Abstract
Background
The spinal cord(SC) is highly relevant to disability in multiple sclerosis(MS), but few studies have evaluated longitudinal changes in quantitative SC-MRI.
Objectives
To characterize relationships between 5-year changes in SC-MRI with disability in MS.
Methods
75 MS patients underwent 3T SC-MRI and clinical assessment(expanded disability status scale[EDSS]) and MS functional composite[MSFC]) at baseline,2, and 5-years. SC-cross-sectional area(CSA) and diffusion-tensor indices(fractional anisotropy(FA), mean, perpendicular, parallel diffusivity(MD,λ⊥,λ‖,)) and magnetization-transfer-ratio(MTR) were extracted at C3-C4. Mixed-effects regression incorporating subject-specific slopes assessed longitudinal change in SC-MRI measures.
Results
SC-CSA and MTR decreased (p=0.009,p=0.03) over 5.1 years. There were moderate correlations between 2-year and 5-year subject-specific slopes of SC-MRI indices follow-up EDSS scores[Pearson’s r with FA=−0.23(p<0.001); MD=0.31(p<0.001);λ=0.34(p<0.001);λ‖=−0.12(p=0.05),MTR=−0.37(p<0.001);SC-CSA=−0.47(p<0.001) at 5 years]; MSFC showed similar trends. 2-year and 5-year subject-specific slopes were robustly correlated(r=0.93–0.97 for FA, λ⊥, SC-CSA, MTR, all p<0.001).
Conclusions
In MS, certain quantitative SC-MRI indices change over 5 years, reflecting ongoing tissue changes. Subject-specific trajectories of SC-MRI index changes at 2 and 5 years are strongly correlated, and highly relevant to follow-up disability. These findings suggest that individual dynamics of change should be accounted for when interpreting longitudinal SC-MRI measures and that measuring short-term change is predictive of long-term clinical disability.
Introduction
The spinal cord (SC) is a clinically eloquent structure commonly affected by multiple sclerosis (MS) and intimately linked to clinical disability.1 As a result, high resolution imaging measurements of the SC are useful to evaluate microstructural changes relevant to clinical disability progression in MS.
Traditionally, lesion-based measures in the SC have been utilized in clinical practice and in investigational settings in MS.2 However, lesion-based measures utilizing conventional T2-based MRI sequences in the SC have limited sensitivity and specificity to clinically-relevant microstructural changes in the SC in MS.3 Numerous studies have demonstrated the clinical relevance of measuring SC atrophy in MS4–6 but a limitation of this measure is that it entails an assessment of the SC after tissue destruction has already taken place.
Other quantitative SC MRI techniques, including diffusion-tensor imaging (DTI) and magnetization-transfer imaging (MTI), have demonstrated more robust correlations with clinical disability than conventional, lesion-based measures.7–9 Furthermore, these measures yield additional information about tissue microstructure, beyond what can be captured by conventional measures of SC lesion count and atrophy alone.1
Although these quantitative SC-MRI measures have demonstrated clear correlations to clinical dysfunction in MS cross-sectionally, before most imaging measures can be utilized in MS clinical practice as a disease monitoring tool, the following criteria must be met on a longitudinal basis: change in the MRI measure must be detectable over time, and there must be a longitudinal relationship between the observed change in the MRI measure and clinical disability progression.10 These are important factors for an imaging measure to have clinical utility as a surrogate endpoint in MS, or to have clinical prognostic value. However, there are exceptions to these rules, including gadolinium-enhancing lesions. Furthermore, reproducibility and feasibility of obtaining the measure are also important considerations.
To date, there have been few longitudinal studies assessing quantitative SC-MRI measures in MS, and most existing longitudinal studies have assessed SC atrophy or lesion count.11,12 To our knowledge, only two existing studies have assessed DTI measures in the SC longitudinally.13,14 One study assessed DTI measures in the cervical SC over a 6-month period during an acute SC relapse and found that baseline radial diffusivity (RD) correlated with recovery at 6 months, and a decrease in RD corresponded to clinical improvement.14 Another study assessed change in SC-CSA and DTI over 2 years, and found that although SC-CSA, FA, and MD changed over time, these measures were not linked to disability change.13
Given the dearth of existing literature on longitudinal change in SC-MRI measures in MS, the objective of this study was to evaluate quantitative SC-MRI measures, including DTI, MTI, and SC atrophy, over 2 and 5 years in a mixed-phenotype cohort of MS patients, and to assess for relationships between change in MRI measure and clinical disability over the follow-up period.
Methods
Study Participants
This study was approved by the Johns Hopkins University Institutional Review Board. All patients provided written informed consent.
Patients with relapsing and progressive MS were recruited from the Johns Hopkins MS Clinic by convenience sampling between 2007 – 2009. MS diagnosis was confirmed by the treating neurologist, according to the 2005 McDonald criteria.15 Within 30-days of MRI, MS functional composite(MSFC) scores and expanded disability status scale(EDSS) scores were obtained.16,17. Patients returned for annual study visits in the first two years. In 2014, patients from the baseline cohort were contacted for a follow-up study visit. Due to the exploratory nature of this cross-sectional study, sample size calculations were not performed.
