Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a form of cardiopulmonary life support frequently utilized in catastrophic lung and or cardiac failure. Patients on ECMO often receive vancomycin therapy for treatment or prophylaxis against Gram-positive organisms. It is unclear if ECMO affects vancomycin pharmacokinetics, thus we modeled the pharmacokinetic behavior of vancomycin according to ECMO-specific variables.
Methods
Adult patients receiving vancomycin and Veno-Arterial-ECMO between 12/1/2016 and 10/1/2017 were prospectively enrolled. Extracorporeal membrane oxygenation settings and four sets of pre- and post-oxygenator vancomycin concentrations were collected for each patient. Compartmental models were built and assessed ECMO flow rates on vancomycin clearance and potential circuit sequestration. Bayesian posterior concentrations of the pre- and post-oxygenator concentrations were obtained for each patient, and summary pharmacokinetic parameters were calculated. Simulations were performed from the final model for efficacy and toxicity predictions.
Results
Eight patients contributed 64 serum concentrations. Patients were a median (interquartile range) age of 58.5 years (50.8–62.3) with a calculated creatinine clearance of 39 mL/min (29.5–62.5) and ECMO flow rates of 3980 mL/min (interquartile range = 3493.75–4132.5). A three-compartment model best fit the data (Bayesian: plasma pre-oxygenation R2 = 0.99, post-oxygenation R2 = 0.99). Vancomycin clearance was not impacted by ECMO flow rate (p = 0.7). Simulations demonstrated that vancomycin 1 g twice daily was rarely sufficient for minimum inhibitory concentrations > 0.5 mg/L. Doses ≥ 1.5 g twice daily often exceeded toxicity thresholds for exposure.
Conclusions
Extracorporeal membrane oxygenation flow rates did not influence vancomycin clearance between flow rates of 3500 and 5000 mL/min and vancomycin was not sequestered in ECMO. Common vancomycin regimens resulted in sub-optimal efficacy and/or excessive toxicity. Individual therapeutic drug monitoring is recommended for patients on ECMO.
1. Introduction
Extracorporeal membrane oxygenation (ECMO) is a form of cardiopulmonary life support that is often utilized in catastrophic lung and/or cardiac failure [1, 2]. Extracorporeal membrane oxygenation is differentiated into two modalities, Veno-Veno (VV) for respiratory support and Veno-Arterial (VA) for cardiac and or respiratory support [1, 2]. The use of VV-ECMO allows for full respiratory support such as with acute respiratory distress syndrome and hypoxic respiratory failure [1], whereas VA-ECMO is used for full cardiac support in conditions such as cardiogenic shock and post-heart transplant support. Extracorporeal membrane oxygenation treatment is often complicated by bleeding, thrombosis, hemolysis, liver dysfunction, renal failure necessitating renal replacement therapy, and infections [3–5]. The most common infections are ventilator-associated pneumonia, blood stream infections, and sepsis [3, 6]. Hence, vancomycin is frequently utilized as empiric or definitive therapy in patients on ECMO.
Extracorporeal membrane oxygenation circuitry comprises a cannula draining blood from the patient’s venous system, a mechanical pump, a heater, an oxygenator, and a cannula back to the patient (e.g., arterial blood supply in VA-ECMO). The extensive circuit means that drugs are often extracorporeal for significant time periods and contract various foreign surfaces. Pharmacokinetics often differ for patients on ECMO because of protein binding to the circuit, larger volumes of distribution, and significant volumes of priming fluids [7–9].
Reports in the literature on the impact of ECMO on vancomycin pharmacokinetics are mixed. Several studies suggest alterations in vancomycin serum concentrations [10–12], whereas other studies have not found significant differences between critically ill patients with ECMO support compared to patients without ECMO support [13–15]. We sought to create a mechanistically relevant model to understand the pharmacokinetic behavior of vancomycin within an ECMO circuit and assess if ECMO flow rates impacted clearance. Further, we employed simulations to understand which (if any) population dosing schemes would result in maximal effectiveness and minimal toxicity for vancomycin.
2. Materials and Methods
2.1. Patient Population
This study protocol was reviewed and approved by the institutional review boards of Northwestern University (IRB#STU00202326) and Midwestern University (IRB#2865). A single-center pharmacokinetic study was conducted between 12/1/2016 and 10/1/2017 at Northwestern Memorial Hospital, an 894-bed academic medical center in Chicago, IL, USA. Patients were prospectively enrolled if they consented, were aged 18 years or older, and were receiving VA-ECMO and vancomycin as per the clinical team’s decision. Exclusion criteria included reasons for altered pharmacokinetic/pharmacodynamic parameters such as pregnancy, burns, morbid obesity with body mass index ≥ 40 kg/m2, and any form of dialysis (example: continuous renal replacement therapy). Those with a predicted life expectancy less than 24 h, vancomycin allergy, or who received large blood transfusions were also excluded. Patient demographics, baseline renal function (defined as estimated creatinine clearance [CrCL, mL/min] calculated using the Cockcroft–Gault equation at the time of study inclusion), urine output (collected over the time period of sample collection), and baseline basic metabolic panel. Infection type (empiric and definitive) and all aspects of vancomycin administration (e.g., dose, administration time, administration duration) were recorded. The VA-ECMO initiation date and time, flow rates, pump rate defined as revolutions per minute, sweep rates (rates of carbon dioxide removal), and oxygenation levels were collected with each vancomycin assay. Paired pre- and post-oxygenator blood draws were obtained at 6, 12, 18 and 24 h. The vancomycin assay (total of bound and unbound vancomycin concentration) was completed by the Clinical Chemistry Laboratory at Northwestern Memorial Hospital (Chicago, IL, USA). The assay was performed on a Beckman Coulter AU5800 analyzer (Danaher Corporation, Brea, CA, USA) using Emit 2000 Vancomycin, a competitive enzyme immunoassay method with a limit of quantification of 2.0 mg/L and precision within 4% [16].
2.2. Extracorporeal Membrane Oxygenation Apparatus
The ECMO system comprised a ROTAFLOW centrifugal pump and CARDIOHELP system (Maquet, Rastatt, Germany) in configuration with the poly-methyl-pentene QUADROX-ID diffusion membrane hollow-fiber oxygenator (Maquet), a Fem-Flex II femoral arterial cannula (Edwards Lifescience, Irvine, CA, USA), and a Bio-Medicus multistage femoral venous cannula (Medtronic, Minneapolis, MN, USA). The ECMO circuit was primed with 600 mL of normal saline.
