Abstract
Background
treatment of breast cancer as one of the most common cancers in the world remains an important area of drug development based on nanoparticulate systems. Effective targeted therapy of affected cells based on ligand conjugate biocompatible polymeric nanoparticles is an attractive perspective in this context.
Objective
In this study, a novel double effect nanoparticle based on Chitosan-Raloxifene conjugate was prepared for adjuvant therapy (hormone and chemo therapy) and drug targeting to breast cancer cells via estrogen receptor (ER).
Methods
Chitosan-raloxifene conjugate was synthesized. Related nanoparticles containing doxorubicin (DOX) were prepared and characterized. Experimental design study was performed to determine the optimum levels of variables in the preparation of nanoparticle. Drug loading, release, nanoparticle stability, and the effect of nanoparticles on cell viability were evaluated. Further, inhibition tests were performed to demonstrate that the function of these novel nanoparticles is mediated via ER.
Results
Chitosan-raloxifene conjugate was successfully synthesized. The prepared nanoparticles showed sizes within 25–35 nm, more than 95% drug loading, about 60% of drug release and desired stability after 24 h. XTT assay on MCF-7 cell line illustrated that these nanoparticles could inhibit the cellular growth up to 60%. The results from inhibition tests revealed that prepared nanoparticles can inhibit cell growth via ER blocking.
Conclusion
This study introduced chitosan-raloxifene nanoparticles containing doxorubicin as a novel targeting agent for adjuvant therapy of breast cancer.
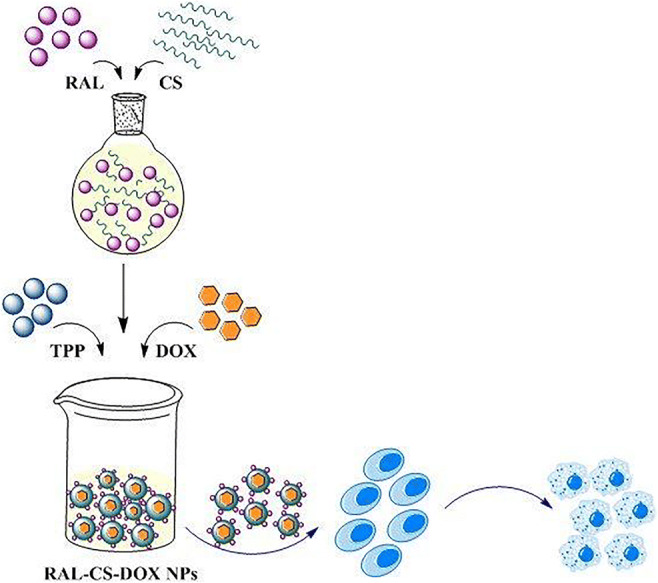
Graphical abstract
Electronic supplementary material
The online version of this article (10.1007/s40199-020-00338-9) contains supplementary material, which is available to authorized users.
Keywords: Chitosan, Raloxifene, Nanoparticles, Targeting vehicle, Breast cancer cells
Introduction
High toxicity of chemotherapeutic drugs in response to their extensive distribution in both healthy and cancerous cells [1, 2] causes severe adverse effects. To overcome this problem, attempts have been made to enhance the efficacy of drugs and reduce their side effects through targeting delivery via nanoparticles (NPs) [3]. Biopolymeric NPs have a high potential to be used as drug carriers. Among them, unique characteristics of chitosan (CS) such as its cationic nature [4] which is based on its primary amino groups, make its NPs one of the best candidates for usage as targeting drug carriers [5, 6]. Furthermore, high affinity of CS for binding to negatively charged biological membranes and some tumor cells contributes to site-specific targeting [7].
Specific targeting ligands such as folic acid [8], galactose [9] and FAP-B [10] have been introduced to CS NPs in order to synthesize an acceptable tumor specific drug carrier which can target overexpressed tumor receptors or new specific ones [6].
The first class of targeted therapy employed to treat breast cancer is hormone therapy [11, 12]. Since nearly 70% of breast cancers express the ER [11, 12], blocking its function can reduce the risk of breast cancer progression. Selective estrogen receptor modulators (SERMs) are synthetic compounds that have agonist/antagonist activity depending on the affected tissue [13, 14]. Raloxifene (RAL) is a second-generation drug of SERMs which has an agonist function on skeleton and cardiovascular system and antagonist effect on uterus and breast [15–17]. Estrogen-dependent proliferation of MCF-7 (human mammary tumor cells) can be inhibited by RAL in vitro. Further, the growth of carcinogen-induced mammary tumors in rats has been blocked via RAL [15].
Thus, by taking advantage of both CS NPs and RAL, a unique delivery system can be developed for targeted breast cancer therapy.
In this study, RAL-CS conjugate NPs (RC NPs) containing DOX were prepared and characterized to be used as a new double effect anticancer system for improving drug targeting to breast cancer cells.