MRI
Cervical SC MRI sequences
All participants underwent a cervical SC-MRI on a 3-tesla Achieva scanner(Philips Medical Systems, Best, The Netherlands) using body coil excitation and two-element phased array surface coil reception.
MT-weighted images consisted of a T2*-weighted, 3D-gradient-echo sequence with a multi-shot echo-planar readout(EPI factor=3), with(MTon) and without(MToff) a 1.5-kHz off-resonance sinc-gauss-shaped radiofrequency magnetization-transfer saturation pulse(620 degree pulse;bandwidth 400Hz), parallel imaging factor=2(TR/TE/α=121ms/12.5ms/9º). There were 30 contiguous 3-mm axial slices spanning C2–C6, and nominal in-plane resolution of 0.6×0.6 mm2.
DTI data were obtained using a multi-slice spin-echo sequence with a single-shot echo-planar readout and a parallel imaging factor of 2(TR/TE=4727ms/63ms). Axial fat-suppressed diffusion-weighted images were obtained in 16 non-coplanar gradient directions(b=500 s/mm2 and one minimally diffusion weighted acquisition(b0~33 s/mm2)). Slice thickness:3 mm;nominal in-plane resolution 1.5×1.5 mm2.
Additional sequences included a sagittal, multi-slice turbo spin echo(TSE factor:20,parallel imaging factor:2) short-tau inversion recovery(STIR) with FOV=250mm, acquired resolution of 1×1×2 mm3(AP,FH,RL) with TR/TE/TI=4227ms/68ms/200ms;total scan time:3 minutes;4 averages.
Image Processing
MTR calculation:MTon was registered to MToff utilizing a 6-degree-of-freedom, rigid-body procedure implemented in FLIRT(Oxford Centre for Functional MRI of the Brain’s Linear Imaging Registration Tool, Oxford, UK) using JIST(Java Image Science Toolkit)18 MTR was calculated using:(MToff−MTon)/MToff.
Each diffusion-weighted image was registered to the initial minimal-b volume using a 6-degree-of-freedom, rigid-body registration, implemented in FLIRT using JIST18 The minimal-b image was deformably registered to MToff and the deformation applied to the DW images.19 All DTI indices were calculated from the eigenvalues of the diffusion tensor which was used to generate the diffusion tensor and maps of fractional anisotropy(FA), mean diffusivity(MD), perpendicular diffusivity(λ), and parallel diffusivity(λ‖).20
SC toolbox, an automated reproducible segmentation tool,21 was applied to the MToff images to delineate regions-of-interest(ROIs) encompassing the axial cross-section of the SC across segments C3-C4 to calculate SC-CSA. Manual segmentation of the SC cross-section was performed across C3-C4 of the FA map, and superimposed on maps of other DTI indices(MD, λ‖, λ) (Figure e-1). Manual segmentation of the MTR maps were performed (Figure e-1). Average values of SC-CSA, FA, MD, λ‖, λ, and MTR were calculated from the ROIs of each index map across all axial slices from C3-C4.
Statistical Analysis
Statistical calculations were performed using STATA Version 15(StataCorp, College Station, TX). Mixed-effects multivariable linear regression including subject-specific intercepts and slopes was used to assess for longitudinal change in SC-MRI indices, using age at the time of MRI as the time variable. This was done so that longitudinal change in MRI measures could be evaluated, while simultaneously controlling for the effect of age, which is a necessary confounder to account for in chronic diseases. Subject-specific slopes were categorized into upper, middle, and lower tertiles. Correlations between subject-specific slopes and follow-up clinical measures(EDSS and MSFC) were assessed in the total group, and in the upper/lower tertiles. Chi-squared tests assessed relationships between upper/lower tertiles of subject-specific slopes and presence/absence of disability progression at follow-up. EDSS progression was defined as an increase of 1.5 when baseline EDSS was ≤1, increase of 1.0 when baseline EDSS was between 1.0–6.0, and increase of 0.5 when the baseline EDSS was ≥6.0.22 MSFC progression was defined as a decrease in the MSFC Z-score at follow-up vs. the baseline value. MSFC Z-scores were calculated using the National MS Society Taskforce reference population.23 Given the exploratory nature of this study, adjustment for multiple comparisons was not performed. Statistical significance was defined as p<0.05.
Results
There were 129 adults with MS in the baseline cohort. Subsequent MRI frequency was heterogeneous over the mean follow-up period of 5.1 years, with the majority of subjects undergoing an MRI at baseline, an interim time-point(mean 1.8 years), and at a mean of 5.1 years(Table 1). Seventy-five of the baseline 129 patients underwent a follow-up MRI at approximately 5 years: 21 patients had withdrawn from the study, 24 were lost to follow-up; 4 were enrolled in other longitudinal research studies and could not return due to time constraints, and 5 were unable to return due to personal circumstances. 75 subjects had MRIs at 3 time-points, 46 had 4 time-points, 12 had 5 time-points, and 3 had 6 time-points. There were no significant differences in age, sex, baseline EDSS, MSFC, disease subtype, or proportion of disease-modifying treatment between the 75 patients who underwent a 5-year follow-up MRI and the 54 patients that were not able to.
