Abstract
Background:
Sacubitril/Valsartan (Sac/Val), a combined angiotensin-II receptor blocker (Val) and neprilysin inhibitor (Sac) in a 1:1 molar ratio, was shown to reduce the risk of cardiovascular death or heart failure (HF) hospitalization in patients with HF and reduced LV ejection fraction (EF). This study examined the effects of Sac/Val on LV structure, function and bioenergetics and on biomarkers of kidney injury and kidney function in dogs with experimental cardiorenal syndrome (CRS).
Methods and Results:
14 dogs with CRS (coronary microembolization-induced HF and renal dysfunction) were randomized to 3 months Sac/Val therapy (100 mg once daily, n=7) or no therapy (control, n=7). LV EF and troponin-I (TnI) as well as biomarkers of kidney injury/function including serum creatinine (sCr) and urinary kidney injury molecule-1 (KIM-1) were measured before and at end of therapy and the change (treatment effect Δ) calculated. Mitochondrial function measures including maximum rate of ATP synthesis (ATPsyn) were measured in isolated cardiomyocytes at end of therapy. In Sac/Val dogs, EF increased compared to controls (6.9±1.4 vs. 0.7±0.6 %, p<0.002) while ΔTnI decreased (−0.16±0.03 vs. −0.03±0.02 ng/ml, p<0.001). Urinary ΔKIM-1 decreased in Sac/Val treated dogs compared to controls (−17.2±7.9 vs. 7.7±3.0 mg/ml, p<0.007) whereas sCr was not significantly different. Treatment with Sac/Val increased ATPsyn compared to control (3,240±121 vs. 986±84 RLU/μg protein, p<0.05).
Conclusions:
In dogs with CRS, Sac/Val improves LV systolic function, improves mitochondrial function and decreases biomarkers of heart and kidney injury. The results offer mechanistic insights into the benefits of Sac/Val in HF with compromised renal function.
Keywords: Heart Failure, Mitochondria, Myocardial Energetics, Ventricular Function
Introduction
Dual blockade of the renin-angiotensin-aldosterone system (RAAS) and the neutral endopeptidase (NEP) pathway in heart failure (HF) was evaluated nearly 20 years ago with the drug omapatrilat through dual angiotensin-converting enzyme (ACE) and NEP inhibitors (1). Even though omapatrilat showed benefits in HF patients, continued development ended due to increased incidence of angioedema (1, 2). Sacubitril/valsartan (Sac/Val) or LCZ696 is a first in class combined angiotensin-II receptor blocker (Val) and neprilysin inhibitor (Sac) in a 1:1 molar ratio. In the Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF), patients with HF and reduced left ventricular (LV) ejection fraction (HFrEF) receiving Sac/Val had 20% lower risk of cardiovascular death, largely due to a decrease in sudden cardiac death compared to patients receiving the ACE inhibitor enalapril (3, 4). Patients treated with Sac/Val also remained in clinical remission longer, had less instances of worsening HF requiring hospitalization and less intensification of therapy, implantation of a device or cardiac transplantation compared to those treated with enalapril (3, 5). The benefits elicited by Sac/Val occurred in patients who at baseline were being treated with guidelines-directed optimal standard of care drugs that included diuretics, ACE inhibitors, angiotensin-receptor blockers (ARB), beta-blockers, aldosterone antagonists, implantable cardioverter-defibrillators, and cardiac resynchronization therapy (3).
From a mechanism of action perspective, a knowledge gap remains as to whether these persuasive benefits of Sac/Val can be attributed to greater normalization of neurohormonal abnormalities than inhibition of the RAAS alone (5). The notion of a “greater normalization” has its basis in many biological effects that RAAS inhibition and neprilysin inhibition have in common. RAAS inhibition in HF leads to 1) reduced sodium and water retention, 2) reduced vasoconstriction, 3) reduced hypertrophy and 4) reduced fibrosis. Along similar lines, neprilysin inhibition in HF can lead to greater 1) natriuresis, 2) vasodilation, 3) aldosterone suppression and 4) inhibition of fibrosis. A dual ARB/NEP inhibitor such as Sac/Val may also positively impact cellular pathways that themselves can elicit important cardiac and renal functional benefits in HF. Several lines of evidence support the notion that benefits of Sac/Val in HF can mostly be the result of enhanced cardio-renal end-organ protection. It is well known that NEP inhibition results in increased levels of natriuretic peptides, bradykinins and adrenomedullin. Increased levels of bradykinin are associated with increased expression of endothelial nitric oxide synthase (eNOS) and reduced levels of inducible NOS (iNOS) (6). Normalization of nitric oxide signaling in HF can have a favorable impact on mitochondrial (MITO) function and, hence, on myocardial energetics (7). Exogenous administration of adrenomedullin in experimental and human HF lowers arterial pressure, reduces left ventricular (LV) filling pressures, improves cardiac output, enhanced angiogenesis, reduces fibrosis, increases tolerance of cells to oxidative stress and hypoxic injury, and augments renal glomerular filtration and sodium excretion, factors that auger well for a favorable effect on both cardiac and renal function (8–12). In the present study, we used a canine model of cardiorenal syndrome (CRS) that combines chronic HF with compromised renal function to explore the mechanisms that potentially underlie the benefits of Sac/Val seen in patients with moderate to severe HF.
Methods
Animal Model
The canine model of intracoronary microembolization-induced chronic HF used in this study was previously described (13). Fourteen healthy, purpose-bred, Class A Dealer mongrel dogs, weighing between 21.0 and 26.7 kg, underwent serial intracoronary microembolizations performed 1 to 2 weeks apart, to produce HF. Embolizations were discontinued when LV ejection fraction (EF), determined angiographically, was <35%. Coronary microembolizations were performed during cardiac catheterization under general anesthesia and sterile conditions. Dogs were induced using a combination of intravenous injections of hydromorphone hydrochloride (0.22 mg/kg) and Acepromazine (0.10 – 0.22 mg/kg). Plane of anesthesia was maintained throughout the study using 1% to 2% isoflurane. To achieve the desired CRS state, dogs with established HF underwent a surgical procedure to create renal dysfunction. The latter was induced by performing a unilateral nephrectomy and by creating a stenosis on the contralateral renal vein sufficient to increase proximal renal venous pressure of 20 to 30 mmHg. This manipulation exposes the kidney to the equivalence of elevated central venous pressure. Dogs were maintained for at least 3 weeks after kidney surgery before the study protocol was initiated (14, 15). The study was approved by Henry Ford Health System Institutional Animal Care and Use Committee and conformed to the National Institute of Health “Guide and Care for Use of Laboratory Animals”. (NIH publication No. 85–23).
Study Protocol
Once CRS was established, the 14 dogs were randomized to 3 months therapy with Sac/Val (100 mg, twice daily, n=7, CRS+Sac/Val) or no therapy at all, controls (CRS-CON), n=7). Hemodynamic, angiographic and echocardiographic measurements were made in all dogs during cardiac catheterization performed a) prior to initiation of oral therapy with Sac/Val or control; b) 2 weeks after initiation of active therapy or control; c) 1 month after initiation of active therapy or control and 4) 3 months after initiation of active therapy or control. Peripheral blood samples to extract plasma and urine samples were collected from all dogs at the same study time points as hemodynamics and used to assess cardiac and renal biomarkers. Tissue samples from the LV free wall were obtained at euthanasia and prepared for MITO studies and for histologic and biochemical evaluations. Seven normal dogs were used to provide normal LV tissue, plasma and urine for comparisons.