Materials and methods
Biological and chemical reagents
Trimethyl chitosan (TMC) (Low Molecular Weight, deacetylation degree >90%) was kindly provided by pharmaceutical research laboratory of Tehran University of medical science. Further, 3-Glycidyloxypropyltrimethoxysilane (3-GPTMS), doxorubicin, Triethylamine (Et3N), Tripolyphosphate (TPP), sodium 3-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate powder (XTT), Phenazine methosulfate (PMS), dimethyl sulfoxide (DMSO), and Penicillin-Streptomycin were purchased from Sigma Aldrich, (USA). Chemical solvents were obtained from Merck, (Germany). Dulbecco’s modified eagle medium (DMEM), Fetal Bovine Serum (FBS), and Trypsin-EDTA were obtained from Gibco (Germany). Estradiol valerate was purchased from Iran hormone company (Iran) and Raloxifene HCL was also kindly provided by this company. All chemical and biological materials were of pharmaceutical grade.
Synthesis and characterization of RAL-CS conjugate (RC conjugate)
Synthesis of RC conjugate was performed as previously described by our group. Briefly, a mixture of RAL (50 mg) and 3-GPTMS as a linker (100 μl) was mixed together and stirred under an argon atmosphere blanket at 60 °C for 30 min. Then, DMF (1 ml) and Et3N (20 μl) were added to the resulting mixture respectively and stirred for 24 h. The yellowish precipitated product (linker-RAL conjugate) was washed with copious amounts of methanol to eliminate the excess of epoxy linker. TMC hydrogel solution (7 ml, 1% (w/v)) was added to the drug-linker mixture and stirred at room temperature for 24 h. Then, organic and aqueous phases were separated by chloroform with the study continued using the aqueous phase. To remove all residual reactants and unreacted linker/RAL, the final mixture was dialyzed against sodium acetate buffer (pH: 4.8–5) for 72 h. The final product was dried at 110 °C and subjected to Fourier transform infrared spectroscopy (FTIR) (Magna-IR 550 Nicolet FTIR spectrometer) and Thermogravimetric analysis (TGA-50,Shimadzu) evaluations as described elsewhere [6].
Preparation of RC NPs
A solution of TPP was prepared in distilled water (1 mg/ml) and RC solution (0.44 mg/ml) in a mixture of solvents (TFA/DMSO ratio 27:1). CS NPs were formed through dropwise addition of RC solution to TPP solution under magnetic stirring (20 min). Various formulations at different TPP to RC weight ratios were prepared (Table 1). After adjusting the pH (=5) using NaOH 1 N, the particle size and zeta potential of NPs were measured.
Table 1.
TPP/RC weight ratios
| Sample code | TPP/RC |
|---|---|
| A | 2 |
| B | 4 |
| C | 8 |
| D | 16 |
| E | 32 |
Preparation of DOX loaded RCNPs (DRC NPs)
The best TPP/RC weight ratios (based on size and zeta potential results) were used to prepare DRC NPs. Briefly, a solution of DOX in water (0.875 mg/ml) was added to RC at a final concentration of 5% (w/w) in relation to RC.
Experimental design study
The optimum levels of variables in NPs preparation were determined via Box-Behnken design which is the most accepted response surface methodology (RSM). RSM allows estimation of the parameters of a quadratic model which requires fewer runs in case of three variables [18].
Box-Behnken design was employed to study all principal effective factors (independent variables) and to assess which factor affects the responses (dependent variables). In this design, 3 factors were examined in 15 trials at high and low levels. Design-Expert (version 7.0.0; Stat-Ease, Inc., Minneapolis, Minnesota, USA) was employed for mathematical modeling and assessment of the responses.
The pH of the solution (X1), DOX/RC weight ratio (X2), and TPP/RC weight ratio (X3) were analyzed at two levels. Y1 and Y2 were studied as responses including particle size and zeta potential respectively. In this design, 12 factorial points with 3 replicates were studied at the central point for calculation of pure error sum of squares.
The mathematical relationship between the response (Y1) and independent variables (X1, X2, X3) is presented in Eq. 1:
| 1 |
According to the responses generated from mathematical formula, two formulations called a1 and a2 (Table 2) were selected to continue the study.
Table 2.
Selected formulations according to experimental design responses
| Formulation code | pH | TPP/RC weight ratio | DOX/RC weight ratio |
|---|---|---|---|
| a1 | 5.69 | 23.34 | 7.35 |
| a2 | 4.62 | 30.36 | 12.07 |
NPs were prepared by these formulations and used for cytotoxicity assay as well as inhibition tests.
Particle size analysis and zeta potential measurement
Size distribution and zeta potential of the NPs were measured using a Zetasizer (Nano ZSP, Malvern Instruments, UK), with a red laser of wavelength λ0 = 633 nm (He-Ne, 4.0 Mw). The analysis was performed in triplicate at a temperature of 25 °C.
Transmission electron microscopy (TEM)
The surface morphological analysis of DRC NPs was carried out via transmission electron microscopy (TEM, Zeiss EM10C, Germany operating at 100 kv). The suspension of NPs was dropped onto formvar carbon coated grid Cu Mesh 300 and dried at room temperature.