Table 1:
Clinical and MRI Characteristics
| All MS | RRMS | Progressive MS | |
|---|---|---|---|
| Subjects, n | 75 | 45 | 30 |
| Mean Age at baseline (sd) | 44 (12) | 38 (10) | 52 (8) |
| % female | 71 | 77 | 63 |
| Mean follow-up time, years (range) | 5.1 years (2.8 – 7.8) | 5.3 (2.9 – 7.7) | 4.7 (2.7 – 7.8) |
| % on DMT | 72 | 93 | 47 |
| Baseline Median EDSS (25th, 75th percentiles) | 3.0 (2.0–5.5) | 2.0 (1.5–3.0) | 6.0 (4.0–6.5) |
| Median Follow-Up EDSS (25th, 75th percentiles) | 3.5 (2.5 – 6.0) | 3.0 (2.0 – 3.5) | 6.0 (3.5 – 6.5) |
| % with progression of EDSS at 5 years | 43 | 43 | 40 |
| Mean Baseline MSFC Z-score (SD) | 0.22 (0.57) | 0.31 (0.63) | 0.07(0.41) |
| Mean Follow-Up MSFC Z-score (SD) | 0.42 (0.65) | 0.52 (0.66) | −0.04 (0.71) |
| % with progression of MSFC at 5 years | 30 | 21 | 40 |
| Mean Baseline FA (SD) | 0.64 (0.06) | 0.64 (0.07) | 0.64 (0.06) |
| Mean Baseline MD (SD) μm2/ms | 1.05 (0.17) | 1.07 (0.11) | 1.04 (0.24) |
| Mean Baseline λ⊥, (SD) μm2/ms | 0.62 (0.13) | 0.61 (0.12) | 0.62 (0.13) |
| Mean Baseline λ‖, (SD) μm2/ms | 1.97 (0.19) | 1.97 (0.19) | 1.97 (0.19) |
| Mean Baseline MTR (SD) | 0.32 (0.05) | 0.33 (0.04) | 0.30 (0.05) |
| Mean Baseline SC-CSA (SD), mm2 | 70.4 (8.1) | 72.3 (6.3) | 67.2 (9.3) |
Clinical characteristics of the study population are described in Table 1. The mean age of MS patients was 44 years, and 71% were female. 60% had relapsing and 40% progressive MS. The baseline median EDSS score was 3.0(IQR 2.0–5.5), median follow-up EDSS score was 3.5(IQR 2.5 – 6.0) at 5.1 years; the mean baseline MSFC Z-score was 0.22(sd=0.57) and 0.4(sd=0.65) at follow-up. 43% of patients demonstrated global disability progression based on an increase in EDSS score and 30% of patients demonstrated MSFC progression over the follow-up period.
Baseline MRI measures are summarized in Table 1. 84% of patients had cervical SC lesions at baseline. The median number of SC lesions was 2 (IQR 1–3) at both baseline and follow-up. 17% of patients (n=13) demonstrated new lesions over 5 years.
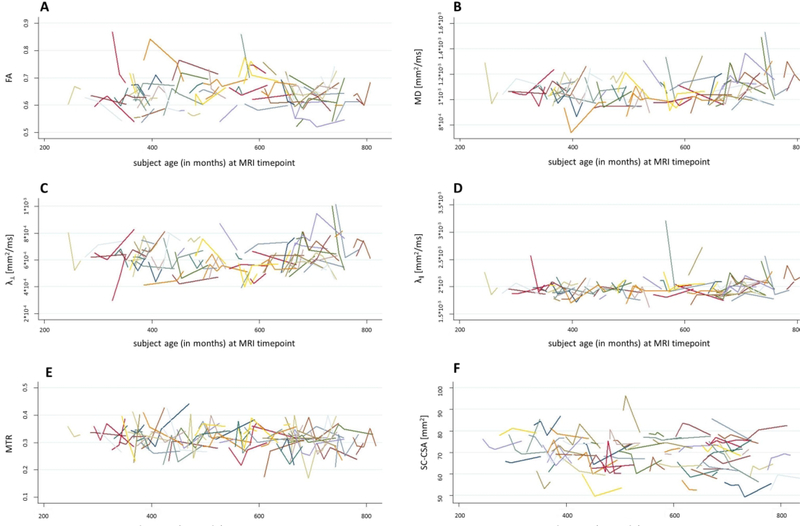
When quantitative SC-MRI measures were assessed longitudinally, there were changes in quantitative SC-MRI measures over 2 and 5 years. Specifically, at 2 years, MTR decreased(p=0.04). At 5 years, MTR(p=0.03) and SC-CSA(p=0.009) decreased (Figure 1, Table 2). There were no significant observable changes in the other quantitative MRI indices at 2 and 5 years (Table 2). As an exploratory analysis, when SC-CSA was included as a covariate in the longitudinal analysis of SC-MTR and DTI indices, the results did not change in a meaningful way with MTR and SC-CSA still demonstrating significant changes at 5 years.