2.3. Pharmacokinetic Models
The non-parametric adaptive grid algorithm [17, 18] within the Pmetrics (version 1.5.0) package (Los Angeles, CA, USA) [18] for R (version 3.5.1, Vienna, Austria) [19] was utilized to conduct the population pharmacokinetic (PK)/pharmacodynamic analysis. Several population PK models with varying physiologically relevant compartments were investigated. The simplest model considered was a two-compartment model representing pre- and post-oxygenator sampling. To facilitate simulations and explore the role of ECMO flow rates, compartmental transfer to the ECMO unit was modeled as a function of flow rate. ‘Sequestration’ was assessed as the rate and extent of drug sequestration from the ECMO unit. Variables known to impact vancomycin pharmacokinetics (e.g., patient body size and CrCL) were considered in the model and assessed with linear regression and Spearman’s Rho. Covariate explorations included: calculated CrCL [20] (via Cockcroft–Gault) and body surface area on vancomycin clearance, ECMO flow rate on inter-compartmental flow constant (Q), and total body weight (TBW) on volume of distribution.
Vancomycin clearance was linearly scaled to both CrCL and body surface area, standardized to 120 mL/min and 1.73 m2, respectively. The ECMO flow rate was standardized to 4000 mL/min, and the volume of distribution was linearly scaled to TBW and standardized to a 70-kg patient. Assay error (standard deviation, SD) was accounted for using an error polynomial as a function of the measured concentration, Y (i.e., SD = C0 + C1Y) with initial C0 and C1 inputs of 2 and 0.15, respectively. The inverse of the observed variance (SD2) was used as the first estimate for observation weighting [18]. Residual error and process noise was estimated using the multiplicative gamma (i.e., error = gamma*SD), which was given a starting value of gamma equal to 3. Final model selection prioritized a mechanistically relevant, yet parsimonious model as defined by Akaike information criteria and – 2 log-likelihood values (compared against a Chi-squared distribution) for appropriate degrees of freedom [18, 21]. Goodness-of-fit and predictive performance of the competing models were evaluated, as previously described [22].
2.4. Non-compartmental Analysis
A non-compartmental analysis of the posterior-predicted vancomycin concentration–time profiles using pre-oxygenator concentrations was conducted to facilitate comparisons of the population-predicted vancomycin parameters from our final model and to compare PK estimates reported in previous studies. The Bayesian posteriors were utilized to calculate 24-h exposure and PK parameters including: area under the curve (AUC), elimination rate constant (K), maximum concentration, clearance, and volume of distribution at steady state. Pharmacokinetic values were estimated using previously described methods with Pmetrics commands ‘makeNCA’ and ‘makeAUC’ within R [18, 23].
2.5. Simulations and Probability of Target Attainment
Simulations of vancomycin plasma concentration–time curves were completed using a multi-modal sampling method from the final model [18, 23]. Covariate ranges for ECMO flow rate simulations were selected to encompass common ranges for ECMO flow rates and the ranges of ECMO rates in the eight patients (i.e., 3500 mL/min, 3820 mL/min [median value], 4000 mL/min, 4500 mL/min, and 5000 mL/min). Our covariates for simulation were fixed to the median values of 84 kg for TBW and 45 mL/min for CrCL. Monte Carlo sampling from the weighted multi-modal distribution generated a novel population of 1000 parameter sets. From each of the 1000 sets of simulated parameters, concentration-time profiles were created for common vancomycin dosing regimens from 2000 to 4000 mg/day (i.e., 1000 mg twice daily, 1500 mg twice daily, and 2000 mg twice daily). An infusion time of 1 h was used for all simulations. Plasma concentrations were generated every half-hour for the first 24 h. Plasma vancomycin concentration-time profiles were not corrected for protein binding (free fraction = 100% assumed), as the majority of clinical assays available measure total vancomycin concentrations [24]. In the probability of target attainment (PTA) analysis, doubling MICs between 0.5 and 8 mg/L were evaluated and a target AUC24/MIC of 400 mg*h/L was selected as the efficacy endpoint based on the new Infectious Diseases Society of America vancomycin guidelines [25]. A toxicity threshold was set at AUC24/MIC of 515 mg*h/L and 550 mg*h/L based on the recent literature identifying these exposures as more nephrotoxic with no clinical efficacy gained [26–28].
3. Results
3.1. Patient Population
A total of eight patients each provided eight samples in a 24-h study period with a median (IQR) age of 58.5 (50.8–62.3) years, weight of 83 (73–88.13) kg, body surface area of 1.82 (1.6–2.2) m2, body mass index of 29.4 (25.23–32.43) kg/m2, and calculated CrCL of 39 (29.5–62.5) mL/min. The median (IQR) ECMO flow rate was 3980 (3493.75–4132.5) mL/min. The median number of vancomycin doses per patient was two doses with the median dose of 1000 mg. Complete patient demographics can be found in Table 1.
Table 1.
Baseline demographics
| Variable (N = 8 patients) | Median (IQR) |
|---|---|
| Age, years | 58.5 (50.8–62.3) |
| Male, % | 5 (63) |
| Height, cm | 171.35 (160.6–175.93) |
| Weight, kg | 83.05 (73–88.13) |
| BMI, kg/m2 | 29.4 (25.3–32.4) |
| BSA, m2 | 1.83 (1.6–2.2) |
| BUN, mg/dL | 31 (21–37.5) |
| Scr, mg/dL | 1.64 (1.14–2.89) |
| CrCL, mL/min | 39 (29.5–62.5) |
| ECMO flow rates, mL/min | |
| T = 6 | 3980 (3493.75–4132.5) |
| T = 12 | 3867.5 (3457.5–4078.75) |
| T = 18 | 3867.5 (3513.75–4078.75) |
| T = 24 | 3795.5 (3451.25–4063.75) |
| Vancomycin concentrations, mcg/mL | |
| Pre-oxygenator, 6-h post-dose | 24.9 (10–34.95) |
| Post-oxygenator, 6-h post-dose | 26.05 (9.825–33.55) |
| Pre-oxygenator, 12-h post-dose | 15.95 (6.575–26.5) |
| Post-oxygenator, 12-h post-dose | 15.45 (6.3–22.325) |
| Pre-oxygenator, 18-h post-dose | 18.5 (12.65–23.6) |
| Post-oxygenator, 18-h post-dose | 17.6 (12.125–23.95) |
| Pre-oxygenator, 24-h post-dose | 13.1 (8.875–17.375) |
| Post-oxygenator, 24-h post-dose | 12.7 (8.9–17.175) |
| Indication, count (%) | |
| Empiric treatment of infection | 7 (87) |
| Definitive treatment of infection | 1 (13) |
| Infectious site, presumed or confirmed, n (%) | |
| Pulmonary system | 4 (50) |
| Vascular | 6 (75) |
| Intraabdominal | 1 (12.5) |
BMI body mass index, BSA body surface area, BUN blood urea nitrogen, CrCL creatinine clearance, ECMO extracorporeal membrane oxygenation, IQR interquartile range, Scr serum creatinine
3.2. Pharmacokinetic Model Selection and Parameters
All 64 vancomycin plasma concentrations were utilized for the PK model build. Vancomycin concentrations are described in Table 1. A three-compartment base model was chosen over a two-compartment base model because of an improved Akaike information criteria score (235.9 vs 337, Table 4 of the Appendix). Creatinine clearance and TBW were found to be significant covariates via univariate linear regression analyses (CrCL vs vancomycin clearance, p = 0.003, TBW vs volume [V1], p < 0.01). Further, upon visual inspection and Spearman Rho calculation, significant relationships (p < 0.01) between TBW and V1 (Rho: 0.59) and CrCL and vancomycin clearance (Rho: 0.61) were also found. Based on this relationship and the well-known impact of these variables on vancomycin clearance [29, 30], clearance and V1 were standardized for TBW and CrCL (example in Fig. 1) and included in the final model. The ECMO flow rate was also included in the final model per original study objective. A complete model build with comparisons can be found in Table 4 of the Appendix.
