Abstract
Background
Platinum-based chemotherapy in non-small cell lung cancer (NSCLC) has been demonstrated as a promising approach by many researchers. However, due to low bioavailability and several side effects, drug targeting to lungs by intravenous administration is not a common route of administration.
Objective
In this study, oxaliplatin loaded polycaprolactone (PCL) nanoparticles were prepared to overcome the limitations of the drug. 33 factorial design was used to evaluate the combined effect of the selected variables on the nanoparticle characteristics and to optimize oxaliplatin loaded PCL nanoparticles.
Methods
The factorial design was used to study the influence of three different independent variables on the response of nanoparticle particle size, polydispersity index (PDI), zeta potential, and encapsulation efficiency. The cellular uptakes of oxaliplatin loaded nanoparticles with different molecular weights of PCL were evaluated. Moreover, optimized nanoparticles were evaluated for their efficacy in non-small lung cancer using the SK-MES-1 cell line.
Results
In factorial design, it is found that the homogenization speed and surfactant ratio represented the main factors influencing particle size and PDI and did not seem to depend on the PCL ratio. While the cytotoxicity of free oxaliplatin and oxaliplatin loaded nanoparticles were similar in low drug doses (2.5 and 25 μg/mL), the cytotoxicity of oxaliplatin loaded nanoparticles on SK-MES-1 cell was found higher in higher doses (p < 0.05). Moreover, oxaliplatin nanoparticles formulated with different molecular weights of PCL did not show significant differences in cellular uptake in 1 h and 2 h. However, the uptake of PCL80000 NPs was found significantly greater than free oxaliplatin at 4 h (p < 0.05).
Conclusion
Hence, the development of oxaliplatin loaded PCL nanoparticles can be a useful approach for effective NSCLC therapy.
Graphical abstract
Development, optimization and in vitro evaluation of oxaliplatin loaded nanoparticles in non-small cell lung cancer
Keywords: Factorial design, Polycaprolactone, Nanoparticles, Oxaliplatin, Non-small cell lung cancer, In vitro
Introduction
Many researchers have demonstrated the widespread use of platinum-based chemotherapy in several forms of cancer, including non-small cell lung cancer (NSCLC), as it is shown that cisplatin-based chemotherapy may increase survival and improve quality of life [1]. However, the treatment of cancer by cisplatin and carboplatin is limited due to their side effects, such as cumulative nephrotoxicity, neurotoxicity, and emetogenesis [2]. For this reason, several less toxic platinum analogs have been synthesized and tested for anticancer activity. Oxaliplatin is a diaminocycloexane platinum which shows more significant activity against various types of tumor. It is shown that oxaliplatin has dose-limiting neurological toxicity, but it lacks nephrological and gastrointestinal toxicity [3].
Even though it’s superior tolerability in comparison to other platin compounds, oxaliplatin is associated with several side effects that limit the range of usable doses. Moreover, effective treatment with conventional chemotherapy demands high drug doses, which may cause unwanted side effects at sites other than those associated with the tumors [4].
The use of controlled drug release systems has many benefits compared to conventional dosage forms. Mainly, the effect of the drug is related to its binding constant and its local concentration on the site of action [5]. Moreover, the efficacy of drugs is inhibited by the efflux of the drug from the target cell, driven by the multidrug drug resistance (MDR), especially in cancer cells. With controlled release systems, the side effect of drugs can be minimized and controlled, and prolonged efficacy can be maintained. Also, permeability and retention of drugs to tumoral tissues are enhanced by nanoparticle administration [6, 7].
Polycaprolactone (PCL) is a biodegradable and biocompatible polymer that is used for the formulation of controlled and targeted delivery systems. PCL controls the release of drugs by extending mean residence time, thus enhancing anti-tumor activity. When PCL nanoparticles are ingested or injected, they can be tailored for desired release profiles and, in some cases, can even provide organ-targeted release [8].
The shape, size range, and drug loading of nanoparticles are essential factors in drug targeting, and these parameters are affected by several factors, including the amount of polymer, preparation method of nanoparticles, percentage of surfactant, applied energy amount, and volume of organic and aqueous phases. It is not easy to show the effect of all parameters and optimize the formulations. Hence, the design of experiments is used for the establishment of a quantitative relationship between the formulation variables and their interactions [9]. Formulations can be optimized with minimum experiments, and the effects can be mathematically modeled by factorial design [10].
In this study, 33 full factorial design was used to evaluate the combined effect of the selected variables on the nanoparticle characteristics and to optimize oxaliplatin loaded polycaprolactone (PCL) nanoparticles. Moreover, optimized nanoparticles were evaluated for their efficacy in non-small lung cancer using the SK-MES-1 cell line. Among the nanoparticle characteristics, particle size controls the residence time in the reticuloendothelial system. Also, it affects the cellular uptake of the drug, zeta potential of the particle, which is an essential parameter for stability and encapsulation efficiency, which should be maximum to carry higher drug content to the desired organ. The factors were homogenization speed, the stabilizer (polyvinyl alcohol (PVA)), and polymer (PCL) concentration. Also, in this study, PCL was selected for the preparation of the nanoparticles due to its biocompatible and biodegradable properties, higher cellular uptake by mucosal surfaces, and sustained release properties [10, 11].
Materials and methods
Materials
Oxaliplatin was a gift from Kocak Farma (Istanbul, Turkey). PCL (average Mn ~10,000), (average Mn ~45,000), and the (average Mn ~80,000), dichloromethane, and PVA (Mowiol 4–88) were purchased from Sigma (Taufkirchen, Germany). The tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade.
Preparation of oxaliplatin loaded nanoparticles
Oxaliplatin loaded nanoparticles were prepared by the emulsification solvent evaporation method with minor modifications using a previously described protocol [12]. Different amounts of PCL (average Mn ~10,000) were dissolved in 3 mL of dichloromethane under magnetic stirring until a clear solution was observed. The inner aqueous phase was prepared by mixing 3 mg oxaliplatin with distilled water. The PCL solution was then slowly added to the inner aqueous phase. The solution was then homogenized using a high shear mixer with a suitable probe (Ultraturrax T18, IKA, Staufen, Germany) at different speeds for 8 min. The obtained first emulsion was slowly added to a 10 mL PVA solution and homogenized again for 8 min. The organic solvent was evaporated under vacuum using a rotary evaporator (R 215, Buchi, Essen, Germany). The nanoparticles were purified by centrifugation at 10,000 RPM for 15 min at 4 °C (MP4R, IEC Centra, Columbus, OH, USA) triplicate and lyophilized (Gamma 2–20, Crist, Osterode am Harz, Germany).
