Abstract
Dopaminergic activity in prefrontal cortex is modulated by the low (Met) and high (Val) activity of the rs4680 Val158Met single nucleotide polymorphism (SNP) in the Catechol-O-Methyltransferase (COMT) gene. While this has been related to working memory maintenance in patients with schizophrenia, the familial pattern, impact across the psychosis spectrum, and the role of this genotype on other aspects of behavior, such as cognitive flexibility, remains unclear. The relationship between COMT Val158Met genotype and both cognitive stability and flexibility were assessed using the Penn Conditional Exclusion Test (PCET) in healthy controls (n = 241), patients with psychotic disorders (n = 542), and their first-degree relatives (n = 613) from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium. Higher rates of perseverative errors (poor flexibility) were associated with the low-activity COMT genotype (Met allele carriers) in probands compared to their first-degree relatives with the same genotype. Probands and first-degree relatives homozygous for the high-activity COMT enzyme (Val/Val) showed elevated rates of regressive errors (poor stability) compared to controls. Conversely, heterozygous relatives had comparable regressive error rates to controls, with probands showing elevated errors in comparison. These findings suggest that impaired suppression of learned response patterns and reduced stability of mental sets may be a familial intermediate cognitive phenotype related to Val COMT allele genotype.
Keywords: psychosis, set shifting, catechol-O-methyltransferase (COMT), Penn Conditional Exclusion Task (PCET), B-SNIP 1
1. Introduction
Global cognitive dysfunction is an enduring feature that is relatively independent of antipsychotic treatments and pervasive across psychotic disorders (Hill et al., 2013b; Hochberger et al., 2016; Keefe et al., 2007). Meta-analytic studies have indicated that unaffected first-degree relatives of individuals with psychotic disorders demonstrate cognitive deficits as well (Bora et al., 2009; Hill et al., 2004; Snitz et al., 2006). Executive function is a broad term that encompasses a wide range of cognitive processes supported primarily by frontostriatal brain systems. Among the executive abilities are two opposing processes: cognitive stability and cognitive flexibility. Cognitive stability is critical for holding information and behavioral plans “on line” over time and for focusing attentional resources on the task at hand, particularly when potential distractors are present. However, when mental representations and plans persist too long, behavior may be inflexible and unresponsive to new contextual information (Cools & D’Esposito, 2001). Cognitive flexibility is necessary when environmental contingencies change, necessitating an adaptive shift in behavior. However, updating too readily could result in distractibility and unstable behavior. A better understanding of the interaction between these complimentary cognitive processes and their associated gene variants in psychotic disorders may be useful in understanding the nature of cognitive dysfunction in psychosis.
Dopamine system dysregulation is an important feature of schizophrenia and psychotic disorders (Bilder et al., 2004; Egan et al., 2001; Lachman et al, 1996; Tunbridge et al., 2006). The catechol-O-methyltransferase (COMT) gene regulates enzymatic breakdown of dopamine, particularly in prefrontal cortex. Specifically, the Val158Met polymorphism results in a valine to methionine amino acid change which reduces prefrontal dopamine availability, particularly in Val homozygotes (Chen et al., 2004). The Met allele has been associated with a more efficient pattern of prefrontal activity during performance of working memory tasks and may be advantageous for other cognitive processes that rely heavily on cognitive stability including spatial verbal working memory, problem solving, inhibition, and numerical computations (Bertolino et al., 2004; Egan et al. 2001; Goldberg et al., 2003; Rosa et al., 2010; Tan et al., 2007). Additionally, first episode psychosis patients with at least one Met allele showed a robust benefit for both cognitive stability and flexibility following antipsychotic treatment (Nelson et al., 2018).
The Val allele has been associated with increased risk of schizophrenia (Egan et al., 2001), putatively by lowering dopamine availability in PFC, particularly in Val homozygotes. This may reflect an antagonistic relationship between prefrontal and striatal dopamine systems. Specifically, relatively high levels of prefrontal dopamine correspond to relatively low levels of striatal dopamine and vice versa (Cools & D’Esposito, 2011; Tunbridge et al., 2006). This is supported by evidence indicating that individuals homozygous for the Val allele have lower concentrations of synaptic dopamine levels in the PFC and higher concentrations in the striatum compared to Met carriers (Akil et al., 2003; Tunbridge et al., 2006). Varying levels of prefrontal and striatal dopamine may set a balance influencing the stability of cognitive representations, with higher striatal DA leading to reduced stability of choice preferences with low prefrontal DA reducing the flexibility of behavioral plans (Bilder et al., 2004; Dodds et al., 2009; Goldberg et al., 2003; Nolan et al., 2004; Rosa et al., 2010). Indeed, Val carriers with chronic schizophrenia showed greater accuracy on a sequencing task that required frequent updating (Hill et al., 2013b). On the other hand, Val homozygotes have been shown to have reduced cognitive stability in treated patients (Nelson et al., 2018). Thus, it may be more challenging for different schizophrenia patients to generate adaptive behaviors depending on whether favorable decisions require flexible or stable internal representations based on their COMT genotype.
