Abstract
INTRODUCTION:
Thromboelastography (TEG) provides a global assessment of hemostasis and may have value for patients with cirrhosis who have multiple hemostatic defects. We sought to examine the characteristics of TEG in hospitalized patients with cirrhosis and its relationship with outcomes.
METHODS:
We performed a cohort study of all adults with cirrhosis hospitalized at Indiana University Hospital between November 2015 and October 2018 with a TEG. We examined the relationships among TEG, traditional measures of hemostasis, liver disease severity, and outcomes, including mortality, discharge to hospice, length of stay, and 30-day readmission.
RESULTS:
A total of 344 patients met inclusion and exclusion criteria. R-value was elevated (≥10 min) in 4.5%, alpha angle was low (<45°) in 9.3%, and maximum amplitude (maximum amplitude) was low (<55 mm) in 72.1%. K-value, alpha angle, and maximum amplitude were all correlated with both platelet count and fibrinogen (absolute rho range 0.52–0.67); R-value and international normalized ratio (INR) were not strongly correlated with traditional measures or TEG, respectively. Patients with bleeding had hypercoagulable profiles, and patients with infection had increased R-value and decreased alpha angle. A total of 35.8% died or were discharged to hospice, and these patients had a greater R-value and smaller alpha angle. However, after adjustment for model for end-stage liver disease (MELD), neither R-value nor alpha angle were associated with discharge outcomes.
CONCLUSIONS:
TEG provides insight into the hemostatic state of patients with cirrhosis beyond that of standard measures of hemostasis. It is associated with liver disease severity and outcomes and may play a role complementary to standard measures of hemostasis in this population.
Keywords: Acute-on-chronic liver failure, Cirrhosis, Thromboelastography (TEG)
INTRODUCTION
Liver cirrhosis manifests in hepatic failure and portal hypertension with sequelae that have well-described implications.1 Among these sequelae are laboratory features of defective hemostasis. Patients with cirrhosis may fail to synthesize coagulation factors, resulting in increased international normalized ratio (INR), and they may have portal hypertension and thrombocytopenia. Therefore, cirrhosis has been considered a bleeding disorder. However, this notion has been challenged in studies assessing postprocedural bleeding.2–7 Furthermore, evidence of decreased endogenous anticoagulant factors (eg, protein C and antithrombin) and increased procoagulants (eg, factor VIII and von Willebrand factor) has led to the recognition of rebalanced hemostasis.8–11
Because conventional laboratory values (ie, INR and platelet count) do not capture the complexity of hemostasis in cirrhosis, a more comprehensive approach is needed. Thromboelastography (TEG) provides an assessment of clot formation, strength, and longevity with measures describing different coagulation components. In cirrhosis, TEG has been studied in transfusions in invasive procedures and nonvariceal hemorrhage.12–18 TEG has also been investigated in acute liver failure, acute-on-chronic liver failure, nonalcoholic fatty liver disease, and alcoholic hepatitis.19–25 Such aspects of liver disease can be present, sometimes simultaneously, when patients with cirrhosis are hospitalized. Hospitalizations of patients with cirrhosis are fraught with morbidity, mortality, and high readmission rates.26–28
TEG may provide insight for the care of hospitalized patients with cirrhosis. We sought to explore the features of TEG in this population through a cohort study examining TEG in routine clinical practice.
METHODS
Study Design and Participants
This study was approved by the Indiana University Institutional Review Board. We reviewed all adults with cirrhosis admitted to Indiana University Hospital between November 2015 and October 2018 with a TEG. Patients were identified through cirrhosis diagnostic codes and electronic TEG orders. All TEGs were performed for routine clinical care and were not obtained for research. Cirrhosis diagnoses were confirmed through chart review, including cirrhosis on histology, or based on clinical, laboratory, and imaging features. We excluded patients admitted for elective procedures or liver transplant surgery. Patients were followed from the admission date to 30 days post discharge. For patients with multiple TEGs, only the first was examined.
Thromboelastography
TEG provides measures corresponding to clot formation and dissolution (Figure 1). The R-value is the latent time prior to the beginning of the clot. The K-value is the time from clot onset until the clot strength reaches a magnitude of 20 mm. Alpha angle is the angle between the starting point and the tangent of the curve. These values measure clot kinetics and acceleration of fibrin formation and cross-linking. The maximum amplitude corresponds to platelet concentration, function and interaction with fibrin. Lastly, LY30 is the percentage reduction in clot strength 30 minutes after the maximum amplitude (fibrinolysis).29,30 We considered values to be abnormal as follows: R-value >10 min, angle <45°, and maximum amplitude <55 mm.31,32 We did not specify K-values as abnormal because of a lack of data defining K-value cutoffs.31 All TEGs were performed on citrated samples with kaolin activation (TEG 5000 Thromboelastograph Hemostasis Analyzer, Haemonetics Corp.).
Figure 1.