Table 4.
Model build comparison
| Model | − 2LL | AIC | Pop pre-oxygenator Bias | Pop pre-oxygenator Imp | Indiv pre-oxygenator Bias | Indiv pre-oxygenator Imp | R2 pre-oxygenator Pop | R2= pre-oxygenator Indiv | R2 post- oxygenator Pop | R2 post- oxygenator Indiv | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base two-compartment model | 326 | 337 | − 0.43 | 10.62 | − 0.37 | 0.92 | 0.34 | 0.95 | 0.13 | 0.94 | – |
| Base three-compartment model | 219.9 | 235.9 | 3.16 | 73.1 | − 0.01 | 1.7 | 0.52 | 0.98 | 0.51 | 099 | 0.08 |
| Base three-compartment sequestration ECMO model | 188.3 | 206.9 | − 1.5 | 75.23 | 0.07 | 1.27 | 0.57 | 0.99 | 0.57 | 0.99 | 0.014 |
| Three-compartment sequestration ECMO model with Normalized creatinine clearance adjusted CL = CL0 * (CrCL/120) |
187.4 | 206 | 0.52 | 42.17 | 0.17 | 1.31 | 0.8 | 0.99 | 0.83 | 0.99 | * |
| Three-compartment model sequestration ECMO model normalized for creatinine clearance and weight adjusted CL = CL0 * (CrCL/120), V1 = V0 * (TBW/70) |
190.3 | 208.9 | 0.74 | 43.26 | − 0.17 | 1.29 | 0.77 | 0.99 | 0.8 | 0.99 | * |
| Three-compartment sequestration ECMO model normalized ECMO flowrate adjusted K13 = (Q2*FLOWrate/4000)/V1 K31 = (Q2*FLOWrate/4000)/V3 |
183.4 | 202 | − 2.69 | 78.14 | 0.04 | 1.53 | 0.61 | 0.99 | 0.60 | 0.99 | * |
| Final model given objectives of study: Three-compartment sequestration ECMO, CL and V1 adjusted model CL = CL0 * (CrCL/120) V3 = V0 * (TBW/70) K13 = (Q2*FLOWrate/4000)/V3 K31 = (Q2*FLOWrate/4000)/V3 |
190.5 | 209.1 | 1.57 | 47.01 | − 0.12 | 1.30 | 0.76 | 0.99 | 0.78 | 0.99 | * |
Bias and imprecision values for post ECMO post oxygenator not shown
ECMO extracorporeal membrane oxygenation, pre-oxygenator before oxygenation by ECMO, post-oxygenator after oxygenation by ECMO, V1 volume central compartment, compartment, V0 volume term, V3 volume in the ECMO compartment, CL total clearance, CL0clearance term, Q2 intercompartment flow rate 2, K13rate constant to ECMO compartment from central compartment, K31 rate constant to central compartment from ECMO compartment, − 2LLlog-likelihood ratio, AICakaike information criterion, Pop population, indiv individual (i.e., Bayesian)
Model comparison did not produce a significant P-value compared to final model. Final model chosen based off objectives and known clinically relevant covariate adjustments
Fig. 1.
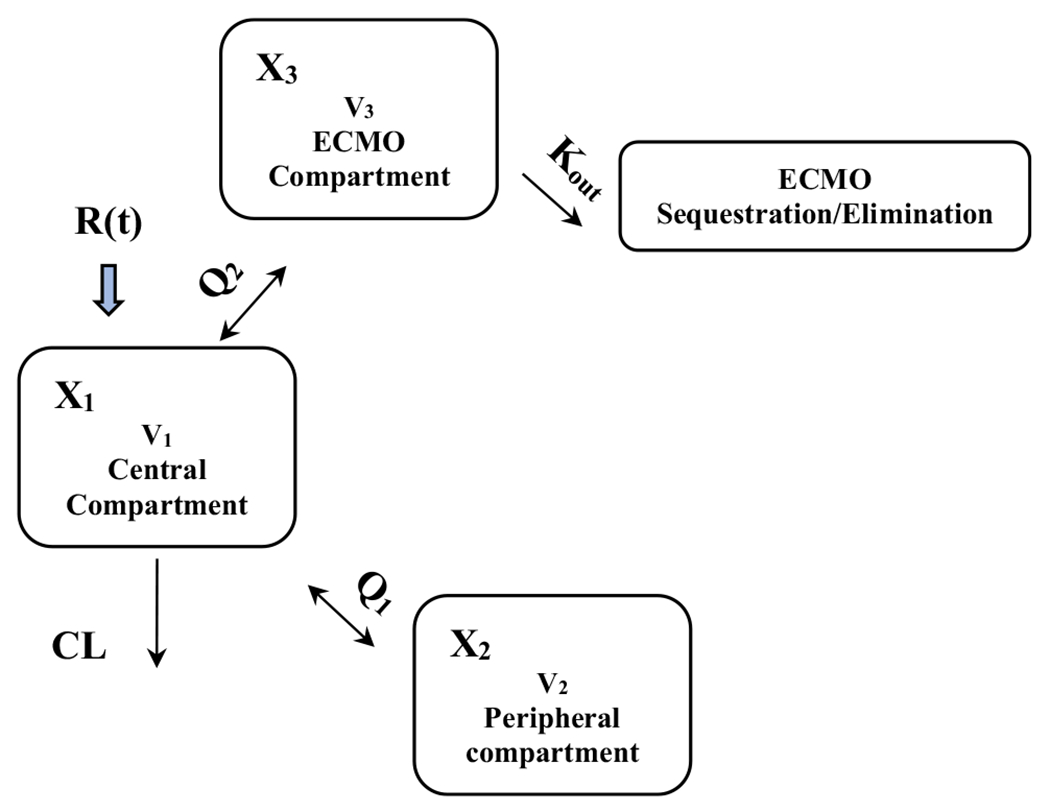
Three-compartment sequestration ECMO, CL and V1 adjusted model
The Bayesian individual posterior fits of the observed data were pre-oxygenator: R2 = 99.3% and post-oxygenator: R2 = 99.6%, with low bias (pre-oxygenator = − 0.12 μg/mL, post-oxygenator = − 0.43 μg/mL) and low imprecision (pre-oxygenator = 1.3 μg2/mL2, post-oxygenator =− 0.9 μg2/mL2). The population PK model fits of the observed data were pre-oxygenator: R2 = 76% and post-oxygenator: R2 = 78%, with low bias (pre-oxygenator = 1.57 μg/mL, post-oxygenator = 1.31 μg/mL) and imprecision (pre-oxygenator = 47 μg2/mL2, post-oxygenator = 42.5 μg2/mL2). The observed vs predicted plots for both fits (pre- and post-oxygenator) from the final model can found in Fig. 2a, b.
