 9).
Figure 9.
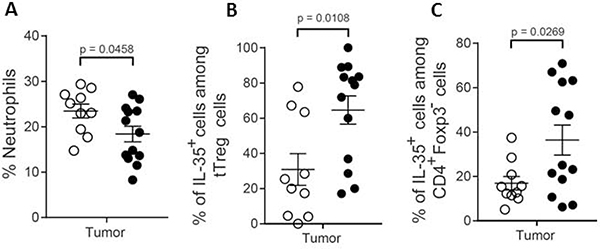
Characterization of tumor-infiltrating immune cells. Luciferase labeled LLC cells (3.5×105) were injected s.c into WT and Hpa-tg mice. Tumors were removed on day 11, dissected and dissociated into a single-cell suspension. Cells (5×105) were then cultured for 48h and the luciferase positive cells subjected to flow cytometry (FACS) analysis to examine the type and level of immune cells infiltrating into the primary tumors. A. Neutrophils, B. IL-35+ cells among tTreg cells, and C. IL-35+ cells among CD4+Foxp3− cells.
Immune cells in the spleen, LNs and blood
While the proportion of F4/80+ macrophages was significantly lower in the spleen and LNs of Hpa-tg vs. WT mice (Fig. 7A, top, left panel), there was no difference in the percentage of neutrophils (Fig. 7A, top, right panel). Increased proportions of both CD11b−CD11c+ conventional dendritic cells (DCs) and CD11b+CD11c+ tolerogenic DCs were found in the spleen of Hpa-tg vs. WT mice (Fig. 7A, bottom, panels). Notably, there was a marked 3-fold elevation in the weights of spleens excised from Hpa-tg vs. WT mice (Suppl. Fig. 2), indicating an altered immune response. Flow cytometric analysis showed a higher proportion of CD19+ cells in the LNs of Hpa-tg than WT mice (Fig. 7B, top, left panel). However, the proportions of Breg cells, which were determined as CD19+CD5+CD1d+ cells, did not differ between the groups (Fig. 7B, top, right panel). IL-35 is a potent anti-inflammatory cytokine that is produced mainly by Treg and Breg cells [39–41] and increased levels of IL-35 have been found in many cancer types, limiting anti-tumor immunity [42, 43]. Analysis of IL-35 positive cells among Breg cells revealed increased levels in the spleen, LNs and blood of Hpa-tg vs. WT mice (Fig. 7B, bottom, left panel) suggesting that Breg cells contribute to the enhanced tumor growth observed in Hpa-tg mice.
T cells play key roles in tumor immunity by altering the expression of surface molecules such as CTLA-4 and PD-1 that negatively regulate T cell function [44]. The proportion of CD4 and CD8 T cells was lower in the spleen and LNs of Hpa-tg as compared to WT mice (Supp. Fig. 3A,B). Moreover, the proportions of PD-1+ and CTLA-4+ cells among CD4 T cells were increased in the spleen and LNs of Hpa-tg mice (Supp. Fig. 3C,D). While there was no difference in the proportion of PD-1+ cells among CD8 T cells (Supp. Fig. 3E), the proportion of CTLA-4+ cells among CD8 T cells was decreased in the blood of Hpa-tg mice (Supp. Fig. 3F). These results suggest that overexpression of heparanase may promote tumor growth by increasing the expression of PD-1 and CTLA-4 on CD4 T cells. Consistent with the increased rate of tumor growth in Hpa-tg mice, we found higher percentages of IL-35+ cells among CD4+Foxp3− and CD8+Foxp3− T cells in Hpa-tg vs. WT mice (Fig. 8A, top panels). We also observed a decrease in IFN-γ+ cells among both CD4+Foxp3− and CD8+Foxp3− T cells in Hpa-tg mice (Fig. 8A, bottom panels). Taken together, these results suggest that overexpression of heparanase may impair the anti-tumor activity of T cells by increasing IL-35 and decreasing IFN-γ production by T cells.
As Treg cells are the main producers of IL-35, we next examined the proportion of IL-35+ cells among Treg cells and found that it was higher in the spleen and LN of Hpa-tg vs. WT mice (Fig. 8B, top, left panel). Thymic derived Treg (tTreg) cells and peripherally induced Treg (pTreg) cells are the two subsets of Treg cells. Helios, used to distinguish between tTreg and pTreg cells [45], is required for the stable inhibitory ability of Treg cells and considered as a marker of tTreg cells. We found that the MFI of Helios in Treg cells was higher in the spleen and LN of Hpa-tg mice and in the blood of WT mice (Fig. 8B, top, right panel). We next determined the proportions of IL-35+ and IFN-γ+ cells among tTreg and pTreg cells, and found that the percentage of IL-35+ cells among both tTreg and pTreg cells was higher in the spleen, LN, and blood of Hpa-tg mice (Fig. 8B, middle panels). In contrast, the proportions of IFN-γ+ cells were lower among tTreg and pTreg cells in the LN and blood of Hpa-tg mice (Fig. 8B, bottom panels). These results illustrate that in WT mice Treg cells switch their phenotype from IL-35 producing anti-inflammatory Treg cells to IFN-γ producing pro-inflammatory Treg cells, thereby promoting anti-tumor immunity. Altogether, it appears that the stability and lack of ability of Treg cells to undergo phenotypic shift contribute to the decreased anti-tumor immunity in Hpa-tg mice.
Tumor-infiltrating immune cells
Hpa-tg tumors exhibited a marked elevation in the percentage of infiltrated IL35 positive cells among both Treg (p = 0.0108) and CD4+Foxp3− (p = 0.0269) cell populations (Fig. 9 middle and right panels). Although there was no difference in the percentage of total Breg cells, the percentage of IL-35 positive B cells was significantly elevated in the Hpa-tg LCC tumors. There was no significant difference in the percentage of CD19 cells nor in the percentage F4/80 positive cells in tumors excised from Hpa-tg and WT mice (not shown). Likewise, there was no difference in the percentage of CD11B−CD11C+ cells. The percentage of CD11b+CD11c+ and CD11b+CD11c+CD8+ was elevated in tumors excised from Hpa-tg vs. WT mice (not shown).