Hemodynamic and Ventriculographic Measurements
All hemodynamic measurements were made during left and right heart catheterizations in anesthetized dogs. The following parameters were evaluated in all dogs: 1) heart rate (HR), 2) mean aortic pressure (mAoP), and 3) LV end-diastolic pressure (LVEDP). Left ventriculograms were obtained with the dog placed on its right side and recorded on digital media at 30 frame/sec during the injection of 20 ml of contrast material (ISOVU-300, Bracco Diagnostics, Inc., Princeton, NJ). Correction for image magnification was made with a radiopaque calibrated markers imbedded in the shaft of the LV ventriculography catheter. LV end-systolic volume (ESV), end-diastolic volume (EDV) were calculated using the area-length method (16). Premature beats and post-extrasystolic beats were excluded from the analysis. LV ejection fraction (EF) was calculated as the ratio of the difference of EDV and ESV to EDV times 100. Stroke volume (SV) was calculated as the difference between EDV and ESV. Cardiac output (CO) was calculated as the product of SV and HR. Systemic vascular resistance (SVR) was calculated as previously described (17).
Echocardiographic and Doppler Measurements
Echocardiographic and Doppler studies were performed using a General Electric VIVID-7 ultrasound system with a 3.5 MHZ transducer and recorded on digital media for off-line analysis. Left ventricular fractional area of shortening (FAS) was measured from 2-dimensional short axis views as the difference between end-diastolic and end-systolic areas divided by end-diastolic area times 100. Trans-mitral inflow velocity waveforms, measured using pulsed-wave Doppler echocardiography, were used to calculate indexes of LV diastolic function namely the ratio of peak mitral inflow velocity during rapid early LV filling (PE) to peak mitral inflow velocity during left atrial contraction (PA), and deceleration time (DT) (7).
Determination of Plasma and Urine Biomarkers
Cardiac biomarkers namely, plasma levels of n-terminal-pro brain natriuretic peptide (nt-pro BNP) and troponin-I (TnI), were determined in EDTA-plasma using the principle of the double antibody sandwich Enzyme-linked immunosorbent assay (ELISA) with commercially available kits (Kamiya Biomedical Co., Seattle, WA and Life Diagnostics Inc., West Chester, PA). Serum creatinine and markers of kidney injury/function namely, urinary kidney injury molecule-1 (KIM-1) and neutrophil gelatinase associated lipocalin (NGAL) and plasma cystatin-C were also measured using ELISA with commercially available kits. KIM-1 and NGAL kits were purchased from abcam (us.technical@abcam.com) and Cystatin-C from Mybiosource (mybioresource.com).
Tissue Collection
At the conclusion of the study, after the final hemodynamic evaluation and while under general anesthesia, the dog chest was opened and the heart rapidly removed and LV tissue prepared for biochemical and histologic evaluation. Fresh LV tissue was used to isolate cardiomyocytes to be used in mitochondrial function assays (7). LV tissue from the mid-ventricular free wall was rapidly minced, flash frozen in liquid nitrogen, and stored ay −70°C until needed for biochemical studies including Western blotting. Tissue from the basal, middle and apical thirds of the LV free wall was prepared and used for histomorphometric evaluations as previously described (7, 18, 19). Left ventricular tissue from 6 normal dogs was prepared in an identical manner and used for comparison. Immediately after the heart was harvested, the abdomen was opened and the left kidney was removed and the tissue prepared for histologic evaluation. Kidney tissue from 7 normal dogs was prepared in an identical manner and used for comparison.
Histomorphometric Measurements
Heart
The volume fraction of replacement fibrosis (VFRF) and interstitial fibrosis (VFIF), myocyte cross-sectional area (MCSA), a measure of cardiomyocyte hypertrophy, capillary density (CD), and oxygen diffusion distance (ODD) were assessed histomorphometrically as previously described (7, 18, 19).
Kidney Tissue:
Tissue blocks from 4 regions across the kidney were prepared. Sections from each block were prepared and stained with trichrome to identify areas of fibrosis. An average of 15 microscopic fields from all 4 kidney regions were selected at random to quantify the total volume fraction of kidney fibrosis (VFKFtotal) consisting of the sum of interfascicular fibrosis, interstitial fibrosis and perivascular fibrosis. In all dogs, VFKFtotal was assessed morphometrically as the percent of total surface area occupied by fibrosis.
Determination of Mitochondrial Function
The following MITO function measures were assessed in freshly, collagenase-isolated, digitonin-permeabilized cardiomyocytes: 1) MITO state-3 respiration was measured using a Clark-type electrode (Strathkelvin Respirometer), 2) MITO membrane potential (Δψm) was measured using the commercially available JC-1 cationic fluorescent dye kit (Sigma, St. Louis, MO), 3) MITO permeability transition pore (mPTP) opening was assessed using calcein and 4) MITO maximal rate of ATP synthesis was determined using the ApoSENSOR™ ADP/ATP ratio bioluminescent assay kit (BioVision, Milpitas, CA) as previously described (7). Mitochondrial Complex-I and complex-IV activities were determined in MITO fractions as previously described (7).
Western Blotting
Western blotting was used to quantify changes in LV tissue protein levels. After separating proteins on 4%–20% SDS-PAGE and transferring on PVDF membrane, blots were treated with specific primary antibodies followed by the corresponding secondary antibody coupled with horse radish peroxidase. The bands on PVDF membrane were developed by Chemiluminescence and band intensity was quantified using a BioRad Model GS-670 imaging densitometer and expressed as densitometric units (du). The following proteins were evaluated: 1) eNOS and iNOS, 2) peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), a necessary protein for MITO biogenesis known to be down-regulated in HF, 3) and cytosolic cytochrome c and caspase 3, promoters of cardiomyocyte apoptosis.
Statistical Analysis
The study sample size was statistically powered for measures obtained at 3 months post therapy. Measurements obtained at 2 weeks and 1 month after initiating therapy were collected to identify the earliest time benefits of Sac/Val therapy can be ascertained. Accordingly, only results obtained at 3 months after initiating therapy will be presented and discussed. Within group comparisons of hemodynamic, ventriculographic, and echocardiographic-Doppler and plasma and urine biomarker measures were made using repeated measures analysis of variance (ANOVA) with alpha set at 0.05. If significance was attained, pairwise comparisons between baseline, pre-treatment and post-treatment measures were made using the Student-Neuman-Keuls (SNK) test with p<0.05 considered significant. To assess treatment effect, the change (Δ) in each measure from pre-treatment to post-treatment (3 months after initiating therapy) within each study arm was calculated and the Δs compared between the two arms using a t-statistic for two means with p≤0.05 considered significant. Histological and biochemical measures between normal dogs (NL), CRS-CON dogs and CRS+Sac/Val treated dogs were compared using one way ANOVA with alpha set at 0.05. If significance was attained by ANOVA, pairwise comparisons were performed using the SNK test with p<0.05 considered significant. All the data in study groups exhibited normal distributions. Data are reported as mean ± standard error of the mean (SEM).
Results
All dogs entered into the study completed the in-life portion of the study and none experienced adverse events.
