Abstract
Background
Diabetes mellitus (DM) is a well-known clinical entity with various late complications. There is a surge of research aiming to use the medical herb in the management of DM.
Objective
This study aimed to investigate whether the alleviation of DM by an isolated compound from Rosa canina is mediated by DNA methylation in STZ-diabetic rats.
Methods
Sixty adult Wistar male rats were classified into control, diabetic and treatment groups. Rats were treated with STZ (40 mg/kg), metformin (500 mg/kg), and oligosaccharide fraction (OF; 10, 20 and 30 mg/kg) isolated from Rosa canina. DNA was extracted from the blood and pancreas to determine DNA methylation using the Global DNA Methylation kit. The expressions of DNA methyltransferases (Dnmts), PDX1, Ins1, GCK and PTP1B2 were determined by using qRT-PCR.
Results
The significant blood glucose-lowering potential of OF was associated with a reduced level of global DNA methylation (p < 0.05). The expression levels of Dnmts 1 and 3α increased in the pancreas and blood from diabetic rats compared to control group which declined by OF treatment (p < 0.05). Paradoxically, the expression of Dnmt 3β augmented in the pancreas and blood of OF group compared to diabetic ones (p < 0.05). Besides, the expressions of Pdx1, PTP1B2, Ins1 and GCK increased in OF-treated rats compared to diabetic groups.
Conclusion
Results revealed that DNA methylation plays a causal role in the effectiveness of the isolated OF. Furthermore, the possible regenerative potential of oligosaccharide in diabetic rats may have contributed to the modulation of DNA methylation.
Graphical abstract
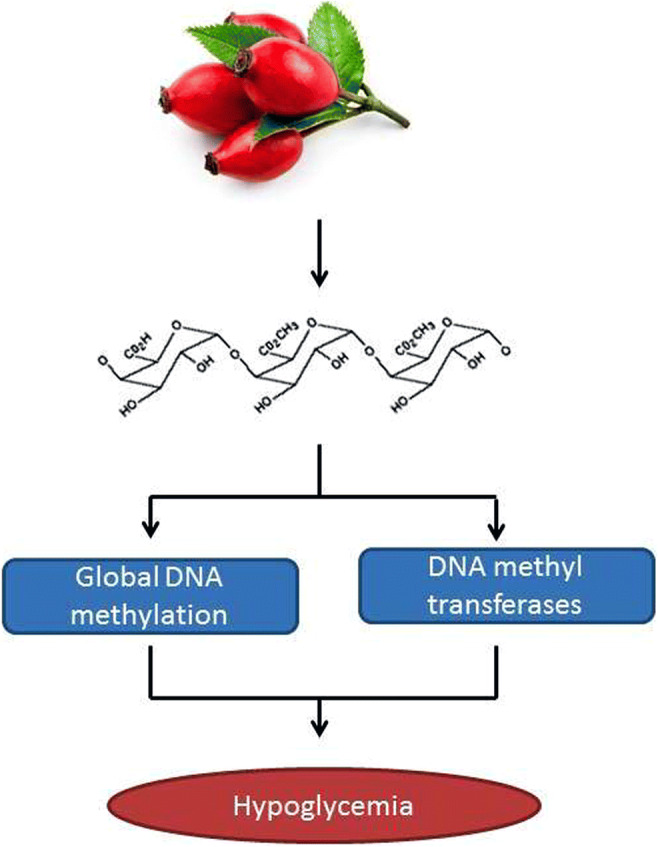
Electronic supplementary material
The online version of this article (10.1007/s40199-020-00363-8) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus, Oligosaccharide, Rosa canina, DNA methylation, DNA methyltransferase, Regeneration
Introduction
Diabetes mellitus (DM) is a group of heterogenic metabolic disorders characterized by glucose intolerance resulting from pancreatic β-cells’ dysfunction. Impairment of pancreatic β-cells is along with prolonged deregulation of insulin and development of macrovascular and microvascular complications. Globally, the prevalence of DM is continuing to rise over 300 million people by 2025 [1]. It is believed that a complex interplay among environmental factors, genetic modifications and epigenetic variations implicates in the progression and the susceptibility of DM. However, the exact molecular mechanism of DM pathogenesis has only partially been understood.
DNA methylation as an epigenetic mechanism is a covalent modification of the DNA sequence with a causal effect on gene expression and genomic stability [2]. DNA methylation is mediated by DNA methyltransferases (DNMTs) that transfer a methyl group from S-adenosyl-methionine to the 5′ position of the cytosine ring of DNA and generate 5-methyl-cytosine [3–5]. In this regard, DNA methylation through modulation of DNA accessibility regulates the expression of associated genes [6]. Over the past few years, extensive studies have revealed the involvement of an altered pattern of DNA methylation in diabetic humans and animal models. For instance, genomic DNA methylation patterns in islets from type 2 diabetic patients showed the increased level of methylated DNA and the decreased expression of related genes compared to non-diabetic ones (13). Hypermethylated CpG sites have been identified in defense system and immune response pathways in type 1 diabetes-affected twins [7]. Owing to the differential methylation pattern in diabetic patients, it seems that targeting of methylation may be an effective approach in the treatment of DM.
Medical herbs have long been used in traditional medicine to treat DM and its complications [8]. Natural products isolated from plants are known to play a causal role in pharmaceutical biology [9]. To reduce the deleterious side effects and enhance the efficacy of medical herbs, it is required to find out the main ingredient and scrutinize their molecular mechanisms in the management of DM. Rosa canina (R. canina; rose hip) is one of the well-known medicinal plants traditionally used for various metabolic and inflammatory diseases with no obvious cytotoxic effects on the growth of cancer cell lines [10, 11]. The anti-diabetic effects of R. canina have been undertaken in a number of studies [12–16]. One of the extracted fraction of R. canina is a novel oligosaccharide composed of the highly methylated and acetylated galacturan and arabinan units [17].