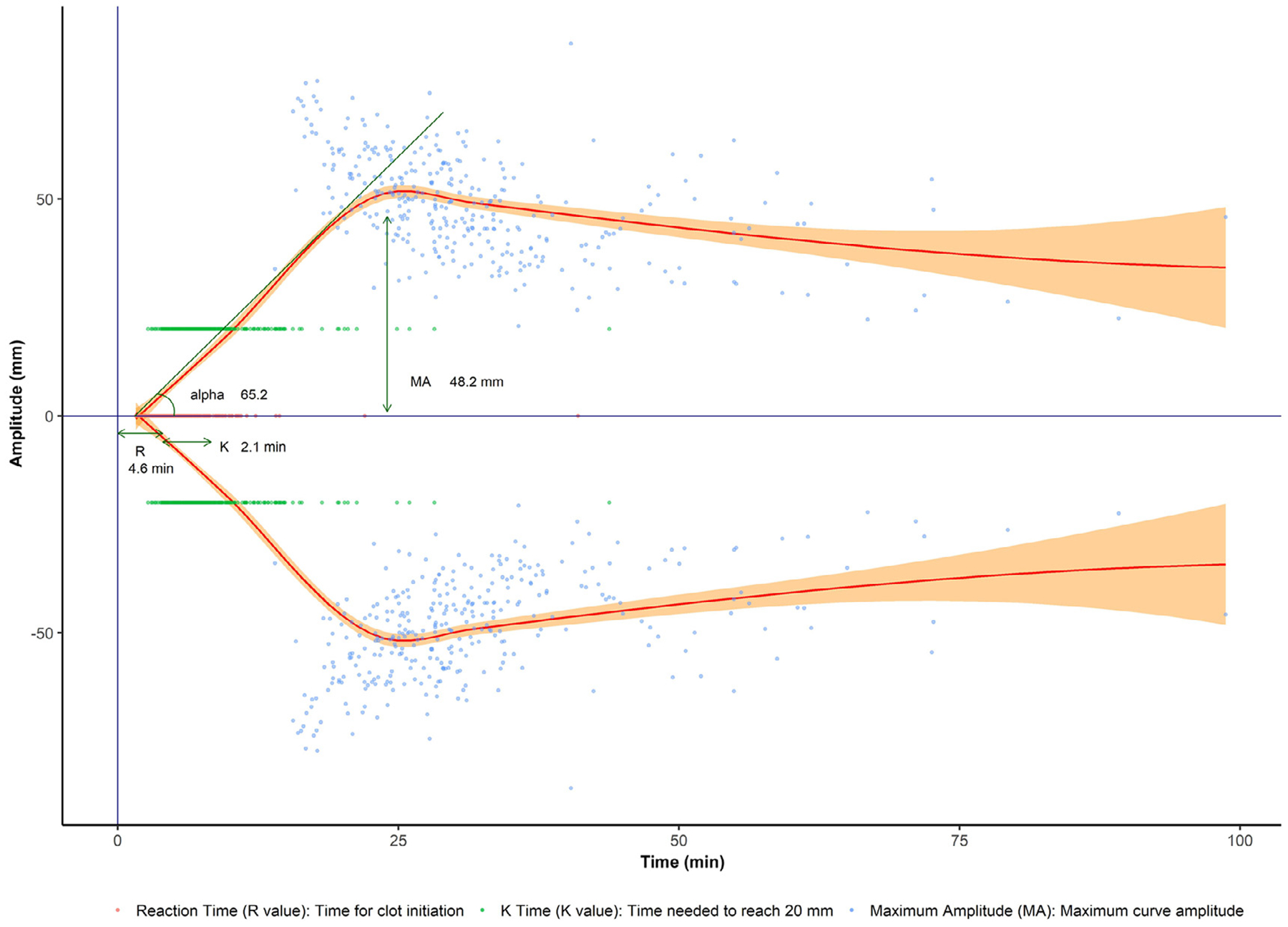
Thromboelastography curve estimate in hospitalized patients with cirrhosis. R-value is the time for clot initiation. K-value is the time needed to reach 20 mm amplitude. Alpha angle is the angle between the starting point and the tangent of the curve. MA is the maximum amplitude.
Outcomes
Outcomes included inpatient and 30-day mortality, discharge to hospice, hospital length of stay, and 30-day readmission.
Variables
We collected demographic and clinical characteristics at admission and at the time of TEG. We recorded patient age, sex, race, liver disease etiology, and location in the hospital (ie, intensive care unit [ICU], floor). We calculated the Charlson Comorbidity Index33 and collected liver-specific complications: hepatic encephalopathy, ascites, portal vein thrombosis, and deep vein thrombosis. We examined the presence of active bleeding or infection at the time of TEG. Laboratory values of interest included hemoglobin, platelet count, leukocyte count, and values to calculate Child-Pugh, model for end-stage liver disease (MELD), and MELD-Na scores.34–36 Vital signs and information on dialysis and mechanical ventilation were collected to assess for acute-on-chronic liver failure.37 We captured inpatient transfusions within 48 hours prior to TEG. Medications (ie, antiplatelet agents, direct oral anticoagulants, warfarin, and heparin) were collected.
Statistical Analysis
Continuous variables were described using means with standard deviations or medians with interquartile ranges (IQRs) and compared using Student t-test, Wilcoxon rank sum test, or analysis of variance. Categorical variables were described with frequencies and percentages and compared using the χ2 test. Spearman correlation was used to examine correlation between TEG and traditional measures of hemostasis. Multivariable logistic regression was used to examine variables associated with death or discharge to hospice. An approximate mean TEG was plotted using ggplot2.38 Two-sided tests were employed throughout, with P value <0.05 considered statistically significant. All analyses were performed using R Studio (version 1.2.1335).39
RESULTS
Study Cohort Characteristics
Of 657 patients, 344 met inclusion and exclusion criteria (Figure 2). The mean age was 54, 55% were males, 90% were white, and 61% were in ICU at the time of TEG (Table 1). The most common etiology of liver disease was alcohol (54%). The mean MELD-Na was 29, 85% had Child-Pugh C cirrhosis, and 37% had acute-on-chronic liver failure. At the time of TEG, 56% had active bleeding (45% gastrointestinal bleed, 4% intra-abdominal, 4% intracranial, 3% epistaxis), 47% had infection (16% pneumonia, 14% spontaneous bacterial peritonitis, 11% spontaneous bacteremia, 10% urinary tract infection), and 11.3% had thrombosis.
Figure 2.