Experimental design
In this study, a 33 factorial design was used to formulate oxaliplatin loaded PCL nanoparticles and to determine the effect of three independent variables (homogenization speed (X1), the concentration of surfactant (X2) and concentration of polymer (X3)) on particle size (Y1), polydispersity index (Y2), zeta potential (Y3) and encapsulation efficiency (%EE) (Y4). Each factor was tried at three levels, selected as −1, 0, and + 1. Investigational conditions of the three factors at each level in our study were given in Table 1. “Design Expert” software was used to create the combinations of these factors at three levels, and the whole design consisted of 27 runs of trials with three replicates of each set of conditions.
Table 1.
The 33 factorial design
| Homogenization Speed (rpm) | PVA Concentration (%) | PCL Concentration (%) | Particle Size (nm) | PDI | Zeta Potential (mV) | EE (%) | |
|---|---|---|---|---|---|---|---|
| 1 | 6400 | 2 | 3 | 1772.13 ± 102.28 | 0.572 ± 0.023 | −14.84 ± 2.11 | 35.91 ± 1.44 |
| 2 | 13,400 | 0.3 | 2 | 1107.56 ± 8.32 | 0.007 ± 0.003 | −19.27 ± 2.78 | 30.10 ± 1.18 |
| 3 | 13,400 | 0.3 | 1 | 1225.10 ± 59.62 | 0.362 ± 0.054 | −19.66 ± 0.44 | 46.50 ± 2.11 |
| 4 | 20,400 | 2 | 3 | 545.01 ± 4.90 | 0.259 ± 0.020 | −17.53 ± 1.38 | 24.90 ± 0.87 |
| 5 | 6400 | 1 | 2 | 1813.03 ± 141.22 | 0.656 ± 0.088 | −12.32 ± 3.74 | 29.14 ± 1.04 |
| 6 | 6400 | 1 | 3 | 2542.84 ± 121.68 | 0.933 ± 0.172 | −4.45 ± 3.81 | 29.88 ± 1.36 |
| 7 | 13,400 | 2 | 3 | 849.19 ± 7.30 | 0.150 ± 0.014 | −13.10 ± 2.51 | 26.20 ± 1.13 |
| 8 | 20,400 | 1 | 2 | 548.44 ± 3.91 | 0.383 ± 0.017 | −9.47 ± 0.79 | 31.18 ± 0.99 |
| 9 | 20,400 | 0.3 | 1 | 500.03 ± 8.17 | 0.400 ± 0.013 | −11.21 ± 0.86 | 27.69 ± 1.14 |
| 10 | 20,400 | 0.3 | 3 | 662.39 ± 10.92 | 0.268 ± 0.030 | −8.98 ± 2.30 | 25.39 ± 1.04 |
| 11 | 13,400 | 2 | 2 | 620.88 ± 3.61 | 0.339 ± 0.021 | −20.34 ± 1.10 | 29.17 ± 0.96 |
| 12 | 20,400 | 2 | 1 | 462.42 ± 5.76 | 0.257 ± 0.039 | −12.04 ± 2.98 | 18.52 ± 0.83 |
| 13 | 20,400 | 0.3 | 2 | 585.60 ± 1.21 | 0.376 ± 0.018 | −17.48 ± 1.50 | 25.80 ± 1.03 |
| 14 | 6400 | 0.3 | 3 | 4177.24 ± 215.19 | 0.992 ± 0.211 | −11.81 ± 3.17 | 19.26 ± 0.96 |
| 15 | 6400 | 1 | 1 | 3033.50 ± 270.13 | 0.925 ± 0.184 | −16.20 ± 0.96 | 19.78 ± 0.84 |
| 16 | 13,400 | 2 | 1 | 699.38 ± 10.24 | 0.306 ± 0.023 | −12.59 ± 0.96 | 22.45 ± 1.02 |
| 17 | 6400 | 2 | 1 | 1526.87 ± 107.30 | 0.642 ± 0.063 | −14.27 ± 2.61 | 15.21 ± 0.65 |
| 18 | 13,400 | 1 | 2 | 662.71 ± 5.66 | 0.278 ± 0.009 | −20.40 ± 0.48 | 19.66 ± 0.93 |
| 19 | 13,400 | 1 | 3 | 1081.40 ± 130.39 | 0.126 ± 0.013 | −17.42 ± 4.31 | 23.28 ± 1.15 |
| 20 | 6400 | 0.3 | 2 | 8293.91 ± 226.72 | 1.186 ± 0.237 | −19.04 ± 0.70 | 18.37 ± 0.95 |
| 21 | 13,400 | 0.3 | 3 | 1591.30 ± 88.39 | 0.234 ± 0.012 | −9.51 ± 2.17 | 18.88 ± 0.82 |
| 22 | 13,400 | 1 | 1 | 732.76 ± 11.94 | 0.440 ± 0.024 | −16.91 ± 2.14 | 25.17 ± 1.12 |
| 23 | 20,400 | 1 | 1 | 453.81 ± 6.90 | 0.314 ± 0.011 | −16.23 ± 3.03 | 17.37 ± 0.88 |
| 24 | 20,400 | 2 | 2 | 417.18 ± 10.32 | 0.303 ± 0.010 | −8.053 ± 0.17 | 30.10 ± 0.74 |
| 25 | 6400 | 0.3 | 1 | 14,961.73 ± 533.69 | 1.575 ± 0.183 | −12.67 ± 3.17 | 57.00 ± 1.39 |
| 26 | 20,400 | 1 | 3 | 379.68 ± 8.81 | 0.293 ± 0.009 | −21.41 ± 4.07 | 34.15 ± 1.24 |
| 27 | 6400 | 2 | 2 | 2289.24 ± 12.36 | 0.653 ± 0.013 | −15.77 ± 1.70 | 23.53 ± 1.02 |
Characterization of nanoparticles
Particle size and zeta potential
Mean particle size, size distribution, and zeta potential of nanoparticles were determined by photon correlation spectroscopy after nanoparticle suspensions were diluted with water (Z3000, Nicomp, Port Richey, FL, USA). The mean particle size was calculated as the z-average diameter and the size distribution of the nanoparticles as the polydispersity index (PDI). For scanning electron microscope (SEM) studies, lyophilized nanoparticles were attached on a surface and covered with gold. Surface morphologies of nanoparticles were observed through a SEM (Zeiss, Jena, Germany) at 20 kV.
