Abstract
Background:
Studies examining the effects of therapeutic interventions after stroke often focus on changes in loss of body function/structure (impairment). However, improvements in activities limitations and participation restriction are often higher patient priorities, and the relationship that these measures have with loss of body function/structure is unclear.
Objective:
This study measured gains across WHO International Classification of Function (ICF) dimensions and examined their interrelationships.
Methods:
Subjects were recruited 11–26 weeks after hemiparetic stroke. Over a 3-week period, subjects received 12 sessions of intensive robot-based therapy targeting the distal arm. Each subject was assessed at baseline and 1-month after end of therapy.
Results:
At baseline, subjects (n=40) were 134.7±32.4 (mean±SD) days post-stroke and had moderate-severe arm motor deficits (arm motor Fugl-Meyer score of 35.6±14.4) that were stable. Subjects averaged 2,579 thumb movements and 1,298 wrist movements per treatment session. After robot therapy, there was significant improvement in measures of body function/structure (Fugl-Meyer score) and activity limitations (Action Research Arm Test, Barthel Index, and Stroke Impact Scale-hand), but not participation restriction (Stroke Specific Quality of Life Scale). Furthermore, while the degree of improvement in loss of body function/structure was correlated with improvement in activity limitations, neither improvement in loss of body function/structure nor in activity limitations was correlated with change in participation restriction.
Conclusions:
After a three-week course of robotic therapy, there was improvement in body function/structure and activity limitations but no reduction in participation restriction.
Keywords: stroke, rehabilitation, outcomes research, recovery, clinical trials
INTRODUCTION
Stroke remains a leading cause of long-term disability worldwide1. Deficits in function of the upper extremity represent a significant contributor to decreased function and quality of life post-stroke2. Interventions such as constraint-induced movement therapy3 and robot-assisted therapy4,5 have emerged as promising approaches to promote return of function beyond that which is regained during spontaneous recovery and from conventional rehabilitation. However, the degree to which specific interventions affect disability and improve function after stroke is still poorly understood6,7.
Numerous outcome measures are available that assess the effect of stroke across all dimensions of the World Health Organization (WHO) International Classification of Function, Disability, and Health (ICF)8,9, complicating selection of appropriate measurement tools when assessing novel rehabilitation-related interventions10,11. Assessments of stroke-related loss of body function/structure (function/structure, previously referred to as impairment) are often preferred given that these measurements are more objective and easier to define1,12. In contrast, measures of activity limitations (activity) and participation restriction (participation), while often higher patient priorities13,14, are frequently qualitative and rely on patient self-reporting, and are therefore less commonly used when assessing novel interventions.
In general, a limited relationship has been found across WHO ICF dimensions such as loss of function/structure and activity12,15,16, and data are conflicting regarding the degree of correlation that exists. For example, a meta-analysis of electromechanical and robot-assisted arm training described high quality evidence that such interventions improve outcomes in both function/structure and activity, but there was limited evaluation of effects on participation17. The Fugl-Meyer (FM) motor scale18,19, a commonly employed measure of function/structure, was shown to demonstrate robust correlation with the Action Research Arm Test (ARAT), a measure of activity, when performed by expert raters19. On the other hand, studies of constraint-induced movement therapy report discrepancies between treatment-related changes in function/structure when compared to activity20. Another report found that a majority of patients demonstrating no measurable upper extremity motor impairment in an assessment of function/structure continued to report deficits as measured by assessments of activity and participation21. This inconsistent relationship may be due to numerous factors having greater influence on outcome as one moves from measurements of function/structure to activity or participation16. For example, multiple studies have shown improvements in patients’ functional independence measure scores (FIM, a measure of activity) over the course of inpatient rehabilitation despite minimal change in FM total score22 or NIHSS23 (both assessments of function/structure), potentially related to use of assistive devices or learning compensatory skills.
In the setting of a clinical trial on intensive robot-assisted therapy, the current study had two primary aims. First, the study aimed to provide a detailed assessment of the effect of robot-assisted therapy across all ICF dimensions. Second, the study also aimed to assess the degree to which therapy-related improvements in function/structure were related to improvements in activity and participation. We hypothesized that robot-assisted therapy would result in clinically and statistically significant gains in function/structure with smaller gains in activity and participation.
METHODS
Subject enrollment
Forty-one individuals 11–26 weeks after stroke onset gave informed consent to participate in a longitudinal study of standardized intensive robot-assisted therapy targeting the distal arm (clinicaltrials.gov, ID# NCT01244243). In one individual, baseline imaging revealed an incidental finding that met exclusion criteria. Therefore, results represent the remaining 40 eligible subjects. Neuroimaging data from a subset of these subjects have previously been reported24. Study procedures were approved by the University of California, Irvine Institutional Review Board.
Inclusion and exclusion criteria were designed to select for individuals with a wide range of motor deficits in whom spontaneous arm motor recovery had reached a plateau. All subjects met the following inclusion criteria: (1) age ≥18 years; (2) hemorrhagic or ischemic stroke with onset 11–26 weeks prior to enrollment; (3) residual arm motor deficits, defined as ARAT score <52 or 9-hole peg test score >25% longer in the affected compared to unaffected hand; and (4) preservation of voluntary movement in the distal upper extremity, as demonstrated by ≥5 degrees of active range of motion in the wrist or index finger metacarpophalangeal joint of the affected side. Individuals were excluded from the study if they had a contraindication to MRI, severe cognitive impairment (Mini Mental Status Examination, MMSE, score < 27), a comorbid diagnosis impacting the function of the affected upper extremity, or unstable arm motor status. Stable arm motor status was defined as change in FM score ≤2 points across two successive baseline assessments that spanned ≥1 week prior to initiation of robot therapy. This was a single treatment-arm study, with no comparison or placebo group.
