Abstract
Background:
Walking abnormalities in people with Parkinson’s disease (PD) are characterized by a shift in locomotor control from healthy automaticity to compensatory prefrontal executive control. Indirect measures of automaticity of walking (e.g., step-to-step variability and dual-task cost) suggest that freezing of gait (FoG) may be associated with reduced automaticity of walking. However, the influence of FoG status on actual prefrontal cortex (PFC) activity during walking remains unclear.
Objective:
To investigate the influence of FoG status on automaticity of walking in people with PD.
Methods:
Forty-seven people with PD were distributed into two groups based on FoG status, which was assessed by the New Freezing of Gait Questionnaire: PD-FoG (n=23; UPDRS-III=35) and PD+FoG (n=24; UPDRS-III=43.1). Participants walked over a 9m straight path (with a 180° turn at each end) for 80s. Two conditions were tested Off medication: single- and dual-task walking (i.e., with a concomitant cognitive task). A portable functional near-infrared spectroscopy system recorded PFC activity while walking (including turns). Wearable inertial sensors were used to calculate spatiotemporal gait parameters.
Results:
PD+FoG had greater PFC activation during both single and dual-task walking than PD-FoG (p=0.031). There were no differences in gait between PD-FoG and PD+FoG. Both groups decreased gait speed (p=0.029) and stride length (p<0.001) during dual-task walking compared to single-task walking.
Conclusions:
These findings suggest that PD+FoG have reduced automaticity of walking, even in absence of FoG episodes. PFC activity while walking seems to be more sensitive than gait measures in identifying reduction in automaticity of walking in PD+FoG.
Keywords: Locomotion, fNIRS, dual-tasking
INTRODUCTION
Freezing of gait (FoG) is one of the most debilitating walking impairment in Parkinson’s disease (PD). FoG is defined as a “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk”.1 It occurs in up to 63% of individuals with PD, with increasing frequency in more advanced stages of the disease.2,3. FoG episodes often result in falls, disability, reduced functional independence and poor quality of life.1,4,5 FoG is often difficult to treat1,6 and the development of enhanced interventions requires a better understanding of the neural basis of FoG.
Although the underlying pathophysiology of FoG is not fully understood,7 it is thought to be associated with reduced automaticity in the control of walking.8–10 A hallmark of healthy walking is automaticity, defined as the ability of the nervous system to successfully control movement with minimal use of executive-attentional resources.11 It has been proposed that many walking abnormalities in people with PD are characterized by a shift in locomotor control from healthy automaticity to compensatory prefrontal executive control.12–17 Behavioral studies demonstrate that people with FoG have increased gait variability (i.e., surrogate measure of gait automaticity where higher variability equates to reduced automaticity) compared to people without FoG. In addition, gait impairments, such as reduced gait speed, are more pronounced in people with FoG when walking during dual-task conditions compared to people without FoG.8,16,18,19 These behavioral findings suggest that automaticity of walking is poorer in PD with FoG compared to PD without FoG. However, these are indirect measures of automaticity of walking and, therefore, more direct neurophysiological measures are needed for a better understanding of the control mechanisms of walking in people with FoG.
Neuroimaging studies suggest that two distinct supraspinal locomotor networks are involved in the control of walking.20–22 The automatic locomotor network is mainly active during steady state walking and it involves direct projections from the primary motor cortex to the central pattern generator circuits.20,21 The executive locomotor network is active for complex walking requiring planning and modulation of locomotion.20–22 In the executive network, locomotor signals originate in the supplementary motor area and the prefrontal cortex (PFC) and are transmitted through the basal ganglia (i.e., striatum, pallidum and subthalamic and mesencephalic locomotor region) before reaching the medullary and pontine reticular formations and the spinal cord.20,21 A feedback loop runs from the spinal cord to the cerebellum and thereby via the thalamus to the cortex in both networks.20,21 In PD, the executive locomotor network is activated as a compensatory mechanism even during single-task walking.21,22 Using this model as a background framework, direct measures of automaticity and executive control can be obtained by recording cortical activity during actual walking. For example, functional near-infrared spectroscopy (fNIRS) systems can be used to record changes in cortical oxy- (HbO2) and deoxygenated hemoglobin (HHb) concentrations, which infer cortical activity.
Measures of PFC activity obtained using fNIRS are reliable23 and can differentiate healthy older adults and clinical populations, such as people with PD.13,23,24 Existing studies focusing on FoG mainly characterize the executive control of turning.25–27 People with PD with FoG have increased PFC activity while turning in place in comparison to non-freezers.26 Increased PFC activity has also been reported immediately before and during FoG episodes when turning 180° during walking.27 In addition, higher PFC activity while turning in place is associated with worse FoG severity and poorer turning performance.26 Such findings suggest greater prefrontal executive control of turning in people with FoG. However, the influence of FoG status on automaticity of walking is still unclear. Based on evidence from gait studies and the increased PFC activity reported for those with FoG in turning studies, we would expect that during walking PFC activity would be greater in people with PD who report FoG compared to non-freezers, even in absence of an actual episode of FoG. Such increased PFC activity would indicate a reduction in automaticity of walking even in absence of an actual FoG episode, and could partly explain the additional gait impairments noticed in people with FoG compared to people without FoG with similar disease severity.
In the current study, we recorded PFC activity and spatiotemporal gait parameters during single and dual-task walking to investigate the influence of FoG status on automaticity of walking in people with PD. As we hypothesized that people with FoG have reduced automaticity of walking in comparison to people without FoG, we expected to observe increased PFC activity and step-to-step variability in people with FoG. We also expected greater deterioration of gait measures under dual-task walking in people with FoG compared to non-freezers. Additionally, this study explored the relationship between PFC activity while walking and spatiotemporal gait parameters and FoG severity.
METHODS
Participants
Participants were recruited from local Neurology clinics via referrals from movement disorder specialist neurologists. Forty-seven people with idiopathic PD were included in the study. Participants were included if they were aged 55–90 years, had a diagnosis of idiopathic PD according to the UK Brain Bank Criteria, were taking anti-parkinsonian medication, and were able to give informed consent to participate, and able to cooperate with the testing. Exclusion criteria included: inability to stand or walk for 2 min at a time, factors affecting gait (e.g., musculoskeletal disorders, hip replacement, uncorrected vision or vestibular problems, etc.) and inability to follow instructions. Participants were grouped according to their perceived FoG status, which was assessed by the New Freezing of Gait Questionnaire28 (NFoGQ): freezers (NFoGQ ≥ 1; PD+FoG) and non-freezers (NFoGQ = 0; PD-FoG). Study procedures were approved by the Oregon Health and Science University Institutional Review Board (eIRB #9903 and #17805), with written informed consent obtained prior to participation.