DOX loading efficiency
The loading efficiency was calculated according to Eq. (2), where the total DOX refers to the concentration of DOX used in the preparation of nanoparticles while free DOX denotes the concentration of the supernatant after ultracentrifugation of loaded nanoparticles at 14000×g for 10 min using centrifugal filter units (30 K, Amicon®, EMD Millipore Co., 152 MA, USA).
| 2 |
Release study
One milliliter of nanoparticle solution was sealed in a dialysis tube (molecular weight cutoff of 12,000–14,000 g/mol) and immersed in 50 ml PBS (pH 7.4) at 37°C for 24 h with a continuous stirring. At predetermined times, an aliquot (1 ml each) was withdrawn and the same volume of fresh medium was added. The concentration of DOX was measured based on the absorbance of DOX at 480 nm using UV- spectrophotometer (2100 Unico, USA).
Stability study
For assessing the stability of DRC NPs, they were diluted in 10 mM PBS (pH 7.4) at 50 mg/ml and placed in an incubator maintained at 37 °C for 72 h. At predetermined times (0, 3, 6, 12, 24, 48, and 72 h), samples were withdrawn and the particle size and zeta potential of the samples determined as described above.
Cell culture
Human breast cancer cell line (MCF-7) was obtained from National Cell Bank of Iran (the Pasteur Institute of Iran, Tehran). The cells were cultured in DMEM media supplemented with 10% (v/v) FBS and 1% Penicillin-Streptomycin at 37 °C in an incubator with 5% CO2.
This was done according to the procedures approved by the Ethics committee of Avicenna Research Institute (ethics code: 92/933).
Assessment of cytotoxicity
One of the most common methods to evaluating the cell viability, cell proliferation and/or cytotoxicity is XTT assay. This method is based on color changes upon the reduction of slightly yellow XTT powder (tetrazolium salt) to its orange formazan derivative. In this study, to evaluate the cytotoxicity of NPs, XTT assay was utilized. In brief, MCF-7 cells were seeded in a flat 96-well culture plate (104 cells per well) and kept in an incubator (37 °C, 5% CO2) to attach to plate. After 24 h, the culture medium was discarded and fresh DMEM (phenol red free) was replaced. According to Table 3, different concentrations of NPs were added to each well in triplicates and then incubated for 24 h. To compare the effect of NPs, culture medium and solvents were considered as controls.
Table 3.
Sample codes and their descriptions for cytotoxicity and inhibition (two-step method) tests
| Sample codes | Description |
|---|---|
| a1 | DRC NPs, TPP/RC: 23.34 |
| a2 | DRC NPs, TPP/RC: 30.36 |
| b1 | a1 without DOX |
| b2 | a2 without DOX |
| c1 | TMC NPs, TPP/TMC: 23.34 |
| c2 | TMC NPs, TPP/TMC: 30.36 |
| d1 | RC conjugate at concentration which used in a1 |
| d2 | RC conjugate at concentration which used in a2 |
| e1 | TMC at concentration which used in a1 |
| e2 | TMC at concentration which used in a2 |
| f1 | DOX at concentration which used in a1 |
| f2 | DOX at concentration which used in a2 |
| g1 | RAL at concentration which used in a1 |
| g2 | RAL at concentration which used in a2 |
XTT and PMS solutions were prepared by dissolving XTT powder in a pre-warmed medium (1 mg/ml) and the PMS in phosphate buffered saline (1.53 mg/ml) respectively. Effective reduction of XTT occurs in the presence of an electron coupling agent such as PMS (phenazine methosulfate).
Here, 5 μl PMS solution was added to 1 ml XTT solution and then 50 μl of this mixture was added to each well and incubated for 2–4 h at 37 °C, 5% CO2. Finally, the absorbance of samples was measured at a wavelength of 450 nm, using a photometer (ELISA reader, BioTek).
Inhibition tests
Inhibition tests on MCF-7 cells were done by two methods as below:
One-step method
The MCF-7 cells were seeded in a 96-well plate at a density of 104 cells/well in DMEM supplemented with 10% FBS and 1% Penicillin-Streptomycin and kept in incubator (37 °C, 5% CO2) to attach. After 24 h, the cells were treated by samples (Table 4) and incubated under the previously mentioned condition for another 24 h. The culture medium and mixture of media and solvent were considered as controls. Then, 50 μl of mixed XTT solution (XTT + PMS) was added to each well with the test continuing as described in part 2.12.
Table 4.
Sample codes and their descriptions for inhibition test (one-step method)
| Sample codes | Description |
|---|---|
| a1 | DRC NPs, TPP/RC: 23.34 |
| a2 | DRC NPs, TPP/RC: 30.36 |
| b1 | a1 without DOX |
| b2 | a2 without DOX |
| c1 | TMC NPs, TPP/TMC: 23.34 |
| c2 | TMC NPs, TPP/TMC: 30.36 |
| m1 | a1 + (estradiol in ethanol, 10−8 M) |
| m2 | a2 + (estradiol in ethanol, 10−8 M) |
| n1 | b1 + (estradiol in ethanol, 10−8 M) |
| n2 | b2 + (estradiol in ethanol, 10−8 M) |
| p1 | c1 + (estradiol in ethanol, 10−8 M) |
| p2 | c2 + (estradiol in ethanol, 10−8 M) |
Two-step method
The cells were seeded in a 96-well plate (104 cells/well). Then, estradiol (10−8 M) was added to each well. The plates were incubated for 24 h at 37 °C in an incubator with 5% CO2. Then, the cells were treated by 50 μl of samples (Table 3) for 24 h. Finally, the XTT assay was performed as described previously.
