Abstract
Postoperative conduction block requiring lifetime pacemaker placement continues to be a considerable source of morbidity for patients undergoing repair of congenital heart defects. Damage to the cardiac conduction system (CCS) during surgical procedures is thought to be a major cause of the conduction block. Intraoperative identification and avoidance of the CCS is thus a key strategy to improve surgical outcomes. In order to avoid conduction tissue damage and mitigate morbidity a number of approaches have been developed. Here, we review the historical and contemporary approaches for identification of conduction tissue during cardiac surgery. The established approach for intraoperative identification is based on anatomical landmarks established in extensive histological studies of the normal and diseased heart. We focus on landmarks to identify the sinus and atrioventricular nodes during cardiac surgery. We also review technologies explored for intraoperative tissue identification including electrical impedance measurements and electrocardiography. We describe new optical approaches, in particular, and optical spectroscopy and fiberoptic confocal microscopy (FCM), for identification of CCS regions and working myocardium during surgery. As a template for translation of future technology developments, we describe research and regulatory pathways to translate FCM for cardiac surgery. We suggest that along with more robust approaches to surgeon training including awareness of fundamental anatomical studies, optical approaches such as FCM show promise in aiding surgeons in repairs of heart defects. In particular, for complex defects, these approaches can complement landmark-based identification of conduction tissue and thus help to avoid injury to the CCS due to surgical procedures.
Keywords: conduction tissue identification, intraoperative imaging, cardiac surgery, congenital, postoperative heart block
Introduction
Primary surgical correction has evolved as the main choice for the treatment of a wide variety of congenital heart defects. These defects are the most common type of birth defects in North America. The worldwide incidence ranges from 19 to 75 per 1000 live births.1 Since the first surgical corrections of congenital heart defects in the 1950s, surgeons were concerned about dysrhythmias that often resulted from these interventions.2 Many of these dysrhythmias did not spontaneously resolve and led to permanent heart block requiring pacemaker implantation to maintain heart function in normal sinus rhythm.3 The need for a postoperative pacemaker varies for each lesion and ranges from 1-3% for simple to more than 15% for complex lesions (Supplemental Table 1). Pacemaker implants place a tremendous lifetime healthcare burden on pediatric patients and their families, and are associated with a major increase in cost to the healthcare system.4, 5 The burden of pacemaker dependence is twofold. First, there is an impact of the pacemaker implant on the ventricular function and quality of life of patients; both of which were shown to decline. Second, there are significant financial implications of pacemaker implantation, i.e. substantial direct and indirect cost accumulations through a lifetime. The risk of postoperative heart block has prompted research into localizing conduction tissue during surgical repairs to avoid trauma to these tissues and development of subsequent postoperative conduction defects. Detailed histological studies established our current understanding of the cardiac conduction system (CCS). More recent anatomical studies using techniques such as micro-computed tomography further elucidated the location of the CCS.6, 7 In addition, various technical approaches were developed to localize the CCS. However, despite the advances in anatomical knowledge and technology, heart block continues to be a major complication in congenital heart disease surgery.3, 8 If nodes and other components of the CCS can be localized during pediatric congenital heart surgery then these can be preserved and the lifetime burden of permanent pacemaker implantation can be avoided.
Here, we review approaches for intraoperative identification of major components of the CCS, in particular the sinus node (SN) and atrioventricular node (AVN). We provide a summary of the current state of the art of identification of these components using anatomical landmarks. We review approaches based on electrical impedance and electrocardiography. We also describe optical approaches, in particular, fiberoptic confocal microscopy (FCM), which is currently undergoing clinical evaluation. Finally, we discuss limitations and suggest new optical techniques that show the promise of intraoperative imaging for identification of the CCS.