Therefore, this study aimed to evaluate whether oligosaccharide fraction isolated from R caninia plays any role in the alteration of the whole methylation genome profile and the expression of DNA methyltransferases in diabetic rats.
Materials and methods
Materials
Streptozotocin (STZ) was purchased from Sigma Aldrich (St Loui, MO), Insulin and Ki67 antibodies, Biotin Blocking System, Liquid DAB + Substrate Chromogen System, EnVision, Dual Link System, and Target Retrieval Solution were provided from DAKO (Denmark). Hydrogen peroxide, Methyl alcohol, Entelan glue, and Ethyl alcohol 99.6% were supplied from Merck (Germany). DNA extraction kit was purchased from SinaPure™DNAKit (Iran), RiboEX ™ RNA extraction kit was provided from GeneAll (Korea), Methylated DNA Quantification Kit, RiboExLS kit from Epigentek (USA), cDNA synthesis kit was obtained from GeneAll (Korea). The RT Premix, qPCR Master Mix, and primers were purchased from Bioneer (Daejeon, Korea).
Extraction of oligosaccharide fraction
Ripe fruits of R. canina were collected from different parts of Kermanshah province in western Iran during Nov-Dec. The collected fruits were dried and its powder was added to de-ionized water to get an extract. The extract was filtered and further subjected to a column of silica gel of G-60. Then, the collected eluent was partitioned two times against a triple volume of ethanol. To further purification, fraction passed over a flash column chromatography with a sephadex LH20 membrane and a column with a silica gel RP-18 membrane to provide oligosaccharide fraction. The purified oligosaccharide was analyzed using a high performance liquid chromatography diode array detector tandem mass spectroscopy (HPLC-DAD MS/MS), infra-red radiation (IR) and nuclear magnetic resonance (NMR) to analyze the structure and the composition of the fraction.
Animals and experimental design
Eight-week-old male Wistar rats, weighing from 200 to 250 g, were purchased from Pasteur Institute (Tehran, Iran). They had free access to food and water and were maintained at a room temperature of 22 °C with 12:12 h light: dark cycle and 45–50% relative humidity. The animal study was reviewed and accepted by ethics committees from the National Institute from Medical Research development (NIMAD) with the number of certification IR NIMAD REC 1396 262 (26-11-2017). Hence, the care and treatment of the animals were done in accordance with the principles of laboratory animal care (National Institutes of Health) and Iran National Committee for Ethics in Biomedical Research (http://ethics.research.ac.ir). The rats were segregated randomly into 6 groups (10 Rats in each group) including untreated (control), diabetic, metformin-treated (positive control) and three treatment groups were taken three different doses of oligosaccharide fraction from Rosa canina, respectively. The control group was given orally water. DM was induced in the animals except for the controls by a single intraperitoneal (i.p.) injection of STZ (40 mg/kg) freshly dissolved in 5 mmol/L citrate buffer (pH 4.5). After 72 h, the level of glucose was measured using a GlucoDr glucometer (allmedicus; Germany) by the sampling of blood from the tail vein of the animals and blood sugar ranging from 250 to 400 mg/dl was considered as positive. The rats in oligosaccharide-treated groups were given three doses (10, 20 and 30 mg/kg) of the isolated oligosaccharide dissolved in distilled water twice daily for 4 and 8 weeks starting two days after STZ injection. Positive controls received metformin (500 mg/kg/day) for the same period. All the treatments were done by oral gavage and stopped after 4 and 8 weeks. Three randomly-selected cured rats in each group were anesthetized by diethyl ether and killed to collect blood and pancreatic samples. Oral glucose tolerance test was assayed by measuring blood glucose after 0, 60, and 90 min administration of 5 mg/kg glucose following 2 h finalization of the treatment period.
Hematoxylin and eosin (H&E) staining
To visualize the regenerative effect of OF in diabetic rats, the pancreatic tissues of 20 mg/kg OF-treated and diabetic rats were dissected and stained by using hematoxylin/eosin (H&E). Paraffin-embedded tissues were provided as 4 μm sections and stained with hematoxylin and eosin (H&E) method. In this method, glass slides containing tissue sections incubated at 70 °C for 2 h. Then, slides rinsed into several jars filled with xylene, graded series of ethanol solutions, hematoxylin, lithium carbonate and eosin. Stained sections were independently evaluated by two pathologists [18].
Immunohistochemistry
Immunohistological staining was performed on formalin-fixed paraffin-embedded tissue sections using antibodies against insulin and Ki67. For this aim, 4 μm tissue sections were deparaffinized at 37 °C for 2 h and Xylene for 24 h. To retrieve antigens, slides immersed in the jar containing Tris buffer (pH = 9) and heated in a water bath at 95 °C for 20 min followed by washing in PBS solution. To quench the intracellular activity of peroxidases, slides were immersed in a solution of 3% Hydrogen peroxide in methanol for 10 min, washed with PBS and placed in jars containing avidin solution for 5 min. After washing with PBS, slides were incubated by primary and secondary antibodies in a humid and dark place at room temperature. The slides were washed in PBS and stained with the substrate-chromogen solution known as 3,3′-diaminobenzidinetetra-hydrochloride (DAB) for 5 min. The counterstaining was performed with hematoxylin for 30 s and washed in water. Then, slides mounted to study under a microscope. Negative controls were exposed to antibody diluent replacing primary antibody [19].
