Introduction
The current opioid epidemic is the largest drug use epidemic in the history of the USA for all racial and ethnic groups [1]. Fatal opioid overdose rates continue to be exceedingly high, with 2017 experiencing an 11% increase in opioid-related fatalities compared to 2016 and more than a 670% increase since the height of the heroin epidemic in 1975 [1, 2]. The rise in fatal opioid overdose since the 1970s heroin epidemic has differentially affected racial groups. In the 1970s, opioid mortality was higher among Black Americans [3, 4]. In recent years, the racial profile of opioid-related fatalities has changed and has been highest among non-Hispanic Whites, with White individuals accounting for 37,113 (78%) and Black individuals for 5513 (12%) of the 2017 opioid-related deaths [2]. These discrepant racial trends in fatal overdose can be partially attributed to the surge in prescription opioids starting in the mid-1990s, which caused a concentrated rise in mortality among Whites while opioid-related mortality among Black individuals remained stable [5]. This divergence is associated with well noted racial disparities in pain management and prescription patterns, with Black patients prescribed opioids at lower rates compared to all other racial/ethnic groups for almost every type of pain visit [5].
While overdose fatality has been highest among White Americans, in recent years, the fatal opioid death rate is increasing more rapidly among Black Americans. From 2016 to 2017, deaths increased by 11% among White and 25% among Black individuals [2]. One factor which may contribute to the recent increase in opioid fatalities among Black compared to White Americans is differential engagement with overdose prevention services including access to, training in, and use of naloxone. This hypothesis is driven by the extensive literature on racial differences in medical care, with Black Americans less likely to report access and utilization of a range of medical procedures and care compared to White Americans [6, 7].
Engagement with overdose prevention, specifically naloxone, is especially critical within the current opioid epidemic, which is characterized by synthetic opioids. Naloxone is a key medication utilized in the treatment of opioid overdoses and works by reversing the depression of the central nervous and respiratory systems caused by opioids. Synthetic opioids, such as fentanyl, are more potent than heroin and may require more than one dose of naloxone to reverse an overdose [8]. Despite naloxone’s important role in the ongoing opioid epidemic, naloxone access varies widely across states [9]. Even within jurisdictions, it is likely that access and utilization vary considerably across geographic areas and may differ by race.
Informed by prior findings of racial disparities in medical care access, the goal of the current study is to examine racial disparities in engagement in overdose prevention in Baltimore, MD, among a community sample of people who inject drugs (PWID). PWID represent a subgroup of people who use opioids and face an elevated risk of overdose. Baltimore City has reported the highest urban opioid overdose rates in the country, reporting 692 overdoses in 2017 [10]. In Baltimore City, the majority of residents self-identify as Black (63%) or White (32%) [11]. As a component of the Baltimore City overdose prevention efforts, the Baltimore City Health Department has responded to the opioid epidemic using a nationally recognized three-pronged model. The Baltimore City model includes increasing access to naloxone and opioid agonist treatment (OAT) as well as introducing campaigns to decrease the stigma of drug dependence. Engagement in overdose prevention may differ due to disparities in the need for overdose prevention services. Therefore, the first aim of this study is to assess whether racial disparities exist in rates of witnessed overdoses, thereby determining whether some racial/ethnic groups face greater needs for overdose response preparedness. The second aim is to examine racial disparities in engagement in overdose prevention through an assessment of disparities in access, training, and use of naloxone.
Methods
Study Design and Population
Study participants were recruited from December 2016 to March 2019 for a randomized clinical trial for an intervention to enhance Hepatitis C (HCV) and HIV prevention and care among people who use substances residing in impoverished neighborhoods in Baltimore, MD. Recruitment was conducted through street-based outreach, word-of-mouth, flyers, advertisements in local newspapers, and community agency referrals. All participants completed written informed consent, which was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. Participants completed face-to-face surveys administered by trained research assistants. The inclusion criteria for the baseline screening visit included being 18 years of age or older and having a history of lifetime injection drug use. Data for the current analysis was restricted to participants who identified as Black or White race and self-reported injection drug use in the past year (n = 372, 57% of the full sample). Naloxone use was assessed among a subsample of study participants who had ever witnessed at least one overdose (n = 345).