Figure 1:
Plots of MRI Measure Changes Over Study Period
Table 2:
Change in MRI Measures Over Time
| MRI Index | p-value at 2 years | p-value at 5 years | Annual rate of change for MRI measures with p<0.05 (over 5.1 years) |
|---|---|---|---|
| FA | 0.54 | 0.68 | n/a |
| MD | 0.14 | 0.07 | n/a |
| λ⊥ | 0.15 | 0.22 | n/a |
| λ‖ | 0.07 | 0.99 | n/a |
| MTR | 0.04 | 0.03 | −1.6%/year; 95% CI [−3.6, −0.5] |
| SC-CSA | 0.07 | 0.009 | −2.0%/year; 95% CI [−3.0, −0.1] |
CI=confidence interval
When correlations between subject-specific slopes of quantitative SC-MRI measure change and disability at follow-up were assessed, there were relationships between subject-specific trajectories of change of all SC-MRI measures and follow-up EDSS. Similarly, with MSFC, relationships were observed between most subject-specific trajectories of SC-MRI measures and follow-up clinical measures, with the exception of λ‖ at 2 years(Tables 3, 4).
Table 3:
Correlations Between Follow-Up EDSS and Subject-Specific Slopes of MRI Measures
| MRI index | Correlation Coefficient with 2-Year Subject-Specific Slope (p-values) | Correlation Coefficient with 5-Year Subject-Specific Slope (p-values) |
|---|---|---|
| FA | −0.23 (p<0.001) | −0.23 (<0.001) |
| MD | 0.28 (p<0.001) | 0.31 (<0.001) |
| λ⊥ | 0.32 (p<0.001) | 0.34 (<0.001) |
| λ‖ | 0.13 (p=0.01) | −0.12 (0.05) |
| MTR | −0.29 (p<0.001) | −0.37 (<0.001) |
| SC-CSA | −0.50 (p<0.001) | −0.47 (<0.001) |
Table 4:
Correlations Between Follow-Up MSFC and Subject-Specific Slopes of MRI Measures
| MRI index | Correlation Coefficient with 2-Year Subject-Specific Slope (p-values) | Correlation Coefficient with 5-Year Subject-Specific Slope (p-values) |
|---|---|---|
| FA | 0.21 (0.003) | 0.18 (0.003) |
| MD | −0.11 (0.11) | −0.33 (<0.001) |
| λ⊥ | 0.20 (0.004) | −0.17 (0.008) |
| λ‖ | 0.04 (0.56) | 0.15 (0.01) |
| MTR | 0.24 (0.005) | 0.30 (<0.001) |
| SC-CSA | 0.34 (<0.001) | 0.22 (0.003) |
As an exploratory analysis: to assess for differences between individuals with slower/faster rates of SC-MRI measure change vs. the study population mean, subject-specific slopes of MRI index change were divided into 3 groups(tertiles) based on rate of change of quantitative SC-MRI measure vs. the study population mean rate of change. Individuals with more rapid rates of pathological change in MRI index vs. the study population mean(i.e. decrease in FA, MTR, SC-CSA, λ‖ and increase in MD and λ⊥) often demonstrated more robust correlations with follow-up clinical measures vs. individuals with slower rates of change vs. the study population mean(Tables e1, e2).
As an exploratory analysis, when relationships were assessed between subject-specific slopes of MRI index change(upper/lower tertiles) and “clinical progression”(based on pre-specified EDSS and MSFC criteria) over the study follow-up period, the majority of SC-MRI measures did not show relationships with EDSS or MSFC progression (Table e3).