DNA extraction and global DNA methylation
To examine the involvement of DNA methylation in the anti-diabetic effect of OF, global DNA methylation of OF-treated and untreated diabetic rats were examined using MethylFlash™ global DNA methylation (5-mc) ELISA easy kit. For this aim, DNA from treated and untreated groups was extracted from pancreas tissue and peripheral blood samples using a standard protocol. Briefly, DNA was extracted by salting out and chloroform, precipitated by ethanol and then, solvated in ddH2O as well as SinaPure™DNA Kit. DNA purity and concentration were evaluated by a NanoDrop 2000/2000c spectrophotometer (Thermo Scientific, USA). The methylation status of DNA was determined using MethylFlash Global DNA Methylation (5-mC) ELISA easy kit as a Methylated DNA Quantification Kit (Epigentek) [20].
RNA isolation, reverse transcription and quantitative real-time quantitative PCR (qRT-qPCR)
Total RNA was isolated from the peripheral blood and isolated pancreas of each rat using the RiboExLS kit (GeneAll, Korea) according to the manufacturer’s protocol. Extracted RNA (2 μg) in a 20-μl reaction volume was reverse transcribed using the Prime ScriptTMTRT kit (Takara BioInc., Otsu, Japan) in the presence of random hexamer. Quantitative real-time polymerase chain reaction (RT-qPCR) was performed with a light cycler instrument (Applied biosystem, Singapore) using SYBR premix Ex Taq technology (Takara BioInc., Otsu, Japan) kit.
Thermal cycling conditions comprised an initial activation step for 30 s at 95 °C followed by 38 cycles, including a denaturation step for 15 s at 60 °C. Melting curves were analyzed to validate a single PCR product of each primer. The following primers were used for SYBR Green-based real-time PCR: GAPDH forward: 5′-GCGTCCACCCGCGAGTACAA-3′ and GAPDH reverse: 5′-ACATGCCGGAGCCGTTGTCG-3′. Dnmt3α forward: CTGGAACACGGCAGAATAGC and Dnmt3α reverse: CGAAGAGGTGGCGGATGAC. Dnmt3β forward: 5′-GGGCCGCTACCACGTTCAGG −3′ and Dnmt3β reverse: 5′-AGGGCCGTCCTGGCTCAAGT −3′; Dnmt1 forward: 5′-CCGGCAACATGGCCTCAGGG-3′ and Dnmt1 reverse: 5′-CCGGCAACATGGCCTCAGGG −3′. Primers for GCK, Ins, Ptp1b2 and Pdx1 were prepared from Qiagen. Comparative quantitation analysis (2–Δ(ΔCt) method) was used to determine fold change in gene expression and normalized to the housekeeping gene, GAPDH, which has been validated as the housekeeping gene for current studies [21, 22].
Statistical analysis
Statistical analyses were carried out by SPSS19 software (SPSS/PC-19.SPSS Inc., Chicago, IL, USA). The data expressed as mean ± SEM. The one-way analysis of variance (ANOVA) followed by Turkey’s post-hoc test was used for the analysis of data. All values were expressed as means±standard deviation (SD). Each experiment was done in triplicate. The p < 0.05 was considered statistically significant.
Results
Determination and evaluation of the active reagent extracted from Rosa canina
After extraction of purified oligosaccharide from ripe fruits of R. canina, structural analyses including HPLC-DAD MS/MS, IR and NMR were performed to distinguish the composition of the oligosaccharide fraction. Results showed that isolated fraction is a pectin derivative composed of a repeated tetrasacchrides with several acetyl and methoxy carbonyl groups (Fig. 1S). The structural and functional characterization of isolated oligosaccharide (Fig. 2S) was reported in the United States patent with the following address (patent No: US9,296,831B2; https://patents.justia.com/inventor/gholamreza-bahrami) [17, 23].
Effect of oligosaccharide fraction (OF) on the body mass and level of plasma glucose in diabetic rats
The effects of oligosaccharide fraction (OF) from R. canina on the whole body weight and blood glucose levels were measured in OF-treated and untreated diabetic rats after 4- and 8-week (4 W and 8 W) treatment (Table 1). The average body mass of the diabetic group was significantly decreased relative to the untreated control groups after 4 and 8 weeks (p < 0.05). Increased body weight in OF-group (20 mg/kg) was significantly higher than the diabetic group after 4 W and 8 W treatment (p < 0.05). At the end of 8 W treatment, rats were fed with ddH2O for two weeks and blood was sampled from rats. Diabetic rats showed a significant increase in the level of blood sugar compared to control group (p < 0.05). The levels of blood glucose significantly decreased in OF-treated groups (20 and 30 mg/kg) and Met group (metformin-treated group) compared to diabetic rats (p < 0.05). The decreased level of blood glucose in OF (20 mg/kg) group implying the hypoglycemic effect of OF at the dose of 20 mg/kg in diabetic rats. The protective effect of OF on the tolerance of oral glucose in diabetic rats was shown in Table 1S.
Table 1.
