Abstract
Background
Combination of curcumin with anti-inflammatory drug like caffeine shows augmented antipsoriatic action compared to curcumin alone and reduce the time taken for treatment of Psoriasis.
Objective
The objective of the present study was to develop nanosponge (NS) based topical gel of curcumin (CUR) and caffeine (CFN) combination that acts as a potential system for the treatment of psoriasis.
Methods
NS composed of dimethyl carbonate (DMC) as crosslinker and beta-cyclodextrin (β-CD) as polymer were prepared by hot melt method and incorporated in topical gels. Factorial design (32) was constructed in a fully randomized manner to study all nine possible experimental runs. The gels were prepared by varying the content of carbopol-934 (gelling agent) (X1) and guar gum (polymer) (X2). The effect of these two independent variables on viscosity (Y1) and in vitro percent drug release (Y2) of prepared gels was evaluated. Other evaluation studies for NS and nanogels were conducted. In vivo animal studies were carried out for optimized formulation using mouse model of imiquimod-induced psoriasis.
Results
The physical and chemical characteristics exhibited by the prepared NS and gels (F1-F9) were found to be optimal. The optimization resulted in achieving formulation N10 with 69.72% in vitro drug release and 12,329.78cp viscosity. Histopathology studies revealed that prepared nanogel has promising anti-psoriatic activity. The results concluded that CUR and CFN combination has reduced the time required for showing anti-psoriatic activity to 10 days when compared to CUR alone that took around 20 days. Moreover, the nanogel has depicted sustained drug release till 12 h.
Conclusions
From the experimental findings it has been concluded that CUR and CFN combination significantly augmented the anti-psoriatic efficacy with respect to individual components and also reduced the time required for onset of effect. Thus, the proposed nanogel would be an imperative drug delivery system for more effective anti-psoriatic therapy.
Graphical abstract
Keywords: Topical drug delivery, Nanosponges, Psoriasis, Curcumin, Caffeine, Topical gel, Imiquimod
Introduction
Psoriasis is a skin disease which is distinguished by massive proliferation, thick inflammatory cell infiltrates, generation of new blood vessels, modifications in lymphatic structure and impaired differentiation of epidermis. It is an autoimmune disorder in which environmental and genetic components plays a major function [1–3].
Majority of drugs available at the moment like Methotrexate, Cyclosporine, Adalimumab, Etanercept (biologics); topical agents like Corticosteroids, Vitamin D-3 derivatives, retinoids, coal tar etc. are been used for treating psoriasis via both systemic and local therapies. Despite the fact that many therapies exist and even more have been proposed, there in treating psoriasis, no single treatment gives complete and satisfactory cure and most of them have adverse effects. In recent times phytopharmaceuticals have gained a lot of attention and interest by the researchers, and they are striving hard to develop something more efficient, safe and reliable for anti-psoriatic therapy. In near future diverse new molecules, viz., phytoconstituents (Curcumin, Capsaicin, Silymarin, Quercetin, Berberine, Beta amyrin etc.) could support the therapies which are now in use. These phytoconstituents have better therapeutic value and fewer side effects. Present work has been carried out using curcumin which is one of the major phytoconstituents having anti psoriatic activity [4, 5].
Curcumin (CRN) is a natural polyphenolic phytochemical, extracted from rhizome of turmeric (Curcuma longa) having many biological and pharmacological activities such as antioxidant, antitumor, anti-inflammatory, anti-psoriatic, anti-carcinogenic, free radical scavenger, to list a few [6]. Its physical properties include some challenging aspects which further makes him a candidate of choice for research and development. It is poorly water soluble, highly photoreactive agent with rapid metabolism and poor absorption that leads to deprived bioavailability. To date, numerous reports have proposed and proven its significant effect and potential in alleviating psoriasis along with properties of numerous receptors to which curcumin binds [7–9]. Furthermore, CRN induced suppression of phosphorylase kinase activity makes it a very sturdy contender for the resolution of human psoriasis [10]. In order to reduce the time needed by CRN to show its activity in treating psoriasis, which greatly impacts on patient’s psychological state, herein an attempt has been made by combining CRN with anti inflammatory drug like caffeine.
Caffeine (CFN) is a methylxanthine moiety capable to hinder the phosphodiesterase (PDE) enzyme; which helps in hydrolysis of cyclic nucleotides resulting in elevated concentrations of intracellular cAMP (cyclic adenosine monophosphate). Cell surface receptors inhibition for adenosine is another proposed mechanism [11]. Reduced intracellular cAMP levels are seen in cutaneous leukocytes of patients with psoriasis. Many researchers have proposed that as a phosphodiesterase inhibitor and methylxanthine, CFN, increases intracellular cAMP levels; which consequently suppress inflammatory pathways and psoriasis progression [12, 13].
Nanotechnology and nanomedicines despite being exceptionally vast research meadows offers solutions to many unsolved puzzles of drug delivery and therapeutics and so a burgeoning bough of science. In order to improve the solubility and stability of CRN, various studies implying nanocarriers in the form of nanoparticles, lipid-based nanospheres, nanocrystals, liposomes and polymer-based delivery systems were reported [14–16]. However, among the available nanoparticle based dosage forms, nanosponges (NS) conquered the lead, since they efficiently solubilize poorly water soluble drugs and at once offer prolonged release. NS can load both hydrophilic and hydrophobic drug molecules because of their inner hydrophobic cavities and external hydrophilic branching. Lately, these nano sized colloidal carriers are gaining much attention particularly for the drug delivery, diagnostic and therapeutic applications [17–19].
In the preparation of NS, cyclodextrins (CDs) are most preferable polymer owing to their potential of solubilizing drug moieties which are water insoluble, and also they propose prolonged drug release. Moreover, bioavailability too can be increased by means of alterations in pharmacokinetic parameters. CDs belong to cyclic glucopyranose oligomers class. They are synthesized by enzymatic action on hydrolyzed starch [17, 18]. Compared to other available nanocarriers, CD NS offer maximum drug loading and has capacity to validate concerns associated to solubility, controlled release, bioavailability and stability of a range of therapeutic moieties [18, 19]. Additionally, natural and semi-synthetic gums or polymers, such as guar gum, xanthan gum, hydroxypropyl methylcellulose, ethylcellulose, hydroxypropylcellulose, and sodium carboxymethylcellulose, are being used as release retarding materials [20].
Our exhaustive search has revealed that till date no study has reported in combination use of CRN and CFN as NS for the treatment of psoriasis. In view of this fact, in the present study, an attempt has been made to develop cyclodextrin NS of CRN and CFN in combination as a new-fangled, potential delivery system for combating psoriasis.
Materials and methods
CRN was obtained from Chaitanya Agro Herbals, Mysuru, India, as a gift sample. CFN, dimethyl carbonate (DMC), guar gum and Carbopol-934 were purchased from Loba Chemie, Mumbai, India. β-CD was procured from Himedia, Mumbai, India. All other reagents and chemicals used were of analytical grade. Ultra-purified water was used for all experiments.
Methodology
Preformulation studies
Ultraviolet Visible (UV-Visible) Spectroscopy
The standard solutions of CUR and CFN (10 μg/mL) were separately scanned in 200 to 800 nm range using methanol as blank. Maximum absorbance wavelengths were determined. From the recorded spectra an isosbestic point was determined; which is the wavelength at which spectra of two drugs cross each other (herein it was noted at 292 nm). Further, diverse serial dilutions were prepared and their absorbances were recorded at isosbestic point wavelength and calibration curve was plotted.
Fourier Transform Infra-Red (FT-IR) Spectroscopy
FT-IR spectrum of CUR, CFN, drug mixture, polymer and Drug mix+polymer were recorded over the range of 4000 to 400 cm−1 by KBr pellet method using a FT-IR spectrophotometer (Shimadzu 8400 S, Tokyo, Japan).