The Wisconsin Card Sorting Test (WCST) and the Penn Conditional Exclusion Task (PCET) are often used to assess executive function including cognitive flexibility. However, in most standard neuropsychological tests of this type, scoring of flexibility and stability deficits are poorly delineated, with both typically falling under the umbrella of perseverative errors (Barceló & Knight, 2002). Rodent models have linked prefrontal cortex dysfunction with difficulty disengaging from a previously reinforced response set and shifting to a new response set (Kim & Ragozzino, 2005; Ragozzino, 2007; Dias Robbins, & Roberts, 1997). This adherence to a prior sorting strategy despite negative feedback has been characterized as a ‘perseverative’ error (Ragozzino, 2007). In contrast, difficulty maintaining a novel response set after initial acquisition of a new sorting principle has been defined as a ‘regressive’ error (Ragozzino, 2007). While perseverative errors are linked to prefrontal function, dysfunction in the dorsal striatum has been linked to regressive errors reflecting disruption of the ability to maintain a new behavioral strategy (Ragozzino 2004; Ragozzino, Raggozino, Mizumori, Kesner 2002).
This distinction between regressive and perseverative errors has been supported by both human and animal studies (Hill et al., 2014; Johnston et al., 2007; Rainer, 2007) and may reflect different points of disruption in fronto-striatal circuitry. Specifically, because the Val genotype is associated with reduced prefrontal DA, it may lead to a reduced ability to maintain cognitive set after switching due to striatal inhibition of prefrontal maintenance (Bertolino et al., 2004; Bilder et al., 2004; Nolan et al., 2004; Rosa et al., 2010). Conversely, because the Met genotype is associated with increased prefrontal DA and reduced striatal activity, it may lead to an inability to switch cognitive set (Rosa et al., 2010; Bilder et al, 2004). Thus, during set shifting tasks, dopamine signaling acts as an opponent process based on the location of the signaling.
This study was designed to assess the relationship between COMT Val158Met genotype and cognitive flexibility (the ability to shift set) and cognitive stability (the maintenance of a newly learned behavioral choice in lieu of the previous response choice) in individuals with psychotic disorders, their first-degree relatives, and healthy controls. Because the Met allele is associated with a higher concentration of prefrontal dopamine and facilitates cognitive flexibility, we hypothesized that Met carriers would have difficulty shifting away from an established response set (more perseverative errors) than their Val/Val counterparts. In contrast, based on the relatively lower concentrations of prefrontal dopamine, we predicted that Val homozygotes may be prone to more frequent updating leading to poor maintenance of a newly acquired behavioral sets (more regressive errors).
2. Methods
2.1. Participants
Participants enrolled in the Bipolar-Schizophrenia Network of Intermediate Phenotypes (BSNIP) project completed the Penn Conditional Exclusion Test (PCET). Demographic and clinical characteristics are presented in Table 1. Probands (n = 542; 34% bipolar with psychosis, 23% schizoaffective disorder, and 43% schizophrenia) had a lifetime diagnosis of a psychotic disorder based on the Structured Clinical Interview for DSM-IV Disorders (SCID) (First, et al., 2002) and were clinically stable with no changes in medication regimen for the prior month. The use of psychotropic medications, chlorpromazine equivalents for antipsychotic treatments, and clinical ratings each accounted for < 5% of the variance in perseverative and regressive errors across all proband diagnostic groups and were therefore not used as covariates in this report. Healthy controls (n = 241) had no personal history of a psychotic disorder or recurrent depression, and no known family history of these disorders. First-degree relatives of the probands (n = 613) were also studied. For more details about B-SNIP recruitment procedures and methodology, see Tamminga et al. (2013).
Table 1.
Demographic data for healthy controls, first-degree relatives, and probands with a history of psychosis.
| Healthy Controls | First-Degree Relatives | Probands | Findings | ||||
|---|---|---|---|---|---|---|---|
| n = 241 | n = 613 | n = 542 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 38.07 | 12.95 | 41.64 | 15.58 | 36.60 | 12.69 | F = 19.20*a |
| Education (years) | 15.10 | 2.49 | 14.26 | 2.68 | 13.27 | 2.28 | F = 46.33*b |
| WRAT-IV: Reading (SS) | 102.91 | 13.25 | 100.35 | 14.96 | 97.00 | 15.20 | F = 15.10*c |
| n | % | n | % | n | % | ||
| Sex | |||||||
| Male | 101 | 41.74% | 187 | 30.51% | 280 | 51.66% | χ2 = 53.50* |
| Female | 141 | 58.26% | 426 | 69.49% | 262 | 48.34% | |
| Race | |||||||
| Caucasian | 148 | 40.11% | 396 | 41.29% | 307 | 37.44% | χ2 = 19.10* |
| African American | 69 | 18.70% | 188 | 19.60% | 205 | 25.00% | |
| Other | 24 | 6.50% | 26 | 2.71% | 30 | 3.66% | |
| COMT Genotype | |||||||
| Val/Val | 87 | 35.95% | 208 | 33.93% | 182 | 33.58% | χ2 = 7.70n.s. |
| Met/Met | 41 | 16.94% | 141 | 23.00% | 96 | 17.71% | |
| Val/Met | 114 | 47.11% | 264 | 43.07% | 264 | 48.71% | |
p ≤ 0.001
healthy controls = probands; healthy control and probands < relatives
probands < relatives < healthy controls
probands < healthy controls and relatives; healthy controls = relatives
In a prior manuscript, we reported behavioral findings using the PCET within proband and relative subgroups (Hill et al., 2014). As there were no test performance differences across disorders or in relatives of the different disorders, we collapsed across diagnostic subgroup for both probands and relatives (bipolar with psychosis, schizoaffective disorder, and schizophrenia). This reduced the number of comparisons across and within COMT genotypes, increasing statistical power for detecting genotype-phenotype associations.