Effect of OF from Rosa canina on the level of Body mass and Blood glucose
| Group | Body mass (g) | Blood Glucose (mg/dl) | ||||
|---|---|---|---|---|---|---|
| 0 | 4 Week | 8 Week | 72 h | 4 Week | 8 Week | |
| Ctrl | 290.5 ± 6.00 | 298.75 ± 6.66 | 324.5 ± 12.69 | 91 ± 2.58# | 94.75 ± 2.17# | 97 ± 1.58# |
| Dia | 281 ± 7.94 | 262.5 ± 3.38* | 231.5 ± 6.56* | 373.5 ± 14.06* | 662.25 ± 22.67* | 759.5 ± 12.18* |
| OF (10 mg/kg) | 290.33 ± 8.67 | 302 ± 10.15# | 325 ± 10.47# | 406.10 ± 12.64* | 242.33 ± 42.33*# | 177 ± 28.75# |
| OF (20 mg/kg) | 286.8 ± 6.94 | 307.71 ± 7.46# | 331.57 ± 19.31# | 365.86 ± 13.46* | 198.43 ± 27.12 # | 122.14 ± 12.11# |
| OF (30 mg/kg) | 290.57 ± 7.44 | 291.71 ± 6.83 | 313.14 ± 20.05 | 364.29 ± 11.70* | 297.29 ± 22.16*# | 209.71 ± 39.63# |
| Met (500 mg/kg) | 283.29 ± 4.27 | 272.71 ± 6.31 | 304.57 ± 21.28 | 402.29 ± 14.55* | 227.57 ± 25.41*# | 205.71 ± 39.49# |
Ctrl: control (No treatment); Dia: Diabetic group; OF: Oligosaccharide fraction-treated diabetic group; Met: Metformin -treated diabetic group (Positive control)
Data are presented as mean ± SE. * p < 0.05 compared to Ctrl group. # p < 0.05 compared to Dia group
Effect of OF on the number of Langerhans islets
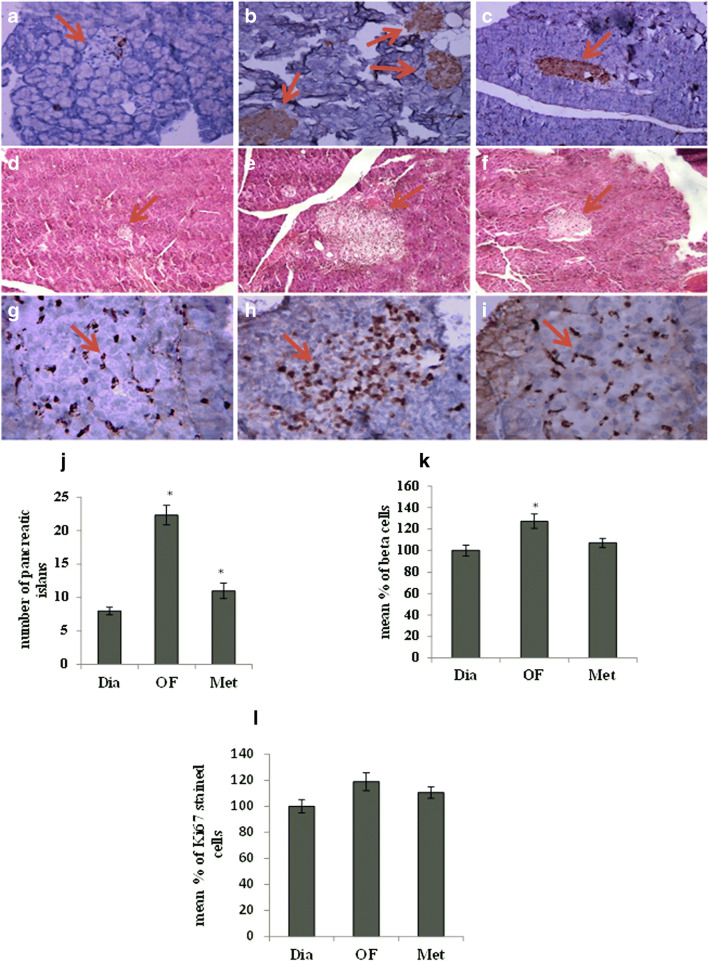
The pancreas from diabetic rats showed a small size relative to untreated control group. Microscopic evaluation of pancreatic tissues clarified a significant decrease in the number of Langerhans islets and β-cells in diabetic group relative to untreated control rats which significantly increased in 20 mg/kg OF group (p < 0.05) in both staining methods. Interestingly, the number of islets and beta cells were not significantly different from diabetic rats (Figs. 1 A-F and J-K). In addition, the proliferative potential of pancreatic cells was evaluated using Ki67 immunohistochemistry, indicating the slight increase in stained cells of pancreatic islets of OF-treated group compared to diabetic rats with no difference statistically (p > 0.05) (Fig. 1 G-I and L).
Fig. 1.