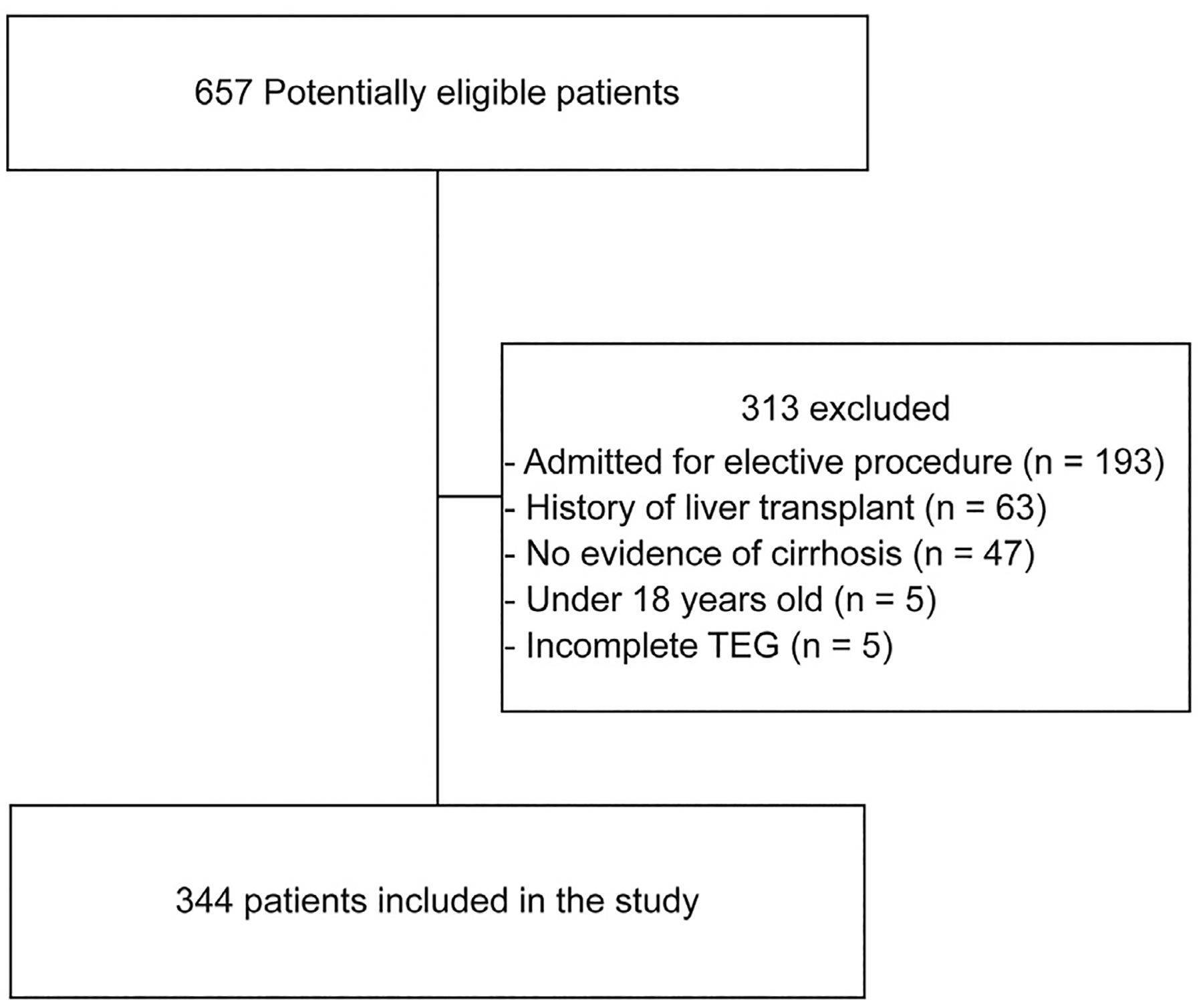
Inclusion and exclusion flow diagram.
Table 1.
Cohort Characteristics and Abnormal Thromboelastography Parameters
| Overall (N = 344) | Normal Angle* (N = 312) | Low Angle* (N = 32) | P Value | Normal MA† (N = 96) | Low MA† (N = 248) | P Value | |
|---|---|---|---|---|---|---|---|
| Age, yrs, mean (SD) | 54.4 (11.1) | 54.5 (11.0) | 53.5 (12.2) | 0.631 | 54.5 (11.8) | 54.4 (10.8) | 0.968 |
| Male, % | 54.9 | 55.4 | 50.0 | 0.555 | 42.7 | 9.7 | 0.005 |
| White race, % | 90.4 | 90.7 | 87.5 | 0.589 | 92.7 | 89.5 | 0.666 |
| TEG Indication, % | 0.002 | 0.146 | |||||
| - Anemia | 13.4 | 13.8 | 9.4 | 14.6 | 12.9 | ||
| - Coagulopathy | 32.0 | 28.8 | 62.5 | 26.0 | 34.3 | ||
| - Other | 5.5 | 5.4 | 6.2 | 5.2 | 5.6 | ||
| - Overt Bleeding | 39.8 | 42.3 | 15.6 | 45.8 | 37.5 | ||
| - Pre-procedure | 6.1 | 6.7 | 0.0 | 8.3 | 5.2 | ||
| - Thrombocytopenia | 3.2 | 2.9 | 6.2 | 0.0 | 4.4 | ||
| Cirrhosis etiology, % | 0.43 | 0.845 | |||||
| - Alcohol | 53.8 | 54.8 | 43.8 | 54.2 | 53.6 | ||
| - NASH | 24.7 | 24.4 | 28.1 | 21.9 | 25.8 | ||
| - HCV | 10.5 | 10.6 | 9.4 | 11.5 | 10.1 | ||
| - Other | 11.0 | 10.3 | 18.8 | 12.5 | 10.5 | ||
| CCI, mean (SD) | 6.7 (2.4) | 6.7 (2.4) | 6.6 (2.4) | 0.798 | 6.8 (2.8) | 6.7 (2.2) | 0.69 |
| PVT, % | 6.7 | 5.8 | 15.6 | 0.034 | 11.5 | 4.8 | 0.027 |
| DVT, % | 4.6 | 3.8 | 12.5 | 0.002 | 6.2 | 4.1 | 0.02 |
| ICU, % | 60.8 | 60.9 | 59.4 | 0.867 | 68.8 | 57.7 | 0.059 |
| Bleeding, % | 56.4 | 59.6 | 25.0 | <0.001 | 69.8 | 51.2 | 0.002 |
| Infection, % | 47.1 | 45.8 | 59.4 | 0.144 | 46.9 | 47.2 | 0.96 |
| ACLF, % | 37.4 | 35.9 | 51.6 | 0.085 | 39.6 | 36.6 | 0.614 |
| Child-Pugh A/B/C, % | 0.6/14.6/84.8 | 0.7/15.4/83.9 | 0.0/6.7/93.3 | 0.386 | 1.1/29.5/69.3 | 0.4/9.1/90.5 | <0.001 |
| MELD score, mean (SD) | 27.8 (10.2) | 27.4 (10.2) | 32.6 (8.8) | 0.007 | 23.9 (9.1) | 29.3 (10.2) | <0.001 |
| MELD-Na score, mean (SD) | 28.8 (10.0) | 28.3 (10.1) | 34.1 (7.8) | 0.002 | 24.9 (9.5) | 30.3 (9.8) | <0.001 |
| INR, mean (SD) | 2.4 (1.3) | 2.4 (1.3) | 2.9 (1.3) | 0.053 | 2.0 (0.8) | 2.6 (1.4) | <0.001 |
| Bilirubin, median (IQR) | 5.6 (2.5, 12.2) | 5.5 (2.3, 12.0) | 7.5 (4.3, 13.3) | 0.118 | 3.3 (1.5, 7.5) | 6.4 (3.2, 13.2) | <0.001 |
| Creatinine, mean (SD) | 2.1 (1.6) | 2.0 (1.5) | 2.5 (2.2) | 0.161 | 1.9 (1.6) | 2.1 (1.6) | 0.232 |
| Fibrinogen, mean (SD) | 150.4 (84.0) | 157.6 (85.4) | 93.9 (41.3) | 0.003 | 230.1 (116.7) | 125.3 (49.3) | <0.001 |
| Platelets, mean (SD) | 95.5 (67.9) | 99.3 (68.9) | 55.8 (40.2) | <0.001 | 157.0 (89.6) | 71.4 (35.1) | <0.001 |
| Albumin, mean (SD) | 2.8 (0.6) | 2.8 (0.6) | 2.9 (0.7) | 0.444 | 2.7 (0.5) | 2.8 (0.6) | 0.095 |
| Hemoglobin, mean (SD) | 8.3 (1.8) | 8.3 (1.8) | 8.6 (1.8) | 0.346 | 8.4 (1.8) | 8.3 (1.8) | 0.529 |
| WBC, mean (SD) | 11.4 (8.3) | 11.6 (8.4) | 9.7 (6.5) | 0.217 | 14.6 (8.9) | 10.1 (7.7) | <0.001 |
| Antiplatelet, % | 9.6 | 10 | 6.2 | 0.497 | 7.4 | 10.5 | 0.381 |
| - Aspirin, % | 9.3 | 9.6 | 6.2 | 0.529 | 7.4 | 10.1 | 0.44 |
| - Clopidogrel, % | 1.5 | 1.3 | 3.1 | 0.409 | 1.1 | 1.6 | 0.698 |