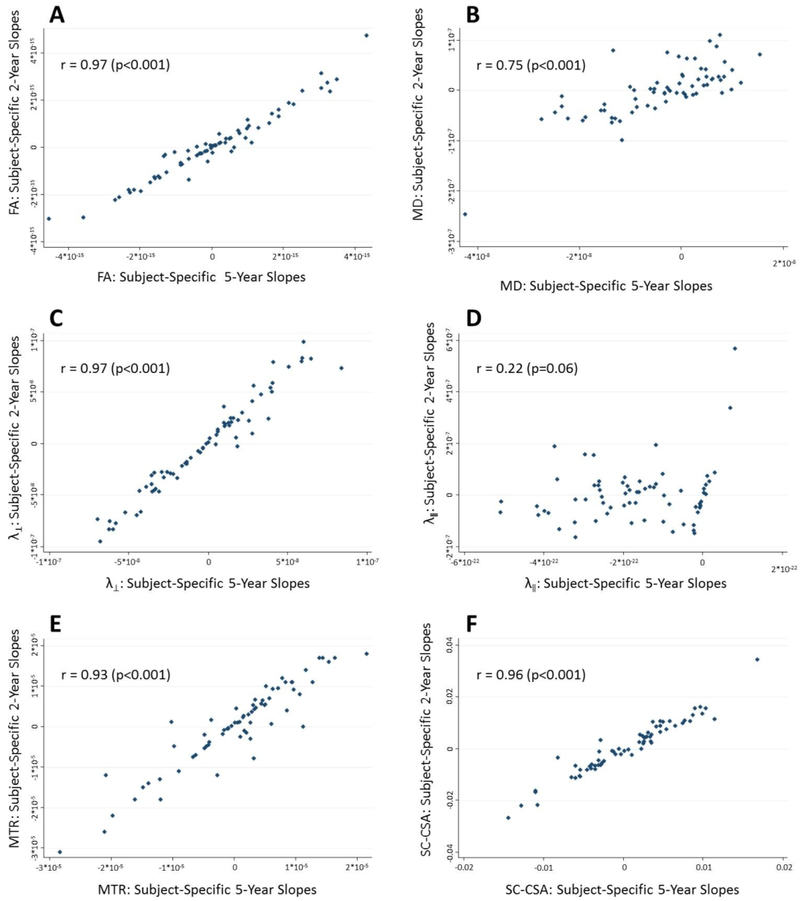
Finally, there were robust correlations between the 2-year trajectories of change of quantitative SC-MRI measures in individual patients and 5-year trajectories of change[r=0.97(p<0.001) for FA; r=0.75(p<0.001) for MD; r=0.97(p<0.001), for λ‖; r=0.93 for MTR(p<0.001);r=0.96(p<0.001) for SC-CSA).(Table 5, Figure 2).
Table 5:
Correlations between 2-year and 5-year subject-specific slopes for SC MRI indices
| MRI Index | Correlation Coefficient (p-values) between 2-year and 5-year subject-specific slopes |
|---|---|
| FA | 0.97 (<0.001) |
| MD | 0.75 (<0.001) |
| λ⊥ | 0.97 (<0.001) |
| λ‖ | 0.22 (0.06) |
| MTR | 0.93 (<0.001) |
| SC-CSA | 0.96 (<0.001) |
Figure 2:
Plots of Correlations between Subject-Specific Trajectories of MRI Measure Change at 2-Years and 5- Years
Discussion
In this study, we found measurable change in quantitative SC-MRI in MS over 2 and 5 years, likely reflecting ongoing, disease-related, microstructural damage. There were correlations observed between subject-specific trajectories of change in SC MRI measures and global disability (EDSS and MSFC) at 2 and 5 years, with stronger correlations observed in individuals with more rapid trajectories of change vs. those with less rapid trajectories of change. Moreover, correlations ranging between r=0.75 – 0.97 were observed between subject-specific trajectories of change at 2 and 5 years, suggesting that short-term change observed in SC-MRI measures correlates with longer-term change. These results provide a framework to utilize quantitative SC-MRI measures that demonstrate change over time and consistent clinical correlations (such as MTR and SC-CSA), as well as other quantitative MRI measures in clinical trials and in clinical practice.
Few studies to date have assessed quantitative SC-MRI measures longitudinally. Agosta et al assessed DTI measures in the SC over 2 years and found there were changes over that time period, but only limited correlations with change in clinical measures at follow-up,13 similar to our findings. However, when we used mixed-effects models to assess subject-specific change, there were correlations with clinical disability at follow-up. Brownlee et al assessed SC-lesion count and atrophy in individuals presenting with clinically isolated syndrome over 5 years and found correlations with clinical disability progression, but this study did not include advanced quantitative measures.11
To our knowledge, this is the first study that has assessed a wide spectrum of quantitative SC-MRI measures over a relatively prolonged follow-up duration of five years. During this time, we observed change in quantitative SC-MRI measures, and found that the observed change was more clearly evident at 5 vs. 2 years, which is to be expected, given the slowly progressive nature of MS, as well as the measurement variability of SC-MRI measures that may preclude detection of subtle change in shorter time periods. The duration of follow-up in this study is of particular value in the context of MS, which is a chronic inflammatory disease that affects patients for decades. As a result, a five-year follow-up period is a clinically meaningful timeframe in the context of MS. Recent studies have demonstrated that particularly for patients with progressive MS, a duration of 2 years may be insufficient to observe benefit of disease-modifying treatments.24 Similarly, in observational studies of people living with MS, follow-up durations extending beyond 2 years allow a more meaningful “sampling” of the disease course in MS, making our observations more relevant in the real-world. It is therefore especially striking, in this context, that subject-specific slopes of MRI changes that were predictive of clinically relevant 5-year changes could be extracted from only 3 scans over a 2-year period.
As expected, we found that quantitative SC-MRI measures changed over 2 and 5 years. These measures are thought to reflect on-going microstructural damage, including demyelination, axonal loss, and gliosis. When individual trajectories of change were evaluated, we found substantial variability across the study population, which is expected, given the diverse study population and the substantial measurement variability associated with SC-MRI measures, due to technical artifacts from the small size of this structure and partial-volume averaging.25,26 Despite these caveats, we found subject-specific trajectories of MRI index change consistently correlated with disability at follow-up, and this was observed across two distinct measures of global disability (EDSS and MSFC). The strength of the correlation was modest for most MRI indices, suggesting that a substantial portion of clinical variability is not captured by these measures.