Morphological characterization and immunohistochemistry staining of rat pancreas. Insulin immunohistochemistry staining in pancreas from diabetic (A), OF (20 mg/ml) (B) and metformin (500 mg/ml) (C)- treated rats after 8 weeks treatment. H&E staining of pancreatic tissues from diabetic (D), OF (20 mg/ml) (E) and metformin (500 mg/ml) (F)-treated rats after 8 weeks treatment. Ki67 immunohistochemistry staining of pancreatic tissues from diabetic (G), OF (20 mg/ml) (H) and metformin (500 mg/ml) (I) -treated rats. The number of islets (J), beta cells (K) and Ki67 (L) stained nucleus was counted under microscope in at least 3 fields. IHC and H&E staining by magnification of 40X in pancreatic tissues
Effect of OF on global DNA methylation
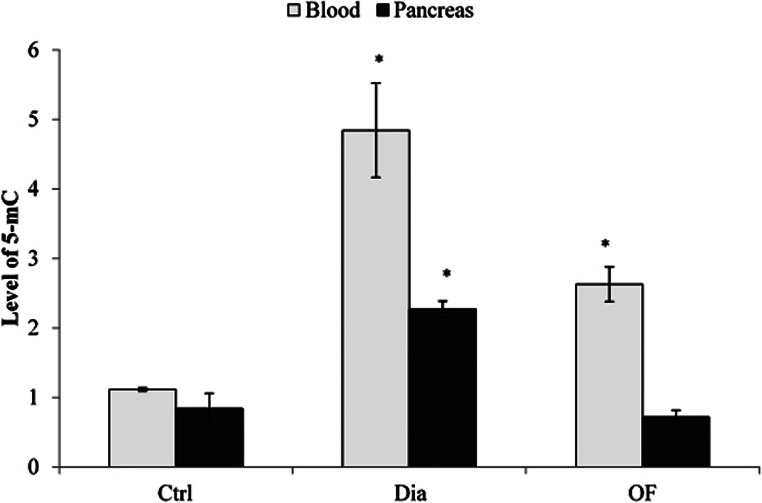
According to Fig. 2, the content of methylated DNA in the blood of diabetic rats increased to 4.84-fold relative to the control group (p < 0.05). However, the 8 W treatment of diabetic rats with 20 mg/kg OF reduced the level of DNA methylation in the blood to 2.63 fold compared to untreated diabetic ones (p < 0.05). In the pancreas, the level of methylated DNA increased to nearly 2.26-fold compared to control group (p < 0.05). Meanwhile, OF treatment led to a 3-fold reduction in the level of DNA methylation relative to diabetic rats.
Fig. 2.
The content of global methylated DNA (5-mC) in the blood and pancreas of control (ctrl), diabetic (Dia) and OF (20 mg/ml)- treated rats. After 8 weeks treatment, DNA was extracted from the blood and pancreas of rats and DNA methylation status was measured using MethylFlash™ global DNA methylation (5-mc) ELISA easy kit. Control, ctrl; diabetic, Dia; and OF, Oligosaccharide fraction. The results are the mean ± SD of triplicate experiment (P < 0.05). *, significantly different from control group
Effect of OF on the expression of DNA methyltransferases
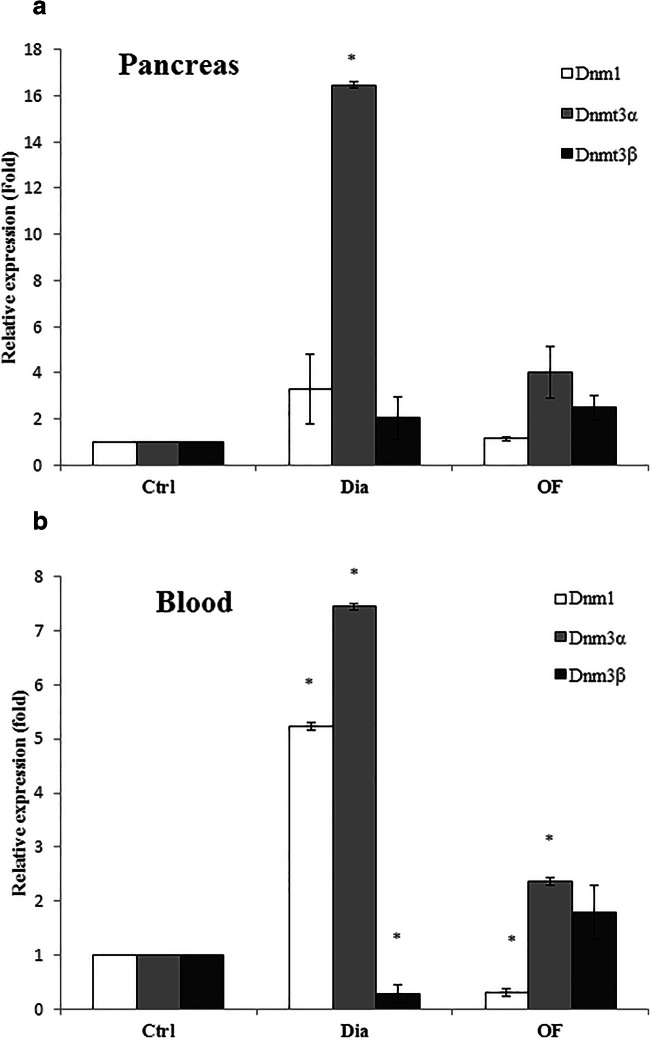
To examine whether the altered methylation profile of DNA is related to the impact of OF on the expression of DNA methyltransferases, the expression of Dnmt1, Dnmt3α and Dnmt3β were analyzed in the pancreas and blood of OF-treated and untreated diabetic groups. As indicated in Fig. 3a, the increased expression of Dnmt3β to about 1.79-fold and decreased expression of Dnmt1 and Dnmt3α to 0.31 and 2.36 fold, respectively, were observed in the blood of OF-treated group relative to diabetic rats (p < 0.05). However, the expression of Dnmt1, Dnmt3α and Dnmt3β reduced nearly to 1.15, 4.01 and 2.51, respectively, in the pancreas of diabetic rats exposed to OF compared to those (3.30, 16.46 and 2.40) in diabetic group (Fig. 3b).
Fig. 3.