| Heparin (Prophylaxis), % | 12.5 | 11.6 | 21.9 | 0.094 | 9.5 | 13.7 | 0.289 |
| Anticoagulation, % | 6.1 | 5.8 | 9.4 | 0.417 | 8.3 | 5.2 | 0.283 |
| - Heparin (Therapeutic), % | 2.0 | 1.3 | 9.4 | 0.002 | 3.1 | 1.6 | 0.373 |
| - Warfarin, % | 2.6 | 2.9 | 0.0 | 0.329 | 4.2 | 2.0 | 0.255 |
| - DOAC, % | 1.5 | 1.6 | 0.0 | 0.47 | 1.1 | 1.6 | 0.698 |
| Length of Stay (days), median (IQR) | 12.0 (5.0, 20.0) | 12.0 (5.0, 20.0) | 9.0 (5.0, 20.8) | 0.413 | 12.0 (7.0, 20.0) | 11.0 (5.0, 20.0) | 0.307 |
| Inpatient Death, % | 29.1 | 27.6 | 43.8 | 0.055 | 26.0 | 30.2 | 0.442 |
| Discharge to Hospice, % | 7.0 | 6.7 | 9.4 | 0.576 | 7.3 | 6.9 | 0.887 |
| 30-Day Readmission, % | 24.7 | 24.0 | 31.2 | 0.368 | 21.9 | 25.8 | 0.448 |
| 30-Day Mortality, % | 33.4 | 31.4 | 53.1 | 0.013 | 32.3 | 33.9 | 0.781 |
Normal alpha angle ≥45°; low alpha angle <45°.
Normal MA ≥55 mm; low MA <55 mm.ACLF = acute-on-chronic liver failure; CCI = Charlson Comorbidity Index; DOAC = direct oral anticoagulant; DVT = deep vein thrombosis; HCV = hepatitis C virus; ICU = intensive care unit; INR = international normalized ratio; IQR = interquartile range; MA = maximum amplitude; MELD = model for end-stage liver disease; NA = sodium; NASH = nonalcoholic steatohepatitis; PVT = portal vein thrombosis; SD = standard deviation; TEG = thromboelastography; WBC = White blood cell count.
TEG Characteristics
TEG was performed on median hospital day 1 (IQR 1–4). TEG values from our cohort are visualized in Figure 1 as an approximate mean TEG curve. Median (IQR) R-value and K-value were 4.6 (3.8–5.7) and 2.1 (1.5–3.1) minutes, respectively. Median alpha angle was 65.2° (56.3–70.7). Median maximum amplitude was 48.2 mm (40.2–56.0), and LY30 was 0.0% (0.0–0.5).
Patients With Abnormal TEG Values
R-Value.
Only 15 patients (4.5%) had an elevated R-value (≥10 min) (Supplementary Table 1, available online). Compared to those with normal R-value, these patients were younger (mean age 48.3 vs 54.7 years, P = 0.03), with higher creatinine (mean 3.6 vs 2.0 g/dL, P <0.001), MELD (mean 35.5 vs 27.5, P = 0.003), MELD-Na (mean 36.2 vs 28.5, P = 0.003), and leukocyte count (mean 17.6 vs 11.1, P = 0.003). There was no difference in prevalence of acute-on-chronic liver failure. R-value was not associated with INR or use of anticoagulants; length of stay, mortality, and readmissions were similar in those with normal and elevated R-values.
Alpha Angle.
A total of 32 patients (9.3%) had a low alpha angle (<45°) (Table 1). Compared to those with a normal angle, these patients were more likely to be receiving therapeutic heparin (9% vs 1%). They were also less likely to be bleeding (25% vs 60%, P <0.001) and more likely to have a thrombus (portal vein thrombosis: 16% vs 6%, P = 0.03; deep vein thrombosis 29% vs 10%, P = 0.002). Patients with low alpha angle had higher MELD scores and INR, and lower platelet count and fibrinogen. They were more likely to die at 30 days (53% vs 31%, P = 0.01).
Maximum Amplitude.