Within Group Differences in Hemodynamics, Ventriculographic and Echocardiographic-Doppler Measures
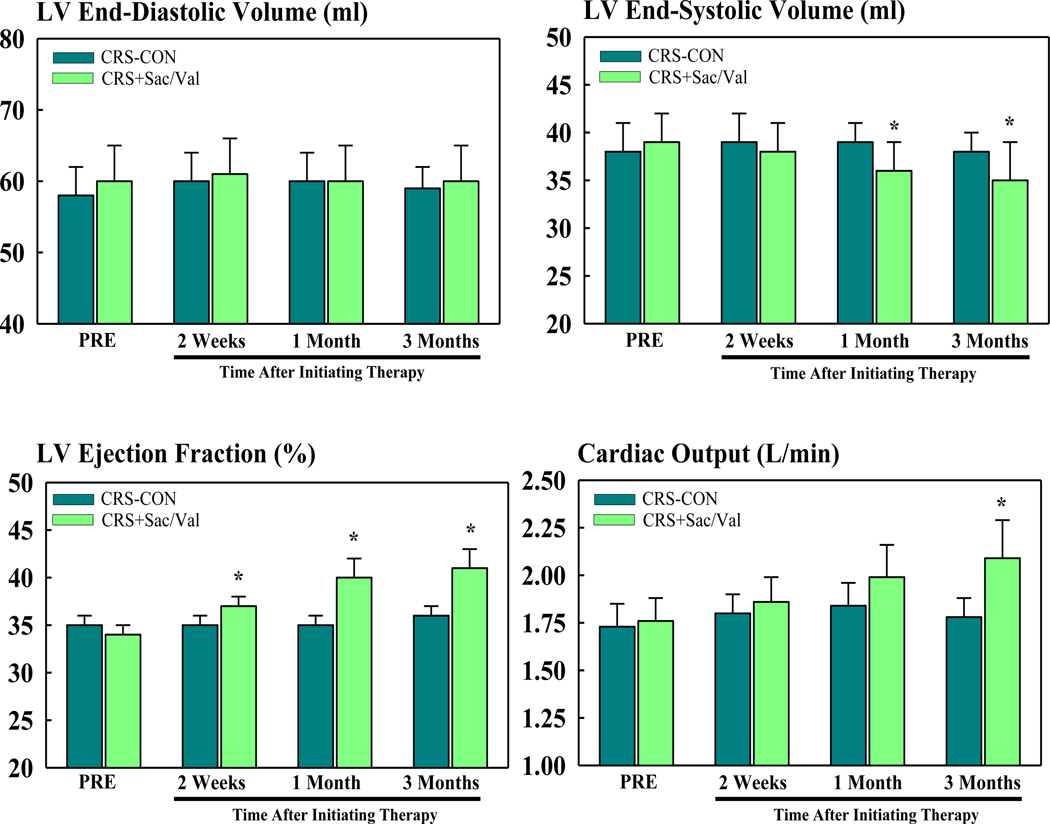
Hemodynamic, ventriculographic, and echocardiographic-Doppler measures are shown in table 1. There were no significant differences between the control and Sac/Val groups at pre-treatment. None of the measures were significantly different in the control group between pre- and post-treatment. In the Sac/Val group, HR, mAoP, LVEDP, and PE/PA were not significantly different between pre- and post-treatment whereas DT, a measure of LV diastolic function, decreased significantly at post-treatment. Therapy with Sac/Val significantly increased SV and CO and decreased SVR. In control dogs, EDV, ESV and EF were unchanged between pre-treatment and post-treatment. Treatment with Sac/Val also had no effect on EDV but significantly decreased ESV and increased LV EF (Figure 1). In general, whenever improvements were seen with Sac/Val therapy at 3 months, they could be identified as early as 2 weeks to one month after initiating therapy.
TABLE 1.
Hemodynamic, ventriculographic, echocardiographic and Doppler measures in untreated cardiorenal syndrome (CRS) control dogs (CRS-CON) and CRS dogs treated with Sac/Val (CRS+Sac/Val).
| CRS-CON (n=7) | CRS + Sac/Val (n=7) | |||||||
|---|---|---|---|---|---|---|---|---|
| PRE | 2W POST | 1M POST | 3M POST | PRE | 2W POST | 1M POST | 3M POST | |
| HR (beats/min) | 86 ± 2 | 87 ± 3 | 88 ± 4 | 85 ± 2 | 87 ± 2 | 83 ± 2 | 83 ± 2 | 85 ± 2 |
| mAoP (mmHg) | 78 ± 3 | 77 ± 2 | 79 ± 2 | 77 ± 3 | 77 ± 3 | 74 ± 3 | 76 ± 4 | 72 ± 3 |
| LVEDP (mmHg) | 13 ± 1 | 14 ± 1 | 15 ± 1 | 15 ± 1 | 13 ± 1 | 12 ± 0 | 13 ± 1 | 12 ± 0 |
| LVEDV (ml) | 58 ± 4 | 60 ± 4 | 60 ± 4 | 59 ± 3 | 60 ± 5 | 61 ± 5 | 60 ± 5 | 60 ± 5 |
| LVESV (ml) | 38 ± 3 | 39 ± 3 | 39 ± 2 | 38 ± 2 | 39 ± 3 | 38 ± 3 | 36 ± 3* | 35 ± 4* |
| LVEF (%) | 35 ± 1 | 35 ± 1 | 35 ± 1 | 36 ± 1 | 34 ± 1 | 37 ± 1* | 40 ± 2* | 41 ± 2* |
| SV (ml) | 20 ± 1 | 21 ± 1 | 21 ± 1 | 21 ± 1 | 20 ± 2 | 22 ± 2* | 24 ± 2* | 24 ± 2* |
| CO (L/min) | 1.73 ± 0.12 | 1.80 ± 0.10 | 1.84 ± 0.12 | 1.78 ± 0.10 | 1.76 ± 0.12 | 1.86 ± 0.13 | 1.99 ± 0.17 | 2.09 ± 0.20* |
| SVR (dynes-sec-cm−5) | 3674 ± 179 | 3498 ± 218 | 3526 ± 262 | 3521 ± 180 | 3594 ± 223 | 3236 ± 167 | 3174 ± 258 | 2878 ± 280* |
| PE/PA | 2.11 ± 0.11 | 2.32 ± 0.11 | 2.43 ± 0.20 | 2.07 ± 0.11 | 2.13 ± 0.19 | 2.06 ± 0.15 | 2.32 ± 0.23 | 2.31 ± 0.27 |
| DT (msec) | 95 ± 4 | 90 ± 6 | 91 ± 5 | 91 ± 5 | 107± 8 | 96 ± 7 | 89 ± 3* | 89 ± 5* |
CRS=cardiorenal syndrome; Sac/Val=sacubitril/valsartan; PRE=before initiating treatment; 2W POST=2 weeks after initiating treatment; 2M POST=2 months after initiating treatment; 3M POST=3 months after initiating treatment; HR=heart rate; mAoP=mean aortic pressure; LV=left ventricular; EDP=end-diastolic pressure; EDV=end-diastolic volume; ESV=end-systolic volume; EF=ejection fraction; SV=stroke volume; CO=cardiac output; SVR=systemic vascular resistance; PE=mitral inflow peak velocity during early filling; PA=mitral inflow peak velocity during left atrial contraction; PE/PA=ratio of PE to PA; DT=deceleration time of early mitral inflow velocity. All data are Mean ± SEM.