Experimental procedures and equipment
All participants were tested in their “Off” medication state, at least 12 h after the last administration of their usual anti-parkinsonian medications. Participant characteristics of age, sex, disease duration, height, and weight were recorded. Disease severity was measured using the Movement Disorders Society (MDS-revised) Unified Parkinson’s disease Rating Scale29 (MDS-UPDRS). PD stage was assessed by the Hoehn and Yahr Rating Scale30 (H&Y). Cognitive function was assessed with the following tests: the Montreal Cognitive Assessment31 (MoCA), the Frontal Assessment Battery32 (FAB), the Royall Clock Drawing Tasks33 (CLOX 1 and 2), and the Trail Making Test (TMT).
Participants walked, at self-selected comfortable pace, back and forth over a 9m straight path, with a 180° turn at each end. Two conditions were tested in a randomized order: single and dual-task walking. Each condition included an initial 20 s of quiet standing (baseline period) followed by 80 s of walking (task period). Participants performed a single trial for each walking condition. The dual-task condition consisted of executing the walking task while performing a concurrent cognitive task (auditory Modified AX-Continuous Performance Task),34 which required participants to press a handheld button after a two-paired letters sequence. The sequence consisted of a cue letter “A” and a probe letter “I” presented sequentially so that the target trail was “AI” and participants were asked to respond as fast as possible after the probe letter. No information about task priority was assigned to participants not to influence the task execution. A research assistant stood by and walked with the participants to avoid eventual falls and ensure their safety. The Modified AX-Continuous Performance Task was also applied while participants were seated in a chair (before the walking part). No FoG episode occurred during the protocol.
A portable 8-channel fNIRS system (OctaMon, Artinis Medical Systems, Elst, The Netherlands) recorded changes in HbO2 and HHb bilaterally in the PFC at a sampling rate of 50 Hz. The fNIRS device consisted of two light detectors and eight light emitters (continuous wave diodes with wavelengths of 760 and 850 nm). Three regular channels (interoptode distance of 35 mm) and one short-separation channel (interoptode distance 15 mm) were used for each hemisphere. Optodes were placed on participants forehead using a headband with predetermined locations (according to the international 10–20 EEG system). A digitizer (Polhemus Patriot 3D digitizer, Colchester, VT, USA) was used to provide 3-dimensional coordinates of anatomical references (Cz, nasion, inion and left and right preauricular points) and positions of optodes.
Eight inertial measurement units (Opal, APDM, Portland, OR, USA) were used to quantify spatiotemporal gait parameters at a sampling rate of 128 Hz. They were located at the sternum and pelvis levels, on the wrists, shanks and both feet of participants. Each inertial sensor consisted of tri-axial accelerometers, gyroscopes, and magnetometers, and were securely fixed to the participant’s body with Velcro straps. The inertial sensors and fNIRS system were synchronized through the Artinis PortaSync.
Data analysis
Processing of fNIRS signals followed current recommendations.22,35 Data from the digitizer was entered into the software package NIRS-SPM (http://www.nitrc.org/projects/nirs_spm),36 which was implemented within MATLAB 2017a (The Mathworks Inc., Natick, MA, USA). The spatial registration routine was used to find the correspondence between the scalp location where the fNIRS measurement was performed and its underlying cortical surface where the source signal was located.37 Cortical regions assessed included the Brodmann areas 9 and 10.
The fNIRS data were preprocessed within custom-made MATLAB algorithms, which consisted of several steps. The initial steps involved functions available in HOMER2 (https://homer-fnirs.org/), and specifically: 1) raw intensity data were converted into optical density, 2) artifacts were removed/attenuated by wavelet filtering;38 3) optical density data were converted into HbO2 and HHb concentrations; 4) remaining artifacts were removed/attenuated by applying the correlation-based signal improvement method.39 The data were then baseline corrected by subtracting the mean of the baseline period (standing) from the entire trial. The following step involved removing superficial hemodynamic response from regular channels.23,40 Briefly, scaling factors were determined by detecting the peaks (positive and negative) of the heart rate within the regular and short-separation channel signals, then dividing them to produce the scaling factors for each pair of channels. These were then used to remove the noise detected within the short-separation reference channels within the regular channels. A low-pass filter with a cut-off frequency of 0.14 Hz removed any remaining high-frequency noise and the six channels were median averaged. Finally, relative changes in HbO2 and HHb concentrations were calculated for both early (median of the first half of the task = initial 40 s) and late (median of the second half of the task = final 40 s) phases of the task, considering straight walk and turns together. The division of the fNIRS signal into early and late phases was motivated by previous studies showing phase-specific PFC activation patterns while walking.35,41–43
Spatiotemporal gait measures were calculated from the inertial sensors using the Mobility Lab software, V2 (APDM, Portland, OR, USA).44 All recorded steps corresponding to walking were included in the analysis. The following gait measures were calculated: gait speed, stride length, foot strike angle and step time variability (coefficient of variation). The accuracy of the Modified AX-Continuous Performance Task was calculated for both seated and walking (i.e., dual-task walking) conditions.
Statistical Analysis
Independent t-tests and Mann-Whitney tests were used to compare demographic and clinical/cognitive variables between groups. Linear mixed-effects models were fit, using the Restricted Maximum Likelihood Estimation (REML), to investigate whether outcomes differed between groups and conditions, while controlling (model #2) or not (model #1) for between-group differences on demographic and clinical/cognitive variables (i.e., MDS-UPDRS-III, H&Y, disease duration, MoCA and TMT-B). The use of covariates in the model allowed us to better isolate the factor of interest (i.e., FoG status) in the analysis. Moreover, comparison between results of the two models would provide evidence on whether or not the covariates influence potential changes on PFC activity and gait in PD-FoG and PD+FoG. REML estimation were used to avoid bias due to our small sample size.45 Each model was adjusted for group (PD-FoG versus PD+FoG), task (single versus dual-task), and the group*task interaction (to test whether groups had different linear trend between tasks). Each model included a random intercept for each participant to account for the repeated measurements within each participant. The association between PFC activity while walking (HbO2) and gait measures was explored, separately per group, using Spearman and Pearson correlation coefficients (according to data type and distribution). For PD+FoG, we also analyzed the association between PFC activity while walking and FoG severity (NFoGQ). The statistical analysis was conducted using Matlab R2019b (The Mathworks Inc., Natick, MA, USA) and SPSS v25 (IBM Inc., IBM Corp., Armonk, NY, USA). Statistical significance was set at p < 0.05.