A total 248 patients (72.1%) had a low maximum amplitude (<55 mm) (Table 1). Compared to those with a normal maximum amplitude, these patients were more likely to be males and less likely to have either thrombosis or bleeding. They were also more likely to have Child-Pugh C cirrhosis, and they had greater MELD scores. In addition to lower platelet counts, these patients had greater INR and lower fibrinogen. Use of antiplatelet agents were similar in those with and without low maximum amplitudes. Mortality, length of stay, and readmissions were also similar.
Correlations Between Measures of Hemostasis
All correlations between TEG and traditional measures of hemostasis were statistically significant (Table 2). R-value and LY30 were not strongly correlated with traditional measures (absolute rho range 0.13–0.37). INR was not strongly correlated with TEG measures (absolute rho range 0.19–0.37). In contrast, K-value, alpha angle, and maximum amplitude all had moderate-to-strong correlations with platelet count and fibrinogen (absolute rho range 0.52–0.67).
Table 2.
Spearman Correlations Between TEG and Traditional Measures of Hemostasis
| INR | Platelet Count | Fibrinogen | |
|---|---|---|---|
| R-value | 0.37 | −0.13 | −0.20 |
| K-value | 0.24 | −0.59 | −0.59 |
| Alpha angle | −0.24 | 0.55 | 0.52 |
| MA | −0.37 | 0.63 | 0.67 |
| LY30 | −0.19 | 0.25 | 0.23 |
INR = international normalized ratio; LY30 = clot lysis at 30 min; MA = maximum amplitude; TEG = thromboelastography.
Measures of Hemostasis in Patients With Bleeding and Infections
Measures of hemostasis according to the presence of active bleeding and infection are shown in Table 3. Patients with bleeding had shorter R-values and K-values, greater alpha angles, and greater maximum amplitudes. INR was lower in those with bleeding; platelet count and fibrinogen were not significantly different. The differences persisted after excluding patients who received transfusions within 48 hours of TEG (n = 112) (Supplementary Table 2, available online). Patients with infection had longer R-value, smaller alpha angle, and greater INR. However, K-value, maximum amplitude, platelet count, and fibrinogen were not significantly different. After excluding patients who received transfusions, R-value and INR remained greater in those with infections, but there was no difference in alpha angle.
Table 3.
Measures of Hemostasis According to the Presence of Bleeding or Infection
| No Bleeding (N = 150) | Bleeding (N = 194) | P Value | No Infection (N = 182) | Infection (N = 162) | P Value | |
|---|---|---|---|---|---|---|
| R-value | 4.8 (4.2, 5.9) | 4.4 (3.6, 5.5) | 0.006 | 4.3 (3.6, 5.2) | 5.0 (4.2, 6.5) | <0.001 |
| K-value | 2.3 (1.6, 3.8) | 1.9 (1.4, 2.8) | 0.002 | 2.0 (1.4, 2.9) | 2.1 (1.5, 3.2) | 0.13 |
| Alpha angle | 61.6 (52.3, 69.0) | 66.7 (60.5, 72.1) | <0.001 | 65.8 (58.3, 71.5) | 63.3 (53.1, 69.5) | 0.027 |
| MA | 44.0 (35.2, 53.1) | 50.2 (43.2, 58.2) | <0.001 | 48.8 (40.9, 56.3) | 47.2 (38.6, 55.7) | 0.349 |
| LY30 | 0.0 (0.0, 0.8) | 0.0 (0.0, 0.4) | 0.64 | 0.0 (0.0, 0.7) | 0.0 (0.0, 0.3) | 0.075 |
| INR | 2.7 (1.3) | 2.2 (1.2) | 0.002 | 2.1 (0.9) | 2.7 (1.6) | <0.001 |
| Platelets | 93.0 (70.2) | 97.4 (66.3) | 0.549 | 96.7 (69.9) | 94.1 (65.8) | 0.725 |
| Fibrinogen | 134.2 (83.7) | 159.0 (83.3) | 0.085 | 157.7 (95.1) | 142.1 (69.0) | 0.257 |
INR = international normalized ratio; LY30 = clot lysis at 30 min; MA = maximum amplitude.
OUTCOMES
The median length of stay was 12 days (IQR 6–20). In-hospital mortality was 29.1% (30-day mortality 33.4%), 7.0% were discharged to hospice, and the 30-day readmission rate was 24.7%. Comparisons between patients that died inhospital or were discharged to hospice and those that were alive at discharge are presented in Table 4. Those who died or who were discharged to hospice were more likely to be in ICU or to have infection or acute-on-chronic liver failure. Patients who died had higher MELD and Child-Pugh scores. They also had higher leukocyte count, lower fibrinogen, longer R-value, smaller alpha angle, and smaller LY30. K-value and maximum amplitude were similar in those discharged alive compared to those who died. In logistic regression adjusting for acute-on-chronic liver failure only, alpha angle was associated with mortality or hospice (beta coefficient −0.98; 95% confidence interval 0.96–1.00). However, after adjusting for MELD, this relationship was not significant. R-value was not associated with mortality or hospice after adjustment for either MELD or acute-on-chronic liver failure.
Table 4.
