Abstract
While radiotherapy is a widely used treatment for many types of human cancer, problems of radio-resistance and side effects remain. Side effects induced by ionizing radiation (IR) arise primarily from its propensity to trigger inflammation and oxidative stress with damage of normal cells and tissues near the treatment area. The highly potent superoxide dismutase mimetic, GC4419 (Galera Therapeutics), rapidly enters cells and is highly effective in dismutating superoxide (O2•−). We performed studies to assess the potency of GC4419 in cancer killing and radio-sensitization in human lung cancer cells and normal immortalized lung cells. Treatment with GC4419 did not alter the radical generation during IR, primarily hydroxyl radical (·OH); however, it quenched the increased levels of O2•− detected in the cancer cells before and following IR. GC4419 triggered cancer cell death and inhibited cancer cell proliferation with no adverse effect on normal cells. Combination of GC4419 with IR augmented the cytotoxic effects of IR on cancer cells compared to monotherapy, while protecting normal cells from IR-induced cell death. DNA fragmentation and caspase-3 activity assays showed that combination of GC4419 with IR enhances cancer cell apoptosis. Moreover, GC4419 increased IR-induced Bax levels with decreased Bcl-2 and elevated Bax/Bcl-2 ratio following treatment. GC4419 increased TrxR activity in the normal cells but decreased activity in cancer cells, conferring increased cancer cell sensitivity to oxidative stress. In conclusion, GC4419 increases the cytotoxic and pro-apoptotic activity of IR in lung cancer cells while decreasing injury in normal cells.
Keywords: free radicals, cancer therapy, radiation therapy, apoptosis, superoxide, hydrogen peroxide, electron paramagnetic resonance, spin trapping
Graphical Abstract
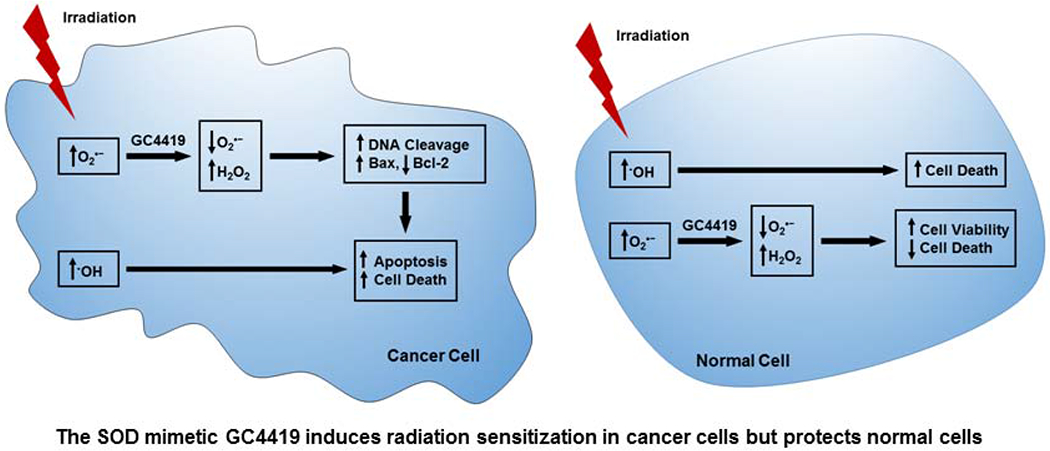
Introduction
Cancer is a major public health problem worldwide and is the second leading cause of death in the United States. In 2017, more than 1,680,000 individuals were newly diagnosed as cancer patients and 600,920 died as a direct effect of cancer in the United States [1]. Lung cancer has the highest incidence and mortality of all cancer, accounting for 25-29% of all estimated cancer death in the US [1]. Non–small-cell lung cancer (NSCLC) is the most widespread and aggressive type of lung cancer. Usually NSCLC is diagnosed in late stages and even when it is diagnosed early, it rapidly metastasizes, leading to frequent systemic relapses and a poor prognosis [2]. The majority of NSCLC patients are not qualified for surgical resection. Instead, ionizing radiation (IR), in combination with chemotherapy is utilized for treatment [3]. During treatment, IR must be localized precisely on tumors to reduce toxicity to the surrounding normal tissues [4]. Despite advances in IR technology, its efficacy remains limited, and the prognosis of patients with lung cancer remains poor [5]. The clinical efficacy of IR is limited by both normal tissue toxicity and tumor resistance. Therefore, there is a need for a selective approach to enhance the dose-dependent effect of IR in killing tumor cells while lowering toxicity to normal cells [6]. Several studies have demonstrated that use of certain sensitizing agents in combination with IR can be more effective than IR alone [7]. Combination therapy may increase the efficacy of IR and reduce its side effects leading to overall improved cancer treatment outcomes.
Superoxide dismutase (SOD) is an important antioxidant enzyme in cells. Three isoforms of SOD exist in the human body. Manganese SOD (MnSOD, SOD2), located in mitochondria, Copper-Zinc SOD (CuZnSOD, SOD1) that is localized in the cytoplasm, cytosol, nucleus, and mitochondria intermembrane space [8, 9] and extracellular SOD (ECSOD, SOD3) [9, 10]. The main function of these enzymes is to catalyze the dismutation of O2•− to H2O2, which is further converted by catalase to water [11]. Several studies have reported lower expression and activity of MnSOD in tumor cells, and that its cellular overexpression inhibits the growth of numerous tumor cell types [12–15]. However, the molecular mechanisms for this activity remain unclear.
The administration of the native SOD enzyme does not show efficacy, as it has large molecular weight and poorly penetrates into the tumor cells [16]. Several low-molecular-mass SOD mimetics (SODm) have been developed to overcome these limitations [17]. A variety of Mn-based metalloporphyrin complexes have been developed and shown to exhibit SODm activity [18]. Several of these porphyrin-based mimetics are effective in decreasing IR-induced damage in normal tissue and radio-sensitizing tumor tissue. In this study, we utilized GC4419 (Fig. 1), a unique highly potent pentaazamacrocyclic Mn (II)-containing SOD mimetic. This class of SODm has advantages over other SODm types in that they possess higher catalytic activity [19, 20], better cell permeability, higher stability and higher selectivity towards O2•− [21, 22]. Combination therapy with GC4419 has shown great promise in cancer treatment for the reduction of IR-induced side effects. Reduction of oral mucositis incidence and severity was observed in phase IIb clinical trials, and a phase III trial is now underway [23, 24].
Fig. 1:
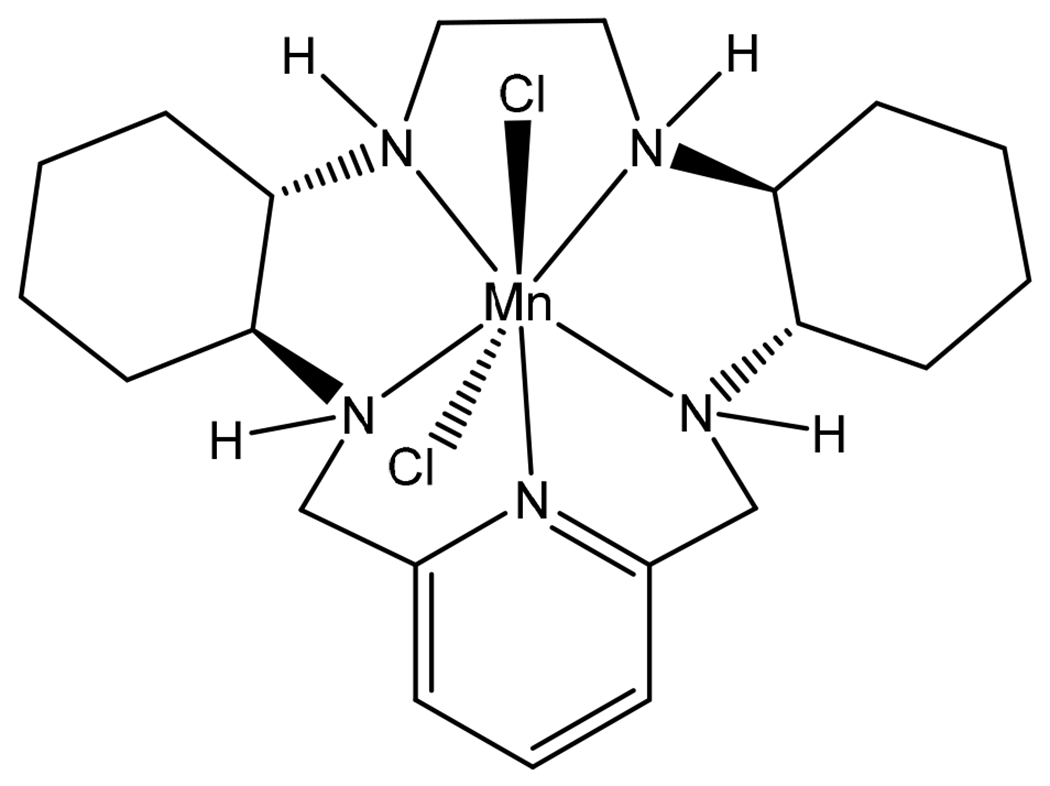
Structure of the superoxide dismutase mimetic GC4419
In this study, we first measure the processes of IR-induced radical generation, and then the subsequent O2•− generation that occurs, in normal immortalized lung cells and lung cancer cells, as well as the effect of GC4419 on these processes. We then investigate the effect of GC4419 on IR-induced cytotoxic activity against lung cancer cell lines. The process of cytotoxicity and cell death was explored with measurement of DNA fragmentation, caspase activity and apoptotic markers. With electron paramagnetic resonance (EPR) spin-trapping, we observe that GC4419 does not alter IR-mediated hydroxyl radical (·OH) generation; however, GC4419 effectively quenches the elevated levels of O2•− generation detected in cancer cells before and after IR. GC4419 is shown to be synergistic with IR, enhancing programmed cell death in tumor cells, while protecting normal cells from IR-induced toxicity.