2.2. Procedures
2.2.1. Neuropsychological assessment.
Participants were administered the PECT and the Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological battery. The BACS composite score, reflecting a single factor of cognitive ability (Hochberger et al., 2016), was computed using age and sex stratified normative data (Keefe et al., 2004) and adjusted for race (Hill et al., 2013b).
2.2.2. Genotyping.
Genomic DNA was isolated from whole blood using standard protocols and genotyped by the Broad Institute using the Illumina Infinium PsychChip array. Quality control (QC) procedures were conducted with PLINK v1.9 (Purcell, 2007) following standardized protocols (Anderson, 2010). No deviations from Hardy-Weinberg equilibrium were observed for the COMT rs4680 SNP in our study sample (p = 0.71). Participants were classified as: Val homozygotes (Val/Val), Met homozygotes (Met/Met), or heterozygotes (Val/Met). The COMT by race interaction effect or differences in allele frequencies across Caucasians or non-Caucasians in our study sample.
2.3. Measures
The PCET is a computerized test that evaluates cognitive set shifting and facilitates standardized administration in multi-site studies (Greenwood et al., 2007; Gur et al., 2010; Irani et al., 2012). Like the WCST, the PCET requires participants to identify a sorting principle based on feedback (Figure 1). When 10 consecutive trials are sorted correctly, the sorting principle changes without warning and the participant is then required to learn the new sorting principle without regressing to the previous response preference.
Figure 1.
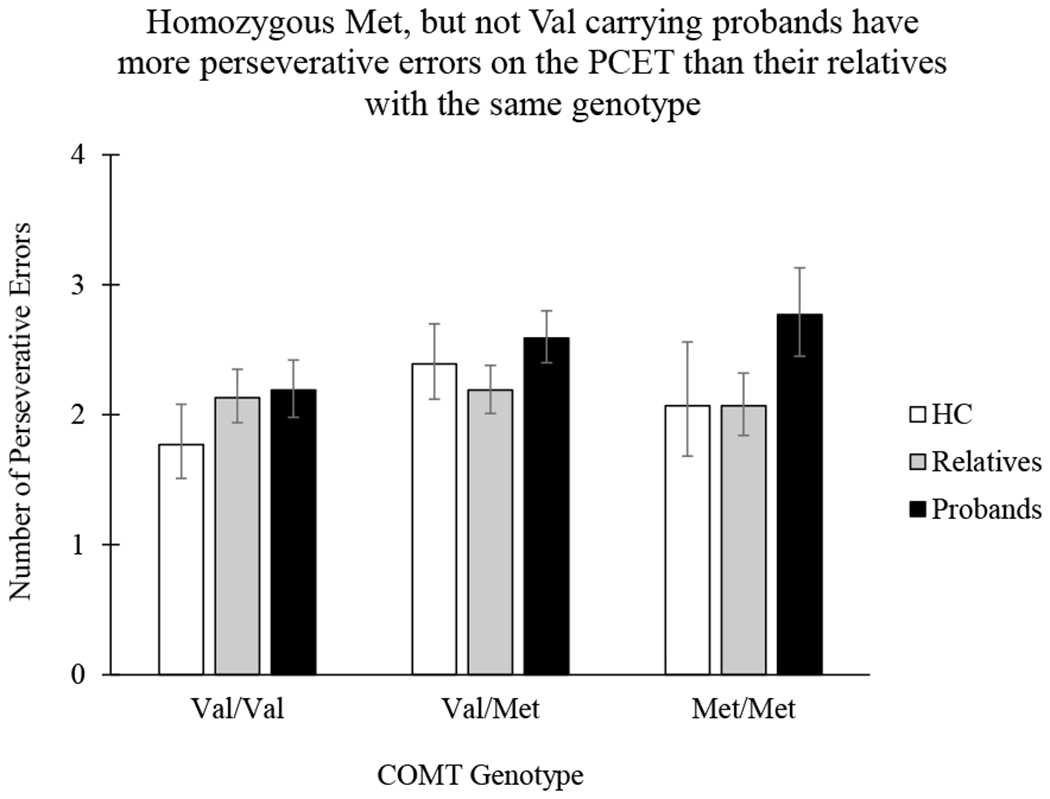
The significant interaction between COMT Val158Met genotype and group (healthy control vs. proband vs. first-degree relatives) for perseverative errors was characterized by selectively elevated perseverative errors for Met/Met probands. Error bars represent a 95% confidence interval.