Statistical analysis
The tests were repeated three times with the data being reported as mean ± SD. Statistical analysis was performed using Student’s t test where the level of statistical significance was considered P < 0.05.
Results
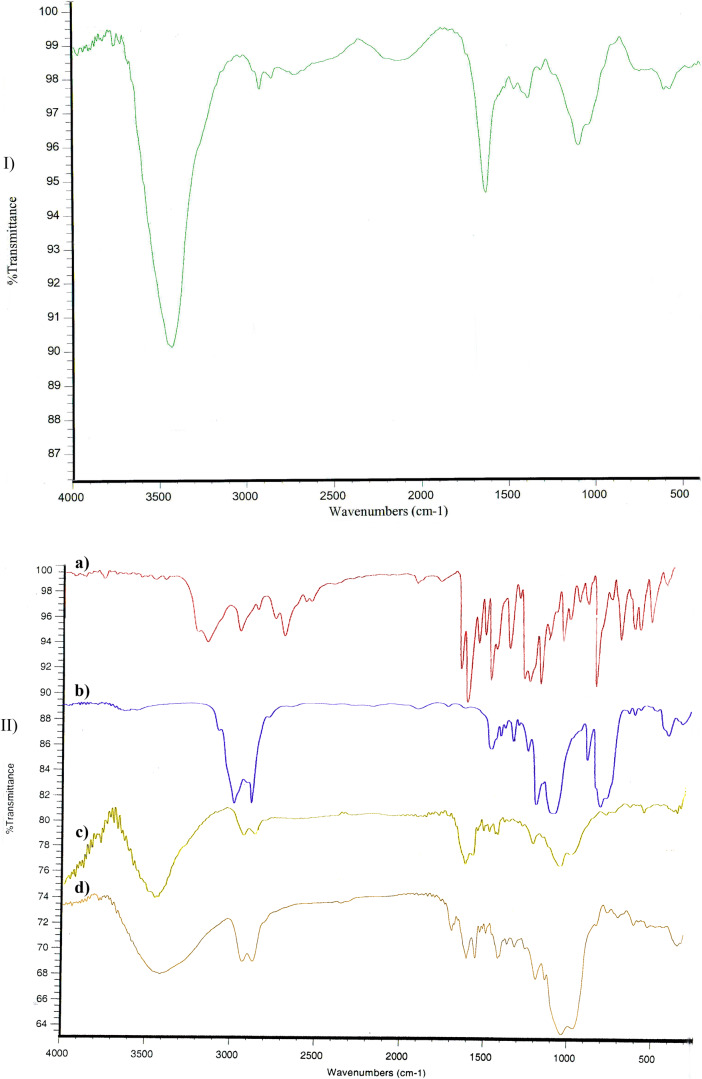
FTIR studies
Figure 1 represents the IR spectra of TMC, RAL, linker, linker-RAL, and RC conjugate. According to Fig. 1-IId, IR spectrum of RC conjugate presented the expected characteristic bands of RAL (3143 and 3205 cm−1) and TMC (1634, and 3431 cm−1).
Fig. 1.
IR Spectra of I) TMC, II) a) RAL, b) linker, c) linker-RAL, d) RC conjugate
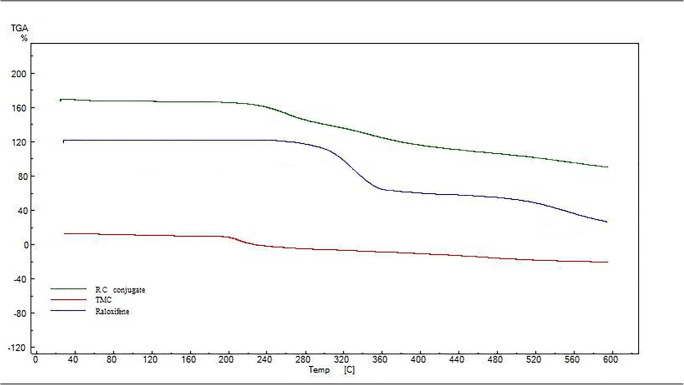
TGA studies
Figure 2 displays the studies on the thermal behavior of the TMC, RAL, and RC conjugate by TGA. The resulting thermograms are according to our previous published data [6]. The thermogram of RC conjugate showed that there is only one-step degradation from 231.05 °C to 325.77 °C with 61.15% weight loss. The start point of this decomposition is approximately between the main decomposition start points of RAL (298 °C) and TMC (201 °C). This pattern is observed in case of decomposition end points (RAL: 351.68 °C, TMC: 274 °C and RC conjugate: 325.77 °C).
Fig. 2.
Thermogram of TMC, RAL and RC conjugate
Preparation and physical evaluations of NPs
Among CS NPs preparation methods, ionic gelation technique is the most attractive method because of non-toxicity due to lack of organic solvents, simplicity, and controllability [19, 20]. The formation of CS NPs is based on electrostatic interaction between the amine group of CS and negatively charged polyanion such as TPP [19–21].