The expression levels of DNA methyltransferases (Dnmt1, Dnmt3α and Dnmt3β). RNA was extracted from the peripheral blood (A) and the pancreas (B) of OF (20 mg/kg)- treated and untreated diabetic rats and the expression of DNA methyltransferases were analyzed relative to control group using quantitative real time PCR (qRT-PCR). The results are the mean ± SD of triplicate experiment (P < 0.05). *, significantly different from control group
Effect of OF on the expression of genes involving in the pancreatic β-cell function
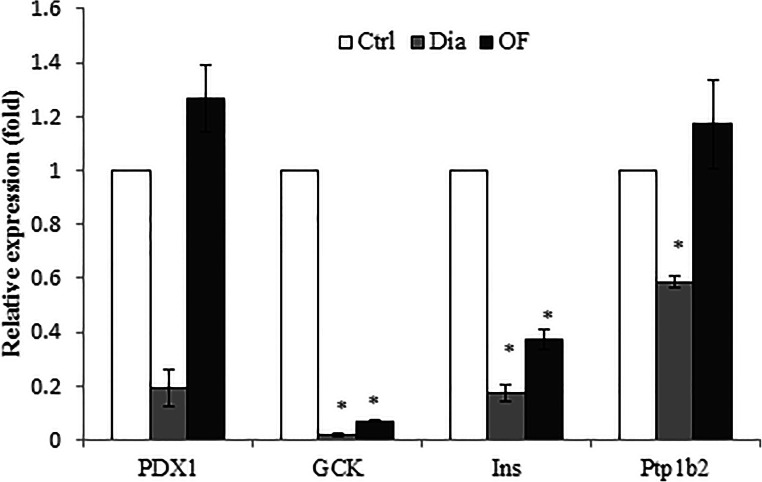
We further tested whether the level of some factors involving in the regeneration and function of β-cells, especially factors associated with regeneration such as Pdx1 and PTP1B2, are changed by administration of 20 mg/kg OF. The expression level of Ins1, GCK, and PTP1B2 significantly decreased in diabetic rats compared to control group (p < 0.05). As shown in Fig. 4, the expression of Pdx1 and PTP1B2 as well as two Pdx1 targets, insulin (Ins1) and glucokinase (GCK) increased to about 10, and 3 fold as well as 1.85 and 1.77 fold in OF-treated group, respectively, relative to diabetic group.
Fig. 4.
The expression level of genes involved in development of pancreatic β-cells in in diabetic and OF (20 mg/kg)-treated diabetic rats. qRT-PCR was performed to evaluate the expression of pancreatic transcription factor (PDX1), glucokinase (GCK), insulin (INS) and Non receptor Protein tyrosine phosphatase 2 (PTP1B2) relative to β-catenin. The results are the mean ± SD of triplicate experiment (P < 0.05). *, significantly different from control group
Discussion
Despite the wealth of studies regarding the pathogenesis of DM and novel therapeutic strategies in recent years, the molecular mechanisms behind the pathogenesis of DM and the anti-diabetic action of drugs have remained largely unknown. In this study, we aimed to introduce the active reagent from the medical herb, R. canina, and capitalized its strength on the global DNA methylation in diabetic rats. After several attempts by using HPLC and mass spectroscopy, results indicated that an oligosaccharide with the modified pectin structure should be the active reagent of Rosa canina. Treatment of STZ-treated diabetic rats for 4 weeks showed the promising reducing blood glucose effects of oligosaccharide fraction at the concentration of 20 mg/kg body weight of rats. Further, pathologic examination of pancreatic tissues implied that the significant blood glucose-lowering potential may be due to the probable regenerative effects of oligosaccharide fraction on the pancreatic β-cells that might be experimentally scrutinized (Fig. 1).
Weight of evidence has supported that the regenerative potential of β-cells is mediated by the induction of survival and proliferative signaling pathways in humans, rodents and mice [24–27]. What is more, epigenetic modifications have been suggested to be associated with DM and its complications in a reversible manner [28, 29]. Owing to the reversible nature of epigenetic mechanisms, it seems that isolated oligosaccharide can alter the methylation profiles of genes involving in the regeneration and even in the metabolism of pancreatic β-cells. In this line, hypermethylation of DNA has been shown to contribute to hyperglycemia and the development of its complications such as cardiomyopathy [30–32].
In this study, the altered global DNA methylation in pancreatic cells was the same for peripheral blood of OF-treated diabetic rats. This provides an opportunity to use the methylated DNA as a clinical biomarker for predicting at-risk patients. Even though, despite the decreased expression levels of Dnmt1 and Dnmt3α in both pancreas and blood, the level of Dnmt3β in the pancreas and peripheral blood of OF-treated diabetic rats decreased and increased, respectively. In this regard, the altered expression of Dnmt3β in the pancreas relative to the blood in OF-treated diabetic rats suggested that oligosaccharide may interact with some upstream signaling factors of DNA methyltransferases. For example, oligosaccharide and/or its degraded products may interact with insulin receptor and/or other receptors involving in the function and regeneration of pancreatic β-cells and target tissues. Different methylation profiles may be due to the involvement of different signaling targets such as proliferative, apoptotic, regenerative and inflammatory factors in the development of DM. It is expected that affected factors in the pancreas may be included genes related to the insulin production, secretion and signaling as well as survival of β-cells but even so, in blood, the inflammatory signals seem to be affected by DNA methylation. Consistence with this study, the altered expression of Dnmt3β as an anti-diabetic mechanism of OF plays the causal role in the altered expression of genes involving in the improvement of β-cell function in streptozotocin (STZ)-induced DM.