Ad hoc analyses were conducted among participants who reported injecting drugs in the past 6 months (n = 340) to examine disparities in engagement with needle exchange. These analyses examined racial disparities in engagement in needle exchange as well as the effect of engagement in needle exchange on naloxone access, training, and use. The analysis of naloxone use was restricted to participants who reported injecting in the past 6 months and witnessing at least one overdose in their lifetime (n = 316).
Measures
Witnessed Overdose and Engagement in Overdose Prevention
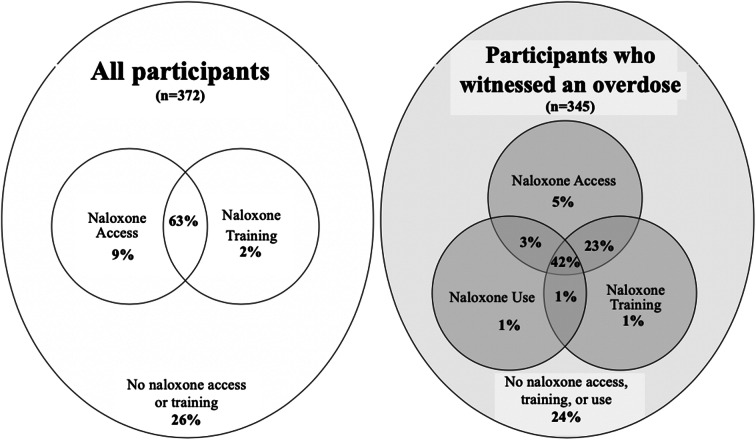
Witnessed overdose was assessed with the question, “in your lifetime how many overdoses have you witnessed?” Three additional questions assessed lifetime engagement in overdose prevention, which included access to, training in, and use of naloxone. Participants were asked to identify if they had ever been (1) prescribed or received a kit containing naloxone, (2) trained to use naloxone, and (3) used naloxone to reverse an opiate overdose (Fig. 1). If participants had not heard of naloxone (n = 16), they were coded as not having accessed, been trained in, or used naloxone.
Fig. 1.
Naloxone access, training, and use among people who inject drugs
Drug Use Characteristics and Demographics
Injection drug use recency compared those who had injected 6 to 12 months ago with those who had injected 4 to 6 months ago or within the past 3 months. Current use of opioid agonist treatment (OAT) included reported use of buprenorphine/naloxone, buprenorphine, methadone, and naltrexone at the time of the study visit. Lifetime number of personal and witnessed overdoses was measured as a continuous variable. Participants who injected drugs within the past 6 months were asked about engagement with needle exchange. Engagement in needle exchange was assessed by participants responding that they had gone to the needle exchange in the past 6 months to get needles for injecting drugs.
Race/ethnicity was self-reported and measured as “White” versus “Black or African-American.” Age was analyzed as a continuous measure. Gender was self-reported as male or female. Education was dichotomized as having completed high school or above compared to grade 11 or less. Participants who experienced homelessness in the past 6 months were compared to those who had not.
Analyses
We first assessed the difference between demographics, drug use characteristics, and engagement in overdose prevention by race using t-test and chi-squared analysis. Next, we conducted bivariate logistic regression analyses of variables, including race, which may be associated with witnessed overdose and overdose prevention. We then built multivariable models; the first model examined the association between race and number of witnessed overdoses using multivariable negative binomial modeling due to the right-skewed distribution of the outcome. The second set of models used multivariable logistic regression models to assess the relationship between race and naloxone access, training, and use. All multivariable models included demographic factors and other variables that may help account for racial differences. Among people who injected drugs in the past 6 months, ad hoc multivariate models also included engagement with needle exchange. Data were analyzed with STATA version 14 with a p-value < 0.05 considered statistically significant [12].
