Abstract
Background
Uncomplicated infections such as candidiasis, bacterial vaginosis (BV), or trichomoniasis are easy to diagnose and treat. However, about 8% of patients will have a more complicated course with failure to respond to treatment or rapid recurrence of symptoms. There are many suggestions in Traditional Persian Medicine like myrtle (Myrtus communis L.) and oak gall (Quercus infectoria G.Olivier) for treatment of vaginitis.
Objectives
A clinical trial was designed to assess the efficacy of a novel herbal suppository, containing myrtle and oak gall (MOGS) in treatment of vaginitis.
Methods
In a parallel randomized clinical trial, 120 women with vaginitis were randomly assigned to MOGS, metronidazole, or placebo. Formulation was simulated from traditional Persian manuscripts and MGOS was prepared after pharmaceutical optimization processing as well as quantification of gallic acid by HPLC. The study was double-blind for MOGS and placebo and single-blind for metronidazole group.
Results
MOGS effectively improved vaginal discharge (p = 0.024 for BV and 0.018 for trichomoniasis) and pH (compared to placebo (p = 0.013) and metronidazole (p = 0.001)). Both MOGS and metronidazole could reverse whiff test. Metronidazole was the best medication for making Nugent score negative (p = 0.005) as well as the best therapy according to laboratory findings to treat BV in comparison with placebo (p = 0.021). While for trichomoniasis, MOGS could improve the disease more successfully (p = 0.001). Both MOGS and metronidazole treated mixed vaginitis (p = 0.002).
Conclusion
MOGS would be a chance for developing new treatment for trichomoniasis.
Graphical abstract
Keywords: Myrtle, Oak gall, Traditional Persian medicine, Suppository
Introduction
One of the most common reasons for visiting a health care provider among women (> 70% of adults) would be vaginal complaints including malodor vaginal discharge, irritation, and itching, which is diagnosed as vaginitis [1, 2]. The prevalence of bacterial vaginosis (BV) in adult women is 20–30%. BV commonly occurs as a result of replacement of Lactobacillus spp. with Gardnerella vaginalis, Mobiluncus curtisii, M. mulieris and/or Mycoplasma hominis. [3, 4]. BV diagnosis is based on the presence of at least three of four Amsel criteria, including pH >4.5, thin and grayish discharge, clue cells, and positive whiff-amine test [5], and Nugent score which is the gold standard for diagnosing and evaluates Gram-stained vaginal discharge smear. This technique is less common than Amsel test due to needing for more resources, time, and experiences [6]. For symptomatic women diagnosed with BV as well as symptomatic pregnant women, Centers for Disease Control and Prevention recommend two different treatments, i.e.: oral metronidazole (7 days) or metronidazole vaginal gel (5 nights), and clindamycin vaginal cream (7 nights) [6]. Another kind of vaginitis among 10–25% of either pregnant or non pregnant women is caused by Trichomonas vaginalis (TV) [7]. Clinical manifestations in trichomoniasis are including yellow discharge, malodor, irritation, dyspareunia and Colpitis macularis or strawberry cervix [8]. While, most of TV symptoms are overlapped with those of BV [9], nearly 50% of TV-infected women are asymptomatic [10]. Diagnostic factors are pH >4.5 (it may be normal) and distinguishing trichomonads in wet prep [11]. Choice therapy for TV is oral metronidazole (7 days) or tinidazole, a 2-g dose [6, 12].
Because of antibiotic resistance, 30% recurrence rate for BV occurs within the first month, 59% within six months, and 69% during 12 months of treatment [13, 14]. Persistent or recurrent TV would be due to inadequate treatment, relapse, or resistance [15]. Moreover, some adverse effects might happen following antibiotic therapy like metallic taste, gastrointestinal disorders and nausea that lead to lower patient’s compliance [16]. Because of such complications, complementary and alternative medicine (CAM) therapy is requested by young women with chronic symptomatic [17]. Natural products including oral and vaginal probiotics like Lactobacilli re-colonization, boric acid, douching, Melaleuca alternifolia (tea tree) essential oil, garlic, and propolis have been used for treatment of vaginitis [18]. According to Traditional Persian Medicine (TPM), different herbal, animal, and mineral materials are recommended for vaginitis in the form of various vaginal drug deliveries [19]. Among TPM recommendations, oak gall (cecidia of Quercus infectoria G.Olivier) [20], and myrtle (Myrtus communis L.) are most popular herbals. They are recommended for vaginitis in a form of cotton-loaded or sitz bath in many Persian manuscripts [21–27]. In this study, the effectiveness of a myrtle and oak gall suppository (MOGS) in the treatment of vaginitis was studied.
Materials and methods
Sample size
Considering ΔP = 0.3, P1/P2 = 1, α = 0.05, β = 0.2, the sample size was calculated based on 40 patients in each group (total of 120 patients) by using statistical software components (SSC) software.
Patients
Women were recruited for the study from the Motahhari outpatient clinic affiliated to Shiraz University of Medical Sciences between July 2017 and May 2018. All volunteers signed informed consent after explaining the study protocol. For illiterate women, informed consent from the legally authorized representatives of participants was provided. This study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1394.219). It was also registered at the Iranian Registry of Clinical Trials website (IRCT2016030526917N1).
Eligible participants met the following inclusion criteria: (1) aged 18 to 55 years old; (2) having complained of burning, itching, discharge or vaginal discomfort with a clinical diagnosis of BV, trichomoniasis or mixed vaginitis in physical examination. The exclusion criteria were use of antiparasitic, antibiotics, immunosuppressive, coumarin anticoagulants, and vaginal drugs over the past two weeks, smoking or alcohol consumption, abnormal uterine bleeding, pregnancy or lactation, liver diseases, central nervous system disease, blood dyscrasia, diabetes, immune deficiency and use of medications, that could interact with MOGS and hypersensitivity to the components of the drugs.
Randomization and allocation
The study was a parallel controlled randomized blinded clinical trial. Participants were enrolled in either MOGS or placebo or conventional drug groups by the block randomization-allocation sequence with a block size of 6. Gynecologist and the patients were blinded to the group allocation of the MOGS or placebo (same packages), but due to the unique packaging of metronidazole vaginal tablets, it was just single-blind (gynecologists were blinded). Moreover, the pathologist was also blinded to the medications that patients had used. Random numbers of participants were administered by a research coordinator from an independent institution, who is not involved in utilizing computer-generated numbers.