Robot therapy
Robot-assisted therapy consisted of 12 treatment sessions across 3 weeks. Sessions occurred 4 days/week for 2 hours/day, totaling 24 total hours of delivered therapy. All subjects completed at least 11 of 12 prescribed robot therapy sessions. This robot has been previously described5 (Figure 1). In brief, the robotic device has 3 degrees-of-freedom, is pneumatically-actuated, and is back-driveable. The 3 degrees are rotational movement of the wrist, thumb, and fingers in the plane of gravity, with the fingers moving as a single unit about the metacarpophalangeal joint. Specifically, the device assists in power grip and release movements in a pattern that combines wrist extension with hand grasp, then wrist flexion with hand release. Robot-assisted joint movement is achieved by a lever design, in which a pneumatic cylinder is mounted at the opposite end of a lever from the limb interface, with a revolute joint in between. Back-driveability of the robot permits the subject to freely drive movements when active assistance is not engaged.
FIGURE 1.
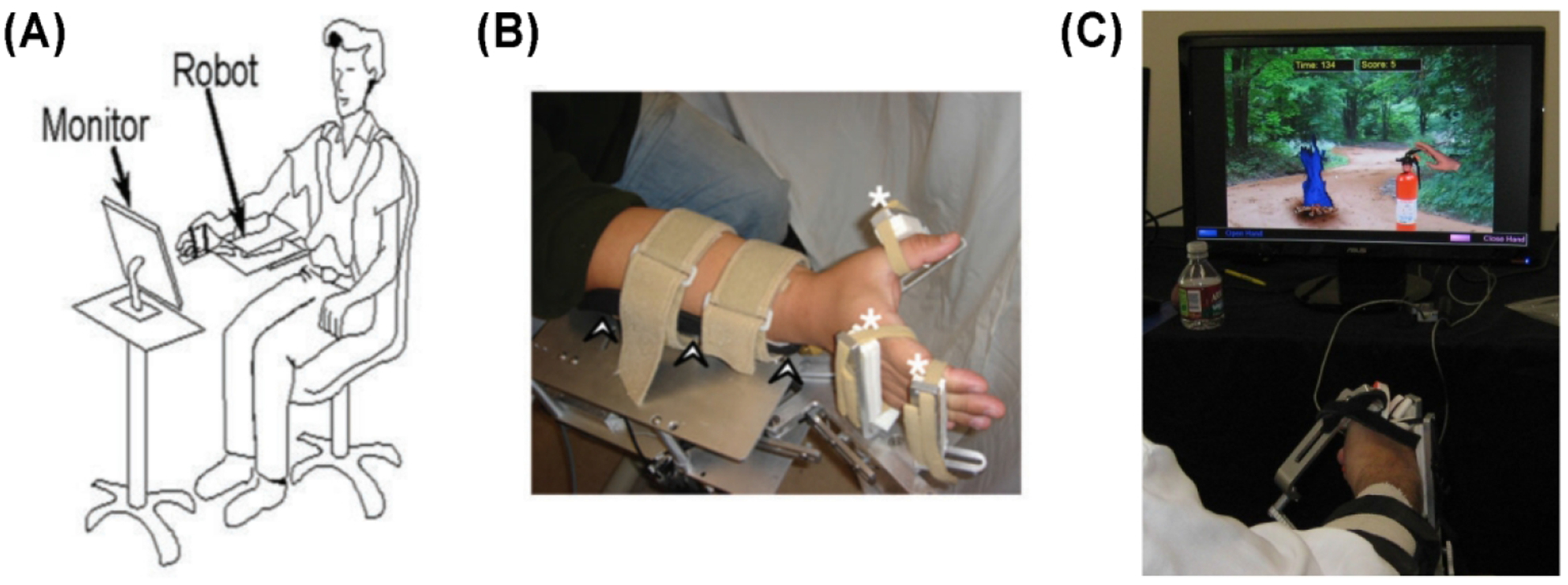
(A) Diagram of subject’s posture, relationship to robot, and relationship to computer monitor during therapy. (B) Demonstration of subject’s hand and arm interfacing with the robot. Arrowheads denote ulnar forearm rest. White asterisks indicate soft Velcro straps connecting the subject’s hand to the robot. (C) Example of virtual reality game, in which hand grasp turned on fire extinguisher while grasp release turned off fire extinguisher during Smokey the Bear game. Figure partially reprinted with permission from previously published material5.
At the start of each session, a trained therapist (licensed occupational therapist or physical therapist) assisted in robot adjustments to accommodate differences in hand sizes across subjects and to ensure maximal biomechanical advantages in robot-assisted movements. The therapist also determined the comfortable passive and active range of motion at each of the three degrees, for each subject, and adjusted the robot’s hard stops accordingly. Movement repetitions were calculated from each subject’s pre-treatment range of motion, such that a movement cycle was counted when measured active movement exceeded a preset threshold of the baseline active range of motion at a specified joint.