The zeta potential and size distribution of two selected formulations were measured using Nano ZSP. The results indicated that the sizes of two selected formulations (according to experimental design studies), a1 and a2, were 34.75 ± 2.1 nm and 26.85 ± 1.89 nm respectively (PDI = 0.3 ± 0.05). In addition, their zeta potentials were 0.17 ± 0.02 mv (a1) and − 0.49 ± 0.04 mv (a2) (width = 4.5 ± 1).
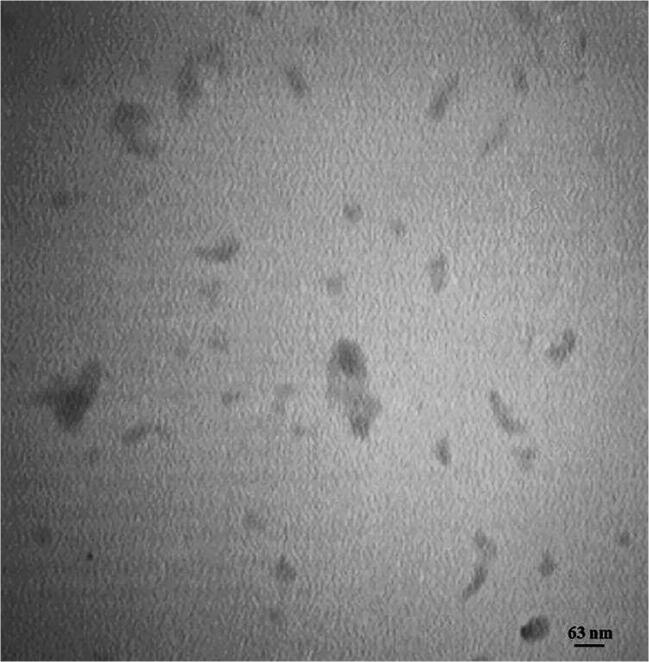
The morphological observation of NPs using TEM revealed spherical/cylindrical NPs with homogeneous dispersity (Fig. 3).
Fig. 3.
TEM photomicrograph of DRC NPs
Loading, release, and stability studies
The loading efficiency of the selected nanoparticles was calculated according to Eq. 2. Dox loading in formulations a1 and a2 was 98% ± 2% and 95% ± 4% respectively.
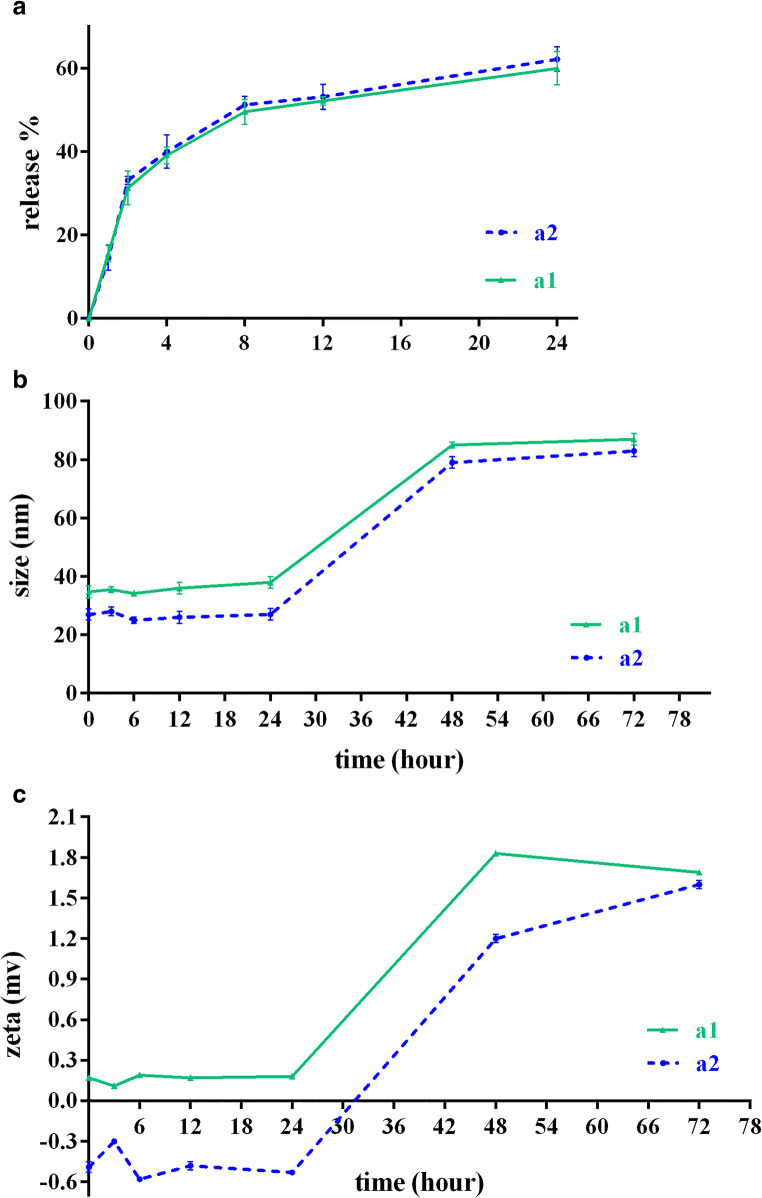
The in-vitro cumulative release profiles of DRC nanoparticles in both a1 and a2 formulations displayed a similar release pattern, indicating that almost 60% of Dox was released after 24 h (Fig. 4a).