Determination of encapsulation efficiency
Encapsulation of oxaliplatin in PCL nanoparticles was determined by previously described high-performance liquid chromatography (HPLC) method [13]. The method was further partially validated after minor changes. 5 mg of lyophilized nanoparticles were dissolved in acetonitrile (10 ml), 10 μL of nanoparticle solution was injected into HPLC with a C18 column (4.6 × 150 mm), and the amount of oxaliplatin was determined by DAD detector at 240 nm (Agilent 1100). The mobile phase was prepared with 5 mM 1-octanesulfonic acid (pH 3.4): methanol (95:5). The HPLC method was validated, and the calibration curve for the quantification of oxaliplatin was linear over the range of standard concentration of oxaliplatin at 0.03–300 μg/mL with a determination coefficient of R2 = 0.9998. Limit of detection (LOD) and limit of quantification (LOQ) were 0.008 μg/mL and 0.023 μg/mL, respectively.
The percent of oxaliplatin encapsulation and drug loading was calculated with the following formula:
The calculated values for three replicate determinations, and their mean values ± SD were reported.
In vitro oxaliplatin release
Drug release studies were performed in vitro in phosphate-buffered saline (PBS) (pH 7.4). A known mass of oxaliplatin loaded nanoparticles was suspended in the PBS (pH 7.4) with sonication in centrifuge tubes. 2 mL of the nanoparticle suspension was sealed in a dialysis tube (12–14 kDa, SpectrumLabs, New Brunswick, NJ, USA) and incubated in 30 mL PBS with stirring by magnetic stirrer at 100 rpm at 37 °C. At predetermined time intervals, 1 mL of the sample was collected from the released media and replaced with fresh PBS. The released oxaliplatin was analyzed by HPLC at 240 nm.
For evaluation of release kinetics, the obtained release data were fitted into first order, zero-order, and Higuchi equations. The selection of the best model was based on the comparisons of the relevant correlation coefficients.
Stability of nanoparticles
The stability of prepared nanoparticles was evaluated by measurement nanoparticle size, size distribution, and zeta potential as a function of storage period (30 days) at room temperature.
Cytotoxicity assay
Cytotoxicity of free oxaliplatin and oxaliplatin loaded nanoparticles were measured by calculation of cell growth inhibition using a tetrazolium dye (MTT) assay. Dulbecco’s Modified Eagle’s Medium (DMEM) was used as a cell growth medium, and a humidified atmosphere (5% CO2) was maintained for cell culture. SK-MES-1 cells were obtained (ATCC HTB-58, Manassas, VA, USA) and seeded on 96 wells. The MTT assay was performed, and the percentage of cell viability was determined after incubating the cells with various concentrations of free oxaliplatin and oxaliplatin loaded nanoparticles for 24 and 48 h.
Cellular uptake of nanoparticles
SK-MES-1 cells were used for studying the cellular uptake of formulations. In this study, 12-well plates were seeded with cells at a density of 5 × 104 per well, and the cells were allowed to attach for 24 h. The medium in each well was exchanged with 1 ml of freshly prepared nanoparticle suspension in the medium, and the plates were incubated for 1, 2, and 4 h. Cells were then washed thrice with ice-cold PBS to remove the nanoparticles, which were not internalized. Cells were then trypsinized. A 100 μL of each cell lysate aliquot was withdrawn and extracted by shaking each sample with 1 ml methanol. The solution was analyzed for oxaliplatin by a HPLC method described previously.
Apoptosis induced by oxaliplatin nanoparticles
Annexin V binding assay was performed to examine if the encapsulation of oxaliplatin in nanoparticles modifies apoptosis. Briefly, SK-MES-1 cells were seeded in flasks, and flasks were co-incubated with free oxaliplatin or oxaliplatin loaded nanoparticle formulations at 37 °C for 24 h. Then the cells floating in the supernatant were combined with the adherent fraction and washed with PBS three times. Cells were incubated with Annexin V-FITC for another 15 min at room temperature in the darkness and immediately analyzed in a flow cytometer (CytoFLEX, Indianapolis, IN, USA).
Statistical analysis
The results of in vitro data were analyzed by statistical software GraphPad Prism using Student’s t test and ANOVA to demonstrate statistical differences (p < 0.05). All results are expressed as mean ± standard deviation.
Results and discussion
Factorial design
The emulsification solvent evaporation method involves the formation of stable droplets of the drug-containing polymer solution (inner phase), emulsification of this phase with another aqueous phase (outer phase), and the subsequent removal of organic solvent from the droplets [14]. Essential factors for drug-loaded nanoparticles are high encapsulation efficiency, suitable zeta potential, particle size, and size distribution. However, there are many factors which can affect the nanoparticle characteristics. Previous studies showed that the nanoparticle preparation by emulsification solvent evaporation method is influenced by the polymer concentration, emulsifier concentration, and applied energy [15]. Hence, 33 factorial design was used for the calculation of the effect of design parameters on the preparation and optimization of nanoparticles. In this study, the effect of homogenization speed (X1), surfactant concentration (X2), and polymer concentration (X3) on particle size (Y1), polydispersity index (Y2), zeta potential (Y3), and encapsulation efficiency (%) (Y4) were investigated. The identification of significant variables was obtained by the elimination of variables having p value >0.05 in the model, and the model was calculated for independent variables [16, 17].
Effect of the independent variables on oxaliplatin loaded nanoparticles
Particle size
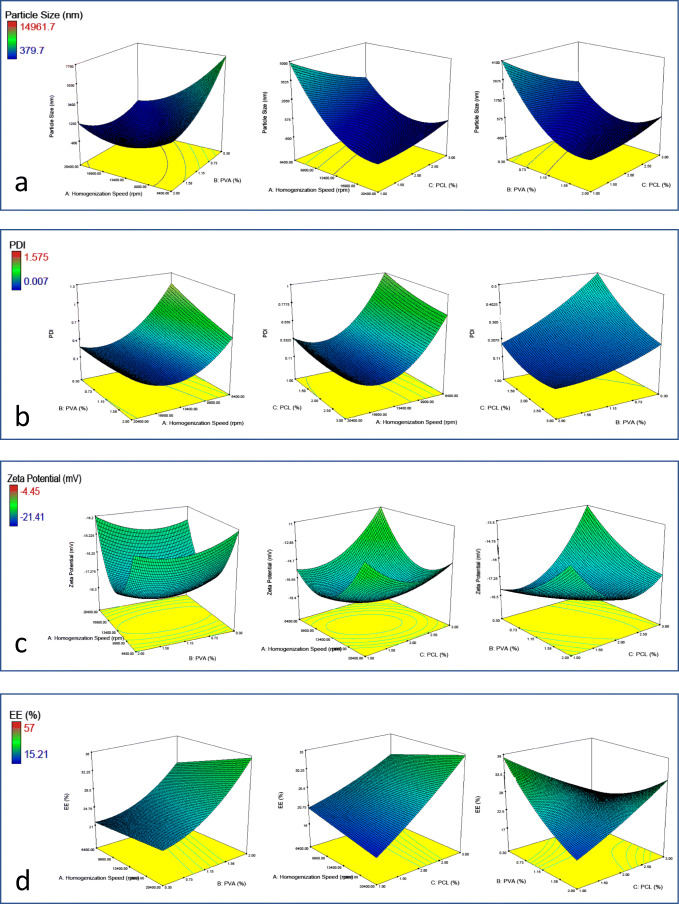
The mean particle size of all the 27 batches of oxaliplatin loaded nanoparticles was shown in Table 1. The mean particle sizes of all formulations were found in the range of 379.68 ± 8.81 to 14,961.73 ± 533.69 nm based on the variables of PCL concentration, PVA concentration, and homogenization speed. The response (Y1) obtained at various levels of two independent variables were subjected to multiple regression to give a quadratic polynomial equation. The effects of each factor and their interactions were statistically significant in the size of nanoparticles prepared with a p < 0.05.