Materials and Methods
Cell Culture, growth curve and calculation of doubling time.
Human lung cancer cells A549, H1299 and normal immortalized lung/bronchial epithelial cells Beas 2b, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). A549 and H1299 cell lines were cultured in ATCC formulated RPMI1640 and F12K Medium, respectively. Media were supplemented with 10% fetal bovine serum and penicillin/streptomycin mix (10,000 units/ml Penicillin + 10,000 μg/ml Streptomycin). Beas 2b cells were grown in bronchial epithelial cell growth medium BEBM™ supplemented with bronchial epithelial cell growth medium bulletKit™ (BEGM™). These media were obtained from Lonza (Lonza; Walkersville, MD). Per ATCC recommendations, cells were used within 15 -20 passages, when they were taken from the liquid nitrogen stock, and frequently checked for any morphological changes and mycoplasma infections, using a microscope and the universal mycoplasma detection kit (ATCC, Manassas, VA, USA). Cells were immediately discarded if any morphological changes or mycoplasma were detected. Cells were grown on cell culture plates and incubated in a humidified atmosphere of 95% air: 5% CO2 at 37°C.
We have determined the rate of proliferation for each cell line in the exponential phase of growth. Cells were seeded in six-well plates at a density of 800-1000 cells/cm2 depending on cell type, incubated in a humidified atmosphere of 95% air: 5% CO2 at 37°C, and counted every day for 5 days. Growth curves were generated by plotting cell numbers against time. The following equation was used to determine the doubling time (DT): DT= 0.693*t/ln (Nt/N0) where t=time in hours, Nt= number of cells at time t, N0= number of cells at the initial seeding time.
Treatment protocol.
Exponentially growing cells were exposed to GC4419 (Galera Therapeutics Inc, Malvern, PA), IR, or a combination of both according to the time line presented in Figure 2. IR was generated using a RS-2000 biological X-ray irradiator (RAD Source) at level zero (floor) with graphite tray with an average rate 1.13 Gy/minute at room temperature. GC4419 was kept in contact with cells until the end of the treatment schedule. In the combination experiments, GC4419 was added and cells were subsequently exposed to IR. At the end of each treatment schedule, cells were washed with phosphate-buffered saline (PBS) and then either harvested by trypsinization (0.05% trypsin was added, cells were incubated at 37°C for 1-2 min, then trypsin was inactivated with 1% serum), or were fixed and stained, depending on the experimental protocol of each assay performed.
Fig. 2:
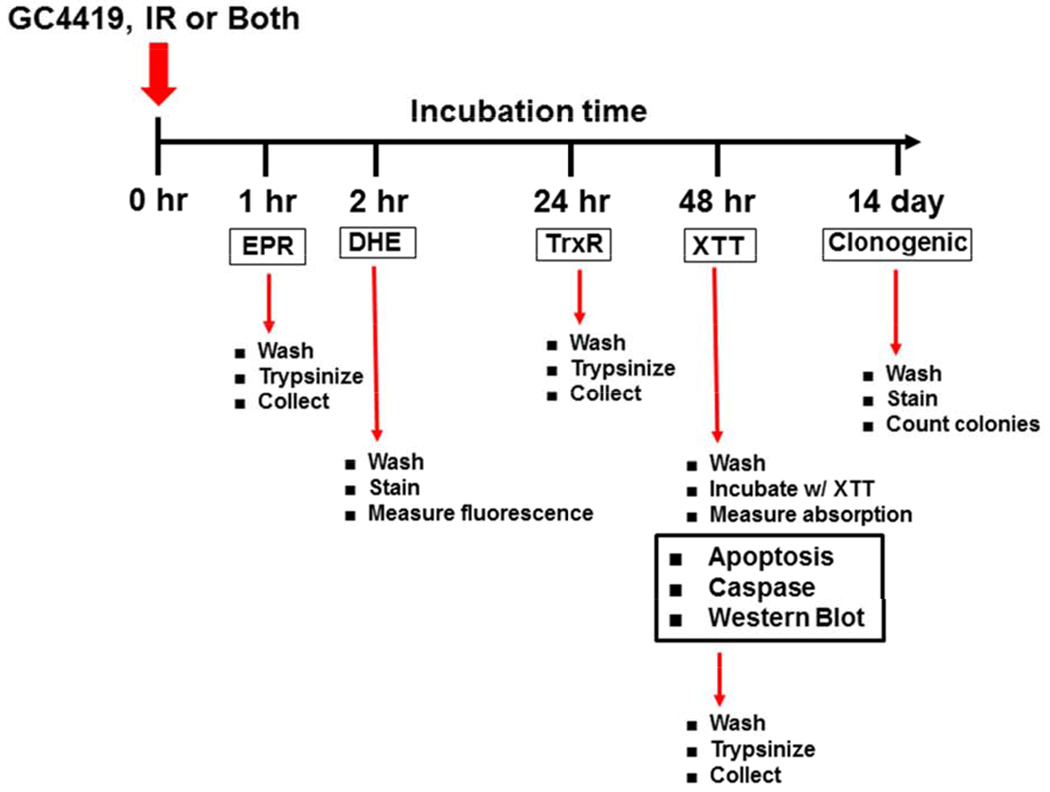
Timeline of GC4419 / IR Treatment and Measurements
Superoxide generating system.
Xanthine oxidase (XO), grade III, from bovine milk was purchased from Sigma-Aldrich, as 0.8 units/mg, in 2.3 M ammonium sulfate, 10 mM sodium phosphate buffer (pH 7.8) containing 1 mM EDTA and 1 mM sodium salicylate. The enzyme was further chromatographically purified with Sephadex G-25 prior to use. O2•− was generated using ~40 mU/ml XO in chelexed PBS buffer, pH 7.4, in the presence of 50 mM 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) and 5 μM diethylenetriaminepentaacetic acid (DTPA), and initiated by adding 200 μM xanthine. Catalase (370 U/ml) was also added to remove the H2O2 generated [25].
EPR measurement of free radical generation.
EPR spectroscopy was performed at room temperature using a Bruker EMX EPR spectrometer at X-band equipped with HS resonator and a quartz flat cell. For the studies of the dose-dependent effect of GC4419 on O2•− generation, spin trapping was performed using 50 mM DMPO in PBS, pH 7.4, with 0.1 mM DTPA. For these measurements, 30 sequential scans were performed over approximately 10 minutes. For the cellular studies, cells were treated with IR in the presence or absence of GC4419 and then, after set times, cells were harvested and cell pellets were re-suspended in PBS with 50 mM DMPO. For the studies of IR-induced radical generation, the spin trap DMPO was added just prior to IR. The EPR acquisition parameters used for the studies of the effects of IR or GC4419 in cells or buffer were: 20 mW microwave power, modulation amplitude of 0.5 G, time constant of 0.08 s, and a scan time of ~ 42 s. Spectra were typically a sum of 10 or 20 scans. The nitrone spin trap DMPO was utilized because of its wide application for studies of oxygen radicals in cellular and biochemical systems and the relatively simple and well characterized spectra of its radical adducts [26]. Relative quantitation of the observed radical adducts was performed by comparing peak amplitudes and spectral intensity obtained by double integration and this was also calibrated against the nitroxide standard, 4-oxo-2,2,6,6-tetramethyl-1-piperidinyloxy (4-oxo-TEMPO). Spectral simulations were performed by component fitting in a manner similar to previous reports [27]. For the simulations shown, WINSIM EPR spectral simulation software was utilized as previously described [28, 29].
Superoxide detection by dihydroethidium (DHE).
To determine the effect of GC4419 and/or IR on reactive oxygen species (ROS) production by normal immortalized and cancer lung cells, cells were seeded on cover slips in each well of 6-well plates and cultured in complete media. After 24 hr, cells were treated with 0 or 10 μM GC4419, 8 Gy IR, or combination, and incubated for 2 hr in a humidified atmosphere of 95% air: 5% CO2 at 37°C. Media was aspirated then the cells attached on cover slips, were washed with PBS before staining with 10 μM of the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) for 5 minutes in the dark at 37°C. Then the cells, were washed with PBS followed by incubation with 10 μM DHE for 30 minutes in dark at 37°C. At 2 hours post-treatment after washing with PBS, coverslips were transferred onto glass slides and subsequently photographed with a confocal microscope (Olympus FV3000) to observe the red fluorescent ethidium formed from the reaction of O2•− with DHE. Three images were taken from different sites in each well at X40 magnification. Fluorescence intensity of DHE in cells co-stained with the nuclear stain DAPI was analyzed with ImageJ software.