Localization Using Anatomical Landmarks
Currently, intraoperative CCS localization is based on an extensive body of work that started in the 1800’s with the pivotal studies of Jan Evangelista Purkinje, Walter Gaskell, Wilhelm His Jr., Sunao Tawara, Arthur Keith and Martin Flack.9 Subsequently, more sophisticated histology techniques were applied to further elucidate location and function as well as the development and maturation of the CCS.6,10–17
These early studies revealed that the SN lies below the epicardial surface within the terminal groove of the right atrium. This terminal groove stretches from the superior to the inferior cavoatrial junction and is superficial to the crista terminalis. The SN typically originates just inferior to the superior cavoatrial junction, but can also extend over the right atrial appendage crest and into the interatrial junction.10, 15 The SN stretches toward the inferior cavoatrial junction. In the adult heart, SN length and width are 10-30 mm and 5-7 mm, respectively.10, 18 There is also variance in the width of the node and its position within the terminal groove (Fig. 1A).10 The majority of the SN in the normal heart lies on the superior vena cava side of the terminal groove centerline. In approximately 10% of cases, the SN has a horseshoe shape and two nodal bodies that extend from the crest of the right atrial appendage into the terminal groove (Fig. 1A).10 A histological section through a SN region is presented in Fig. 2A. The SN in the terminal groove is accompanied by paranodal tissue, which lies subendocardially and on the superior vena cava side of the crista terminalis.13 The paranodal tissue connects the SN and the surrounding atrial tissue, and comprises nodal and atrial cells. While the SN cannot be seen with the naked eye, connective tissue tends to be embedded within the nodal body and in neighboring regions. This connective tissue can cause a white opaque patch of tissue visible on the epicardial surface of the terminal groove in some cases.19 Also, superficial pads of adipose tissue can overlay the most superior portions of the SN. Surgeons sometimes use the opaque patch and superficial to help identify the SN region. Blood supply to the SN is provided by the SN artery which originates from either the right coronary artery, the left coronary artery, or both. This requires surgeons to recognize that crucial SN blood supply can originate from the superior cavoatrial junction or the tissue on the vena cava side of the terminal groove inferior to the SN.
Figure 1.
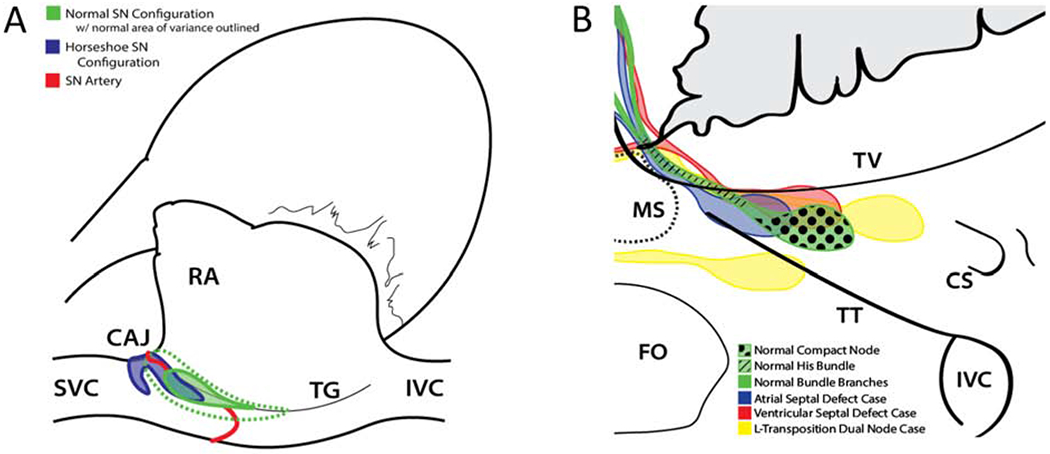
Anatomical landmarks and location variance of the SN and AVN. Anatomical structures are shown from a surgical perspective. (A) Normal and horseshoe SN configuration as well as possible locations of the SN artery. Variance of the normal SN location is outlined by green dotted line. (B) Triangle of Koch region comparing normal AVN node location to location in congenital defect cases. CS, coronary sinus; CAJ, superior cavoatrial junction; FO, fossa ovalis; RA, right atrial appendage; IVC, inferior vena cava; MS, membranous septum; SVC, superior vena cava; TG, terminal groove; TT, tendon of Todaro; TV, tricuspid valve annulus. Images were based on prior work10, 21–23.
Figure 2.

Example Masson’s trichrome sections from SN and AVN. (A) Section from a human infant 6 days of age after birth, sectioned parallel to the crista terminalis. Green outline indicates SN region. (B) Section from a premature human infant, 7 months gestational age, sectioned from coronary sinus to membranous septum. Green outline indicates AVN region. AVN, Atrioventricular Node; SN, Sinus Node; RA, right atrium; SVC, Superior Vena Cava; CFB, central fibrous body; IVS, interventricular septum.
The AVN and associated cell zones are located within the anatomical landmark known as the triangle of Koch. The node is about 2-7 mm long and 1-5 mm wide.20 The triangle of Koch is formed by the annulus or attachment of the septal leaflet of the tricuspid valve, the orifice of the coronary sinus, and the difficult to visualize tendon of Todaro. The tendon of Todaro is a fibrous offshoot of the Eustachian valve, approximately level with the middle of the sinus septum, which extends towards the membranous septum.10, 12 Commonly, the compact AVN body originates approximately two thirds of the way from the coronary sinus to the membranous septum and tapers into the vertex of the triangle of Koch where nodal tissue transitions into the His bundle (Fig. 1B). The His bundle penetrates below or through the inferior border of the central fibrous body and into the interventricular septum where it splits into the left and right bundle branches. In normal hearts, the compact AVN is associated with left and right nodal extensions housing the slow and fast pathways on the coronary sinus side, as well as transitional tissue and ring structures near the compact node and encircling the orifices of both atrioventricular valves.6, 14, 21 A histological section through the AVN region is presented in Fig. 2B. The AVN is localized subendocardially, in part, beneath atrial overlay cells.