Characteristics of Patients Based on Hospital Outcomes
| Alive at Discharge (N = 221) | Inpatient Death/Hospice (N = 123) | P Value | |
|---|---|---|---|
| Age | 54.6 (10.7) | 54.2 (11.8) | 0.739 |
| Males, % | 55.2 | 54.5 | 0.896 |
| White, % | 92.3 | 87 | 0.233 |
| Cirrhosis etiology, % | 0.376 | ||
| - Alcohol | 52.5 | 56.1 | |
| - NASH | 27.6 | 19.5 | |
| - HCV | 10.0 | 11.4 | |
| - Other | 10.0 | 13.0 | |
| CCI | 6.6 (2.4) | 6.9 (2.4) | 0.205 |
| PVT, % | 8.6 | 3.3 | 0.057 |
| DVT, % | 13.6 | 7.3 | 0.079 |
| ICU, % | 54.3 | 72.4 | 0.001 |
| Bleeding, % | 53.4 | 61.8 | 0.132 |
| Infection, % | 40.3 | 59.3 | <0.001 |
| ACLF, % | 25.2 | 58.8 | <0.001 |
| Child Pugh A/B/C, % | 1.0/21.1/78.0 | 0.0/3.3/96.7 | <0.001 |
| MELD score | 24.7 (8.9) | 33.3 (9.9) | <0.001 |
| MELD-Na score | 25.9 (9.2) | 34.0 (9.3) | <0.001 |
| R-value | 4.3 (3.6, 5.3) | 5.1 (4.2, 6.5) | <0.001 |
| K-value | 2.0 (1.4, 2.9) | 2.2 (1.5, 3.2) | 0.122 |
| Alpha angle | 65.6 (57.5, 71.4) | 63.4 (54.5, 69.8) | 0.041 |
| MA | 48.9 (40.7, 56.8) | 46.5 (39.6, 55.3) | 0.241 |
| LY30 | 0.0 (0.0, 0.7) | 0.0 (0.0, 0.2) | 0.038 |
| INR | 2.2 (1.2) | 2.8 (1.4) | < 0.001 |
| Platelets | 97.1 (67.9) | 92.7 (68.2) | 0.562 |
| Total Bilirubin | 4.2 (2.0, 9.3) | 8.2 (4.7, 17.7) | < 0.001 |
| Creatinine | 1.8 (1.4) | 2.6 (1.8) | < 0.001 |
| Fibrinogen | 164.9 (98.8) | 132.0 (55.8) | 0.017 |
| Albumin | 2.8 (0.6) | 2.7 (0.6) | 0.256 |
| Hemoglobin | 8.4 (1.7) | 8.2 (1.9) | 0.318 |
| WBC | 9.6 (6.5) | 14.6 (10.0) | < 0.001 |
| Length of Stay (days) | 12.0 (6.0, 20.0) | 11.0 (4.5, 20.0) | 0.381 |
ACLF = acute-on-chronic liver failure; CCI = Charlson Comorbidity Index; DVT = deep vein thrombosis; HCV = hepatitis C virus; ICU = intensive care unit; INR = international normalized ratio; LY30 = clot lysis at 30 min; MA = maximum amplitude; MELD = model for end-stage liver disease; Na = sodium; NASH = nonalcoholic steatohepatitis; PVT = portal vein thrombosis; WBC = white blood cell count.
DISCUSSION
This cohort study is the largest assessing TEG in routine practice for hospitalized patients with cirrhosis. We described TEG in relevant settings (ie, bleeding and infection), examined correlations between TEG and traditional hemostasis measures, and assessed relationships among TEG, liver disease severity, and outcomes.
Clot initiation and propagation were relatively normal as the 25th and 75th percentiles for R-value, K-value, and alpha angle were within the previously reported range for healthy individuals.40 Clot formation and propagation is likely to be preserved because of rebalanced hemostasis in cirrhosis where deficiencies in platelets and clotting factors II, VII, IX, and X are met by increased von Willebrand factor (vWF) and factor VIII and deficiencies in anticoagulant factors (ie, protein C, protein S, and antithrombin).11,41 In contrast to this normal clot initiation, 72% had a low maximum amplitude. This finding is largely attributable to thrombocytopenia, which was present in 83% of our cohort. In addition, maximum amplitude is also dependent on the fibrin network, which is impaired in cirrhosis as a result of the decreased production of fibrinogen and dysfibrinogenemia.42 Notably, maximum amplitude was correlated with both fibrinogen and platelet count. Despite the severity of liver disease in our study (mean MELD 28), TEG profiles resembled a normal population more closely than another study of patients with cirrhosis.13 However, the prior study had strict criteria for inclusion (ie, INR >1.8, platelet count <50) and exclusion (ie, bleeding, thrombosis, anticoagulation). Therefore, our study may be more representative of hospitalized patients with end-stage liver disease.
Patients with cirrhosis have a large burden of comorbidities and often have indications for anticoagulation and antiplatelets. Interestingly, these agents were not associated with either abnormal R-value or maximum amplitude. A lack of response of maximum amplitude to antiplatelets has been described previously.43,44 However, our findings contrast with data supporting an effect of anticoagulants on R-value and maximum amplitude.45,46 Our study may have been underpowered to detect such differences because prolonged R-value and use of anticoagulation were rare. Unlike R-value and maximum amplitude, a low alpha angle was more likely to be present with therapeutic heparin, consistent with heparin’s role in preventing fibrin generation.47 This finding may also explain the paradoxical lower alpha angle in patients with thrombosis, reflecting thrombosis treatment rather than an innate hypocoagulability. Of note, we also found a nonsignificant trend in the association between prophylactic heparin and alpha angle.
The dynamic relationship among TEG and INR, platelet count, and fibrinogen remains incompletely understood. In our results, correlations between TEG values and traditional measures of hemostasis were mostly moderate to weak. This weak correlation has been shown previously48,49 and highlights the unique information provided by TEG. It also indicates that TEG may have value as a complement to, rather than a substitute for, traditional labs. In this light it is important to understand how TEG behaves in the setting of bleeding or infection.
Patients with bleeding had more hypercoagulable profiles on both TEG and traditional labs of hemostasis. Patients with bleeding might be exhibiting a compensatory mechanism that manifests as increased coagulation to regain hemostasis. In a study that compared patients with cirrhosis who were bleeding to normal controls, patients who were bleeding had a lower maximum amplitude and longer K-value only, but similar R-value and alpha angle, suggesting that some TEG parameters are insensitive to active bleeding.49 In addition, multiple studies have documented a lack of relationship between traditional markers of hemostasis and the risk of bleeding in this population.25,49 The value of these markers is yet to be determined in terms of bleeding risk.
In contrast to bleeding, patients with infections in our study had impairments in clot initiation and propagation (R-value and alpha angle). These findings are consistent with previous work in patients with cirrhosis showing impaired coagulation on TEG correlating temporally with infections.50 This impairment can be attributed to circulating endogenous heparinoids, which are increased in the setting of infection.51–53 Our study further supports the notion that the rebalanced hemostasis in cirrhosis is fragile and can deteriorate in the setting of infection. TEG may be a valuable tool to assess hemostasis in cirrhosis, particularly in infections.
Overall, patients with abnormal TEG parameters consistently had greater MELD scores, a finding that has previously been demonstrated in patients undergoing liver transplant.54 Our results extend these findings to patients with cirrhosis hospitalized for reasons other than transplant because we excluded patients admitted for transplant. This relationship can be explained in part by the inclusion of INR as a measure of coagulopathy within the MELD score. However, the coagulopathy detected by TEG appears to be related to other components of liver disease severity as well, including an association between bilirubin and alpha angle and an association between creatinine and R-value. TEG may, therefore, reflect liver disease severity independent of its relationship with INR.