Table 2 shows that changes in process variables dramatically changed the particle size. Previous studies have shown that selection of PVA as an emulsifier generally resulted in uniform small-sized particles, but in our study, it is found that microparticles (>1000 nm) are formed when 0.3% concentration of PVA is used. Also, a decrease in mean particle size is observed with the higher PVA concentrations. The stabilizing effect of emulsifiers can explain this phenomenon. The stabilizer ratio plays a critical role in the emulsification process and stabilization of nanoparticles in the emulsification solvent evaporation method. In low concentrations, an insufficient amount of emulsifier could not stabilize the nanoparticles and some of the aggregate. So larger particle sizes occur [18]. Generally, the higher concentrations of PVA decrease the particle size of nanoparticles, but also adding PVA to the formulation causes an increase in the viscosity of the outer phase in the emulsification process, which causes larger particles. Hence, the change in concentration of PVA from 1% to 2% did not affect the particles as much as the increase from 0.3% to 1% [19].
Table 2.
Parameters of the response surfaces obtained from a 33 factorial design
| Particle size | PDI | Zeta potential | Encapsulation efficiency | |||||
|---|---|---|---|---|---|---|---|---|
| F | p value | F | p value | F | p value | F | p value | |
| Source | Value | Prob > F | Value | Prob > F | Value | Prob > F | Value | Prob > F |
| Model | 5.739 | 0.0010* | 12.99 | < 0.001* | 0.90 | 0.5407 | 1.50 | 0.2249 |
| X1-Homogenization Speed | 17.976 | 0.0006* | 58.47 | < 0.001* | 0.001 | 0.9747 | 0.11 | 0.7433 |
| X2-PVA | 8.909 | 0.0083* | 8.19 | 0.0108* | 0.004 | 0.9478 | 1.47 | 0.2422 |
| X3-PCL | 1.284 | 0.2729 | 3.99 | 0.0621 | 0.35 | 0.5609 | 0.01 | 0.9189 |
| X1X2 | 9.686 | 0.0063* | 8.66 | 0.0091* | 0.06 | 0.7989 | 0.20 | 0.6566 |
| X1X3 | 2.930 | 0.1051 | 0.81 | 0.3794 | 1.83 | 0.1937 | 0.92 | 0.3505 |
| X2X3 | 2.397 | 0.1400 | 1.17 | 0.2949 | 1.72 | 0.2066 | 10.28 | 0.0052* |
| X12 | 4.017 | 0.0612 | 31.36 | < 0.001* | 2.86 | 0.1086 | 0.00 | 0.9961 |
| X22 | 2.961 | 0.1034 | 0.20 | 0.6594 | 0.11 | 0.7374 | 0.49 | 0.4918 |
| X32 | 0.106 | 0.7487 | 0.35 | 0.5625 | 1.07 | 0.3148 | 0.05 | 0.8299 |
*Significant differences (p < 0.05)
The influence of PCL on mean particle size was also investigated. In the range of 1% and 3% ratio of PCL content, the effect of PCL concentration on particle size was not found significant (p > 0.05). Moreover, it was observed that the particle size was increased with the increasing amount of PCL. This phenomenon was also observed in several studies [20–22]. The higher polymer concentration in the first emulsion increases the viscosity of the dispersed phase, which causes a poor dispersibility of polymer solution into the aqueous phase. The high viscosity shows resistance to shear forces in the emulsification process results in bigger droplet sizes and particles [20].
As the effect of emulsifier concentration, homogenization speed also caused a negative effect on the mean particle size of the nanoparticles. Increasing the application energy results in greater disruption of the emulsion and, consequently, a tendency to particle size decrease [23] (Fig. 1c). By contrast, the interaction of PCL or PVA concentration with homogenization speed produced a positive effect on the nanoparticle size at high concentrations of PCL or PVA in the quadratic model (Fig. 1a). The data obtained from the experiments were subjected to multiple regression analysis, and results were fitted in the equation:
Fig. 1.
Response surface plots for oxaliplatin loaded PCL NPs: effect of homogenization speed, PVA concentration, and PCL concentrations on (a) particle size, b PDI, c zeta potential and d encapsulation efficiency (%)
The model coefficients of analysis are shown in Table 2. The effect of terms X1, X2, and X1*X2 were found significant due to having p value <0.05 of these parameters in the selected model [24].
Polydispersity index
PDI is an essential parameter that gives information about the physical stability of nanoparticles. For an ideal nanoparticle formulation, The PDI must be very low. The threshold PDI value of homogeneity is generally accepted as 0.5 [25]. However, lower PDI (<0.2) shows the homogeneity. The PDI value of all formulations was found in the range of 0.007 ± 0.003 to 1.186 ± 0.237, which was variable to indicate the homogeneity of all formulations (Table 1).