Fig. 2.
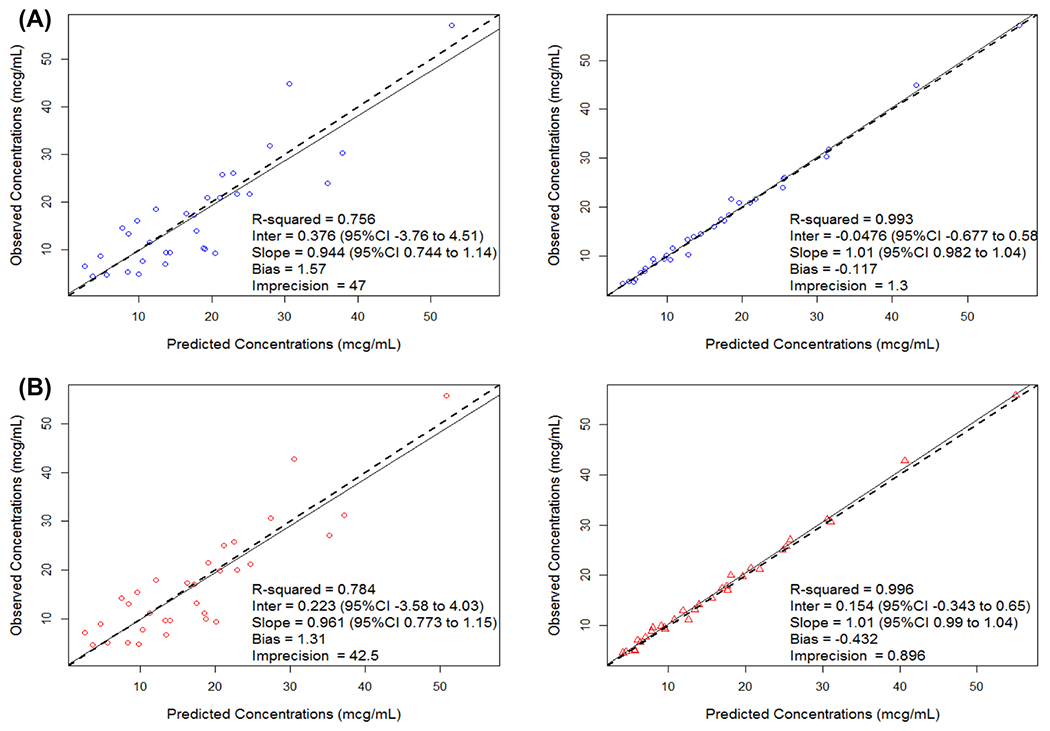
Goodness-of-fit plot for population (left figure) and Bayesian (right figure) predicted to observed vancomycin concentrations (mcg/ml) for Pre (a) and Post (b) oxygenation
The weighted parameter values and variability measures (i.e., median, IQR) for the final population PK model are summarized in Table 2. The final model parameters included a volume term for TBW covariate adjustment (V0), volume in the peripheral compartment (V2), volume in the ECMO compartment (V3), clearance term for CrCL covariate adjustment (CL0), inter-compartment flow rate 1 (Q1), inter-compartment flow rate 2 (Q2), and ECMO drug sequestration (Kout). Briefly, the final model’s median (coefficient of variation %) parameter values for V0, V2, V3, CL0, Q1, Q2, and Kout were 13.4 L (74.18%), 32.72 L (18.97%), 0.22 L (107.43%), 6.89 L/h (35.42%), 9.1 L (40.01%), 8.78 L (62.89%), and 0.79 h−1 (68.64%), respectively.
Table 2.
Final model median population parameter value summaries from the final extracorporeal membrane oxygenation model
| PK parameter | Mean | CV% | Weighted medians | 95% Credibility interval |
|---|---|---|---|---|
| V0 (L) | 13.4 | 74.18 | 13.3981 | 11.1484–15.4135 |
| V2 (L) | 32.72 | 18.97 | 32.7183 | 26.68–39.5534 |
| V3 (L) | 0.22 | 107.43 | 0.2181 | 0.183–0.4659 |
| CL0 (L/h) | 6.89 | 35.42 | 6.8829 | 4.0225–9.0576 |
| Q1 (L/h) | 9.1 | 40.01 | 9.0957 | 6.0399–13.6675 |
| Q2 (L/h) | 8.78 | 62.89 | 8.7761 | 3.2698–14.7805 |
| Kout (h−1) | 0.79 | 68.64 | 0.7909 | 0.3467–1.2313 |
CL0 clearance term, CV% coefficient of variation percent, Kout extracorporeal membrane oxygenation drug sequestration/elimination, PK pharmacokinetic, Q1 intercompartment flow rate 1, Q2 intercompartment flow rate 2, V0 volume term, V2 volume in the peripheral compartment, V3volume in the extracorporeal membrane oxygenation compartment
3.3. Simulations and Probability of Target Attainment Across SDD Minimum Inhibitory Concentrations
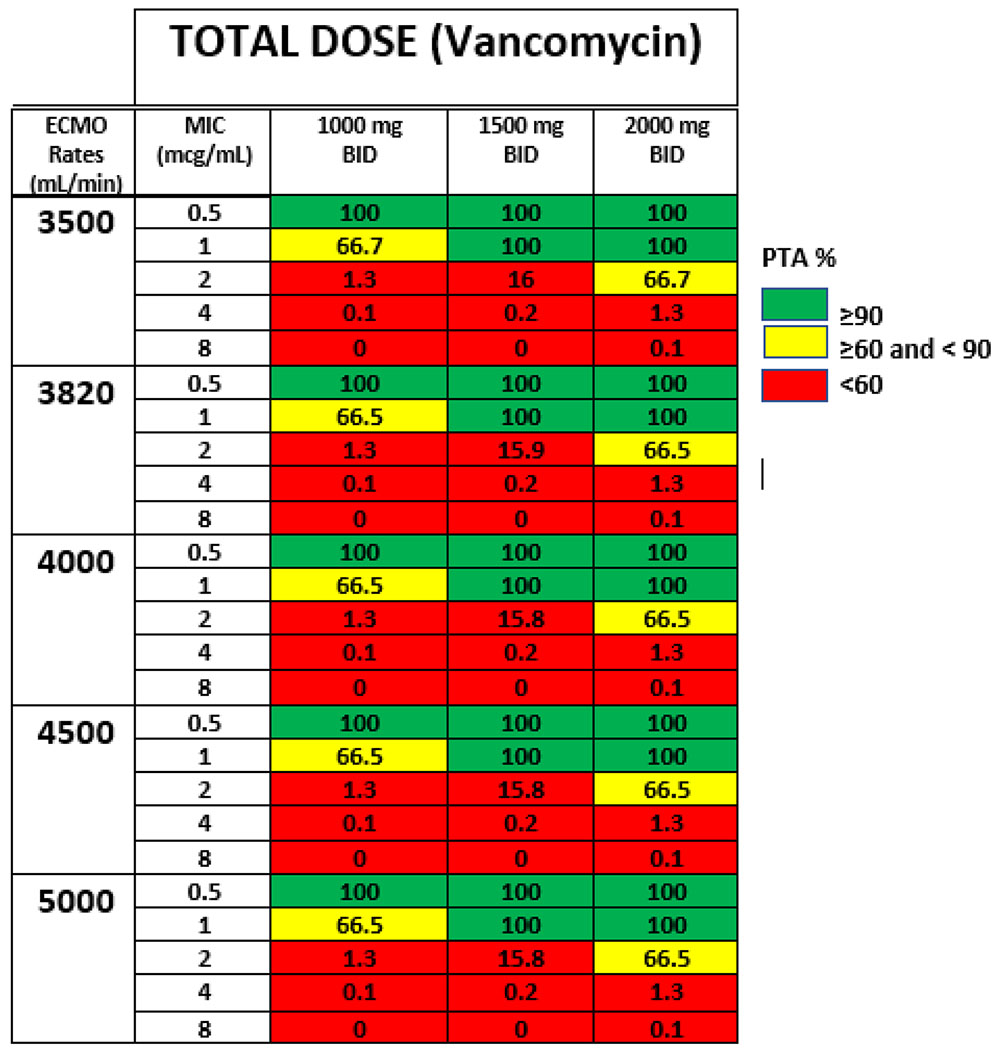