Fig. 4.
a In vitro release profiles of doxorubicin from RC NPs; b and c Stability profiles of DOX loaded RC NPs in PBS (pH 7.4) at 37 °C. Values are shown as mean ± SD; n = 3
On the other hand, Dox was rapidly released from nanoparticles; nearly 30% of the incorporated Dox was released within 2 h.
The physical stabilities of the nanoparticles suspended in PBS (pH 7.4) were evaluated over 3 days at 37 °C. As shown in Fig. 4b, c, the particle sizes and zeta potentials of both formulations were maintained well for a day. However, after 24 h, the size of nanoparticles increased gradually, which can be due to particle aggregation. Furthermore, the zeta potentials of both formulations were changed after 24 h.
Cytotoxicity studies
The results obtained from cytotoxicity assay (Fig. 5a) indicated that cytotoxicity of RC NPs at a weight ratio of RC/TPP: 30.36 (b2) was about 60% which is significantly more than that of TMC NPs at the same ratio (c2). On the other hand, the intact polymeric RC (d1, d2) showed no toxicity on cells.
Fig. 5.
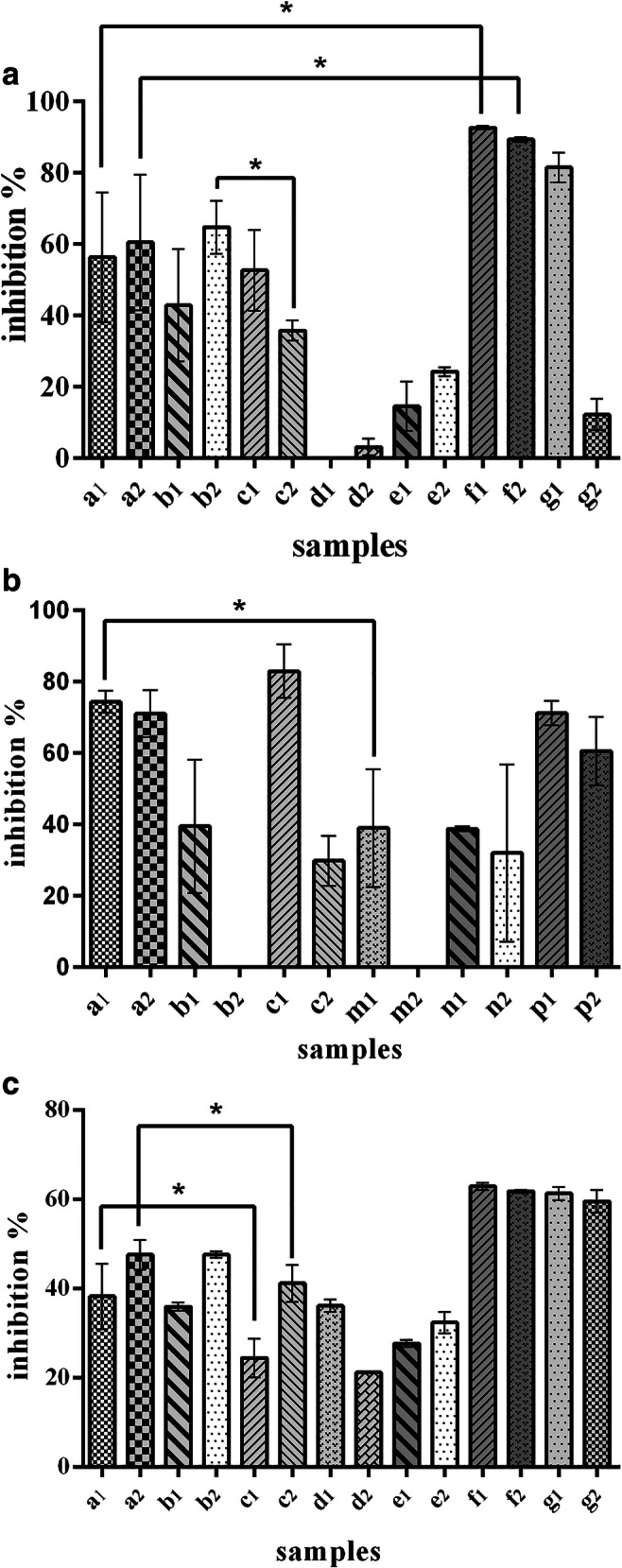
a Effect of DOX, RAL, RC conjugate, RC NPs and DRC NPs at different concentrations on inhibition of MCF-7 cells growth (sample definition is according to Table 3). Values are shown as mean ± SD; n = 3. Statistical analysis was performed using Student’s t test, P < 0.05 b Cytotoxicity effects of various samples on MCF-7 cells during one-step method of inhibition test (sample definition is according to Table 4). Values are shown as mean ± SD; n = 3. Statistical analysis was performed using Student’s t test, P < 0.05. c cytotoxicity effects of various samples on MCF-7 cells during two-step method of inhibition test (sample definition is according to Table 3). Values are shown as mean ± SD; n = 3. Statistical analysis was performed using Student’s t test, P < 0.05
In addition, DRC NPs (a1, a2) led to a significantly lower cell death rate compared to free DOX (f1, f2)(about 60% vs 80%).