Differential Scanning Calorimetry (DSC) Analysis
Thermograms of CUR, CFN drug mix and polymer were recorded using Shimadzu DSC-60 differential scanning calorimeter. For analysis samples were placed in aluminium pans, hermetically sealed and heated at 20 °C /min rate, over a range of 40 to 300 °C temperature. By purging nitrogen 40 mL/minute flow rate, atmosphere surrounding sample pans was maintained as inert.
-
2.
Synthesis of β-CD NS
An array of synthetic methods are available for synthesizing NS, we have adopted classical ‘hot melt method’ in present research vocation. Using β-CD as a polymer and DMC as a cross linking agent, in different ratios, three types of NS formulations were prepared (NS1 1:2; NS2 1:4 and NS3 1:8). Excess amount of DMC was melted at about 90 °C and to this hot melt anhydrous β-CD was added. The reaction mixture was then stirred for 5 h with continuous heating and stirring using a magnetic stirrer (Whirlmatic Spectra Lab, Mumbai, India). The substrate mixture (β-CD and DMC) was allowed to react for 5 h so as to ensure completion of crosslinking reaction amongst them; resulting in formation of NS. Later, the mixture was allowed to cool followed by filtration. The obtained solid mass was then grounded in a mortar and Soxhlet extracted with ethanol, to remove either impurities or unreacted excessive DMC. Post purification, NS were stored at 25 °C until further use [21, 22].
Loading of drugs into NS
The precisely weighed amounts of prepared NS (100 mg) with varied crosslinking ratios were suspended in water (20 mL) and sonicated for few minutes to avoid the formation and presence of any aggregates. In this aqueous suspension, excess amount of drug mixture was dispersed. The resultant suspension was maintained under constant stirring for 24 h for allowing complexation between CD NS and incorporated drugs. After complexation reaction, the uncomplexed drug was separated out from nanosuspension via centrifugation (at 2000 rpm for 10 min). The obtained colloidal supernatant was then freeze dried (Modulyo freeze drier, Edwards, UK) to get drug loaded NS; which were then stored into covered vacuum desiccator at ambient temperature till further studies [23]. In Table 1 detail of NS prepared with different ratios of polymer and crosslinker are quoted.
Table 1.
Details of different batches of NS prepared
| Sl. No. | Formulation code |
Drug (mg) |
Polymer: Crosslinker (mg) |
Polymer: Crosslinker Ratio |
|---|---|---|---|---|
| 1. | NS1 | 150 | 100:200 | 1:2 |
| 2. | NS2 | 150 | 100:400 | 1:4 |
| 3. | NS3 | 150 | 100:800 | 1:8 |
Solubility studies
Solubility studies of pure CUR, pure CFN, CUR+ β-CD, CFN+ β-CD and CUR + CFN + β-CD NS were carried out by adding excess amount of each individual drug or drug-polymer mixture to 20 mL of water. The contents were stirred for 48 h at 25 ± 0.5 °C. Post equilibrium, the samples were filtered and analyzed using UV-spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at 419 nm (for CUR), at 272 nm (for CFN) and at 292 nm (the isosbestic point of CUR and CFN).
Entrapment efficiency (EE)
Determining the drug amount embedded in NS is of major significance, because it determines the release characteristics and consequently the therapeutic potency. To calculate the EE, accurately weighed amount of NS (150 mg) was dissolved in methanol, sonicated for 15 min to break the NS complex and for complete solvation of drugs followed by centrifugation. The obtained supernatant was then filtered, diluted suitably using 7.4 pH phosphate buffer solution (PBS), and analyzed using the UV-spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) at 292 nm. All measurements were performed in triplicate under ambient environmental conditions. EE was calculated by using the below mentioned formula:
| 1 |
Where, Ca: actual drug content in NS; Cth: theoretical drug content
In vitro drug release studies
These studies were conducted for all the NS formulations adopting dialysis bag method and using phosphate buffer solution (PBS) pH 7.4 as a media for mimicking skin conditions. Briefly, 100 mg of the freeze-dried NS were sealed in a dialysis bag (MW cut-off 12,500 Da, Himedia, Mumbai, India) and suspended in 10 mL of the buffer system. Incubator shaker (Mini Galaxy E) set at 50 rpm and maintained at 37 ± 1 °C was used for conducting release study. From the release media or buffer system, 2 mL aliquots were withdrawn at predetermined time intervals upto 12 h. Volume of the aliquots withdrawn was replaced with the fresh release medium so as to constantly maintain the release medium volume and sink condition. Drug content in the withdrawn samples was quantified spectrophotometrically at 292 nm (UV-1800, Shimadzu, Kyoto, Japan). To reveal drug release mechanism and to contrast release profiles disparities among formulations, data obtained from timely drug release were used. Further, release data were analyzed by means of diverse mathematical models to know release kinetics.
Based on the above results, NS formulation (NS2) with maximum EE and in vitro drug release was selected and further detailed characterization and studies were done for it.
-
4.
Characterization of NS2
NS2 was characterized by performing scanning electron microscopy (SEM) analysis, particle size analysis (PSA), Zeta potential determination, FT-IR spectroscopy and DSC analysis.
Scanning electron microscopy (SEM)
The morphology and surface texture, topography of NS2 was observed by scanning electron microscope (S-3400 N type II model, Hitachi Ltd., Tokyo, Japan).
FT-IR and DSC analysis
FT-IR spectroscopy and DSC analysis were carried out for prepared NS2 formulation as aforementioned.
Particle size analysis (PSA) and Zeta potential measurement
The mean particle size, size distribution, and Zeta potential or surface charge of the resulting NS were determined by dynamic light scattering (DLS) technique using Nanotrac Wave II Q, Microtrac, USA. The samples were analyzed thrice, to minimize the relative error.
-
5.
Experimental design for formulations of NS gels
In the present work a 2-factor and 3-level full factorial design (32 FFD) was implied to obtain statistically significant and optimized formulation ingredients. Design Expert® software, version 11.0 (Stat-Ease Inc., Minneapolis, MN, USA) was used to create design. The two independent variables viz. amount of gelling agent (X1) and amount of polymer (X2) were optimized using Design of Experiment (DoE) at three different levels: low (−1), medium (0) and high (+1). Viscosity (cP) (R1) and in vitro drug release at 12 h (% cumulative drug release) (R2) were selected as response variables. On a scale of diverse parameters, prepared NS formulations were evaluated and characterized.
Preparation of NS based gel
Precisely weighed amount of Carbopol-934 was soaked in water (around 5 mL) for 2 h and neutralized with triethanolamine (TEA) and stirred continuously. Guar gum and drug loaded NS (equivalent to topical doses of drugs) were dissolved in propylene glycol. This mixture was then transferred to the carbopol mixture and mixing was done for further 20 min. The dispersion was kept aside for 60 min, for complete hydration and swelling of gel components. Before performing viscosity studies, all the prepared gel samples were allowed to equilibrate for at least 24 h at room temperature [24]. Table 2 represents formulation chart of prepared NS based gel formulations.
Table 2.
Formulation chart of different NS gel formulations
| Formulation code | Carbopol-934 (%) |
Guar gum (%) | Propylene glycol (%) | Propyl paraben (%) | TEA (mL) |
|---|---|---|---|---|---|
| N1 | 0.4 | 0.4 | 0.5 | 0.02 | 0.1 |
| N2 | 0.4 | 0.5 | 0.5 | 0.02 | 0.1 |
| N3 | 0.4 | 0.6 | 0.5 | 0.02 | 0.1 |
| N4 | 0.5 | 0.4 | 0.5 | 0.02 | 0.1 |
| N5 | 0.5 | 0.5 | 0.5 | 0.02 | 0.1 |
| N6 | 0.5 | 0.6 | 0.5 | 0.02 | 0.1 |
| N7 | 0.6 | 0.4 | 0.5 | 0.02 | 0.1 |
| N8 | 0.6 | 0.5 | 0.5 | 0.02 | 0.1 |
| N9 | 0.6 | 0.6 | 0.5 | 0.02 | 0.1 |
Determination of pH
Digital pH meter (Mettler Toledo MP 220, Greifensee, Switzerland) was used to determine pH of the prepared gels. The samples were analyzed in triplicate. If slight deviations in pH were noted, it was adjusted to skin pH using dropwise addition of triethanolamine solution [23].