Furthermore, we found that individual patients with more rapid rates of change(vs. the study population mean rate of change) often demonstrated stronger relationships with clinical disability at follow-up vs. those with lower than average rates of change. These observations highlight the importance of incorporating information on subject-specific change when assessing quantitative imaging measures in a chronic, highly variable disease such as MS. In this study, the use of mixed-effects models, which allows for clinically-relevant subject-specific MRI change to be captured while taking into account the magnitude of change in the entire study group is of particular value. Simply assessing mean values of “grouped” quantitative MRI data, which is what is typically done in clinical trials and in observational studies, may result in losing valuable information that is of high relevance from a clinical standpoint.4,27 This observation is not only applicable to SC-MRI measures, but to all quantitative measures assessed in the brain and retina,28 and potentially even quantitative biological biomarkers, such as serum neurofilament levels29. Particularly for SC-MRI measures, there is substantial measurement variability that limits their potential for clinical application in individual patients.25 The ability to identify individuals who clearly have a more rapid trajectory of change vs. the population mean may be a practical way to apply measures that can be limited by variability in daily clinical practice.
As mentioned above, we found that subject-specific 2-year trajectories were strongly correlated with 5-year trajectories of change, with observed correlations as high as 0.97 with FA and MTR. This finding is of significant practical utility, and, if confirmed, will likely extrapolate to other quantitative imaging measures in MS with less measurement variability. Utilizing short-term trajectories of change to predict longer-term disability can have significant practical implications in day-to-day clinical practice. For example, in an MS patient who is changing disease-modifying treatment, measuring short-term trajectories of validated MRI measures would be helpful to rapidly assess for treatment response, allowing for personalized treatment optimization. Prior studies have demonstrated that in large groups of MS patients, the rate of brain volume loss in early years correlate with longer-term disability at 8–10 years.30 The use of individualized trajectories of change in the short-term to predict longer-term clinical disability helps pave the way toward personalized, precision medicine.31 One exception to this strong correlation was the axial diffusivity (λ‖), a potential indicator of neuronal damage.32,33 This suggests that the changes in λ‖ may be too unreliable with small sample sizes, or that not enough time has elapsed over the disease course to detect axonal damage.
Finally, although we found correlations between change in MRI measures and follow-up disability measures, we did not find consistent correlations between the magnitude of MRI measure change and EDSS and MSFC progression. There are likely a number of reasons for this observation, including the small sample size, measurement variability of SC MRI measures, that not enough time has elapsed to detect clinical disability, or that there are floor and ceiling effects in the ability to detect disability. Perhaps the most important reason for this observation, however, is intrinsic limitations in the global clinical disability measures of EDSS and MSFC34,35. Both of these scales can be insensitive to subtle clinical decline. Furthermore, in our analysis, we were only able to categorize “progression” as a binary outcome, which likely resulted in a reduction in our ability to detect a correlation. Further study will be important to establish this important link.
This study has a number of limitations. First, of the baseline 129 patients, only 75 were able to return for a follow-up MRI scan, increasing the risk of selection bias. However, there were no differences in baseline clinical characteristics between those included in this study vs. those that were not able to participate, and the clinical characteristics of our study population were in line with what one would expect in a chronic MS population. Furthermore, despite this limitation, the results are still of interest and of practical utility as a step towards utilizing quantitative MRI measures in personalized clinical medicine, particularly in light of the fact that there is such a dearth of longitudinal studies on quantitative SC-MRI. Second, the sample size is relatively small and there is no comparative healthy control population, therefore conclusions drawn should be interpreted with caution. Despite the small sample size, however, the results we obtained are relatively consistent across two distinct measures of global disability (EDSS and MSFC), which lend credence to our speculations. Finally, although we included clinical measures that are conventionally utilized in MS clinical trials and investigational settings, we were not able to obtain system-specific, quantitative clinical measures assessing sensorimotor function in these patients,36 which would have been quite useful particularly in the context of SC-MRI measures, and may have allowed for the establishment of a link between change in MRI measure and change in disability over time. Future studies will be necessary to evaluate this possibility.
In conclusion, we found that quantitative SC-MRI measures change over both 2 and 5 years in MS patients, and that individual trajectories of change in these measures are highly relevant to clinical disability at 2 and 5 years. This study highlights the importance of including information on individual, subject-specific dynamics of change compared to average values when interpreting longitudinal SC-MRI measures, and that measuring short-term change is predictive of long-term clinical disability. These findings, if validated in larger studies, could expand the clinical utility of quantitative SC-MRI measures, and other quantitative biomarkers in MS.