The SN and, to a greater extent, the AVN are known to change locations in hearts with congenital defects. The only documented change in the location of the SN is the juxtaposition of the atrial appendages and left atrial isomerism.10 The AVN location, however, often changes in hearts with congenital defects (Fig. 1B). In cases with perimembranous ventricular septal defects, where portions of the defect rim are formed by the central fibrous body or membranous septum, the compact AVN lies caudal to the normal AVN position within the triangle of Koch. The His bundle in these cases is almost always shifted to the coronary sinus side of the triangle of Koch and the bundle branches stretch around the rim of the septal defect. In cases of atrioventricular canal malformations, the compact node rests in a much more apical position closer to the membranous septum.10 In extreme cases like transposition of the great vessels and because the AVN and His bundle develop from the atrioventricular canal myocardium of the trabecular septum, the AVN is often split into two compact nodal bodies and two bundles of His that straddle the pulmonary valve before converging and penetrating into ventricular tissue.22 These cases are illustrated in Fig. 1B. Other forms of variance in AVN location depended on the congenital defect and were characterized through histological studies.21
During procedures to correct congenital heart defects, surgeons currently rely solely on their knowledge of the location of the CCS components in relation to key anatomical landmarks. Comprehensive research and high-resolution histological studies established guides to CCS location in normal and congenitally deformed hearts.10, 21, 23 These guides are summarized in today’s gold standard surgical anatomy references. The decrease in morbidity and mortality associated with surgical repair of congenital heart defects since the mid-1900s is a testament to the accuracy of these guides and their increasing familiarity among cardiothoracic surgeons.24, 25
While these findings are important for physician training and surgical planning, the applied methods themselves are not usable during the operative procedure for identifying the CCS. Inadvertent trauma to the CCS during surgical operations decreased significantly because of reliable anatomical landmarks. However, a degree of variance is associated with the location of the components of the CCS in each patient. Even after identification of the terminal groove and superior cavoatrial junction, surgeons must treat the entire atrial appendage crest, interatrial junction, and terminal groove as if it contains conduction tissue or blood vessels supplying the SN.10 Also, after correctly identifying the triangle of Koch, surgeons cannot exactly discern the beginning of the compact AVN relative to the coronary sinus, the size of the left and right nodal extensions, and where the His bundle and bundle branches reside.21 Surgeons cautiously account for the variation in nodal tissue location in their surgical procedures, which is reflected in increased numbers of residual lesions left.26
Localization Using Electrical Impedance and Electrocardiography
Early work on intraoperative CCS localization was based on measurements of either electrical impedance or electrocardiograms (Supplemental Table 2). For both approaches, electrodes were locally applied to the tissue to measure electrical signals. Measurements were presented instantaneously to the operator. Impedance measurements were performed using handheld unipolar and bipolar electrode probes to apply alternating electrical current and measuring voltage in the tissue. Differences between the impedance of nodal tissues and ventricular myocardium were applied to generate auditory signals to inform a surgeon.27, 28 Nodal tissues were associated with reduced impedance. The approach was explored in canine models and patients with heart defects.
While technical complexity of impedance-based localization is small (see references in Supplemental Table 3 for technical details), its major limitation is that the measured impedance is a complex function of the spatial three-dimensional distribution of tissues, which is highly heterogeneous in nodal regions (Fig. 2). The heterogeneity hampers reliable localization of CCS components. It is thus not surprising that work on this approach ceased after its introduction in the 1960s.
Intraoperative localization of components of the CCS based on local electrocardiograms use the electrical activity and conduction of tissue.29–33 Commonly, bipolar measurements with handheld electrodes were performed. Characteristic electrocardiograms for different tissues, e.g. right atrium and bundle of His, were established in the canine and human heart. Variants of this approach integrated atrial pacing to assure antegrade conduction.34–36 Most studies of electrocardiogram-based localization of CCS components demonstrated technical feasibility. A study on 15 pediatric and adult patients with congenital heart disease presented a success rate of 67% for localization of the distal His bundle and right bundle branch.29 In a study on 10 patients undergoing repair of endocardial cushion defects 9 slight and 1 major deviations of the His bundle locations were identified.33 While these findings strongly support the use of intraoperative electrocardiogram-based localization, clinical trials assessing patient outcomes in surgeries with and without localization were not performed.