Homogeneity
After placing the gels in the container, all formulations were tested for homogeneity (aggregates presence and appearance) by inspecting visually.
Spreadability studies
Spreadability is a mean of measuring the extent at which the semisolid formulations gets readily spread onto the administration site post application of little shear. Spreadability of the NS gel formulation was determined using wooden block-glass slide apparatus. Herein, approximately 20 g of weight was applied to upper sliding glass slide and estimation of time needed for complete detachment of upper movable slide from lower fixed slide was done. Higher spreadability is expressed by least time needed for separation of two slides. To calculate spreadability, below formula was used.
| 2 |
Where, S = spreadability, M = weight (in grams) tied to upper moving slide, L = length (in cm) of glass slides, and T = time (in sec) taken to separate the two slides completely from each other.
Viscosity studies
All measurements were carried out by viscometer (DV-II+, Brookfield engineering laboratories, Inc., MA, USA) with spindle No. 6 at 10 rpm and at temperature of 37 ± 0.5 °C. The rheological properties of the formulated NS gels were studied at different rpm and the viscosity was recorded in cP.
In vitro drug diffusion studies
NS based gels were permeated through a commercial semipermeable cellophane membrane (Fischer Scientific Co., London, England) using Franz diffusion apparatus (Perme Gear Inc., Bethlehem, PA, USA) with a donor chamber and water jacketed receptor chamber maintained at 37 ± 0.5 °C. 1 g of gel was placed carefully on the cellophane membrane, which was placed between the donor and receptor compartments. The receptor compartment contained PBS pH 7.4, while the donor compartment was empty and open to atmosphere. The contents of the receptor section were kept at 37 ± 0.5 °C with continuous stirring at rate of 25 rpm, using a magnetic stirrer. Aliquots (2 mL) were withdrawn at regular intervals from the receptor compartment and an equal volume of fresh preheated receptor medium was replaced so as to maintain the constant volume of media and the sink condition. Collected samples were spectrophotometrically analyzed using UV visible spectrophotometer (1800, Shimadzu, Japan) at 292 nm and the amount of drug released from gel was calculated.
Check point analysis and optimization of design
Optimized NS gel was obtained from optimum values of factors, desirability and overlay plot of DoE. Viscosity (R1) and in vitro drug release (R2) were selected as response variables. To investigate the relationship between factors and responses, desirability and an overlay plot were constructed. The optimized formulation (N10) was prepared and evaluated for viscosity and in vitro drug release. Further studies like ex vivo permeation and in vivo studies were also carried out using this optimized formulation.
Mice grouping and treatment
8–11 weeks old healthy BALB/c mice of either sex were purchased from Adita Biosys Pvt. Ltd., Tumkuru, India. Experimental study procedure and handling of the experimental animals were approved by Institutional Animal Ethics Committee (IAEC), JSS College of Pharmacy, JSS Academy of Higher Education and Research, Mysuru (approval No. P12–282/2018). A topical dose of 62.5 mg Imiquad® (5% IMQ marketed cream; Glenmark Pharmaceuticals Ltd., India) was applied daily to the shaved dorsal skin/region of mice for 8 consecutive days for development of psoriasis like skin features. In Table 3, details allied to animal grouping and treatments given to them are mentioned.
Table 3.
Animal groupings for in vivo studies and given treatments
| Groups | Treatments |
|---|---|
| Group 1 (G1; Control group) | Vaseline treatment |
| Group 2 (G2; Negative control group) | IMQ application and no treatment |
| Group 3 (G3; Test group) | IMQ application and treated with NS based optimized gel |
| Group 4 (G4; Marketed group) | IMQ application and treated with marketed CRN product |
-
b)
Visual inspection
In specified groups after applying IMQ marketed gel for 8 days, psoriasis like flakes and minor lesions were observed. After treatment with NS based optimized test gel sample and marketed product, visual inspection was done on daily basis and observations were noted.
-
c)
Skin irritation studies
Acute skin irritation (owing to formulation components or any other means) was evaluated in the mice after they have been shaved for the efficacy study. Gel samples were applied to the shaved skin and the appearance, any signs of edema and/or erythema were evaluated at 1st, 24th, 48th, and 72nd hour after application.
-
d)
Histopathology studies
In 10% neutral buffered formalin, selected tissue samples were fixed and followed by dehydration in a graded alcohol series. It is then embedded in paraffin blocks. Sections of the embedded tissues were taken at 4 μm thickness using a rotary microtome and were stained with hematoxylin and eosin (HE) stains. These stained tissues were then observed under an optical microscope equipped with a digital camera system and images were captured and analyzed seeking help of an adept pathologist.
-
e)
Scoring severity of skin inflammation
An ideal scoring system was developed based on the clinical Psoriasis Area and Severity Index (PASI), to score the inflammation severity of the back/dorsal skin. Erythema and scaling of skin were taken as two parameters and were independently scored on a scale from 0 to 4 as: 0- none; 1- slight; 2- moderate; 3- marked; 4- very marked.
Ex vivo permeation studies
Animals were purchased from licensed supplier Adita Biosys Pvt. Ltd., Tumkuru, India. Skins of healthy BALB/c mice were collected by sacrificing them and were used for ex vivo permeation studies. Topical gels containing pure CRN and NS based gel (N10) were prepared and permeated through excised dorsal skin of mouse. Procedure similar to that of in vitro drug diffusion studies was followed.
Estimation of drug retained in the skin layers
The skin was removed from the diffusion apparatus after completion of experiments. For estimating undiffused drug, the surface of skin specimen was washed 10 times with 1 mL distilled water and the drug content in the washings was determined spectrophotometrically. Diffused drug in the receptor compartment was estimated in the similar way. For estimation of drug retained inside the skin, epidermis and dermis layers were effectively separated implying classical heat method. The skin specimen was placed in a sealed bag and placed in water bath maintained at 52 °C for 30 s. Post 30 s, the dermis and epidermis were separated by peeling [27]. The separated dermis and epidermis layers were minced with a sterile surgical scalpel and placed in 10 mL methanol and vortexed for 5 min. The tissue suspensions were then centrifuged at 10,000 rpm for 15 min followed by supernatant filtration. Filtered supernatants of dermis and epidermis tissue suspensions were further extracted with methanol and filtered again. The filtrate was lastly serially diluted and analyzed using UV spectrophotometry.
Stability studies
Stability studies were conducted for 6 months for optimized formulation according to ICH guidelines. The storage requirements were 25 °C/60% RH, 30 °C/60% RH and 40 °C/75% RH. Changes in physical appearance and drug content of formulations were observed at standard time intervals.
Results
Preformulation studies:
Ultraviolet Visible (UV-Visible) Spectrophotometry
Maximum absorbance wavelengths (λ max) of CUR and CFN in methanol are quoted in Table 4 and the overlay plot of two drugs is shown in Fig. 1. As indicative from the overlay plot, the isosbestic point of two drugs was found to be 292 nm. The calibration curve plotted has reflected that both drug concentrations followed linear regression with absorbance; with a regression coefficient, slope and Y-intercept of 0.998, 0.0287 and 0.0064, respectively. The developed analytical method was precise, specific and robust one.
Table 4.
Maximum wavelengths of CUR and CFN mixture
| Solvent | Drug | Wavelengths of maximum absorbance, λ max (nm) | |
|---|---|---|---|
| Observed | Reported | ||
| Methanol | Curcumin | 419 | 421 |
| Caffeine | 272 | 273 | |
Fig. 1.