The results of the target attainment analysis using the pre-oxygenator concentrations are shown in Table 6 of the Appendix. All vancomycin dosing regimens produced PTAs above 90% at a MIC of 0.5 mg/L. Conversely, at MICs of 1 mg/L and above, only 1500 mg and 2000 mg twice daily produced a PTA > 90%. At MICs of 2 mg/L and above, no regimen was able to produce a favorable PTA of > 90%. The ECMO flow rate did not meaningfully impact the PTA for each regimen or MIC as the PTA for an AUC24/MIC ≥ 400 mg*h/L was similar between all rates (Fig. 3).
Table 6.
The probability of toxic exposures as predicted by MCS for each simulated regimen at various EMCO rates
| Dosing regimen | ECMO rate (mL/min) | Probability of toxicity (%) (AUCtotal ≥ 550) (mean AUC mg*h/L, SD) | Probability of toxicity (%) (AUC total ≥ 515) (mean AUC mg*h/L, SD) |
|---|---|---|---|
| 1000 mg BID | |||
| 3500 | 12 (452.05, SD: 114.21) | 19.8 (452.05, SD: 114.21) | |
| 3820 | 11.9 (451.83, SD: 114.14) | 19.6 (451.83, SD: 114.14) | |
| 4000 | 11.9 (451.73, SD: 114.11) | 19.5 (451.73, SD: 114.11) | |
| 4500 | 11.8 (451.02, SD: 114.02) | 19.4 (451.47, SD: 114.02) | |
| 5000 | 11.8 (451.26, SD: 113.95) | 19.2 (451.26, SD: 113.95) | |
| 1500 mg BID | |||
| 3500 | 82.4 (678.08, SD: 171.33) | 92.1 (678.08, SD: 171.33) | |
| 3820 | 82.3 (677.75, SD: 171.21) | 92.1 (677.75, SD: 171.21) | |
| 4000 | 82.2 (677.59, SD: 171.16) | 92.1 (677.59, SD: 171.16) | |
| 4500 | 82.1 (677.2, SD: 171.03) | 92.1 (677.2, SD: 171.03) | |
| 5000 | 82 (676.88, SD: 170.92) | 92.1 (676.88, SD: 170.92) | |
| 2000 mg BID | |||
| 3500 | 99.9 (904.1, SD: 228.43) | 100 (904.1, SD: 228.43) | |
| 3820 | 99.9 (903.66, SD: 228.28) | 100 (903.66, SD: 228.28) | |
| 4000 | 99.9 (903.44, SD: 228.21) | 100 (903.44, SD: 228.21) | |
| 4500 | 99.9 (902.93, SD: 228.03) | 100 (902.93, SD: 228.03) | |
| 5000 | 99.9 (902.51, SD: 227.89) | 100 (902.51, SD: 227.89) | |
ECMO extracorporeal membrane oxygenation, BID twice a day, MCS Monte Carlo simulation, AUC area under the curve, SD standard deviation
Fig. 3.