Each robot-assisted therapy session began with robot-assisted passive stretching, then grasp-release exercises. A wide range of real objects was placed in the hand, and the subject was asked to describe features such as texture or temperature. Next, and occupying most of the session were repeated grasp-release movements of the affected hand coupled to video games (e.g., squeezed mustard onto a hot dog consumed by Homer Simpson, or hand closure in the robot squeezed a fire extinguisher to douse a forest fire, with approval of Smokey The Bear, Figure 1C). These games emphasized control of hand movement range, speed, and timing. For each movement, the subject-initiated hand grasp, and if the subject did not complete the full movement, the pneumatic cylinder was activated to assist in completing hand grasp. In most games, movement was initiated in response to a simple computer-generated cue; in others, movement onset time was self-initiated, in an attempt to activate supplementary motor area25; and in others, choice of movement was guided by cues that corresponded to regularly-changing rules displayed on the screen, in an attempt to activate dorsal premotor cortex26. The therapist was at the patient’s side at all times to monitor and adjust robot-subject interface, clarify gameplay, and address any questions that arose. In total, the robot recorded an average of 11,278 finger movements, 28,970 thumb movements, and 15,759 wrist movements across the 3 weeks of therapy; average movement repetitions per day were 954 for finger movements, 2,579 for thumb movements, and 1,298 for wrist movements. For some games, hand grasp required movement of the fingers and thumb only, while other games required movement of the wrist and thumb. As a result, the number of movement repetitions was greatest for the thumb.
Study design
Clinical assessments were performed at three time points: (1) ≥2 weeks prior to therapy [first baseline visit], (2) ≥1 week prior to therapy and at least 7 days after first baseline visit [second baseline visit], and (3) 1 month following completion of therapy. Performance of two baseline assessments prior to initiation of therapy was done in order to ensure subjects had reached a stable plateau in motor recovery of the affected distal upper extremity.
Clinical outcome measures
All clinical assessments were performed by one of two physical therapists blinded to the subjects’ performance during therapy. The primary outcome measures were the Upper Extremity FM Assessment18 and the ARAT27, which have good reliability, reproducibility, and responsiveness to therapy19.
Assessments were categorized per the ICF dimensions of stroke recovery8,28 as defined by the RehabMeasures Database at the Shirley Ryan AbilityLab (https://www.sralab.org/rehabilitation-measures). Measures of loss of body function/structure were the FM arm motor scale, grip strength, and pinch strength. Measures of activity limitations were ARAT, Box and Blocks Test, Barthel Index29, and the Stroke Impact Scale30,31 hand motor domain (SIS-hand). One measure of participation restriction was used, the Stroke Specific Quality of Life Scale32 (SS-QOL). The FM, ARAT, and Box and Blocks Test were scored at both baseline visits. Due to logistical issues, the SS-QOL was added after the tenth subject was enrolled.
Statistical analysis
For FM, ARAT, and Box and Blocks assessments, evaluation of robot therapy-related improvement was calculated with respect to the mean of the two baseline assessments. For all other measures, therapy-related improvement was calculated with respect to scores at the first baseline visit.
Paired testing was used to determine the statistical significance of therapy-related improvement. Measures that were normally distributed or could be transformed to a normal distribution were evaluated using the paired t-test, while non-normally distributed measures were evaluated using the Wilcoxon signed-rank test. Effect size was determined using the Cohen’s d metric for parametric measures and the Mann-Whitney U Test for nonparametric measures. Bivariate analyses were used to determine the degree of correlation between the changes in clinical measures using Pearson’s correlation for normally distributed measures and Spearman’s rank correlation for non-normally distributed measures. All analyses were two-tailed with alpha=0.05, Bonferroni corrected, and were performed using MATLAB 8.5.0 (Mathworks, Natick, MA).
RESULTS
Subject characteristics
Demographics for the 40 subjects are summarized in Table 1. Subjects were mainly right-hand dominant. Across the 40 subjects, the index infarct was in the left hemisphere in 21 and in the right hemisphere in 19. Ten individuals had more than one infarct. There was significant variability in size and location of infarcts (33.1±50.0 cc, mean±SD). Mean time between the two baseline assessments was 15.6±6.0 days (range 7–30 days). FM, ARAT, and Box and Blocks Test scores did not change across the two baseline assessments (p>0.05). Baseline scores appear in Table 2.