Overlay spectrum of CUR and CFN mixture in Methanol
FT-IR analysis
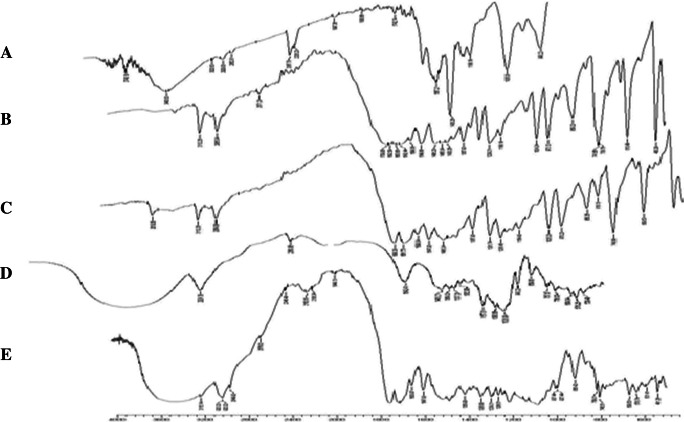
The FT-IR spectra of pure drugs (CUR and CFN), pure drug mixture, polymer and physical mixture of drugs and polymer were recorded. The functional groups interpreted for physical mixture of drugs and polymer was found to be in correlation with pure drug mixture peaks. As shown in Fig. 2, the prominent peaks of drug mixture, i.e. 2920.32 cm−1 corresponding to Alkane C-H stretching; 1358.90 cm−1 corresponding to Alkene C-H bending; 1285.60 cm−1 corresponding to Amine C-N-stretching and 1600.97 cm−1 corresponding to Amide N-H- bending were noticed in the physical mixture FT-IR spectra.
DSC Analysis
Fig. 2.
FT-IR spectra of (a) Curcumin; (b) Caffeine; (c) Polymer; (d) Drug+polymer physical mixture; (e) NS2 formulation
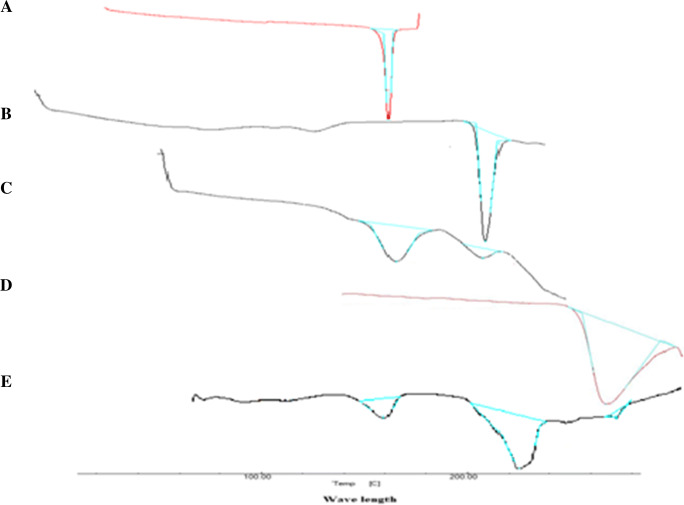
The DSC thermograms of drugs (CUR and CFN), drug mixture, polymer, and drug-polymer physical mixture are depicted in Fig. 3. The endotherm A that appeared at around 175.37 °C represents the melting point of CUR (183 °C theoretically). The second endotherm B at 227.40 °C represents the melting point of CFN (235 °C theoretically). Drug mixture exhibited endothermic peaks of CUR and CFN at 171 °C and 225 °C, respectively. Endotherm D can be attributed to melting point of polymer at 272 °C. The physical mixture of drugs and polymer exhibited the endothermic peak of polymer at 276 °C for β-cyclodextrin.
-
2.
Evaluation of NS
Entrapment efficiency (EE)
Fig. 3.
Overlay DSC thermograms of (a) Curcumin; (b) Caffeine; (c) Polymer; (d) Drug+polymer physical mixture; (e) NS2 formulation
The drug entrapment efficiencies noted for different NS formulations are given in Table 5. It is the amount of drug entrapped in the CD NS cage and was calculated for all the NS formulations. EE of NS batches have ranged from 50.26% to 61.14%. It was clearly evident that EE has changed significantly when drug and polymer ratios have been altered, and also that the EE was chiefly dependent on the amount of crosslinker used in the formulation.
Table 5.
Entrapment efficiencies of prepared NS
| Sl. No. | Formulation code | Entrapment efficiency (%) (Mean ± SD*) |
In vitro drug release studies(%) (Mean ± SD*) |
|---|---|---|---|
| 1 | NS1 | 50.26 ± 0.053 | 71.67 ± 0.021 |
| 2 | NS2 | 61.14 ± 0.028 | 87.32 ± 0.078 |
| 3 | NS3 | 57.65 ± 0.015 | 79.56 ± 0.043 |
*SD- Standard deviation, n = 3
In vitro drug release studies
The NS formulations were subjected to in vitro release studies. The drug release percentages obtained for formulations NS1 to NS3 are given in Table 5. It was found that formulation containing polymer and crosslinker in 1:4 ratio (NS2) has shown maximum in vitro drug release as compared to other formulations. This could be attributed to the highest degree of crosslinking between β-CD and DMC, which permits the encapsulation of drugs in the inner structure of the NS. Additionally, release profiles of all the formulations have been subjected to diverse release kinetics mathematical models; which revealed that Higuchi model is the best fit model for NS1, NS2, NS3 with a regression coefficient (r2) values of 0.925, 0.929, 0.924, respectively.
Based on the above results, NS2 formulation was found to have maximum EE and in vitro drug release. Hence further characterization studies were carried out on this formulation.
-
3.
Characterization of NS2
Scanning electron microscopy (SEM)
SEM analysis was done for the prepared NS to check the morphology and surface texture of the same. As expected, NS were observed to be roughly spherical in shape with uneven surface and spongy nature (Fig. 4).
FT-IR analysis of NS
Fig. 4.

SEM micrograph of optimized NS2 formulation
FT-IR spectroscopy of NS2 formulation was carried out for confirming drug mixture entrapment in the NS. As shown in Fig. 2, the major peaks of drug mix, i.e. 3510.56 cm−1 corresponding to phenolic OH group stretch, 3112.26 cm−1 corresponding to Amine N-H stretch and 740.69 cm−1 corresponding to Alkene = C-H bending were absent in the spectra of NS2 formulation. These outcomes suggest that drug mixture was successfully entrapped into the NS.
DSC analysis of NS
The DSC thermogram of NS formulation (NS2) is presented in Fig. 3. An endothermic peak at 272 °C was seen corresponding to β-CD melting; which indicates that drug mixture was completely entrapped inside the nanocage of β-CD.
Particle size analysis (PSA), Zeta potential measurement
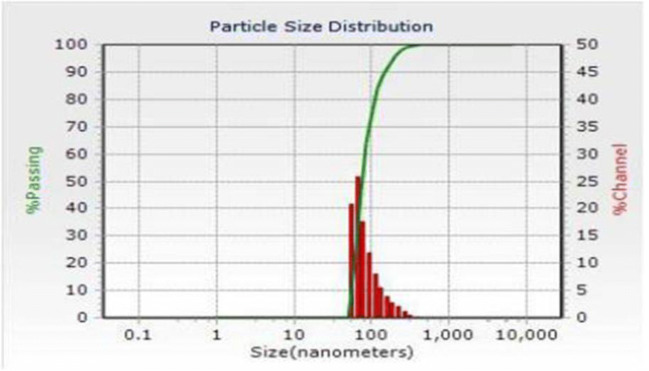
The average particle size of prepared NS formulations was found to be in the range of 170 nm to 200 nm with polydispersity indices (PDI) in range of 0.291 ± 0.073 to 0.395 ± 0.026; representing uniformity in distribution of NS particles. Particle size distribution pattern of the NS2 formulation is given in Fig. 5.
Fig. 5.