RESULTS
Participant characteristics
All demographic characteristics and clinical scale scores are reported in Table 1. There were no differences between groups for age, gender, height, weight, FAB, TMT_A, TMT_B-A, CLOX1 and CLOX2. PD+FoG had more severe motor symptoms (MDS-UPDRS-III) and advanced stage (H&Y), longer disease duration and worse cognition (MoCA and TMT_B) than PD-FoG.
Table 1.
Participants’ characteristics
| Variable | PD-FoG | PD+FoG | pValue |
|---|---|---|---|
| NFoGQ | 0 ± 0 | 12.2 ± 5.8 | <0.001 |
| Gender (male / female) | 18 / 5 | 16 / 8 | 0.380 |
| MDS-UPDRS-III (score) | 35.0 ± 10.3 | 42.4 ± 13.2 | 0.038* |
| H&Y (I / II / III) | 1 / 19 / 3 | 0 / 16 / 8 | 0.09* |
| Disease duration (years) | 7.2 ± 5.2 | 10.1 ± 6.1 | 0.072t |
| MoCA (score) | 27.2 ± 3.7 | 26.0 ± 3.1 | 0.020* |
| FAB (score) | 14.6 ± 3.1 | 14.6 ± 3.0 | 0.940 |
| TMT_A(s) | 36.5 ± 15.6 | 51.9 ± 57.7 | 0.395 |
| TMT_B (s) | 83.5 ± 38.5 | 116.5 ± 56.1 | 0.035* |
| TMT B-A (s) | 46.9 ± 30.4 | 64.5 ± 44.9 | 0.125 |
| CLOX1 (score) | 12.1 ± 2.0 | 12.2 ± 1.4 | 0.761 |
| CLOX2 (score) | 13.9 ± 0.9 | 13.3 ± 1.6 | 0.208 |
| Age (years) | 70.8 ± 7.6 | 70.3 ± 4.7 | 0.756 |
| Height (cm) | 169.6 ± 12.8 | 167.9 ± 13.2 | 0.461 |
| Weight (kg) | 84.4 ± 21.9 | 78.8 ± 14.8 | 0.580 |
CLOX: Royall Clock Drawing Tasks; FAB: Frontal Assessment Battery; H&Y: Hoehn and Yahr Rating Scale; MDS-UPDRS: Movement Disorders Society - Unified Parkinson’s Disease Rating Scale; MoCA: Montreal Cognitive Assessment; NFoGQ: New Freezing of Gait Questionnaire; PD-FoG: people with Parkinson’s disease without freezing of gait; PD+FoG: people with Parkinson’s disease with freezing of gait; TMT: Trail Making Test.
PFC activity
Freezers showed greater PFC activity than nonfreezers when controlling for severity of disease and cognitive status (i.e., MDS-UPDRS-III, H&Y, disease duration, MoCA and TMT-B). Results of the two linear mixed-effects models for relative HbO2 and HHb are reported in Table 2. No significant results were observed for fNIRS outcomes in model #1, which did not control for covariates. While controlling for covariates (model #2), PD+FoG showed higher HbO2 in the late phase of walking compared to PD-FoG (p = 0.031; see Figure 1), regardless of walking condition. In addition, HbO2 and HHb, both in the early and late phases, were similar between single- and dual-task. Lastly, no significant group*task interactions were found in model #2.
Table 2.
Results of the statistical models applied for fNIRS outcomes, while controlling (model #2) or not (model #1) for covariates, which included MDS-UPDRS-III, H&Y stage, disease duration, MoCA and TMT-B.
| Variable | Estimate | SE | pValue | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Model 1 | |||||
| Group effects | |||||
| HbO2 early | −0.078 | 0.108 | 0.472 | −0.294 | 0.137 |
| HHb early | 0.060 | 0.060 | 0.321 | −0.059 | 0.179 |
| HbO2 late | −0.239 | 0.140 | 0.092 | −0.518 | 0.040 |
| HHb late | 0.116 | 0.082 | 0.157 | −0.046 | 0.279 |
| Task effects | |||||
| HbO2 early | −0.108 | 0.094 | 0.251 | −0.294 | 0.078 |
| HHb early | 0.064 | 0.048 | 0.185 | −0.031 | 0.160 |
| HbO2 late | −0.143 | 0.114 | 0.215 | −0.370 | 0.084 |
| HHb late | 0.100 | 0.064 | 0.125 | −0.028 | 0.228 |
| Group*task | |||||
| HbO2 early | 0.054 | 0.059 | 0.364 | −0.064 | 0.173 |
| HHb early | −0.035 | 0.031 | 0.258 | −0.096 | 0.026 |
| HbO2 late | 0.082 | 0.073 | 0.259 | −0.062 | 0.227 |
| HHb late | −0.045 | 0.041 | 0.275 | −0.127 | 0.036 |
| Model 2 | |||||
| Group effects | |||||
| HbO2 early | −0.107 | 0.111 | 0.338 | −0.328 | 0.114 |
| HHb early | 0.073 | 0.062 | 0.243 | −0.051 | 0.197 |
| HbO2 late | −0.314 | 0.143 | 0.031 | −0.598 | −0.029 |
| HHb late | 0.146 | 0.084 | 0.086 | −0.021 | 0.314 |
| Task effects | |||||
| HbO2 early | −0.105 | 0.094 | 0.269 | −0.292 | 0.082 |
| HHb early | 0.063 | 0.048 | 0.195 | −0.033 | 0.159 |
| HbO2 late | −0.135 | 0.115 | 0.242 | −0.598 | −0.029 |
| HHb late | 0.097 | 0.064 | 0.137 | −0.031 | 0.225 |
| Group*task | |||||
| HbO2 early | 0.053 | 0.06 | 0.381 | −0.066 | 0.172 |
| HHb early | −0.034 | 0.031 | 0.267 | −0.095 | 0.027 |
| HbO2 late | 0.079 | 0.073 | 0.283 | −0.066 | 0.225 |
| HHb late | 0.044 | 0.041 | 0.291 | −0.125 | 0.038 |
CI: confidence interval; HbO2: oxygenated hemoglobin; HHb: deoxygenated hemoglobin; SE: standard error.
Figure 1.