Intervention
The MOGS and placebo were prepared, standardized, and packaged at the Department of Phytopharmaceuticals (Traditional Pharmacy), School of Pharmacy, Shiraz University of Medical Sciences. Myrtle and oak gall samples were collected from Noorabad Mamsani, Fars province, and Khoramabad, Lorestan province, Iran, respectively. Herbarium samples were prepared and sent to the Herbarium Center of School of Pharmacy, Shiraz University of Medical Sciences. Herbarium samples were identified as Myrtus communis L. (no. 782) and Quercus infectoria G.Olivier (no. 786) by botanist S. Khademian.
For preparing extract, freeze-dried powder of 10% aq. extract of myrtle and oak gall powder were prepared. This formulation and corresponding dosage form was a simulation taken from Persian manuscripts [21–27]. HPLC analysis for quantification was carried out on a Knauer technologies model apparatus attached to Eurospher 100–5 C18 column (250 × 4.6 mm with pre-column) and connected to a photodiode array (PDA) detector. The column was equilibrated in 100% A (water with phosphoric acid, pH = 3.2)-0% B (acetonitrile), and elution was carried out with the following gradient: 100–100% A (0–3 min), 100–97% A (3–9 min), 97–94% A (9–16 min), 94–89% A (16–23 min), 89–55% A (23–33 min), 55–100% A (33–40 min). Based on the validated HPLC method, the content of gallic acid in one suppository was 276.81 ± 4.89 μg.
During optimization processing, 16 different runs were designed by Design-Expert statistical software with different two factors (PEG 600/3350 and extract concentration %) which were examined according to the responses (disintegration time, mechanical strength and particle size). In optimum condition, PEG 600 was added to the extract and homogenized by probe sonication device; and then, melted PEG 3350 was added to the mixture. This mixture was molded and sealed in polypropylene plastic molds. Placebo was prepared merely with PEG 3350 and 600.
Patients used metronidazole, MOGS, or placebo according to the same method, i.e.: a vaginal suppository for seven nights before going to bed. Before the treatment and after one week (as a follow up) the laboratory tests and physical examinations were carried out (Fig. 1). PEG 3350 (Kimiagaran-e-Emrooz, Iran), PEG 600 (Merck, Germany), Metronidazole vaginal tablets (Parsdarou, Iran), Potassium hydroxide (Merck®, Germany), pH- indicator strips (Merck®, Germany) and fixator (PadtanTeb, Iran) were used at the preparing process or clinic.
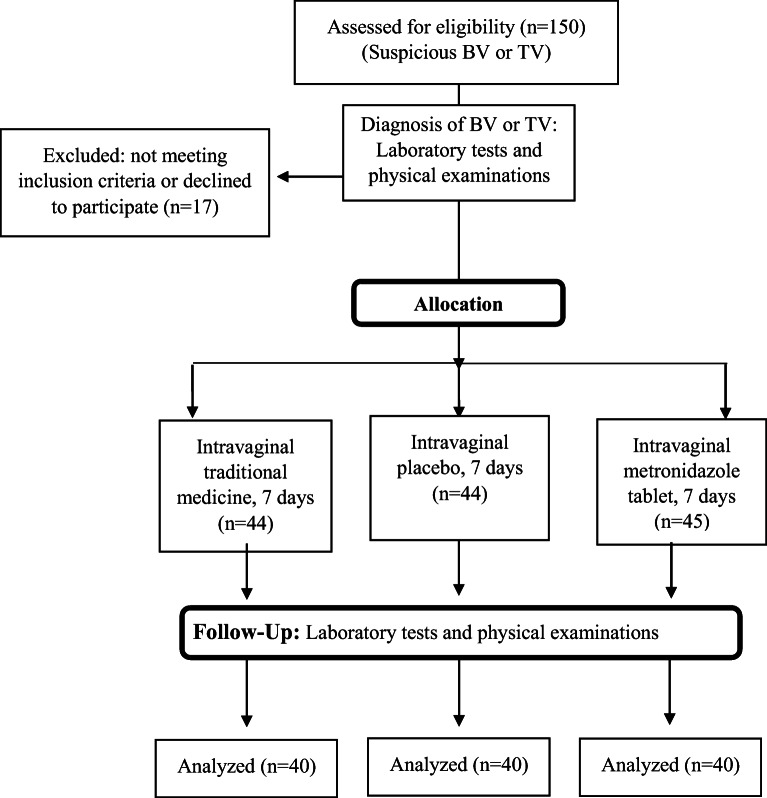
Fig. 1.
CONSORT flowchart of parallel RCT for vaginitis patients
Outcome measures
The effect of metronidazole/MOGS/placebo in the treatment of vaginitis was considered as the primary target criteria. Also, consistency of the diagnoses between laboratory and gynecologist was measured.
Statistical analysis
Considering the design of this study, the Chi-squared test (or Fisher’s exact test as applicable) used to compare the effect of MOGS vs. placebo vs. metronidazole. One way ANOVA and chi-square tests were used to compare quantitative and qualitative variables between the groups, respectively. Cohen’s κ was run to determine if there is an agreement between the diagnosis of gynecologists and laboratory. Quantitative and qualitative variables were described by mean ± S.D. and frequency (%), respectively. Kruskal-Wallis, Wilcoxon signed-rank and McNemar also were used for further analyses. All statistical analyses were performed using SPSS® version 22.0 (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered to be statistically significant.
Naranjo scale for adverse drug reaction (ADR)
Adverse drug reactions (ADR) were recorded according to Naranjo scale [28]. For that, after answering 10 questions, each ADR can get a point and categorize in one class of interpretation: definite, probable, possible and doubtful.
Results
Patients’ details
The enrollment of the patients has been shown in the CONSORT flowchart (Fig. 1). Among a total of 150 patients, 120 patients (40 patients in metronidazole arm, 40 patients in MOGS and 40 patients in the placebo arm) completed the therapeutic protocol. Seventeen patients were excluded at first stage, because they did not meet inclusion criteria or declined to participate. While, 13 patients were excluded according to the study protocol, because they did not arrive on time to the clinic for follow up or could not full fill the study. Patients took medicines correctly based on self-reporting. Tables 1 and 2 presented the distribution of quantitative and qualitative demographic factors such as age, age at marriage, BMI, number of intercourse per week, number of pregnancy, number of childbirth, job, husband education, consumption of oral probiotic products or OCP, and Pap smear. Regarding these tables, patients’ average age was about 39.12 ± 10.88 years, and the marriage age was 19.79 ± 5.03. They reported a 6-day average menstrual duration and around 26.35 ± 4.48 kg/m2 BMI. No statistically significant differences in demographic characteristics, gynecological history and characteristics of vaginitis were observed among three arms of the study (Tables 1 and 2). As a limitation, the rate of recurrence of bacterial vaginitis before the treatment in 3 different groups of randomization is not available.
