Abstract
Background:
Mood disorders and problematic substance use co-occur and confer reciprocal risk for each other. Few studies use analytic approaches appropriate for testing whether specific features of one disorder confer risk for the other.
Methods:
445 participants (59.8% female, Mean age = 20.3 years) completed measures of depression and hypo/mania symptoms and substance use-related impairment; 330 had complete data at follow-up. Of these, 28% reported a history of depression, 4% of bipolar spectrum disorder, 11% of substance use disorder, and 55% reported substance-related impairment. Symptoms and domains of substance-related impairment were modeled in cross-sectional and cross-lagged panel network models.
Results:
Impulsive and interpersonal impairment were most highly comorbid with mood symptoms. Suicidal ideation, sadness, decreased need for sleep, and guilt were the symptoms most highly comorbid with impairment. Interpersonal impairment due to substance use was the strongest cross-construct predictor of mood symptoms and suicidal ideation was most predictive of impairment. Social, intrapersonal, and physical impairment due to substance use were most predicted by previous mood symptoms and decreased need for sleep, guilt, and euphoria were most strongly predicted by past impairment.
Limitations:
Measures do not assess all mood symptoms, participants with low reward sensitivity were excluded, only self-report measures were used, and some variables were single-items.
Conclusions:
Components of these syndromes that confer cross-construct risk might not be the same components that are predicted by the other construct. The bidirectional relationship between mood symptoms and problematic substance use might be better conceptualized at the element, rather than diagnostic, level.
Keywords: Comorbidity, depression, hypomania, bipolar disorder, substance use
Introduction
Mood disorders and problematic substance use frequently are comorbid (Kessler et al., 1997). This co-occurrence is associated with worse treatment outcomes, more severe course of illness, and heightened risk of suicide (Tolliver & Anton, 2015). Compared to individuals with no mood disorders, those with unipolar depression were twice as likely, and people with a bipolar spectrum disorder (BSD) nearly seven times as likely, to have a history of substance use (Kessler et al., 1997). Importantly, research suggests that the relationship between problematic substance use and mood symptoms is bidirectional in nature (Pacek et al., 2013; Salloum & Thase, 2000). This is consistent with theories that suggest that one reason for this comorbidity is that features of one disorder increase risk for the other (Strakowski & DelBello, 2000). Consistent with research suggesting that not all symptoms of a given diagnosis have the same risk factors (Fried et al., 2014), it is possible that not all mood symptoms confer equal risk for all types of problems secondary to substance use, and vice-versa. To explore this possibility, this project employed network analyses with cross-sectional and longitudinal data to investigate the interplay between symptoms of depression, hypomania/mania (henceforth referred to as hypo/mania), and substance use-related impairment.
Mood Symptoms and Substance Use: Reciprocal Risk
Epidemiological findings consistently indicate that mood and substance use disorders (SUDs) are highly comorbid; however, there are limited prospective studies investigating reciprocal risk between the syndromes. Some evidence suggests that adolescent-onset of SUDs significantly increases risk for developing a mood disorder, particularly BSDs (Kenneson et al., 2013), whereas work in adult samples has found that depression can be both a risk factor and consequence of SUDs (Kessler et al., 2005). Several theories linking mood disorders and SUDs as risk factors for one another exist.
For example, the cumulative failure model posits that negative outcomes related to substance use engagement (i.e., failure in social and academic/work domains) lead to subsequent depression by undermining self-confidence (Patterson & Stoolmiller, 1991). Similarly, substance use among adolescents may impair brain development, and thus, lead to cognitive impairment, which precedes mood symptoms (Peeters et al., 2014). For instance, the association of BSDs with decreased cortical gray matter volumes and neurocognitive impairment may be exacerbated by the effects of substance abuse on neurodevelopmental processes (Balanzá-Martínez et al., 2015). It is also plausible that withdrawal or substance use-related impairment (e.g., interpersonal) could trigger depression symptoms such as guilt, suicidal ideation, or dysphoria (Kessler, 2004; Markou & Kenny, 2002; Rappeneau & Bérod, 2017; Swendsen et al., 2010). Additionally, a history of substance use is associated with earlier onset of BSDs and has been hypothesized to be a potential trigger for individuals with pre-existing vulnerabilities to BSDs, potentially via social and/or biological consequences (e.g., interpersonal difficulties, social/circadian rhythm dysregulation, etc.; Cardoso et al., 2016; Strakowski & Delbello, 2000). Most of these theories do not focus on the frequency of substance use as a risk factor; rather, risk is conferred by the impairment that results from problematic substance use.
Conversely, pre-existing mood symptoms may predict subsequent problematic substance use via difficulties in peer relationships (including irritability and peer deviance), and substance use may emerge as a coping mechanism for interpersonal distress (Hussong et al., 2011). Indeed, depression symptoms associated with difficulties in social adjustment and poor coping skills are most predictive of substance use (Sanchez et al., 2015; Thornton et al., 2012) and individuals with extreme low or high affect might use substances to regulate or amplify that affect (Blum et al., 2000; Bowirrat & Oscar-Berman, 2005; Volkow et al., 2003). Additionally, the impulsivity, reward hypersensitivity, and decreased need for sleep associated with hypo/manic episodes might increase risk for engaging in problematic substance use by increasing exposure to alcohol and drugs at parties, bars, etc. Thus, evidence suggests the relationship between mood symptoms and substance use impairment might be best conceptualized at the element (symptom) level rather than diagnostic level. Specifically, there might be differential relationships between symptoms of mood disorders and specific types of impairment due to substance use.
Network Theory of Comorbidity
Investigation of comorbidity at the element level can inform the etiological and nosological understanding of mood symptoms and problematic substance use. Recent advances in network science allow for a nuanced investigation of the relationships between these phenomena. Contrary to the more traditional common-cause conceptualization of psychopathology, network theory argues that symptoms are not the result of a single latent factor. Rather, symptom co-occurrence may reflect symptom-to-symptom relationships, a perspective seen in several theoretical models of comorbidity (e.g., Strakowski & DelBello, 2000). Consistent with this theory, network analyses enable element-wise (e.g., symptom, domain of impairment) investigations of psychopathology. This facilitates transdiagnostic research in that, unless a researcher specifies otherwise, network models treat all variables as separate entities, encouraging interpretations at the level of the behavior/cognition/symptom rather than the diagnostic category. Inherently transdiagnostic, this perspective can test the theories described above and improve our understanding of the nature of comorbidity (Cramer et al., 2010).
The Present Study
Although the theory that some features of mood disorders might increase risk for problematic substance use, and vice-versa, is not new, recent advances in network modeling allow for unprecedented tests of this theory. This study simultaneously modeled relationships between five distinct domains of substance-related impairment (interpersonal, intrapersonal, social, impulsive, physical) and thirteen mood symptoms from both ends of the mood spectrum (depression and hypo/mania) using contemporaneous and prospective models. Impairment was chosen over other measures of substance use (e.g., frequency/intensity) to maximize clinical relevance and comparability between participants (equal frequency and/or intensity can affect people with various body types and lifestyles differently). This approach allowed for exploration of which mood symptoms are most associated with specific domains of substance-related impairment, and vice-versa. In particular, identification of central symptoms (e.g., symptoms with high bridge expected influence, in/out prediction, described below) might highlight potential treatment targets or elements specifically likely to predict, or be predicted by, symptoms of the other disorder (Elliott et al., 2020).