=p<0.05 vs. PRE.
Figure 1.
Bar graphs (mean ± SEM) depicting changes in left ventricular (LV) end-diastolic volume (EDV) (top left), end-systolic volume (top right), ejection fraction (bottom left) and cardiac output (bottom right) in untreated control cardiorenal syndrome (CRS-CON) dogs (n=7) and in CRS dogs treated with sacubitril/valsartan (CRS+Sac/Val) (n=7). Data time points are before initiating therapy (PRE) and 2 weeks, 1 month and 3 months after initiating therapy. *=p<0.05 vs. PRE.
Within Group Differences in Plasma and Urine Biomarkers
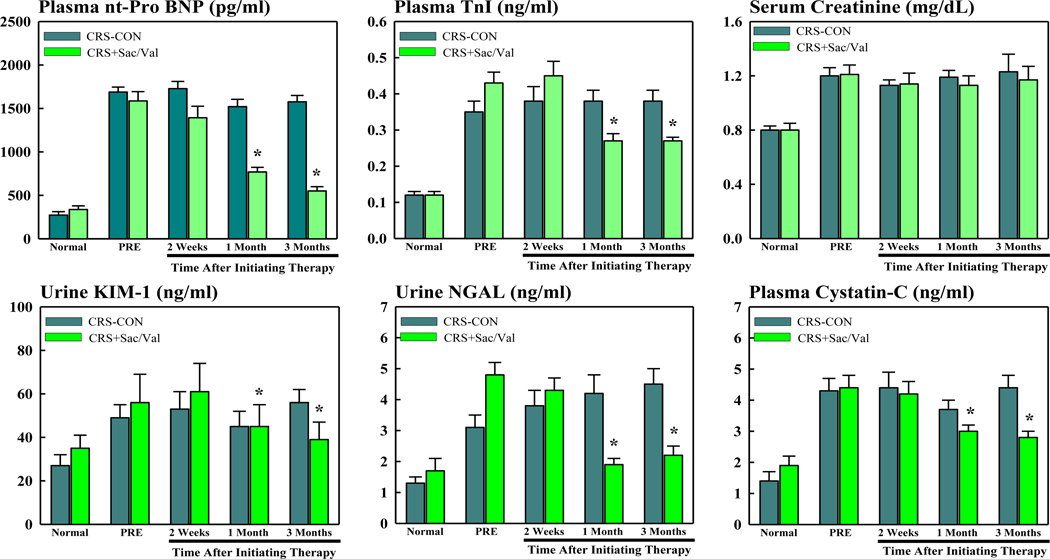
Plasma and urine biomarkers are shown in table 2. At pre-treatment, plasma levels of nt-pro BNP and TnI increased significantly in controls compared to normal dogs and remained elevated throughout the 3 months treatment period. At pre-treatment, plasma levels of nt-pro BNP and TnI were also significantly increased in dogs randomized to treatment with Sac/Val compared to normal dogs. In this treatment group, levels of nt-pro BNP and TnI decreased gradually over the treatment period and were significantly lower at post- compared to pre-treatment (Figure 2).
TABLE 2.
Cardiac and kidney biomarkers in untreated cardiorenal syndrome (CRS) control dogs (CRS-CON) and CRS dogs treated with Sac/Val (CRS+Sac/Val).
| CRS-CON (n=7) | |||||
|---|---|---|---|---|---|
| Baseline | PRE | 2W POST | 1M POST | 3M POST | |
| Creatinine (mg/dL) | 0.80±0.03 | 1.20±0.06‡ | 1.13±0.04‡ | 1.19±0.05‡ | 1.23±0.13‡ |
| BUN (mg/dL) | 12±1 | 19±2‡ | 19±1‡ | 18±2‡ | 19±2‡ |
| Plasma nt-Pro BNP (pg/ml) | 273±39 | 1689±57‡ | 1728±83‡ | 1521±84‡ | 1576±73‡ |
| Plasma TnI (ng/ml) | 0.12±0.01 | 0.35±0.03‡ | 0.38±0.04‡ | 0.38±0.03‡ | 0.38±0.03‡ |
| Urine KIM-1 (ng/ml) | 27±5 | 49±6‡ | 53±8‡ | 45±7‡ | 56±6‡ |
| Urine NGAL (ng/ml) | 1.3±0.2 | 3.1±0.4‡ | 3.8±0.5‡ | 4.2±0.6‡* | 4.5±0.5‡* |
| Plasma Cystatin-C (ng/ml) | 1.4±0.3 | 4.3±0.4‡ | 4.4±0.5‡ | 3.7±0.3‡ | 4.4±0.4‡ |
| CRS-Sac/Val (n=7) | |||||
|---|---|---|---|---|---|
| Baseline | PRE | 2W POST | 1M POST | 3M POST | |
| Creatinine (mg/dL) | 0.80±0.05 | 1.21±0.07‡ | 1.14±0.08‡ | 1.13±0.07‡ | 1.17±0.10‡ |
| BUN (mg/dL) | 12±1 | 20±2‡ | 18±1‡ | 17±1‡ | 20±1‡ |
| Plasma nt-Pro BNP (pg/ml) | 337±42 | 1587±107‡ | 1393±133‡ | 769±53‡* | 550±50* |
| Plasma TnI (ng/ml) | 0.12±0.01 | 0.43±0.03‡ | 0.45±0.04‡ | 0.27±0.02‡* | 0.27±0.01‡* |
| Urine KIM-1 (ng/ml) | 35±6 | 56±13‡ | 61±13‡ | 45±10 | 39±8 |
| Urine NGAL (ng/ml) | 1.7±0.4 | 4.8±0.4‡ | 4.3±0.4‡ | 1.9±0.2* | 2.2±0.3* |
| Plasma Cystatin-C (ng/ml) | 1.9±0.3 | 4.4±0.4‡ | 4.2±0.4‡ | 3.0±0.2* | 2.8±0.2* |
Baseline, normal state prior to induction of heart failure; PRE=before initiating treatment; 2W POST=2 weeks after initiating treatment; 2M POST=2 months after initiating treatment; 3M POST=3 months after initiating treatment; Sac/Val=sacubitril/valsartan; BUN=blood urea nitrogen; nt-Pro-BNP=n-terminal-pro brain natriuretic peptide; TnI=troponin-I; KIM-1= kidney injury molecule-1; NGAL=neutrophil gelatinase associated lipocalin. All data are Mean ± SEM.
=p<0.05 vs. BL;
=p<0.05 vs. PRE.
Figure 2.
Bar graphs (mean ± SEM) depicting changes in plasma and urine biomarkers in untreated control cardiorenal syndrome dogs (CRS-CON) (n=7) and in CRS dogs treated with sacubitril/valsartan (CRS+Sac/Val) (n=7). Top left: plasma n-terminal pro-brain natriuretic peptide (nt-pro BNP); Top Middle: plasma troponin-I (TnI); Top Right: serum creatinine; Bottom Left: urine kidney injury molecule-1 (KIM-1); Bottom Middle: urine neutrophil gelatinase associated lipocalin (NGAL); and Bottom Right: plasma cystatin-C. Data time points are when dog was normal, prior to inducting CRS, when dog has experimental CRS but before initiating therapy (PRE) and 2 weeks, 1 month and 3 months after initiating therapy. *=p<0.05 vs. PRE.