Electrocardiogram-based localization is based on technology established in cardiology (see references in Supplemental Table 3 for technical details), but a limitation is the requirement of surgical protocols that maintain electrical activity. Electrocardiogram-based localization cannot be applied with protocols requiring cardioplegia and leading to cessation of electrical activity.
Optical Localization of the CCS
Optical systems for localization were introduced in the 1990s. Several systems applied ultraviolet light for excitation of fluorophores intrinsic in the tissue and measurement of spectra of the fluorescent light emission.37–40 Optical fibers coupled the tip of the spectroscopic probe to the light sources and detectors. The systems were evaluated using canine hearts as well as fresh and fixed samples from human hearts. While the optical systems differed in their specific wavelength for excitation and wavelengths of measured spectra, differences of the spectra between nodal tissue regions and working myocardium were sufficiently large for their discrimination. These findings reflect that the distribution of light scatterers, e.g. nuclei and mitochondria, and light absorbers, e.g. hemoglobin and lipids, is tissue specific. However, none of the introduced optical approaches was explored in in-vivo animal models or translated in clinical trials.
From a translational perspective, spectroscopic optical approaches are the least advanced. Their evaluation in the in-situ heart is still pending. Similar as for impedance-based approached, technical complexity of spectroscopy-based system is small, but the spectra are a complex function of the spatial distribution of tissues, which hinders reliable tissue identification and localization.
A recently introduced approach for intraoperative tissue localization applies FCM (Fig. 3).41 This optical imaging modality requires extrinsic fluorophores, e.g. Fluorescite® excited at a wavelength of 488 nm, to produce real-time sequences of two-dimensional images of tissue microstructure. These sequences were used to discriminate between tissue types. As for other types of confocal microscopy, FCM allows optical sectioning and can produce images from within a sample. In contrast to conventional confocal microscopes used in research laboratories, objectives are detached from the FCM system using coherent optical fiber bundles. In these bundles, the fibers are aligned in such a manner that images can be directly transmitted from one to the other end. One end of the fiber bundle is attached to the confocal microscope system comprising a light source and detector. The other end of the bundle is attached to a microobjective. The fiber bundle can have lengths up to several meters, which facilitates application in the operating room (OR).
Figure 3.

Technologies used to localize conduction tissue during heart surgery. (A) Electrical probe for application of current to tissue and measurement of voltage to determine impedance. (B) Electrical probe for measurement of electrical activity of the CCS at tissue surface. (C) FCM probe for excitation of fluorophores in tissue and collection of emitted light. (D) Probes are handheld for pointwise measurements during surgery. (E) System for processing of measurements. (F) Electrical impedance, (G) electrograms and (H) microscopic images identify tissue types.
Initial work applied rodent models to evaluate if FCM can be used to discriminate between nodal tissues and working myocardium.41 The investigations were based on 3D reconstructions of tissue microstructure using confocal microscopy and immunofluorescence labelling. From these reconstructions, characteristic striated and reticular patterns for these working myocardium and nodal tissues, respectively, were identified (Fig. 4). Also, the focal length of the micro-objective was optimized to account for depth of nodal tissues. Example images from the living rodent heart are presented in Fig. 5A–C. Sensitivity and specificity of the classification of these images was similarly high for both, human experts and automated methods.42 This initial work suggests that while technical complexity of FCM is high and its depth coverage is small, an advantage of FCM is that the produced images directly reflect the tissue distribution.
Figure 4.

Conventional confocal microscopy of rodent (A-H) atrial working myocardium (AWM) and (IP) SN region. The tissues were fixed and labeled for (A,I) nuclei using 4’,6-diamidino-2-phenylindole (DAPI), (B, J) extracellular matrix using wheat germ agglutinin (WGA) conjugated to the fluorophore CF488A, antibodies for (C, K) hyperpolarization-activated cyclic nucleotide-gated potassium channel 4 (HCN4), and (D, L) sarcomeric α-actinin. Secondary antibodies for HCN4 and sarcomeric α-actinin were conjugated to Alexa Fluor 633 and Alexa Fluor 555, respectively. HCN4 served as a marker for nodal myocytes. Sarcomeric α-actinin served as a marker for cardiomyocytes. AWM exhibited densely packed, aligned myocytes with characteristic striated patterns of the extracellular space. (E) Overlaid image of DAPI, WGA and sarcomeric α-actinin in AWM. (E) Overlaid image of DAPI, WGA and HCN4 in AWM. Reconstruction of region marked in (E) presented (G) with and (H) without epicardial layer. (M) Overlaid image of DAPI, WGA and sarcomeric α-actinin in SN region at a depth of 26.6 pm illustrating regular AWM arrangement. (N) Overlaid images of DAPI, sarcomeric α-actinin and HCN4 in SN region at a depth of 5.6 μm illustrating reticulated microstructure of nodal myocytes. (O) Reconstruction of marked region in (M) showing the layered tissue structure in SN region. (P) Reconstruction of SN and AWM layer below the epicardial layer. The nodal region exhibited characteristic reticulated patterns of the extracellular space. Myocyte density was smaller than in AWM and their orientation was irregular. Scale bar in (A) applies to (A-F) and (I-N). Figure from Huang et al, 201341.