Assessment of cell viability and growth inhibition.
The XTT assay measures the cellular metabolic activity which reflects the number of viable cells, and was used to provide information on cytotoxicity and the inhibition of cell proliferation or growth. This colorimetric assay is based on the reduction of a yellow tetrazolium salt (sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy6-nitro)benzene sulfonic acid hydrate or XTT) to an orange formazan dye by metabolically active cells. Cells were seeded in 96-well plates at approximately 10 x 103 cells per well in a final volume of 100 μL. The number of cells to be seeded was determined for each cell type and treatment protocol in order to keep the cells sub confluent and the color absorbance within the linear portion of the growth curve (0.1 to 1.0 OD). The number of cells seeded in control and corresponding treatment groups were identical. After overnight incubation, cells were treated with GC4419 (5, 10 and 20 μM), IR (2, 4 and 8 Gy), or combination, and maintained for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37°C. At the end of the treatment period, media was removed and 100 μL of phenol-free medium containing 250 μg XTT and 100 μM phenazine methosulfate was added to each well. Cells were then incubated at 37°C for 2 hr. The color absorbance at 450 nm was measured using a SpectraMax M5 Microplate Reader (Molecular Devices, CA, USA), and the background absorbance at 690 nm was subtracted. The percentage of viable cells was calculated as the absorbance of the treated group divided by the absorbance of the untreated group X 100. Results were expressed as % of untreated control.
Assessment of clonogenic survival.
Clonogenic assay was done, with minor modifications, according to Franken et al 2006 [30]. Exponentially growing cells were plated in 60 mm culture dishes at a density of 2500 per cm2, either treated or left untreated and incubated in a humidified atmosphere of 95% air: 5% CO2 at 37°C for 24 hr. Cells were trypsinized and trypsin was inactivated with growth media containing serum. Cells were centrifuged at 1200 rpm, suspended in fresh media, counted, replated at various densities, and allowed to grow undisturbed in complete media in a humidified atmosphere of 95% air: 5% CO2 at 37°C for 14 days. The initial cell density was determined depending on the plating efficiency (PE) of each of the cell lines. After fourteen days incubation, medium was removed, cells were washed with PBS, fixed with 70% cold ethanol for 5 min, air dried, and stained with 0.5% crystal violet. Each group of 50 cells or more was counted as a colony. PE was calculated as the number of colonies observed divided by the number of cells seeded. Colony survival fraction (SF) was calculated as the colonies counted divided by the number of cells plated with a correction for the plating efficiency (SF= (colonies counted)/[ number of cells seeded x (PE /100)].
DNA fragmentation assay.
Apoptotic cell proportion was evaluated using a Cell Death Detection ELISAPlus Kit (Roche Applied Science, Mannheim, Germany), according to the manufacturer’s instructions. Briefly, cells were seeded onto 100 mm plates. Following overnight incubation, cells were treated with GC4419 and/or IR for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37°C, cells were then trypsinized and harvested, total DNA was extracted and transferred to a streptavidin-coated plate. After a 2 hr incubation at room temperature with a mixture of peroxidase conjugated anti-DNA and biotin-labelled anti-histone, the plate was washed thoroughly and incubated with 2,2’-azino-di-[3-ethylbenzthiazolinesulfonate] diammonium salt. The absorbance was measured at 405 nm with a reference wavelength of 490 nm using a SpectraMax M5 Microplate Reader (Molecular Devices, CA, USA).
Caspase-3 activity assay.
Caspase-3 activity was measured using a Caspase-3/CPP32 Colorimetric Kit, according to the manufacturer’s instructions (Life Technologies Corp., MD, USA). In brief, cells in appropriate density were plated in 100 mm cell culture plates overnight, then treated with GC4419, IR, or combination and incubated for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37°C. After treatment, cells were trypsinized and centrifuged, cell pellets were collected and 50 μL lysis buffer was added. Total cellular proteins were estimated using Bio-Rad DC protein assay kit (Bio-Rad laboratories, CA). Samples were diluted to 100 μg total protein in 50 μL lysis buffer. 50 μL of 2x reaction buffer and 5 μL of 4 mM DEVD-pNA substrate were added to 50 μL sample, containing 100 μg total protein. Samples were incubated in the dark at 37°C for 2 hr. The absorbance at 400 nm was measured for different groups, and caspase-3 activity was calculated relative to untreated control.
Western blot analysis.
Cells were seeded in 100 mm cell culture plates and after 24 hr cells were treated with GC4419, IR, and combination and incubated for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37°C. Cells were then trypsinized and centrifuged, the cell pellets were washed with ice-cold PBS then cells were lysed in RIPA buffer containing phosphatase and protease inhibitor cocktail. Cells were then subjected to sonication for 3 cycles of 5 seconds each, on ice over 30 minutes and centrifuged using an Eppendorf centrifuge 5417R (Hermle Labortechnik, Germany) at 14000 rpm for 15 minutes at 4 °C. Supernatant was collected and protein concentration was determined using Bio-Rad DC protein assay kit. The cell homogenates were loaded onto 4-20 gradient SDS-PAGE gels and separated electrophoretically. Proteins were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 1 hr at 37°C with Tris-buffered saline and Tween (TBST) containing 5% nonfat dried milk, and then were incubated with the following primary antibodies: mouse and rabbit monoclonal anti-β-actin 1:5000 (3700s, 8457s, Cell Signaling, USA), rabbit polyclonal anti-Bax 1:1000 (2772s, Cell Signaling, USA) and mouse monoclonal anti-Bcl-2 1:500 (SC-23960, Santa Cruz, USA) overnight at 4°C. Primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies anti-mouse IgG 1:5000 and anti-rabbit IgG 1:5000 (Cell Signaling, USA), and visualized using the enhanced chemiluminescence method. The band density was quantified using ImageJ software.
Thioredoxin Reductase (TrxR) activity.
TrxR activity was measured using a Thioredoxin Reductase Assay Kit (Abcam, Cambridge, England). Briefly, cells in appropriate density were plated in 100 mm cell culture plates. After overnight incubation, cells were treated with GC4419, IR, and combination and incubated in a humidified atmosphere of 95% air: 5% CO2 at 37°C. 24 hr post treatment, ~ 2 x 106 cells were harvested, and homogenized in 100 - 200 μL cold assay buffer on ice. The homogenate was centrifuged at 10,000 x g for 15 min at 4°C and the supernatant collected for assay and stored on ice till assay. Total protein was estimated using a Bio-Rad DC protein assay kit (Bio-Rad laboratories, CA). For the TrxR activity assay, 2-50 μL of sample was added to each well of 96 well plates, and 2 sets of matched samples were tested, with or without TrxR inhibitor. TrxR inhibitor was added to one set of samples for testing background enzyme activity, and the other set of samples without TrxR inhibitor for testing total reduction of 5, 5’-dithiobis (2-nitrobenzoic) acid (DTNB). The reaction mixture was added to each well including DTNB and NADPH and the absorbance was kinetically measured for 20 min at 412 nm using a SpectraMax M5 Microplate Reader (Molecular Devices, CA, USA). The activity was calculated according to the manufacturer’s instructions.
Statistical analysis.
Results are expressed as the mean ± standard error. Statistical differences among groups were evaluated by ANOVA using SigmaPlot. P-values < 0.05 were considered to be statistically significant.
Results
Measurement of the potency of GC4419 in dismutating superoxide.
Experiments were performed to evaluate the efficacy of GC4419 in the dismutation of O2•−. Spin trapping of O2•− generated from the xanthine/XO system, with catalase present to scavenge H2O2, was performed using the spin trap DMPO. While in the absence of GC4419 a prominent signal of DMPO-OOH was seen from trapped O2•−, in the presence of 2, 5, 10 and 20 μM GC4419 this signal was quenched by 66%, 74%, 82%, or 95%, respectively (Fig. 3). Thus, GC4419 was shown to be an effective SOD, quenching the O2•− detected by > 80% at and above 10 μM concentrations.
Fig. 3: EPR spin trapping of superoxide (O2•−) by DMPO with increasing [GC4419].