2.3.1. Defining perseverative and regressive errors.
Perseverative errors in set-shifting tasks (such as the WCST) are typically defined using multiple criteria, and, consequently, represent a heterogenous index capturing numerous types of errors (Heaton et al., 1993; Barceló & Knight, 2002; Hill et al., 2014). The current report set out to delineate this heterogeneity by parsing errors into two main types that occur after the first-category shift and are defined with respect to category rule acquisition: (1) Perseverative errors: persistent use of the prior category rule in the face of negative feedback until the first, correct choice for the new category rule has been selected, and (2) Regressive errors: a return to the prior response rule after acquisition in which the first unambiguous switch to the new, correct category rule is positively reinforced (Hill et al., 2014; Ragozzino et al., 2002; Ragozinno & Choi, 2004). Although similar to a “loss of cognitive set” this definition of regressive errors is distinct in that it requires that the response pattern shift to the previously acquired response rule. Consistent with prior reports (Hill et al., 2014; Nelson et al., 2018) analysis of errors was limited to those during Category 2 given the difficulty evaluating regressive errors after two category rules have been learned, and the absence of a competing prior response rule during Category 1. A subset of probands were unable to complete the first sorting principle (n = 57) and were excluded from the primary analysis. There were no significant group differences in demographics (age, sex, race, education, WRAT-IV reading standard score), COMT genotype, or global cognitive functioning (BACS composite z-score) across patients who did and did not complete the first sorting principle (see Table 2).
Table 2.
Probands who did and did not complete PCET Category 1 were similar demographically and had a similar distribution of COMT genotype.
| Did Not Complete Category 1 | Completed Category 1 | Findings | |||
|---|---|---|---|---|---|
| n = 57 | n = 437 | ||||
| Mean | SD | Mean | SD | ||
| Age (years) | 36.32 | (11.54) | 36.26 | (12.68) | F = 0.18 |
| Education (years) | 12.87 | (2.50) | 13.25 | (2.27) | F = 1.32 |
| Wide-Range Achievement Test-IV: Reading (SS) | 95.67 | (14.48) | 96.45 | (15.11) | F = 0.14 |
| BACS Composite (z-score) | −1.10 | (1.44) | −1.21 | (1.35) | F = 0.36 |
| n | % | n | % | ||
| Sex | |||||
| Male | 35 | 61.4% | 224 | 51.3% | χ2 = 2.08 |
| Female | 22 | 38.6% | 213 | 48.7% | |
| Race | |||||
| Caucasian | 30 | 52.6% | 251 | 57.4% | χ2 = 0.61 |
| Afr. American | 23 | 40.4% | 163 | 37.3% | |
| Other | 4 | 7.0% | 23 | 5.3% | |
| COMT Genotype | |||||
| Val/Val | 19 | 33.3% | 148 | 33.9% | χ2 = 1.49 |
| Met/Met | 31 | 54.4% | 209 | 47.8% | |
| Val/Met | 7 | 12.3% | 80 | 18.3% | |
All differences were non-significant at p < 0.05
2.4. Statistical Analyses
The Brief Assessment of Cognition in Schizophrenia (BACS) composite score was included as a covariate in all analyses to control for generalized cognitive functioning across groups (see Table 1). As is common with set shifting measures, both error rates (raw counts) were not normally distributed (Hill et al., 2014). As such, the primary analyses used generalized linear models designed to account for Poisson distributions, with separate models for error type (perseverative, regressive) with COMT genotype (Val/Val, Met/Met, and Val/Met) and general diagnostic group (HC, Proband Relative) as factors. All main and interaction effects were probed using pairwise contrasts with a Bonferroni correction for multiple comparisons.
3. Results
3.1. Perseverative Errors and COMT
Controlling for global cognitive ability (BACS score), Poisson regression analysis revealed a significant main effect of COMT genotype on perseverative error rate (χ2[2] = 12.95, p = 0.002), such that heterozygous Met carriers had more perseverative errors than homozygous Val carriers (, p = 0.001, 95%CIDiff [0.12,0.60]). In addition, there was a significant interaction between group (HC vs. Proband vs. Relative) and COMT genotype for perseverative errors (χ2[4] = 9.48, p = 0.05) (Figure 1). The interaction was characterized by similar rates of perseverative errors for probands and relatives homozygous for the Val allele (, p = 1.00, 95%CIDiff [−0.43, 0.55]), while homozygous Met probands had significantly more perseverative errors than their Met relatives (, p = 0.032, 95%CIDiff [0.03, 1.39]) (Figure 2).
Figure 2.