Table 1.
Quantitative demographic information of parallel RCT of MGOS vs metronidazole vs placebo on vaginitis patients
| Variables | Metronidazole (mean ± S.D.) | Placebo (mean ± S.D.) |
MOGS (mean ± S.D.) |
p value |
|---|---|---|---|---|
| Age (years) | 40.58 ± 11.14 | 37.38 ± 10.81 | 39.40 ± 10.68 | 0.415 |
| Age at marriage (years) | 19.63 ± 4.93 | 20.20 ± 5.85 | 19.55 ± 4.30 | 0.821 |
| BMI (kg/m2) | 25.61 ± 4.26 | 27.62 ± 5.46 | 25.83 ± 3.72 | 0.158 |
| Husband age (years) | 45.85 ± 12.24 | 40.57 ± 10.99 | 43.73 ± 10.31 | 0.134 |
| Number of intercourse per week | 1.41 ± 0.98 | 1.53 ± 1.71 | 1.47 ± 1.21 | 0.920 |
| Number of pregnancy | 2.40 ± 1.36 | 2.45 ± 2.04 | 2.70 ± 1.59 | 0.695 |
| Number of childbirth | 2.10 ± 1.37 | 1.65 ± 1.49 | 2.20 ± 1.44 | 0.193 |
| Number of cesarean section | 0.30 ± 0.69 | 0.28 ± 0.64 | 0.50 ± 0.82 | 0.311 |
| Number of abortion | 0.28 ± 0.55 | 0.80 ± 1.62 | 0.48 ± 0.68 | 0.088 |
| Number of living children | 2.08 ± 1.37 | 1.60 ± 1.43 | 2.25 ± 1.39 | 0.102 |
| Duration of menstrual bleeding (days) | 6.73 ± 2.08 | 6.58 ± 2.49 | 6.57 ± 2.34 | 0.952 |
BMI, Body Mass Index; MOGS, Myrtle and oak gall suppository
Table 2.
Qualitative demographic information of parallel RCT of MGOS vs metronidazole vs placebo on vaginitis patients
| Variables | Metronidazole number (%) | Placebo number (%) | MOGS number (%) | p value |
|---|---|---|---|---|
| Job | 0.834 | |||
|
-Self-employment -Employee -University student / Housekeeper |
1 (2.5%) 5 (12.5%) 34 (85.0%) |
3 (7.5%) 6 (15.0%) 31 (77.5%) |
3 (7.5%) 5 (12.5%) 32 (80.0%) |
|
| Education | 0.904 | |||
|
-Illiterate -Literacy -Third grade of middle school -High school diploma -College education |
1 (2.5%) 11 (27.5%) 9 (22.5%) 11 (27.5%) 8 (20.0%) |
2 (5.0%) 9 (22.5%) 8 (20.0%) 11 (27.5%) 10 (25.0%) |
1 (2.5%) 7 (17.5%) 6 (15.0%) 16 (40.0%) 10 (25.0%) |
|
| Husband job | 0.554 | |||
|
-Self-employment -Employee -University student / Housekeeper -No husband: died or divorced |
27 (67.5%) 11 (27.5%) 1 (2.5%) 1 (2.5%) |
24 (60.0%) 11 (27.5%) 0 (0.0%) 5 (12.5%) |
26 (65%) 12 (30.0%) 0 (0.0%) 2 (5.0%) |
|
| Husband education | 0.257 | |||
|
-Illiterate -Literacy -Third grade of middle school -High school diploma -College education -No husband: died or divorced |
0 (0.0%) 6 (15.0%) 8 (20.0%) 16 (40.0%) 9 (22.5%) 1 (2.5%) |
1 (2.5%) 6 (15.0%) 7 (17.5%) 6 (15.0%) 15 (37.5%) 5 (12.5%) |
1 (2.5%) 5 (12.5%) 5 (12.5%) 15 (37.5%) 12 (30.0%) 2 (5.0%) |
|
| Menstrual interval | 0.579 | |||
|
-Regular -Irregular interval, spotting -No, postmenopausal or hysterectomy |
18 (45.0%) 14 (35.0%) 8 (20.0%) |
19 (47.5%) 15 (37.5%) 6 (15.0%) |
21 (52.5%) 9 (22.5%) 10 (25.0%) |
|
| Menstrual during study | 0.231 | |||
|
-Yes -No |
4 (10.0%) 36 (90.0%) |
7 (17.5%) 33 (82.5%) |
2 (5.0%) 38 (95.0%) |
|
| Bleeding after intercourse | 0.289 | |||
|
-No -Yes -No intercourse |
38 (95.0%) 1 (2.5%) 1 (2.5%) |
32 (80.0%) 3 (7.5%) 5 (12.5%) |
34 (85.0%) 4 (10.0%) 2 (5.0%) |
|
| Consumption of probiotic products* | 1.000 | |||
|
-No -Yes |
6 (15.0%) 34 (85.0%) |
6 (15.0%) 34 (85.0%) |
7 (17.5%) 33 (82.5%) |
|
| Pap smear | 0.508 | |||
|
-Normal -Abnormal |
30 (93.8%) 2 (6.3%) |
28 (84.8%) 5 (15.2%) |
28 (84.8%) 5 (15.2%) |
|
| OCP consumption | 0.068 | |||
|
-use -not use |
0 (0.0%) 40 (100.0%) |
2 (5.0%) 38 (95.0%) |
5 (12.5%) 35 (87.5%) |
Data are presented as number (%); * Oral consumption has been considered; MOGS: Myrtle and oak gall suppository
Sign and symptom
Table 3 shows signs and symptoms of vaginitis patients before and after intervention by metronidazole, placebo and MOGS. There were no significant differences among malodor discharge, malodor discharge after intercourse, vaginal irritation, dysuria and lower abdominal pain before the intervention (p value>0.05). However, there was a significant difference among the groups regarding itching and dyspareunia (p value = 0.045 and 0.049, respectively). Itching was more incidence reported from the beginning in the patients who were planned to take MOGS (25 patients) in comparison with metronidazole and placebo groups (14 and 18 patients, respectively). Number of patients (27 patients) reported dyspareunia in metronidazole group in comparison to placebo and MOGS groups (18 and 20 patients, respectively). After completing intervention, a significant difference was observed for malodor discharge (metronidazole better than placebo), and malodor discharge after intercourse (both metronidazole and MOGS better than placebo). In each treatment group, there was a reduction in the number of patients who suffered from malodor, malodor after intercourse, dyspareunia and lower abdominal pain after one week. MOGS and metronidazole groups showed a better relieve on irritation compared to placebo group, during one week (Table 3). Results of Table 4 showed that MOGS is the best medication in comparison with metronidazole and placebo for treating discharge in BV and TV patients.