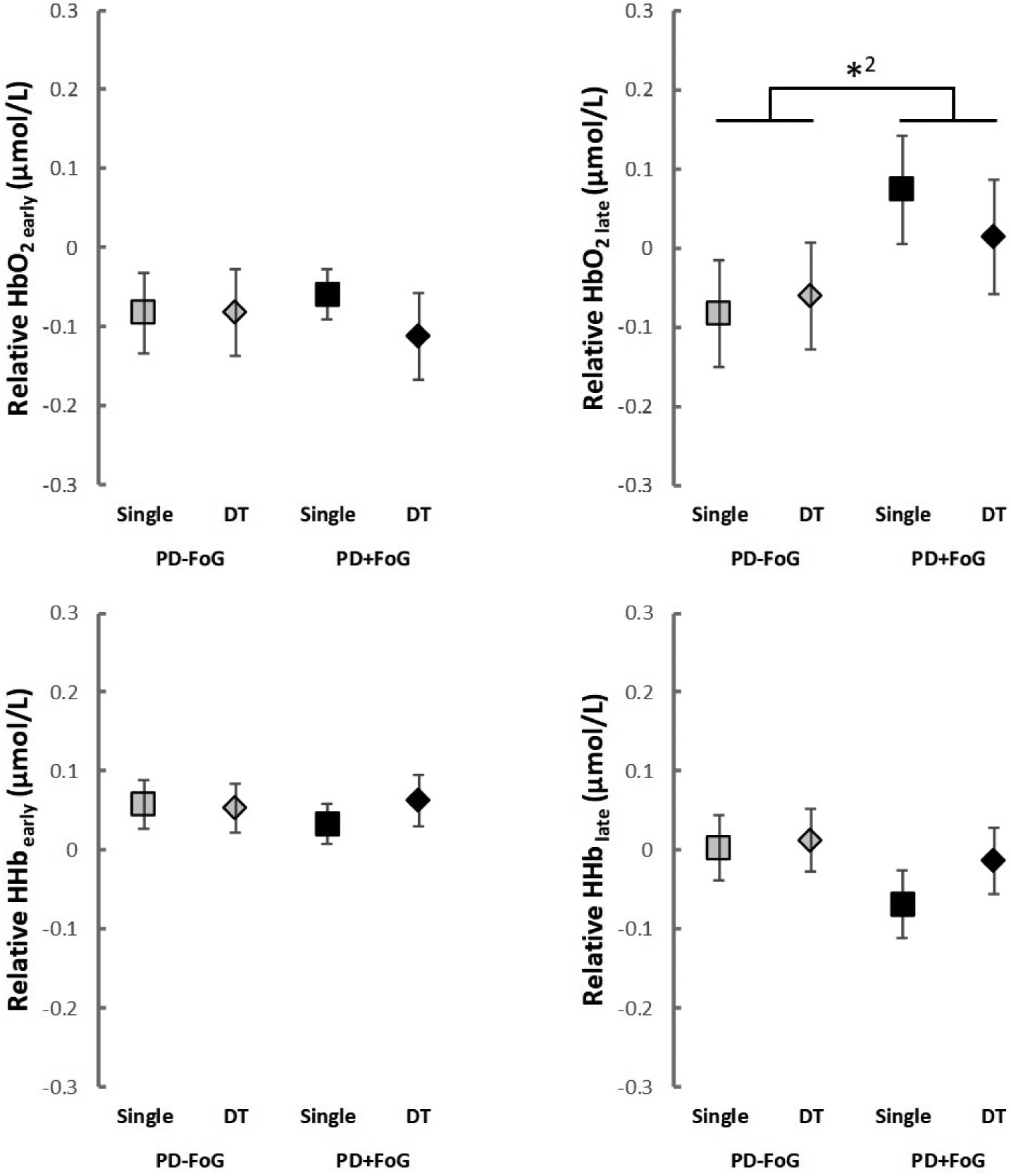
Relative HbO2 and HHb (mean and standard error) for both groups in each condition. DT: dual-task; HbO2: oxygenated hemoglobin; HHb: deoxygenated hemoglobin; PD-FoG: people with Parkinson’s disease without freezing of gait; PD+FoG: people with Parkinson’s disease with freezing of gait; *2: significant group main effect (model #2)
Gait
Freezers and nonfreezers showed very similar gait characteristics, when results were controlled for severity of disease and cognitive status (i.e., MDS-UPDRS-III, H&Y, disease duration, MoCA and TMT-B). Results of the two linear mixed-effects models for spatiotemporal gait parameters are reported in Table 3. Model #1 revealed a group main effect for step time variability only (p = 0.044). PD+FoG showed greater step time variability than PD-FoG (Figure 2). However, such difference was no longer significant in model #2, which revealed no significant group main effect (Table 3). Both models revealed task main effects for gait speed (model #1: p = 0.026; model #2: p = 0.029), stride length (model #1: p < 0.001; model #2: p < 0.001) and foot strike angle (model #1: p = 0.012; model #2: p = 0.013). In fact, both groups had slower gait speed, shorter stride length and lower foot strike angle in the dual-task compared to single task (Figure 2). No significant group*task interactions were observed for gait outcomes.
Table 3.
Results of the statistical models applied for gait outcomes, while controlling (model #2) or not (model #1) for covariates, which included MDS-UPDRS-III, H&Y stage, disease duration, MoCA and TMT-B.
| Variable | Estimate | SE | pValue | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Model 1 | |||||
| Group effects | |||||
| Speed | 0.011 | 0.062 | 0.862 | −0.112 | 0.133 |
| Foot strike angle | −0.330 | 1.568 | 0.833 | −3.447 | 2.785 |
| Stride length | 0.039 | 0.057 | 0.498 | −0.075 | 0.154 |
| Step time variability | −1.609 | 0.787 | 0.044 | −3.173 | −0.045 |
| Task effects | |||||
| Speed | −0.063 | 0.028 | 0.026 | −0.119 | −0.008 |
| Foot strike angle | −1.553 | 0.609 | 0.012 | −2.764 | −0.343 |
| Stride length | −0.077 | 0.021 | <0.001 | −0.119 | −0.034 |
| Step time variability | −0.394 | 0.455 | 0.389 | −1.298 | 0.510 |
| Group*task | |||||
| Speed | 0.014 | 0.018 | 0.436 | −0.021 | 0.049 |
| Foot strike angle | 0.250 | 0.388 | 0.521 | −0.521 | 1.021 |
| Stride length | 0.019 | 0.014 | 0.173 | −0.008 | 0.046 |
| Step time variability | 0.401 | 0.290 | 0.170 | −0.174 | 0.976 |
| Model 2 | |||||
| Group effects | |||||
| Speed | −0.075 | 0.055 | 0.173 | −0.183 | 0.034 |
| Foot strike angle | −1.434 | 1.622 | 0.379 | −4.66 | 1.792 |
| Stride length | −0.044 | 0.049 | 0.368 | −0.141 | 0.053 |
| Step time variability | −0.803 | 0.722 | 0.269 | −2.238 | 0.632 |
| Task effects | |||||
| Speed | −0.062 | 0.028 | 0.029 | −0.118 | −0.007 |
| Foot strike angle | −1.548 | 0.612 | 0.013 | −2.764 | −0.332 |
| Stride length | −0.075 | 0.021 | <0.001 | −0.118 | −0.033 |
| Step time variability | −0.392 | 0.455 | 0.391 | −1.300 | 0.513 |
| Group*task | |||||
| Speed | 0.013 | 0.018 | 0.452 | −0.022 | 0.049 |
| Foot strike angle | 0.247 | 0.389 | 0.527 | −0.526 | 1.020 |
| Stride length | 0.018 | 0.014 | 0.191 | −0.009 | 0.045 |
| Step time variabilitv | 0.400 | 0.290 | 0.170 | −0.176 | 0.977 |
CI: confidence interval; SE: standard error.