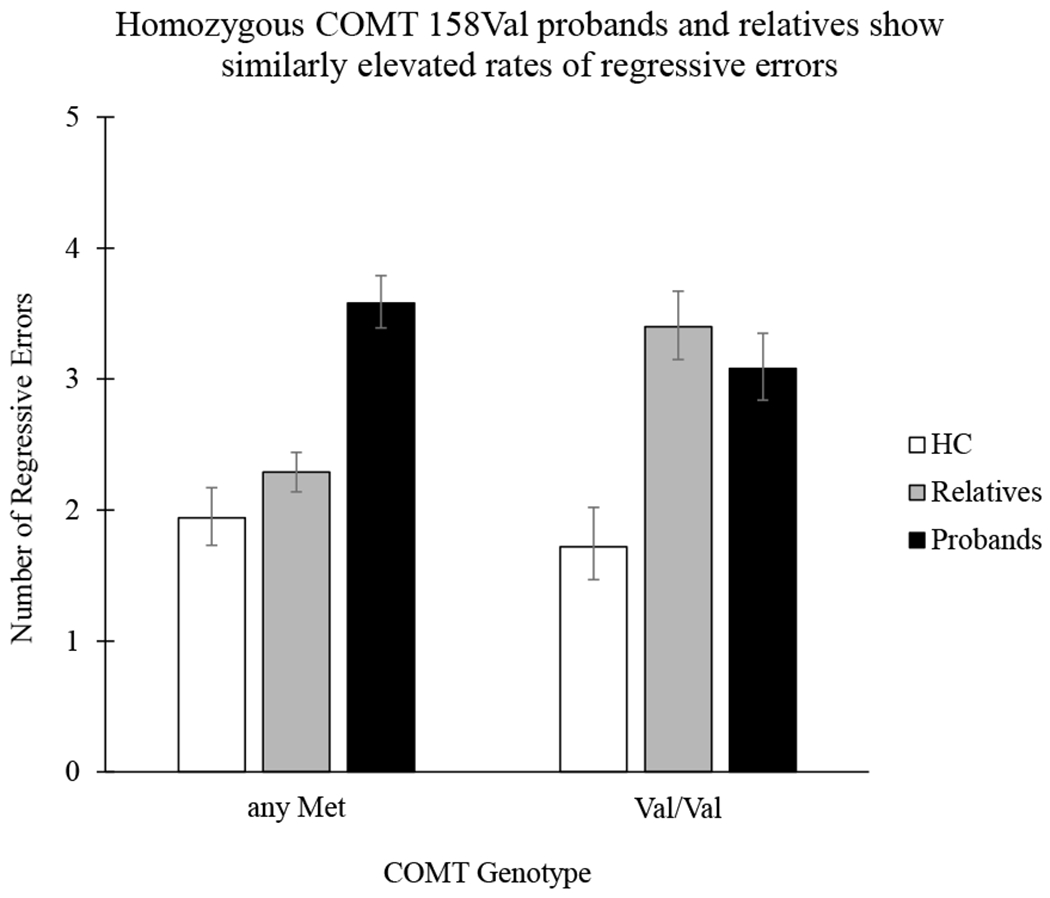
The interaction between COMT genotype and group (healthy control vs. proband vs. first-degree relatives) on regressive error rate. Homozygous Val probands and relatives evidenced similar performance (and elevated errors compared to controls) whereas heterozygous relatives’ performance was comparable to controls, with only probands showing elevated errors in comparison. Error bars represent a 95% confidence interval. Please note, homozygous Met and heterozygous groups were collapsed for illustrative purposes.
3.2. Regressive Errors and COMT
Controlling for global cognitive ability, Poisson regression analyses revealed a significant main effect of COMT genotype on regressive error rate (χ2[2] = 16.54, p < 0.001), such that homozygous Val (, p = 0.001, 95%CIDiff [0.19,0.86]) and heterozygous Val (, p < 0.001, 95%CIDiff [0.24,0.87]) carriers had more errors than Met homozygotes. In addition, there was a significant group by genotype interaction (χ2[4] = 65.51, p < 0.001) (Figure 2). The interaction was clarified by comparing genotype groups across diagnostic groups. Findings indicated that probands homozygous for the Val allele had comparable performance to same genotype relatives (, p = 1.00, 95%CIDiff [−0.91, 0.28]), with both probands (, p < 0.001, 95%CIDiff [0.74, 1.97]) and relatives (, p < 0.001, 95%CIDiff [1.06, 2.30]) committing significantly more regressive errors than controls. However, probands heterozygous for the Met allele had significantly more regressive errors than both Met/Met relatives (, p < 0.001, 95%CIDiff [0.98, 1.96]) and controls (, p < 0.001, 95%CIDiff [1.08, 2.24]).