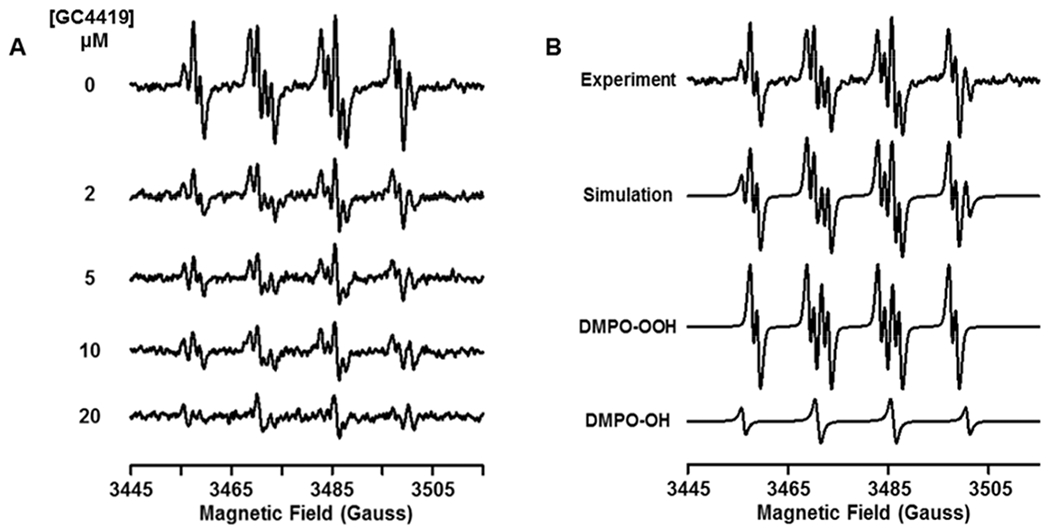
O2•− was generated by the xanthine oxidase/xanthine system in the presence of 50 mM DMPO with the GC4419 concentrations noted of 0, 2, 5, 10 and 20 μM (A). A strong DMPO-OOH signal is seen that is quenched with increasing [GC4419]. A small signal of DMPO-OH is also present. Computer simulation of the experimental spectrum with 0 μM GC4419 as a combination of DMPO-OOH and DMPO-OH adducts (B). The following parameters were determined from simulation of the experimental EPR spectrum: DMPO-OOH: aN = 14.15 G, aHβ = 11.31 G and aHγ = 1.24 G, weight: 76%; DMPO-OH: aN = 15.0 G, aH = 14.5 G, weight: 24%. EPR system scan parameters are: microwave power = 20 mW, modulation amplitude = 0.50 G, sweep width = 70 G, receiver gain = 1 x 105, time constant = 81.92 ms, scan time 21 s with 5 scans coadded.
Measurement and characterization of IR-induced free radical generation in the presence and absence of GC4419.
Experiments were performed to assess the effects of GC4419 on the process of IR-induced free radical generation and if there may be any quenching of this process. Six well plates with 1 mL of PBS alone, or PBS and cells, were subjected to 8 Gy IR in the presence of 50 mM of the spin trap DMPO, and 0, 5, 10 or 20 μM of GC4419. The spin trap containing buffer was then transferred to an EPR flat cell for measurement. IR generated a prominent 1:2:2:1 quartet signal of DMPO-OH and a much weaker signal with 5 distinct peaks seen of DMPO-H (Fig. 4). As the DMPO-OH signal can either be derived directly from trapping of ·OH or by the decay of the DMPO-OOH adduct formed from the trapping of O2•−, additional experiments were performed in the presence of 5% ethanol, that can distinguish ·OH through the formation of the DMPO-CH3C·HOH adduct. As shown in Figure 5, in the presence of ethanol, the DMPO-OH signal largely decreased with formation of the CH3C·HOH adduct. In the presence of GC4419 at 10 μM concentration, there was no discernible change in the observed EPR signals either in the presence or absence of ethanol. In addition, at doses from 5 μM to 20 μM GC4419, no significant change in radical generation was seen (data not shown). Thus, GC4419 did not significantly quench the IR-produced radical generation as detected by spin trapping.
Fig. 4: EPR spin-trapping of radical generation with x-ray irradiation.
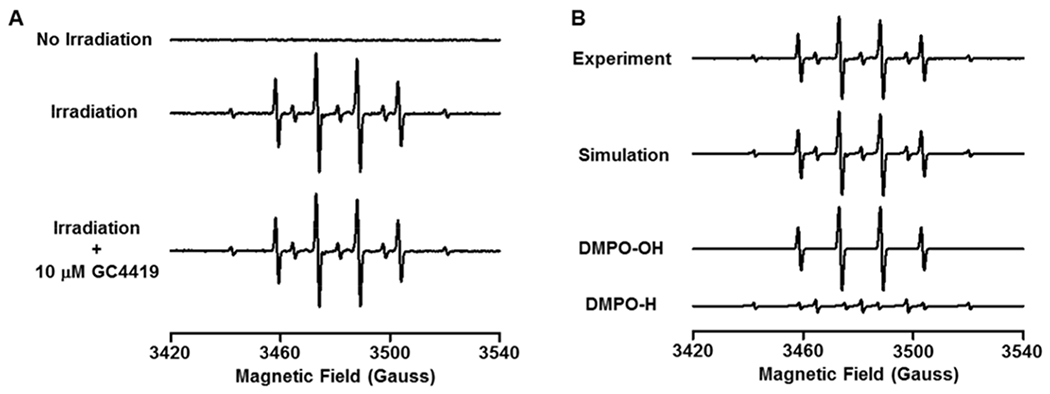
Spin-trapping was performed using 50 mM DMPO in PBS, pH 7.4, with and without 10 μM GC4419. A: No signal was detected without IR, but with 8 Gy a large 1:2:2:1 quartet DMPO-OH adduct signal is seen with a much smaller signal due to a DMPO-H adduct. GC4419 had no effect on the signal, confirming that it was from ·OH and ·H adducts, not superoxide. B: shows computer simulation of the experimental spectrum exhibiting good fit with a mixture of DMPO-OH and DMPO-H adducts. The following parameters were determined by EPR spectral simulation: DMPO-OH: aN ~ 14.96 G, aH ~ 14.79 G, Weight: 75%; DMPO-H: aN ~ 16.5 G, aH(2) ~ 22.5 G, Weight: 25%. EPR acquisition parameters were: microwave power = 20 mW, modulation amplitude = 0.50 G, sweep width = 120 G, receiver gain = 1 x 105, time constant = 82 ms and 10 scans of 42 s coadded.
Fig. 5: EPR Spin-trapping of radical generation with x-ray irradiation in the presence of 5% ethanol.
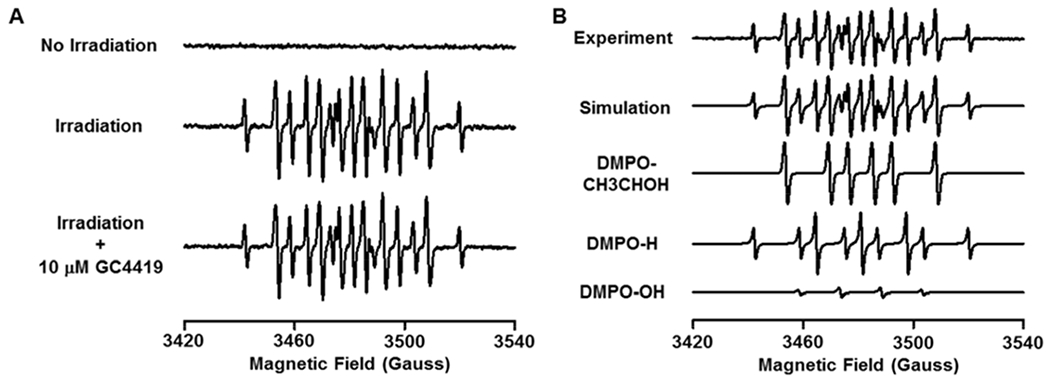
Spin trapping was performed with 50 mM DMPO and 5% ethanol in PBS buffer, pH 7.4 with and without 10 μM GC4419 added. A: No signal was detected without IR, but with 8 Gy, a large signal that is a combination of three spin adducts is seen. GC4419 treatment had no effect on the signal, confirming that it was not generated by superoxide. B: Computer simulation of the experimental spectrum showing good fit with a mixture of DMPO-CH3C·HOH, DMPO-H and DMPO-OH adducts. The following parameters were determined by EPR spectral simulation: DMPO-CH3C·HOH: aN ~ 15.91 G, aH ~ 22.98 G, Weight: 52 %; DMPO-H: aN ~ 16.48 G, aH(2) ~ 22.49 G, Weight: 39%; DMPO-OH: aN ~ 15.07 G, aH ~ 14.57 G, Weight: 9%. EPR acquisition parameters were as in Fig. 4.
This radical generation was shown to consist largely of ·OH and ·H formed from the splitting of H2O. No DMPO-OOH adduct was detected immediately following IR, and even with nitrone spin traps such as 5-diethoxyphosphoryl-5-methyl-1-pyrroline-n-oxide (DEPMPO) or 5-diisopropoxyphosphoryl-5-methyl-1-pyrroline N-oxide (DIPPMPO), that form more stable −OOH adducts [31–33], no such adduct was detected (data not shown). Since ·H was trapped by the nitrone spin trap, this would prevent secondary formation of O2•− [34]. The process of IR-induced radical generation was similar with IR of buffer alone or buffer in the presence of cells. Thus, these measurements demonstrated that IR generated primarily ·OH, with minimal trapping of O2•−, and that GC4419 does not alter the IR-induced ·OH radical generation required for tumor cell killing.
Effects of IR and GC4419 on superoxide generation in A549 and H1299 cancer cell lines and Beas 2b normal immortalized cells.