Discussion
Evidence accumulating in the last two decades has critically staged heparanase at the heart of tumor progression and metastasis [10, 12, 13]. This led to the development of heparanase inhibitors, some of which (i.e., PG545= Pixatimod) [46] are being evaluated in advanced clinical trials alone and in combination with other drugs [47]. Less attention was, nonetheless, directed toward deciphering the protumorigenic role of systemic heparanase contributed by the host and/or expressed by host cells that constitute the tumor microenvironment [22, 23]. Investigating this topic, we have reported that heparanase-neutralizing monoclonal antibodies attenuate the growth of human lymphoma cells by targeting heparanase activity contributed by cells of the tumor microenvironment [21], but the nature of these cells has not been characterized. Focusing on macrophages, we and others have reported that heparanase is intimately involved in cytokine gene regulation [24, 25, 30, 38]. We have further noted that heparanase and chemotherapy synergize in driving macrophage activation, polarization and migration, resulting in enhanced tumor growth, thus extending the repertoire of host heparanase functions in tumorigenicity and drug resistance [24, 48]. In the current research, we investigated the impact of host heparanase on tumor initiation, growth and metastasis using, among other systems, a genetic model of mice over-expressing (Hpa-tg) heparanase and characterizing the profile of immune cells and systemic factors that appear to mediate the pro-tumorigenic effect exerted by the host.
Tumor initiation
Investigating the susceptibility of Hpa-tg mice to chemical carcinogenesis (DMBA), we have noted that tumor initiation was increased substantially following overexpression of heparanase. Unlike WT mice, tumor formation was not restricted to the mammary glands, but rather appeared widespread in the liver, lungs, and other internal organs (Fig. 1B), resulting in extensive disease. The molecular mechanism underlying enhanced tumor initiation/promotion by heparanase in response to intragastric administration of DMBA is not entirely clear, but likely involves co-operation of heparanase with oncogenes (i.e., Myc) other than Ras shown previously to play an important role in the two-step DMBA/TPA skin cancer model [18].
Tumor growth
While the role of host heparanase in tumor metastasis awaits in-depth investigation (see below), its pro-tumorigenic function in the microenvironment of the primary tumor is emerging [21, 24, 25]. Consistently, our results indicate that tumor growth is affected by the levels of heparanase in the host. Thus, Panc02, B16, El-4 and LLC cells developed bigger tumors once implanted in Hpa-tg mice (Fig. 2A-D), and this was associated with increased cell proliferation (BrdU; 2E, F) and decreased cell death (cleaved caspase 3; 2E, G). Strikingly, smaller tumors were developed by LLC and El-4 cells transplanted in Hpa-KO mice (Supp. Fig. 1). Immunostaining revealed the recruitment of heparanase-positive host cells to tumors developed by Panc02 cells in Hpa-tg mice (Fig. 3A, upper panels), associating with increased heparanase activity in these tumors (Fig. 3B, upper panel), in accordance with increased tumor weight (Fig. 2A). In contrast, heparanase-positive host cells were not detected in tumors developed by B16 melanoma cells in Hpa-tg mice (3A, lower panels), nor was an evident increase in heparanase activity in these tumors (3B, lower panel) despite being bigger (Fig. 2B). These results led us to propose that in some tumor models [i.e., Panc02, EL4 (present study), Raji lymphoma [21]] enhanced tumor growth in Hpa-tg mice is mainly due to recruitment of heparanase-positive host cells [49], whereas in other models (i.e., B16 melanoma), tumor enhancement may be due primarily to systemic factors that are elevated in the plasma of Hpa-tg mice. Indeed, we found that the level of several proteins is increased markedly in the plasma of Hpa-tg mice (Table 3 in Fig. 4), and were able to validate the induction of some of these mediators by real-time PCR and immunostaining (Fig. 4A). Moreover, we could demonstrate that this elevation is functional and contributes significantly to cell migration and invasion (Fig. 4B,C). Among others, we found increased levels of SPARC (Secreted Protein Acidic and Cysteine Rich), MIP2, RANKL, and IL17BR in the plasma of Hpa-Tg mice (Table 3 in Fig. 4), proteins that are highly implicated in tumor growth, metastasis, and osteolysis [50–52]. Increased RANKL levels in the plasma of Hpa-tg mice agrees with previous reports showing that heparanase upregulates the expression and secretion of RANKL in myeloma cells and patients, leading to enhanced osteolysis [53]. Of note, MIP-2 was among the factors most prominently elevated in Hpa-tg plasma (Table 3 in Fig. 4) and tissues (colon, liver, bone marrow) as revealed at the protein and mRNA levels (Fig. 4). Furthermore, we have demonstrated that MIP-2 expression was markedly reduced in macrophages isolated from Hpa-KO vs. control mice [24, 25] whereas exogenous addition of heparanase prominently stimulated MIP-2 expression [24]. Collectively, these results strongly suggest that MIP-2 expression is tightly regulated by heparanase.
Tumor metastasis
Heparanase activity has been shown to correlate with the metastatic potential of cancer cells since the early days of heparanase research [54, 55]. This correlation gained a substantial preclinical and clinical support soon after cloning of the heparanase gene and the development of molecular tools that enable silencing of heparanase or its over-expression, as well as the development of heparanase inhibitors [10, 13, 56, 57]. Heparanase is thus considered as an intrinsic component of the ‘seed’ (i.e., the metastatic cell). Less attention was nonetheless given to the role of heparanase in the ‘soil’ [17].
The results obtained with LLC (Fig. 5) and Panc02 (Fig. 6) cells suggest that heparanase also plays a critical role in creating an environment that is favorable for metastatic tumor cell colonization and growth. Strikingly, once inoculated intra-venous, LLC (Fig. 5) and Panc02 (Fig. 6) cells developed a far greater number of tumor metastases in Hpa-tg than WT mice, associating with reduced survival (Fig. 6B). A similar result was noted in a spontaneous metastasis model (LLC, Fig. 5B). Furthermore, replacement of the bone marrow of WT mice with bone marrow of Hpa-tg mice was sufficient to recapitulate this phenotype. Thus, all Hpa-tg-WT mice died by 50 days following Panc02 cell inoculation while all WT-WT mice were still alive (Fig. 6C). This effect may be attributed in part to the elevated levels of MIP-2 found in the bone marrow (Fig. 4) of Hpa-tg vs. WT mice and reported previously to accelerate tumor metastasis [58–60]. It should be noted that in this model system, tumors do develop also in WT-WT mice but appear less aggressive and were decorated with a significantly fewer number of macrophages compared with tumors developed in Hepa-tg-WT mice (Fig. 6D).