To elucidate the interplay between mood symptoms and domains of substance use-related impairment, the current project asked: 1) Do discrete domains of substance-related impairment have differential concurrent relationships with specific mood symptoms?; 2) Which mood symptoms most strongly predict future substance use-related impairment, and vice-versa?; and 3) Which mood symptoms are most strongly predicted by past substance use-related impairment, and vice-versa?
Methods
Participants and Procedures
Participants were drawn from the Teen Emotion and Motivation (TEAM) Project (Alloy et al., 2012), a prospective, longitudinal study of the onset and course of BSDs. Participants were selected via a two-phase screening procedure from the greater Philadelphia area (Alloy et al., 2012). In Phase I, 9,991 students aged 14–19 from Philadelphia public high schools and local universities were screened. They completed two self-report measures of reward sensitivity: the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) Scales (Carver & White, 1994) and Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSRQ; Torrubia et al., 2001). Students who scored in the top 15th percentile on both the BAS-Total (BAS-T) of the BIS/BAS and the Sensitivity to Reward Scale (SR) of the SPSRQ composed the high BAS (high reward sensitivity) group, and those who scored between the 40th and 60th percentiles on both measures composed the moderate BAS (moderate reward sensitivity) group of the main study sample. Participants with low BAS sensitivity were excluded because it is associated with vulnerability to unipolar depression (Depue & Iacono, 1989) and Project TEAM was designed to examine vulnerability to first onset of BSDs based on the BAS/reward hypersensitivity model of BSDs. The screening sample was representative of adolescents aged 14–19 in the Philadelphia area on race, sex, and age (Alloy et al., 2012).
Participants in the high or moderate BAS groups were invited to a Phase II screening and were administered an expanded Schedule for Affective Disorders and Schizophrenia—Lifetime diagnostic interview (SADS-L, Alloy et al., 2008; Endicott & Spitzer, 1978) by interviewers blind to participants’ BAS risk group status; 424 high BAS and 241 moderate BAS participants completed Phase II. The exp-SADS-L interview was expanded to allow both DSM-IV-TR (American Psychiatric Association, 2000) and Research Diagnostic Criteria (RDC; Endicott & Spitzer, 1978) diagnoses. Participants not fluent in English were excluded.
Participants completed measures of impairment due to substance use and symptoms of depression and hypo/mania at Time 1 (T1) and Time 2 (T2; mean/median months apart = 10.7/7.6, SD = 8.2, range = 1.3 – 60.9 months, see Supplemental Figure 1 for a histogram). The analytic sample included 445 participants (281 high BAS and 164 moderate BAS, 99 with a history of a BSD, Mage = 20.3 years at T1, SD = 1.7 years). The sample was 59.8% female, 60.9% White, 21.5% Black, 10.1% Asian, 3.4% Latino/a, 2.7% Multiracial, and 0.2% Native American; 1.8% did not report their race. The sample of 330 participants with complete follow-up measures at T2 did not differ from those with T2 missing data on BAS group, gender, substance-related impairment, or mood symptoms (all ps > .05). However, the participants with complete data at T2 were younger (mean difference = .39 years, p = .03) and less likely to be White and more likely to be Black (G2(5) = 12.24, p = .03).
Measures
Depression symptoms.
Depression symptoms were assessed using the Beck Depression Inventory-1A (BDI-1A; Beck & Steer, 1993). The BDI-1A is a 21-item measure assessing depression symptoms in the past 30 days. Items are rated on a scale of 0–3 (higher scores indicating more severe symptoms). It has shown acceptable psychometric properties (Beck & Steer, 1993). The mean BDI-1A score at T1 was 5.43, SD = 6.25. Due to power constraints, eight items most closely matching eight DSM-5 depression criteria were included in this project: items 1 (sadness), 4 (anhedonia), 5 (guilt), 9 (suicidal ideation), 11 (irritability), 13 (indecisiveness), 17 (fatigue), and 18 (decrease in appetite). The BDI-1A lacks items measuring psychomotor retardation/agitation or increased need for sleep, so these symptoms were not modeled. Additionally, decrease in sleep was not modeled as a depression symptom because a similar, but more specific, item was included in the hypo/mania measure.
Several DSM-5 criteria for depression are multi-faceted and measured by several items on the BDI-1A so we used the following rationale when selecting which items best apply to a DSM criterion: Item 4 was chosen for anhedonia because it measures global dissatisfaction, whereas Items 12 and 21 specifically measure loss of interest in people and sex, respectively. Increase/decrease in both appetite and weight are a single symptom in the DSM-5, but the BDI-1A only measures decreases in appetite and weight. Item 18 (appetite) was chosen because weight change is more likely to be secondary to other factors (e.g., change in appetite, exercise, etc.). Guilt and worthlessness make up the same DSM-5 criterion, but guilt (Item 5) was chosen because no questions specifically measured worthlessness.
Hypo/mania symptoms
Symptoms of hypo/mania were assessed using the Altman Self-Rating Mania Scale (ASRM; Altman et al., 1997). The ASRM has five items rated on 5-point Likert scales (0–4; higher scores indicating more severe symptoms over the past 30 days). The five symptoms assessed are: inflated self-confidence, talkativeness, euphoria, reduced need for sleep, and increased activity. The measure has demonstrated strong convergent validity with clinical interviews and other measures of hypo/mania (Altman et al., 2001). All five items were used in analyses. The mean ASRM score at T1 was 4.77, SD = 3.79.
Impairment due to substance use
Impairment due to substance use was assessed using the Short Inventory of Problems-Revised (SIP-R; Blanchard et al., 2003). The SIP-R consists of 15 items that assess problems associated with substance use over the past 30 days. The measure yields five subscales (interpersonal, intrapersonal, social role, impulsive, and physical) and has demonstrated good convergent validity (Kiluk et al., 2013). The five subscales were used in the analyses and each had a range of 3–12 (higher scores indicate more impairment).
Diagnostic interviews
Expanded versions of the Schedule for Affective Disorders and Schizophrenia—Lifetime (SADS-L; Endicott & Spitzer, 1978) and —Change (SADS-C; Endicott & Spitzer, 1978) were administered during the screening for the parent study and at follow-up visits, respectively (see Alloy et al., 2008; 2012 for more details). They are semi-structured diagnostic interviews used to assess lifetime and current Axis I psychiatric disorders. They were designed to generate both DSM-IV-TR and RDC diagnoses, to increase items and improve the probes in the mood disorder sections, and to diagnose and evaluate medical history, family history, and organic rule-out conditions (Alloy et al., 2012). Before administering the interviews, post-doctoral fellows, doctoral students, and research assistants in clinical psychology received approximately 200 hours of extensive training. These interviews have demonstrated high inter-rater reliability (Alloy et al., 2000).