Table 3.
Signs and symptoms of vaginitis patients before and after treatment by metronidazole, placebo and MOGS
| Variable | Metronidazole number (%) | Placebo number (%) | MOGS number (%) | p value |
|---|---|---|---|---|
| Malodor discharge | ||||
| Before | 25 (62.5%) | 29 (72.5%) | 26 (65.0%) | 0.614 |
| c | c | |||
| After | 4 (10.0%) | 14 (33.3%) | 8 (20.0%) | 0.024 |
| p value | 0.000 | 0.000 | 0.000 | |
| Malodor discharge after intercourse | 0.302 | |||
| Before | 20 (50.0%) | 19 (47.5%) | 17 (42.5%) | |
| c | ac | a | ||
| After | 0 (0.0%) | 4 (10.0%) | 0 (0.0%) | 0.000 |
| p value | 0.000 | 0.000 | 0.000 | |
| Itching | b | b | ||
| Before | 14 (35.0%) | 18 (45.0%) | 25 (62.5%) | 0.045 |
| After | 7 (17.5%) | 8 (20.0%) | 5 (12.5%) | 0.657 |
| p value | 0.065 | 0.013 | 0.000 | |
| Dyspareunia | c | c | 0.049 | |
| Before | 27 (67.5%) | 18 (45.0%) | 20 (50.0%) | |
| After | 1 (2.5%) | 1 (2.5%) | 5 (12.5%) | 0.031 |
| p value | 0.000 | 0.000 | 0.000 | |
| Vaginal irritation | ||||
| Before | 15 (37.5%) | 14 (35.0%) | 20 (50.0%) | 0.343 |
| After | 6 (15.0%) | 7 (17.5%) | 6 (15.0%) | 0.939 |
| p value | 0.012 | 0.118 | 0.001 | |
| Dysuria | ||||
| Before | 10 (25.0%) | 10 (25.0%) | 11 (27.5%) | 0.957 |
| After | 3 (7.5%) | 3 (7.5%) | 5 (12.5%) | 0.787 |
| p value | 0.039 | 0.039 | 0.109 | |
| Lower abdominal pain | ||||
| Before | 21 (52.5%) | 27 (67.5%) | 23 (57.5%) | 0.381 |
| After | 10 (25%) | 12 (30.0%) | 11 (27.5%) | 0.882 |
| p value | 0.007 | 0.001 | 0.004 | |
Data are presented as number (%); a: Differences between placebo and MOGS groups, b: Difference between metronidazole and MOGS groups, c: Difference between metronidazole and placebo groups; MOGS: Myrtle and oak gall suppository
Table 4.
Trend of discharge changes before and after treatment for BV and TV patients of parallel RCT of MGOS vs metronidazole vs placebo
| Laboratory diagnosis | Groups | Discharge* | p value | Dif-discharge (mean ± S.D.) | |
|---|---|---|---|---|---|
| Before (mean ± S.D.) | After (mean ± S.D.) |
||||
| BV = yes (n = 31) | Metronidazole (n = 11) | 2.36 ± 0.81 | 1.55 ± 0.82 | 0.024b | −0.82 ± 0.87 |
| Placebo (n = 9) | 2.33 ± 1.12 | 1.44 ± 1.01 | 0.033a | −0.89 ± 0.93 | |
| MOGS (n = 11) | 2.18 ± 1.08 | 0.64 ± 0.50 | 0.011ab | −1.55 ± 1.21 | |
| p value | 0.901 | 0.024 | – | 0.280 | |
| TV = yes (n = 106) | Metronidazole (n = 37) | 2.03 ± 0.957 | 1.35 ± 0.753 | 0.000 b | −0.68 ± 0.852 |
| Placebo (n = 37) | 2.32 ± 0.973 | 1.46 ± 0.803 | 0.000 a | −0.86 ± 0.918 | |
| MOGS (n = 32) | 2.16 ± 1.019 | 0.97 ± 0.740 | 0.000 ab | −1.19 ± 0.965 | |
| p value | 0.304 | 0.018 | 0.103 | ||
*Considered scores are 0: no discharge, 1: mild, 2: moderate and 3: severe; a: Differences between placebo and MOGS groups, b: Differences between metronidazole and MOGS groups; BV, Bacterial vaginosis; Dif, difference; MOGS, Myrtle and oak gall suppository; TV, Trichomonas vaginitis
Laboratory findings
Laboratory findings of the patients with vaginitis are presented in Tables 5 and 6, before and after intervention by metronidazole, placebo and MOGS. No statistically significant differences in laboratory diagnosis of vaginitis, at the time of enrollment including pH (abnormal for all groups), whiff test, clue cells, Nugent score, Candida albicans, Gardnerella morphotype, and Trichomonas vaginalis were observed among three groups (Tables 5 and 6). After medical treatments, pH, whiff test, and Nugent score were changed. Both MOGS and metronidazole compared to the placebo, could decrease pH and reverse whiff test results in the patients. Metronidazole showed the best effect on the Nugent score and increasing normal flora.
Table 5.