4. Discussion
This is the first study to investigate the relationship between COMT genotype and two translationally informed indicators of cognitive flexibility and stability in patients with psychotic disorder and their first-degree relatives. In line with the primary hypotheses, findings indicated that COMT moderated regressive error type – both homozygous and heterozygous Val carriers had more errors than homozygous Met carriers. Furthermore, findings indicated a group by rs4680 COMT genotype interaction characterized by differential effects on cognitive stability and flexibility. Specifically, Met carrying probands had higher rates of perseverative errors than their Met carrying first-degree relatives (who did not differ from Met carrying controls). In terms of regressive errors, relatives homozygous for the Val allele performed as poorly as their proband counterparts. Proband regressive error rate across genotype was consistently worse than same-genotype controls, whereas only homozygous Val and homozygous Met relatives were worse than controls. Additionally, this pattern could not be attributed to global cognitive ability. Thus, the moderating role of COMT on set shifting, and differential impact of genotype, goes beyond generalized cognitive dysfunction and may uniquely or with some specificity impact the maintenance and updating of behavioral plans and mental representations.
4.1. Continuity and Specificity of COMT Across Groups and Within the Frontostriatal Network
Efficient modulation of set shifting via frontostriatal networks involves two distinct processes: cognitive flexibility supported primarily by prefrontal cortex and cognitive stability supported via striatal function (Frank et al., 2001; Johnston et al., 2007; Kim & Ragozzino, 2005; Ragozzino, 2007). These circuits are heavily dependent upon dopamine signaling with increased tonic cortical D1 promoting maintenance (stability) via reciprocal reductions in subcortical phasic D2 firing, resulting in a suppression of updating which promotes flexibility (Bilder et al., 2004; Dodds et al., 2009; Goldberg et al., 2003; Nolan et al., 2004; Rosa et al., 2010). Consistent with our hypothesis, Val and Met alleles were related to stability and flexibility in different ways. Whereas the Met allele was related to reduced flexibility (more perseverative errors), particularly in probands, the Val allele was associated with reduced stability of newly learned plans relative to previously preferred ones (more regressive errors). These findings were consistent with research suggesting that the COMT gene indirectly modulates dopamine firing (and thus flexibility and stability) through its effect on neocortical dopamine catabolism (Bilder et al., 2004; Matsumoto et al., 2003; Tunbridge et al., 2006), resulting in differential enhancement of frontal and striatal components of frontostriatal networks with consequent specific changes in behavioral flexibility.
Met carriers, with enhanced cortical dopamine compared to their Val counterparts, may have difficulty processing information adaptively to develop novel problem-solving strategies. Additionally, bursts of striatal dopamine signaling during set-shifting are thought to promote updating and flexibility by inhibiting prefrontal maintenance (Rosa et al., 2010; Bilder et al., 2004). In contrast, Val homozygotes, who may have increased striatal dopamine as a downstream effect of the high activity COMT allele, exhibit difficulty with stability and maintenance of previously reinforced behavior. Dopamine has been shown to exert a modulatory “inverted-u-shaped curve” effect on set shifting (Cools & D’Esposito, 2011) and exacerbate dopaminergic abnormalities resulting in a shifting or narrowing of the response curve secondary to illness-specific or neurobiological factors in patients.
4.2. COMT and Set Shifting in Probands and First-Degree Relatives
Although probands showed elevated rates of regressive errors compared to controls, their performance compared to their first-degree relatives varied as a function of COMT genotype. Among first-degree relatives there was a selective impairment for maintaining a newly acquired behavioral set only for Val homozygous relatives. Interestingly, first-degree relatives who were homozygous for the Val allele did not differ from controls with the same genotype in terms of global cognitive ability. Regressive errors have been identified as an area of focal cognitive impairment in psychotic disorders, representing a deficit not accounted for by overall level of cognitive impairment (Hill et al., 2014; Reilly & Sweeney, 2014). In addition, the COMT Val allele has been linked to the expression of core psychosis phenotypes (Goghari & Sponheim, 2008), and, in earlier studies, an increased risk for developing a psychotic disorder overall (Egan et al., 2001). Furthermore, Val homozygosity was over-represented in the more severely impaired B-SNIP Biotype I patients who present with pronounced cognitive and imaging impairments (Wolfe et al, 2018). Perhaps the Val allele may be more useful as a familial risk indicator for psychosis (Egan et al., 2001), with homozygous expression of Val contributing to a more severe form of psychosis with greater penetrance in first-degree relatives. Additional familial and high-risk research is needed to evaluate the potential utility of Val homozygosity as an early indicator of psychosis risk. Given prior findings of selective impairment in cognitive stability following antipsychotic treatment in Val homozygotes (Nelson et al., 2018), it will also be important to investigate treatment responsivity of Val homozygotes and the potential pharmacogenetic implications.
Finally, heterozygous and homozygous Met probands were impaired compared to their same-genotype relatives (and controls). This suggests that the Met allele may uniquely contribute to, or interact with, illness-specific factors to disrupt cognitive stability and flexibility. Although further research is needed, there are several promising mechanisms that could underlie this interaction, notably Met moderated reductions in striatal DA activity compounded by similar illness-related reductions in both DA and frontostriatal function (Bertolino et al., 2004; Egan et al., 2001; Goldberg et al., 2003; Rosa et al., 2010).