For initial measurements to detect the O2•− generation in normal immortalized and cancer lung cells after treatment with IR and/or GC4419 with cellular microscopy, the probe DHE, which reacts with O2•− to form the fluorescent product ethidium, was used [35]. Both A549 and H1299 cancer cell lines exhibited basal fluorescence intensity that was largely quenched by the SOD mimetic GC4419, while the levels in normal immortalized Beas 2b cells were much lower and nearly totally quenched by GC4419 (Fig. 6). With IR treatment of A549 and H1299 cells following 2 hours, the fluorescence intensity was seen to increase by ~ 2-fold. In the Beas 2b cells following IR, there was also a clear increase in DHE-derived fluorescence, but this remained about 2.5-fold below the values seen in the cancer cell lines. In both basal and post IR cells, the treatment with 10 μM GC4419 largely quenched the observed fluorescence, confirming that this fluorescence was O2•−-derived.
Fig. 6: Detection of superoxide with using the fluorescence probe DHE.
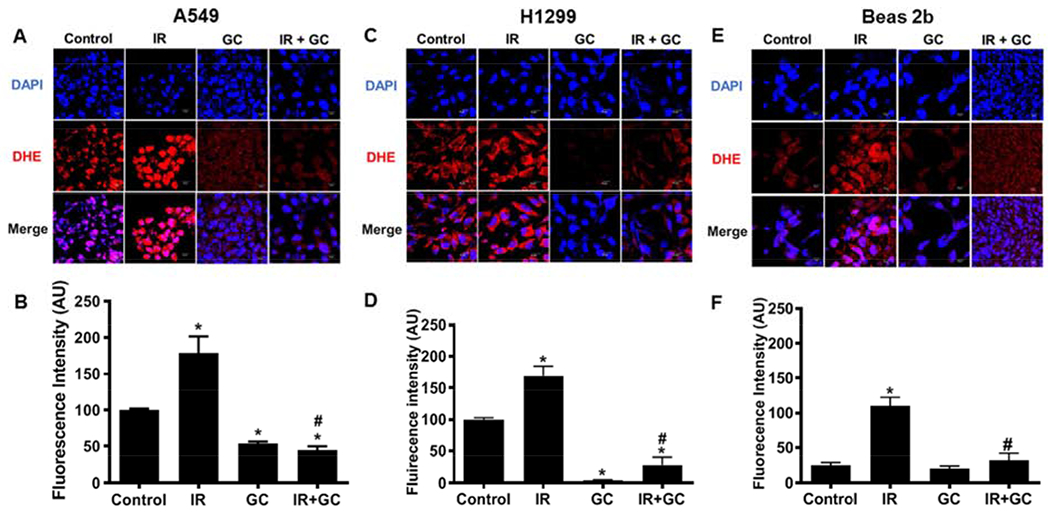
A549, H1299 and Beas 2b cells grown on a coverslip were either left untreated or treated with GC4419 (GC;10 μM) alone, IR (8Gy) alone or in combination, then maintained in a humidified atmosphere of 95% air: 5% CO2 at 37 0C. At 120 minutes post IR, the drug was washed out and attached cells were incubated for 30 minutes with DHE that reacts with superoxide giving rise to red fluorescence. Cells were imaged (A, C, E) by confocal microscopy. The quantitation of the images are shown in (B, D, F). GC4419 largely quenched the observed fluorescence confirming that it was superoxide-derived. The results of triplicate measurements are shown with mean ± SEM. * significant from control (p<0.001), # significant from IR (p< 0.001).
EPR spin trapping of basal and IR-induced radical generation in cancer cells and normal cells and the effects of GC4419.
In order to more specifically measure the O2•− generation that occurs in these cell lines before and following IR, EPR spin trapping was performed (Fig. 7). In the presence of DMPO, at baseline the normal immortalized A549 cells gave rise to a clear basal signal with peak ratio of 1:2:2:1 indicative of DMPO-OH and this was largely abolished after treatment with the SOD mimetic GC4419 (10 μM), demonstrating that the observed signal was derived from trapping of O2•−. Following treatment of A549 cells with 8Gy IR, there was a two-fold increase in the observed DMPO-OH signal compared to the untreated cells (Fig. 7 A, B). Again, treatment of the A549 cells with GC4419 before IR largely abolished the observed spin trap signal. These results indicate that following IR, O2•− generation is increased in these cancer cells and that GC4419 quenches this radical generation. In the Beas 2b cells, only a small DMPO-OH signal was seen, detectable just above the noise level, and following IR this signal increased over 2-fold, but remained ~2-fold lower than in similarly irradiated cancer cells (Fig. 7 A). Both the basal signal and the larger signal following irradiation were nearly totally quenched by GC4419 (10 μM), confirming the efficacy of this mimetic in quenching O2•− levels and their trapping in these cells (Fig. 7 B).
Fig. 7: EPR spin trapping of free radical generation in A549 lung cancer or Beas 2b normal immortalized cells and effects of IR and the SOD mimetic GC4419.
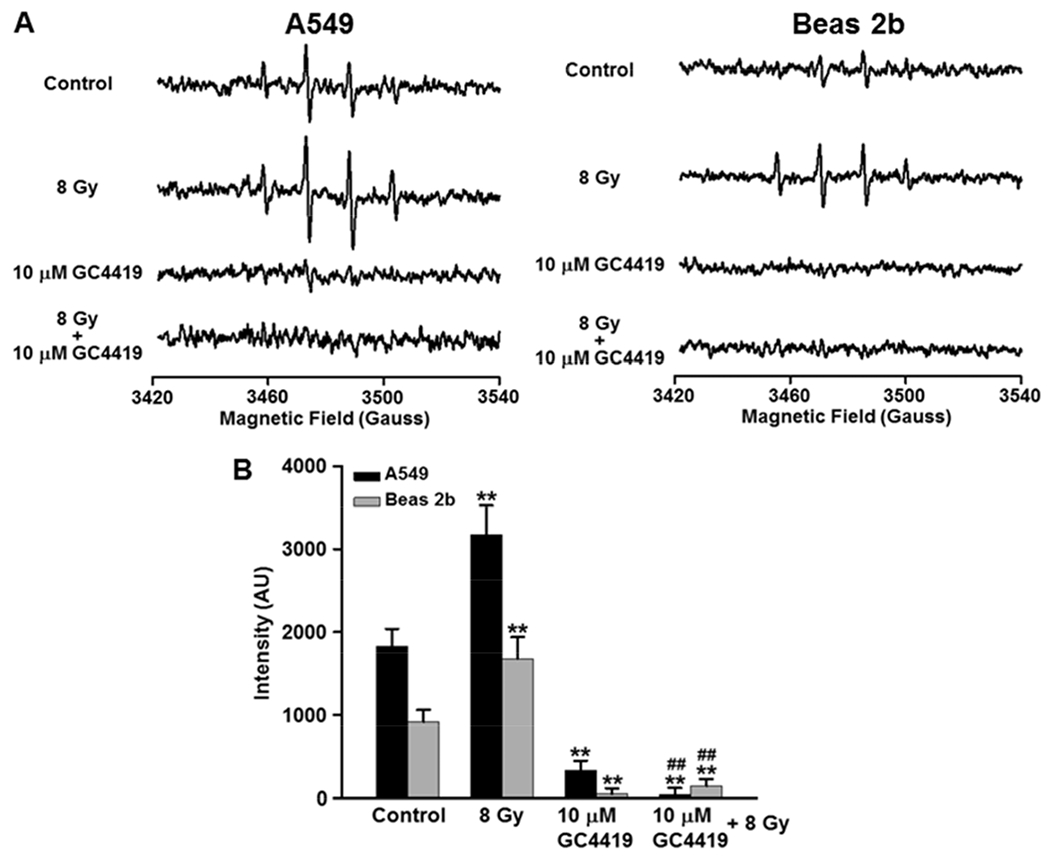
A: EPR spectra measured from A549 and Beas 2b cells either left untreated (Control), treated with GC4419 (10 μM), 8Gy IR, or combination, then maintained in a humidified atmosphere of 95% air: 5% CO2 at 37 0C. After ~60 min incubation drug was washed out and cells were trypsinized, resuspended in the presence of the spin trap DMPO and EPR measurements performed. B: Bar graph of the signal quantitation from a series of repeat measurements performed in both cell lines. The results are expressed as the mean ± SEM. ** significant from control (p < 0.01), ## significant from 8 Gy (p < 0.01).
GC4419 increases the cell growth inhibitory effect of IR in cancer cell lines and protects normal immortalized cells.
Initial studies were performed to evaluate the dose-dependent effects of IR or GC4419 concentration on the growth of A549, H1299 and Beas 2b cells. Cells were exposed to different IR doses (2, 4, and 8 Gy) and different concentrations of GC4419 (5, 10, and 20 μM) with the number of viable cells measured after 48 hr by XTT assay (Fig. 8 A, B). In A549 and H1299 cancer cells, the number of viable cells following IR and GC4419 decreased in a dose-dependent manner compared to that in the matched untreated controls.
Fig. 8: Dose-dependent effect of IR or GC4419 on the inhibition of cell growth of lung cancer (A549 and H1299) or normal immortalized cells (Beas 2b).