TABLE 1:
Subject demographics
| Gender | 11 F / 29 M | |
| Age, mean (SD) | 58.0 (13.7) years | |
| Time since last stroke, mean (SD) | 134.7 (32.4) days | |
| Ethnicity | Hispanic | 3 |
| Non-Hispanic or Latino | 37 | |
| Race | Asian | 7 |
| Black or African American | 5 | |
| White | 24 | |
| Co-morbid medical conditions | Hypertension | 21 N/ 19 Y |
| Hyperiipidemia | 21 N / 19 Y | |
| Diabetes meilitus | 29 N / 11 Y | |
| Atrial fibrillation | 35 N / 5 Y | |
| History of prior stroke | 30 N/10Y |
TABLE 2:
Clinical assessments at baseline and post-treatment
| Baseline mean (SD) | Post-treatment mean (SD) | Change mean (SD) | Test statistiC† (p-value) | |
|---|---|---|---|---|
| Measures of loss of body function/struclture | ||||
| Fugl-Meyer | 35.6 (14.4) | 39.4 (15.0) | 3.79 (3.32) | 5.14† (<0.0001) |
| Grip strength (kg) | 6 (6.5) | 8.2 (8.8) | 2.25 (4.54) | 3.86* (<0.0001) |
| Pinch strength (kg) | 1.9 (1.7) | 2.6 (2.1) | 0.64 (1.06) | 3.69* (<0.0001) |
| Measures of activity limitations | ||||
| ARAT | 25.1 (18.7) | 29.2 (19.6) | 4.11 (5.86) | 4.10*(<0.0001) |
| Box and Blocks | 13.2 (15.5) | 17.0 (17.8) | 3.81 (5.51) | 4.29* (<0.0001) |
| Barthel Index | 88.5 (9.1) | 93.3 (8.1) | 4.75 (6.2) | 3.89† (0.0001) |
| SIS-hand | 2.08 (1.03) | 2.6 (1.2) | 0.47 (0.76) | 3.28† (0.0001) |
| Measures of participation restriction | ||||
| SS-QOL | 3.8 (0.57) | 3.6 (0.6) | 0.10† (0.50) | 1.08† (0.29) |
Student’s t-test t-Ratio is reported for normally distributed measures.
Wilcoxon signed-rank z-score is reported for non-normally distributed measures.
Outcomes after robot therapy
Subjects demonstrated statistically significant improvement in all measures of function/structure and activity after robot therapy, from baseline to the post-therapy assessment (Table 2). The group did not show statistically significant change in SS-QOL, the only measure of participation. Across all measures, the largest effect sizes were noted for improvement in FM (d=0.57), Box and Blocks Test (d=0.48), and ARAT (d=0.46); a smaller effect size was seen for SIS-hand (d=0.37).
Correlation across ICF dimensions
Correlations for improvements in measures both within and across ICF dimensions are detailed in Table 3. Briefly, modest correlations were demonstrated between measures within each ICF dimension. However, the strongest correlations were found between measures of function/structure and activity. For example, improvements in FM were robustly correlated with improvements in ARAT (Figure 2A, r=0.69, p<0.0001) and Box and Blocks Test (Figure 2B, r=0.55, p=0.0002). Similarly, improvement in grip strength was correlated with improvement in Box and Blocks Test score (r =0.52, p=0.0005). Change in SIS-hand, a patient-reported measure of activity, did not correlate with improvements in FM or ARAT.
TABLE 3:
Correlations between clinical measures
| Correlation coefficient | P | |||
|---|---|---|---|---|
| Within ICF dimension correlations | ||||
| Measures of loss of body function/structure (function/structure) | ||||
| Fugl-Meyer | Grip strength | 0.26 | 0.11 | |
| Fugl-Meyer | Pinch strength | 0.34 | 0.03 | |
| Grip strength | Pinch strength | 0.50 | 0.001* | |
| Measures of activity limitations (activity) | ||||
| ARAT | Box and Blocks | 0.50 | 0.0009* | |
| ARAT | SIS-hand | 0.21 | 0.20 | |
| Box and Blocks | SIS-hand | 0.41 | 0.008* | |
| Across ICF dimension correlations | ||||
| Function/structure | Activity | |||
| Fugl-Meyer | ARAT | 0.69 | < 0.0001* | |
| Fugl-Meyer | Box and Blocks | 0.55 | 0.0002* | |
| Fugl-Meyer | SIS-hand | 0.16 | 0.32 | |
| Grip strength | ARAT | 0.17 | 0.29 | |
| Grip strength | Box and Blocks | 0.52 | 0.0005* | |
| Grip strength | SIS-hand | 0.24 | 0.13 | |
| Pinch strength | ARAT | 0.37 | 0.02 | |
| Pinch strength | Box and Blocks | 0.31 | 0.05 | |
| Pinch strength | SIS-hand | 0.35 | 0.03 | |
| Function/structure | Participation | |||
| Fugl-Meyer | SS-QOL | 0.34 | 0.07 | |
| Grip strength | SS-QOL | 0.11 | 0.56 | |
| Pinch strength | SS-QOL | 0.12 | 0.51 | |
| Activity | Participation | |||
| ARAT | SS-QOL | 0.24 | 0.20 | |
| Box and Blocks | SS-QOL | 0.32 | 0.08 | |
| SIS-hand | SS-QOL | 0.22 | 0.25 | |
denotes correlations reaching statistical significance, with p<0.05, corrected for multiple comparisons
FIGURE 2.