Heparanase can affect the site of cell seeding (‘soil’) in several ways. It can modify the soil enzymatically (i.e., alter the structural integrity of the ECM) and/or affect its pro-adhesion function [61, 62]. Enzymatically, heparanase releases HS-bound growth factors and chemokines that attract metastatic cancer cells and support their survival and growth, thereby driving the metastatic process. This is important because the ECM provides not only a physical scaffold for cells in the tumor microenvironment but also has a dynamic role in the spread of cancer, especially as the adhesion of a cell to the ECM is key to its movement out of and into the tumor microenvironment [5, 8]. Also, higher levels of factors that are released systemically following heparanase overexpression may affect the homing of tumor cells to specific sites. Among the systemic candidate mediators that were identified are SPARC, CXCL2, MIP2, RANKL, IL17BR, and FGFR5 shown by antibody protein array to be elevated in the plasma of Hpa-tg as opposed to WT mice (Table 3 in Fig. 4).
Immune cells
Immune cells constitute a major cellular component of the tumor microenvironment, infiltrating the tumor tissue and, depending on the type of tumor and immune cells, can either stimulate or suppress tumor growth [26]. Likewise, leukocyte-heparanase can promote tumor survival and growth [21, 24] but also can enhance tumor immune surveillance and tumor cell clearance [63, 64]. Importantly, tumor cells can utilize their own heparanase as well as heparanase from the tumor microenvironment with similar outcomes on tumor progression [21]. Expression of heparanase by leukocytes is inducible by various cell activation stimuli [31, 65, 66], promoting leukocyte migration [67, 68], cell rolling and adhesion [69–71], upregulation of pro-inflammatory cytokines [38, 49], and activation of innate immune cells [24, 25, 30]. Having demonstrated the significance of host heparanase in tumor initiation, growth and metastasis, we applied FACS analysis [68] to identify the type of host immune cells that may support tumor progression in Hpa-tg vs. WT mice. Briefly, we determined the percentage of immune cell populations in the spleen, inguinal lymph nodes (LN) and blood taken from tumor-bearing WT and Hpa-tg mice. Subsequently, we examined the type and level of immune cells that infiltrated the primary LLC2 tumors grown in the flank of WT v. Hpa-tg C57BL mice. Among other effects, our results indicate that overexpression of heparanase in Hpa-tg mice impairs the anti-tumor ability of T cells by increasing IL-35 and decreasing IFN-γ produced by T cell subpopulations. Analysis of IL-35 positive cells among Breg cells revealed increased levels in the spleens, LNs and blood of Hpa-tg vs. WT mice (Supp. Table 1) suggesting that Breg cells contribute to the enhanced tumor growth observed in Hpa-tg mice. It was also found that in WT but not Hpa-tg mice Treg cells switched their phenotype from IL-35 producing anti-inflammatory cells to IFN-γ producing pro-inflammatory Treg cells, suggesting that the stability and lack of ability of Treg cells to undergo phenotypic shift contributed to a decreased anti-tumor immunity in Hpa-tg mice. It further appeared that overexpression of heparanase facilitates the recruitment of CD19+ cells to lymph nodes located in close proximity to the tumor, where they are influenced by the immunosuppressive tumor microenvironment to differentiate into IL-35+ Breg cells. It was also noted that overexpression of heparanase may promote tumor growth by increasing the expression of PD-1 and CTLA-4 on CD4 T cells (Suppl. Fig. 3). Finally, analysis of the tumor-infiltrating immune cells revealed a marked elevation in the percentage of IL35 positive cells among the Treg, Breg and CD4+Foxp3− cell populations (Fig. 9) infiltrating the Hpa-tg LCC tumors.
It should be noted that the above described immune cell analyses were restricted to LLC tumor-bearing mice. Similar studies applying the other tumor models that were investigated (DMBA carcinogenesis, panc02 carcinoma, EL4 lymphoma, B16 melanoma) are needed to ascertain and generalize the notion that the observed accelerated tumor growth is attributed to an overall impaired anti-tumor activity of the Hpa-tg immune cells (e.g., increased levels of IL-35 producing T cells and decreased levels of IFN-γ producing T cell subpopulations). Also, the pattern of immune cell distribution in tumor-free Hpa-tg vs. wild type mice needs to be analyzed. As previously reported the role of heparanase in various immune cell types is multi-faceted, with effects on immune cell gene regulation, differentiation, and migration/invasion [1]. Heparanase enters the nucleus of activated T lymphocytes, regulates histone methylation, and co-localizes with RNA polymerase II at promoters of actively transcribed immune genes, including IFNγ, IL-2 and TNFα [35, 72]. Heparanase is also vital for macrophage activation, polarization and function, including cytokine production and tumor cell infiltration [25, 30, 38]. Likewise, expression of heparanase by dendritic [67], NK [64] and T [63] cells is vital for their recruitment and ability to migrate, resulting in overall depletion of immune system defenses [1]. It is therefore not surprising that leukocyte-derived heparanase can either assist or impede tumor progression, depending on the setting [26]. Applying several experimental systems we have demonstrated the pro-tumorigenic effects of host systemic and immune cell heparanase, yet given the complexity of the immune system it should be emphasized that we are just beginning to uncover the influence of heparanase on the pro- and/or anti-tumor immune response [26]. Together, these results support an overall tumor-promoting effect exerted by immune cell subpopulations residing in the Hpa-tg vs. WT mice and tumors. Collectively, our results emphasize the significance of heparanase in mediating the pro-tumorigenic interactions between the tumor, the tumor microenvironment, immune cells and systemic factors generated by the host.
Experimental procedures
Cells and cell culture
Mouse Lewis lung carcinoma (LLC2) [73], PANC02 pancreatic ductal adenocarcinoma [74], EL-4 lymphoma [21, 25] and B16-F10 melanoma [57, 75] were purchased from the American Type Culture Collection. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with glutamine, pyruvate, antibiotics and 10% fetal calf serum (Biological Industries, Beit Haemek, Israel) in a humidified atmosphere containing 5% CO2 at 37°C.