Statistical Analyses
Contemporaneous network estimation
In network models, variables are referred to as “nodes”, and “edges” are associations between nodes controlling for all other nodes (Epskamp & Fried, 2016). First, to explore cross-sectional associations among domains of substance-related impairment and mood symptoms, we estimated a Graphical Gaussian Model (GGM), using the R package qgraph (Version 1.6.5; Epskamp et al., 2012). All five subscales of the SIP-R, all five items of the ASRM, and the eight BDI-1A items listed above were included as nodes. Because several items were heavily skewed and nodes included both continuous and ordinal variables, Spearman correlations were used to estimate the network (Epskamp & Fried, 2018).
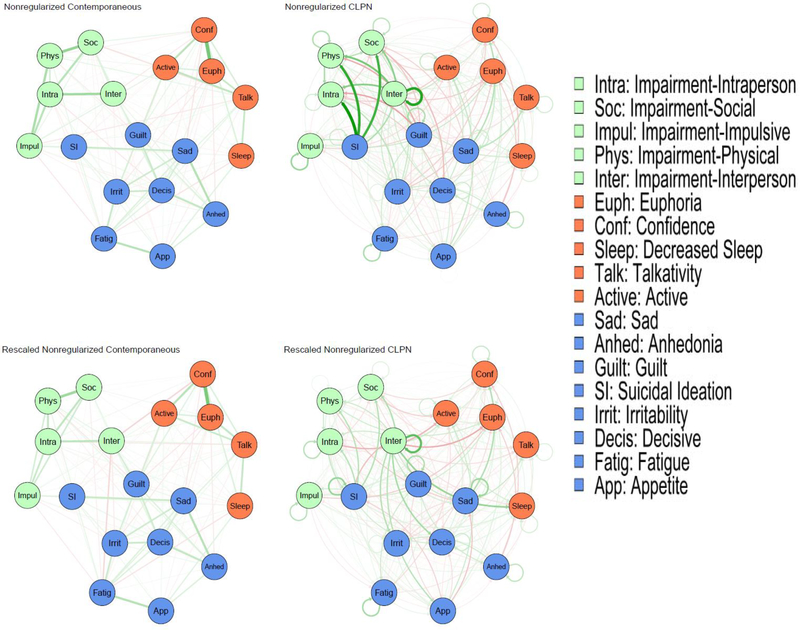
To address concerns about the performance of regularization techniques in psychopathology research (Williams et al., 2019), models (including temporal models) were estimated using nonregularized partial correlations. As described below, the nodes used had different measurement ranges, which could bias parameters. Thus, models also were estimated with all nodes rescaled to match the smallest Likert scaling (0–3) in the dataset. Models also were estimated with regularization as a sensitivity analysis, to evaluate which associations are robust to this procedure (Supplemental Methods/Results). All models used a modified Fruchterman-Reingold algorithm (the default option in the graph package that provides a clear visualization of the network) to place more strongly connected nodes closer to each other, but the location of nodes should not be used to interpret properties of these nodes (Epskamp & Fried, 2018).
As the primary aim of this study was to explore how mood symptoms influence impairment due to substance use, and vice-versa, we estimated one-step bridge expected influence (BEI) using the R package networktools (Version 1.2.3; Jones, 2018). Specifically, one-step BEI quantifies the sum of the edge weights between a given node (e.g., a mood symptom) and all nodes from other constructs (e.g., substance use impairment domain) with which it is associated. As the focus of this study was the relationship between mood symptoms and substance use-related impairment, and not the relationship between depression and hypo/mania symptoms, all mood symptoms were categorized as one construct. In a scenario where edges represent causal connections, nodes with high BEI would be the most likely to influence other communities or be activated by nodes from a nearby community, and consequently, spread activation within its own community (Jones, Ma, et al., 2019). Before interpretation, it is necessary to test centrality parameter stability using a case dropping procedure to test the proportion of the sample that can be dropped from the analysis and result in a .7 correlation between centrality estimates (i.e., BEI) of the original sample and the new sample, resulting in a correlation-stability coefficient. This bootstrapping procedure was executed in the R package bootnet (Version 1.3; CRAN link: http://cran.r-project.org/package=bootnet). A correlation-stability coefficient of .25 (25% of the sample dropped) is considered adequately stable and at least .50 is considered good stability (Epskamp et al., 2017). It is important to note that, because these networks use cross-sectional data, no interpretations of directionality can be made. BEI estimates were correlated with node variances to evaluate the extent to which they might be driven by measurement characteristics. Large, positive correlations indicate measurement characteristics might be driving estimates and alternative models should be considered.
Temporal networks
We also examined cross-lagged panel networks of mood symptoms and impairment due to substance use at T1 and T2 (Rhemtulla et al., 2018) in R package glmnet (Version 3.0–2; Friedman et al., 2010). This technique estimates the effects of nodes at T1 on all other nodes at T2, controlling for auto-regressive effects (i.e., regressing each node at T2 on itself at T1). Following Rhemtulla et al. (2018), we first computed within- and between-timepoint coefficients and auto-regressive coefficients with regularized regressions. Cross-lagged (i.e., T1 to T2) edges are directed, meaning it is possible to estimate both in-prediction and out-prediction. In-prediction summarizes the proportion of variance for a given node at T2 that is accounted for by nodes at T1. Out-prediction refers to the effect a given node at T1 has on nodes at T2 (computed as a sum of squared outgoing standardized regression coefficients). As the primary aim of the longitudinal models was to explore the effect of mood symptoms at T1 predicting specific domains of substance use-related impairment at T2, and the effect of substance use-related impairment at T1 predicting mood symptoms at T2, cross-construct effects were calculated, which exclude paths connecting nodes within the same community (e.g., the effect of mood symptoms at T1 predicting mood symptoms at T2). Similar to BEI, we tested the correlation between out-prediction and in-prediction estimates with node variances at T1 and T2, respectively, and estimated regularized versions of the models (Supplemental Methods/Results). The qgraph package was used to plot all networks. Identical maximum edge weight values and layouts were imposed for all networks to ease visual comparison.
Results
Preliminary Analyses
Descriptive statistics for all nodes in the nonregularized networks and sample characteristics can be found in Tables 1 and 2, respectively. Correlations between ASRM, BDI-1A, and SIP-R total scores at both time points are provided in Supplemental Table 1. 27.8% met criteria for a depressive disorder, 4.3% met for criteria for a BSD, and 10.6% met criteria for a SUD at one or both visits used in these analyses. 55.2 % of the sample reported some substance-related impairment (SIP-R total score above the minimum value) during at least one timepoint. Approximately 78.4% of the sample reported regularly using (defined as at least “several times per month”) alcohol, 38.5% regularly used marijuana, 28.9% regularly used tobacco, 6.1% regularly used amphetamines without a prescription, 2.9% regularly used cocaine, 1.8% regularly used hallucinogens, 1.6% regularly used tranquilizers, 1.1% regularly used opiates without a prescription, .5% regularly used inhalants, and 0% reported regular use of barbiturates or angel dust. The full range of scores was endorsed in this sample for all nodes except impulsive impairment due to substance use (range = 3–9 out of a maximum 3–12). A comparison of contemporaneous networks estimated using the full sample at T1 and the participants with data at T2 using R package NetworkComparisonTest (Version 2.2.1; Borkulo et al., 2016) did not find any significant differences in global network strength, structural invariance (the difference in the maximum difference between edge-weights within each network), or BEI (all p’s > .05).