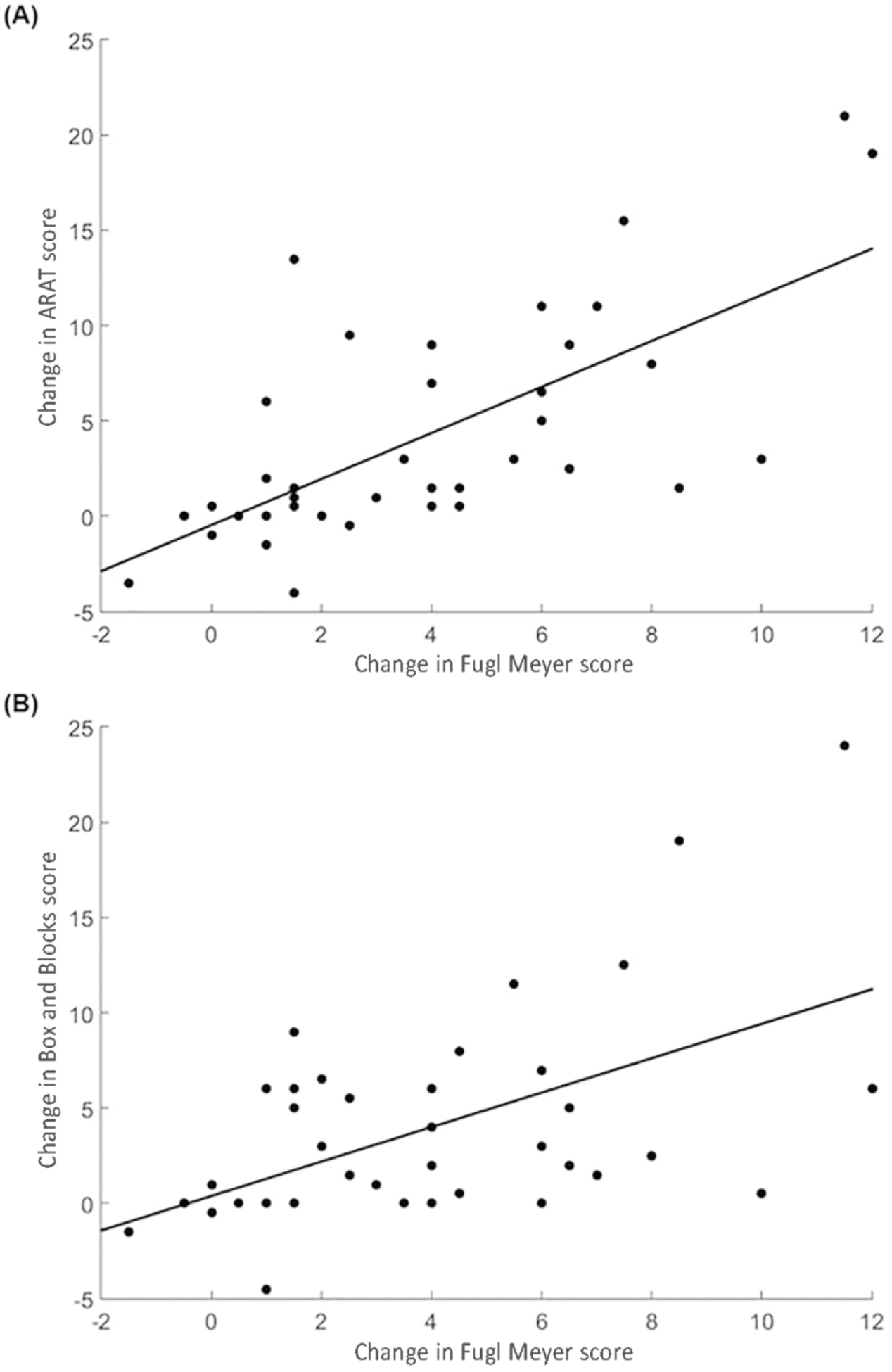
Scatterplot demonstrating the relationship between change in Upper Extremity Fugl-Meyer Assessment score and change in (A) Action Research Arm Test (r = 0.69, p < 0.0001) and (B) Box and Blocks Test (r = 0.55, p =0.0002). Change after robot-assisted therapy was calculated as the absolute change in score from baseline (mean of two assessments) to 1-month post-therapy assessment.
Changes in SS-QOL were not correlated with improvements in any measure of function/structure or activity, including FM, ARAT, and SIS-hand.
DISCUSSION
Stroke-related deficits in upper extremity function remain a significant contributor to disability worldwide, with persistent deficits directly linked to activity limitations, participation restriction, poorer quality of life, and decreased subjective well-being1,21,33. Novel interventions, including robot-assisted therapy, have been developed to reduce stroke-related disability. However, available data provide a limited understanding of the degree to which such interventions produce changes across ICF dimensions. Furthermore, there have been limited studies that directly examine the degree to which treatment-related gains in one ICF dimension are associated with gains across other ICF dimensions.
The current study found that subjects who completed a course of robot-based therapy demonstrated statistically significant improvements in stroke-related loss of body function/structure and activity limitations, but not in participation restriction. Specifically, robot-assisted therapy was associated with statistically significant gains across all measures of function/structure and activity, including both primary endpoints: FM (a measure of function/structure) and ARAT (a measure of activity). Estimates of the minimal clinically important difference (MCID) for the FM and ARAT have been estimated to be 10% of the scale maximum18,34, or 6.6 and 5.7 points respectively. Using this estimate, 17.5% of the study patients achieved MCID on the FM, and 32.5% on the ARAT, after robot-assisted therapy. Lo et al4 have suggested that for some subjects with chronic stroke, gains below MCID might be clinically meaningful. SIS-hand, which is a patient-reported measure of activity, also showed a statistically significant improvement after therapy, though effect size was notably smaller. Improvements in measures of function/structure were robustly correlated with measures of activity, consistent with prior studies that found measures of function/structure to be significantly correlated with measures of activity, including Barthel Index, FIM, and SIS-1635,36. Together, the current results provide an in-depth examination of the degree to which clinical improvements after robot-based therapy extend across ICF dimensions.