Animals
Transgenic mice overexpressing human heparanase (Hpa-tg), heparanase knockout (Hpa-KO) [76], and wildtype (WT) C57BL/6 mice were maintained in the animal facility, located at the Biomedical Center, Uppsala University (Uppsala, Sweden) and the Rappaport Faculty of Medicine (Technion, Haifa). The experiments were performed in accordance with the Technion’s Institutional Animal Care and Use Committee (IL-080–08-2018; OPRR-A5026–01) and the local ethics committee (C105/15) at Uppsala Court, Sweden.
Cancer models
For experimental metastasis, LLC2 (1×106) or Panc02 (1.5×105) cells suspended in 100 μl of PBS were injected into the tail vein of 6–8 week old C57BL WT or Hpa-tg mice. Body weights were measured twice a week. The animals were sacrificed 17 days after inoculation and the lungs were fixed in Bouin’s solution for analysis of tumor foci colonization in the lungs [57, 77]. For flank inoculation, LLC2, EL-4, B16-F10 or Panc02 cells suspended in 100 μl of PBS were injected into the right flank of 6–8 week old C57BL WT, Hpa-KO or Hpa-tg mice [24, 25]. Xenografts size was determined by externally measuring tumors in 2 dimensions using a digital caliper. Tumor volume (V) was determined by the equation V=L × W2 × 0.5, where L is the length and W the width of the xenograft [78]. Each experiment was repeated at least twice, with 6–10 mice per group.
DMBA treatment
Two or four administrations of 7, 12-dimethylbenz(a)anthracene (DMBA, Sigma; 50 μg/0.1 ml acetone) were given (p.o; 1 or 1.5 mg/mouse) once a week to Balb/c WT and Hpa-tg mice. Mice were examined weekly to detect tumor development. Mice were sacrificed when tumors exceeded 1000 mm3 or when mice turned morbid. Tumors were excised and fixed in formalin for histological evaluation [78]. Each experiment was repeated at least twice, with at least 6 mice per group.
Bone marrow transplantation
Bone marrow (BM) transplantation was performed, as previously described [25, 79]. Briefly, BM cells were obtained by flushing femurs and tibias of 8-week-old donor C57BL/6 WT and Hepa-KO mice. The BM cells (5×106 per recipient) were transplanted by tail vein injection into lethally irradiated C57BL/6 WT recipients [1,000 cGy total body irradiation (250 cGy/minute)] using Elekta Precise (ElektaOncology Systems) linear accelerator 6MeV photon beam radiation]. Experiments were performed six weeks after BM cell reconstitution [25, 79].
Heparanase activity
Preparation of dishes coated with sulfate labeled ECM [80] and determination of heparanase enzymatic activity (i.e., release of sulfate labeled HS degradation fragments) were carried out essentially as described [21, 75, 81, 82]. Briefly, tumors were excised, homogenized and lysed by three freeze/thaw cycles and the resulting extracts were incubated (24 hours, 37°C, pH 6.0) with 35S-labeled ECM. The incubation medium was analyzed by gel filtration on Sepharose CL-6B. Fractions of 0.2 ml are eluted with PBS and measured for their radioactivity [75, 80]. Degradation fragments of HS side chains are eluted from Sepharose 6B at 0.5 < Kav < 0.8 (peak II, fractions 17–30). Nearly intact sulfate labeled HSPGs are eluted just after the void volume (Kav < 0.2, peak I, fractions 1–10). These high molecular weight products are released by proteases that cleave the HSPG core protein [75]. Each experiment was performed three times and the variation in elution positions (Kav values) did not exceed ± 15%.
Histology and immunohistochemistry
Histological examination and immunostaining of formalin-fixed, paraffin-embedded 5-micron sections were performed essentially as described [24, 25]. Briefly, slides were deparaffinized, rehydrated and endogenous peroxidase activity was quenched (30 min) by 3% hydrogen peroxide in methanol. Slides were then subjected to antigen retrieval by boiling (20 min) in 10 mM citrate buffer, pH 6. Following washes and blocking with 10% normal goat serum (NGS) in PBS, slides were incubated (20 h, 4°C) with the indicated antibody diluted in blocking solution. Slides were then extensively washed and incubated with a secondary reagent (Envision kit) according to the manufacturer’s (Dako, Glostrup, Denmark) instructions. Color was developed with the AEC reagent (Dako, Glostrup, Denmark), and sections were counterstained with hematoxylin and mounted [24, 25, 78]. BrdU incorporation was performed as previously described [82].
Real-time PCR analyses
Total RNA was extracted with TRIzol (Sigma) and RNA (1 μg) was amplified using the one-step PCR amplification kit, according to the manufacturer’s (ABgene, Epsom, UK) instructions. Real time-PCR analyses were performed using ABI PRISM 7000 Sequence Detection System employing SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) [24, 25].
Protein array
Plasma samples collected from WT and Hpa-tg C57BL mice were analyzed using Biotin Label-based Antibody Array (RayBio®; Cat. number AAM-BLG-1–2) performed according to manufacturer instruction [25].
Wound healing (scratch) assay
Panc02 or B16-F10 were seeded in an ImageLock 96-well plate at a density of 30,000 cells/well and cultured for 24 h until cells were adherent. All wells were inflicted by a wound across the cell layer using WoundMaker™, washed with PBS and supplemented with fresh DMEM serum-free medium containing 1% plasma from Hpa-tg or WT mice. Cell migration was monitored using an IncuCyte Zoom microscope (Essen Bioscience, MI, USA), with image acquisition every two h for 46 h or 34 h. The IncuCyte Zoom image analysis software was used to quantify wound closure [25].
Cell migration and invasion
Invasion and migration assays were performed using modified Boyden chambers with polycarbonate Nucleopore membrane (Corning, Corning, NY), essentially as described [25, 83]. Briefly, filters (6.5 mm in diameter, 8 μm pore-size) were coated with Matrigel (BD Biosciences; 30 μl; cell invasion) or fibronectin (Biological Industries; 1 mg/ml; cell migration). Cells (5 × 105) in 100 μl of serum-free medium were seeded in triplicate on the upper part of each chamber, and the lower compartment was filled with 600 μl medium supplemented with 10% FCS. After incubation for 6 h at 37°C in a 5% CO2 incubator, non-migrating/invading cells on the upper surface of the filter were wiped with a cotton swab, and migrating/invading cells on the lower surface of the filter were fixed, stained with 0.5% crystal violet (Sigma) and counted by examination of at least seven microscopic fields.