Table 1.
Non-regularized Node Descriptives
| Node | T1 Mean (Variance) | T2 Mean (variance) | Stabilityŧ | BEI | CC In (proportion variance) | CC Out (∑β2) | |||
|---|---|---|---|---|---|---|---|---|---|
| Orig | Resc | Orig | Resc | Orig | Resc | ||||
| 1. Intrapersonal imp. | 3.49 (1.48) | 3.42 (1.45) | .53 | .01 | .04 | .38 | .07 | .06 | .16 |
| 2. Social Role imp. | 3.41 (.99) | 3.34 (1.00) | .37 | .00 | .00 | .30 | .08 | .05 | .11 |
| 3. Impulsive imp. | 3.64 (.90) | 3.42 (.67) | .45 | .18 | .22 | .06 | .03 | .06 | .10 |
| 4. Physical imp. | 3.42 (.94) | 3.34 (.96) | .47 | .12 | .14 | .31 | .06 | .04 | .11 |
| 5. Interpersonal imp. | 3.14 (.52) | 3.13 (.46) | .62 | .15 | .12 | .11 | .02 | .24 | .67 |
| 6. Euphoria | 1.07 (1.06) | .98 (1.01) | .24 | .02 | .10 | .05 | .09 | .01 | .00 |
| 7. Increased confidence | 1.12 (1.18) | 1.06 (1.21) | .32 | −.01 | −.08 | .02 | .04 | .01 | .00 |
| 8. Decreased need for sleep | .52 (.75) | .45 (.62) | .21 | .11 | .13 | .02 | .11 | .01 | .00 |
| 9. Talkativeness | .74 (.90) | .57 (.73) | .29 | −.06 | −.04 | .01 | .02 | .01 | .00 |
| 10. Increased activity | 1.32 (1.53) | 1.19 (1.47) | .33 | .05 | .07 | .04 | .06 | .00 | .00 |
| 11. Sadness | .33 (.33) | .26 (.25) | .30 | .13 | .18 | .00 | .05 | .04 | .00 |
| 12. Anhedonia | .25 (.30) | .18 (.21) | .28 | .10 | .08 | .01 | .02 | .02 | .00 |
| 13. Guilt | .20 (.27) | .16 (.19) | .27 | .11 | .18 | .01 | .09 | .26 | .03 |
| 14. Suicidal ideation | .08 (.10) | .06 (.06) | .33 | .14 | .05 | .00 | .04 | 1.98 | .26 |
| 15. Irritability | .36 (.34) | .24 (.23) | .21 | −.10 | −.10 | .03 | .06 | .15 | .02 |
| 16. Indecisiveness | .28 (.34) | .22 (.29) | .22 | .10 | .13 | .02 | .07 | .10 | .02 |
| 17. Increased fatigue | .49 (.42) | .41 (.39) | .34 | −.10 | −.19 | .01 | .02 | .04 | .01 |
| 18. Decreased appetite | .22 (.26) | .17 (.24) | .09 | −.02 | .03 | .02 | .06 | .05 | .01 |
Note:
Temporal stability was estimated using Pearson correlations for domains of substance use-related impairment and Spearman rank-order correlations for mood symptoms. Italicized numbers indicate in-prediction/out-prediction associations that were negative (in-prediction is proportion variance accounted for, out-prediction is a sum of squared standardized coefficients, neither of which indicate direction of association). T1 = Time 1 (Full Sample), T2 = Time 2, BEI = bridge expected influence, CC In = cross-construct in-prediction, CC Out = cross-construct out-prediction, Orig = original scaling, Resc = rescaled, imp. = impairment.
Table 2.
Summary of Sample Characteristics
| Variable | Time 1 (N = 445) | Time 2 (N = 330) |
|---|---|---|
| M (SD) and range for continuous variables or % for categorical variables | ||
| Age | 20.32 (1.67) | 21.11 (1.71) |
| Sex | ||
| Female | 59.8 % | 60.7 % |
| Race | ||
| White | 62.0 % | 57.7 % |
| Black | 21.5 % | 24.1 % |
| Latinx | 3.4 % | 3.4 % |
| Asian | 10.1 % | 11.1 % |
| Native American | 0.2 % | 0.3 % |
| Multiracial | 2.7 % | 3.4 % |
| Diagnostic History | ||
| Depression | 20.0 % | 16.4 % |
| Hypo/mania | 3.4 % | 2.1 % |
| Substance Use Disorder | 8.4 % | 6.4 % |
Note. M = Mean, SD = Standard deviation
Contemporaneous Networks of Mood Symptoms and Impairment due to Substance Use
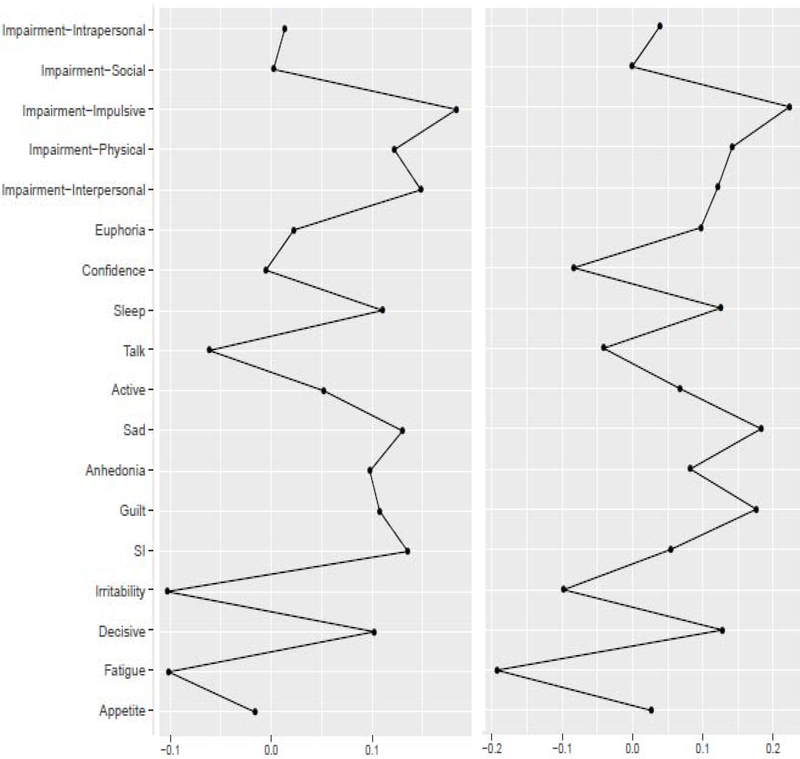
Bootstrapped correlation-stability analyses found acceptable stability for BEI (28% for the original, 36% for the rescaled networks). Figure 1 presents the nonregularized networks, which visualize the associations between the nodes. The domains of substance use-related impairment with the highest BEI on concurrent mood symptoms were impulsive and interpersonal impairment (Table 1, Figure 2). The mood symptoms with the highest BEI on concurrent substance use-related impairment were suicidal ideation, sadness, decreased need for sleep, and guilt. BEI had a nonsignificant, negative correlation with node variance with a small effect size (rs = −.15, p = .56). Significance is difficult to interpret because the sample size is constrained to the number of nodes; but the lack of a positive association suggests that BEI is not an artifact of node variability.