In untreated control CRS dogs, urinary KIM-1 and NGAL and plasma cystatin-C were all significantly increased in at pre-treatment compared to normal dogs and remained significantly elevated throughout the 3 months follow-up period. Serum creatinine and BUN (blood urea nitrogen) both increased significantly in control CRS dogs compared to normal dogs and remained elevated throughout the 3 months follow-up period (Table 2).
In Sac/Val treated CRS dogs. urine levels of KIM-1 and NGAL and plasma cystatin-C were all significantly increased at pre-treatment compared to normal dogs and all decreased gradually during the treatment period and were significantly lower at post-treatment compared to pre-treatment (Figure 2). In Sac/Val treated CRS dogs, serum creatinine and BUN also increased significantly at pre-treatment compared to normal dogs. Serum creatinine and BUN remained elevated throughout the 3 months treatment with Sac/Val (Table 2). In general, whenever improvements were seen with Sac/Val therapy at 3 months, they could be identified as early as 2 weeks to 1 month after initiating therapy.
Between Groups Differences (Treatment Effect)
Between-group comparisons of the change (Δ) between pre-treatment and post-treatment (3 months) measurements are shown in table 3. Compared to CRS-CON, long-term therapy with Sac/Val had no effect on HR, mAoP, LVEDP, PE/PA, DT, creatinine and BUN. Treatment with Sac/Val significantly increased EF, CO, and SV and significantly decreased ESV, nt-proBNP, TnI, plasma and urine levels of KIM1, NGAL and cystatin-C (Table 3).
TABLE 3.
Comparisons of treatment effect (Δ) between untreated cardiorenal syndrome (CRS) control dogs (CRS-CON) and CRS dogs treated with Sac/Val (CRS+Sac/Val).
| CRS-CON (n=7) | CRSF+Sac/Val (n=7) | P-value | |
|---|---|---|---|
| Δ HR (beats/min) | −0.9 ± 3.8 | −1.4 ± 2.7 | NS |
| Δ mAoP (mmHg) | −5 ± 4 | −7 ± 2 | NS |
| Δ LVEDP (mmHg) | 1.3 ± 1.1 | −0.4 ± 0.3 | NS |
| Δ LVEDV (ml) | 0.6 ± 1.3 | 0.1 ± 0.9 | NS |
| Δ LVESV (ml) | −0.1 ± 0.9 | −3.9 ± 1.4 | 0.041 |
| Δ LVEF (%) | 0.7 ± 0.6 | 6.9 ± 1.4 | 0.002 |
| Δ SV (ml) | 0.7 ± 0.5 | 4.0 ± 0.8 | 0.004 |
| Δ CO (L/min) | 0.04 ± 0.09 | 0.34 ± 0.09 | 0.036 |
| Δ SVR (dynes-sec-cm−5) | −153 ± 70 | −716 ± 149 | 0.005 |
| Δ PE/PA | −0.04 ± 0.13 | 0.08 ± 0.26 | NS |
| Δ DT (msec) | −4 ± 6 | −19±6 | NS |
| Δ Creatinine (mg/dL) | 0.03 ± 0.10 | −0.04 ± 0.08 | NS |
| Δ BUN (mg/dL) | −0.29 ± 1.44 | −0.14 ± 1.24 | NS |
| Δ Plasma nt-Pro BNP (pg/ml) | −113 ± 88 | −1037 ± 128 | 0.001 |
| Δ Plasma TnI (ng/ml) | 0.03 ± 0.02 | −0.16 ± 0.03 | 0.001 |
| Δ Urine KIM-1 (ng/ml) | 7.7 ± 3.0 | −17.2 ± 7.0 | 0.007 |
| Δ Urine NGAL (ng/ml) | 1.38 ± 0.43 | −2.59 ± 0.46 | 0.001 |
| Δ Plasma Cystatin-C (ng/ml) | 0.15 ± 0.11 | −1.57 ± 0.55 | 0.010 |
Histomorphometric Findings
Heart:
Compared to normal dogs, untreated CRS control dogs showed significant increases in VFRF, VFIF, ODD and MCSA and only modest reduction of CD (Table 4). Compared to untreated control, dogs treated with Sac/Val showed significant reduction in VFRF and VFIF but only modest non-significant effects on CD, ODD and MCSA (Table 4).
TABLE 4.
Histomorphometric findings in LV myocardium of normal dogs (NL), untreated cardiorenal syndrome (CRS) control dogs (CRS-CON) and CRS dogs treated with Sac/Val (CRS+Sac/Val).
| Normal (n=6) | CRS-CON (n=7) | CRS+Sac/Val (n=7) | |
|---|---|---|---|
| VFRF (%) | 0.0 | 13.8 ± 0.9* | 10.8 ± 1.3*† |
| VFIF (%) | 3.5 ± 0.07 | 10.6 ± 0.4* | 9.0 ± 0.5*† |
| CD (capillaries/mm2) | 2910 ± 80 | 2768 ± 124 | 2804 ± 107 |
| ODD (μm) | 8.8 ± 0.2 | 10.1 ± 0.4* | 10.6 ± 0.3* |
| MCSA (μm2) | 410 ± 10 | 565 ± 17* | 538 ± 18* |
| VFKFtotal | 0.31 ± 0.06 | 7.39 ± 0.47* | 2.47 ± 0.22*† |
Sac/Val=sacubitril/valsartan; VFRF=volume fraction of replacement fibrosis; VFIF=volume fraction of interstitial fibrosis; CD=capillary density; ODD=oxygen diffusion distance; MCSA=myocyte cross-sectional area. VFKFtotal=Total volume fraction of kidney fibrosis.
=p<0.05 vs. NL;
=p<0.05 vs. CRS-CON.
Kidney:
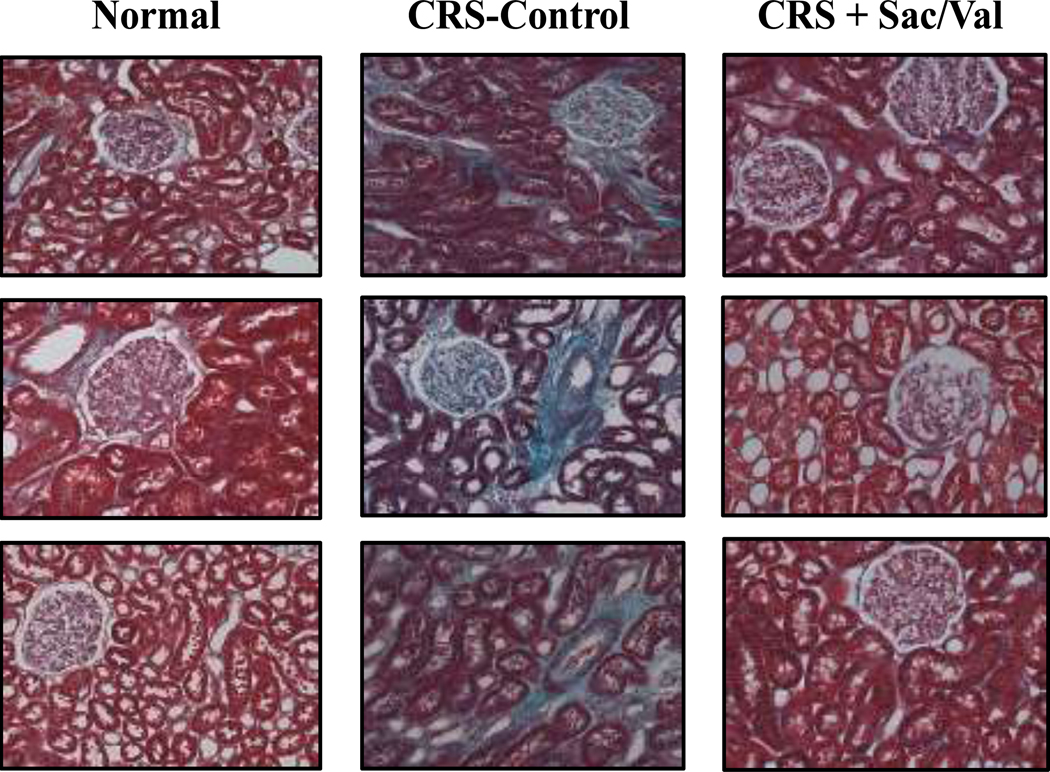
Compared to normal dogs, untreated CRS control dogs showed significant increases in VFKFtotal (Table 4, Figure 4). Compared to untreated control, dogs treated with Sac/Val showed significant reduction in VFKFtotal (Table 4, Figure 4).