Particle size distribution pattern of the NS2 formulation
Zeta potential plays a vital function in the interaction of formulation with biological system and it has been reported and proven in various studies till date. It shows the charge type that is present on the NS surface and also provides idea of stability of the prepared formulation in a colloidal suspension. Zeta potential of NS formulations was found to be in range 14.6 ± 1.1 mV to −28.35 mV, and it has been increased with an increase in the concentration of crosslinker in the formulation.
-
4.
Evaluation of NS based gels
From the software, nine formulation runs were obtained. Evaluation studies like pH, homogeneity, viscosity, spreadability and in vitro drug release studies were performed for NS based gels and the results obtained are represented in Table 6. By fixing gelling agent concentration and polymer concentration as independent variables, viscosity and in vitro drug release were taken as two dependent variables or responses for experimental design approach (32 full factorial design) using Design Expert® software to obtain an optimized formulation. This formulation was then further subjected for ex vivo permeation and in vivo studies.
Table 6.
Results of evaluation parameters for NS based gels
| Formulation code | pH | Homogeneity | Spreadability# | Viscosity (cP) |
In vitro drug release (%) |
|---|---|---|---|---|---|
| N1 | 5.2 | Clear, translucent | +++ | 9747 ± 0.22 | 76.63 ± 1.12 |
| N2 | 5.5 | Clear, translucent | +++ | 9958 ± 0.13 | 75.24 ± 0.98 |
| N3 | 5.3 | Clear, translucent | +++ | 10,246 ± 0.18 | 73.82 ± 0.93 |
| N4 | 4.7 | Clear, translucent | +++ | 11,785 ± 0.23 | 70.45 ± 1.03 |
| N5 | 5.4 | Clear, translucent | +++ | 12,316 ± 0.11 | 69.32 ± 0.76 |
| N6 | 5. 1 | Clear, translucent | +++ | 12,954 ± 0.16 | 68.51 ± 0.94 |
| N7 | 4. 8 | Clear, translucent | +++ | 13,625 ± 0.27 | 66.42 ± 0.57 |
| N8 | 6.0 | Clear, translucent | +++ | 14,128 ± 0.21 | 65.14 ± 0.34 |
| N9 | 5.1 | Clear, translucent | +++ | 14,934 ± 0.19 | 63.95 ± 1.07 |
# + − Poor; ++ − Intermediate; +++ − Good
pH determination
pH values of all gels were in the range of 4.5–6.0; which indicated that gel pH were well within the safety range and near to that of skin pH [28]. Notable increase or decrease in the pH values may lead to skin irritation, however, in present study such probability was ruled out by recorded results.
Homogeneity
All the prepared NS based gels were visually inspected and evaluated for homogeneity. The prepared gels were observed to be clear, translucent and homogenized, without any lumps or aggregates.
Spreadability studies
All the prepared NS based gel formulations have exhibited good spreadability (Table 6).
Viscosity studies
The viscosity of all the NS based gel formulations was noted in the range of 9000–13,000 cP and it was found to be dependent on polymeric concentration in NS based gel formulations (Table 6).
In vitro drug diffusion studies
These studies provide essential data about imitative action of the formulation during in vivo application. The results indicated that a sustained drug release was offered by formulations over extended period of time (Table 6 and Fig. 6).
-
5.
Experimental design for formulations of NS gels
Fig. 6.

In vitro drug release profiles of formulations N1-N9
The results noted for chosen responses for the software suggested nine formulation runs are presented in Table 7. The viscosity of all runs was ranged between 9000 and 13,000 cP and the in vitro drug release (%) was observed in range of 66.42 ± 0.57% to 76.63 ± 1.12%.
Table 7.
32 Full factorial design layout and responses noted for NS based gel formulations
| Formulation run | X1# (%) |
X2# (%) |
R1# (cP)* |
R2# (%)* |
|---|---|---|---|---|
| N1 | −1 | −1 | 9747 ± 0.22 | 76.63 ± 1.12 |
| N2 | −1 | 0 | 9958 ± 0.13 | 75.24 ± 0.98 |
| N3 | −1 | +1 | 10,246 ± 0.18 | 73.82 ± 0.93 |
| N4 | 0 | −1 | 11,785 ± 0.23 | 70.45 ± 1.03 |
| N5 | 0 | 0 | 12,316 ± 0.11 | 69.32 ± 0.76 |
| N6 | 0 | +1 | 12,954 ± 0.16 | 68.51 ± 0.94 |
| N7 | +1 | −1 | 13,625 ± 0.27 | 66.42 ± 0.57 |
| N8 | +1 | 0 | 14,128 ± 0.21 | 65.14 ± 0.34 |
| N9 | +1 | +1 | 14,934 ± 0.19 | 63.95 ± 1.07 |
| Factors and their coded levels | Low (−1) | Medium (0) | High (+1) | |
| X1 and X2 | 0.4 | 0.5 | 0.6 | |
#X1- Concentration of gelling agent Carbopol-934; X2- Concentration of guar gum; R1- Viscosity; R2- In vitro drug release, *Mean ± SD, n = 3
Regression analysis
The regression equations depicted relative effect of the independent variables X1: concentration of gelling agent (%) and X2: concentration of guar gum (%) on dependent variables R1: viscosity (cP) and R2: in vitro drug release (%). From the equations, it was clearly established that as the concentration of gelling agent and guar gum increases, viscosity increases and in vitro drug release decreases.
The optimization resulted in achieving predicted values of 12,329.78 ± 0.24 cP viscosity and 69.72 ± 0.83% in vitro drug release for optimized formulation N10. The regression coefficient (r2) values were found to be 0.93 and 0.97 for viscosity and in vitro drug release, respectively.
Final equation in coded factors terms
To develop predictions regarding the responses pertaining to levels of each factor, the equation in coded factors terms can be used. The equation for response R1 has shown that both the factors have significant positive effect on viscosity of the NS based gel formulations.
| 3 |
The equation for response R2 has showed that factor A has negative effect; whereas factor B has positive effect on in vitro drug release of the NS based gel formulations.
| 4 |
Response-surface analysis
The major aim of optimization is to discover the optimized levels of the variables affecting the process, so that easy and reproducible production of a product with preferred characteristics is possible. Optimum response region can be identified using the response-surface of selected responses with limitation (in vitro drug release and viscosity). Figure 7a and b shows 3D surface plot that reveals relationship between factors and responses.
Fig. 7.
a) 3D surface plots of R1: Viscosity; b) R2: In vitro drug release as a function of gelling agent concentration and polymer concentration c) Contour plot representing overall desirability function of optimized formulation (N10); d) Overlay plot for optimization of NS based gel formulation
Analysis of variance (ANOVA)
Analysis of Variance showed that viscosity and in vitro drug release results obtained exhibited statistically significant difference (p < 0.05).
Check point analysis and optimization of design
Final formulation was prepared from optimum values of factors X1 and X2, desirability and overlay plot value of DoE (Fig. 7c and d). Preparation of optimized formulation (N10) was done for check point analysis and evaluated for viscosity and in vitro drug release. The optimized formulation has shown response variables as R1 = 12,315.64 cP and R2 = 68.57%. In the check point analysis, values of observed and predicted responses were analyzed and compared. The relative error was calculated as given in Table 8. Desirability value of 0.956 with quite low relative errors was recorded; suggesting precision, specificity and robustness of the design implied.
Table 8.