Figure 1. Nonregularized networks of mood symptoms and impairment due to substance use.
Note: CLPN = Cross-lagged panel network. Green edges in the networks depict positive associations, red edges represent negative associations, and thicker/more saturated edges depict stronger associations. Edges in the cross-lagged panel networks present cross-time effects and denote the direction of prediction with arrows. Circular paths are auto-regressive associations.
Figure 2:
Nonregularized one-step bridge expected influence (original scale- left; rescaled- right).
Concordance of ordinal rankings of BEI estimates between the original and rescaled nonregularized models was good (rs = .85, p < .001). In the rescaled models, the ordinal ranking of domains of substance use-related impairment with the highest BEI on concurrent mood symptoms remained the same except that interpersonal impairment dropped to third below physical impairment (Table 1, Figure 2). The mood symptoms with the highest BEI on concurrent substance use-related impairment were largely the same: sadness, guilt, indecisiveness, and decreased need for sleep. BEI had a nonsignificant, negative correlation with node variance with a small effect size (rs = −.18, p = .45).
Temporal Networks
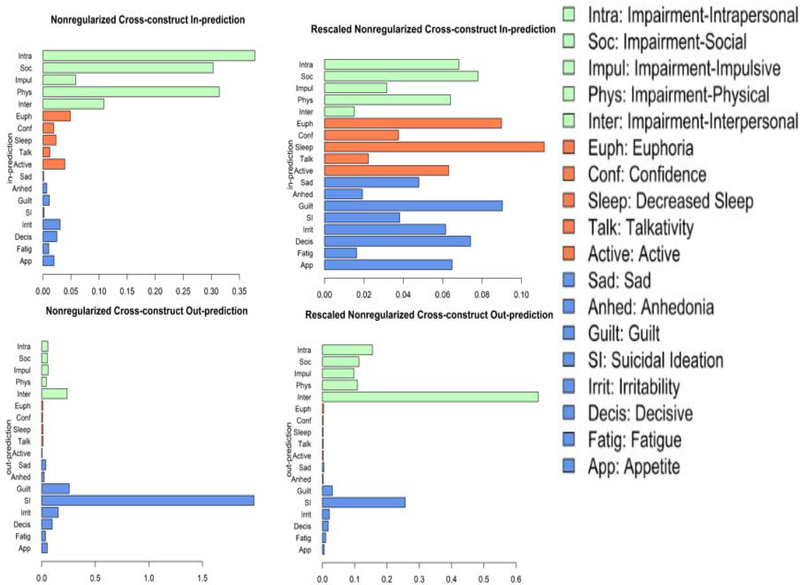
Cross-construct out-prediction
Figure 1 also shows the cross-lagged panel networks. Figure 3 shows cross-construct (mood ➔ substance-related impairment, substance-related impairment ➔ mood) estimates of in-prediction and out-prediction (Table 1). Interpersonal impairment due to substance use was the strongest predictor of future mood symptoms. Suicidal ideation had, by far, the greatest out-prediction to future substance use-related impairment, followed by guilt (which, unlike the rest of the results described, had a negative cross-construct association). Hypo/mania symptoms had negligible prediction of substance use-related impairment. Out-prediction had a significant, negative correlation with node variance with a large effect size (rs = −.58, p = .01).
Figure 3. Cross-lagged panel network estimates of centrality for nonregularized networks with original (left) and rescaled (right) nodes.
Note. Cross-Construct in-prediction estimates (top) for a given node at T2 by all nodes in the other construct at T1 (i.e., excludes any path connecting nodes from the same construct) and out-prediction estimates (bottom) for a given node at T1 to all nodes in the other construct at T2. Larger values indicate greater centrality.
Concordance of ordinal rankings of out-prediction estimates between the nonregularized models was good (rs = .82, p < .01). In the rescaled model, interpersonal impairment due to substance use still had, by far, the greatest out-prediction to future mood symptoms. Further, suicidal ideation and guilt were still the mood symptoms with the strongest out-prediction to substance use impairment and hypo/mania symptoms still had negligible out-prediction. Out-prediction had a significant, negative correlation with node variance with a large effect size (rs = −.73, p < .001).
Cross-construct in-prediction
Intrapersonal, physical, and social substance use-related impairment were predicted most strongly by past mood symptoms in the nonregularized model with the original variables (Table 1). Mood symptoms had notably smaller in-prediction estimates compared to the domains of substance-related impairment. However, euphoria, increased activity, and irritability had the highest in-prediction of the mood symptoms. In-prediction had a significant, positive correlation with node variance with a large effect size (rs = .65, p < .01).
Concordance of ordinal rankings of in-prediction estimates between the nonregularized models was very low (rs = .28, p = .26). In the rescaled model, social, intrapersonal, and physical impairment due to substance use still had the highest in-prediction, but their ranking changed. Decreased need for sleep, guilt, and euphoria (which, unlike the rest of the results described, had a negative cross-construct association), had the highest mood symptom in-prediction. Unlike the above models, mood symptoms in the rescaled model had comparable in-prediction estimates to substance use-related impairment. In-prediction had a nonsignificant, positive correlation with node variance with a small effect size (rs = .08, p = .75).
Discussion
The current study extends previous theories suggesting that discrete mood symptoms are differentially related to risk factors (e.g., history of depression, recent stress, childhood stress, Fried et al., 2014), and that particular mood symptoms are more strongly related to substance use-related impairment than others (Sanchez et al., 2015; Thornton et al., 2012) by using network analysis and modeling symptoms from across the mood spectrum. Specifically, 1) impulsive and interpersonal impairment were the domains most strongly concurrent with mood symptoms, and 2) suicidal ideation, sadness, decreased need for sleep, and guilt were the mood symptoms most highly concurrent with impairment due to substance use in contemporaneous networks. Prospective modeling found that 3) interpersonal impairment due to substance use was the strongest predictor of future mood symptoms and that 4) suicidal ideation and guilt were the mood symptoms that most strongly predicted future substance use-related impairment, and all hypo/mania symptoms had negligible prediction to future impairment due to substance use.
There was a significant, positive correlation between node variance and in-prediction estimates in the nonregularized model with originally-scaled variables, indicating that these estimates might be driven by measurement properties. There was no evidence of this issue with the rescaled variables; thus, in-prediction estimates will be discussed for the rescaled model. This model found that 5) social, intrapersonal, and physical impairment were the domains of substance use-related impairment most strongly predicted by previous mood symptoms. Finally, 6) decreased need for sleep, guilt, and euphoria were the mood symptoms most strongly predicted by previous substance use-related impairment. These results are discussed in the context of theory below. Major discrepancies between the nonregularized and regularized models (Supplemental Methods/Results) also will be discussed.
How Do Specific Mood Symptoms Relate to Domains of Substance Use-related Impairment?