Figure 4.
Trichrome stained micrographs of kidney cortex fields from 3 normal dogs (left panels), 3 untreated cardiorenal syndrome dogs (CRS-Control) (middle panels) and 3 cardiorenal dogs treated with sacubitril valsartan (CRS+Sac/Val) (right panels). Magnification X20. Micrographs show increased overall fibrosis in untreated control dogs compared to normal dogs and a lesser degree of fibrosis in Sac/Val treated dogs compared to untreated controls.
Mitochondrial Function
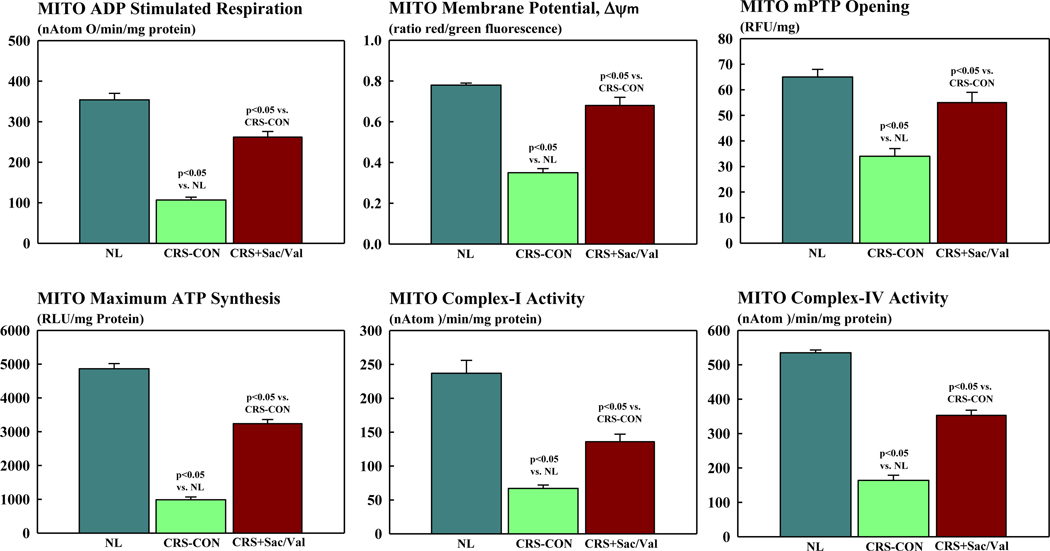
Compared to normal dogs, MITO ADP-stimulated respiration (state-3 respiration), Δψm, mPTP opening and maximum rate of ATP synthesis were all decreased in CRS control dogs (Figure 3). Similarly, enzymatic activities of complex-I and complex-IV of the electron transport chain (ETC) were significantly reduced in control dogs compared to normal dogs. Treatment with Sac/Val significantly increased all MITO function measures to near normal levels. Furthermore, treatment with S/Val significantly increased enzymatic activities of both complex-I and IV also to near normal levels (Figure 3).
Figure 3.
Bar graphs (mean ± SEM) depicting mitochondrial (MITO) function in cardiomyocytes of left ventricular myocardium of normal (NL) dogs (n=5), untreated control dogs with cardiorenal syndrome (CRS-CON) (n=7) and CRS dogs treated with sacubitril/valsartan (CRS+Sac/Val) (n=7). Top Left: mitochondrial adenosine diphosphate (ADP) stimulated respiration. Top Middle: mitochondrial membrane potential. Top Right: mitochondrial permeability transition pore (mPTP) opening. Bottom Right: mitochondrial maximum rate of adenosine triphosphate (ATP) synthesis; Bottom Middle: mitochondrial complex-activity; Bottom Right: mitochondrial complex-IV activity. Probability values are comparisons between normal dogs, CRS-CON dogs and CRS+Sac/Val treated dogs.
Proteins that Regulate MITO Respiration, MITO Biogenesis and Apoptosis
Proteins that regulate MITO biogenesis and respiration were dysregulated in LV myocardium of control dogs compared to normal dogs (Table 5). Compared to normal dogs, protein levels of eNOS and PGC-1α were significantly down-regulated in CRS control dogs while iNOS protein levels were significantly up-regulated. Treatment with Sac/Val normalized levels of both NOS isoforms and PGC-1α (Table 5). Cytosolic levels of cytochrome c were significantly increased in control dogs as were tissue levels of active caspase-3. Treatment with Sac/Val normalized the expression of both pro-apoptotic proteins (Table 5).
TABLE 5.
Protein levels of nitric oxide synthases, growth factors, mitochondrial biogenesis factors, and apoptosis mediating factors in LV myocardium of normal dogs (NL), untreated cardiorenal syndrome (CRS) control dogs (CRS-CON) and CRS dogs treated with Sac/Val (CRS+Sac/Val).
| Normal (n=7) | CRS-CON (n=7) | CRS+Sac/Val (n=7) | |
|---|---|---|---|
| eNOS (du) | 1.81 ± 0.20 | 0.47 ± 0.04* | 1.35 ± 0.11† |
| iNOS (du) | 0.37 ± 0.04 | 1.70 ± 0.02* | 0.62 ± 0.07*† |
| PGC-1α (du) | 2.12 ± 0.29 | 0.47 ± 0.05* | 1.08 ± 0.14*† |
| Active Caspase-3 or p17 du) | 1.27 ± 0.15 | 3.87 ± 0.13* | 2.78 ± 0.14*† |
| Cytosolic Cytochrome c (du) | 0.58 ± 0.14 | 4.35 ± 0.23* | 1.78 ± 0.18*† |
Sac/Val=sacubitril/valsartan; eNOS=endothelial nitric oxide synthase; iNOS=inducible nitric oxide synthase; mitochondrial; PGC-1α= peroxisome proliferator-activated receptor coactivator-1α; du=densitometric units.
=p<0.05 vs. Normal;
=p<0.05 vs. CRS-CON.