Figure 5.

FCM images of living cardiac tissues in (A-C) rodent and (D-F) human. FCM of (A) atrial working myocardium, (B) SN and (C) AVN region labeled with a dextran-fluorophore conjugate as marker of the extracellular space. Living tissues exhibited similar microstructural patterns as in fixed tissues using extracellular matrix labeling presented in Fig. 4. Atrial working myocardium and nodal tissues were associated with striated and reticulated microstructure, respectively. (D) FCM images acquired from the atrium of a pediatric patient during surgical repair of secundum atrial septal defect present a striated pattern. The extracellular space of the tissue was labeled using Fluorescite®. Images acquired at (E) SN and (F) AVN regions show reticulated microstructure. The regions were identified using anatomical landmarks. Scale bar in (A) applies to (B) and (C). Scale bar in (D) applies to (E) and (F). Images from studies described in (A-C) Huang et al, 201341 and (D-F) Kaza et al, 202045.
Translation of Technologies for Intraoperative Conduction Tissue Identification
Translation of technology for intraoperative CCS identification from the laboratory into the clinic require a unique perspective on safety, regulation and trial design. Commonly, the translation of these technologies will require working knowledge of the quality systems, design control and risk management as mandated by the US Food and Drug Administration (FDA) and International Standards Organization (ISO). These requirements are outlined in the quality system requirements (ISO 13485 and FDA 21CFR820), design control (ISO 13485 7.3 and FDA 21CFR820.30) and risk management (ISO 14971). For devices not already approved for human use by regulatory agencies and deemed to be significant risk by an Institutional Review Board (IRB), the device will require an Investigational Device Exemption (IDE) from the FDA prior to use in humans.
In an example for clinical translation, a clinical trial was designed to evaluate FCM for use during cardiac surgery. The FCM device was previously approved by various regulatory agencies for imaging in pulmonary, gastrointestinal and urogenital applications. These approvals along with subsequent reimbursement codes for healthcare providers led to several years of experience in, for instance, gastrointestinal endoscopy, where FCM has an excellent safety record and proven to increase diagnostic accuracy.43 For use in cardiac surgery, the FDA device was assessed for safety in animal studies.44 The research team worked with the device manufacturer, IRB and FDA on off-label use of FCM and a fluorophore in pediatric cardiac surgery. This approach involving engineers, clinicians, scientists, regulatory experts and the FDA, resulted in a mutually agreed process to conduct a clinical trial.45 Technical parameters are described in Supplemental Table 3. Example FCM images from the heart of pediatric patients in this trial are presented in Fig. 5D–F. The FCM system was easily integrated to the surgical workflow. Time spent for localization of nodal tissue was less than 3 min, which is short compared to cardiopulmonary bypass times (88-158 min) and cross-clamp times (24-48 min) in pediatric heart surgery.46
Discussion and Conclusions
Advancements in the treatment of patients with congenital heart disease resulted in significantly improved outcomes and complication-free survival. However, we still have an unmet need with regards to acquired conduction defects necessitating pacemaker placement. CCS location in common defects is well established, but even with this knowledge a subset of patients develops postoperative conduction defects requiring pacemaker placement. This incidence of postoperative conduction defects is increased in children with complex defects such as heterotaxy syndrome and congenitally corrected transposition. Tertiary referral centers treat an increasing number of patients with complex congenital cardiac defects. Despite advances in understanding of the CCS in congenitally deformed hearts, these patients remain at significant risk for conduction defects requiring pacemaker placement. While the etiology of postoperative conduction defects is debated, first principles suggest that they are related to the surgical intervention. Developing technologies to determine CCS location and strategies to avoid injury to the CCS is expected to have a significant impact on surgical outcomes.
Several technologies were explored for aiding clinicians in CCS localization of the CCS and avoiding injury, but none are currently used routinely in heart surgery. We explained this lack of translation in part by limitations of the technologies. A major limitation of all presented technologies is that clinical trials comparing patient outcomes using localization technologies versus outcomes in a control group have not been performed. As for other biomedical technologies undergoing clinical translation, such clinical trials will be crucial to establish the benefits and risks of localization technology.