Check point analysis of optimized NS formulation (N10)
| Formulation N10 |
R1 (Cp) |
R2 (%) |
Desirability |
|---|---|---|---|
| Predicted | 12,329.87 | 69.72 | 0.956 |
| Observed | 12,315.64 | 68.57 | |
| Relative error | 0.014 | 1.18 |
In vivo studies
Visual inspection
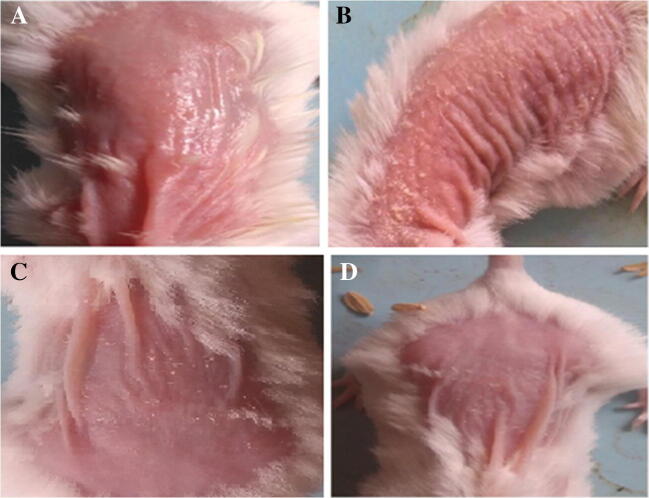
Representative images of dorsal skin of mice treated with IMQ and those treated with the test NS gel samples are shown in Fig. 8. In Fig. 8a, image of mice treated with Vaseline (control) can be seen. In Fig. 8b, image of mice treated with IMQ can be seen with visible, clear white flakes on dorsal back skin of mice. However in Fig. 8c and d, marked reduction in that scaling can be noted after treatment with test NS gel sample (after 10 days of application) and marketed curcumin gel (after 20 days of application), respectively.
Fig. 8.
Images of dorsal skin of mice (a) Treated with vaseline (control); (b) Treated with IMQ; (c) Reduction in scaling after treatment with NS; (d) Reduction in scaling after treatment with marketed formulation
Also, a group of researcher has conducted work on curcumin for its topical application; where they have conducted in vivo studies on mouse models. The results stated that it took around 20 days to observe decrease in psoriatic lesions in mice when topical curcumin was applied [29]. In this respect the prepared NS based gel well served the purpose of augmented anti-psoriatic treatment in a short time span.
-
b)
Skin irritation studies
Post application of the optimized NS formulation onto the skin, any signs of appearance of edema and/or erythema were evaluated at the end of 1, 24, 48, and 72 h. The observations noted are quoted in Table 9.
Table 9.
Observations of skin irritation studies
| Time (h) | Edema or erythema |
|---|---|
| 1 | No sign |
| 24 | No sign |
| 48 | No sign |
| 72 | No sign |
-
c)
Histopathology studies
After initiation of the in vivo studies, IMQ-treated mice’s dorsal/back skin started to show signs of very mild thickening, erythema, and scaling between days 2–4 after the IMQ treatment. From day 4 onward, inflammation was noticeable and got constantly increased in severity upto day 8. These symptoms gradually reduced in groups treated with test sample (G3) and marketed formulation (G4). Maximum reduction of signs was seen in 10 days in case of G3, whereas it took around 20 days to get similar results in the group G4.
Histopathological slides stained with HE-stain were microscopically observed (Fig. 9) and assessed taking help of an adept pathologist.
-
d)
Scoring severity of skin inflammation
Fig. 9.
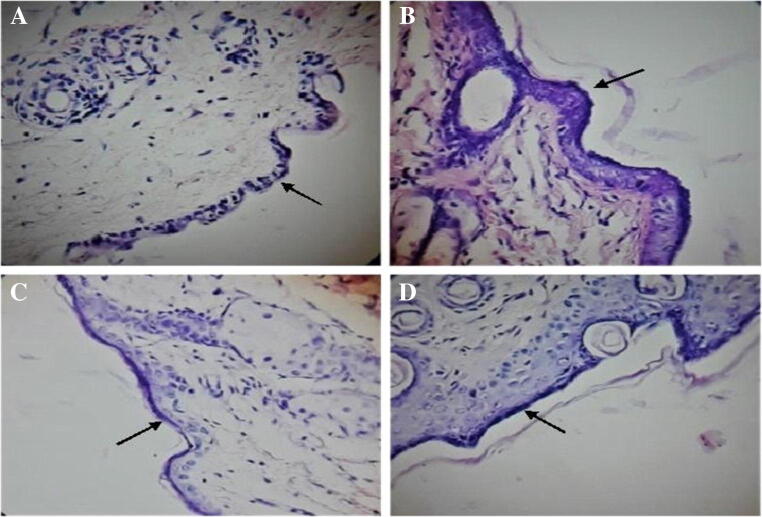
Representative HE (hematoxylin and eosin) stained histological slides of mice dorsal skin of (a) control group, (b) IMQ treated group, (c) IMQ induced and NS gel treated group and (d) IMQ induced and marketed product treated group
Based on the clinical PASI score, erythema and scaling were independently scored on 0 to 4 scale as aforementioned in methodology section. The scoring results noted for diverse groups are presented in Table 10.
Table 10.
Psoriasis area and severity index (PASI) score observations
| Day | Group 1* | Group 2* | Group 3* | Group 4* |
|---|---|---|---|---|
| 0 | 0 | 4 | 4 | 4 |
| 2 | 0 | 4 | 3 | 3 |
| 4 | 0 | 4 | 2 | 2 |
| 6 | 0 | 4 | 1 | 2 |
| 7 | 0 | 4 | 0 | 2 |
| 13 | – | – | – | 0 |
*0-none; 1- slight; 2- moderate; 3- marked; 4- very marked
Ex vivo permeation study
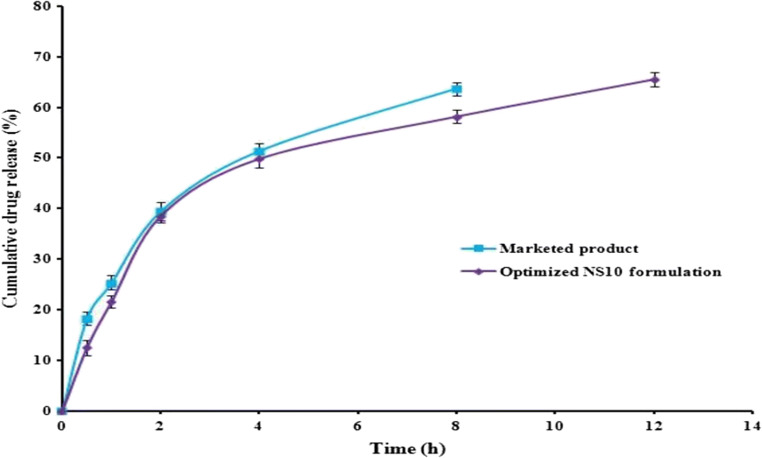
Marketed CRN gel has shown drug release for 8 h and after that no release was noticed; whereas NS10 formulation has shown drug release till 12 h (max 65.48%). The percent drug release of marketed formulation was noted to be around 63.74% at the end of 8 h and after that no release was seen. The drug release profiles obtained for ex vivo permeation studies are presented in Fig. 10. Furthermore, both the marketed CRN gel and optimized NS10 formulation have followed Higuchi kinetic model for drug release with a regression coefficients of 0.989 and 0.969, respectively.
Estimation of drug retained in the skin layers
Fig. 10.
Drug release profiles of marketed CRN formulation and optimized NS based gel formulation obtained during ex vivo permeation studies
The concentration of drug in skin layers was found to be less. Obtained results clearly state in case of optimized N10 formulation, most of the drug was found to be in epidermal layer of the skin. In dermis layer very minimal amount of drug was present. In case of marketed formulation, more amount of drug diffused into epidermis layer. The drug release profiles obtained for drug retention in skin layers are presented in Table 11.
Table 11.
Retention of drug in skin layers
| Formulation | Epidermis | Dermis | Diffused drug |
|---|---|---|---|
| Optimized N10 | 55.18 ± 0.61 | 32.61 ± 0.75 | 16.91 ± 0.82 |
| Marketed | 84.12 ± 0.91 | 8.76 ± 0.75 | 7.08 ± 0.83 |
Stability studies
As per ICH Guidelines, stability studies were conducted for optimized NS based gel formulation for a period of 6 months and storage conditions were 25 °C/60% RH, 30 °C/60% RH and 40 °C/75% RH. The optimized formulation was analyzed for changes in physical appearance and drug content at regular time intervals during the study period. Results obtained are shown in Table 12, which indicated that there was no significant change in appearance and drug content of NS formulation after subjection to stress testing for the 6 months period.