Results
The study sample was predominately male (67%), unemployed (92%), and had a high school education or above (66%; see Table 1). The sample was comprised of 55% Black and 45% White participants. The mean age was 44 years. Most of the sample reported injecting drugs in the past three months (85%) and were currently using OAT (63%). Participants had, on average, witnessed 9 overdoses in their lifetime and personally overdosed 3 times. Of the 16 participants who had not heard of naloxone, the majority (94%, n = 15) were Black. Approximately three-quarters of participants had access to naloxone (72%), and more than half (65%) had been trained on how to use naloxone. Among participants who witnessed an overdose, about half (47%) reported having used naloxone on an individual who was experiencing an overdose. Race was not significantly associated with current enrollment in OAT or engagement with needle exchange services. In bivariate analysis, White participants were significantly younger (< 0.001), completed more education (< 0.001), more likely to be homeless (< 0.001), and had personally experienced more overdoses (< 0.001) compared to Black participants.
Table 1.
T-test and chi-square models of demographics, drug use characteristics, and engagement in overdose prevention among people who inject drugs by race
| Characteristic | Number (%) | p-value | ||
|---|---|---|---|---|
| Total (N = 372) | Black (N = 203) | White (N = 169) | ||
| Sex, Male | 249 (67) | 132 (65) | 117 (69) | 0.391 |
| Age, mean (SD) | 44 (11) | 49 (10) | 38 (9) | < 0.001 |
| Education, graduated high school | 245 (66) | 116 (57) | 129 (76) | < 0.001 |
| Homelessness in the past 6 months | 214 (58) | 95 (47) | 119 (70) | < 0.001 |
| Injection drug recency | ||||
| 6 to 12 months ago | 32 (9) | 19 (9) | 13 (8) | 0.499 |
| 4 to 6 months ago | 25 (7) | 16 (8) | 9 (5) | |
| In past 3 months | 315 (85) | 168 (83) | 147 (87) | |
| Opioid agonist treatment | 234 (63) | 120 (59) | 114 (67) | 0.097 |
| Engagement in needle exchange (n = 340) | 169 (50) | 94 (51) | 75 (48) | 0.580 |
| Number of witnessed overdoses, mean (SD) | 9 (16) | 9 (19) | 8 (13) | 0.707 |
| Number of personal overdoses, mean (SD) | 3 (4) | 2 (3) | 4 (5) | < 0.001 |
| Naloxone access | 268 (72) | 130 (64) | 138 (82) | < 0.001 |
| Trained to use naloxone | 243 (65) | 117 (58) | 126 (75) | 0.001 |
| Used naloxone (n = 345) | 162 (47) | 67 (36) | 95 (60) | < 0.001 |
No racial differences were found in the incidence rate of witnessed overdose in the bivariate and multivariate analyses (see Table 2). In the adjusted model, the incidence rate of witnessed overdose was higher among older study participants adjusted incidence rate ratio (aIRR) 1.02; 95% CI 1.01–1.03), participants reporting recent injection drug use (aIRR 2.25; 95% CI 1.49–3.40 for past 3 months compared to past 6 to 12 months), and participants who had experienced more personal overdoses (aIRR 1.11; 95% CI 1.07–1.14).
Table 2.