When the effect of variables on PDI was investigated, it was found that X1, X2, and X1*X2 and X12 were significant effects (p < 0.05) (Table 2). It was observed that higher PDI values were formed at low homogenization speeds (Fig. 1b). Moreover, PDI was affected by PVA concentration inversely. The negative effect could be attributed to the effect of stabilizers, which prevent the emulsion droplets form coalescing to each other and form uniform particles. Batches with higher PVA as stabilizer provided better PDI values. The polymer chains of the stabilizer might have provided relatively better coverage and, thus, stabilization to the emulsion droplets permitting a homogeneous nanoparticle distribution [26]. The equation after fitting the data subjected to multiple-regression analysis is:
Zeta potential
Zeta potential gives information about the stability of colloidal dispersions. Generally, the higher zeta potential nanoparticles show the better colloidal stability of the system owing to the repulsion effect between the particles. The zeta potential values of all formulations ranged from −4.45 ± 3.81 to −21.41 ± 4.07 (Table 1). It was observed that none of the variables have a significant effect on the zeta potential of formulations (p > 0.05) (Table 2). It was seen that as the PCL concentration was increased from 1% to 3%, there was an increase in the zeta potential value (Fig. 1c). This is because the polymer is anionic, and increasing its concentration increases the total charge on the particle [11]. The calculated equation can be written as:
Encapsulation efficiency
The model coefficients estimated by multiple linear regression for encapsulation efficiency were shown in Table 2. All of the terms, including model having p value >0.05, were insignificant in contributing to the prediction of encapsulation efficiency. In formulations with high amounts of PCL, the encapsulation efficiency was increased with the increasing amount of surfactant and homogenization speed. Unless in low PCL concentrations, encapsulation efficiency was decreased with the increasing amount of surfactant and homogenization speed. A similar trend of encapsulation efficiency was observed in the effect of the surfactant ratio. An increase in encapsulation efficiency (Fig. 1d) was observed on increasing the amount of surfactant from 0.3% to 2. The model equation can be written as:
Determination of optimal formulation
The optimal formulation of oxaliplatin loaded PCL nanoparticles was analyzed using ANOVA, and each parameter was evaluated using the F test. As optimum nanoparticles must have desired particle size and zeta potential with a low PDI and high encapsulation values, the design was optimized in accordance with these criteria. The results of the Design Expert analysis of oxaliplatin nanoparticles was shown in Table 3.
Table 3.
The experimental and predicted values of the optimum formulation
| X1:X2:X3 | Response variable | Experimental value | Predicted value | Prediction error (%) |
|---|---|---|---|---|
| 20,400: 0.3: 3 | Y1 (Particle Size) | 558.30 | 577.00 | 3.24 |
| Y2 (PDI) | 0.364 | 0.340 | −2.40 | |
| Y3 (Zeta Potential) | −15.70 | −13.78 | −13.93 | |
| Y4 (Encapsulation Efficiency) | 28.08 | 21.55 | −30.30 |
Characterization of formulations
After the optimization, the selected formulation was prepared using three types of PCL ((average Mn ~10,000) (PCL10000 NPs) (average Mn ~45,000) (PCL45000 NPs) and the (average Mn ~80,000) (PCL80000 NPs)) to obtain the polymer molecular weight effect on in vitro characteristics of oxaliplatin loaded nanoparticles.
As shown in Table 4, the molecular weight of the polymer was only affected the particle size of oxaliplatin loaded PCL nanoparticles. It was found that particle size was increased with the increase in the molecular weight of PCL from 10,000 to 45,000. It was mentioned that higher molecular weighted polymers form higher viscous polymer solutions, which limits the effect of homogenization energy and causes bigger emulsion droplets. Otherwise, polymers with low molecular weight tend to form smaller emulsion droplets resulting in smaller particles [27]. On the contrary, the particle size of nanoparticles was decreased with the increase in the molecular weight of PCL from 45,000 to 80,000 (p < 0.05). This result was also observed in similar studies. For instance, Budhian et al. were also prepared nanoparticles with the emulsification solvent evaporation method using different preparation procedures. When they used homogenizer, they observed the particle size of nanoparticles was increased with the change of molecular weight of nanoparticles from 14 kDa to 63 kDa, but the effect was not continued after 63 kDa [28].
Table 4.
Oxaliplatin loaded nanoparticles prepared with different Mn of PCL
| Particle size (nm) | PDI | Zeta potential (mV) | Drug loading (%) | Encapsulation efficiency (%) | |
|---|---|---|---|---|---|
| PCL10000 NPs | 558.30 ± 7.44* | 0.364 ± 0.140 | −15.7 ± 1.35 | 1.32 ± 0.18 | 28.08 ± 0.67 |
| PCL45000 NPs | 714.51 ± 10.73* | 0.315 ± 0.104 | −16.6 ± 0.49 | 1.56 ± 0.22 | 32.81 ± 0.33 |
| PCL80000 NPs | 617.81 ± 11.68* | 0.332 ± 0.079 | −17.7 ± 1.41 | 1.45 ± 0.13 | 29.17 ± 0.74 |
*Significant difference from other formulations (p < 0.05)
The effect of polymer molecular weight on the polydispersity index, zeta potential, and encapsulation efficiency was not found significant and also did not follow a regular pattern. The encapsulation efficiency of nanoparticles was increased from 28.08 ± 0.67% to 32.81 ± 0.33%, as the molecular weight was increased from about 10,000 to 45,000 Da; then, a decrease (p < 0.05) was observed to 29.17 ± 0.74% as the molecular weight was increased to 80,000 Da (Table 4). The physicochemical properties of the drug and polymer are key factors affecting the encapsulation efficiency of nanoparticles. When the molecular weight of polymer increases, interactions between hydrophobic molecular chains of polymer and drug increases. An increase in the encapsulation efficiency was observed. Nevertheless, in higher polymer molecular weight, an increase in the viscosity might have decreased the diffusion rate of solvent into the external aqueous phase resulting in lower encapsulation of drugs [27].
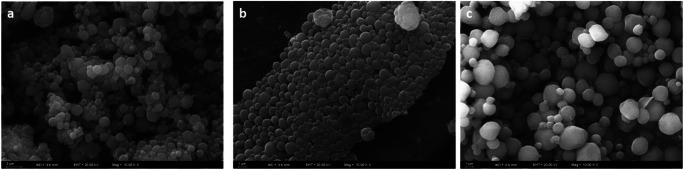
The particle size measurements were confirmed by SEM that allowed both the determination of the particle size and the shape of the particles (Fig. 2). These average diameters were similar to those that were observed with particles reported in the particle size measurement section and previous studies with the same preparation method [29].
Fig. 2.
SEM images of PCL10000 NPs, PCL45000 NPs and PCL80000 NPs
In vitro drug release studies
One of the main factors affecting the drug release from polymeric nanoparticles is the molecular weight of the polymer. The term molecular weight expresses the chain length of the polymer and gives information about the hydrophilicity/lipophilicity of the polymer. Longer chain lengths increase the lipophilicity of the polymer, which results in a decrease in the degradation rate [27]. Both the drug release rate and release kinetics of drugs can be modified by the use of the same polymer with different molecular weights.