Antibodies and Flow cytometry
Spleen, inguinal lymph nodes (LN) and blood were collected from WT and Hpa-tg mice, 17 days after subcutaneous flank inoculation of 1×106 LLC cells. Single-cell suspensions from spleen and LN were prepared as previously described [68, 84] and single-cell suspension from the tumor was prepared as described earlier [25, 79]. The red cells were lysed by the addition of 0.2 M NH4Cl. Cells were then washed twice with RPMI and stained with the following surface antibodies: FITC-conjugated CD4 (RM4–5, eBioscience, San Diego, CA, USA), APC-H7-conjugated CD8 (53–6.7, BD Biosciences, San Jose, CA, USA), BV605-conjugated CD5 (53–7.3, BD Biosciences), APC-H7-conjugated CD19 (1D3, BD Biosciences), FITC-conjugated CD1d (1B1, BioLegend, San Diego, CA, USA), APC-conjugated PD-1 (29F.1A12, BioLegend), FITC-conjugated CD11b (M1/70, BioLegend), BV605-conjugated CD11c (N418, BioLegend), BV421-conjugated F4/80 (BM8, BioLegend), BV421-conjugated Ly6G (1A8, BioLegend), APC-conjugated CD49d (DATK32, BioLegend) and PE-conjugated CXCR4 (L276F12, BioLegend). The cells were then permeabilized and fixed with Fixation/Permeabilization buffer (eBioscience). The following antibodies were used to stain the permeabilized cells: PE-Cy7-conjugated Foxp3 (FJK-16s, eBioscience), APC-conjugated Ebi3 (355022, R&D Systems, Minneapolis, MN, USA), PE-conjugated IL-12p35 (27537, R&D Systems), PacificBlue-conjugated Helios (22F6, BioLegend), BV605-conjugated IFN-γ (XMG1.2, BioLegend), PE-conjugated CTLA-4 (UC10–4B9, BioLegend). Fluorescence minus one (FMO) controls were applied. The cells were subjected to flow cytometry on LSRFortesa (BD) using DivaDacker software (BD) at BioVis (Uppsala University, Sweden) and the files were analyzed on FlowLogic (Inivai Technologies, Australia) [68, 84]. Anti-heparanase polyclonal antibody #773 has been described previously [78]. Anti-Ki67 monoclonal antibodies were purchased from Abcam (Cambridge, UK). Anti-cleaved caspase 3 anti-MIP2, anti-BrdU, and anti-F4/80 were purchased from Cell Signaling (Beverly, MA).
Statistical analysis
For parametric comparison, Student’s t-test was performed to compare means between two groups. Repeated measures ANOVA was used for tumor growth curves. Non-parametric Mann-Whitney U-test/Wilcoxon sign-rank test (for paired comparison) was used to compare two groups. GraphPad Prism 10.0 was used for statistical analyses. The threshold for statistical significance was set at α=0.05 (*P<0.05, **P<0.01 and ***P<0.001).
Supplementary Material
Highlights.
Mice overexpressing heparanase (Hpa-tg) were applied to reveal the contribution of host heparanase to tumor growth and metastasis.
Xenograft tumors transplanted in Hpa-tg mice exhibited accelerated tumor progression and shorter survival time.
Accelerated tumor growth was attributed to increased levels of pro-tumorigenic factors in the plasma and bone marrow of the Hpa-tg mice.
Host heparanase impaired the overall anti-tumor activity of immune T-cell subpopulations.
Heparanase mediates the pro-tumorigenic crosstalk between the tumor, tumor microenvironment, immune cells and host factors.
Acknowledgments
Funding information. This study was generously supported by research grants awarded to R.S and I.V by the National Institutes of Health (CA211752) and the United States-Israel Binational Science Foundation (BSF). It was also supported by grants from the Israel Science Foundation (grant 601/14), the ISF-NSFC joint research program (grant No. 2572/16 awarded to I.V and S-M.Y), and the Israel Cancer Research Fund (ICRF, awarded to I.V). I. Vlodavsky is a Research Professor of the ICRF. The work was further supported by grants from the Swedish Research Council (2015–02595), Swedish Cancer Foundation (150815) and National Nature Science Foundation of China (81673924, 81774039), Beijing Nature Science Foundation (7172095). Tahira Batool was PhD student on Erasmus Mundus (Expert4Asia) scholarship.