Temporal models provided insight into how mood and substance use psychopathology bidirectionally confer risk for one another over time at the symptom/impairment domain level. Specifically, interpersonal substance-related impairment had the highest out-prediction, suggesting interpersonal issues stemming from problematic substance use are a potentially important driver of mood symptoms. This is consistent with a cumulative failure model (Patterson & Stoolmiller, 1991), which posits that negative outcomes secondary to problematic substance use (e.g., interpersonal conflict) can lead to subsequent mood symptoms. Thus, it could be beneficial to supplement treatments for problematic substance use with interpersonal skills and conflict resolution training to help avoid interpersonal impairment and future mood symptoms, which may reciprocally increase risk for further substance use.
Social, intrapersonal, and physical impairment due to substance use were the most strongly associated with previous mood symptoms. Individuals with negative self-concept who use substances to the point of impairment (potentially as a coping mechanism) might internalize their problematic substance use as a reflection of their flaws (intrapersonal impairment). Further, depression is associated with changes in appetite and goal-oriented behavior (e.g., exercising, fulfilling work/other obligations) that might be exacerbated by problematic substance use (e.g., weight gain or loss, feeling hungover and missing planned exercise/work). These results support the use of behavioral activation and self-compassion informed therapies to avoid behavioral issues (e.g., missing work, skipping exercise) or internalization of mistakes while intoxicated with clients with mood disorders who use substances.
Suicidal ideation was, by far, the strongest mood predictor of future substance-use related impairment. As suicidal ideation typically is associated with negative self-concept, it is unsurprising that intrapersonal impairment due to substances had one of the highest cross-construct in-predictions for substance-related impairment. Thus, this study provides support for problematic substance use as a coping mechanism for suicidal ideation, which results in additional impairment. This provides further evidence that suicidal ideation should be actively monitored and targeted when present. No hypo/mania symptoms had notable out-prediction to substance-related impairment. As hypo/mania is typically more episodic than depression, there might have been stronger association between these constructs at a shorter time scale. Guilt had a negative association with future substance use, suggesting that individuals who feel greater guilt are less likely to engage in problematic substance use, ostensibly to avoid intrapersonal discomfort.
This conclusion is supported by the finding that substance use-related impairment at T1 predicted greater guilt at T2. Decreased need for sleep also had high in-prediction; suggesting that problematic substance use was associated with dysregulated social/circadian rhythms, an established risk factor for mood disorders (Alloy et al., 2017). Thus, approaches from interpersonal and social rhythms therapy (Frank et al., 1997) might help stabilize social/circadian rhythms in those with problematic substance use to prevent mood psychopathology. Euphoria also had high in-prediction from substance-related impairment, but the association was negative, suggesting more impairment predicted less euphoria. Importantly, unlike the other results discussed here, both euphoria and decreased need for sleep had no in-prediction in the regularized models (in fact, all hypo/mania symptoms had less than 1% of their variance explained by previous impairment due to substance use in the regularized models), suggesting that the nonregularized estimates were the result of many tiny associations. Given the relatively episodic nature of hypo/mania, the associations between substance use-related impairment at T1 and T2 might have been stronger and/or more positive in a sample with shorter time-to-follow-up. In the regularized model, guilt had the highest in-prediction, followed by decreased appetite and irritability. Notably, irritability is a symptom of both depression and hypo/mania.
The differences in which nodes had the highest in- vs. out-prediction suggest that potential feedback loops may not be simplistic dyads, but instead may operate through a more complex pathway. Generally, mood symptoms (other than suicidal ideation and, to a lesser extent, guilt) largely seemed to operate as down-stream effects of, rather than risk factors for, problematic substance use. This was particularly true for hypo/mania symptoms, which strictly operated as down-stream sequelae rather than predictors of substance-related impairment. These differences highlight the importance of longitudinal data in network analyses.
Some results of the temporal models dovetail with the contemporaneous models, underscoring benefits of network research utilizing cross-sectional and longitudinal data. Interpersonal impairment ranked high in BEI with mood symptoms in the cross-sectional data and had the highest out-prediction of the impairment domains. This highlights interpersonal impairment as highly comorbid with current, and a risk factor for future, mood symptoms. Similar conclusions can be drawn for suicidal ideation. Guilt had high BEI, in-prediction, and out-prediction, suggesting guilt and problematic substance use are both comorbid and implicated in etiological pathways to each other. The most notable discrepancy between the contemporaneous and temporal models is that impulsive impairment due to substance use had the strongest BEI with mood symptoms, but did not have high cross-construct in-prediction/out-prediction in the temporal models. This suggests that, compared to some of the other cross-construct associations reported, the relationship between impulsive impairment due to substance use and mood symptoms might operate on a shorter time scale than used in this study (a possibility discussed in further detail below).
Strengths, Limitations, and Future Directions
This study had several notable strengths. First, it featured a diverse sample of late adolescents/young adults, a group at heightened risk for mood psychopathology and problematic substance use (Alloy et al., 2006; 2012; Hankin et al., 1998). Second, we included both depression and hypo/mania symptoms, increasing relevance for both the etiology and nosology of the co-occurrence of mood and substance use problems. Third, focusing on substance use-related impairment, rather than substance use frequency/intensity, increases the clinical relevance of these findings. Further, different frequencies and intensities of use influence individuals of different body types and lifestyles differently, which would reduce generalizability. However, there might be additional information gained from examining other metrics of substance use. Fourth, the inclusion of cross-sectional and longitudinal analyses maximizes the clarity of the information gained from the study. However, it is important to consider how the prospective associations here might be moderated by time-to-follow-up. For example, the construct of hypo/mania typically is considered to be less temporally stable than depression, so hypo/mania symptoms might have been more likely to predict problematic substance use at shorter time scales than used in this study. Thus, future research with different time-lags (e.g., ecological momentary assessment) is needed. Further, studies explicitly testing whether these associations are moderated by time would be of value to the field. Finally, we presented results for several alternative modeling techniques, demonstrating consistency of several key results and increasing relevance as network methodology advances.
Despite these strengths, results should be considered in light of several limitations. First, the BDI-1A did not include any items measuring psychomotor retardation or agitation. The ASRM also was not an exhaustive measure of hypo/mania symptoms. In addition to missing potentially important content, this also reduces the ability for the network analyses to account for potential conflation of impairment and mood symptomatology. For example, we can be confident that general guilt in the context of depression was not conflated with intrapersonal impairment due to substance use because an item measuring generalized guilt was modeled. However, the impulsive domain of impairment due to substance use might capture variance accounted for by hypo/mania-related increases in reckless behavior because we were unable to model this symptom separately. Future research should extend this study using a dataset that captures a wider range of mood symptomatology. Second, this sample was selected for high and moderate reward sensitivity. Whereas this increased the likelihood of including participants with clinically relevant hypo/mania symptoms, it might reduce the generalizability of these results to individuals with low reward sensitivity, a risk factor for depression and substance use. Third, self-report measures pose the risk of under-reporting (e.g., individuals experiencing hypo/mania might be less likely to report impairment than euthymic or depressed individuals). Fourth, although investigating these questions in a non-clinical sample selected for high and moderate reward sensitivity has benefits (described above), extension of these analyses to a clinical sample is important. Fifth, there was considerable variability in time to follow-up (see Supplemental Figure 1 suppl for a histogram). As mentioned above, temporal dynamics are an important methodological consideration for any longitudinal study. To evaluate whether outliers in time-to-follow-up might be driving effects, the models above were re-estimated removing five participants with months between T1 and T2 > 3 SD than the mean. Results were almost identical (rs ≥ .99 for ordinal ranking of in- and out-prediction between the original and outliers-removed samples). Still, future research would benefit from more temporally consistent designs. Additionally, having more than two time points could help investigate more nuanced interplay between these constructs (e.g., impulsivity➔ interpersonal impairment due to substance use➔ guilt). Finally, analyses included some single items originally designed to be part of larger measures, which have lower test-retest reliability than aggregate variables. However, high temporal stability is not necessarily ideal. Ideal temporal stability should be anchored in the theoretical stability of the subject of measurement. Thus, it is not necessarily surprising or problematic that specific symptoms might be less stable than the total scores reflecting general depression or hypo/mana severity, which collapse many different symptoms (each with different temporal stabilities) into a general measure of symptomatology. However, evaluation of best practice for using single items from established questionnaires in network analysis is needed.