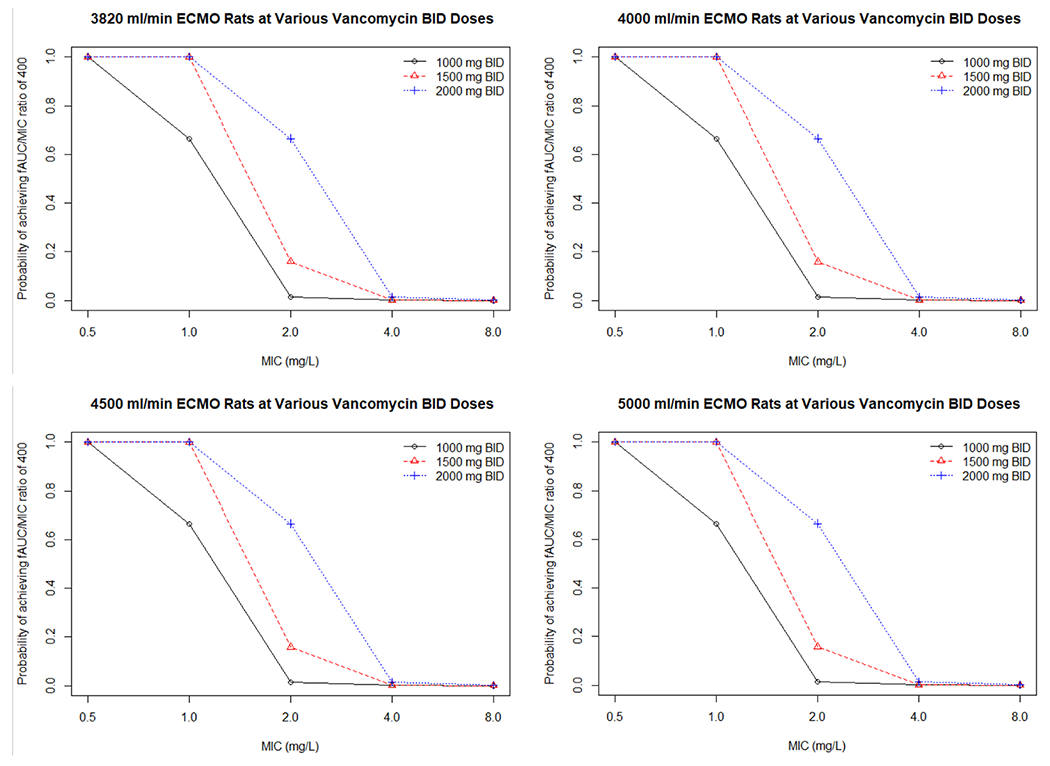
Probability of target attainment for different vancomycin doses with various ECMO rates. * The ECMO rate does not impact the probably of achieving an AUC/MIC ratio of 400 (mg*h/L) at various doses. ECMO extracorporeal membrane oxygenation, BID twice a day, AUC area under the curve, MIC minimum inhibitory concentration
The probability of reaching the toxicity threshold can be found in Table 6 of the Appendix. Briefly, simulations showed that only the regimen of 1000 mg twice daily (2000 mg/day) produced a favorable mean AUC24/MIC and the lowest probability (≤ 20%) of reaching toxicity thresholds of ≥ 550 and 515 mg*h/L. Doses of 1500 mg twice daily and 2000 mg twice daily produced a high probability (> 80%) of reaching the toxicity thresholds specified. The complete population parameter value covariance matrix can be found in Table 7 of the Appendix.
Table 7.
Population parameter value covariance matrix
| V0 | V2 | V3 | CL0 | Q1 | Q2 | K34 | |
|---|---|---|---|---|---|---|---|
| V0 | 131.437 | – | – | – | – | – | – |
| V2 | − 11.495 | 38.522 | – | – | – | – | – |
| V3 | − 2.191 | 0.722 | 0.15 | – | – | – | – |
| CL0 | 5.427 | −1.67 | −0.071 | 5.436 | – | – | – |
| Q1 | 22.95 | −19.79 | −0.33 | 3.895 | 15.142 | – | – |
| Q2 | − 42.634 | − 11.593 | 1.209 | 1.142 | 2.798 | 26.788 | – |
| Kout | − 3.424 | 0.06 | 0.028 | −0.3 | − 0.869 | 0.933 | 0.296 |
V0 volume term, V2 volume peripheral compartment, V3 volume in the ECMO compartment, CL0 clearance term, Q1 intercompartment flow rate 1, Q2 intercompartment flow rate 2, K31 rate constant to central compartment from ECMO compartment, Kout ECMO drug sequestration/elimination
4. Discussion
We found that vancomycin clearance was not impacted by ECMO flow rate, and the ECMO circuit resulted in minimal sequestration of vancomycin. Further, our simulations suggest that a population dosing approach is not sufficient for either attainment of efficacy or avoidance of toxicity. Thus, these data indicate that individual therapeutic drug monitoring should be performed on patients receiving vancomycin while on ECMO. Future work will be needed to determine if our proposed model can be utilized as a Bayesian prior to minimize the number of samples required to determine the vancomycin AUC. Patients receiving ECMO therapy are at a high risk for methicillin-resistant Staphylococcus aureus catheter-related bloodstream infections and/or nosocomial infections. Thus, vancomycin is commonly utilized as either empiric or definitive therapy [31, 32].
Recent data have better delineated the therapeutic window for vancomycin in the setting of methicillin-resistant S. aureus bacteremia. Effectiveness for vancomycin exists when the AUC24/MIC ratio approaches 400 mg*h/L; however, lower exposures may be efficacious [25, 27]. The toxicity window for vancomycin is also becoming clearer. Vancomycin AUC24/MIC should remain below ~515 mg*h/L [26–28] to prevent proximal tubular necrosis [33–35]. Our simulations suggest that commonly utilized vancomycin doses and the application of population PK approaches will struggle to keep patients precisely within an AUC24/MIC window of 400 mg*h/L. It is also important to note that the threshold for efficacy depends on MIC whereas the toxicity threshold only considers exposure (i.e., AUC). Thus, MICs ≤ 1 mg/L are needed to simultaneously meet the requirement of AUC24/MIC ~ 400 mg*h/L and AUC24/MIC < 515 mg*h/L.
Previous studies have looked at vancomycin pharmacokinetics in patients on ECMO. A study by Wu and colleagues focused on adults that received a minimum of four vancomycin doses [7]. The authors enrolled both VV, VA, and Veno-Veno-Arterial patients in the study with either a centrifugal pump or a roller pump. The authors included 11 patients, demonstrating a mean clearance of 1.18 mL/min/kg, and a mean volume of distribution at steady state of 0.84 L/kg. When compared to the mean clearance and volume of distribution at steady state of a matched cohort of critically ill patients not on ECMO, their values were 1.45 mL/min/kg and 0.81 L/kg, respectively. Interestingly, it was noted that when a centrifugal pump was used, the vancomycin elimination rate was not affected; however, when a roller pump was used, patients’ vancomycin clearance was significantly lower in patients with ECMO roller pumps compared to centrifugal pumps [7]. Overall, the authors hypothesized that the difference in volume of distribution at steady state and clearance was due to priming fluid and the patient acuity rather than drug sequestration in the circuit. Similarly, Donadello and colleagues described continuous infusion vancomycin population pharmacokinetics in critically ill patients. Patients were enrolled within the first 24 h of vancomycin administration and were ± ECMO and ± continuous renal replacement therapy [14]. The authors included a total of 11 patients on ECMO (five VA ECMO, six VV ECMO) and demonstrated that no adjustment in vancomycin dosing was required for patients receiving ECMO therapy compared to patients not on ECMO with a similar acuity of illness. Multiple other studies have demonstrated that the presence of ECMO did not result in different trough concentrations between similar two patient populations [7, 11, 13, 14].
The utility of trough vancomycin concentrations may be insufficient to explain the full PK relationship and thus AUC is a better predictor for kidney injury [36, 37]. Pharmacokinetic parameters from our study are similar to those reported in the literature. Vancomycin clearance (CL0) was 6.89 L/h, which was similar to the findings of Wu and colleagues (5.9 L/h), and slightly faster than Donadello et al. and Moore et al. (2.4 L/h and 2.8 L/h, respectively) [13, 14]. The median volume of distribution was 0.52 L/kg, which similarly fell in between what is reported in the literature (0.25 L/kg, 0.7L/kg, and 0.84 L/kg) [7, 13, 14]. These findings underscore the importance for patient individualized dosing of vancomycin and utilization of loading doses to rapidly achieve the goal AUC, while therapeutic drug monitoring should be performed to avoid iatrogenic kidney injury.