Novel optical technology may be a tool that can be used routinely during complex surgeries to avoid CCS damage. We envision the use of optical technologies to help surgeons estimate the CCS location and develop strategies to avoid these regions during repair. An important barrier to the adoption of new technology is the complexity of use and potential impediment to work flow during these surgical procedures. A recent clinical trial demonstrated that FCM can be integrated into the OR with minimal disruption to the work flow.45 We suggest that this localization technology will usher in an era of real-time tissue cartography, which has the potential to limit the number of patients who develop postoperative conduction defects. Also, knowledge of crucial anatomical landmarks combined with a method to determine patient specific CCS location will likely reduce residual lesions and further decrease morbidity and mortality.
Our review of technical developments for intraoperative CCS localization revealed the recent focus on optical technologies, in particular, spectroscopy and confocal microscopy. In part, this is explained by the major advances in optical technologies, which revolutionized diagnosis and treatment of diseases in many clinical disciplines. Further advances such as multiphoton and light sheet microscopy as well as optical coherence tomography are still not explored for intraoperative CCS localization. These modalities promise to resolve some of the limitations of optical spectroscopy and confocal microscopy. In particular, conventional optical spectroscopy lacks depth resolution and confocal microscopy provides only images of superficial tissues. In contrast, the novel modalities provide high spatial resolution in the depth of tissue. Other emerging modalities that could be considered for intraoperative CCS localization include hyperspectral and photoacoustic imaging. While clinical imaging modalities such as magnetic resonance tomography, computed tomography, fluoroscopy and ultrasound imaging have been established for many clinical applications, their further development will be necessary for CCS localization during cardiac surgery. Obstacles for application of these modalities for intraoperative localization are, in particular, spatial resolution and tissue contrast.
Beyond improvements in patient care, a critical motivation to introduce new technology in medicine is reduction of the economic burden of disease. The cost of a new technology to identify and avoid conduction tissue during pediatric open-heart surgery should be compared to the cost of lifetime pacemaker management. As one example, introduction of FCM technology requires the purchase of a $250,000 instrument. Procedures require a probe that costs $7500 and can be reused up to 20 times, which requires however reprocessing. With conservative estimates for other costs (reprocessing, operating room time, dye) and assuming the instrument will last 4 years and be used twice a month, it is difficult to imagine that the per-case cost will exceed $5,000. If this results in 50% fewer cases of permanent heart block, the comparison would be roughly $10,000 in increased procedure costs to eliminate one pacemaker implantation. The estimation suggests a large cost saving, because procedures related to pacemaker implantation and maintenance already lead to higher costs. However, clinical trials evaluating the impact of a technology to identify conduction tissue during pediatric open-heart surgery on patient outcomes will be required to fully assess cost savings and account for risks of the technology.
Conduction block after surgical repair of congenital defects is an unfortunate, but perhaps preventable surgical complication. The incidence of permanent block has decreased significantly from the 1950s until the early 2000s mostly due to landmark-based descriptions of the CCS. Since then there has been little change, despite further anatomical descriptions and attempts to localize conduction tissue during surgery. Optical technologies were recently introduced and are currently evaluated to localize conduction tissue. This may be the light at the end of a long tunnel - a light that will improve outcomes and quality of life for pediatric patients.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R56 HL128813 and R01 HL135077). We acknowledge support by the Nora Eccles Treadwell Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors Kaza, Sachse and Hitchcock have been granted patents and are applying for patents associated with techniques discussed in this paper.