An additional consideration is that the in-prediction estimates of the originally-scaled nonregularized model had a large, positive correlation with node variance, suggesting that these results might be driven by measurement properties. However, this concern is ameliorated by the fact that the nonregularized rescaled model’s in-prediction estimates had a nonsignificant, small correlation with node variance (rs = .08). Thus, these results were interpreted, in conjunction with the regularized models run as sensitivity analyses. No other centrality estimates in the nonregularized, originally-scaled networks had correlations with node variance that were cause for concern.
Further work is needed to evaluate the replicability and utility of these results. Although there is growing evidence for the replicability of network models (Faelens et al., 2019; Jones, Williams, et al., 2019; Moriarity et al., 2020), to date, there have been no attempted replications of cross-lagged panel network models. Thus, replication of these results is necessary to verify the substantive conclusions as well as to support continued use of this modeling procedure. Further, although there is preliminary support that expected influence has prognostic utility (Elliott et al., 2020), work evaluating the utility of in-prediction and out-prediction for clinical outcomes is necessary to determine the importance of our longitudinal models. As network methods continue to evolve, it will be crucial to simultaneously evaluate their replicability and utility. Additionally, although this paper focuses on impairment, similar analyses in datasets with other facets of problematic substance use (e.g., frequency, intensity per occasion of use) can help create a clearer picture about the co-occurrence and bidirectional associations between these constructs. For example, it would be interesting to see if intensity of substance use was more or less associated with mood symptoms than substance-related impairment. Further, analyses separated by what particular substance an individual uses could produce insight into substance-specific pathways between substance use and mood symptoms.
Conclusion
Mood disorders and problematic substance use have high rates of comorbidity (Kessler et al., 1997) and their co-occurrence predicts worse treatment outcomes, functional impairment, and risk of suicide (Tolliver & Anton, 2015). Consequently, elucidating the nature of the bidirectional association between these pathologies is an important public health concern. Importantly, this project suggests that the components of these syndromes that confer risk for the other might not be the same components that are predicted by the other. Thus, the results from this study have implications for both the etiology and classification of mood disorder/ problematic substance use comorbidity.
Supplementary Material
Highlights.
Suicidal ideation was the mood symptom most comorbid with problematic substance use
Impulsive substance-related impairment was the most comorbid with mood symptoms
Suicidal ideation was the strongest predictor of future problematic substance use
Interpersonal substance-related impairment predicted future mood symptoms
Mood symptoms are primarily outcomes, not predictors, of problematic substance use
Acknowledgments
Funding: This research was supported by National Research Service Award F31MH122116 and funding from the Civic Foundation via the Rosalyn and Stephen Weinstein Summer Graduate Research Award (Daniel P. Moriarity) and by National Institute of Mental Health Grants MH077908 and MH102310 (Lauren B. Alloy).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, & Hogan ME (2008). Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders : prospective prediction of bipolar mood episodes. Bipolar Disorders, 10, 310–322. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Panzarella C, & Rose DT (2006). Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology, 115(1), 145–156. 10.1037/0021-843X.115.1.145 [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner C. a, Liu RT, Grant D. a, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, & Abramson LY (2012). High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. Journal of Abnormal Psychology, 121(2), 339–351. 10.1037/a0025877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Ng TH, Titone MK, & Boland EM (2017). Circadian Rhythm Dysregulation in Bipolar Spectrum Disorders. Current Psychiatry Reports, 19(4), 21 10.1007/s11920-017-0772-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, & Davis JM (1997). The Altman self-rating Mania scale. Biological Psychiatry, 42(10), 948–955. 10.1016/S0006-3223(96)00548-3 [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker D, Peterson JL, & Davis JM (2001). A comparative evaluation of three self-rating scales for acute mania. Biological Psychiatry, 50(6), 468–471. [DOI] [PubMed] [Google Scholar]
- Balanzá-Martínez V, Crespo-Facorro B, González-Pinto A, & Vieta E (2015). Bipolar disorder comorbid with alcohol use disorder :focus on neurocognitive correlates. Frontiers in Psychiatry, 6(108), 1–9. 10.3389/fphys.2015.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, & Steer RA (1993). Manual for the Beck Depression Inventory. Psychological Corporation. [Google Scholar]
- Blanchard KA, Morgenstern J, Morgan TJ, & Labouvie EW (2003). Assessing Consequences of Substance Use : Psychometric Properties of the Inventory of Drug Use Consequences. Psychology of Addictive Behaviors, 17(4), 328–331. 10.1037/0893-164X.17.4.328 [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VI, Miller D, Lubar JO, Chen TJH, & Comings DE (2000). The reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. Journal of Psychoactive Drugs, 32, 1–112. 10.1080/02791072.2000.10736099 [DOI] [PubMed] [Google Scholar]
- Borkulo C Van, Boschloo L, Kossakowski J, Tio P, Schoevers R, Borsboom D, & Waldorp L (2016). Comparing network structures on three aspects: A permutation test. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, & Oscar-Berman M (2005). Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. American Journal of Medical Genetics - Neuropsychiatric Genetics, 132 B(1), 29–37. 10.1002/ajmg.b.30080 [DOI] [PubMed] [Google Scholar]
- Cardoso TDA, Bauer IE, Jansen K, Suchting R, Zunta-soares G, Quevedo J, Glahn DC, & Soares JC (2016). Effect of alcohol and illicit substance use on verbal memory among individuals with bipolar disorder. Psychiatry Research, 243, 225–231. 10.1016/j.psychres.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Conway KP, Compton W, Stinson FS, & Grant BF (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry, 67(2), 247–258. [DOI] [PubMed] [Google Scholar]
- Cramer AOJ, Waldorp LJ, Van der Maas HLJ, & Borsboom D (2010). Comorbidity: A network perspective. Behavioral and Brain Sciences, 33, 137–193. [DOI] [PubMed] [Google Scholar]
- Depue RA, & Iacono WG (1989). Neurobehavioral aspects of affective disorders. Annual Review of Psychology, 40, 457–492. 10.1146/annurev.ps.40.020189.002325 [DOI] [PubMed] [Google Scholar]