Discussion
To our knowledge, this is the first study of the effects of Sac/Val in a large animal model in which both cardiac and renal dysfunction co-exist. The results of this study indicate that in dogs with moderate CRS, long-term monotherapy with Sac/Val improves LV systolic function. The magnitude of improvement is similar to that observed in canine HF model after long-term therapy with beta-blockers (20). Improved LV systolic function was associated with reductions in nt-pro BNP and TnI and was not associated with significant changes in HR or mAoP but significantly decreased SVR; the latter likely the result of increased cardiac output. These benefits occurred alongside improvements of MITO function evidenced by improved organelle respiration and maximum rate of ATP synthesis. Long-term monotherapy with Sac/Val, however, did not elicit a significant improvement of LV diastolic function.
In this CRS model, therapy with Sac/Val had only a modest but nonetheless significant effect on interstitial and replacement fibrosis. This benefit, however, was not nearly as robust as that seen previously with aldosterone antagonists in dogs with HF (21). Treatment with Sac/Val also did not elicit significant beneficial effects on other structural LV cellular remodeling measures including cardiomyocyte hypertrophy, capillary density and oxygen diffusion distance. Previous studies in which long-term therapy with valsartan alone was used in dogs with HF also showed no substantial benefits on cellular components of structural remodeling (22). Absence of substantial improvements in LV structural components likely contributed to a lack of improvement of LV compliance and passive LV filling and, hence, to absence of overall improvement of LV diastolic function. In patients with HFrEF Sac/Val did not significantly reduce central aortic stiffness (23) but did reduce nt-pro BNP and the later, was weakly but significantly correlated with markers of cardiac volume and function (24).
The CRS dog model used in this study is characterized by elevated serum levels of creatinine and BUN and by a marked increase of biomarkers of kidney injury including urinary KIM-1 and NGAL and plasma cystatin C (14, 15). Long-term treatment with Sac/Val significantly reduced all biomarkers of kidney injury but had no effect on kidney functional measure of creatinine and BUN. While it may be true that therapy with Sac/Val in this model of CRS did not improve overall kidney function, it certainly did not worsen overall kidney function. The marked reduction in biomarkers of kidney injury may signal prevention of ongoing kidney injury. This is supported by the observation of reduced kidney fibrosis in dogs treated with Sac/Val compared to untreated controls as evidenced by a significant reduction of VFKFtotal. Additional studies are needed to fully elucidate the merits of mitigating or reversing the rise of kidney injury biomarkers in the setting of chronic HF.
The present study, to our knowledge, is also a first large animal study to document the effects of long-term therapy with Sac/Val on mitochondrial function of the failing left ventricle. In this study, chronic treatment with Sac/Val improved energy availability in the failing myocardium as evidenced by increased maximum rate of ATP synthesis. In addition to improving MITO respiration, MITO membrane potential and reducing opening of the mPTP, treatment with Sac/Val also normalized the activity of complex-I and complex-IV thus improving oxidative phosphorylation through a more efficient electron transfer along the ETC. Mitochondria are a major source of production of reactive oxygen species (ROS) (7). Excess ROS formation can lead to tissue injury, cardiomyocyte degeneration and programmed cell death or apoptosis. Increased ROS can contribute to increase opening of the mPTP (25). We previously showed that HF is associated with opening of the mPTP with an attendant increase in the level of cytochrome c in the cytosol and consequently an increase in cardiomyocyte apoptosis (26–28). In the present study, long-term therapy with Sac/Val attenuated mPTP openings and significantly decreased the levels of cytosolic cytochrome c and active caspase-3 thus suppressing a major signaling pathway for apoptosis. Preventing ongoing loss of functional cardiac units by attenuating apoptosis and necrosis with the latter evidenced by reduced plasma TnI levels, augers well as a contributor to long-term prevention of worsening LV function characteristic of the HF state.
A key mechanistic component of NEP inhibition lies in the prevention of the degradation of bradykinins that can increase the expression of eNOS and the elaboration of nitric oxide. The latter leads to vasodilation and consequently cardiac unloading and improved left ventricular (LV) function albeit in the short-term. Cardiac unloading, in and of itself, if reasonably long-lasting, can have a beneficial effect on the LV by mediating “functional recovery”. In the present study, however, no appreciable reductions of preload and afterload were present and, therefore, improvement of LV systolic function could not be solely attributed to cardiac unloading. There is, however, an excellent potential for long-term benefit to increasing eNOS activity and expression. In HF, eNOS is markedly down-regulated as seen in this study. Heart failure, regardless of etiology, is associated with increased sympathetic drive. The increase in norepinephrine can lead to downregulation of eNOS, decreased levels of cyclic guanosine monophosphate, and finally to downregulation of PGC-1α (29). Because PGC-1α is an important co-transcriptional regulator of MITO biogenesis, its downregulation can have a major adverse impact on MITO biogenesis and, therefore, lead to disruption of needed organelle turnover. Results from the present study indicate that long-term therapy with Sac/Val is associated with normalization of eNOS expression and expression of PGC-1α. This can have a beneficial impact on physiologic mitochondria turnover and, hence, on myocardial and, potentially, renal bioenergetics. Heart failure is also associated with an enhanced inflammatory state evidenced by an increase in many pro-inflammatory cytokines (30). This pro-inflammatory state can lead to increased expression of iNOS which, in turn, can result in suppression of mitochondrial respiration (31–33). Results from the present study showed that long-term therapy with Sac/Val in dogs with CRS was also associated with normalization of iNOS along with normalization of MITO respiration and overall mitochondrial function compared to untreated control dogs.
There are several limitations to the present study that warrant consideration. One limitation is the lack of a valsartan only arm to assess the possible additive or incremental benefits of neprilysin inhibition on top of valsartan. An active drug control arm, namely CRS dogs treated with either an ACE inhibitor alone or an ARB alone would have helped provide more insight into the reason why serum creatinine did not change after treatment with Sac/Val. ACE inhibitors and ARBs can cause intrarenal efferent vasodilation thus decreasing filtration pressure. This may have elicited a different serum creatinine response compared to the no therapy at all control. Another limitation is the absence of studies on the renal effects of Sac/Val in the absence of cardiac disease. Results from such studies would have clarified whether the effects of Sac/Val on kidney biomarkers reflect improvement of cardiac output or a direct effect of Sac/Val on the kidney. The study, as originally designed, did not allow for detailed assessment of the kidney as was done with the heart and, in particular, assessment of MITO function in the kidney. An assessment of MITO function in renal epithelial cells would have provided important knowledge as to the beneficial effects of S/Val or lack thereof, on the energetic state of the dysfunctional kidneys. Finally, the model of CRS used in this study manifests moderate renal dysfunction and is by no means perfect in replicating the classical CRS pathophysiology whereby injury in one organ, for instance the heart, results in failure of the other organ, namely the kidney. In the clinical setting, the classical model is likely temporally driven in that HF can manifest for years before renal failure develops. This long-term, time-dependent disease state progression is difficult to replicate under experimental laboratory conditions.
In conclusion, chronic therapy with Sac/Val in dogs with experimental CRS improves LV systolic function but not diastolic function. The improvements of systolic function were independent of changes of HR, preload and afterload and, as such, not primarily mediated through modification of central hemodynamics. Instead, functional improvements are likely the result of improved myocardial bioenergetics elicited by improved MITO function. In addition to improving LV systolic function, Sac/Val also reduced plasma and urine biomarkers of kidney injury potentially setting the stage for longer term preservation of renal function. This is consistent with observations in patients with chronic kidney disease and patients with HF and preserved EF in whom treatment with Sac/Val was effective in delaying the progression of renal dysfunction (34, 35). Further studies are needed to explore the role of biomarkers of kidney injury on prevention of progressive renal dysfunction in the setting of advanced HF and renal compromise.