Unadjusted and adjusted incidence rate ratio (IRR) and odds ratio (OR) models of witnessed overdose and overdose prevention among people who inject drugs
| Characteristics | Witnessed overdose (n = 372) | Access to naloxone (n = 372) | Naloxone training (n = 372) | Naloxone use (n = 345) | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted IRR (95% CI) | Adjusted IRR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Male (ref.: female) | 1.13 (0.88–1.44) | 1.11 (0.87–1.41) | 1.23 (0.75–2.01) | 1.47 (0.86–2.54) | 1.22 0.77–1.93 | 1.48 (0.89–2.46) | 1.26 (0.80–1.98) | 1.80* (1.07-3.04) |
| Age, mean (SD) | 1.02* (1.01–1.03) | 1.02* (1.01–1.03) | 0.99 (0.97–1.01) | 1.01 (0.99–1.04) | 0.99 (0.97–1.01) | 1.01 (0.99–1.04) | 0.97* (0.95–0.99) | 0.99 (0.97–1.02) |
| White (ref.: Black) | 0.93 (0.74–1.18) | 1.06 (0.83–1.36) | 2.50* (1.54–4.05) | 2.36* (1.29–4.30) | 2.15* (1.38–3.36) | 1.93* (1.11–3.37) | 2.64* (1.70–4.08) | 2.02* (1.17-3.50) |
| Graduated high school (ref.: grade 11 or less) | 0.94 (0 .74–1.21) | 1.10 (0.87–1.39) | 1.38 (0. 86–2.20) | 1.15 (0.68–1.94) | 1.44 (0.92–2.24) | 1.26 (0.77–2.06) | 1.54 (0.98–2.41) | 1.31 (0.79–2.17) |
| Homeless | 0.87 (0.69–1.10) | 1.08 (0.84–1.38) | 1.17 (0.74–1.84) | 1.19 (0.70–2.02) | 1.35 (0.88–2.08) | 1.47 (0.89–2.43) | 1.73* (1.12–2.69) | 1.79* (1.06-3.01) |
| Injection drug recency (ref: 6 to 12 months ago) | ||||||||
| 4 to 6 months ago | 1.63 (0.89–3.00) | 1.82 (1.01–3.29) | 0.68 (0.23–2.04) | 0.59 (0.18–1.91) | 0.74 (0.26–2.13) | 0.62 (0.20–1.90) | 1.18 (0.33–4.18) | 0.91 (0.23–3.62) |
| In past 3 months | 2.30* (1.50–3.54) | 2.25* (1.49–3.40) | 1.25 (0.57–2.75) | 1.53 (0.66–3.57) | 1.39 (0.66–2.92) | 1.62 (0.74–3.57) | 3.23* (1.34–7.79) | 3.17* (1.23-8.14) |
| Opioid agonist treatment | 0.94 (0.74–1.19) | 0.98 (0.78–1.24) | 3.32* (2.08–5.31) | 3.45* (2.08–5.74) | 2.9 * (1.86–4.52) | 3.11* (1.93–5.02) | 1.63* (1.04–2.54) | 1.81* (1.10-2.98) |
| Number of personal overdoses | 1.10* (1.07–1.14) | 1.11* (1.07–1.14) | 1.15* (1.06–1.26) | 1.09 (1.00–1.20) | 1.11* (1.04–1.19) | 1.06 (0.98–1.14) | 1.12* (1.05–1.19) | 1.06 (0.99–1.14) |
| Number of witnessed overdoses | – | 1.03* (1.00–1.06) | 1.02 (0.99–1.05) | 1.03* (1.00–1.05) | 1.02 (0.99–1.04) | 1.05* (1.02–1.08) | 1.04* (1.01–1.07) | |
*p value < 0.05
Race was associated with all measures of overdose prevention in both the bivariate analyses and multivariate models. White participants had significantly higher odds of naloxone access (aOR 2.36; 95% CI 1.29–4.30), naloxone training (aOR 1.93; 95% CI 1.11–3.37), and naloxone use (aOR 2.02; 95% CI 1.17–3.50) compared to Black participants. Current enrollment in OAT increased odds of naloxone access (aOR 3.45; 95% CI 2.08–5.74), naloxone training (aOR 3.11; 95% CI 1.93–5.02), and naloxone use (aOR 1.81; 95% CI 1.10–2.98).