Figure 2.
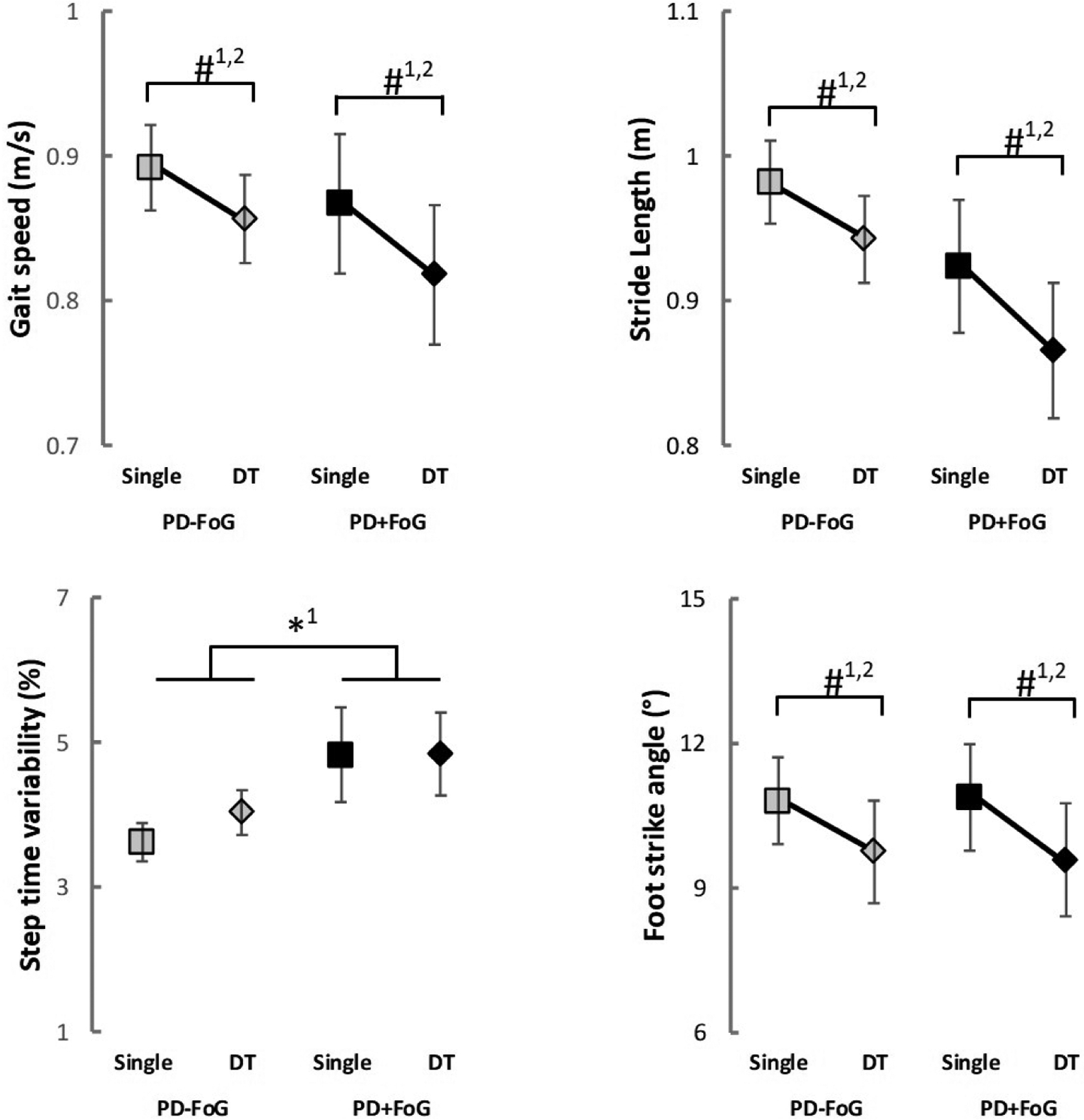
Mean and standard error of gait outcomes for both groups in each condition. DT: dual-task; PD-FoG: people with Parkinson’s disease without freezing of gait; PD+FoG: people with Parkinson’s disease with freezing of gait; *: significant group main effect; # significant task main effect; 1: model #1; 2: model #2
Concurrent cognitive task
Freezers and nonfreezers showed similar performance on the cognitive dual task. Due to technical issues with the equipment (e.g., signals from button and inertial sensor not synchronized), the accuracy of the concurrent cognitive task was not evaluated in all participants. The accuracy is reported for 17 PD-FoG (mean ± standard error of the mean; seated: 0.91 ± 0.04; walking: 0.80 ± 0.07) and 15 PD+FoG (seated: 0.86 ± 0.07; walking: 0.79 ± 0.08). Both statistical models revealed no significant main effect or group*task interaction (p>0.05).
Correlations
During dual-task walking, greater HbO2 late was associated with less severe FoG (r = −0.526, p = 0.011) and greater HbO2 early was associated with lower step time variability (r = −0.463, p = 0.023) in PD+FoG; see Figure 3. For PD+FoG, no significant correlations were observed for HbO2 during single-task walking. No significant correlations were observed for PD-FoG (p > 0.05).
Figure 3.