Table 12.
Stability study data of NS based gel formulation
| Stability testing conditions | Sampling interval (months) |
Physical appearance | % Drug content of NS based gel formulation* |
|---|---|---|---|
| 25 °C /60 ± 5% RH | 0 | No change | 91.87 ± 0.32 |
| 3 | No change | 90.18 ± 0.21 | |
| 6 | No change | 89.21 ± 0.35 | |
| 30 °C /60 ± 5% RH | 0 | No change | 91.87 ± 0.32 |
| 3 | No change | 89.67 ± 0.52 | |
| 6 | No change | 88.75 ± 0.28 | |
| 40 °C /75 ± 5% RH | 0 | No change | 91.87 ± 0.32 |
| 3 | No change | 88.92 ± 0.64 | |
| 6 | No change | 88.11 ± 0.52 |
*Mean ± SD, n = 3
Discussion
Preformulation studies
Ultraviolet Visible (UV-Visible) Spectroscopy
From the results, linear correlation among concentration and absorbance is indicated in the range of 0-30 μg/mL of CUR and CFN mixture in methanol.
FT-IR analysis
Obtained results state that no interactions between drug mixture and polymer occurred because no change in the characteristic peaks was seen. Hence drug mixture (CUR and CFN) and selected polymer were compatible with each other.
DSC Analysis
Drug peaks in physical mixture are not sharp, indicating the complete entrapment of drug mixture in the polymeric cage of NS system.
-
2.
Evaluation of NS
Entrapment efficiency (EE)
The highest EE was found for the NS2 formulation; where larger quantity of drug was encapsulated due to optimum cross linking in the polymeric cage [22]. From the drug encapsulation studies, it was also established that DMC acts as an efficient crosslinker for the formulation of NS. This is due to insufficient nanochannels or nanopores formation in NS1 that leads to lower level of inclusion and non-inclusion complexation while higher extent of crosslinking was seen in case of NS3formulation that resulted in inability of drug to enter into the nanochannels [16, 19].
In vitro drug release studies
The lesser drug release in case of NS1 could be because of lesser extent of inclusion and non-inclusion complexation seen in NS1 due to insufficient nanochannels or nanopores formation. Whereas in case of NS3 formulation the limited release can be attributed to quite higher extent of crosslinking due to excess crosslinker presence in the formulation. This resulted in restricted binding sites for drug into the NS polymeric cage and inability of drug to enter into the nanochannels [16, 19].
-
3.
Characterization of NS2
Scanning electron microscopy (SEM)
The SEM micrographs revealed that formed NS were having several fine surface voids; most likely as a result of solvent diffusion. Moreover, no residual, intact crystals of drugs were seen on NS surface, indicating precise encapsulation of drugs into the NS and formation of NS matrix by drug-CD inclusion and/or non-inclusion complexes.
FT-IR analysis of NS
From the obtained FT-IR spectroscopy results, the drug mixture entrapment in the polymer system of NS can be confirmed. Also other characteristic peaks like Alkane C-H stretch, Alkene C-H bending, Amine C-N- stretch and Amide N-H bending; which are present in the pure drug mix and drug and polymer physical mixture were observed in the NS2 formulation spectrum. This indicates the presence of drug in the NS formulation in encapsulated form.
DSC analysis of NS
No sharp peaks pertaining to drugs were noticed; which shows that drug mixture is successfully and precisely entrapped in the polymeric system of CD NS.
Particle size analysis (PSA), Zeta potential measurement
Size of a particle is a very important parameter in NS performance, because drug release rate, extent and drug absorption are majorly affected by it. As the particle size decreases, interfacial area available for drug diffusion increases and thus improvement in the drug release can be seen. Particle size distribution pattern of optimized NS formulation is depicted in Fig. 5. The Ostwald ripening probability was conquered because of narrow size distribution noted in case of the NS formulation [27]. This could be credited to carbonate group’s presence in NS structure that provides physical stability to NS particles (by exerting electrostatic repulsion in colloidal nanosuspension), and thus avoids aggregations. Also, the decrease in particle size leads to increased surface area and ultimately to higher Zeta potential; which consequently again imparts higher physical stability of the NS formulations.
-
4.
Evaluation of NS based gels
pH determination
Notable increase or decrease in the pH values may lead to skin irritation, however, in present study such probability was ruled out by recorded results; which depicted formulation pH compatible with the skin.
Spreadability studies
Spreadability is one of the vital characters for an ideal gel. The findings of spreadability studies explained that prepared formulations easily spreaded on applying small amount of shear. The spreadability was noted to be inversely proportional to the viscosity of gels and it got decreased as the carbopol-934 and guar gum amounts have been increased.
In vitro drug diffusion studies
All formulations exhibited initial burst release in the initial hours; which might be due to unentrapped drugs in the gel matrix, followed by prolonged release of entrapped drugs from NS core over extended period of time. Due to steady erosion of NS and continuous drug diffusion into the external polymer matrix, drug release from NS based gel occurs in timely manner. Optimum drug concentration required for controlling symptoms immediately was provided by initial burst release, which was followed by prolonged release that would help in maintaining the therapeutic concentration required for overall psoriasis treatment for a longer duration.
The major aim of optimization is to discover the optimized levels of the variables affecting the process, so that easy and reproducible production of a product with preferred characteristics is possible. Optimum response region can be identified using the response-surface of selected responses with limitation (in vitro drug release and viscosity). Figure 7a and b shows 3D surface plot that reveals relationship between factors and responses.
Analysis of Variance (ANOVA)
Analysis of Variance showed that viscosity and in vitro drug release results obtained exhibited statistically significant difference (p < 0.05).
Check point analysis and optimization of design
Final formulation was prepared from optimum values of factors, overlay plot and desirability of DoE. Preparation of optimized formulation (N10) was done for check point analysis and evaluated for viscosity and in vitro drug release. The optimized formulation has shown response variable as R1 = 12,315.64 cP and R2 = 68.57%. In the check point analysis, values of observed and predicted responses were analyzed and compared. The error was calculated as given in Table 8. Desirability value of 0.956 with low relative errors was recorded.
Reliability of the optimization procedure to prepare formulation as per 32 factorial designs which is followed in the present study was confirmed by above value. A and B factors with the composition of 0.52% and 0.49% are best suitable for drug delivery as NS based topical gel. Figure 7c and d represents desirability plot and overlay plot of the optimized NS based gel formulation, respectively.
In vivo studies
Visual inspection
Flakes formed in Fig. 8b are indicative of skin scaling as happens in psoriatic condition. Curative action in Fig. 8c can be attributed to presence of CRN and CFN loaded NS in the gel; that inhibited the IMQ-induced flakes formation and thickening of both the epidermal and subcutaneous tissue. Hence, potency of prepared and optimized NS based topical gel in reducing major signs and interventions of psoriasis was visually established.
-
b)
Skin irritation studies
Results of the study have proved the compatibility and non-irritant nature of the prepared optimized NS based gel.
-
c)
Histopathology studies
When compared with the control group (Fig. 9a), the IMQ-treated dorsal back skin section exhibited amplified epidermal and subcutaneous tissue thickness (Fig. 9b). Hyperplasia of basal and suprabasal keratinocytes are the reason behind this increased thickness. Furthermore, abnormal differentiation of keratinocytes with evident parakeratosis (nuclei in the Stratum corneum) was seen. CRN and CFN NS based gel inhibited the thickness increased due to IMQ-induction in both epidermal and subcutaneous tissues (Fig. 9c). In the control group, no abnormal phenotype was noticed. The keratinocytes layer thickness in each group stained skin section is indicated with the arrows in Fig. 9. Since Group 1 was treated only with Vaseline and no other chemicals were used, no unusual phenotype was noticed in the control group. In case of Group 2, marked changes were observed since the animal subjects were treated with IMQ. In Groups 3 and Group 4 slight changes were observed since treatment with test sample and marketed product was given after treating with IMQ. These results further confirmed that prepared test sample containing CRN and CFN NS based gel inhibited the thickness of skin which was increased due to IMQ-induction in both epidermal and subcutaneous tissues.