The ad hoc models of participants who reported injecting drugs within the past 6 months additionally adjusted for engagement with needle exchange services (results not shown). Engagement with needle exchange increased the odds of naloxone access (aOR 2.23; 95% CI 1.30–3.83; n = 340), training (aOR 2.53; 95% CI 1.52–4.21; n = 340), and use (aOR 2.36; 95% CI 1.43–3.91; n = 316).
Discussion
This study examined racial differences in engagement in overdose prevention in a sample of PWID, a high-risk population. In this study population, the vast majority of study participants reported witnessing at least one overdose and, on average, participants had witnessed multiple overdoses in their lifetime. The experience of witnessing an overdose did not differ by race in this study, indicating that White and Black participants had an equal need for naloxone access, training, and use. This finding is aligned with Bohnert, Tracy, and Galea (2012) who found no differences in witnessed overdose by race among PWID in a New York City community-based sample [13].
The likelihood of witnessing an overdose did not significantly differ by race indicating an equal need for overdose response preparedness. Yet, this study’s findings identified significant racial inequalities in engagement in overdose prevention with Black participants reporting less engagement. Baltimore City has ongoing public health intervention efforts to ensure widespread distribution of naloxone throughout the City, yet there is a disparity in engagement in naloxone training, access, and use identified in this study, which warrants further investigation and action by public health practitioners. While this study did not find differential engagement in OAT or the needle exchange by race, access to other sources of naloxone in the city may help explain the racial disparities in naloxone access, training, and use. Other primary sources of naloxone training in Baltimore are clinics and pharmacies [14]. Racial disparities in engagement with these services may be explained by a number of factors.
Stigma is a potential driver of racial disparities in engagement with overdose prevention services [15]. A recent study in Australia found that PWID who felt stigmatized because of their drug use were less likely to access preventive services [16]. A qualitative study from the USA suggests heightened substance use stigma and discrimination among Black individuals who use substances [17]. The study found that the double stigma of racial prejudice and substance use negatively affected the use of substance use treatment services [17]. Double stigma among Black PWID may also differentially impact engagement in overdose prevention services.
Medical mistrust may be another potential explanation of these findings. Previous research on racial differences in medical care has documented historical maltreatment and a lack of trust in and comfort with health care services among Black populations [6, 18]. While mistrust of the medical system is experienced by some Black Americans, research indicates that this attitude can be modified [6]. Medical mistrust may also influence engagement with overdose prevention services. An ethnographic study by Merrill and colleagues found that medical mistrust may be a particularly important issue for people who use opioids and their providers, as they may display mutual mistrust in each other [19]. Research on cultural competence training suggests that providers can improve their self-awareness and the quality of their relationships with patients [18]. A step towards addressing racial disparities in overdose prevention may be to ensure cultural appropriateness of overdose prevention services through engaging diverse providers and promoting cultural competence training.
Public health practitioners should be vigilant that services are equally accessible to diverse populations. Other strategies to increase naloxone training and use among Black PWID or other groups with lower rates of uptake include social network interventions, which have been identified as a strategy to diffuse behavior change to hard-to-reach populations [20]. A potential avenue to engage Black PWID is through peer educators distributing naloxone and providing training on how to use it.
While stigma and medical distrust have been identified as sources of racial disparities in general health care utilization research, engagement in overdose prevention services may be uniquely affected by policing practices. Police harassment and arrests of PWID is a long-standing issue in Baltimore, as in many cities [21]. Current policies in Baltimore, such as the Good Samaritan Law, protect PWID who attempt to help an overdose victim from prosecution if found engaging in some illegal activities on the scene of an overdose [22]. Yet, a large body of research illuminates that harassment and arrests by law enforcement are disproportionately focused on PWID and Black Americans [23–26]. This may, in turn, negatively impact interest in engaging in harm reduction both for self and others if there is a perception of an increased risk of criminalization. Future research should examine how fear of police practices may dissuade PWID from engaging in fatal overdose prevention services. Public health practitioners should also work to normalize use of overdose prevention services for both people who use and do not use opioids. By marketing these services for a larger audience, engagement in overdose prevention services does not need to identify a client as a person who uses drugs. For example, Black churches have been utilized as partners in health promotion for related topics, including HIV/AIDS prevention and drug use [27, 28]. Church partnerships can mitigate issues such as drug use stigma and police harassment by offering education and naloxone kits to all congregants, not just PWID.