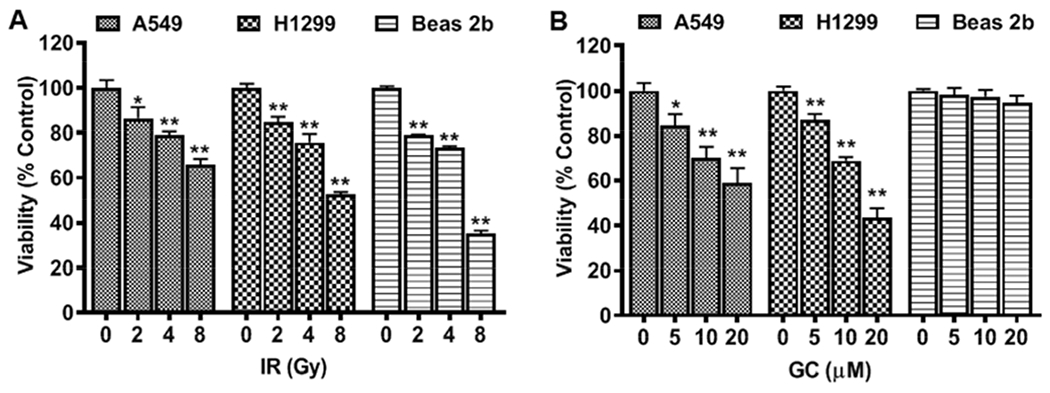
Cells were either untreated or treated with IR (A) or GC4419 (GC; B), then maintained for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37 0C, drug was washed out, and XTT assay performed to measure the cell growth inhibition. The results shown are depicted as percent of viable cells compared to matched untreated control with 0 Gy IR and 0 μM GC. Values shown are the mean ± SEM of four experiments. * significant from control (p < 0.05), ** (p < 0.001).
On the other hand, GC4419 did not affect the cell growth of Beas 2b cells. IR decreased cell growth of all three cell lines at all doses studied. As seen with the 8 Gy dose, the two cancer cell lines were more resistant to high dose IR, with higher survival levels seen in the A549 and H1299 cells than in the Beas 2b cells.
In these measurements, IR was seen to dose-dependently decrease the number of viable cells of A549 cells with lower viability percent relative to untreated cells of 85 ± 5 %, 79 ± 2 % and 66 ± 3 % after 2, 4 and 8 Gy treatment, respectively (Fig. 8 A). A similar effect was seen after treatment of H1299 cells with 2, 4 and 8 Gy with viability percent decreased to 85 ± 2 %, 76 ± 3.8% and 53 ± 1 %, respectively, compared to untreated cells. In addition, treatment of Beas 2b with 2, 4 and 8 Gy reduced values to 79 ± 1 %, 74 ± 1% and 35 ± 2%, respectively, compared to untreated cells. GC4419, at a 5 μM concentration, decreased the number of viable cells of both cancer cell lines with viability values of 84 ± 5% and 87 ± 3% in A549 and H1299 cells, respectively (Fig. 8 B). With higher GC4419 concentration of 10 μM with A549 and H1299 cells, further decreases were seen with values of 70 ± 5% and 69 ± 2%, respectively. With 20 μM GC4419, the viability values decreased even further to 59 ± 7% and 43 ± 4%, respectively. In contrast, treatment of the Beas 2b cells with 5, 10 or 20 μM GC4419 did not significantly affect cell growth with viability values of 98 ± 3%, 97 ± 3 % and 95 ± 4 %, respectively.
In order to assess the possible synergy of GC4419 with IR in cancer treatment, low dose (2Gy) or high dose (8Gy) IR was performed in the presence of varying concentrations of GC4419 (0, 5, 10, 20 μM), with viability percent measured 48 hr post IR (Fig. 9). With IR in the presence of the GC4419, a dose-dependent increase in IR-induced cytotoxicity was observed in both A549 and H1299 cells compared to cells treated with IR or GC4419 alone. With 2 Gy of IR alone, 20 μM GC4419 decreased the viability percent of A549 cells from 87 ± 5 % to 65 ±1 % (*p < 0.001) (Fig. 9 A). Similarly, for the H1299 cells, a decrease from 85 ± 2% to 64 ± 1% was seen (*p < 0.001). With 8 Gy, 20 μM GC4419 decreased the viability percent of A549 cells from 66 ± 3 % to 34 ± 4 % (*p < 0.001) and for the H1299 cells from 53 ± 1 % to 42 ± 1 % (*p < 0.001) (Fig. 9 B).
Fig. 9: Dose-dependent effect of GC4419 on IR-induced cell growth inhibition in A549, H1299 and Beas 2b cells.
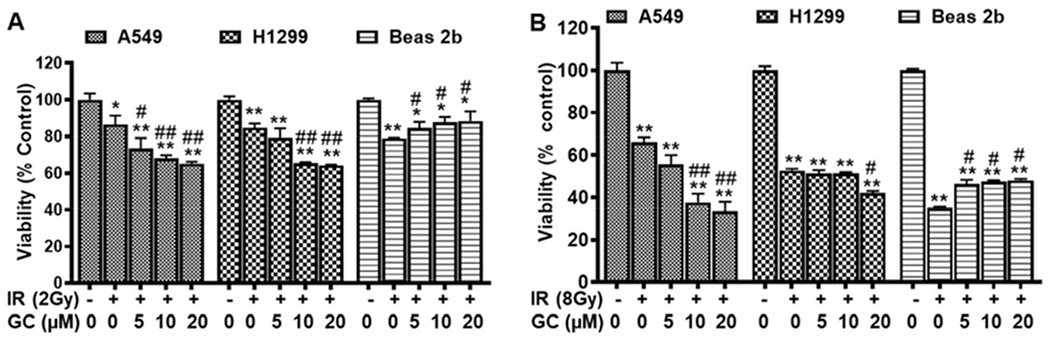
Cells were either left untreated or treated with IR (2 Gy in A or 8 Gy in B) alone or in combination with GC4419 (GC; 5, 10, 20 μM), then maintained in a humidified atmosphere of 95% air: 5% CO2 at 37 0C for 48 hr. The decline in cell viability is shown, with IR doses. Cell growth inhibition was determined by the XTT assay. Results are expressed as the mean ± SEM of four experiments. * significant from control (p < 0.05), ** (p < 0.001), # significant from IR (2 Gy or 8 Gy) (p < 0.05), ## (p < 0.001).
In contrast to this, GC4419 conferred dose-dependent protection on the Beas 2b cells. At 20 μM concentration, GC4419 decreased the cytotoxic effect of IR on Beas 2b cells with an increase in viability percent at the 2 Gy dose from 79 ± 1% to 88 ± 5 % and from 35 ± 1 % to 48 ± 1 % at the 8Gy dose (*p < 0.001) (Fig. 9 A, B). Thus, GC4419 selectively increased the IR-induced cell killing of both cancer cell lines, while protecting the normal cells. Both at lower dose and high dose, combination treatment with GC4419 rendered the cancer cell lines more susceptible to IR-induced injury.
In order to further characterize the effects of GC4419 on longer-term cancer cell survival and growth, clonogenic assays were performed at the 2 Gy dose with colony formation measured after 14 days. As shown in Figure 10, we observed that IR decreased colony number and survival fraction in all three cell lines, both cancer and normal cells, with clonogenic survival fractions of 53 ± 3 % and 58 ± 3 % in A549 and H1299 lung cancer cells, respectively, and 73 ± 2 % (*p < 0.001) in Beas 2b cells. GC4419 alone had only a modest effect on the A549 cells with a survival fraction of 82 ± 6 %, while a larger effect was seen in the H1299 cells with a survival fraction of 50 ± 2%. In both of these cancer cells lines, the combination of IR and GC4419 treatment decreased survival fraction more than IR alone. In contrast, GC4419 protected the normal cells, with preserved colony numbers compared to IR alone, with an increase in survival fraction from 73 ± 2 % to 90 ± 2 %. The PE and SF for A549, H1299, and Beas 2b cells in the absence of treatment were calculated as described under “Materials and Methods”. PE was found to be 53 %, 30 %, and 39 %, for A549, H1299, and Beas 2b cells, respectively. SF for untreated A549, H1299, and Beas 2b cells was calculated in relation to PE and found to be 97.3%, 91.1%, and 95.4%, respectively. Collectively, these clonogenic assays showed that GC4419 enhanced the anti-cancer activity of IR with protection of normal cells.
Fig. 10: Effect of IR, GC4419 or combination on colony formation of cancer and normal immortalized lung cells.