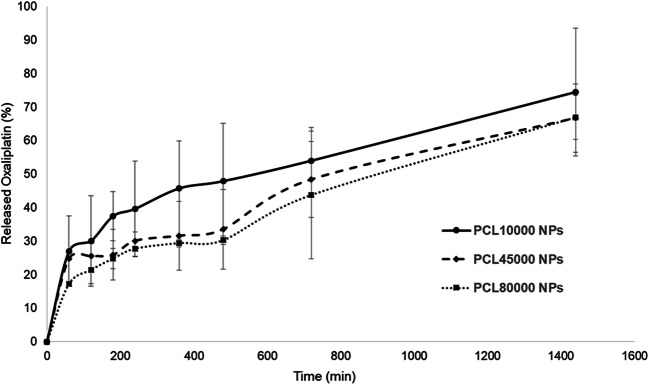
In all the formulations, initial burst release occurred in 120 min, which is caused by the presence of the drug in the surface of nanoparticles (Fig. 3). Similar release profiles of drugs from PCL nanoparticles were observed in several studies. For example, Chawla et al. found that about 68% of tamoxifen was released from PCL nanoparticles in the first hour [30]. Moreover, different release patterns in different time intervals can be explained by the drug release phenomenon from polymeric nanoparticles. In this phenomenon, it was explained that the early phase of drug release is controlled by the diffusion in the polymer matrix, while during the later phases, the release is mediated through both diffusion of the therapeutic agent and degradation of the polymer matrix itself [31].
Fig. 3.
The released oxaliplatin from of PCL10000 NPs, PCL45000 NPs and PCL80000 NPs
An increase in molecular weight from 10,000 to 80,000 Da significantly decreased the release rate of oxaliplatin. PCL10000 NPs released 74.48 ± 19.09% of total drug in 1440 min. On the other hand, PCL45000 NPs and PCL80000 NPs showed only 66.69 ± 10.24% and 66.86 ± 6.54% release of the total drug in 1440 mins, respectively, at a nearly constant rate (Fig. 3). Degradation of polymers plays an important role in drug release from polymeric matrices. The degradation rate is a time-dependent process and constantly increases with time. Commonly, degradation of polymers depends on the molecular weight, and polymers with low molecular weight have faster degradation rates [27].
Moreover, an increase in the molecular weight increases the lipophilicity, which limits the drug release [32]. In addition to molecular weight, the particle size of the nanoparticles affects the drug release. Larger particle sizes limit the penetration of buffer due to the surface area/volume ratio, thus slower the drug release [27].
Release kinetics was evaluated by fitting obtained data into first order, zero-order, and Higuchi equations. Based on the results, oxaliplatin release from nanoparticles followed the Higuchi equation and related correlation coefficients were better than both zero-order and first-order kinetics (Table 5).
Table 5.
Correlation coefficients of different mathematical models and release rate constant (k) for oxaliplatin from nanoparticles
| Formulation | Correlation coefficients (r2) | |||
|---|---|---|---|---|
| First order | Second order | Higuchi | *k (mg/min) | |
| PCL10000 NPs | 0.87 | 0.94 | 0.97 | 2.18 |
| PCL45000 NPs | 0.92 | 0.94 | 0.97 | 1.79 |
| PCL80000 NPs | 0.95 | 0.96 | 0.98 | 1.68 |
*k denotes release rate constant according to Higuchi model
Stability of nanoparticles
Poor stability of nanoparticles would lead to changing the characteristics of the drug delivery system, resulting in large side effects and small therapeutic effects, which is a major obstacle for nanoparticles need to overcome [33]. Particle size and PDI of nanoparticles were 563.24 ± 5.83 nm and 0.387 ± 0.041 for PCL10000 NPs, 740.22 ± 37.50 nm and 0.308 ± 0.096 for PCL45000 NPs and 637.81 ± 34.84 nm and 0.386 ± 0.115 for PCL80000 NPs. As expected, it was found that the results were not different from prepared nanoparticles (p > 0.05). These results suggested that prepared nanoparticles remained their stability for at least 30 days.
Cytotoxicity assay
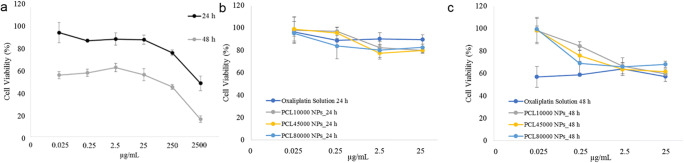
MTT assay was performed on SK-MES-1 cells to evaluate the effect of the oxaliplatin on cell viability for 24 h and 48 h. It was shown a concentration-dependent effect on the cell viability of cells. Especially, only a slight change in the viability of cells was observed when drug concentration ranged from 0.025 to 25 μg/mL for 24 h and 48 h (Fig. 4a) (p > 0.05). However, with the concentrations higher than 25 μg/mL, a significant (p < 0.05) decrease in cell viability was observed. The results were in accordance with the literature [7].
Fig. 4.
Cytotoxicity profiles of (a) free Oxaliplatin and PCL10000 NPs, PCL45000 NPs and PCL80000 NPs in (b) 24 h and c 48 h
The cytotoxicity effect of oxaliplatin loaded nanoparticles on the SK-MES-1 cell line also evaluated for 24 h and 48 h (Fig. 4b and c). At the end of 24 h, it was observed that the effect of all formulations was increased with the increase of drug doses. However, the effect of all formulations was similar in all drug doses (p > 0.05) (Fig. 4b).
At the end of 48 h, the decrease in cell viability with oxaliplatin and oxaliplatin loaded PCL nanoparticles in the studied concentration range was found to be between 56.88–63.87% and 59.64–99.49%, respectively. Conversely, the effect of all formulations was similar in high drug doses (2.5 and 25 μg/mL). In lower doses, the effect of free oxaliplatin on the SK-MES-1 cell line was found more cytotoxic (p < 0.05). Also, the effect of nanoparticles prepared by the different molecular weight of PCL was not found significant at any time point (p > 0.05) (Fig. 4c).
In vitro cytotoxicity profiles demonstrated that PCL10000 NPs, PCL45000 NPs, and PCL80000 NPs, which contains the same oxaliplatin concentration exhibited similar cytotoxicity with oxaliplatin solution (Fig. 4b and c). As the drug is sequestered inside the nanoparticles, the release of oxaliplatin from nanoparticles takes a while, and low drug concentrations in wells show less cytotoxic effect. However, it was shown in preclinical studies that particulate oxaliplatin systems have higher anticancer activity than oxaliplatin solutions. The clearance of drug-loaded nanoparticles was mediated by the reticuloendothelial system (RES), which changes the systemic circulation half-life and pharmacokinetics of drugs. The application of the same concentration of drug and drug-loaded nanoparticles in wells creates artificial cytotoxicity results strongly favoring drug solution [34].