Laboratory findings of vaginitis before and after treatment by metronidazole, placebo and MOGS
| Variable | Metronidazole number (%) | Placebo number (%) | MOGS number (%) | p value |
|---|---|---|---|---|
| pH- Abnormal, >4.5 | ||||
| Before | 40 (100.0%) | 40 (100.0%) | 40 (100.0%) | – |
| b | a | ab | ||
| After | 25 (62.5%) | 24 (64.9%) | 14 (35.0%) | 0.013 |
| p value | 0.000 | 0.000 | 0.000 | |
| Whiff test- Positive | ||||
| Before | 30 (75.0%) | 30 (75.0%) | 29 (72.5%) | 0.957 |
| c | ac | a | ||
| After | 5 (12.5%) | 13 (35.1%) | 2 (5.0%) | 0.001 |
| p value | 0.000 | 0.001 | 0.000 | |
| Clue cells- Presence | ||||
| Before | 5 (12.5%) | 7 (17.5%) | 7 (17.5%) | 0.779 |
| After | 0 (0.0%) | 5 (12.5%) | 4 (10.0%) | 0.075 |
| p value | 0.063 | 0.687 | 0.508 | |
| Candida albicans- Presence | ||||
| Before | 4 (10.0%) | 4 (10.0%) | 4 (10.0%) | 1.000 |
| After | 2 (5.0%) | 3 (7.5%) | 5 (12.5%) | 0.601 |
| p value | 0.625 | 1.000 | 1.000 | |
| Gardnerella- Presence | ||||
| Before | 4 (10.0%) | 7 (17.5%) | 6 (15.0%) | 0.619 |
| After | 0 (0.0%) | 5 (12.5%) | 4 (10.0%) | 0.075 |
| p value | 0.125 | 0.687 | 0.727 | |
| Trichomonas- Presence | ||||
| Before | 6 (15.0%) | 4 (10.0%) | 2 (5.0%) | 0.387 |
| After | 0 (0.0%) | 3 (7.5%) | 1 (2.5%) | 0.322 |
| p value | 0.031 | 1.000 | 1.000 | |
Data are presented as number (%); a: Differences between placebo and MOGS groups, b: Differences between metronidazole and MOGS groups, c: Difference between metronidazole and placebo groups; MOGS, Myrtle and oak gall suppository
Table 6.
Trend of Nugent score changes for BV patients in a parallel RCT of MGOS vs metronidazole vs placebo
| Laboratory diagnosis | Groups | Nugent score* (mean ± S.D.) |
p value | Dif-Nugent (mean ± S.D.) | |
|---|---|---|---|---|---|
| Before | After | ||||
| BV = yes (n = 31) | Metronidazole (n = 11) | 2.00 ± 0.00 | 0.55 ± 0.52 | 0.003c | −1.45 ± 0.52 |
| Placebo (n = 9) | 2.00 ± 0.00 | 1.67 ± 0.71 | 0.180c | −0.33 ± 0.71 | |
| MOGS (n = 11) | 2.00 ± 0.00 | 1.18 ± 0.75 | 0.014 | −0.82 ± 0.75 | |
| p value | 1.000 | 0.005 | – | 0.005 | |
*Considered scores are 0: negative, normal flora; 1: uncertain; 2: positive, abnormal flora; c: Differences between metronidazole and placebo groups; BV, Bacterial vaginosis; Dif, difference; MOGS, Myrtle and oak gall suppository
Laboratory diagnosis
According to Table 7, there were no significant differences among three arms of the study in laboratory diagnosis at the first visit. As many of BV patients may have super/co-infection with fungi, it was not a concern to omit such patients from the study. The results showed that distribution of fungal super/co-infection in 3 different groups of the study was not statistically significant (Tables 5 and 7).
Table 7.
Laboratory diagnosis of vaginitis patients involved in RCT before and after treatment by metronidazole, placebo and MOGS
| Variable | Metronidazole number (%) | Placebo number (%) | MOGS number (%) | p value |
|---|---|---|---|---|
| BV | ||||
| Before | 11 (27.50%) | 9 (22.50%) | 11 (27.50%) | 0.840 |
| c | c | |||
| After | 3 (7.50%) | 13 (32.50%) | 9 (22.50%) | 0.021 |
| p value | 0.057 | 0.289 | 0.581 | |
| TV | ||||
| Before | 37 (92.50%) | 37 (92.50%) | 32 (80.00%) | 0.167 |
| b | a | ab | ||
| After | 18 (45.00%) | 22 (55.00%) | 6 (15.00) | 0.001 |
| p value | 0.000 | 0.000 | 0.000 | |
| Mixed | ||||
| Before | 8 (47.1%) | 6 (35.3%) | 3 (17.6%) | 0.272 |
| After |
c 0 (0.00%) |
ac 8 (20.00%) |
a 1 (2.50%) |
0.002 |
| p value | 0.008 | 0.687 | 0.625 | |
| Fungi | ||||
| Before | 3 (7.50%) | 4 (10.00%) | 4 (10.00%) | 1.000 |
| After | 2 (5.00%) | 3 (7.50%) | 5 (12.50%) | 0.601 |
| p value | 1.000 | 1.000 | 1.000 | |
| a | a | |||
| Total Cure | 19 (47.50%) | 13 (32.50%) | 26 (65.00%) | 0.014 |
Data are presented as number (%); a: Differences between placebo and MOGS groups, b: Differences between metronidazole and MOGS groups, c: Difference between metronidazole and placebo groups; BV, Bacterial vaginosis; MOGS, Myrtle and oak gall suppository; TV, Trichomonas vaginitis
Consistency and agreement of diagnosis
Table 8 shows comparison between diagnosis of vaginitis and curing from the viewpoint of gynecologist and laboratory (as a gold standard) results. For measuring agreement, Cohen’s Kappa statistic test was performed between gynecologist and laboratory diagnosis. Furthermore, sensitivity and specificity were calculated. Sensitivity presents the chance that: if the gynecologist diagnosed that a vaginitis is cured, it is confirmed by laboratory results. While, specificity shows the chance that: if presence of vaginitis is diagnosed by clinicians, it is confirmed by laboratory tests.
Table 8.