Acknowledgments
Sources of Funding
This study was supported, in part, by a research grant from Novartis Corporation and by a Grant from National Heart, Lung, and Blood Institute RO1HL132154-03
Disclosures
Dr. H.N. Sabbah received research grants from Novartis Corporation and has received travel expenses and honoraria for attending Novartis scientific advisory boards. Drs. K. Zhang, R.C. Gupta, J. Xu, and V. Singh-Gupta have no disclosures to make.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation. 2002;106:920–926. [DOI] [PubMed] [Google Scholar]
- 2.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR for the PRADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 4.Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Riskala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin-receptor-neprulysin inhibitor LCZ696 compared to enalapril on mode of death in heart failure patients. Eur Heart J 2015;36;1990–1997. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile M, Andersen K, Arango JL, Arnold JM, Bělohlávek J, Böhm M, Boytsov S, Burgess LJ, Cabrera W, Calvo C, Chen CH, Dukat A, Duarte YC, Erglis A, Fu M, Gomez E, Gonzàlez-Medina A, Hagège AA, Huang J, Katova T, Kiatchoosakun S, Kim KS, Kozan Ö, Llamas EB, Martinez F, Merkely B, Mendoza I, Mosterd A, Negrusz-Kawecka M, Peuhkurinen K, Ramires FJ, Refsgaard J, Rosenthal A, Senni M, Sibulo AS Jr, Silva-Cardoso J, Squire IB, Starling RC, Teerlink JR, Vanhaecke J, Vinereanu D, Wong RC; PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 6.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K. Chronic therapy with elamipretide (MTP-131), a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Circ Heart Fail. 2016;9: e002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu P, Shindo T, Iwata H, Iimuro S, Takeda N, Zhang Y, Ebihara A, Suematsu Y, Kangawa K, Hirata Y, Nagai R. Protective effects of endogenous adrenomedullin on cardiac hypertrophy, fibrosis, and renal damage. Circulation 2004;109:1789–1794. [DOI] [PubMed] [Google Scholar]
- 9.Li LL, Peng C, Zhang M, Liu Y, Li H, Chen H, Sun Y, Zhu C, Zhang Y. Mesenchymal stem cells overexpressing adrenomedullin improve heart function through antifibrotic action in rats experiencing heart failure. Mol Med Rep 2018;17:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, Sakamaki F, Oya H, Kyotani S, Nakanishi N, Goto Y, Masuda Y, Miyatake K, Kangawa K. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 2000;101:498–503. [DOI] [PubMed] [Google Scholar]
- 11.Niu P, Shindo T, Iwata H, Ebihara A, Suematsu Y, Zhang Y, Takeda N, Iimuro S, Hirata Y, Nagai R. Accelerated cardiac hypertrophy and renal damage induced by angiotensin II in adrenomedullin knockout mice. Hypertens Res 2003;26:731–736. [DOI] [PubMed] [Google Scholar]
- 12.Rademaker MT, Cameron VA, Charles CJ, Lainchbury JG, Nicholls MG, Richards AM. Adrenomedullin and heart failure. Regulatory Peptides 2003;112:51–60. [DOI] [PubMed] [Google Scholar]
- 13.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S,, Hawkins ET, Goldstein S . A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. [DOI] [PubMed] [Google Scholar]
- 14.Brewer R, Wang M, Zhang K, Gupta RC, Rastogi S, Paone G, Sabbah HN. A canine model of chronic heart failure and renal insufficiency (Cardiorenal Syndrome). J Am Coll Cardiol 2012;59; A236. [Google Scholar]
- 15.Sabbah HN, Gupta RC, Lanfear D. Characterization of kidney function in a canine model of cardiorenal syndrome. Europ J. Heart Fail 2019;21:577. [Google Scholar]
- 16.Dodge HT, Sandler H, Baxley WA, Hawley RR. Usefulness and limitations of radiographic methods for determining left ventricular volume. Am J Cardiol. 1966;18:10–24. [DOI] [PubMed] [Google Scholar]
- 17.Sabbah HN, Imai M, Cowart D, Amato A, Carminati P, Gheorghiade M. Hemodynamic properties of a new-generation positive luso-inotropic agent for the acute treatment of advanced heart failure. Am J Cardiol. 2007;99:41A–46A. [DOI] [PubMed] [Google Scholar]
- 18.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997; 99:1926–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation. 2000; 102:1990–1995. [DOI] [PubMed] [Google Scholar]
- 20.Sabbah HN, Shimoyama H, Kono T, Gupta RS, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation 1994; 89:2852–2859. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki G, Morita H, Mishima T, Sharov VG, Todor A, Tanhehco EJ, Rudolph AE, McMahon EG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with eplerenone, a novel aldosterone receptor blocker, on the progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation 2002;106:2967–2971. [DOI] [PubMed] [Google Scholar]
- 22.Tanimura M, Sharov VG, Shimoyama H, Mishima T, Levine TB, Goldstein S, Sabbah HN. Effects of AT1-receptor blockade on progression of left ventricular dysfunction in dogs with heart failure. Am J Physiol 1999;276:H1385–H1392. [DOI] [PubMed] [Google Scholar]
- 23.Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, McCague K. Abbas CA, Rocha R, Mitchell CF, for the EVALUATE-HF Investigators. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction a randomized clinical trial. JAMA 2019;322:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD for the PROVE-HF Investigators. Association of change in n-terminal pro–B-Type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochimica et biophysica acta. 2009. November;1787:1402–15. [DOI] [PubMed] [Google Scholar]
- 26.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–2367. [DOI] [PubMed] [Google Scholar]
- 28.Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M, Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol 1996;148:141–149. [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah HN, Gupta RC, Singh-Gupta V, Zhang K. Abnormalities of mitochondrial dynamics in the failing heart: Normalization following long-term therapy with elamipretide. Cardiovasc Drugs and Therap 2018;32:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev 2010;15:331–341. [DOI] [PubMed] [Google Scholar]
- 31.Brown GC, Borutaite V. Nitric oxide and mitochondrial respiration in the heart. Cardiovasc Res. 2007;75:283–290. [DOI] [PubMed] [Google Scholar]
- 32.Brown GC. Nitric oxide and mitochondrial respiration. Biochemica et Biophysica Acta. 1999;1411:351–369. [DOI] [PubMed] [Google Scholar]
- 33.Garcia JA, Ortiz F, Miana J, Doerrier C, Fernandez-Ortiz M, Rusanova I, Escames G, Garcia JJ, Acuña-Castroviejo D. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem. 2017;73:235–244. [DOI] [PubMed] [Google Scholar]
- 34.Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H.LCZ696 (Sacubitril/Valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res. 2017;9:5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 35.Voors AA, Gori M, Liu LCY, Claggett B, Zile MR, Pieske B, McMurray JJV, Packer M, Shi V, Lefkowitz MP, Solomon SD, and the PARAMOUNT Investigators. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eurp J Heart Fail 2015;17:510–517. [DOI] [PubMed] [Google Scholar]