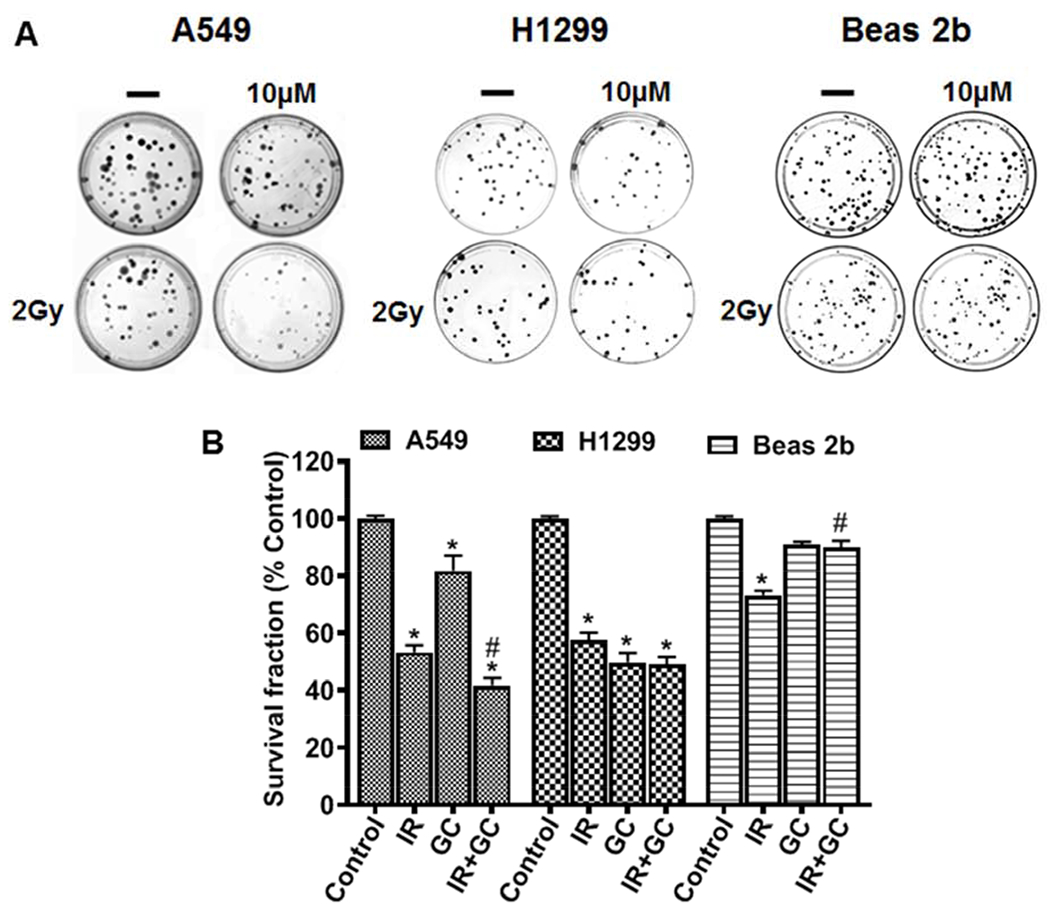
Cells were either untreated or treated with 2 Gy (IR), or GC4419 (GC; 10 μM), or combination. Following 24 hr of treatment, cells were trypsinized, re-plated, maintained undisturbed in a humidified atmosphere of 95% air: 5% CO2 at 37 0C for 14 days, and the clonogenic assay was performed. Cells were stained and imaged (A). Colonies were counted and the survival fraction was calculated and expressed as % of untreated control (B). *significant from control (p<0.05), #Significant from IR (p<0.05).
GC4419 increases the pro-apoptotic activity of IR in A549 and H1299 cancer cell lines.
To investigate how GC4419 increases IR-induced lung cancer cell death, the pro-apoptotic effects of GC4419 and /or IR were investigated by assaying DNA fragmentation. Treatment of A549 cells with 2 Gy induced a 40% increase in DNA fragmentation to 1.4 -fold of the levels in untreated cells, while treatment with 2 Gy and 10 μM GC4419 increased DNA fragmentation to 1.8 – fold of the levels in untreated cells) (*p < 0.001) (Fig. 11 A).
Fig. 11: Apoptotic effects of IR, GC4419, and combination in lung cancer and normal immortalized cells.
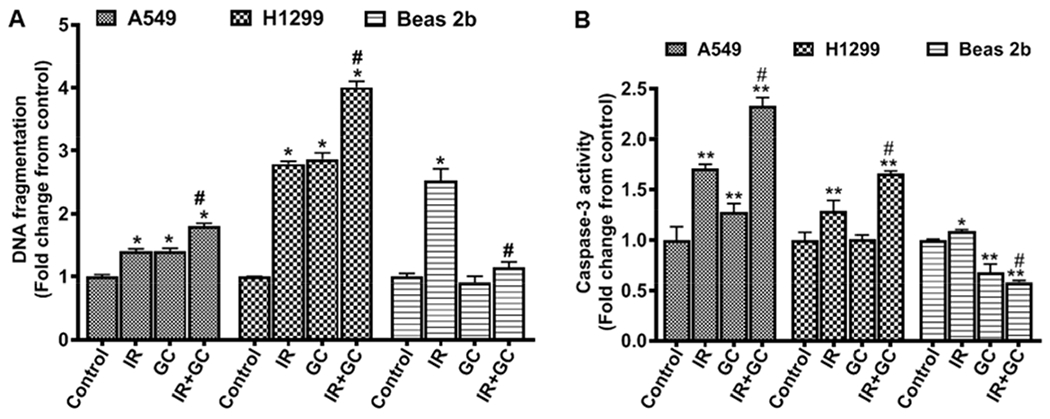
Lung cancer cells A549 and H1299 or normal immortalized cells Beas 2b were either left untreated or treated with 2 Gy (IR), 10 μM GC4419 (GC;), or combination then maintained for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37 0C . Cells were washed, trypsinized, and DNA fragmentation (A) and Caspase-3 activity (B) were performed. Results are presented as mean ± SEM of three independent experiments. *significant from control (p < 0.05), ** significant from control (p<0.001), # significant from IR (p<0.001).
Treatment of H1299 cells with 2 Gy induced an even more marked increase to 2.8-fold the levels in untreated control cells, while with GC4419 a similar 2.9-fold increase was seen, and with the combination of GC4419 and 2 Gy a marked 4-fold increase in DNA fragmentation compared to untreated controls was seen (*p < 0.001) (Fig. 11 A). In contrast to the GC4419-induced cancer cell cytotoxicity, treatment of the Beas 2b lung cells with IR combined with 10 μM GC4419 decreased DNA fragmentation from 2.5 -fold to 1.1 - fold of the levels in untreated control cells (*p < 0.001) (Fig. 11 A). Thus, while co-treatment with GC4419 sensitized the cancer cells, it protected the normal cells from IR-induced DNA fragmentation.
Activation of the caspase cascade plays an important role in apoptosis. The processes of caspase activation are preceded by release of cytochrome c from the mitochondrial inter-membrane space into the cytosol. Since GC4419 increased the cytotoxicity and DNA cleavage with IR of A549 and H1299 lung cancer cells, we further investigated whether caspase-3 activation also occurred. 2 Gy IR induced an increase in caspase-3 activity in A549 and H1299 lung cancer cells of 71 ± 4% and 29 ± 1%, respectively, compared to untreated controls (*p < 0.001). Co-treatment with GC4419 (10 μM) and IR increased the caspase-3 activity by 133 ± 8% and 66 ± 2% in both A549 and H1299 cells, respectively (*p < 0.001), compared to untreated control cells (Fig. 11 B). In contrast to the increase seen in the cancer cell lines, in the Beas 2b cells, caspase-3 activity was decreased to 58 ± 2 % of the levels in untreated control cells after co-treatment with GC4419 and IR (*p < 0.001). Furthermore, GC4419 alone decreased the caspase activity of these cells to 67 ± 8% of the levels in untreated control cells (*p < 0.001) (Fig. 11 B).
The Bcl-2 family proteins are critical regulators of the mitochondrial outer membrane permeabilization that is associated with apoptosis. Bcl-2 proteins include pro-apoptotic Bax or anti-apoptotic Bcl-2 [36, 37]. To further investigate the effect of GC4419 and/or IR on apoptosis, we examined their effects on the expression of the pro-apoptotic and anti-apoptotic proteins, Bax and Bcl-2 (Fig. 12). We found that 2 Gy IR increased expression of Bax by 63 ± 7% and 58 ± 10% in A549 and H1299 cells, respectively (*p < 0.001) (Fig. 12 B). In addition, 2 Gy IR also increased Bax expression of Beas 2b by 51 ± 7% (*p < 0.001). GC4419 (10 μM) treatment alone also increased the expression of Bax by 68 ± 5% and 50 ± 12% in A549 and H1299 cells, respectively, compared to untreated controls (*p < 0.001). In contrast, the expression of Bax after treatment of the Beas 2b cells with GC4419 was significantly decreased to 31 ± 4% of the levels in untreated control cells (*p < 0.001). GC4419 in combination with IR further increased the expression of Bax in both cancer cell lines with 120 ± 10% and 88 ± 6% increase, respectively, in A549 and H1299 cells compared to untreated cells (p < 0.001). In contrast, combination treatment of Beas 2b cells decreased Bax expression by ~ 20% compared to IR treatment alone (p < 0.05). For the anti-apoptotic protein Bcl-2 after treatment with IR, the expression was decreased to 64 ± 2 %, 51 ± 3 % and 55 ± 2 % of untreated control values for A549, H1299 and Beas 2b, respectively (Fig. 12 C). GC4419 decreased the expression of Bcl-2 in A549 and H1299 cells to 58 ± 3 % and 76 ± 6 %, respectively, of values in untreated controls (*p < 0.001). The Beas 2b cells showed no significant change of Bcl-2 expression after treatment with GC4419. With combination of IR with GC4419, Bcl-2 expression was decreased with values of 56 ± 2 % and 53 ± 2 % of control levels, respectively, in A549 and H1299 cells (*p < 0.001), but not significantly different from the levels with 2 Gy IR. In contrast, this combination treatment increased the expression of Bcl-2 in the Beas 2b cells to 1.13 ± 0.15 of the levels in untreated controls, an increase of ~ 58% from the level with 2 Gy IR. From this data, the Bax/Bcl-2 ratios were seen to greatly increase with treatment (2 Gy IR, GC4419, or in combination) in the cancer cell lines. In the normal cells, the ratio increased with 2Gy IR, but decreased in the GC4419 (10 μM) treatment (Fig. 12 D). No statistically significant change in the Bax/Bcl-2 ratio was present with the combination treatment in the Beas 2b cells relative to control, but there was a significant decrease relative to 2 Gy IR alone. Overall, these data indicate that combination treatment exerts its cytotoxicity through induction of apoptosis in cancer cells with protection of normal cells from IR-induced apoptosis.