References
- 1.Mendis S, Puska P, Norrving B, World Health Organization., World Heart Federation., World Stroke Organization. Global atlas on cardiovascular disease prevention and control. Geneva: World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; 2011. [Google Scholar]
- 2.Lillehei CW, Gott VL, Hodges PC Jr., Long DM, Bakken EE. Transitor pacemaker for treatment of complete atrioventricular dissociation. J Am Med Assoc April 30 1960;172:2006–2010. [DOI] [PubMed] [Google Scholar]
- 3.Gross GJ, Chiu CC, Hamilton RM, Kirsh JA, Stephenson EA. Natural history of postoperative heart block in congenital heart disease: implications for pacing intervention. Heart Rhythm May 2006;3:601–604. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JB, Czosek RJ, Knilans TK, Meganathan K, Heaton P. Postoperative heart block in children with common forms of congenital heart disease: results from the KID Database. J Cardiovasc Electrophysiol December 2012;23:1349–1354. [DOI] [PubMed] [Google Scholar]
- 5.Czosek RJ, Bonney WJ, Cassedy A, et al. Impact of cardiac devices on the quality of life in pediatric patients. Circ Arrhythm Electrophysiol December 2012;5:1064–1072. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson RS, Atkinson A, Kottas P, et al. High resolution 3-Dimensional imaging of the human cardiac conduction system from microanatomy to mathematical modeling. Sci Rep August 3 2017;7:7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephenson RS, Jones CB, Guerrero R, Zhao J, Anderson RH, Jarvis JC. High-Resolution Contrast-Enhanced Micro-Computed Tomography to Identify the Cardiac Conduction System in Congenitally Malformed Hearts: Valuable Insight From a Hospital Archive. JACC Cardiovasc Imaging November 2018;11:1706–1712. [DOI] [PubMed] [Google Scholar]
- 8.Garcia RU, Safa R, Karpawich PP. Postoperative complete heart block among congenital heart disease patients: Contributing risk factors, therapies and long-term sequelae in the current era. Progress in Pediatric Cardiology 2018;49:66–70. [Google Scholar]
- 9.Silverman ME, Grove D, Upshaw CB Jr. Why does the heart beat? The discovery of the electrical system of the heart. Circulation June 13 2006;113:2775–2781. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RH, Ho SY, Becker AE. The surgical anatomy of the conduction tissues. Thorax June 1983;38:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RH, Yanni J, Boyett MR, Chandler NJ, Dobrzynski H. The anatomy of the cardiac conduction system. Clin Anat January 2009;22:99–113. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson AJ, Logantha SJ, Hao G, et al. Functional, anatomical, and molecular investigation of the cardiac conduction system and arrhythmogenic atrioventricular ring tissue in the rat heart. J Am Heart Assoc December 19 2013;2:e000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler N, Aslanidi O, Buckley D, et al. Computer three-dimensional anatomical reconstruction of the human sinus node and a novel paranodal area. Anat Rec (Hoboken) June 2011;294:970–979. [DOI] [PubMed] [Google Scholar]
- 14.Dobrzynski H, Anderson RH, Atkinson A, et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol Ther August 2013;139:260–288. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima T, Sasaki H. Gross anatomy of the human cardiac conduction system with comparative morphological and developmental implications for human application. Ann Anat February 20 2011;193:1–12. [DOI] [PubMed] [Google Scholar]
- 16.Lev M The normal anatomy of the conduction system in man and its pathology in atrioventricular block. Annals of the New York Academy of Sciences 1964;111:817–829. [DOI] [PubMed] [Google Scholar]
- 17.Merideth J, Titus JL. The anatomic atrial connections between sinus and A-V node. Circulation. Vol 371968:566–579. [DOI] [PubMed] [Google Scholar]
- 18.Csepe TA, Zhao J, Hansen BJ, et al. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog Biophys Mol Biol January 2016;120:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nooma K, Saga T, Iwanaga J, et al. A novel method with which to visualize the human sinuatrial node: Application for a better understanding of the gross anatomy of this part of the conduction system. Clin Anat March 2020;33:232–236. [DOI] [PubMed] [Google Scholar]
- 20.Widran J, Lev M. The dissection of the atrioventricular node, bundle and bundle branches in the human heart. Circulation December 1951;4:863–867. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RH, Spicer DE, Hlavacek AM, Cook AC, Backer CL. Wilcox’s surgical anatomy of the heart: Cambridge University Press; 2013. [Google Scholar]
- 22.Baruteau AE, Abrams DJ, Ho SY, Thambo JB, McLeod CJ, Shah MJ. Cardiac Conduction System in Congenitally Corrected Transposition of the Great Arteries and Its Clinical Relevance. J Am Heart Assoc December 21 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiello VD. Understanding the morphology of the specialized conduction tissues in congenitally malformed hearts. World J Pediatr Congenit Heart Surg April 2015;6:239–249. [DOI] [PubMed] [Google Scholar]
- 24.Emkanjoo Z, Mirza-Ali M, Alizadeh A, et al. Predictors and frequency of conduction disturbances after open-heart surgery. Indian pacing and electrophysiology journal 2008;8:14. [PMC free article] [PubMed] [Google Scholar]