- Elliott H, Jones PJ, & Schmidt U (2020). Central Symptoms Predict Posttreatment Outcomes and Clinical Impairment in Anorexia Nervosa: A Network Analysis. Clinical Psychological Science, 8(1), 139–154. 10.1177/2167702619865958 [DOI] [Google Scholar]
- Endicott J, & Spitzer R (1978). A diagnostic interview The schedule for affective disorders and schizophrenia. Archives of General Psychiatry, 35(7), 837–844. [DOI] [PubMed] [Google Scholar]
- Epskamp S, Borsboom D, & Fried EI (2017). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50(1), 195–212. 10.3758/s13428-017-0862-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epskamp S, & Fried EI (2018). A Tutorial on Regularized Partial Correlation Networks. Psychological Methods, 23(4), 617–634. [DOI] [PubMed] [Google Scholar]
- Epskamp S, Schmittmann VD, & Borsboom D (2012). qgraph: Network visualizations of relationships in psychometric data. Journal of Statistical Software, 48(4), 1–18. [Google Scholar]
- Faelens L, Hoorelbeke K, Fried E, De Raedt R, & Koster EHW (2019). Negative influences of Facebook use through the lens of network analysis. Cambridge University Press, 53(9), 1689–1699. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Frank E, Hlastala S, Ritenour A, Houck P, Tu XM, Monk TH, Mallinger AG, & Kupfer DJ (1997). Inducing lifestyle regularity in recovering bipolar disorder patients: Results from the maintenance therapies in bipolar disorder protocol. Biological Psychiatry, 41(12), 1165–1173. 10.1016/S0006-3223(96)00241-7 [DOI] [PubMed] [Google Scholar]
- Fried EI, Nesse RM, Zivin K, Guille C, & Sen S (2014). Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychological Medicine, 44, 2067–2076. 10.1017/S0033291713002900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, & Tibshirani R (2010). Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software, 33(1), 1–22. [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. 10.1037/0021-843X.107.1.128 [DOI] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, & Boeding S (2011). An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors, 25(3), 390–404. 10.1037/a0024519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PJ (2018). Networktools: Tools for identifying important nodes in networks. R Package Version 1.1.1. [Google Scholar]
- Jones PJ, Ma R, & McNally RJ (2019). Bridge Centrality: A Network Approach to Understanding Comorbidity. Multivariate Behavioral Research, 0(0), 1–15. 10.1080/00273171.2019.1614898 [DOI] [PubMed] [Google Scholar]
- Jones PJ, Williams DR, & Mcnally RJ (2019). Sampling variability is not nonreplication: A Bayesian reanalysis of Forbes, Wright, Markon, & Krueger. OSF Preprints. [DOI] [PubMed] [Google Scholar]
- Kenneson A, Funderburk JS, & Maisto SA (2013). Substance use disorders increase the odds of subsequent mood disorders. Drug and Alcohol Dependence, 133(2), 338–343. 10.1016/j.drugalcdep.2013.06.011 [DOI] [PubMed] [Google Scholar]
- Kessler RC (2004). The epidemiology of dual diagnosis. Biological Psychiatry, 56, 730–737. 10.1016/j.biopsych.2004.06.034 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, & Anthony C (1997). Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry, 54, 313–321. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Dreifuss JA, Weiss RD, Morgenstern J, & Carroll KM (2013). The Short Inventory of Problems – Revised (SIP-R): Psychometric properties within a large, diverse sample of substance use disorder treatment seekers. Psychol Addict Behav, 27(1), 307–314. 10.1037/a0028445.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, & Kenny PJ (2002). Neuroadaptations to chronic exposure to drugs of abuse: Relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotoxicity Research, 4(4), 297–313. 10.1080/10298420290023963 [DOI] [PubMed] [Google Scholar]
- Moriarity DP, Horn SR, Kautz MM, Haslbeck JMB, & Alloy LB (2020). How handling extreme C-reactive protein (CRP) values and regularization influences CRP & depression symptom networks. PsyArXiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Martins SS, & Crum RM (2013). The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: results from a national sample. 148, 188–195. 10.1016/j.jad.2012.11.059The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, & Stoolmiller M (1991). Replications of a dual failure model for boys’ depressed mood. Journal of Consulting and Clinical Psychology, 59(4), 491–498. [DOI] [PubMed] [Google Scholar]
- Peeters M, Vollebergh WAM, Wiers RW, & Field M (2014). Psychological changes and cognitive impairments in adolescent heavy drinkers. Alcohol and Alcoholism, 49(2), 182–186. 10.1093/alcalc/agt162 [DOI] [PubMed] [Google Scholar]
- Rappeneau V, & Bérod A (2017). Reconsidering depression as a risk factor for substance use disorder: Insights from rodent models. Neuroscience and Biobehavioral Reviews, 77, 303–316. 10.1016/j.neubiorev.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Rhemtulla M, van Bork R, & Cramer AOJ (2018). Cross-lagged network models. Multivariate Behavior Research, Preprint. [Google Scholar]
- Salloum IM, & Thase ME (2000). Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disorders, 3, 269–280. [DOI] [PubMed] [Google Scholar]
- Sanchez K, Walker R, Campbell ANC, Greer TL, Hu M, Grannemann BD, Nunes EV, Madhukar H, Sanchez K, Walker R, Campbell ANC, Tracy L, Hu M, Grannemann BD, Nunes EV, Trivedi MH, Sanchez K, Walker R, Campbell ANC, … Trivedi MH (2015). Depressive symptoms and associated clinical characteristics in outpatients seeking community-based treatment for alcohol and drug problems. Substance Abuse, 36, 297–303. 10.1080/08897077.2014.937845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski SM, & Delbello MP (2000). The co-occurence of bipolar and substance use disorders. Clinical Psychology Review, 20(2), 191–206. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, Sampson N, & Kessler RC (2010). Mental disorders as risk factors for substance use, abuse and dependence: Results from the 10-year follow-up of the National Comorbidity Survey. Addiction, 105(6), 1117–1128. 10.1111/j.1360-0443.2010.02902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LK, Baker AL, Lewin TJ, Kay-lambkin FJ, Kavanagh D, Richmond R, Kelly B, & Johnson MP (2012). Addictive Behaviors Reasons for substance use among people with mental disorders. Addictive Behaviors, 37(4), 427–434. 10.1016/j.addbeh.2011.11.039 [DOI] [PubMed] [Google Scholar]
- Tolliver BK, & Anton RF (2015). Assessment and treatment of mood disorders in the context of substance abuse. Dialogues in Clinical Neuroscience, 17(2), 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, & Caseras X (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Volkow ND, Fowler JS, & Wang G (2003). The Addicted Human Brain: Insights from Imaging Studies Find the Latest Version: The Addicted Human Brain: Insights from Imaging Studies. The Journal of Clinical Investigation, 111(10), 1444–1451. 10.1172/JCI200318533.Imaging [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Rhemtulla M, Wysocki AC, & Rast P (2019). On Nonregularized Estimation of Psychological Networks. Multivariate Behavioral Research, 54(5), 719–750. 10.1080/00273171.2019.1575716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.