Abbreviations
- ECM
extracellular matrix
- HPSE
heparinase
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycans
- DMBA
7,12-Dimethylbenz[a]anthracene
- RANKL
receptor activator of nuclear factor-B ligand
- MIP-2
macrophage-inflammatory protein 2
- Hpa-tg mice
mice over-expressing heparinase
- WT
wild type
- BM
bone marrow
- LLC
Lewis lung carcinoma
Footnotes
Declarations of interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gaskin SM, Soares Da Costa TP, Hulett MD, Heparanase: Cloning, Function and Regulation, Adv. Exp. Med. Biol. 1221 (2020) 189–229. [DOI] [PubMed] [Google Scholar]
- [2].Ilan N, Bhattacharya U, Barash U, Boyango I, Yanku Y, Gross-Cohen M, Vlodavsky I, Heparanase-The message comes in different flavors, Adv. Exp. Med. Biol. 1221 (2020) 253–283. [DOI] [PubMed] [Google Scholar]
- [3].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75–76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sanderson RD, Bandari SK, Vlodavsky I, Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling, Matrix Biol. 75–76 (2019) 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Theocharis AD, Karamanos NK, Proteoglycans remodeling in cancer: Underlying molecular mechanisms, Matrix Biol 75–76 (2019) 220–259. [DOI] [PubMed] [Google Scholar]
- [6].Vlodavsky I, Ilan N, Sanderson RD, Forty Years of Basic and Translational Heparanase Research, Adv. Exp. Med. Biol. 1221 (2020) 3–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Iozzo RV, Gubbiotti MA, Extracellular matrix: The driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hammond E, Khurana A, Shridhar V, Dredge K, The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics, Front. Oncol 4 (2014) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khanna M, Parish CR, Heparanase: Historical Aspects and Future Perspectives, Adv. Exp. Med. Biol. 1221 (2020) 71–96. [DOI] [PubMed] [Google Scholar]
- [10].Rivara S, Milazzo FM, Giannini G, Heparanase: a rainbow pharmacological target associated to multiple pathologies including rare diseases, Future Med. Chem. 8 (2016) 647–80. [DOI] [PubMed] [Google Scholar]
- [11].Purushothaman A, Hurst DR, Pisano C, Mizumoto S, Sugahara K, Sanderson RD, Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype, J. Biol. Chem. 286 (2011) 30377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vlodavsky I, Gross-Cohen M, Weissmann M, Ilan N, Sanderson RD, Opposing functions of heparanase-1 and heparanase-2 in cancer progression, Trends Biochem. Sci. 43 (2018) 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N, Heparanase: From basic research to therapeutic applications in cancer and inflammation, Drug Resistance Updates 29 (2016) 54–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Giannini G, Battistuzzi G, Rivara S, The Control of Heparanase Through the Use of Small Molecules, Adv. Exp. Med. Biol. 1221 (2020) 567–603. [DOI] [PubMed] [Google Scholar]
- [15].Noseda A, Barbieri P, Roneparstat: development, preclinical and clinical studies, Adv. Exp. Med. Biol. 1221 (2020) 523–538. [DOI] [PubMed] [Google Scholar]
- [16].Chhabra M, Ferro V, PI-88 and related heparan sulfate mimetics, Adv. Exp. Med. Biol. 1221 (2020) 473–491. [DOI] [PubMed] [Google Scholar]
- [17].Arvatz G, Shafat I, Levy-Adam F, Ilan N, Vlodavsky I, The heparanase system and tumor metastasis: is heparanase the seed and soil?, Cancer Metastasis Rev. 30 (2011) 253–68. [DOI] [PubMed] [Google Scholar]
- [18].Boyango I, Barash U, Naroditsky I, Li JP, Hammond E, Ilan N, Vlodavsky I, Heparanase cooperates with Ras to drive breast and skin tumorigenesis, Cancer Res. 74 (2014) 4504–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramani VC, Zhan F, He J, Barbieri P, Noseda A, Tricot G, Sanderson RD, Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma, Oncotarget 7 (2016) 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shteingauz A, Boyango I, Naroditsky I, Hammond E, Gruber M, Doweck I, Ilan N, Vlodavsky I, Heparanase enhances tumor growth and chemoresistance by promoting autophagy, Cancer Res. 75 (2015) 3946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weissmann M, Arvatz G, Horowitz N, Feld S, Naroditsky I, Zhang Y, Ng M, Hammond E, Nevo E, Vlodavsky I, Ilan N, Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis, Proc. Natl. Acad. Sci. USA 113 (2016) 704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Blonska M, Agarwal NK, Vega F, Shaping of the tumor microenvironment: Stromal cells and vessels, Semin. Cancer Biol. 34 (2015) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yamauchi M, Gibbons DL, Zong C, Fradette JJ, Bota-Rabassedas N, Kurie JM, Fibroblast heterogeneity and its impact on extracellular matrix and immune landscape remodeling in cancer, Matrix Biol. (2020) PMID: 32442601. [DOI] [PubMed] [Google Scholar]
- [24].Bhattacharya U, Gutter-Kapon L, Kan T, Boyango I, Barash U, Yang SM, Liu J, Gross-Cohen M, Sanderson RD, Shaked Y, Ilan N, Vlodavsky I, Heparanase and Chemotherapy Synergize to Drive Macrophage Activation and Enhance Tumor Growth, Cancer Res 80(1) (2020) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gutter-Kapon L, Alishekevitz D, Shaked Y, Li JP, Aronheim A, Ilan N, Vlodavsky I, Heparanase is required for activation and function of macrophages, Proc. Natl. Acad. Sci. USA 113 (2016) E7808–E7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mayfosh AJ, Baschuk N, Hulett MD, Leukocyte Heparanase: A Double-Edged Sword in Tumor Progression, Front. Oncol. 9 (2019) 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Elkin M, Role of heparanase in macrophage activation, Adv. Exp. Med. Biol. 1221 (2020) 445–460. [DOI] [PubMed] [Google Scholar]
- [28].Li JP, Vlodavsky I, Heparin, heparan sulfate and heparanase in inflammatory reactions, Thromb. Haemost. 102 (2009) 823–8. [DOI] [PubMed] [Google Scholar]