Ex vivo permeation study
A sustained release of drugs was established from the developed NS based topical gel formulation.
Estimation of drug retained in the skin layers
From the results it can be concluded that NS helps in localizing the drug within the skin and produces local effect on the psoriatic lesions. Lesser deposition of drug in dermis layer showed that drug did not enter into blood and further into systemic circulation through blood vessels present in the dermis. Similarly, in case of marketed formulation, drug hasn’t entered into dermis layer and thus enhanced degree of localized effect and also overruled any probability of side effects due to entry into systemic circulation.
Conclusions
CD based NS of CRN and CFN were successfully synthesized and characterized in great detail. From all the results, it was evident that NS synthesized with 1:4 ratios of polymer: crosslinker exhibits superior characteristics and better performance. Incorporation of CRN and CFN NS in topical gel executed therapeutically better effects in treating psoriasis than the conventional marketed formulation. Sustained drug release was achieved till the end of 12 h by prepared NS based topical gel. The prolonged release of drugs serves the purpose of synthesizing and loading NS into the topical gel formulation. Moreover, promising results of in vivo studies and ex vivo permeation studies have inveterate the improved potential of NS based gel to effectively alleviate psoriasis. Thus, the prepared CD NS based topical gel might be proposed as a promising carrier for an effective local treatment of psoriasis.
Acknowledgements
The author(s) express deep sense of gratitude towards JSS Academy of Higher Education and Research (JSSAHER), Mysuru, and University of Mysore, Mysuru, for provision of obligatory facilities to carry out present research work and Genespy Pvt. Ltd., Mysuru, for their valuable support in conducting histopathological analysis.
Author’s contribution
All the authors have contributed equally in reparation of the manuscript.
Funding information
This study did not receive any funds.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Michelle AL, Anne MB, James GK. Pathogenesis and therapy of psoriasis. Nature. 2007;445(22):866–872. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 2.Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019;20(6):1475. doi: 10.3390/ijms20061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehncke WH. Etiology and pathogenesis of psoriasis. Rheum Dis Clin N Am. 2015;41(4):665–675. doi: 10.1016/j.rdc.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Khanna NM. Turmeric - Nature’s precious gift. Curr Sci. 1999;76:1351–1356. [Google Scholar]
- 5.Golnaz S, Minoo A, Parvin M, Jinous A, Kosar R, Mehdi R. Topical turmeric Microemulgel in the Management of Plaque Psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14(3):865–876. [PMC free article] [PubMed] [Google Scholar]
- 6.Yunus P, Omid F, Stephen LA, Muhammed M, Alexandra EB, Thomas PJ, et al. Evidence of curcumin and curcumin analogue effects in skin diseases: a narrative review. J Cell Physiol. 2018;234(2):1165–1178. doi: 10.1002/jcp.27096. [DOI] [PubMed] [Google Scholar]
- 7.Vollono L, Falconi M, Gaziano R, et al. Potential of Curcumin in Skin Disorders. Nutrients. 2019;11(9):2169. doi: 10.3390/nu11092169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nardo VD, Gianfaldoni S, Tchernev G, et al. Use of Curcumin in Psoriasis. Open Access Maced. J Med Sci. 2018;6(1):218–220. doi: 10.3889/oamjms.2018.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varma SR, Sivaprakasam TO, Mishra A, Prabhu S, Rangesh RM. Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur J Pharmacol. 2017;813:33–41. doi: 10.1016/j.ejphar.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Baden HP, Gifford AM. Isometric contraction of epidermis and stratum corneum with heating. J Investig Dermatol. 1970;54(4):298–293. doi: 10.1111/1523-1747.ep12258594. [DOI] [PubMed] [Google Scholar]
- 11.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Boushey HA. Bronchodilators and other agents used in asthma. In: Katzung BG, editor. Basic and clinical pharmacology. 7. Los Altos (CA): Appleton & Lange; 1998. pp. 330–337. [Google Scholar]
- 13.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer.10; 2011. [DOI] [PMC free article] [PubMed]
- 14.Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris. J Dermatolog Treat. 2005;16(4):234–237. doi: 10.1080/09546630510011801. [DOI] [PubMed] [Google Scholar]
- 15.Yang HB, Song W, Chen LY. Differential expression and regulation of prohibition during curcumin-induced apoptosis of immortalized human epidermal HaCaT cells. Int J Mol Med. 2014;33(3):507–514. doi: 10.3892/ijmm.2014.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alashqar M, Goldstein N. Caffeine in the treatment of atopic dermatitis and psoriasis: a review. From Gene to Clinic International Congress, London: Psoriasis; 2017. [Google Scholar]
- 17.Osmani RAM, Hani U, Bhosale RR, Kulkarni PK, Shanmughanathan S. Nanosponge carriers- an archetype swing in cancer therapy: a comprehensive review. Curr Drug Targets. 2017;18(1):108–118. doi: 10.2174/1389450116666151001105449. [DOI] [PubMed] [Google Scholar]
- 18.Osmani RAM, Kulkarni PK, Shanmuganathan S, Hani U, Srivastava A, Prerana M, et al. A 32 full factorial design for development and characterization of a nanosponge-based intravaginal in situ gelling system for vulvovaginal candidiasis. RSC Adv. 2016;6:18737–18750. [Google Scholar]
- 19.Sai VC, Priti PP, Kisan RJ, Gajendra G, Vilasrao JK. Cyclodextrin-based nanosponges: a propitious platform for enhancing drug delivery. Expert Opin Drug Deliv. 2014;11:111–120. doi: 10.1517/17425247.2014.865013. [DOI] [PubMed] [Google Scholar]
- 20.Venkatalakshmi R, Jason Y. Development and evaluation of mouth dissolving tablets using natural super Disintegrants. J Young Pharm. 2017;9(3):332–335. [Google Scholar]
- 21.Sarfaraz A. Formulation and evaluation of clobetasopropionate loaded nanoemulsion gel containing tea tree oil. World J Pharm Pharm Sci. 2016;5(10):616–628. [Google Scholar]
- 22.Darandale SS, Vavia PR. Cyclodextrin-based nanosponges of curcumin: formulation and physicochemical characterization. J Incl Phenom Macrocycl Chem. 2013;75:315–322. [Google Scholar]
- 23.Osmani RAM, Aloorkar NH, Ingale D, Kulkarni PK, Hani U, Bhosale RR. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J. 2015;23(5):562–572. doi: 10.1016/j.jsps.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osmani RAM, Bhosale RR, Hani U, Vaghela R, Kulkarni PK. Cyclodextrin based nanosponges: impending carters in drug delivery and nanotherapeutics. Curr Drug Ther. 2015;10(1):3–19. [Google Scholar]
- 25.Kent S, Kristen MS, Marina RY, Kevin MY, Liselotte J, Gil Y. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016;157:2536–2543. doi: 10.1097/j.pain.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.William RS, Kellie AM, Andrew JS, Doina D, Yi F, Xianying X. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 2017;9(24):1–21. doi: 10.1186/s13073-017-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honary S, Zahir F. Effect of zeta potential on properties of Nano-drug delivery systems- a review. Trop J Pharm Res. 2013;12:255–264. [Google Scholar]
- 28.Chatterjee S, Hui PC, Kan CW, Wang W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci Rep. 2019;9(1):1–3. doi: 10.1038/s41598-019-48254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang D, Luo L, Jiang W, Lu Q, Rong M, Lai R. Curcumin shows excellent therapeutic effect on psoriasis in mouse model. Biochimie. 2016;123:73–80. doi: 10.1016/j.biochi.2016.01.013. [DOI] [PubMed] [Google Scholar]