Cellular uptake
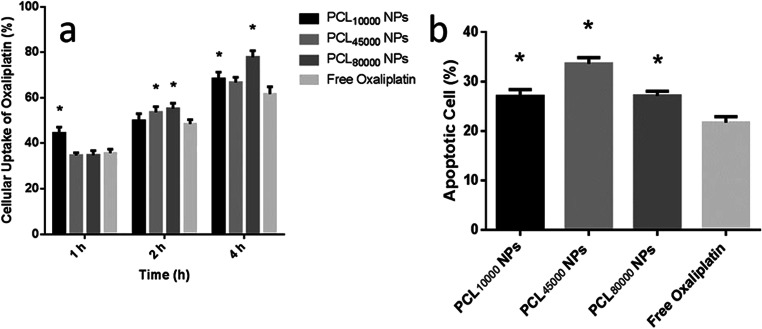
The mechanisms of cellular uptake and efflux of platin derivatives are still not fully understood. Nevertheless, it is thought that passive diffusion is the main pathway of drug uptake due to the uptake proceeded linearly with time [2]. In this study, Cellular uptake of oxaliplatin for both NPs and the free drug was also linear with time for up to 4 h (p < 0.05) (Fig. 5a). Moreover, SK-MES-1 cell uptake of oxaliplatin was dependent on the administration type as nanoparticle administration enhanced the cellular uptake of drugs. The 1 h and 4 h uptake of PCL10000 NPs and PCL80000 NPs were found significantly higher than that of free oxaliplatin (p < 0.05) (Fig. 5a).
Fig. 5.
a Cellular uptake and b cell death analysis of free oxaliplatin and oxaliplatin loaded nanoparticles (*significantly different from free oxaliplatin (p < 0.05))
In previous studies, it was observed that the cellular uptake of the drug-loaded nanoparticles might be related to the size and zeta potential of the nanoparticles [35]. However, oxaliplatin loaded nanoparticles formulated with a different molecular weight of PCL did not show differences in cellular uptake in 1 h and 2 h. Only the uptake of PCL80000 NPs was found greater than PCL10000 NPs and PCL45000 NPs at 4 h (p < 0.05). Although the decrease in the mean size of the oxaliplatin loaded nanoparticles with decreasing polymer molecular weight was also observed in our study, the cellular uptake of oxaliplatin did not show correlated results as in previous studies. The reason may be the high polydispersity index of formulations. As the formulations differ in mean particle size, they contained a considerable number of particles in the same size range.
Cell death analysis
The results in Fig. 5b demonstrate that incubation of SK-MES-1 cells with oxaliplatin causes PS translocation from the inner plasma membrane to the outer cell surface detectable by the Annexin V, which could be attributed to events in the apoptotic cycle [36]. The apoptotic cells were observed in cells treated with all the three nanoparticle formulations and free oxaliplatin, but the percentage of apoptotic cells varied with each formulation. As shown in Fig. 5b, the apoptotic cells incubated with free oxaliplatin was 21.73 ± 1.20%. Likewise, the number of apoptotic cells distribution increased to 27.04 ± 1.39%, 33.62 ± 1.24%, and 27.10 ± 0.95% when the SK-MES-1 cells were incubated with PCL10000 NPs, PCL45000 NPs, and PCL80000 NPs respectively. Therefore, oxaliplatin loaded PCL nanoparticles induced more cancer cell apoptosis when compared with the free oxaliplatin (p < 0.05). The apoptotic signaling pathway is regulated by complex molecules in the network, which involves the expression changes of distinct pro-apoptotic and anti-apoptotic proteins. The oxaliplatin is a potent inhibitor of survivin and induced apoptosis in cancer cells. However, Vivek et al. demonstrated that oxaliplatin loaded nanoparticles induced apoptosis not only by inhibiting survivin but also through intrinsic apoptotic signaling pathway [7].
Conclusion
In the present study, the preparation and characterization of PCL nanoparticles of oxaliplatin as an anticancer drug was carried out. For simultaneous analysis of the influence of different factors on the properties of the nanoparticles and to find optimum formulations, the formulation was optimized using a 33 factorial design. Homogenization speed and surfactant ratio represented the main factors influencing particle size and PDI and did not seem to depend on the PCL ratio. It was found that particle size was increased with the increase in the molecular weight of PCL from 10,000 to 45,000. In all the oxaliplatin loaded nanoparticle formulations, initial burst release occurred in 120 min, which is caused by the surface presence of the drug. An increase in molecular weight from 10,000 to 80,000 Da significantly decreased the release rate of oxaliplatin. The effect of oxaliplatin loaded nanoparticles on cytotoxicity of the SK-MES-1 cell line also evaluated for 24 h. It was found that the cytotoxicity of oxaliplatin on the SK-MES-1 cells was enhanced when loaded on PCL nanocarrier. Moreover, PCL nanoparticles enhanced the cellular uptake and apoptosis of oxaliplatin. In general, the results show that the PCL nanoparticles may be considered as a promising carrier system for controlled release and targeted delivery of oxaliplatin with possible clinical application in NSCLC therapy.
Acknowledgements
This study was supported by University of Health Sciences (Project Number: 2019/010).
Contributions
Dr. Ozgur Esim, Prof. Yalcin Ozkan, and Prof. Ayhan Savaser conceived of the presented idea, designed and directed the project, carried out some experiments, and wrote the manuscript. Prof. Yalcin Ozkan and Prof. Sibel A.Ozkan supervised and directed the project. Nuray Yildirim and Dr. Ozgur Esim prepared and characterized the nanoparticles. Meral Sarper performed cell studies. Dr. Nurgul K.Bakirhan performed the HPLC experiments.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franciosi V, Barbieri R, Aitini E, Vasini G, Cacciani GC, Capra R, Camisa R, Cascinu S. Gemcitabine and oxaliplatin: a safe and active regimen in poor prognosis advanced non-small cell lung cancer patients. Lung Cancer. 2003;41(1):101–106. doi: 10.1016/s0169-5002(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Monnet I, Brienza S, Hugret F, Voisin S, Gastiaburu J, Saltiel J, et al. Phase II study of oxaliplatin in poor-prognosis non-small cell lung cancer (NSCLC) Eur J Cancer. 1998;34(7):1124–1127. doi: 10.1016/s0959-8049(98)00007-0. [DOI] [PubMed] [Google Scholar]
- 4.Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine. 2010;6(1):179–190. doi: 10.1016/j.nano.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Cevenini A, Celia C, Orrù S, Sarnataro D, Raia M, Mollo V, Locatelli M, Imperlini E, Peluso N, Peltrini R, de Rosa E, Parodi A, del Vecchio L, di Marzio L, Fresta M, Netti PA, Shen H, Liu X, Tasciotti E, Salvatore F. Liposome-embedding silicon microparticle for Oxaliplatin delivery in tumor chemotherapy. Pharmaceutics. 2020;12(6):559. doi: 10.3390/pharmaceutics12060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy LH, Bazile D. Drug delivery design for intravenous route with integrated physicochemistry, pharmacokinetics and pharmacodynamics: illustration with the case of taxane therapeutics. Adv Drug Deliv Rev. 2014;71:34–57. doi: 10.1016/j.addr.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Vivek R, Thangam R, Nipunbabu V, Ponraj T, Kannan S. Oxaliplatin-chitosan nanoparticles induced intrinsic apoptotic signaling pathway: a “smart” drug delivery system to breast cancer cell therapy. Int J Biol Macromol. 2014;65:289–297. doi: 10.1016/j.ijbiomac.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff MA, Hutmacher DW. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–1256. [Google Scholar]