Inhibition tests
One-step method
In this study, NPs were designed to target ER. Thus, this receptor at the surface of MCF-7 cells was blocked by estradiol as the main ligand.
Accordingly, estradiol and prepared NPs were added to culture medium simultaneously (according to Table 4 to evaluate their effects on cell death). The results indicated that with estradiol and NPs added to media (m1), cell growth inhibition decreased significantly compared to absence of estradiol (a1).
Particularly, a greater reduction in cell growth inhibition was observed when the TPP/RC weight ratio increased (m2 compared to m1). On the other hand, in case of TMC NPs, in the presence of estradiol at both weight ratios (p1 and p2), no significant reduction in inhibition effect was observed when compared to absence of estradiol (c1 and c2) (Fig. 5b).
Two-step method
To confirm the results obtained from one-step method of inhibition test, the second method was carried out. In this method, estradiol as a cell growth inducer for MCF-7 cells (due to the existence of ER on these cells surfaces) was initially added to the culture medium (24 h prior to treatment). The obtained results (Fig. 5c) indicated that DRC NPs (a1, a2) as compared to TMC NPs (c1, c2), had significantly greater inhibitory effects on cell growth.
Discussion
The ability of chitosan to conjugate with specific targeting agents makes its nanoparticles very effective vehicles for delivery of chemotherapeutic drugs to tumor cells. As most breast cancers express ER, molecules with an antagonist effect on this receptor such as RAL can reduce the risk of breast cancer by blocking the ER function. Thus, in this study, chitosan-raloxifene nanoparticles containing doxorubicin were prepared as a new double effect vehicle for drug targeting to breast cancer cells.
After successful conjugation of RAL to TMC, their nanoparticles containing DOX were prepared. The pH of the solution, DOX/RC weight ratio, and TPP/RC weight ratio were the most important independent variables affecting the size and zeta potential of nanoparticles.
The best TPP/RC ratios in the nanoparticle formation selected by experimental design study (a1:23.34 and a2:30.36) showed desirable physical, morphological, and biological characteristics.
The selected nanoparticles (formulations a1 and a2) showed small sizes and small values of zeta potentials. These near-zero zeta potentials could lead to particle aggregation and thus formulation instability, which was observed after 24 h of stability study.
Other researchers have also shown that different factors can affect the size and zeta potential of chitosan nanoparticles. Using experimental design study, Abdel-Hafez et al. described that the optimum levels of pH, stirring rate, acetic acid concentration, and CS:TPP ratio can lead to small and mono-dispersed chitosan nanoparticles [18]. Some researchers found that the ratio of chitosan to other polymeric/small molecules, which contributes to nanoparticle formation, is very critical in producing the best nanoparticles [19–21].
The cytotoxicity of selected nanoparticles was assessed via XTT assay on MCF-7 cells (Fig. 5a). It was found that the presence of RAL in NPs structure (b2) could considerably increase the cytotoxicity in comparison with TMC NPs (c2). Cytotoxicity results showed that polymeric RC cannot mediate cell toxicity; however, its nanoparticles can lead to cell death clearly. This result can be due to an increase in the effective area of NPs compared to intact RC, which can consequently increase the probability of cell contact and cell penetration.
As indicated, the cytotoxicity of free DOX was greater than that of DRC NPs. Strong and complete trapping of DOX inside a1 and a2 nanoparticles (more than 95% loading efficiency) and lack of complete release over the 24-h period (about 60%) may lead to this observation. Further, DOX and RAL can develop hydrogen bonding via OH/NH2 groups and static π-π bonding moieties through aromatic rings in their molecular structure, which may lead to unavailability of bioactive parts of DOX thus lowering its biological effects. Interestingly, Janes et al. obtained similar results about chitosan-DOX nanoparticles and free DOX. When they found that free DOX has greater cytotoxicity than DOX loaded chitosan nanoparticles, they concluded that this may be attributed to two reasons: drug-chitosan tight interaction and/or partial damage to the molecular structure of DOX during its complexation with chitosan [21].
Inhibition experiments were performed using two methods. In the first method, one step, the target receptor at the cell surface was blocked using the free ligand and then the cells were treated by the prepared nanoparticulate delivery system. It was expected that NPs would not be able to perform their functions after blocking the receptors as strongly as before.
The obtained results indicated that the main effect of RC NPs containing DOX on breast cancer cells is exerted through the estrogen receptor. This conclusion is due to ER’s occupation by estradiol causing inability of RC NPs to play its role. However, under the same conditions, the cytotoxicity effect of TMC nanoparticles was not reduced, which confirms this mechanism.
In the second method of inhibition experiment, two step, when cells were growing in the presence of estrogen (24 h prior to the experiment), adding RAL (as an estrogen receptor blocker present in a1 and a2 NPs) to the medium led to a competition between these molecules; consequently, cell death increased via blocking some ER receptors by RAL during this competition.
Thus, it seems that the prepared nanoparticles could block estrogen receptors at the surface of the cancerous cells, and after releasing doxorubicin, they could lead to targeted toxicity.