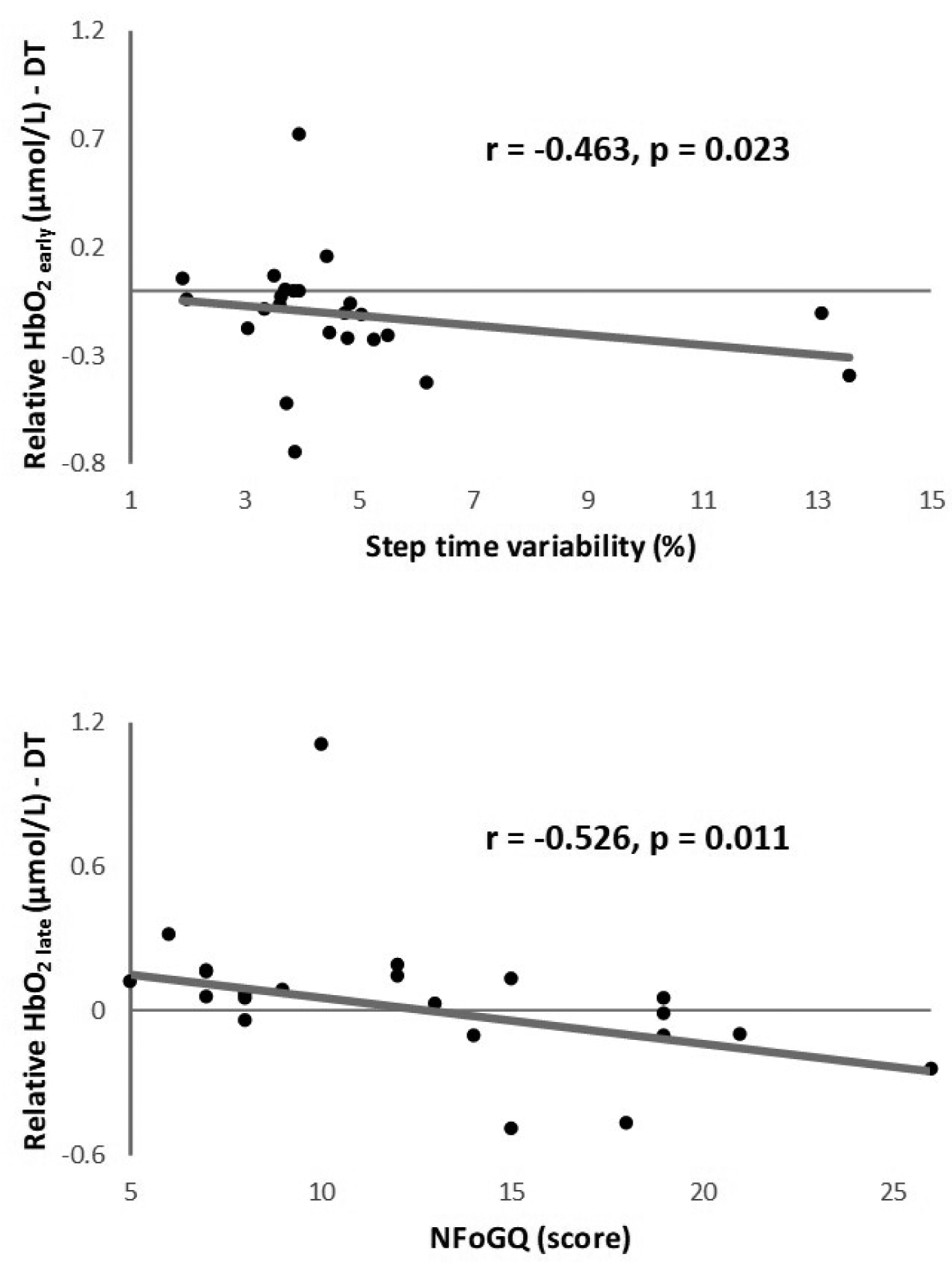
Significant correlations observed in PD+FoG. DT: dual-task; HbO2: oxygenated hemoglobin; NFoGQ: New Freezing of Gait Questionnaire
DISCUSSION
To the best of our knowledge, this is the first study applying fNIRS to examine the influence of FoG status on PFC activity during single and dual-task walking in people with PD. To better isolate the influence of FoG status itself rather than disease progression, we controlled the analysis for between-group differences on demographic and clinical/cognitive variables. Our main results, when controlling for covariates, were: 1) PD+FoG had greater PFC activity while walking (i.e., higher HbO2 late levels) than PD-FoG, regardless of walking condition; 2) Spatiotemporal gait measures were similar in PD+FoG compared to PD-FoG, and both groups showed worse performance in dual-task compared to single-task; 3) higher PFC activity while walking was associated with less severe FoG and less variable gait, only in the PD+FoG group. These findings suggest that greater contribution of the executive locomotor network is required in PD+FoG due to impaired movement automaticity. Thus, our hypothesis of more pronounced reduction in automaticity of walking in PD+FoG was confirmed by PFC activity (measured by fNIRS) outcomes, but not by gait measures.
NIR light illuminates reduction in automaticity of walking in freezers
This study suggests that PFC activity, measured through mobile fNIRS, can identify reduction in automaticity of walking in PD+FoG. Overall, our findings corroborate previous studies showing that automaticity of walking is reduced in people with PD and even more in those with FoG.1,16 The observed deterioration of gait parameters under the dual-task condition in both groups suggests that walking is not an automatic task for people with PD,11,16 regardless of FoG status. Moreover, the increased PFC activity (HbO2 late level) observed in PD+FoG suggests further reduced automaticity of walking in this group, which is in line with existing fNIRS studies on turning.26,27 PD+FoG required the allocation of additional prefrontal executive resources for the control of walking, which is likely a compensatory mechanism to maintain gait performance comparable to non-freezers.
In fact, in keeping with these findings, spatiotemporal gait parameters were unable to differentiate PD+FoG and PD-FoG, while controlling for covariates, even under the dual-task condition. Thus, fNIRS outcomes may be more sensitive than gait outcomes in identifying reduction in automaticity of walking in PD+FoG. Since PFC activation is argued to be part of a compensatory mechanism to maintain a certain level of task performance,22 it is also possible that changes in PFC activity precede further gait impairments in people with FoG. Such interpretation is supported by the literature. Changes in cortical activity have been proposed as a preclinical sign of (future) gait impairments and falls in healthy middle-aged adults46 and high-functioning older adults.47 Longitudinal studies are needed to confirm (or not) if this line of interpretation applies to people with FoG.