Acknowledgments
Study Funding: National Multiple Sclerosis Society (RG 5093-A-5); NIH P41EB015909; R01NS082347
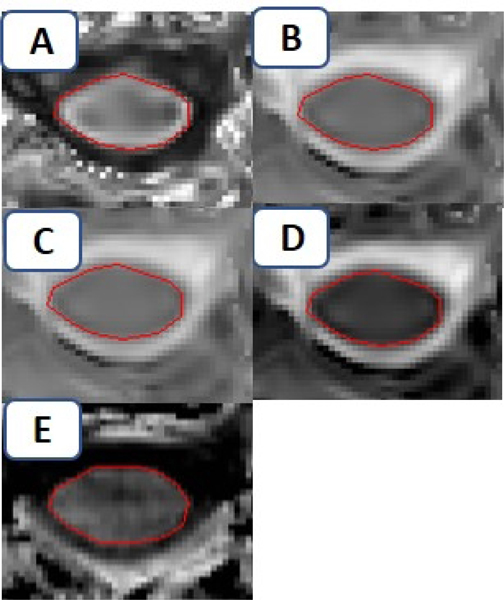
Figure e-1:
Manual delineation of region-of-interest on A) map of fractional anisotropy; which is then super-imposed on map of B) mean diffusivity [MD] and C) λ‖, D) λ⊥. E) Manual delineation of region-of-interest on map of magnetization-transfer ratio [MTR]
Table e1:
Correlations between Follow-Up EDSS and Subject-Specific Slopes of MRI Measures (Upper and Lower Tertiles)
| Upper and lower tertiles of subject-specific slopes for individual MRI measures | Subject-Specific Slope Tertiles | Correlation Coefficient with Follow-Up EDSS |
|---|---|---|
| FA | Upper | 0.10 (0.40) |
| Lower | −0.38 (<0.002) | |
| MD | Upper | 0.37 (0.001) |
| Lower | 0.47 (<0.001) | |
| λ⊥ | Upper | 0.25 (0.01) |
| Lower | 0.01 (0.91) | |
| λ‖ | Upper | −0.19 (0.08) |
| Lower | −0.04 (0.68) | |
| MTR | Upper | −0.37 (<0.001) |
| Lower | −0.53 (<0.001) | |
| SC-CSA | Upper | 0.22 (0.04) |
| Lower | −0.47 (<0.001) |
Table e2:
Correlations between Follow-Up MSFC and Subject-Specific Slopes of MRI Measures (Upper and Lower Tertiles)
| Upper and lower tertiles of subject-specific slopes for individual MRI measures | Subject-Specific Slope Tertiles | Correlation Coefficient with Follow-Up MSFC |
|---|---|---|
| FA | Upper | 0.10 (0.38) |
| Lower | 0.09 (0.41) | |
| MD | Upper | −0.50 (<0.001) |
| Lower | −0.03 (0.80) | |
| λ⊥ | Upper | −0.21 (0.05) |
| Lower | 0.02 (0.88) | |
| λ‖ | Upper | 0.18 (0.10) |
| Lower | 0.59 (<0.001) | |
| MTR | Upper | 0.44 (<0.001) |
| Lower | 0.28 (<0.001) | |
| SC-CSA | Upper | −0.03 (0.78) |
| Lower | 0.21 (0.05) |
Table e3.
Relationships between EDSS and MSFC Progression (yes/no) and Subject-Specific Slopes of MRI Measures (Upper and Lower Tertiles)
| Upper/lower tertiles of subject-specific slopes for individual MRI measures | Chi-squared p-values | |
|---|---|---|
| EDSS | MSFC | |
| FA | 0.96 | 0.92 |
| MD | 0.31 | 0.88 |
| λ⊥ | 0.70 | 0.09 |
| λ‖ | 0.002 | 0.03 |
| MTR | 0.39 | 0.21 |
| SC-CSA | 0.18 | 0.02 |
Footnotes
Disclosures: The authors declare no relevant conflicts of interest.