Conclusions
Overall, it was observed that this novel delivery system can be used both as a targeted delivery system for ER+ breast cancer cells (via estrogen receptor) and as a double effect system for both chemo and hormone therapy. The results obtained from this study confirmed that RC NPs containing DOX can be applied in in-vivo studies as a potential cancer therapy system in future.
Electronic supplementary material
(DOCX 16 kb)
Acknowledgements
The authors would like to acknowledge Iran National Science Foundation (INSF) for financial support (grant number 91004795), and Mrs. Saadat for her valuable assistance.
Compliance with ethical standards
Conflict of interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zohreh Mohammadi and Fatemeh Yazdi Samadi contributed equally to this work.
References
- 1.Chidambaram M, Manavalan R, Kathiresan K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J Pharm Pharm Sci. 2011;14(1):67–77. doi: 10.18433/J30C7D. [DOI] [PubMed] [Google Scholar]
- 2.Yousefpour P, Atyabi F, Vasheghani-Farahani E, Movahedi A-AM, Dinarvand R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int J Nanomedicine. 2011;6:1977. doi: 10.2147/IJN.S21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Islam MS, Haque P, Rashid TU, Khan MN, Mallik AK, Khan MNI, Khan M, Rahman MM. Core–shell drug carrier from folate conjugated chitosan obtained from prawn shell for targeted doxorubicin delivery. J Mater Sci Mater Med. 2017;28(4):55. doi: 10.1007/s10856-017-5859-x. [DOI] [PubMed] [Google Scholar]
- 4.Naruphontjirakul P, Viravaidya-Pasuwat K, Editors. Development of doxorubicin—Core Shell chitosan nanoparticles to treat Cancer. Proceedings of the international conference on biomedical engineering and technology; IACSIT Press: Singapore; 2011.
- 5.Bernkop-Schnürch A, Dünnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81(3):463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Samadi FY, Mohammadi Z, Yousefi M, Majdejabbari S. Synthesis of raloxifene–chitosan conjugate: a novel chitosan derivative as a potential targeting vehicle. Int J Biol Macromol. 2016;82:599–606. doi: 10.1016/j.ijbiomac.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 7.Qi L-F, Xu Z-R, Li Y, Jiang X, Han X-Y. In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J Gastroenterol: WJG. 2005;11(33):5136–5141. doi: 10.3748/wjg.v11.i33.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jing H, Guo Z, Guo W, Yang W, Xu P, Zhang X. Synthesis and characterization of folic acid modified water-soluble chitosan derivatives for folate-receptor-mediated targeting. Bioorg Med Chem Lett. 2012;22(10):3418–3424. doi: 10.1016/j.bmcl.2012.03.102. [DOI] [PubMed] [Google Scholar]
- 9.Mourya V, Inamdar NN. Chitosan-modifications and applications: opportunities galore. React Funct Polym. 2008;68(6):1013–1051. doi: 10.1016/j.reactfunctpolym.2008.03.002. [DOI] [Google Scholar]
- 10.Mohammadi Z, Abolhassani M, Dorkoosh F, Hosseinkhani S, Gilani K, Amini T, et al. Preparation and evaluation of chitosan–DNA–FAP-B nanoparticles as a novel non-viral vector for gene delivery to the lung epithelial cells. Int J Pharm. 2011;409(1):307–313. doi: 10.1016/j.ijpharm.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Gil EMC. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40(7):862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Pritchard K. Endocrine therapy: is the first generation of targeted drugs the last? J Intern Med. 2013;274(2):144–152. doi: 10.1111/joim.12065. [DOI] [PubMed] [Google Scholar]
- 13.Wood AJ, Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- 14.Fan P, Jordan VC. Acquired resistance to selective estrogen receptor modulators (SERMs) in clinical practice (tamoxifen & raloxifene) by selection pressure in breast cancer cell populations. Steroids. 2014;90:44–52. doi: 10.1016/j.steroids.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L. A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update. 2000;6(3):212–224. doi: 10.1093/humupd/6.3.212. [DOI] [PubMed] [Google Scholar]
- 16.Muchmore DB. Raloxifene: a selective estrogen receptor modulator (SERM) with multiple target system effects. Oncologist. 2000;5(5):388–392. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- 17.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295(2):431–437. [PubMed] [Google Scholar]
- 18.Abdel-Hafez SM, Hathout RM, Sammour OA. Towards better modeling of chitosan nanoparticles production: screening different factors and comparing two experimental designs. Int J Biol Macromol. 2014;64:334–340. doi: 10.1016/j.ijbiomac.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68(3):513–525. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Gazori T, Khoshayand MR, Azizi E, Yazdizade P, Nomani A, Haririan I. Evaluation of alginate/chitosan nanoparticles as antisense delivery vector: formulation, optimization and in vitro characterization. Carbohydr Polym. 2009;77(3):599–606. doi: 10.1016/j.carbpol.2009.02.019. [DOI] [Google Scholar]
- 21.Janes KA, Fresneau MP, Marazuela A, Fabra A. Alonso MaJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73(2):255–267. doi: 10.1016/S0168-3659(01)00294-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)