Comparison between gynecologist and laboratory diagnosis and curing of vaginitis in a parallel RCT of MGOS vs metronidazole vs placebo
| Groups Diagnosis |
Metronidazole | Placebo | MOGS | Total | |
|---|---|---|---|---|---|
| BV | Kappa* | 0.13 ± 0.09 | 0.03 ± 0.09 | 0.03 ± 0.10 | 0.07 ± 0.06 |
| specificity | 57.57% | 44.83% | 58.33% | 53.80% | |
| sensitivity | 64.28% | 59.09% | 45.00% | 55.36% | |
| TV | Kappa | 0.70 ± 0.08 | 0.62 ± 0.09 | 0.62 ± 0.09 | 0.65 ± 0.05 |
| specificity | 100.00% | 94.44% | 82.35% | 90.54% | |
| sensitivity | 76.36% | 81.82% | 83.33% | 77.63% | |
| Mix | Kappa | 0.28 ± 0.11 | 0.20 ± 0.10 | 0.00 ± 0.08 | 0.19 ± 0.06 |
| specificity | 76.39% | 65.15% | 75.00% | 72.43% | |
| sensitivity | 75.00% | 64.28% | 25.00% | 61.54% | |
| Cure | Kappa | 0.75 ± 0.10 | 0.66 ± 0.11 | 0.13 ± 0.16 | 0.57 ± 0.07 |
| specificity | 76.19% | 77.78% | 35.71% | 67.74% | |
| sensitivity | 100.00% | 100.00% | 76.92% | 89.65% | |
*Kappa value measured as mean±S.D.; BV: Bacterial vaginosis; MOGS: Myrtle and oak gall suppository; TV, Trichomonas vaginitis; Laboratory diagnosis considered as gold standard
Comparison between Amsel criteria and Nugent score
We considered Nugent scoring system plus gram staining in this study, because it has been introduced as the gold standard for the diagnosis of BV in literature. However, many limitations have been reported to this diagnostic method. Therefore, we decided to add a second bedside diagnostic method i.e.: Amsel’s criteria. Based on Amsel criteria, MOGS was able to treat the BV patients more effectively than metronidazole; however, based on the Nugent score metronidazole was more effective than MOGS (Table 9). Due to the kappa value (near zero), there was no agreement between these two diagnostic methods. Total sensitivity and specificity were 58.93% and 77.08%, respectively, and it means that Amsel criteria weren’t sufficient for the diagnosis of BV curing (Table 10).
Table 9.
Comparison between BV patients based on different diagnostic methods in a parallel RCT of MGOS vs metronidazole vs placebo
| Variable | Metronidazole number (%) | Placebo number (%) | MOGS number (%) | p value |
|---|---|---|---|---|
| Amsel | ||||
| Before | 20 (50.0%) | 21 (52.5%) | 24 (60.0%) | 0.646 |
| a | a | |||
| After | 3 (7.5%) | 7 (17.5%) | 0 (0.0%) | 0.016 |
| p value | 1.000 | 0.412 | – | |
| Nugent score | ||||
| Before | 0.456 | |||
| Uncertain | 11 (27.5%) | 8 (20.0%) | 14 (35.0%) | |
| Positive | 11 (27.0%) | 9 (22.5%) | 11 (27.5%) | |
| After | c | c | 0.035 | |
| Uncertain | 25 (62.5%) | 13 (32.5%) | 17 (42.5%) | |
| Positive | 3 (7.5%) | 13 (32.5%) | 9 (22.5%) | |
| p value | 0.306 | 0.003 | 0.337 | |
Data are presented as number (%); a: Differences between placebo and MOGS groups, c: Differences between metronidazole and placebo groups; BV, Bacterial vaginosis; MOGS, Myrtle and oak gall suppository
Table 10.
Comparison between two diagnostic methods of BV in a parallel RCT of MGOS vs metronidazole vs placebo
| Groups Diagnosis |
Metronidazole | Placebo | MOGS | Total | |
|---|---|---|---|---|---|
| BV | Kappa* | 0.03 ± 0.07 | 0.00 ± 0.07 | 0.01 ± 0.07 | 0.00 ± 0.04 |
| Specificity | 83.33% | 72.97% | 75.86% | 77.08% | |
| Sensitivity | 71.43% | 63.64% | 45.00% | 58.93% | |
*Kappa value measured as mean±S.D.; BV, Bacterial vaginosis; MOGS, Myrtle and oak gall suppository; Nugent score considered as gold standard
Naranjo scale for ADR
Only two chief complaints were reported by patients: lower abdominal pain and discharge.
Discussion
The increased resistance and hypersensitivity to conventional antibiotics for the treatment of vaginitis made us seek for new treatments from TPM. Myrtle (Myrtus communis L.) and oak gall (Quercus infectoria G.Olivier) were selected from Persian manuscripts among various natural choices for treatment of vaginitis [21–27]. There are reports on the positive effects of myrtle and oak gall on vaginitis. Recently, effects of ethanolic extract of dried oak gall on TV have shown 100% inhibition of the parasitic growth [29]. Moreover, Bhalerao (2013) reported anti-vaginitis effect of an oral Ayurvedic formulation containing gall of Q. infectoria [30]. In a clinical trial, satisfactory results of oral metronidazole plus Q. brantii vaginal cream compared to oral metronidazole plus placebo vaginal cream have been reported [31]. On the other hand, the results of one clinical trial show a statistically significant difference between a group receiving Myrtus communis L. and Berberis vulgaris L. compared to another group receiving metronidazole gel alone [32]. In addition, anti-inflammatory [33, 34] and wide range of antimicrobial activities [35–38] of these two medicinal herbs can support such anti-vaginitis activities. Therefore, a vaginal suppository (MOGS) was prepared following pharmaceutical optimization and quantification of gallic acid by HPLC.
A clinical trial was designed for evaluating the efficacy of MOGS as a novel herbal suppository containing myrtle and oak gall in the treatment of BV, TV or mixed type of vaginitis. In a parallel randomized clinical trial, 120 women suffering from vaginitis were randomly assigned to MOGS, metronidazole vaginal tablets, or placebo group by block randomization method. Considering the fact that there were no statistical differences between groups in quantitative and qualitative demographic variables, the study was well randomized. Results of the intervention revealed that metronidazole could eliminate malodor discharge better than placebo, and also both metronidazole and MOGS compared to placebo were more effective in improving malodor discharges after intercourse. Furthermore, our results showed that MOGS and metronidazole could relieve irritation more effective than placebo during a week. MOGS was (in comparison to metronidazole and placebo) the best chance to treating discharge in both BV and TV patients. Moreover, both MOGS and metronidazole could decrease pH, and reverse Whiff test results compared to placebo. Metronidazole showed the best effect on the Nugent score and increasing normal flora. Most of BV- and TV-cured women were in the metronidazole and MOGS groups, respectively. Both metronidazole and MOGS successfully treated mixed vaginitis in comparison to placebo.