- [29].Goldberg R, Meirovitz A, Hirshoren N, Bulvik R, Binder A, Rubinstein AM, Elkin M, Versatile role of heparanase in inflammation, Matrix Biol. 32 (2013) 234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blich M, Golan A, Arvatz G, Sebbag A, Shafat I, Sabo E, Cohen-Kaplan, Petcherski S, Avniel-Polak S, Eitan A, Hammerman H, Aronson D, Axelman E, Ilan N, Nussbaum G, Vlodavsky I, Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression, Arterioscler. Thromb. Vasc. Biol. 33 (2013) e56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Cohen-Kaplan V, Petcherski S, Avniel-Polak S, Eitan A, Hammerman H, Aronson D, Axelman E, Ilan N, Nussbaum G, Vlodavsky I, Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice, J. Clin. Invest. 121 (2011) 1709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zcharia E, Metzger S, Chajek-ShaulL T, Aingorn H, Elikn M, Friedmann Y, Weinstein T, Jin-Ping L, Lindahl U, Vlodavsky I, Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior, FASEB J. 18 (2004) 252–263. [DOI] [PubMed] [Google Scholar]
- [33].Cohen I, Pappo O, Elkin M, San T, Bar-Shavit R, Hazan R, Peretz T, Vlodavsky I, Abramovitch R, Heparanase promotes growth, angiogenesis and survival of primary breast tumors, Int. J. Cancer 118 (2006) 1609–17. [DOI] [PubMed] [Google Scholar]
- [34].Sun X, Zhang G, Nian J, Yu M, Chen S, Zhang Y, Yang G, Yang L, Cheng P, Yan C, Ma Y, Meng H, Wang X, Li JP, Elevated heparanase expression is associated with poor prognosis in breast cancer: a study based on systematic review and TCGA data, Oncotarget 27 (2017) 43521–43535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].He YQ, Sutcliffe EL, Bunting KL, Li J, Goodall KJ, Poon IK, Hulett MD, Freeman C, Zafar A, McInnes RL, Taya T, Parish CR, Rao S, The endoglycosidase heparanase enters the nucleus of T lymphocytes and modulates H3 methylation at actively transcribed genes via the interplay with key chromatin modifying enzymes, Transcription 3 (2012) 130–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Song T, Spillmann D, Transcriptomic analysis reveals cell apoptotic signature modified by heparanase in melanoma cells, J. Cel. Mol. Med. 23 (2019) 4559–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang F, Jia J, Lal N, Zhang D, Chiu AP, Wan A, Vlodavsky I, Hussein B, Rodrigues B, High glucose facilitated endothelial heparanase transfer to the cardiomyocyte modifies its cell death signature, Cardiovascular Res 112 (2016) 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goodall KJ, Poon IK, Phipps S, Hulett MD, Soluble heparan sulfate fragments generated by heparanase trigger the release of pro-inflammatory cytokines through TLR-4, PLoS One 9 (2014) e109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S, IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases, Nature 507(7492) (2014) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Vignali DA, Kuchroo VK, IL-12 family cytokines: immunological playmakers, Nat. Immunol. 13 (2012) 722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE, Interleukin-35 induces regulatory B cells that suppress autoimmune disease, Nat. Med. 20 (2014) 633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Turnis ME, Sawant DV, Szymczak-Workman AL, Andrews LP, Delgoffe GM, Yano H, Beres AJ, Vogel P, Workman CJ, Vignali DA, Interleukin-35 limits anti-tumor immunity, Immunity 44 (2016) 316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pylayeva-Gupta Y, Molecular Pathways: Interleukin-35 in Autoimmunity and Cancer, Clin. Cancer Res. 22 (2016) 4973–4978. [DOI] [PubMed] [Google Scholar]
- [44].Brunner-Weinzierl MC, Rudd CE, CTLA-4 and PD-1 Control of T-Cell Motility and Migration: Implications for Tumor Immunotherapy, Front. Immunol 9 (2018) 2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ, Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity, Proc. Natl. Acad. Sci. USA 113 (2016) 6248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Weissmann M, Bhattacharya U, Feld S, Hammond E, Ilan N, Vlodavsky I, The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action, Matrix Biol. 77 (2019) 58–72. [DOI] [PubMed] [Google Scholar]
- [47].Dredge K, Brennan TV, Hammond E, Lickliter JD, Lin L, Bampton D, Handley P, Lankesheer F, Morrish G, Yang Y, Brown MP, Millward M, A Phase I study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours, Br. J. Cancer 118 (2018) 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD, Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior, Matrix Biol. 65 (2018) 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hermano E, Meirovitz A, Meir K, Nussbaum G, Appelbaum L, Peretz T, Elkin M, Macrophage polarization in pancreatic carcinoma: role of heparanase enzyme, J. Nat. Cancer Inst. 106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gonzalez-Suarez E, Sanz-Moreno A, RANKL as a therapeutic target in cancer, The FEBS J 283(11) (2016) 2018–33. [DOI] [PubMed] [Google Scholar]
- [51].Jansen MP, Sieuwerts AM, Look MP, Ritstier K, Meijer-van Gelder ME, van Staveren IL, Klijn JG, Foekens JA, Berns EM, HOXB13-to-IL17BR expression ratio is related with tumor aggressiveness and response to tamoxifen of recurrent breast cancer: a retrospective study, J. Clin. Oncol 25 (2007) 662–8. [DOI] [PubMed] [Google Scholar]
- [52].Tai IT, Tang MJ, SPARC in cancer biology: its role in cancer progression and potential for therapy, Drug Resistance Updates 11 (2008) 231–46. [DOI] [PubMed] [Google Scholar]
- [53].Yang Y, Ren Y, Ramani VC, Nan L, Suva LJ, Sanderson RD, Heparanase enhances local and systemic osteolysis in multiple myeloma by upregulating the expression and secretion of RANKL, Cancer Res. 70 (2010) 8329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nakajima M, Irimura T, DiFerrante D, DiFerrante N, Nicolson GL, Heparan sulfate degradation: relation to tumor invasion and metastatic properties of Mouse B 16 Melanoma sublines., Science 220 (1983) 611–613. [DOI] [PubMed] [Google Scholar]
- [55].Vlodavsky I, Fuks Z, Bar-Ner M, Ariav Y and Schirrmacher V, Lymphoma cells mediated degradation of sulfated proteoglycans in the subendothelial extracellular matrix: relation to tumor cell metastasis., Cancer Res.43 (1983) 2704–2711. [PubMed] [Google Scholar]
- [56].Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, Brandt R, Ferro V, Bytheway I, PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models, Br. J. Cancer 104 (2011) 635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Edovitsky E, Elkin M, Zcharia E, Peretz T, Vlodavsky I, Heparanase gene silencing, tumor invasiveness, angiogenesis, and metastasis, J Natl Cancer Inst 96(16) (2004) 1219–1230. [DOI] [PubMed] [Google Scholar]