Despite the relationship between TEG and liver disease severity, few studies have examined TEG as a potential predictor of outcomes for patients with cirrhosis. One study of ambulatory patients with cirrhosis showed no difference in long-term transplant-free survival with respect to any TEG measures.25 In our study, 36% of patients died or were discharged to hospice, reflecting the severity of illness of hospitalized patients with cirrhosis at a tertiary care center. When compared to those who survived their hospital stay, those that died or were discharged to hospice had longer R-values and smaller alpha angles. However, these relationships were nullified by well-established markers of inpatient cirrhosis mortality (ie, MELD and acute-on-chronic liver failure), suggesting a limited role for TEG in short-term mortality prediction.
This single-center inpatient study has several limitations, including limited external validity because of heterogeneity in TEG availability and use at different centers. Multicenter or population-based studies assessing TEG characteristics and outcomes in similar populations are needed to confirm our findings and enhance generalizability. Another limitation is the study reliance on TEGs obtained for routine clinical care, which prevented a more systematic assessment that could be achieved with a prospective protocol. However, the benefit of this type of data collection is that we were able to examine the performance of TEG in routine clinical care, which may better inform clinicians for real-world practice. In contrast to these limitations, this study benefits from a large sample size (ie, the largest description of TEG in real-world use for patients with end-stage liver disease) with robust postdischarge follow-up. We also employed detailed, granular data collection, including in-depth assessments of medications, the presence of acute-on-chronic liver failure, and the presence of thromboses, including portal vein thrombosis.
CONCLUSIONS
TEG provides a unique insight into the hemostatic state of patients with cirrhosis that cannot be ascertained based on standard measures of hemostasis alone. Overall, patients with cirrhosis have preserved initiation and propagation of clots, with impaired maximum clot strength. Infections significantly impact coagulation in this population, though bleeding does not necessarily reflect more severe impairment in coagulation. TEG is associated with both liver disease severity and clinical outcomes and may play a role complementary to standard measures of hemostasis in this population. Further work is needed to better establish how TEG may fit into routine clinical care and how it may fill gaps in our understanding of end-stage liver disease.
Supplementary Material
CLINICAL SIGNIFICANCE.
Hospitalized patients with cirrhosis have preserved clot initiation and propagation but impaired maximal clot strength.
Thromboelastography does not correlate well with traditional measures of hemostasis in this population.
Thromboelastography is associated with liver disease severity in this population but not with outcomes.
Funding:
This work was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK109202. The sponsor had no role in the study design, collection, analysis, and interpretation of the data or in the writing of the report. The work was independent of the funding.
Footnotes
Conflicts of Interest: NC reports serving as a consultant and receiving research grants from several pharmaceutical companies. HS, KRP, MG, APD, LN, SK, ESO report none.
References
- 1..D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44(1):217–31. 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Ewe K Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci 1981;26(5):388–93. 10.1007/bf01313579. [DOI] [PubMed] [Google Scholar]
- 3.McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99(5):1396–400. 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 4.Diaz LK, Teruya J. Liver biopsy. N Engl J Med 2001;344(26):2030. [PubMed] [Google Scholar]
- 5.Terjung B, Lemnitzer I, Dumoulin FL, et al. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion 2003;67(3):138–45. 10.1159/000071293. [DOI] [PubMed] [Google Scholar]
- 6.Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatol Baltim Md 2004;40(2):484–8. 10.1002/hep.20317. [DOI] [PubMed] [Google Scholar]
- 7.Segal JB, Dzik WH, Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion (Paris) 2005;45(9):1413–25. 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Tripodi A, Primignani M, Mannucci PM. Abnormalities of hemostasis and bleeding in chronic liver disease: the paradigm is challenged. Intern Emerg Med 2010;5(1):7–12. 10.1007/s11739-009-0302-z. [DOI] [PubMed] [Google Scholar]
- 9.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences1: Hypercoagulability in cirrhosis. J Thromb Haemost 2011;9(9):1713–23. 10.1111/j.1538-7836.2011.04429.x. [DOI] [PubMed] [Google Scholar]
- 10.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365(2):147–56. 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 11.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 2010;116(6):878–85. 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 12.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg 1985;64(9):888–96. [PMC free article] [PubMed] [Google Scholar]
- 13.De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial: thrombelastography-guided blood product. Hepatology 2016;63(2):566–73. 10.1002/hep.28148. [DOI] [PubMed] [Google Scholar]
- 14.Rocha LL, Pessoa CMS, Neto AS, et al. Thromboelastometry versus standard coagulation tests versus restrictive protocol to guide blood transfusion prior to central venous catheterization in cirrhosis: study protocol for a randomized controlled trial. Trials 2017;18(1):85 10.1186/s13063-017-1835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey C, Saluja V, Gaurav K, Tandon M, Pandey V, Bhadoria A. K time & maximum amplitude of thromboelastogram predict post-central venous cannulation bleeding in patients with cirrhosis: A pilot study. Indian J Med Res 2017;145(1):84 10.4103/ijmr.IJMR_749_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabate A, Blasi A. Thromboelastography and blood product usage in cirrhosis with severe coagulopathy. Hepatology 2017;65(4):1413–4. 10.1002/hep.29061. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography guided blood component use in patients with cirrhosis with nonvariceal bleed ing: a randomized controlled trial. Hepatology 2020;71(1):235–46. 10.1002/hep.30794. [DOI] [PubMed] [Google Scholar]