Taken with the current findings, there remain substantial inconsistencies in the empirical literature regarding the connection between SNPs, dopamine signaling, and the concepts of cognitive stability and flexibility. Notably, although Rosa et al. (2010) and others have found support for the role of the Met allele in promoting maintenance, evidence for the Val allele promoting flexibility is far less consistent. Substantial heterogeneity in operationalizing and isolating cognitive flexibility and stability (over the distinct construct of cognitive control) may partially contribute to this inconsistency; however, it is more likely due to the complexity of dopaminergic interactions with frontostriatal functions and cognition compared to the relative simplicity of the model being evaluated (Bilder et al., 2004; Nelson et al., 2018; Nolan et al., 2004; Rosa et al., 2010). Regardless further research is needed to both refine the tonic-phasic model as well as further clarify any unique impact of the Val allele.
4.3. Limitations
There are several factors that may limit the interpretation and generalization of the present findings. First, by focusing on category two responses, the analysis was limited to participants who completed the first response set (Category 1) on the PCET. Thus, the performance of patients with more severe deficits (those who could not complete the first category) could not be assessed. Second, both error types were defined with respect to set acquisition, and given the significantly larger number of trials post-acquisition, there were more opportunities to commit regressive errors and thus there were psychometric advantages for detecting effects related to regressive vs. perseverative errors. Although the constructs cognitive stability and flexibility being reflected in perseverative and regressive errors on set shifting measures has been the topic of several reports in both human and animal model literature as they relate to COMT (Hill et al., 2014; Johnston et al., 2007; Rainer, 2007; Ragozzino, 2007; Ragozzino 2004; Ragozzino, Raggozino, Mizumori, Kesner 2002), no formal psychometric analysis of construct validity has been performed to date. Given the conflicting reports in the literature, the present findings should be interpreted with caution until a clear picture regarding the impact of SNP variants in COMT on cognitive stability and flexibility emerges. Finally, well powered gene association studies typically require large sample sizes (e.g., n ≥ 1,000) (Gauderman, 2002). Although the number of total participants was large for a phenotyping study, the ability to conduct differential association studies across diagnostic groups was limited. Overlap across confidence intervals for select effects also indicates relatively small effect sizes, necessitating caution in interpreting the current findings.
4.4. Future Directions
Research findings regarding the impact of COMT on cognitive alterations associated with psychotic disorders has been inconsistent. One potential contributing factor to discordant findings in the literature may be the different aspects of executive function that have been assessed. One advantage of the current study was in the direct examination of COMT genotype in relation to two complimentary aspects of executive function (cognitive stability and flexibility) that have been linked to distinct regions in frontostriatal systems. Future research might focus on further validating the relationship between the Met allele and cognitive flexibility as well as the role of Val allele in cognitive stability. Further research is needed to examine the degree to which the present findings generalize across different diagnoses within the psychosis spectrum. As the severity of current psychotic symptoms was limited, future studies may benefit from examining the impact of COMT on cognitive stability and flexibility during acute phases of psychotic disorders and in relation to antipsychotic therapy.
5.1. Acknowledgements
This study was supported in part by NIMH grants MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888. The writing of this manuscript was also supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
5.3 Role of the Funding Source
The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.2 Conflicts and Disclosures
Dr. Tamminga is on the board of IntraCellular Technology and an ad hoc consultant for Takeda, Pierre Fabre, and Atiphony. The other authors have no conflicts of interest at this time. The other authors have nothing to disclose.
6. References
- Anderson CA, Pettersson FH, Clarke GM, Cardon LR, Morris AP, & Zondervan KT (2010). Data quality control in genetic case-control association studies. Nature Protocols, 5 (9), 1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceló F, & Knight RT (2002). Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia, 40 (3), 349–356. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, … & Nardini M (2004). Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. American Journal of Psychiatry, 161 (10), 1798–1805. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, & Grace AA (2004). The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology, 29 (11). [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M & Pantelia C (2009). Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders, 113, 1–20. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang X, O’Neill AF, Walsh D, & Kendler KS (2004). Variants in the catechol-o-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Molecular Psychiatry, 9 (10), 962. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, … Tamminga CA (2016). Identification of distinct psychosis biotypes using brain-based biomarkers. American Journal of Psychiatry, 173 (4), 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, & D’Esposito M (2011). Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biological psychiatry, 69 {12), 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1997). Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. Journal of Neuroscience, 17 (23), 9285–9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Clark L, Dove A, Regenthal R, Baumann F, Bullmore E, … & Müller U (2009). The dopamine D2 receptor antagonist sulpiride modulates striatal BOLD signal during the manipulation of information in working memory. Psychopharmacology, 207 (1), 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, … & Weinberger DR (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, 98 (12), 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, & Grattan LM (1993). Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia, 31 (1), 17–28. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured Clinical Interview for DSMIV-TR Axis I Disorders. New York State Psychiatric Institute. [Google Scholar]
- J. M, Loughry B, & O’Reilly RC (2001). Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cognitive, Affective, and Behavioral Neuroscience, 1 (2), 137–160. [DOI] [PubMed] [Google Scholar]