This study is not without limitations. The study participants were recruited by street outreach and were not a random sample. They were primarily White or Black race, and the small number of participants from other races and ethnicities limited our ability to analyze differences among other racial groups. Additionally, all information was self-reported and may be subject to recall and social desirability biases. We were also wary of over adjusting for factors that may be linked to race due to long-standing patterns of racial inequality, segregation, and racism, which could obscure the relationship between race and health outcomes. For example, employment was not included in the model, which in prior studies has been found to be associated with access to health care. In this sample, unemployment was high (92%) and did not differ by race. Including employment status in the models did not qualitatively change study findings. As there were racial differences in factors such as age and homelessness in this sample, we felt it prudent to keep these variables, demographic factors, and other factors that may be linked to race in the model. Although race was slightly attenuated in the multivariate models, it remained strongly associated with naloxone access, training, and use.
The racial differences in naloxone access, training, and use evident in these findings suggest that research in other communities should investigate how social and structural factors may undermine engagement with overdose prevention and create racial inequalities. This research can inform a public health response dedicated to ensuring equitable access to and adoption of life-saving overdose prevention strategies.
Acknowledgments
Study participants, NIH grant DA040488 supported this research. We thank Roeina Love, Tonya Johnson, Denise Mitchell, Charles Moore, Marlesha Bates, Abby Winiker and Joanne Jenkins for their assistance and support in data collection. The funders had no role in the study design, analyses, interpretation, or writing. None of the authors have financial conflicts of interests.
Compliance with Ethical Standards
All participants completed written informed consent, which was approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.James K, Jordan A. The opioid crisis in black communities. J Law, Med Ethics. 2018;46(2):404–421. doi: 10.1177/1073110518782949. [DOI] [PubMed] [Google Scholar]
- 2.Scholl S, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;67:1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander MJ, Kiang MV, Barbieri M. Trends in black and white opioid mortality in the United States, 1979-2015. Epidemiology. 2018;29(5):707–715. doi: 10.1097/EDE.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 5.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA - J Am Med Assoc. 2008;299(1):70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 6.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(SUPPL. 1):146–161. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- 7.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(SUPPL. 1):108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 8.Fairbairn N, Coffin PO, Walley AY. Naloxone for heroin, prescription opioid, and illicitly made fentanyl overdoses: challenges and innovations responding to a dynamic epidemic. Int J Drug Policy. 2017;46:172–179. doi: 10.1016/j.drugpo.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman PR, Hankosky ER, Lofwall MR, Talbert JC. The changing landscape of naloxone availability in the United States, 2011–2017. Drug Alcohol Depend. 2018;191:361–364. doi: 10.1016/j.drugalcdep.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maryland Department of Health. Unintentional drug- and alcohol-related intoxication deaths in Maryland Annual Report 2017. Baltimore, Maryland: Maryland Department of Health; 2018.
- 11.United States Census Bureau. Quick Facts (2018): Baltimore City, Maryland. https://www.census.gov/quickfacts/fact/table/baltimorecitymarylandcounty/AGE295218. Accessed 19 Aug 2019.
- 12.StataCorp. Stata Statistical Software: Release 14. 2014.