- 18.Olson JC. Thromboelastography guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized controlled trial. Clin Liver Dis 2019;13(4):102–5. 10.1002/cld.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012;56(1):129–36. 10.1016/j.jhep.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol 2012;8 (8):513–20. [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Donald P, Vasudevan A, Angus P, et al. Coagulation in acutely ill patients with severe chronic liver disease: insights from thromboelastography. J Crit Care 2017;38:215–24. 10.1016/j.jcrc.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Potze W, Siddiqui MS, Boyett SL, et al. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J Hepatol 2016;65 (5):980–7. 10.1016/j.jhep.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Premkumar M, Bihari C, Saxena P, et al. Heparin-like effect associated with risk of bleeding, sepsis, and death in patients with severe alcohol-associated hepatitis. Clin Gastroenterol Hepatol 2020;18 (2):486–495.e3. 10.1016/j.cgh.2019.04.057. [DOI] [PubMed] [Google Scholar]
- 24.Goyal S, Jadaun S, Kedia S, et al. Thromboelastography parameters in patients with acute on chronic liver failure. Ann Hepatol 2018;17 (6):1042–51. 10.5604/01.3001.0012.7205. [DOI] [PubMed] [Google Scholar]
- 25.Hugenholtz GCG, Lisman T, Stravitz RT. Thromboelastography does not predict outcome in different etiologies of cirrhosis. Res Pract Thromb Haemost 2017;1(2):275–85. 10.1002/rth2.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge PS, Runyon BA. Treatment of patients with cirrhosis. N Engl J Med 2016;375(8):767–77. 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj JS, Reddy KR, Tandon P, et al. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatol Baltim Md 2016;64(1):200–8. 10.1002/hep.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orman ES, Ghabril M, Emmett TW, Chalasani N. Hospital readmissions in patients with cirrhosis: a systematic review [e-pub ahead of print]. J Hosp Med. doi: 10.12788/jhm.2967, accessed Month Day, Year. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis Int J Haemost Thromb 2001;12(5):327–37. 10.1097/00001721-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci 2009;40(2):119–23. 10.1016/j.transci.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang S-C, Shieh J-F, Chang K-Y, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc 2010;42 (7):2590–3. 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 32.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88 (2):312–9. 10.1213/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60(8):646–9. 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 35.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatol Baltim Md 2001;33(2):464–70. 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 36.Londôno M-C, Cárdenas A, Guevara M, et al. MELD score and serum sodium in the prediction of survival of patients with cirrhosis awaiting liver transplantation. Gut 2007;56(9):1283–90. 10.1136/gut.2006.102764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67 (6):2367–74. 10.1002/hep.29773. [DOI] [PubMed] [Google Scholar]
- 38.Create elegant data visualisations using the grammar of graphics. Available at:https://ggplot2.tidyverse.org/. Accessed February 4, 2020.
- 39.RStudio j Open source & professional software for data science teams. Available at:https://rstudio.com/. Accessed April 6, 2019.
- 40.Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol 2009;42(12):1210–7. . [DOI] [PubMed] [Google Scholar]
- 41.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol 2013;11(9):1064–74. 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Besser M, MacDonald S. Acquired hypofibrinogenemia: current perspectives. J Blood Med 2016;7:217–25. 10.2147/JBM.S90693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka KA, Szlam F, Kelly AB, Vega JD, Levy JH. Clopidogrel (Plavix) and cardiac surgical patients: implications for platelet function monitoring and postoperative bleeding. Platelets 2004;15(5):325–32. 10.1080/09537100410001710236. [DOI] [PubMed] [Google Scholar]
- 44.Orlikowski CE, Payne AJ, Moodley J, Rocke DA. Thrombelastography after aspirin ingestion in pregnant and non-pregnant subjects. Br J Anaesth 1992;69(2):159–61. 10.1093/bja/69.2.159. [DOI] [PubMed] [Google Scholar]
- 45.Gantioqui J, Stevic I, Atkinson H, Chan AKC. Rivaroxaban and dabigatran did not affect clotting profiles in plasma reconstituted with varying levels of autologous platelets to the same degree as heparin when evaluated using thromboelastography. Blood Coagul Fibrinolysis Int J Haemost Thromb 2018;29(6):521–7. 10.1097/MBC.0000000000000751. [DOI] [PubMed] [Google Scholar]
- 46.Solbeck S, Jensen AS, Maschmann C, Stensballe J, Ostrowski SR, Johansson PI. The anticoagulant effect of therapeutic levels of dabigatran in atrial fibrillation evaluated by thrombelastography (TEG®), Hemoclot Thrombin Inhibitor (HTI) assay and Ecarin Clotting Time (ECT). Scand J Clin Lab Invest 2018;78(1–2):25–30. 10.1080/00365513.2017.1408138. [DOI] [PubMed] [Google Scholar]
- 47.Balandina AN, Serebriyskiy II, Poletaev AV, et al. Thrombodynamics —a new global hemostasis assay for heparin monitoring in patients under the anticoagulant treatment. PLoS One 2018;13(6):e0199900 10.1371/journal.pone.0199900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G-Y, Ou Yang X-L, Wu J-H, et al. [Comparison of thromboelastography and routine coagulation tests for evaluation of blood coagulation function in patients]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015;23 (2):546–51. 10.7534/j.issn.1009-2137.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 49.Hum J, Amador D, Shatzel JJ, et al. Thromboelastography better reflects hemostatic abnormalities in cirrhotics compared with the international normalized ratio[e-pub ahead of print]. J Clin Gastroenterol. doi: 10.1097/MCG.0000000000001285, accessed Month Day, Year. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papatheodoridis GV, Patch D, Webster GJ, Brooker J, Barnes E, Burroughs AK. Infection and hemostasis in decompensated cirrhosis: a prospective study using thrombelastography. Hepatol Baltim Md 1999;29(4):1085–90. 10.1002/hep.510290437. [DOI] [PubMed] [Google Scholar]
- 51.Zambruni A, Thalheimer U, Coppell J, et al. Endogenous heparin-like activity detected by anti-Xa assay in infected cirrhotic and non-cirrhotic patients. Scand J Gastroenterol 2004;39(9):830–6. 10.1080/00365520410004433. [DOI] [PubMed] [Google Scholar]
- 52.Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol 2002;37(4):463–70. 10.1016/s0168-8278(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 53.Senzolo M, Riddell A, Tuddenham E, Burroughs AK. Endogenous heparinoids contribute to coagulopathy in patients with liver disease. J Hepatol 2008;48(2):371–2. 10.1016/j.jhep.2007.11.003 [author reply 372–373]. [DOI] [PubMed] [Google Scholar]
- 54.Kohli R, Shingina A, New S, et al. Thromboelastography parameters are associated with cirrhosis severity. Dig Dis Sci 2019;64(9):2661–70. 10.1007/s10620-019-05597-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