Several limitations to our work exist. Our study was single center with a small sample size and utilized a single ECMO methodology (i.e., VA) and a pump for all patients. Additionally, flow rates in our study ranged from approximately 3500 mL/min to 4100 mL/min. While the flow rates were somewhat constrained, this range is common in ECMO and no impact on vancomycin clearance was observed. Last, it is not uncommon that patients supported on ECMO also receive renal replacement therapy; however, we sought to capture the effects of the circuit and to remove any confounders, thus this modeling may not depict what may occur in patients receiving concomitant renal replacement therapy and ECMO. Despite these limitations, our study is unique in that we sampled vancomycin concentrations pre- and post-oxygenators and have fit a representative PK structural model.
5. Conclusions
We found that vancomycin was not sequestered in ECMO, and vancomycin clearance was not significantly impacted by ECMO flow rate between 3500 and 5000 mL/min. Simulations from our model indicate that patients should receive a vancomycin loading dose and have individual therapeutic drug monitoring performed as common vancomycin doses are predicted to result in low efficacy and unnecessary toxicity.
Key Points.
Multiple recommendations exist for dosing vancomycin in patients receiving extracorporeal membrane oxygenation (ECMO).
We sought to create a relevant systems model to explain vancomycin transit through an ECMO circuit.
ECMO variables did not impact vancomycin pharmacokinetics; minimal vancomycin sequestration was observed.
Vancomycin can be dosed using traditional therapeutic drug monitoring approaches (i.e. venous blood sampling and standard clinical pharmacokinetic modeling).
Acknowledgments
Funding Marc H. Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award number R21AI149026. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding for assays in this study were paid for utilizing discretionary research funds from the Scheetz Laboratory. All other efforts by the study authors were donated or part of normal work activities.
Appendix
See Fig. 4 and Tables 4, 5, 6 and 7.
Fig. 4.
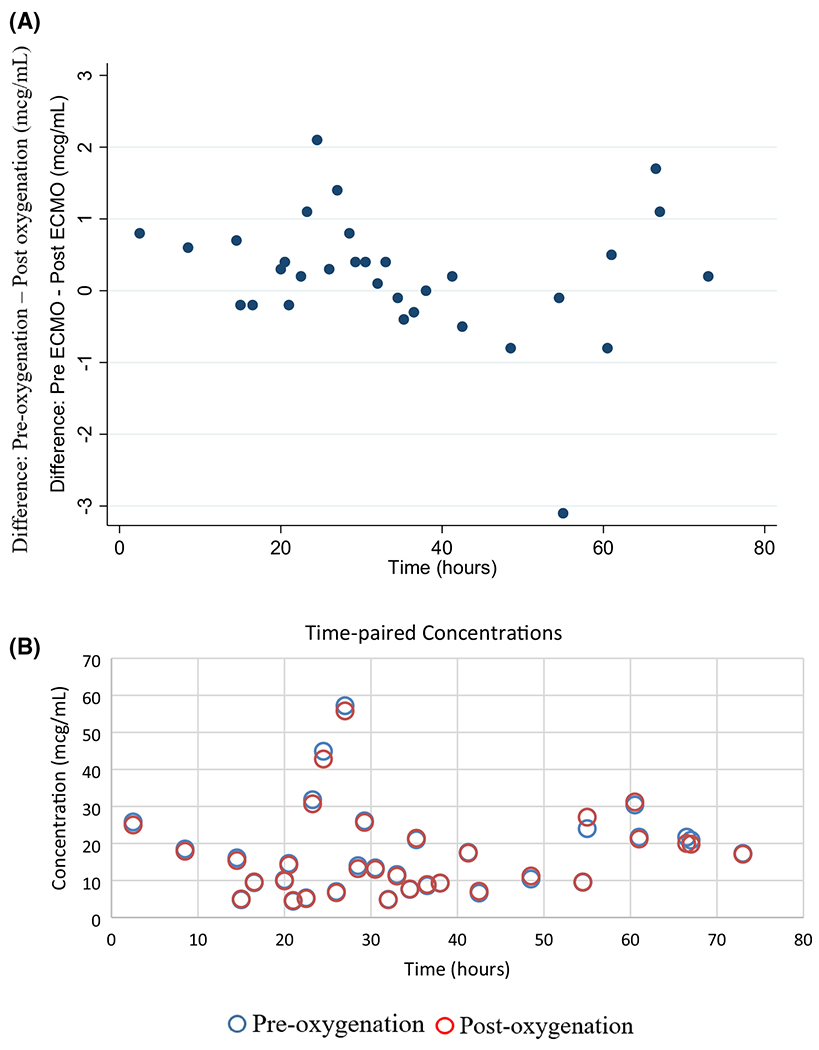
Pre and post oxygenation vancomycin concentration differences over time (a) and pre and post oxygenation time-paired concentrations (b). Pre-oxygenator = before oxygenation by ECMO, Postoxygenator = after oxygenation by ECMO
Table 5.
The probability of target attainment for different dosing regimens and ECMO rates
 |
PTA probability of target attainment, ECMO extracorporeal membrane oxygenation, BID twice a day, MIC minimum inhibitory concentration
Concentrations did not significantly differ when comparing the time-matched pre- and post-oxygenator concentrations (mean difference – 0.22 mg/L, 0.90 mg/L SD, p = 0.18) indicating little sequestration (Fig. 4 of the Appendix). Results of the non-compartmental analysis from the Bayesian posterior-predicted concentrations (i.e., pre-oxygenator concentrations) for the eight patients are summarized in Table 3. Briefly, within the eight study patients, the median (IQR) clearance and volume of distribution at steady-state values were 3.4 (1–3.87) L/h and 43.91 (40.65–51.4) L, respectively.
Table 3.
Non-compartmental pharmacokinetic analysis for the eight patients from the Bayesian posterior-predicted concentrations
| Patient | AUC (mg*h/L) | K (h−1) | Cmax (mcg/mL) | CL (L/h) | Vdss (L) |
|---|---|---|---|---|---|
| 1 | 366.01 | 0.017 | 22.68 | 0.91 | 53.44 |
| 2 | 371.2 | 0.058 | 106.01 | 3.79 | 41.26 |
| 3 | 438.06 | 0.019 | 43.9 | 0.87 | 44.64 |
| 4 | 307.40 | 0.083 | 51.61 | 4.47 | 45.28 |
| 5 | 394.46 | 0.065 | 45.89 | 3.90 | 40.45 |
| 6 | 551.04 | 0.033 | 57.76 | 1.32 | 37.22 |
| 7 | 227.6 | 0.05 | 53.82 | 3.35 | 54.13 |
| 8 | 533.5 | 0.076 | 51.55 | 3.46 | 43.19 |
| Median (IQR) | 382.83 (351.36–461.92) | 0.054 (0.02–0.07) | 51.58 (44.39–56.77) | 3.4 (1–3.87) | 43.91 (40.65–51.4) |
AUC area under the curve, Cmax maximum concentration, CL clearance, IQR interquartile range, K rate elimination constant, Vdss volume of distribution at steady state
Values rounded to nearest hundreds place, K rounded to nearest thousands place
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s40262-020-00902-1) contains supplementary material, which is available to authorized users.