Current findings are similar to previously published large-scale clinical trials in which an experimental intervention produced significant gains in measures of function/structure and activity, but not in measures of participation3,37,38. This difficulty to generate changes in participation likely reflects both the multitude of factors that contribute to participation in society and the complex interaction of such factors1. Previous studies have identified demographic variables, socioeconomic status, injury characteristics, medical and psychiatric comorbidities, social support, and functional status as contributors to deficits in post-stroke participation2,39. Given that participation can be influenced by so many variables, including those related to both the function/structure and activity dimensions40, it may be that detection of meaningful improvements in participation requires a longer time period in order to develop and thus an extended follow-up period, consistent with the observation from longitudinal studies that quality of life after stroke can take months to years to manifest41. In line with this, Wolf and colleagues found initial changes in SIS functional domains were only followed by improvements in SIS participation domains after a period of 12–24 months beyond when the intervention was completed42.
The high number and density of movement repetitions provided by the robot represent several strengths of the current study. First, standardization of treatment by the robot minimizes variability in conventional rehabilitation interventions which could confound the relationship between improvements after robot-assisted therapy across ICF dimensions43. Second, robot-based interventions often deliver a higher intensity of movement repetitions compared to conventional interventions43,44. In one study, conventional therapist-administered interventions provided an average of 32 repetitions/session45, which is too few movements to induce neural plasticity46 and an impediment to improving behavior, particularly in higher order ICF dimensions including participation47. In contrast, patients in the current study averaged 2,579 thumb movements and 1,298 wrist movements per treatment session, exceeding the 600–700 repetitions per day48 in rats and the 92449 movements/day in primates considered necessary to realize functional benefits with stroke rehabilitation. Third, each hand movement in the robot was coupled to video games. Games promote patient involvement in health care50,51 and motivate patients to engage in enjoyable play behavior that involves therapeutically relevant movements52,53. Use of games alters cognitive context54 and, compared to rote movement repetition, increases activity in cognitive networks in patients with stroke55.
After robot-assisted therapy, improvements in function/structure and activity were not associated with improvements in participation. Recently published results from two large-scale clinical trials also reported a dissociation between treatment-related motor gains and change in measures of participation16,38. Lang and colleagues38 found that while the majority of subjects reported overall perception of meaningful change with treatment, as a group they did not demonstrate statistically significant improvement in measures of participation. A cross-sectional study31 comparing SIS with SS-QOL also reported significant dissociation, with SIS subscores being more responsive to treatment as compared to SS-QOL subscores. On the other hand, Roth and Lovell56 reported a correlation between FIM scores >80 at one year post-stroke and increased community/home participation as defined by the Frenchay Activities Index. Together, these results highlight an ongoing need for robust, responsive, and specific measures of participation, particularly when evaluating the effect of novel interventions for reducing stroke disability. Strikingly, in a review of the 116 instruments on www.rehabmeasures.org28 designated for stroke, only 15 instruments were strictly participation measures. The overwhelming majority of those instruments assess only three or four items to characterize overall quality of life, and thus provide coarse gradation across patients. Underscoring the limited attention to participation in studies of stroke rehabilitation therapies, a report by Salter and colleagues showed only 25% of randomized controlled trials of stroke rehabilitation in the last four decades included an assessment of participation57. These prior studies indicate the ongoing interest and the paucity of knowledge regarding the translation between improvements in function/structure and participation, changes that are more clinically meaningful to patients.
Several limitations are associated with the current study. First, subjects were studied 11–26 weeks post-stroke. As such, it is unknown the degree to which the current findings can be extended to patients who initiate therapy at an earlier or later time post-stroke. In addition, while the literature reports 95% of patients with upper extremity deficits reach recovery plateau by 11 weeks post-stroke58, the improvements demonstrated after robot-assisted therapy (Table 2) may not be entirely attributable to the robotic intervention and instead may be confounded by a degree of natural recovery. Any contributions of natural recovery to the current results are likely attenuated in this study, however, as serial baseline assessments indicated that subjects had reached stable arm motor status at the time of enrollment. The primary endpoint of the study was at one-month post therapy, which was an additional study limitation. It may be that any long-term changes in participation and their relationship over time59 with structure/function and activities, were not captured as a result of this one-month focus. Finally, reflecting the paucity of meaningful participation measures reported in the stroke literature, the SS-QOL was the only participation measure used in the current study. Although other measures of participation are available, the SS-QOL has been validated in stroke patients in multiple studies60,61 and includes measures of quality of life as they relate to upper extremity function, making it especially suited for the current study.
Though the generalizability of these results to robot-assisted therapy beyond the upper extremity is yet unknown, studies showing correlations between function/structure of the lower extremity62 and activity using the Barthel index35,62, SIS-1636, and FIM22,36 suggest that further studies in other key components of movement are warranted and may show similar relationships.
Conclusions
Novel interventions to reduce stroke disability are in development63. The current results demonstrate robot-assisted therapy, specifically, supports improvements in stroke-related loss of body function/structure, as well as activity limitations. The results also underscore concerns regarding the incomplete relationships between therapy-related improvement in function/structure and activity (often the primary endpoints in clinical trials), and improvements in participation, which are often prioritized by patients1. Ultimately, this study highlights the need64 for further development of instruments that provide accurate, specific, reliable, reproducible, and granulated assessments of participation restriction after stroke.