Although near-infrared light seems appropriate to illuminate reduction in movement automaticity in PD+FoG, careful definition of outcomes is required. The fact that between-group difference for HbO2 was only observed in the late phase of the task might relate to a sustained pattern of PFC activation throughout the task by PD+FoG. Several previous studies have reported PFC activation in the initial part of walking corresponding to gait initiation and acceleration phase.41–43,48 In healthy individuals, the initial increased PFC activity tend to be attenuated during later periods of walking, suggesting a more automatic control.41–43,48 On the hand, PFC activation has been shown to be sustained throughout the walking task in clinical populations, such as post-stroke patients.41 Thus, it is possible to interpret that PD+FoG had a more sustained PFC activation pattern throughout the task compared to PD-FoG. Alternatively, one may also speculate that a potential fatigability component of our task played a role. PD+FoG usually report increased perceived fatigue49 and, therefore, they are more susceptible to fatigue during a motor task. Fatigue negatively impacts performance in locomotor tasks50 and therefore may require more higher level attention to control locomotion. It is possible that PD+FoG increased PFC activity (relative to PD-FoG) to deal with the increasing fatigue level. Since this explanation is speculative, we encourage future studies designed (e.g., including fatigue outcomes or protocol to induce fatigue) to further explore the influence of fatigue on PFC activity in PD+FoG.
Both groups prioritized the concurrent cognitive task
Our findings demonstrated that while accuracy in the concurrent cognitive task was similar between seated and dual-task conditions for both groups, gait parameters deteriorated during dual-task walking. Thus, participants prioritized the concurrent cognitive task in detriment of gait performance, even not having received a specific instruction to do so. Such prioritization may have occurred due to a low hazard estimation by patients,51 as they were in a controlled environment and had a research assistant ensuring their safety. However, the adoption of the so called “posture-second” strategy may go at the expense of maintaining balance during daily activities in the real world.52 Since PFC activity was also similar between single and dual-task walking, it is possible that participants allocated most of the available prefrontal cognitive resources for the performance of the cognitive task in the dual-task condition. As a consequence, gait deteriorated for not having the required level of prefrontal cognitive resources.
PFC activity during dual-task walking is associated with FoG severity and gait variability in PD+FoG
In PD+FoG, we observed negative correlations between relative HbO2 during dual-task walking and NFoGQ score and step time variability. Specifically, freezers with greater PFC activity during dual-task walking had less severe FoG (perceived, self-reported) and lower step time variability. These findings suggest that those with less severe FoG may have more prefrontal resources available or a relatively more effective compensatory prefrontal executive control of gait (compared to those with more severe FoG). In addition, we observed no significant correlations involving PFC activity during single-task walking. This finding combined with the significant correlations involving PFC activity during dual-task walking reinforces the important role of the PFC in the control of locomotion during more demanding tasks.
Clinical implications
Findings suggest that PFC should be targeted for the development of enhanced interventions aiming to improve gait in PD+FoG. Cortical non-invasive brain stimulation offers a potential method to achieve this end.7 In fact, there is preliminary evidence that transcranial magnetic and direct current stimulation applied over the PFC improved turning53 and clinical symptoms of FoG.54
Key study strength and limitation
A key strength of this study is the robust data analysis methods for fNIRS processing, especially the use of short-separation channels to remove the superficial hemodynamic response from the fNIRS signal. This approach reduces the likelihood of false positive results55,56 and, therefore, is recommended.22,35 On the other hand, our study is limited by assessing the PFC only. Since PD leads to a broad cortical dysfunction,57 future studies should assess multiple cortical areas while walking for a more complete understanding of PD- and FoG-related cortical control mechanisms.
CONCLUSION
In conclusion, people with PD who report FoG have reduced automaticity of walking, based on PFC activity, in comparison to people without FoG, even when they have no actual FoG episodes. Higher PFC activity in people with FoG may be compensatory to maintain gait similar to people without FoG. PFC activity while walking seems to be more sensitive than spatiotemporal gait parameters in identifying changes in automaticity in people with PD who report FoG.
Acknowledgements
The authors thank all participants for generously donating their time to participate. We also thank Georgeann Booth and Makena Strand for helping with data collection and study coordination, and Dr. Fay B Horak for helpful discussion.
Funding
This work has been supported by grants from the National Institute of Health via Career Development Award 5R00HD078492-04 (PI Mancini) and a PCO Pilot Grant Award (PI Mancini).
Footnotes
Conflict of interest
Nothing to report
References
- 1.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. The Lancet Neurology. 2011;10(8):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Lloret S, Negre-Pages L, Damier P, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA neurology. 2014;71(7):884–890. [DOI] [PubMed] [Google Scholar]
- 3.Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism & related disorders. 2015;21(3):254–258. [DOI] [PubMed] [Google Scholar]
- 4.Amboni M, Stocchi F, Abbruzzese G, et al. Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study. Parkinsonism & related disorders. 2015;21(6):644–649. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Movement disorders : official journal of the Movement Disorder Society. 2004;19(8):871–884. [DOI] [PubMed] [Google Scholar]
- 6.Vercruysse S, Gilat M, Shine JM, Heremans E, Lewis S, Nieuwboer A. Freezing beyond gait in Parkinson’s disease: a review of current neurobehavioral evidence. Neuroscience and biobehavioral reviews. 2014;43:213–227. [DOI] [PubMed] [Google Scholar]
- 7.Weiss D, Schoellmann A, Fox MD, et al. Freezing of gait: understanding the complexity of an enigmatic phenomenon. Brain : a journal of neurology. 2020;143(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbossche J, Deroost N, Soetens E, et al. Freezing of gait in Parkinson’s disease: disturbances in automaticity and control. Frontiers in human neuroscience. 2012;6:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijders AH, Takakusaki K, Debu B, et al. Physiology of freezing of gait. Annals of neurology. 2016. [DOI] [PubMed] [Google Scholar]
- 10.Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(8):1000–1004. [DOI] [PubMed] [Google Scholar]
- 11.Clark DJ. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Frontiers in human neuroscience. 2015;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuart S, Vitorio R, Morris R, Martini DN, Fino PC, Mancini M. Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: A structured review. Maturitas. 2018;113:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maidan I, Nieuwhof F, Bernad-Elazari H, et al. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults: An fNIRS Study. Neurorehabilitation and neural repair. 2016;30(10):963–971. [DOI] [PubMed] [Google Scholar]