- [58].Kwon Y, Kim Y, Eom S, Kim M, Park D, Kim H, Noh K, Lee H, Lee YS, Choe J, Kim YM, Jeoung D, MicroRNA-26a/−26b-COX-2-MIP-2 loop regulates allergic inflammation and allergic inflammation-promoted enhanced tumorigenic and metastatic potential of cancer cells, J. Biol. Chem. 290 (2015) 14245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Menten P, Saccani A, Dillen C, Wuyts A, Struyf S, Proost P, Mantovani A, Wang JM, Van Damme J, Role of the autocrine chemokines MIP-1alpha and MIP-1beta in the metastatic behavior of murine T cell lymphoma, J. Leu. Biol 72(4) (2002) 780–9. [PubMed] [Google Scholar]
- [60].Sosnoski DM, Krishnan V, Kraemer WJ, Dunn-Lewis C, Mastro AM, Changes in cytokines of the bone microenvironment during breast cancer metastasis, Int. J. Breast Cancer 2012 (2012) 160265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Levy-Adam F, Feld S, Suss-Toby E, Vlodavsky I, Ilan N, Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans, PLoS ONE 3 (2008) e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Levy-Adam F, Ilan N, Vlodavsky I, Tumorigenic and adhesive properties of heparanase, Semin. Cancer Biol. 20 (2010) 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, Ittmann MM, Marchetti D, Dotti G, Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes, Nat. Med. 21 (2015) 524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Putz EM, Mayfosh AJ, Kos K, Barkauskas DS, Nakamura K, Town L, Goodall KJ, Yee DY, Poon IK, Baschuk N, Souza-Fonseca-Guimaraes F, Hulett MD, Smyth MJ, NK cell heparanase controls tumor invasion and immune surveillance, J. Clin. Invest. 127 (2017) 2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [65].Chen G, Wang D, Vikramadithyan R, Yagyu H, Saxena U, Pillarisetti S, Goldberg IJ, Inflammatory cytokines and fatty acids regulate endothelial cell heparanase expression, Biochemistry 43 (2004) 4971–7. [DOI] [PubMed] [Google Scholar]
- [66].de Mestre AM, Khachigian LM, Santiago FS, Staykova MA, Hulett MD, Regulation of inducible heparanase gene transcription in activated T cells by early growth response 1, J. Biol. Chem. 278 (2003) 50377–85. [DOI] [PubMed] [Google Scholar]
- [67].Poon IK, Goodall KJ, Phipps S, Chow JD, Pagler EB, Andrews DM, Conlan CL, Ryan GF, White JA, Wong MK, Horan C, Matthaei KI, Smyth MJ, Hulett MD, Mice deficient in heparanase exhibit impaired dendritic cell migration and reduced airway inflammation, Eur. J. Immunol. 44 (2014) 1016–30. [DOI] [PubMed] [Google Scholar]
- [68].Digre A, Singh K, Abrink M, Reijmers RM, Sandler S, Vlodavsky I, Li JP, Overexpression of heparanase enhances T lymphocyte activities and intensifies the inflammatory response in a model of murine rheumatoid arthritis, Sci. Rep 7 (2017) 46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O, Molecular behavior adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH, J. Exp. Med. 181 (1995) 1929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Changyaleket B, Chong ZZ, Dull RO, Nanegrungsunk D, Xu H, Heparanase promotes neuroinflammatory response during subarachnoid hemorrhage in rats, J. Neuroinflammation 14 (2017) 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lever R, Rose MJ, McKenzie EA, Page CP, Heparanase induces inflammatory cell recruitment in vivo by promoting adhesion to vascular endothelium, Am. J. Physiol. 306 (2014) C1184–90. [DOI] [PubMed] [Google Scholar]
- [72].Parish CR, Freeman C, Ziolkowski AF, He YQ, Sutcliffe EL, Zafar A, Rao S, Simeonovic CJ, Unexpected new roles for heparanase in Type 1 diabetes and immune gene regulation, Matrix Biol. 32 (2013) 228–33. [DOI] [PubMed] [Google Scholar]
- [73].Gingis-Velitski S, Loven D, Benayoun L, Munster M, Bril R, Voloshin T, Alishekevitz D, Bertolini F, Shaked Y, Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice, Cancer Res. 71 (2011) 6986–96. [DOI] [PubMed] [Google Scholar]
- [74].Meirovitz A, Hermano E, Lerner I, Zcharia E, Pisano C, Peretz T, Elkin M, Role of heparanase in radiation-enhanced invasiveness of pancreatic carcinoma, Cancer Res. 71 (2011) 2772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michal I, Spector L, Pecker I, Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis, Nat. Med. 5 (1999) 793–802. [DOI] [PubMed] [Google Scholar]
- [76].Zcharia E, Jia J, Zhang X, Baraz L, Lindahl U, Peretz T, Vlodavsky I, Li JP, Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases, PLoS ONE 4 (2009) e5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Elkin M, Vlodavsky I, Tail vein assay of cancer metastasis, Curr. Prot. Cell Biol Chapter 19 (2001) 19.2.1–19.2.7. [DOI] [PubMed] [Google Scholar]
- [78].Boyango I, Barash U, Fux L, Naroditsky I, Ilan N, Vlodavsky I, Targeting heparanase to the mammary epithelium enhances mammary gland development and promotes tumor growth and metastasis, Matrix Biol. 65 (2018) 91–103. [DOI] [PubMed] [Google Scholar]
- [79].Voloshin T, Alishekevitz D, Kaneti L, Miller V, Isakov E, Kaplanov I, Voronov E, Fremder E, Benhar M, Machluf M, Apte RN, Shaked Y, Blocking IL1beta pathway following paclitaxel chemotherapy slightly inhibits primary tumor growth but promotes pontaneous metastasis, Mol. Cancer Therap. 14 (2015) 1385–94. [DOI] [PubMed] [Google Scholar]
- [80].Vlodavsky I, Preparation of extracellular matrices produced by cultured corneal endothelial and PF-HR9 endodermal cells, Curr. Protoc. Cell Biol. Chapter 10 (2001) Unit 10 4. [DOI] [PubMed] [Google Scholar]
- [81].Barash U, Lapidot M, Zohar Y, Loomis C, Moreira A, Feld S, Goparaju C, Yang H, Hammond E, Zhang G, Li JP, Ilan N, Nagler A, Pass HI, Vlodavsky I, Involvement of heparanase in the pathogenesis of mesothelioma: Basic aspects and clinical applications, J. Nat. Cancer Inst. 110 (2018) 1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Barash U, Zohar Y, Wildbaum G, Beider K, Nagler A, Karin N, Ilan N, Vlodavsky I, Heparanase enhances myeloma progression via CXCL10 downregulation, Leukemia 28 (2014) 2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Albini A, Benelli R, The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation, Nat. Protoc 2 (2007) 504–11. [DOI] [PubMed] [Google Scholar]
- [84].Liu G, Chen Y, Qi F, Jia L, Lu XA, He T, Fu Y, Li L, Luo Y, Specific chemotherapeutic agents induce metastatic behaviour through stromal- and tumour-derived cytokine and angiogenic factor signalling, J. Pathol. 237 (2015) 190–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.