Footnotes
CONFLICTS OF INTEREST
Dr. Cramer serves as a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, SanBio, Fujifilm Toyama Chemical Co., and TRCare.
REFERENCES
- 1.Winstein CJ, Stein J, Arena R, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169. [DOI] [PubMed] [Google Scholar]
- 2.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36(7):1480–1484. [DOI] [PubMed] [Google Scholar]
- 3.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. [DOI] [PubMed] [Google Scholar]
- 4.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131(Pt 2):425–437. [DOI] [PubMed] [Google Scholar]
- 6.Kus S, van de Ven-Stevens LA, Coenen M, Berno S, Kollerits B, Cieza A. What is our knowledge of functioning and disability in hand conditions based on? Arch Phys Med Rehabil. 2011;92(8):1326–1332. [DOI] [PubMed] [Google Scholar]
- 7.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. [DOI] [PubMed] [Google Scholar]
- 8.McDougall J, Wright V, Rosenbaum P. The ICF model of functioning and disability: incorporating quality of life and human development. Dev Neurorehabil. 2010;13(3):204–211. [DOI] [PubMed] [Google Scholar]
- 9.Organization WH. International Classification of Functioning, Disability and Health (ICF). Published 2018. Updated 3/02/2018. Accessed 7/21/2020.
- 10.Barak S, Duncan PW. Issues in selecting outcome measures to assess functional recovery after stroke. NeuroRx. 2006;3(4):505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31(6):1429–1438. [DOI] [PubMed] [Google Scholar]
- 12.Burridge JH, Turk R, Notley SV, Pickering RM, Simpson DM. The relationship between upper limb activity and impairment in post-stroke hemiplegia. Disabil Rehabil. 2009;31(2):109–117. [DOI] [PubMed] [Google Scholar]
- 13.Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-term outcome poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. 2011;92(11):1802–1808. [DOI] [PubMed] [Google Scholar]
- 14.Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4(3):200–205. [DOI] [PubMed] [Google Scholar]
- 15.Lewthwaite R, Winstein CJ, Lane CJ, et al. Accelerating Stroke Recovery: Body Structures and Functions, Activities, Participation, and Quality of Life Outcomes From a Large Rehabilitation Trial. Neurorehabil Neural Repair. 2018;32(2):150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winstein CJ, Wolf SL, Dromerick AW, et al. Effect of a Task-Oriented Rehabilitation Program on Upper Extremity Recovery Following Motor Stroke: The ICARE Randomized Clinical Trial. JAMA. 2016;315(6):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2018;9:CD006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. [DOI] [PubMed] [Google Scholar]
- 19.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27(8):732–741. [DOI] [PubMed] [Google Scholar]
- 20.Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013;27(2):99–109. [DOI] [PubMed] [Google Scholar]
- 21.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelton FD, Volpe BT, Reding M. Motor impairment as a predictor of functional recovery and guide to rehabilitation treatment after stroke. Neurorehabil Neural Repair. 2001;15(3):229–237. [DOI] [PubMed] [Google Scholar]
- 23.Roth EJ, Heinemann AW, Lovell LL, Harvey RL, McGuire JR, Diaz S. Impairment and disability: their relation during stroke rehabilitation. Arch Phys Med Rehabil. 1998;79(3):329–335. [DOI] [PubMed] [Google Scholar]
- 24.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77(1):132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passingham RE. The frontal lobes and voluntary action. Oxford University Press; 1993. [Google Scholar]
- 26.Dodakian L, Sharp KG, See J, et al. Targeted engagement of a dorsal premotor circuit in the treatment of post-stroke paresis. NeuroRehabilitation. 2013;33(1):13–24. [DOI] [PubMed] [Google Scholar]
- 27.Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87(12):1605–1610. [DOI] [PubMed] [Google Scholar]
- 28.Moore JL, Raad J, Ehrlich-Jones L, Heinemann AW. Development and use of a knowledge translation tool: the rehabilitation measures database. Arch Phys Med Rehabil. 2014;95(1):197–202. [DOI] [PubMed] [Google Scholar]
- 29.Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke. 2011;42(4):1146–1151. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. [DOI] [PubMed] [Google Scholar]
- 31.Lin KC, Fu T, Wu CY, Hsieh YW, Chen CL, Lee PC. Psychometric comparisons of the Stroke Impact Scale 3.0 and Stroke-Specific Quality of Life Scale. Qual Life Res. 2010;19(3):435–443. [DOI] [PubMed] [Google Scholar]
- 32.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30(7):1362–1369. [DOI] [PubMed] [Google Scholar]
- 33.Wyller TB, Sveen U, Sodring KM, Pettersen AM, Bautz-Holter E. Subjective well-being one year after stroke. Clin Rehabil. 1997;11(2):139–145. [DOI] [PubMed] [Google Scholar]
- 34.van der Lee J, Beckerman H, Lankhorst G, Bouter L. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med. 2001;33(3):110–113. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Mayo NE, Higgins J, Salbach NM, Finch L, Wood-Dauphinee SL. The Stroke Rehabilitation Assessment of Movement (STREAM): a comparison with other measures used to evaluate effects of stroke and rehabilitation. Phys Ther. 2003;83(7):617–630. [PubMed] [Google Scholar]