It has been suggested that Dnmt1 participates in the maintenance of methylated DNA and Dnmt1 involves in global methylation of the genome in mice with ICF syndrome, while Dnmt3α and 3β implicate in de novo methylation of DNA and its Dnmt3β inactivation results in the demethylation of a group of repeated sequences and CpG islands in X chromosome [33]. This clarifies the fact that Dnmt3β acts in a specific methylation manner that may affect the DNA methylation profile and function of a chosen number of targeted factors. However, Dnmt1 is visualized in higher content and activity compared to Dnmt3α and Dnmt3β [34]. Based on our results, OF administration is associated with a decrease in the level of Dnmt3β and an increase in the levels of Dnmt1 and Dnmt3α, suggesting that the specific groups of genes were affected by methylation through activation of each DNA methyltransferases. Regarding the similar DNA methylation profile in the blood and pancreas of control, treated and untreated diabetic rats, monitoring the altered DNA methylation and the expression of DNA methyltransferases in the blood seems to be considered as a promising biomarker in DM.
Increased number of islets in OF- treated rats provides evidence that OF is likely able to induce the regenerative potential of β-cells in the pancreas. There is mounting evidence that regeneration and the function of β-cells in the adult pancreas mediate, in part, by pancreatic and duodenal homeobox 1 (Pdx1) and alteration in its expression is associated with the changes in the expression of target genes such insulin 1 (Ins1) and glucokinase (GCK). Protein tyrosine phosphatase 1 B (Ptp1b) is also a key regulator of insulin signaling through dephosphorylation and inactivation of insulin receptor [35–37]. Therefore, the potential involvement of oligosaccharide in regeneration of islet cells and expression of some genes related to the function and regeneration of β-cells including Pdx1 and PTP1B2 and their targets, Ins1 and GCK, were evaluated in the pancreas of OF-treated and un-treated diabetic rats. The increased expression levels of the Pdx1, PTP1B2 and/or Ins1 and GCK genes may be related to the hypomethylation effect of OF in streptozotocin-induced DM. However, further investigations are needed to evaluate the methylated genes involved in the regeneration of the pancreas by OF administration. In this context, we intend to evaluate the effect of oligosaccharide on the methylation of some genes involving in the regeneration of β-cells.
Conclusion
In conclusion, our findings indicate for the first time that the decreased blood glucose induced by isolated oligosaccharide from R. canina in the streptozotocin (STZ)-induced DM may be due to altered expression of genes involving in the plasticity and functionality of β-cells. What is more, the regenerative effect of oligosaccharide seems to be affected by the alteration in the methylation status of genes and/or change in the expression of DNA methyltransferases. This study opens new horizons to uncover the molecular mechanisms of the isolated oligosaccharide as a patent medicine.
Electronic supplementary material
(DOCX 1394 kb)
Acknowledgments
The authors appreciate the financial support of this investigation by Kermanshah University of Medical Sciences, Kermanshah, Iran. This work was supported by the research council of Kermanshah University of Medical Sciences [grant number 96499] and the National Institute for Medical Research Development (NIMAD) [grant number 963534].
Abbreviations
- DM
Diabetes mellitus
- DNMT
DNA methyltransferase
- Ins1
Insulin1
- GCK
Glucokinase
- H&E
Hematoxylin and eosin
- HPLC-DAD MS/MS
high performance liquid chromatography diode array detector tandem mass spectroscopy
- IHC
Immunohistochemistry
- IR
infra-red radiation
- NMR
nuclear magnetic resonance
- Pdx1
Pancreatic and duodenal homeobox1
- PTP1B2
Protein tyrosine phosphatase 1 B2
- OF
Oligosaccharide fraction
- STZ
Streptozotocin
Author’s contribution
Dr. Soraya Sajadimajd and Prof. Gholamreza Bahrami conceived of the presented idea, designed and directed the project, carried out some experiments, and wrote the manuscript. Dr. Gholamreza Bahrami supervised and directed the project. Bahareh Mohammadi and Samira Keshavarzi carried out isolation and analysis of the isolated oligosaccharide. Dr. Seyed Hamid Madani and Dr. Shahram Miraghaee performed histological experiments. Razieh Hatami carried out animals’ care and treatments. Prof. Mozafar Khazaei provided equipment in evaluating gene expression.
Funding information
This research was funded by National Institute for Medical Research Development, grant number 963534.
Compliance with ethical standards
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Human and animal rights
All animals were treated under the principles of laboratory animal care (National Institutes of Health) and approved by ethics committees from the National Institute from Medical Research Development (NIMAD) with the number of certification IR NIMAD REC 1396 262 (26-11-2017).
Footnotes
Highlight
• An active biomolecule was isolated and characterized as Oligosaccharide fraction.
• Oligosaccharide fraction from R. canina showed the blood glucose lowering potential.
• Natural product from R. canina induced hypomehylation in diabetic rat.
• Extracted fraction from R. canina may affect the regeneration of β-cells.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH. Global Burden of Disease. Projections of mortality and burden of disease, 2002–2030. 2009. 2017.