- 9.Derakhshandeh K, Erfan M, Dadashzadeh S. Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: factorial design, characterization and release kinetics. Eur J Pharm Biopharm. 2007;66(1):34–41. doi: 10.1016/j.ejpb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Bhavsar MD, Tiwari SB, Amiji MM. Formulation optimization for the nanoparticles-in-microsphere hybrid oral delivery system using factorial design. J Control Release. 2006;110(2):422–430. doi: 10.1016/j.jconrel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Sinha V, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 2004;278(1):1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 12.Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26(5):1025–1058. doi: 10.1007/s11095-008-9800-3. [DOI] [PubMed] [Google Scholar]
- 13.Luo FR, Yen T-Y, Wyrick SD, Chaney SG. High-performance liquid chromatographic separation of the biotransformation products of oxaliplatin. J Chromatogr B Biomed Sci Appl. 1999;724(2):345–356. doi: 10.1016/s0378-4347(98)00565-9. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Gaete C, Bustos GL, Godoy RR, Saez CK, Novoa GP, Fernández EM, Tsapis N, Fattal E. Successful factorial design for the optimization of methylprednisolone encapsulation in biodegradable nanoparticles. Drug Dev Ind Pharm. 2013;39(2):310–320. doi: 10.3109/03639045.2012.676049. [DOI] [PubMed] [Google Scholar]
- 15.Esim O, Savaser A, Kurbanoglu S, Ozkan CK, Ozkan SA, Ozkan Y. Development of assay for determination of eletriptan hydrobromide in loaded PLGA nanoparticles. J Pharm Biomed Anal. 2017;142:74–83. doi: 10.1016/j.jpba.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Mehta AK, Yadav KS, Sawant KK. Nimodipine loaded PLGA nanoparticles: formulation optimization using factorial design, characterization and in vitro evaluation. Current drug delivery. 2007;4(3):185–193. doi: 10.2174/156720107781023929. [DOI] [PubMed] [Google Scholar]
- 17.Esim O, Ozkan CK, Kurbanoglu S, Arslan A, Tas C, Savaser A, et al. Development and in vitro/in vivo evaluation of dihydroergotamine mesylate loaded maltodextrin-pullulan sublingual films. Drug Dev Ind Pharm. 2019:1–8. [DOI] [PubMed]
- 18.S-s F, Huang G. Effects of emulsifiers on the controlled release of paclitaxel (Taxol®) from nanospheres of biodegradable polymers. J Control Release. 2001;71(1):53–69. doi: 10.1016/s0168-3659(00)00364-3. [DOI] [PubMed] [Google Scholar]
- 19.Vandervoort J, Ludwig A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. Int J Pharm. 2002;238(1–2):77–92. doi: 10.1016/s0378-5173(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 20.Mainardes RM, Evangelista RC. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int J Pharm. 2005;290(1):137–144. doi: 10.1016/j.ijpharm.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Quintanar-Guerrero D, Fessi H, Allémann E, Doelker E. Influence of stabilizing agents and preparative variables on the formation of poly (D, L-lactic acid) nanoparticles by an emulsification-diffusion technique. Int J Pharm. 1996;143(2):133–141. [Google Scholar]
- 22.Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 2002;83(3):389–400. doi: 10.1016/s0168-3659(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 23.Leong T, Wooster T, Kentish S, Ashokkumar M. Minimising oil droplet size using ultrasonic emulsification. Ultrason Sonochem. 2009;16(6):721–727. doi: 10.1016/j.ultsonch.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Esim O, Savaser A, Ozkan C, Bayrak Z, Tas C, Ozkan Y. Effect of polymer type on characteristics of buccal tablets using factorial design. Saudi Pharmaceutical Journal. 2018;26(1):53–63. doi: 10.1016/j.jsps.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yüksel A, Şahin-Yeşilçubuk N. Encapsulation of structured lipids containing medium-and long chain fatty acids by complex coacervation of gelatin and gum arabic. J Food Process Eng. 2018;41(8):e12907. [Google Scholar]
- 26.De Jaeghere F, Allémann E, Doelker E, Gurny R, Cerny R, Galli B, et al. pH-dependent dissolving nano-and microparticles for improved peroral delivery of a highly lipophilic compound in dogs. Aaps Pharmsci. 2001;3(1):92–99. doi: 10.1208/ps030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittal G, Sahana D, Bhardwaj V, Kumar MR. Estradiol loaded PLGA nanoparticles for oral administration: effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119(1):77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Budhian A, Siegel SJ, Winey KI. Haloperidol-loaded PLGA nanoparticles: systematic study of particle size and drug content. Int J Pharm. 2007;336(2):367–375. doi: 10.1016/j.ijpharm.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 29.Leroueil-Le Verger M, Fluckiger L, Kim Y-I, Hoffman M, Maincent P. Preparation and characterization of nanoparticles containing an antihypertensive agent. Eur J Pharm Biopharm. 1998;46(2):137–143. doi: 10.1016/s0939-6411(98)00015-0. [DOI] [PubMed] [Google Scholar]
- 30.Chawla JS, Amiji MM. Biodegradable poly (ε-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249(1–2):127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 31.Panyam J, Dali MM, Sahoo SK, Ma W, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolide) nano-and microparticles. J Control Release. 2003;92(1–2):173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 32.Braunecker J, Baba M, Milroy GE, Cameron RE. The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int J Pharm. 2004;282(1–2):19–34. doi: 10.1016/j.ijpharm.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo X, Lei T, Miao L, Chu W, Li X, Luo L, Gou J, Zhang Y, Yin T, He H, Tang X. Disulfiram-loaded mixed nanoparticles with high drug-loading and plasma stability by reducing the core crystallinity for intravenous delivery. Journal of colloid interface science. 2018;529:34–43. doi: 10.1016/j.jcis.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10(3):350–357. [PubMed] [Google Scholar]
- 35.Huang M, Khor E, Lim L-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res. 2004;21(2):344–353. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- 36.Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, Moghimi SM. Low and high molecular weight poly (l-lysine) s/poly (l-lysine)–DNA complexes initiate mitochondrial-mediated apoptosis differently. FEBS Lett. 2005;579(27):6191–6198. doi: 10.1016/j.febslet.2005.09.092. [DOI] [PubMed] [Google Scholar]