- Gauderman WJ (2002). Sample size requirements for association studies of gene-gene interaction. American Journal of Epidemiology, 155 (5), 478–484. [DOI] [PubMed] [Google Scholar]
- Goghari VM, & Sponheim SR (2008). Differential association of the COMT Val158Met polymorphism with clinical phenotypes in schizophrenia and bipolar disorder. Schizophrenia Research, 103 (1–3), 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D and Weinberger DR, 2003. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry, 60 (9), 889–896. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1994). Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences, 6 (4), 348–357. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, … & Schork NJ (2007). Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Archives of general psychiatry, 64( 11), 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, … & Gur RE (2010). A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. Journal of neuroscience methods, 187(2), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, & Curtiss G (1993). Wisconsin Card Sorting Test (WCST): Manual: Revised and Expanded. Psychological Assessment Resources (PAR). [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA (2004). Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophrenia Research, 1 (68): 49–63. [DOI] [PubMed] [Google Scholar]
- Hill SK, Bjorkquist O, Carrathers T, Roseberry JE, Hochberger WC, Bishop JR (2013a). Sequential processing deficits in schizophrenia: Relationship to neuropsychology and genetics. Schizophrenia Research, 151, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS … & Sweeney JA (2013b) Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: Findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. The American Journal of Psychiatry, 170 (11), 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Ragozzino ME…Sweeney JA (2014). Regressing to prior response set after switching implicates striatal dysfunction across psychotic disorders: Findings from the B-SNIP study. Schizophrenia Bulletin, 41 (4), 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberger WC, Hill SK, Nelson CLM, Reilly JL, Keefe RSE, Pearlson GD, Keshavan MS, Tamminga CA, Clementz BA, Sweeney JA (2016). Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophrenia Research, 170 (1), 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani F, Brensinger CM, Richard J, Calkins ME, Moberg PJ, Bilker W, … & Gur RC (2012). Computerized neurocognitive test performance in schizophrenia: A lifespan analysis. The American Journal of Geriatric Psychiatry, 20(1), 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, & Everling S (2007). Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron, 53(3), 453–462. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA (2007) Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. American Journal of Psychiatry, 164 (7): 1061–1071. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, & Hawkins K (2008). Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophrenia Research,102 (1), 108–115. [DOI] [PubMed] [Google Scholar]
- Kim J, & Ragozzino ME (2005). The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of learning and memory, 83(2), 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996). Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics, 6, 243–250. [DOI] [PubMed] [Google Scholar]
- Lin SH, Liu CM, Hwang TJ, Hsieh MH, Hsiao PC, Faraone SV, … & Chen WJ (2013). Performance on the Wisconsin Card Sorting Test in families of schizophrenia patients with different familial loadings. Schizophrenia Bulletin, 39 (3), 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, … & Kleinman JE (2003). Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology, 28 (8), 1521–1530. [DOI] [PubMed] [Google Scholar]
- Manoach DS (2003). Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophrenia Research, 60 (2), 285–298. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Amsbaugh HM, Reilly JL, Rosen C, Marvin RW, Ragozzino ME, … & Hill SK (2018). Beneficial and adverse effects of antipsychotic medication on cognitive flexibility are related to COMT genotype in first episode psychosis. Schizophrenia Research, 202, 212–216. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, & Volavka J (2004). Catechol O-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. American Journal of Psychiatry, 161(2), 359–361. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, De Bakker PI, & Daly MJ (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics, 81, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP (2002). Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience, 116 (1), 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D (2004). Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learning and Memory, 11 (1), 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME (2007). The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York academy of sciences, 1121 (1), 355–375. [DOI] [PubMed] [Google Scholar]
- Rainer G (2007). Behavioral flexibility and the frontal lobe. Neuron, 55(3), 321–323. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Sweeney JA (2014). Generalized and specific neurocognitive deficits in psychotic disorders: Utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophrenia bulletin, 40 (3), 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa EC, Dickinson D, Apud J, Weinberger DR, & Elvevag B (2010). COMT Val158Met polymorphism, cognitive stability and cognitive flexibility: an experimental examination. Behavioral and Brain Functions, 6 (1), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, & Carter CS (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophrenia Bulletin, 32(1), 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNP & Variation Suite™ (Version 8.x) [Software], Bozeman, MT: Golden Helix, Inc. Available from http://www.goldenhelix.com. [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, … & Sweeney JA (2013). Clinical phenotypes of psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP). The American Journal of Psychiatry, 10. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, & Weinberger DR (2007). Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex, 17 (suppl_1), i171–i181. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, & Weinberger DR (2006). Catechol-O-Methyltransferase, Cognition, and Psychosis: Val158Met and Beyond. Biological Psychiatry, 60 (2), 141–151. [DOI] [PubMed] [Google Scholar]
- Wolfe M, Gotra M, Miller S Clementz, Gershon ES, Keefe RSE, Keshavan MS, Pearlson GD, Sweeney JA, Tamminga CA & Hill SK (2018, June). Distribution of Catechol-O-methyl transferase Genotype Across Psychosis Classification Systems. Poster session presented at the annual meeting of the American Academy of Clinical Neuropsychology, San Diego, CA. [Google Scholar]