Conflict of interest Ahmed Mahmoud Sean N. Avedissian, Abbas Al-Qamari, Tiffany Bohling, and Michelle Pham have no conflicts of interest that are directly relevant to the content of this article. Marc H. Scheetz received a research grant with Nevakar and has a patent (US2019/0099500 A1) pending.
References
- 1.Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015. 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratnani I, Tuazon Di, Zainab A, Uddin F. The role and impact of extracorporeal membrane oxygenation in critical care. Methodist Debakey Cardiovasc J. 2018;14(2):110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel AM, Lew DF, Kao LS, Lally KP. Defining risk for infectious complications on extracorporeal life support. J Pediatr Surg. 2011. 10.1016/j.jpedsurg.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Biffi S, Di Bella S, Scaravilli V, Peri AM, Grasselli G, Alagna L, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017. 10.1016/j.ijantimicag.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Begg EJ, Barclay ML, Kirkpatrick CJM. The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol. 1999. 10.1046/j.1365-2125.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu MS, Chiu KM, Huang YT, Kao KL, Chu SH, Liao CH. Risk factors for nosocomial infection during extracorporeal membrane oxygenation. J Hosp Infect. 2009. 10.1016/j.jhin.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Wu CC, Shen LJ, Hsu LF, Ko WJ, Wu FLL. Pharmacokinetics of vancomycin in adults receiving extracorporeal membrane oxygenation. J Formos Med Assoc. 2016. 10.1016/j.jfma.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Shekar K, Roberts JA, Mcdonald CI, Fisquet S, Barnett AG, Mullany DV, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012. 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010. 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaker RD, Dipiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005. 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoie E, Swigart S, Leuschen M, Willett L, Bolam D, Goodrich P, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–5. [PubMed] [Google Scholar]
- 13.Moore JN, Healy JR, Thoma BN, Peahota MM, Ahamadi M, Schmidt L, et al. A population pharmacokinetic model for vancomycin in adult patients receiving extracorporeal membrane oxygenation therapy. CPT Pharmacometrics Syst Pharmacol. 2016. 10.1002/psp4.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donadello K, Roberts JA, Cristallini S, Beumier M, Shekar K, Jacobs F, et al. Vancomycin population pharmacokinetics during extracorporeal membrane oxygenation therapy: a matched cohort study. Crit Care. 2014. 10.1186/s13054-014-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Yang JH, Park HJ, In YW, Lee YM, Cho YH, et al. Trough concentrations of vancomycin in patients undergoing extracorporeal membrane oxygenation. PLoS ONE. 2015. 10.1371/journal.pone.0141016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulter Beckman. AU5800 series clinical chemistry analyzers 2019. https://www.beckmancoulter.com/en/products/chemistry/au5800. Accessed 12 May 2020.
- 17.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models. Proc IEEE Symp Comput Med Syst. 2001. 10.1109/CBMS.2001.941750. [DOI] [Google Scholar]
- 18.Neely MN, Van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Team RC. R: a language and environment for statistical computing. J Comput Graph Stat. 2015. [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976. 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed 2013. 10.1002/9781118548387. [DOI] [Google Scholar]
- 22.Rhodes NJ, Gardiner BJ, Neely MN, Grayson ML, Ellis AG, Lawrentschuk N, et al. Optimal timing of oral fosfomycin administration for pre-prostate biopsy prophylaxis. J Antimicrob Chemother. 2015. 10.1093/jac/dkv067. [DOI] [PubMed] [Google Scholar]
- 23.Goutelle S, Bourguignon L, Maire PH, Van Guilder M, Conte JE, Jelliffe RW. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother. 2009. 10.1128/AAC.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stove V, Coene L, Carlier M, De Waele JJ, Fiers T, Verstraete AG. Measuring unbound versus total vancomycin concentrations in serum and plasma: methodological issues and relevance. Ther Drug Monit. 2015. 10.1097/FTD.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 25.Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2020;77(11):835–864. 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 26.Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019. 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lodise TP, Rosenkranz SL, Finnemeyer M, Evans S, Sims M, et al. The emperor’s new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with MRSA bloodstream infections (PROVIDE). Clin Infect Dis. 2019. 10.1093/cid/ciz460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avedissian SN, Pais GM, O’Donnell JN, Lodise TP, Liu J, Prozialeck WC, et al. Twenty-four hour pharmacokinetic relationships for intravenous vancomycin and novel urinary biomarkers of acute kidney injury in a rat model. J Antimicrob Chemother. 2019. 10.1093/jac/dkz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Álvarez R, Cortés LEL, Molina J, Cisneros JM, Pachón J. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. 2016. 10.1128/AAC.03147-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts JA, Taccone FS, Udy AA, Vincent J- L, Jacobs F, Lipman J. Vancomycin dosing in critically ill patients: robust methods for improved continuous-infusion regimens. Antimicrob Agents Chemother. 2011. 10.1128/aac.01708-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, et al. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017. 10.1097/CCM.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 32.Kim DW, Yeo HJ, Yoon SH, Lee SE, Lee SJ, Cho WH, et al. Impact of bloodstream infections on catheter colonization during extracorporeal membrane oxygenation. J Artif Organs. 2016. 10.1007/s10047-015-0882-5. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell JN, Ghossein C, Rhodes NJ, Peng J, Lertharakul T, Pham CK, et al. Eight unexpected cases of vancomycin associated acute kidney injury with contemporary dosing. J Infect Chemother. 2017. 10.1016/j.jiac.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell JN, Rhodes NJ, Lodise TP, Prozialeck WC, Miglis CM, Joshi MD, et al. 24-hour pharmacokinetic relationships for vancomycin and novel urinary biomarkers of acute kidney injury. Antimicrob Agents Chemother. 2017. 10.1128/AAC.00416-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downes KJ, Hayes M, Fitzgerald JC, Pais GM, Liu J, Zane NR, et al. Mechanisms of antimicrobial-induced nephrotoxicity in children. J Antimicrob Chemother. 2019. 10.1093/jac/dkz325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014. 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O’Donnell JN, Pais G, et al. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother. 2016. 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