References
- 1.Oh J, Saidha S, Chen M, et al. Spinal cord quantitative MRI discriminates between disability levels in multiple sclerosis. Neurology 2013; 80(6): 540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bot JC, Barkhof F, Polman CH, et al. Spinal cord abnormalities in recently diagnosed MS patients: added value of spinal MRI examination. Neurology 2004; 62(2): 226–33. [DOI] [PubMed] [Google Scholar]
- 3.Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002; 15(3): 239–45. [DOI] [PubMed] [Google Scholar]
- 4.Rocca MA, Horsfield MA, Sala S, et al. A multicenter assessment of cervical cord atrophy among MS clinical phenotypes. Neurology 2011; 76(24): 2096–102. [DOI] [PubMed] [Google Scholar]
- 5.Klein JP, Arora A, Neema M, et al. A 3T MR imaging investigation of the topography of whole spinal cord atrophy in multiple sclerosis. AJNR Am J Neuroradiol 2011; 32(6): 1138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casserly C, Seyman EE, Alcaide-Leon P, et al. Spinal Cord Atrophy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J Neuroimaging 2018; 28(6): 556–86. [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Zackowski K, Chen M, et al. Multiparametric MRI correlates of sensorimotor function in the spinal cord in multiple sclerosis. Mult Scler 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti B, Rocca MA, Rovaris M, et al. A diffusion tensor MRI study of cervical cord damage in benign and secondary progressive multiple sclerosis patients. J Neurol Neurosurg Psychiatry 2010; 81(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 9.Charil A, Caputo D, Cavarretta R, Sormani MP, Ferrante P, Filippi M. Cervical cord magnetization transfer ratio and clinical changes over 18 months in patients with relapsing-remitting multiple sclerosis: a preliminary study. Mult Scler 2006; 12(5): 662–5. [DOI] [PubMed] [Google Scholar]
- 10.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69(3): 89–95. [DOI] [PubMed] [Google Scholar]
- 11.Brownlee WJ, Altmann DR, Alves Da Mota P, et al. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult Scler 2017; 23(5): 665–74. [DOI] [PubMed] [Google Scholar]
- 12.Tsagkas C, Magon S, Gaetano L, et al. Preferential spinal cord volume loss in primary progressive multiple sclerosis. Mult Scler 2018: 1352458518775006. [DOI] [PubMed] [Google Scholar]
- 13.Agosta F, Absinta M, Sormani MP, et al. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain 2007; 130(Pt 8): 2211–9. [DOI] [PubMed] [Google Scholar]
- 14.Freund P, Wheeler-Kingshott C, Jackson J, Miller D, Thompson A, Ciccarelli O. Recovery after spinal cord relapse in multiple sclerosis is predicted by radial diffusivity. Mult Scler 2010; 16(10): 1193202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 2005; 58(6): 840–6. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–52. [DOI] [PubMed] [Google Scholar]
- 17.Rudick RA, Polman CH, Cohen JA, et al. Assessing disability progression with the Multiple Sclerosis Functional Composite. Mult Scler 2009; 15(8): 984–97. [DOI] [PubMed] [Google Scholar]
- 18.Rohde GK, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Trans Med Imaging 2003; 22(11): 1470–9. [DOI] [PubMed] [Google Scholar]
- 19.Chen M CA, Wheeler MB, Liu X, Prince JL. Multi-Channel Enhancement of the Adaptive Bases Algorithm. 16th Annual Meeting of the Organization for Human Brain Mapping 2010; Barcelona, Spain; 2010. [Google Scholar]
- 20.Smith SA, Jones CK, Gifford A, et al. Reproducibility of tract-specific magnetization transfer and diffusion tensor imaging in the cervical spinal cord at 3 tesla. NMR Biomed 2010; 23(2): 207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Leener B, Levy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017; 145(Pt A): 24–43. [DOI] [PubMed] [Google Scholar]
- 22.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(6): 545–56. [DOI] [PubMed] [Google Scholar]
- 23.Rudick R, Antel J, Confavreux C, et al. Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol 1997; 42(3): 379–82. [DOI] [PubMed] [Google Scholar]
- 24.Giovannoni G, Cutter G, Sormani MP, et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord 2017; 12: 70–8. [DOI] [PubMed] [Google Scholar]
- 25.Hickman SJ, Miller DH. Imaging of the spine in multiple sclerosis. Neuroimaging Clin N Am 2000; 10(4): 689–704, viii. [PubMed] [Google Scholar]
- 26.Chen M, Carass A, Oh J, et al. Automatic magnetic resonance spinal cord segmentation with topology constraints for variable fields of view. Neuroimage 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362(5): 387–401. [DOI] [PubMed] [Google Scholar]
- 28.Saidha S, Sotirchos ES, Oh J, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol 2013; 70(1): 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89(22): 2230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher E, Rudick RA, Cutter G, et al. Relationship between brain atrophy and disability: an 8-year follow-up study of multiple sclerosis patients. Mult Scler 2000; 6(6): 373–7. [DOI] [PubMed] [Google Scholar]
- 31.Giovannoni G. Personalized medicine in multiple sclerosis. Neurodegener Dis Manag 2017; 7(6s): 13–7. [DOI] [PubMed] [Google Scholar]
- 32.Farrell JA, Zhang J, Jones MV, et al. q-space and conventional diffusion imaging of axon and myelin damage in the rat spinal cord after axotomy. Magn Reson Med 2010; 63(5): 1323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landman BA, Farrell JA, Smith SA, Reich DS, Calabresi PA, van Zijl PC. Complex geometric models of diffusion and relaxation in healthy and damaged white matter. NMR Biomed 2010; 23(2): 152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen JA, Reingold SC, Polman CH, Wolinsky JS, International Advisory Committee on Clinical Trials in Multiple S. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 2012; 11(5): 467–76. [DOI] [PubMed] [Google Scholar]
- 35.Goodkin DE, Cookfair D, Wende K, et al. Inter- and intrarater scoring agreement using grades 1.0 to 3.5 of the Kurtzke Expanded Disability Status Scale (EDSS). Multiple Sclerosis Collaborative Research Group. Neurology 1992; 42(4): 859–63. [DOI] [PubMed] [Google Scholar]
- 36.Newsome SD, Wang JI, Kang JY, Calabresi PA, Zackowski KM. Quantitative measures detect sensory and motor impairments in multiple sclerosis. J Neurol Sci 2011; 305(1–2): 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]