- 14.Maidan I, Bernad-Elazari H, Giladi N, Hausdorff JM, Mirelman A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease? Brain topography. 2017;30(4):531–538. [DOI] [PubMed] [Google Scholar]
- 15.Gilat M, Bell PT, Ehgoetz Martens KA, et al. Dopamine depletion impairs gait automaticity by altering cortico-striatal and cerebellar processing in Parkinson’s disease. NeuroImage. 2017;152:207–220. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis. 2015;82:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell T, Potvin-Desrochers A, Lafontaine AL, Monchi O, Thiel A, Paquette C. Cerebral Metabolic Changes Related to Freezing of Gait in Parkinson Disease. J Nucl Med. 2019;60(5):671–676. [DOI] [PubMed] [Google Scholar]
- 18.Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res. 2003;149(2):187–194. [DOI] [PubMed] [Google Scholar]
- 19.Peterson DS, Fling BW, Mancini M, Cohen RG, Nutt JG, Horak FB. Dual-task interference and brain structural connectivity in people with Parkinson’s disease who freeze. Journal of neurology, neurosurgery, and psychiatry. 2015;86(7):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.la Fougere C, Zwergal A, Rominger A, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. NeuroImage. 2010;50(4):1589–1598. [DOI] [PubMed] [Google Scholar]
- 21.Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: A systematic review. Neuroscience and biobehavioral reviews. 2015;57:310–327. [DOI] [PubMed] [Google Scholar]
- 22.Herold F, Wiegel P, Scholkmann F, Thiers A, Hamacher D, Schega L. Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics. 2017;4(4):041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart S, Belluscio V, Quinn JF, Mancini M. Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson’s Disease. Frontiers in Neurology. 2019;10(536). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orcioli-Silva D, Vitório R, Nóbrega-Sousa P, et al. Levodopa Facilitates Prefrontal Cortex Activation During Dual Task Walking in Parkinson Disease. Neurorehabilitation and neural repair. 2020:1545968320924430. [DOI] [PubMed] [Google Scholar]
- 25.Stuart S, Mancini M. Prefrontal Cortical Activation With Open and Closed-Loop Tactile Cueing When Walking and Turning in Parkinson Disease: A Pilot Study. Journal of neurologic physical therapy : JNPT. 2020;44(2):121–131. [DOI] [PubMed] [Google Scholar]
- 26.Belluscio V, Stuart S, Bergamini E, Vannozzi G, Mancini M. The Association between Prefrontal Cortex Activity and Turning Behavior in People with and without Freezing of Gait. Neuroscience. 2019;416:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maidan I, Bernad-Elazari H, Gazit E, Giladi N, Hausdorff JM, Mirelman A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures. Journal of neurology. 2015;262(4):899–908. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30(4):459–463. [DOI] [PubMed] [Google Scholar]
- 29.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 30.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. [DOI] [PubMed] [Google Scholar]
- 31.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. [DOI] [PubMed] [Google Scholar]
- 32.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621–1626. [DOI] [PubMed] [Google Scholar]
- 33.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. Journal of neurology, neurosurgery, and psychiatry. 1998;64(5):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002;17(3):235–272. [PubMed] [Google Scholar]
- 35.Vitorio R, Stuart S, Rochester L, Alcock L, Pantall A. fNIRS response during walking - Artefact or cortical activity? A systematic review. Neuroscience and biobehavioral reviews. 2017;83:160–172. [DOI] [PubMed] [Google Scholar]
- 36.Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44(2):428–447. [DOI] [PubMed] [Google Scholar]
- 37.Tsuzuki D, Dan I. Spatial registration for functional near-infrared spectroscopy: from channel position on the scalp to cortical location in individual and group analyses. NeuroImage. 2014;85 Pt 1:92–103. [DOI] [PubMed] [Google Scholar]
- 38.Molavi B, Dumont GA. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiological measurement. 2012;33(2):259–270. [DOI] [PubMed] [Google Scholar]
- 39.Cui X, Bray S, Reiss AL. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. NeuroImage. 2010;49(4):3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodwin JR, Gaudet CR, Berger AJ. Short-channel functional near-infrared spectroscopy regressions improve when source-detector separation is reduced. Neurophotonics. 2014;1(1):015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? NeuroImage. 2007;37(4):1338–1345. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki M, Miyai I, Ono T, Kubota K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. NeuroImage. 2008;39(2):600–607. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M, Miyai I, Ono T, et al. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. NeuroImage. 2004;23(3):1020–1026. [DOI] [PubMed] [Google Scholar]
- 44.Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C. Validity of Mobility Lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiological measurement. 2019;40(9):095003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNeish D, Stapleton LM. Modeling Clustered Data with Very Few Clusters. Multivariate Behav Res. 2016;51(4):495–518. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell T, Starrs F, Soucy JP, Thiel A, Paquette C. Impaired Sensorimotor Processing During Complex Gait Precedes Behavioral Changes in Middle-aged Adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2019;74(12):1861–1869. [DOI] [PubMed] [Google Scholar]
- 47.Verghese J, Wang C, Ayers E, Izzetoglu M, Holtzer R. Brain activation in high-functioning older adults and falls: Prospective cohort study. Neurology. 2017;88(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. NeuroImage. 2015;112:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawada M, Wada-Isoe K, Hanajima R, Nakashima K. Clinical features of freezing of gait in Parkinson’s disease patients. Brain Behav. 2019;9(4):e01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbieri FA, Santos PC, Lirani-Silva E, Vitorio R, Gobbi LT, van Dieen JH. Systematic review of the effects of fatigue on spatiotemporal gait parameters. J Back Musculoskelet Rehabil. 2013;26(2):125–131. [DOI] [PubMed] [Google Scholar]
- 51.Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Movement disorders : official journal of the Movement Disorder Society. 2012;27(6):765–770. [DOI] [PubMed] [Google Scholar]
- 52.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. Journal of the neurological sciences. 2006;248(1–2):196–204. [DOI] [PubMed] [Google Scholar]
- 53.Lee SY, Kim MS, Chang WH, Cho JW, Youn JY, Kim YH. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci. 2014;32(6):743–753. [DOI] [PubMed] [Google Scholar]
- 54.Dagan M, Herman T, Harrison R, et al. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2018;33(4):642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato T, Nambu I, Takeda K, et al. Reduction of global interference of scalp-hemodynamics in functional near-infrared spectroscopy using short distance probes. NeuroImage. 2016;141:120–132. [DOI] [PubMed] [Google Scholar]
- 56.Tachtsidis I, Scholkmann F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics. 2016;3(3):030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takakusaki K Functional Neuroanatomy for Posture and Gait Control. Journal of movement disorders. 2017;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