According to kappa value (Table 8), there was no agreement between gynecologist and laboratory on BV diagnoses, moderate agreement for TV, mild for mixed vaginitis and moderate for curing diagnoses. This finding shows a diagnostic gap between gynecologists and laboratory. Totally, if gynecologist diagnosis was the cure for BV, it would be correct 55.36% (sensitivity), and if her diagnosis was vaginitis, it would be correct 53.80% (specificity). Total sensitivity and specificity for TV and mixed vaginitis were more adequate than BV’s (77.63%, 90.54% and 61.54%, 72.43%, respectively). Concerning gynecologists’ diagnosis in the second visit and after taking the medications, for metronidazole and placebo sensitivity of cure diagnosis was 100%, while it was 76.92% for MOGS. Considering the total specificity of 64.74%, laboratory tests are suggested before starting the clinical treatment of all patients with vaginitis. Based on Amsel criteria, MOGS was able to treat the BV patients more effectively than metronidazole. However, based on the Nugent score (as a gold standard), metronidazole was more effective than MOGS. There was no agreement between these two diagnostic methods. Total sensitivity and specificity of Amsel criteria in comparison with Nugent score (as a gold standard) were 58.93% and 77.08%, respectively. It means that Amsel criteria are not sufficient for the diagnosis of cure for BV (Table 10). As we noticed in this study, although Amsel criteria is an inexpensive and convenient method for BV diagnosis, it has poor sensitivity and not always reliable. Therefore, the alternative Nugent scoring is suggested [39, 40].
Lower abdominal pain and discharge were the chief complaints and according to Naranjo scale questions, their scores were − 2 belong to the last class of interpretation (doubtful). Lower abdominal pain and discharge were probably because of the vaginitis and dissolving dosage form in the body or its leakage.
As a probable mechanism for MOGS activity, it can be concluded from literature, that gallic acid and phenolic compounds (i.e.: hydrolysable tannins constitute of myrtle or oak gall) can damage membrane and peptidoglicans of cells, and interrupt the amino acids needed for microbial growth [41]. Myrtucommulone, a myrtle active component, also can inhibit production of prostaglandins and improve wound healing and vaginitis [42].
Conclusion
The current clinical trial showed us clinical efficacy of a standardized preparation of TPM suppository (PEG base containing myrtle and oak gall) particularly for TV compared to metronidazole, without major complications and side effects. All in all, MOGS would be a chance for running larger size clinical trials to achieving new treatment for at least Trichomonas vaginitis.
Abbreviations
- ADR
Adverse Drug Reaction
- BMI
Body Mass Index
- BV
Bacterial vaginosis
- CAM
Complementary and Alternative Medicine
- Dif
difference
- MOGS
Myrtle and Oak Gall suppository
- PEG
Polyethylene glycol
- TPM
Traditional Persian Medicine
- TV
Trichomonas vaginitis
Authors’ contributions
Design of the study, B.N.J., A.D., A.A., P.B. and A.M.; Lab work and acquisition clinical data, S.F.A.; Drafting the article and revising, S.F.A., B.N.J., M.T., A.D., A.A. and A.M.; Supervision, B.N.J., A.D., P.B., A.Z., A.A. and A.M. All authors are in agreement with the content of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Festschrift in honor of late Professor Dr. Seyed Hadi Samsam-Shariat, Isfahan School of Pharmacy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mills BB. Vaginitis: beyond the basics. Obstet Gynecol Clin N Am. 2017;44:159–177. doi: 10.1016/j.ogc.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Physician. 2018;97(5):321–329. [PubMed] [Google Scholar]
- 3.Bautista CT, Wurapa E, Sateren WB, Morris S, Hollingsworth B, Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res. 2016;3. 10.1186/s40779-016-0074-5. [DOI] [PMC free article] [PubMed]
- 4.van Schalkwyk J, Yudin MH, Allen V, Bouchard C, Boucher M, Boucoiran I, et al. Vulvovaginitis: screening for and management of trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis. J Obstet Gynaecol Can. 2015;37:266–274. doi: 10.1016/S1701-2163(15)30316-9. [DOI] [PubMed] [Google Scholar]
- 5.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 6.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendation and Reports. 2015;64(3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Ifeanyi OE, Chinedum OK, Chijioke UO. Trichomonas vaginalis: complications and treatment. International Journal of Current Research in Medical Sciences. 2018; 10.22192/ijcrms.2018.04.05.012.
- 8.Wølner-Hanssen P, Krieger JN, Stevens CE, Kiviat NB, Koutsky L, Critchlow C, DeRouen T, Hillier S, Holmes KK. Clinical manifestations of vaginal trichomoniasis. JAMA. 1989;261:571–576. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]
- 9.Singh J, Kalia N, Kaur M. Recurrent vulvovaginal infections: etiology, diagnosis, treatment and management. In: Singh P, editor. Infectious diseases and your health. Singapore: Springer; 2018. pp. 257–289. [Google Scholar]
- 10.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards T, Burke P, Smalley H, Hobbs G. Trichomonas vaginalis: clinical relevance, pathogenicity and diagnosis. Crit Rev Microbiol. 2016:1–12. 10.3109/1040841X.2014.958050. [DOI] [PubMed]
- 12.Muzny CA, Schwebke JR. The clinical spectrum of Trichomonas vaginalis infection and challenges to management. Sex Transm Infect. 2013;89:423–425. doi: 10.1136/sextrans-2012-050893. [DOI] [PubMed] [Google Scholar]
- 13.Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. Journal of Lower Genital Tract Diseas. 2014; 10.1097/LGT.0b013e31829156ec. [DOI] [PubMed]
- 14.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 15.Obiero J, Rulisa S, Ogongo P, Wiysonge CS. Nifuratel-Nystatin combination for the treatment of mixed infections of bacterial vaginosis, vulvovaginal candidiasis, and trichomonal vaginitis. The Cochrane Database of Systematic Reviews. 2018. 10.1002/14651858.CD013012.
- 16.Mahboubi M. Systematic review: the potency of Zataria multiflora Boiss. in treatment of vaginal infections. Infectio. 2018; 10.22354/in.v22i2.712.