- 2.Jones P. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012. [DOI] [PubMed]
- 3.Edwards JR, Yarychkivska O, Boulard M, Bestor TH. DNA methylation and DNA methyltransferases. Epigenetics Chromatin. 2017;10(1):23. doi: 10.1186/s13072-017-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 5.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Ponnaluri VC, Ehrlich KC, Zhang G, Lacey M, Johnston D, Pradhan S, et al. Association of 5-hydroxymethylation and 5-methylation of DNA cytosine with tissue-specific gene expression. Epigenetics. 2017;12(2):123–138. doi: 10.1080/15592294.2016.1265713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaminsky ZA, Tang T, Wang S-C, Ptak C, Oh GH, Wong AH, et al. DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet. 2009;41(2):240–245. doi: 10.1038/ng.286. [DOI] [PubMed] [Google Scholar]
- 8.Al-Rowais NA. Herbal medicine in the treatment of diabetes mellitus. Saudi medical journal. 2002;23(11):1327–1331. [PubMed] [Google Scholar]
- 9.Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Aloe vera linn an overview. International Journal of Pharmaceutical Sciences Review and Research. 2010;4(2):106–110. [Google Scholar]
- 10.Nathan M, Scholten R. The complete german commission E monographs: Therapeutic guide to herbal medicines. Annals of Internal Medicine. 1999;130(5):459. [Google Scholar]
- 11.Marty AT. The complete German commission E monographs. JAMA. 1999;281(19):1852–1853. [Google Scholar]
- 12.Orhan N, Aslan M, Hosbas S, Deliorman OD. Antidiabetic effect and antioxidant potential of Rosa canina fruits. Pharmacogn Mag. 2009;5(20):309. [Google Scholar]
- 13.Taghizadeh M, Rashidi AA, Taherian AA, Vakili Z, Sajad Sajadian M, Ghardashi M. Antidiabetic and antihyperlipidemic effects of ethanol extract of Rosa canina L. fruit on diabetic rats: an experimental study with histopathological evaluations. Journal of evidence-based complementary & alternative medicine. 2016;21(4):NP25–NP30. doi: 10.1177/2156587215612626. [DOI] [PubMed] [Google Scholar]
- 14.Fattahi A, Niyazi F, Shahbazi B, Farzaei MH, Bahrami G. Antidiabetic mechanisms of Rosa canina fruits: an in vitro evaluation. Journal of evidence-based complementary & alternative medicine. 2017;22(1):127–133. doi: 10.1177/2156587216655263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen SJ, Aikawa C, Yoshida R, Kawaguchi T, Matsui T. Anti-prediabetic effect of rose hip (Rosa canina) extract in spontaneously diabetic Torii rats. J Sci Food Agric. 2017;97(12):3923–3928. doi: 10.1002/jsfa.8254. [DOI] [PubMed] [Google Scholar]
- 16.Andersson U, Berger K, Högberg A, Landin-Olsson M, Holm C. Effects of rose hip intake on risk markers of type 2 diabetes and cardiovascular disease: a randomized, double-blind, cross-over investigation in obese persons. Eur J Clin Nutr. 2012;66(5):585–590. doi: 10.1038/ejcn.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahrami G. Herbal extract composition for the treatment of diabetes and a method of extracting the same. Google Patents; 2016.
- 18.Luna L, Gridley M. US Armed Forces Institute of Pathology. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology 3rd ed New York: Blakiston Division McGraw-Hill. 1968.
- 19.Leong AY, Wright J. The contribution of immunohistochemical staining in tumour diagnosis. Histopathology. 1987;11(12):1295–1305. doi: 10.1111/j.1365-2559.1987.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurdyukov S, Bullock M. DNA methylation analysis: choosing the right method. Biology. 2016;5(1):3. doi: 10.3390/biology5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiang L, Wu T, Zhang H, Lu N, Hu R, Wang Y, et al. HIF-1α is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating notch signaling pathway. Cell Death & Differentiation. 2012;19(2):284–294. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–8. [DOI] [PubMed]
- 23.Rahimi M, Sajadimajd S, Mahdian Z, Hemmati M, Malekkhatabi P, Bahrami G, et al. Characterization and anti-diabetic effects of the oligosaccharide fraction isolated from Rosa canina in STZ-induced diabetic rats from Rosa canina. Carbohydr Res. 2020;107927. [DOI] [PubMed]
- 24.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by β-cell regeneration. J Clin Invest. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302(5648):1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- 26.Chong AS, Shen J, Tao J, Yin D, Kuznetsov A, Hara M, et al. Reversal of diabetes in non-obese diabetic mice without spleen cell-derived ß cell regeneration. Science. 2006;311(5768):1774–1775. doi: 10.1126/science.1123510. [DOI] [PubMed] [Google Scholar]
- 27.Suri A, Calderon B, Esparza TJ, Frederick K, Bittner P, Unanue ER. Immunological reversal of autoimmune diabetes without hematopoietic replacement of ß cells. Science. 2006;311(5768):1778–1780. doi: 10.1126/science.1123500. [DOI] [PubMed] [Google Scholar]
- 28.Mishra PK, Tyagi N, Kundu S, Tyagi SC. MicroRNAs are involved in homocysteine-induced cardiac remodeling. Cell Biochem Biophys. 2009;55(3):153–162. doi: 10.1007/s12013-009-9063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease? Transl Res. 2011;157(4):253–264. doi: 10.1016/j.trsl.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P. Baker EK et al. Diabetes: Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that co-exist on the lysine tail; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagiannis TC, Maulik N. Factors influencing epigenetic mechanisms and related diseases. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA; 2012. [DOI] [PubMed]
- 32.Nikoshkov A, Sunkari VG, Savu O, Forsberg E, Catrina S-B, Brismar K. Epigenetic DNA methylation in the promoters of the Igf1 receptor and insulin receptor genes in db/db mice. Epigenetics. 2011;6(4):405–409. doi: 10.4161/epi.6.4.14791. [DOI] [PubMed] [Google Scholar]
- 33.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 34.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9(16):2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 35.Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278(2):739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- 36.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6(6):1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 37.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45(10):1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1394 kb)