- 36.Ward I, Pivko S, Brooks G, Parkin K. Validity of the stroke rehabilitation assessment of movement scale in acute rehabilitation: a comparison with the functional independence measure and stroke impact scale-16. PM R. 2011;3(11):1013–1021. [DOI] [PubMed] [Google Scholar]
- 37.Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle-cerebral-artery stroke: a randomised trial. Lancet. 1999;354(9174):191–196. [DOI] [PubMed] [Google Scholar]
- 38.Lang CE, Strube MJ, Bland MD, et al. Dose response of task-specific upper limb training in people at least 6 months poststroke: A phase II, single-blind, randomized, controlled trial. Ann Neurol. 2016;80(3):342–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King RB. Quality of life after stroke. Stroke. 1996;27(9):1467–1472. [DOI] [PubMed] [Google Scholar]
- 40.De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993;24(8):1178–1181. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Determinants of quality of life in stroke survivors and their informal caregivers. Stroke. 2005;36(4):803–808. [DOI] [PubMed] [Google Scholar]
- 42.Wolf SL, Winstein CJ, Miller JP, et al. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lum P, Reinkensmeyer D, Mahoney R, Rymer WZ, Burgar C. Robotic devices for movement therapy after stroke: current status and challenges to clinical acceptance. Top Stroke Rehabil. 2002;8(4):40–53. [DOI] [PubMed] [Google Scholar]
- 44.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peurala SH, Kantanen MP, Sjogren T, Paltamaa J, Karhula M, Heinonen A. Effectiveness of constraint-induced movement therapy on activity and participation after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2012;26(3):209–223. [DOI] [PubMed] [Google Scholar]
- 48.Jeffers MS, Karthikeyan S, Gomez-Smith M, et al. Does Stroke Rehabilitation Really Matter? Part B: An Algorithm for Prescribing an Effective Intensity of Rehabilitation. Neurorehab Neural Re. 2018;32(1):73–83. [DOI] [PubMed] [Google Scholar]
- 49.Plautz E, Milliken G, Nudo R. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74(1):27–55. [DOI] [PubMed] [Google Scholar]
- 50.Brox E, Fernandez-Luque L, Tøllefsen T. Healthy Gaming – Video Game Design to promote Health. Applied Clinical Informatics. 2011;2:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson D, Baranowski T, Buday R, et al. Serious Video Games for Health How Behavioral Science Guided the Development of a Serious Video Game. Simulation & gaming. 2010;41(4):587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.http://www.rwjf.org/content/rwjf/en/research-publications/find-rwjf-research/2011/03/advancing-the-field-of-health-games.html. Robert Wood Johnson Foundation. Advancing the Field of Health Games: A Progress Report on Health Games Research. Robert Wood Johnson Foundation, 2011. Accessed 7/21/2020. [Google Scholar]
- 53.Przybylski A, Rigby C, Ryan R. A Motivational Model of Video Game Engagement. Rev Gen Psychol. 2010;14:154–166. [Google Scholar]
- 54.Dennis A, Bosnell R, Dawes H, et al. Cognitive context determines dorsal premotor cortical activity during hand movement in patients after stroke. Stroke. 2011;42(4):1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dodakian L, Campbell Stewart J, Cramer SC. Motor imagery during movement activates the brain more than movement alone after stroke: a pilot study. J Rehabil Med. 2014;46(9):843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth EJ, Lovell L. Community skill performance and its association with the ability to perform everyday tasks by stroke survivors one year following rehabilitation discharge. Top Stroke Rehabil. 2007;14(1):48–56. [DOI] [PubMed] [Google Scholar]
- 57.Salter KL, Foley NC, Jutai JW, Teasell RW. Assessment of participation outcomes in randomized controlled trials of stroke rehabilitation interventions. Int J Rehabil Res. 2007;30(4):339–342. [DOI] [PubMed] [Google Scholar]
- 58.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–398. [DOI] [PubMed] [Google Scholar]
- 59.Davis AM, Perruccio AV, Ibrahim S, et al. The trajectory of recovery and the inter-relationships of symptoms, activity and participation in the first year following total hip and knee replacement. Osteoarthritis Cartilage. 2011;19(12):1413–1421. [DOI] [PubMed] [Google Scholar]
- 60.Hsueh IP, Jeng JS, Lee Y, Sheu CF, Hsieh CL. Construct validity of the stroke-specific quality of life questionnaire in ischemic stroke patients. Arch Phys Med Rehabil. 2011;92(7):1113–1118. [DOI] [PubMed] [Google Scholar]
- 61.Kerber KA, Brown DL, Skolarus LE, et al. Validation of the 12-item stroke-specific quality of life scale in a biethnic stroke population. J Stroke Cerebrovasc Dis. 2013;22(8):1270–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao HF, Hsueh IP, Tang PF, Sheu CF, Hsieh CL. Analysis and comparison of the psychometric properties of three balance measures for stroke patients. Stroke. 2002;33(4):1022–1027. [DOI] [PubMed] [Google Scholar]
- 63.Lin DJ, Finklestein SP, Cramer SC. New Directions in Treatments Targeting Stroke Recovery. Stroke. 2018;49(12):3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salter K, Jutai JW, Teasell R, Foley NC, Bitensky J. Issues for selection of outcome measures in stroke rehabilitation: ICF Body Functions. Disabil Rehabil. 2005;27(4):191–207. [DOI] [PubMed] [Google Scholar]