- 17.Nyirjesy P, Robinson J, Mathew L, Lev-Sagie A, Reyes I, Culhane JF. Alternative therapies in women with chronic vaginitis. Obstet Gynecol. 2011;117:856–861. doi: 10.1097/AOG.0b013e31820b07d5. [DOI] [PubMed] [Google Scholar]
- 18.Felix TC, de Brito Röder DVD, dos Santos Pedroso R. Alternative and complementary therapies for vulvovaginal candidiasis. Folia Microbiol (Praha) 2018;64:133–141. doi: 10.1007/s12223-018-0652-x. [DOI] [PubMed] [Google Scholar]
- 19.Askari SF, Mohagheghzadeh A, Azadi A, Namavar Jahromi B, Tansaz M, Badr P. A brief review on vaginal drug delivery in traditional Persian medicine. Traditional and Integrative Medicine. 2018;3(4):224–230. [Google Scholar]
- 20.Askari SF, Azadi A, Namavar Jahromi B, Tansaz M, MirzapourNasiri A, Mohagheghzadeh A, Badr P. A comprehensive review about Quercus infectoria G. Olivier Gall Research Journal of Pharmacognosy. 2019;7(1):69–77. [Google Scholar]
- 21.Ghaeni Heravi SM. Qarabadin-e salehi. Badr P, Mohagheghzadeh a, shams Ardekani MR, editors. Chogan pub: Tehran; 2013. [Google Scholar]
- 22.Aghili Shirazi MH. Qarabadin-e kabir. Research Institute for Islamic and Complementary Medicine: Tehran; 2007. [Google Scholar]
- 23.Azam KM. Qarabadin-e azam. Almai: Tehran; 2014. [Google Scholar]
- 24.Avicenna H. The canon of medicine. Sharafkandi a, translator. Tehran: Soroush Press; 1997. [Google Scholar]
- 25.Tonkaboni MM. Tohfat almomenin. Rahimi R, shams Ardekani MR, Farjadmand F, editors. Tehran: Shahid Beheshti University of Medical Sciences; 2007. [Google Scholar]
- 26.Aghili MH. Makhzan aladvia. Shams Ardakani MR, Rahimi R, Farjadmand F, editors. Tehran: Tehran University of Medical Sciences pub; 2009. [Google Scholar]
- 27.Mansouri SM. Kefaye Mansouri. Research Institute for Islamic and Complementary Medicine: Tehran; 2003. [Google Scholar]
- 28.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts E, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 29.Ardestani MM, Aliahmadi A, Toliat T, Dalimi A, Momeni Z, Rahimi R. Antimicrobial activity of Quercus infectoria gall and its active constituent, gallic acid, against vaginal pathogens. Traditional and Integrative Medicine. 2019;4(1):12–21. [Google Scholar]
- 30.Bhalerao S, Mohite S, Kulkarni S, Deo V, Shrikhande B. Effect of Lucronil® tablets in the patients with leucorrhea: an open clinical trial. International Journal of Science and Research. 2016;5(10):1338–1342. [Google Scholar]
- 31.Zare A, Moshfeghy Z, Zarshenas MM, Namavar Jahromi B, Akbarzadeh M, Sayadi M. Quercus brantii Lindl. Vaginal cream versus placebo on Bacterial Vaginosis: A randomized clinical trial. Quercus brantii Lindl Vaginal cream versus placebo on bacterial vaginosis: a randomized clinical trial Journal of Herbal Medicine. 2019;16:100247. 10.1016/j.hermed.2018.11.003.
- 32.Masoudi M, Miraj S, Rafieian-Kopaei M. Comparison of the effects of Myrtus communis L, Berberis vulgaris and metronidazole vaginal gel alone for the treatment of bacterial vaginosis. J Clin Diagn Res. 2016. 10.7860/JCDR/2016/17211.7392. [DOI] [PMC free article] [PubMed]
- 33.Viana AFSC, da Silva FV, Fernandes HDB, Oliveira IS, Braga MA, Nunes PIG, et al. Gastroprotective effect of (−)-myrtenol against ethanol-induced acute gastric lesions: possible mechanisms. J Pharm Pharmacol. 2016;68:1085–1092. doi: 10.1111/jphp.12583. [DOI] [PubMed] [Google Scholar]
- 34.Kaur G, Hamid H, Ali A, Alam MS, Athar M. Antiinflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol. 2004;90:285–292. doi: 10.1016/j.jep.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Darogha SN. Antibacterial activity of Quercus infectoria extracts against bacterial isolated from wound infection. Journal of Kirkuk University Scientific Studies. 2009;4(1):20–30. [Google Scholar]
- 36.Basri D, Ha F, Jantan I. Antibacterial activity of the galls of Quercus infectoria. Malaysian Journal of Science. 2005;24(1):257–262. [Google Scholar]
- 37.Alem G, Mekonnen Y, Tiruneh M, Mulu A. In vitro antibacterial activity of crude preparation of myrtle (Myrtus communis) on common human pathogens. Ethiop Med J. 2008;46(1):63–69. [PubMed] [Google Scholar]
- 38.Mansouri S, Foroumadi A, Ghaneie T, Najar AG. Antibacterial activity of the crude extracts and fractionated constituents of Myrtus communis. Pharm Biol. 2001;39:399–401. doi: 10.1076/phbi.39.5.399.5889. [DOI] [Google Scholar]
- 39.Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1996;88:573–576. doi: 10.1016/0029-7844(96)00233-5. [DOI] [PubMed] [Google Scholar]
- 40.Modak T, Arora P, Agnes C, Ray R, Goswami S, Ghosh P, Das NK. Diagnosis of bacterial vaginosis in cases of abnormal vaginal discharge: comparison of clinical and microbiological criteria. The Journal of Infection in Developing Countries. 2011;5(5):353–360. doi: 10.3855/jidc.1153. [DOI] [PubMed] [Google Scholar]
- 41.Mahmoudvand H, Badparva E, Baharvand Z, Salehi LH. Anti-trichomonas vaginalis activities and apoptotic effects of some Iranian medicinal plants. Trop Biomed. 2018;35(2):347–353. [PubMed] [Google Scholar]
- 42.Khalilzadeh S, Eftekhar T, Rahimi R, Mehriardestani M, Tabarrai M. An evidence-based review of medicinal plants used for the treatment of vaginitis by Avicenna in “the Canon of Medicine”. Galen Medical Journal. 2019; 10.31661/gmj.v8i0.1270. [DOI] [PMC free article] [PubMed]