- 13.Bohnert ASB, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1–3):168–173. doi: 10.1016/j.drugalcdep.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dayton L, Gicquelais RE, Tobin K, Davey-Rothwell M, Falade-Nwulia O, Kong X, Fingerhood M, Jones AA, Latkin C. More than just availability: who has access and who administers take-home naloxone in Baltimore, MD. PLoS One. 2019;14(11):1–11. doi: 10.1371/journal.pone.0224686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe C, Santos G, Vittinghoff E, Wheeler E, Davidson P, Coffin P. Predictors of participant engagement and naloxone utilization in a community-based naloxone distribution program. Addiction. 2015;110:1301–1310. doi: 10.1111/add.12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Hippel C, Brener L, Horwitz R. Implicit and explicit internalized stigma: relationship with risky behaviors, psychosocial functioning and healthcare access among people who inject drugs. Addict Behav. 2018;76(July 2017):305–311. doi: 10.1016/j.addbeh.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 17.Scott MC, Wahl OF Substance abuse stigma and discrimination among African American male substance users. Stigma Res Action. 2011;1(1):60–66. doi: 10.5463/sra.v1i1.3. [DOI] [Google Scholar]
- 18.Cooper LA, Beach MC, Johnson RL, Inui TS. Delving below the surface: understanding how race and ethnicity influence relationships in health care. J Gen Intern Med. 2006;21(SUPPL. 1):21–27. doi: 10.1111/j.1525-1497.2006.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrill J, Rhodes L, Deyo R, Marlatt A, Bradley K. Mutual mistrust in the medical care of drug users. J Gen Intern Med. 2002;17:327–333. doi: 10.1080/13200968.1996.11077211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latkin CA, Knowlton AR. Social network assessments and interventions for health behavior change: a critical review. Behav Med. 2015;41(3):90–97. doi: 10.1080/08964289.2015.1034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beletsky L, Cochrane J, Sawyer AL, Serio-Chapman C, Smelyanskaya M, Han J, Robinowitz N, Sherman SG. Police encounters among needle exchange clients in Baltimore: drug law enforcement as a structural determinant of health. Am J Public Health. 2015;105(9):1872–1879. doi: 10.2105/AJPH.2015.302681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baltimore City Health Department. Baltimore city health commissioner signs new standing order for opioid overdose reversal medication. https://health.baltimorecity.gov/news/press-releases/2017-06-01-baltimore-city-health-commissioner-signs-new-standing-order-opioid. Published 2017. Accessed 14 Jul 2019.
- 23.Davis C, Burris S, Kraut-Becher J, Lynch K, Metzger D. Effects of an intensive street-level police intervention on syringe exchange program use in Philadelphia, Pa. Am J Public Health. 2005;95(1):233–236. doi: 10.2105/AJPH.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jürgens R, Csete J, Amon JJ, Baral S, Beyrer C. People who use drugs, HIV, and human rights. Lancet. 2010;376(9739):475–485. doi: 10.1016/S0140-6736(10)60830-6. [DOI] [PubMed] [Google Scholar]
- 25.Dottolo AL, Stewart AJ. “Don’t ever forget now, you’re a Black man in America”: intersections of race, class and gender in encounters with the police. Sex Roles. 2008;59(5–6):350–364. doi: 10.1007/s11199-007-9387-x. [DOI] [Google Scholar]
- 26.Brunson R. “Police don’t like black people”: African-american young men’s accumulated police experiences. Criminol Public Policy. 2007;6(1):71–102. doi: 10.1111/j.1745-9133.2007.00423.x. [DOI] [Google Scholar]
- 27.Sutherland M, Hale CD, Harris GJ. Community health promotion: the church as partner. J Prim Prev. 1995;16(2):201–216. doi: 10.1007/BF02407340. [DOI] [PubMed] [Google Scholar]
- 28.Szaflarski M, Vaughn LM, Chambers C, et al. Engaging religious institutions to address racial disparities in HIV/AIDS: a case of academic-community partnership. Int J Res Serv Commun Engage. 2014;2(1):95–114. [PMC free article] [PubMed] [Google Scholar]