- 25.Titus JL, Daugherty GW, Kirklin JW, Edwards JE. Lesions of the atrioventricular conduction system after repair of ventricular septal defect. Relation to heart block. Circulation July 1963;28:82–88. [DOI] [PubMed] [Google Scholar]
- 26.Nathan M, Karamichalis JM, Liu H, et al. Surgical technical performance scores are predictors of late mortality and unplanned reinterventions in infants after cardiac surgery. J Thorac Cardiovasc Surg November 2012;144:1095–1101 e1097. [DOI] [PubMed] [Google Scholar]
- 27.Bernhard WF, Grass AM. A method for localization of the cardiac conduction system during open-heart surgery. N Engl J Med November 30 1961;265:1079–1083. [DOI] [PubMed] [Google Scholar]
- 28.Bormes WA, Gorman WC, Kayser KL, Lepley D. The identification of the conduction system of the heart by audiofrequency conductimetry. Surgery 1961;50:50–57. [Google Scholar]
- 29.Krongrad E, Malm JR, Bowman FO Jr., Hoffman BF, Waldo AL. Electrophysiological delineation of the specialized A-V conduction system in patients with congenital heart disease. II. Delineation of the distal His bundle and the right bundle branch. Circulation June 1974;49:1232–1238. [DOI] [PubMed] [Google Scholar]
- 30.Stuckey JH, Hoffman BF, Amer NS, Cranfield PF, Cappelletti RR, Domingo RT. Localization of the bundle of His with a surface electrode during cardiotomy. Surg Forum 1960;10:551–554. [PubMed] [Google Scholar]
- 31.Zarnstorff WC, Gott VL, Dutton RC, Rowe GG. A Cardiac Conduction System Localizer. The Journal of Thoracic and Cardiovascular Surgery 1964;47:122–128. [PubMed] [Google Scholar]
- 32.Dick M 2nd, Van Praagh R, Rudd M, Folkerth T, Castaneda AR. Electrophysiologic delineation of the specialized atrioventricular conduction system in two patients with corrected transposition of the great arteries in situs inversus (I,D,D). Circulation Jun 1977;55:896–900. [DOI] [PubMed] [Google Scholar]
- 33.Wolff GS, Alley RD. Intraoperative identification of the conduction system in repair of endocardial cushion defect. Ann Thorac Surg January 1977;23:39–44. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser GA, Waldo AL, Beach PM, Bowman FO Jr., Hoffman BF, Malm JR. Specialized cardiac conduction system. Improved electrophysiologic identification technique at surgery. Arch Surg Dec 1970;101:673–676. [DOI] [PubMed] [Google Scholar]
- 35.Siegel L, Mahoney EB, Manning JA, Stewart S. An audible alarm system to facilitate the intraoperative identification of cardiac conduction tissue. The Journal of Thoracic and Cardiovascular Surgery 1974;68:241–247. [PubMed] [Google Scholar]
- 36.Lincoln C, Butler P, Logan-Sinclair R, Anderson RH. A cardiac conduction monitor and probe for intraoperative identification of conduction tissue. Br Heart J September 1979;42:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aziz D, Caruso A, Aguirre M, Gmitro AF. Fluorescence response of selected tissues in the canine heart: an attempt to find the conduction system. Diagnostic and Therapeutic Cardiovascular Interventions II 1992:166–175. [Google Scholar]
- 38.Bagdonas S, Zurauskas E, Streckyte G, Rotomskis R. Spectroscopic studies of the human heart conduction system ex vivo: implication for optical visualization. J Photochem Photobiol B August 21 2008;92:128–134. [DOI] [PubMed] [Google Scholar]
- 39.Perk M, Flynn GJ, Gulamhusein S, et al. Laser induced fluorescence identification of sinoatrial and atrioventricular nodal conduction tissue. Pacing Clin Electrophysiol August 1993;16:1701–1712. [DOI] [PubMed] [Google Scholar]
- 40.Venius J, Bagdonas S, Zurauskas E, Rotomskis R. Visualization of human heart conduction system by means of fluorescence spectroscopy. J Biomed Opt October 2011;16:107001. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Kaza AK, Hitchcock RW, Sachse FB. Identification of nodal tissue in the living heart using rapid scanning fiber-optics confocal microscopy and extracellular fluorophores. Circ Cardiovasc Imaging September 2013;6:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Sachse FB, Hitchcock RW, Kaza AK. Sensitivity and Specificity of Cardiac Tissue Discrimination Using Fiber-Optics Confocal Microscopy. PLoS One 2016;11:e0147667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Mansour MR, Caycedo-Marulanda A, Davis BR, et al. SAGES TAVAC safety and efficacy analysis confocal laser endomicroscopy. Surg Endosc May 13 2020. [DOI] [PubMed] [Google Scholar]
- 44.Mondal A, Lackey J, Saeed M, et al. An Imaging Protocol to Discriminate Specialized Conduction Tissue During Congenital Heart Surgery. Semin Thorac Cardiovasc Surg Autumn 2019;31:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaza AK, Mondal A, Piekarski B, Sachse FB, Hitchcock RW. Intraoperative localization of cardiac conduction tissue regions using real-time fiberoptic confocal microscopy: First in human trial. Eur J Cardiothorac Surg 20201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal HS, Wolfram KB, Saville BR, Donahue BS, Bichell DP. Postoperative complications and association with outcomes in pediatric cardiac surgery. J Thorac Cardiovasc Surg August 2014;148:609–616 e601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