Fig. 12: Western blot detection of pro- and anti-apoptotic markers.
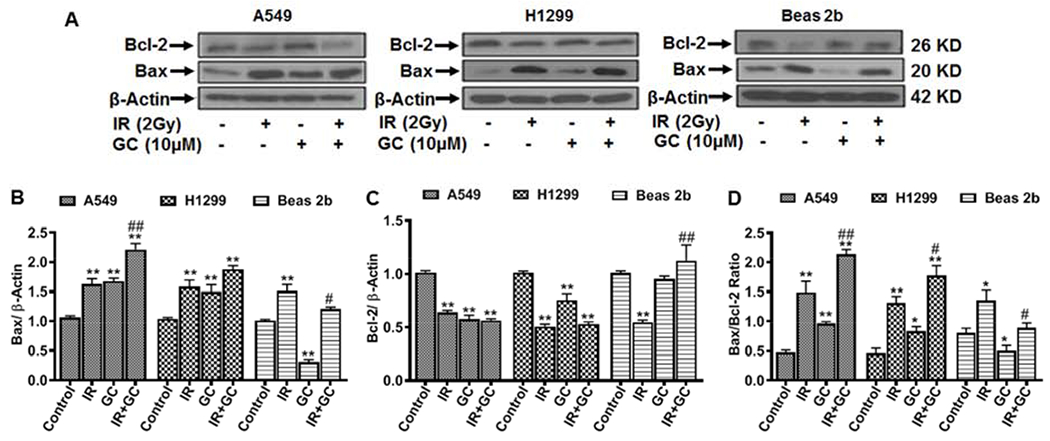
Cells were either left untreated, treated with 10 μM GC4419 (GC), 2 Gy (IR), or combination, then maintained for 48 hr in a humidified atmosphere of 95% air: 5% CO2 at 37 0C. Cells were washed, trypsinized, and expression of Bax and Bcl-2 was measured in cell lysate. A: western blots of Bax and Bcl-2. Bax (B), Bcl-2 (C), and Bax/Bcl-2 ratio (D) as measured from the band density of the corresponding blot in (A). Results shown as mean ± SEM from three independent experiments. * significant from control (p < 0.05), ** significant from control (p<0.001), # significant from IR (p<0.05), ## significant from IR (p< 0.001).
Effects of GC4419 on thioredoxin reductase activity in cancer cell lines and normal immortalized cells.
TrxR plays a major role in the regulation of the cellular redox state [38]. Higher expression and activity of TrxR is correlated with prevention of apoptosis, acceleration of tumor growth and resistance of cancer cells to chemotherapy. IR significantly increased the activity of TrxR in A549 and H1299 cells by 16 ± 4% and 18 ± 5%, respectively, compared to untreated cells (*p < 0.05) (Fig. 13). In contrast, IR of Beas 2b cells decreased TrxR activity to 41 ± 15% of that in untreated cells (*p < 0.001) (Fig. 13). GC4419 (10 μM) increased the TrxR activity by 40 ± 4%, 31 ± 7% and 99.5 ± 3.6% in A549, H1299 and Beas 2b, respectively, compared to that in untreated cells. Co-treatment of the A549 cells with GC4419 and IR reversed the increase in TrxR activity with IR alone to 75 ± 4 % of values in untreated, while in H1299 cells the activity declined to only 24 ± 3 % above untreated control levels. In contrast, in Beas 2b cells with GC4419 co-treatment, over 4-fold increase to 229 ± 25 % of control values was seen compared to IR alone. These data suggest that GC4419 may increase the radiation protection of normal cells compared to cancer cells through an increase in TrxR activity.
Fig. 13: Effects of GC4419, IR, and combination on TrxR activity in lung cancer and normal immortalized cells.
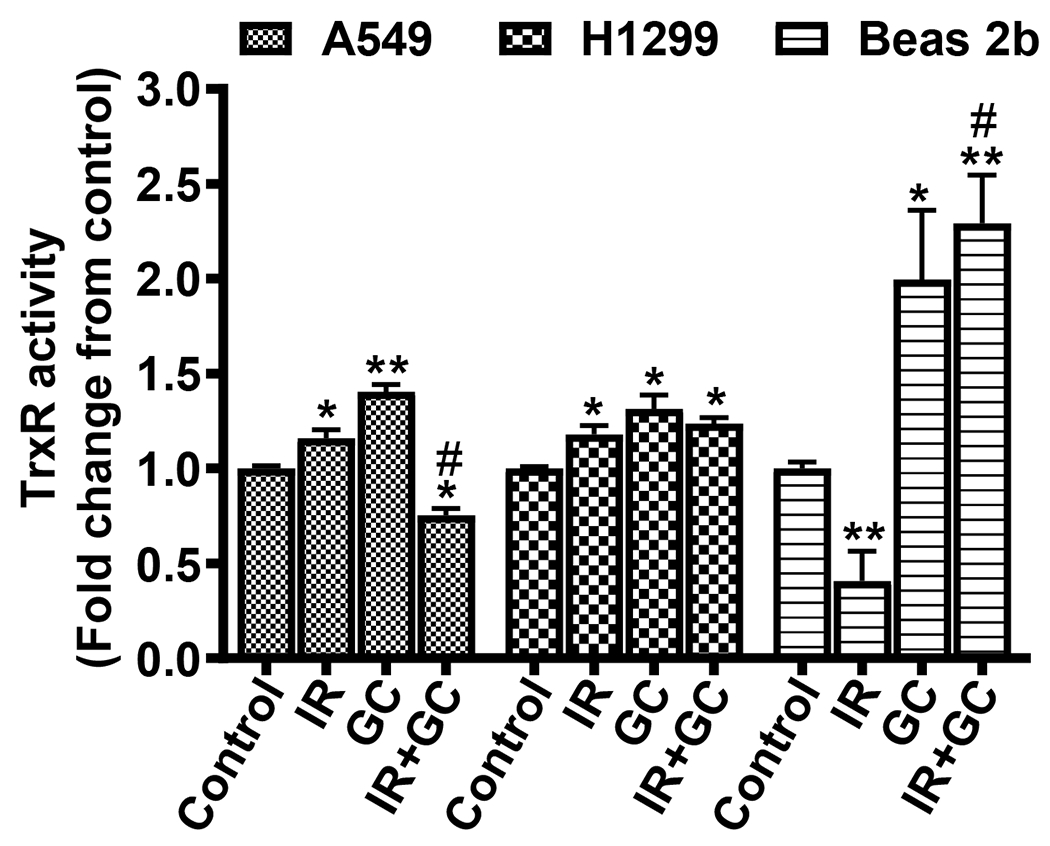
Cells were either left untreated, treated with 10 μM GC4419 (GC), or 2 Gy (IR), and combination then maintained for 24 hr in a humidified atmosphere of 95% air: 5% CO2 at 37 0C. Cells were washed, trypsinized and the enzyme activity was measured. The results are expressed as the mean ± SEM from three independent experiments. * significant from control (p < 0.05), ** significant from control (p<0.001), # significant from IR (p<0.001).
Discussion
Ionizing radiation is an important treatment of non-small cell lung carcinoma. Although IR techniques have been improved, prognosis for IR therapy in NSCLC remains extremely poor and is limited by normal tissue tolerance and inherent tumor radio-resistance, tumor invasion, metastasis and relapse. Thus, there remains a need to identify ways to enhance the lethality of IR to cancer cells while protecting the surrounding normal tissue. Novel therapeutic agents that promote the radiosensitivity of cancer cells while protecting normal cells would be of great value in cancer radiotherapy. The new high potency SOD mimetic GC4419 that has high cell permeability, high stability and high selectivity towards O2•− has been proposed as such an agent that can serve to enhance IR-induced tumor killing while protecting normal surrounding tissues [21, 22]. GC4419 is of particular interest as it has been shown to be well tolerated for human clinical use with combination therapy. In cancer radiotherapy, GC4419 has been shown to have great promise for the reduction of IR-induced side effects. Reduction of oral mucositis incidence and severity was observed in phase IIb clinical trials, and a phase III trial is now underway [23, 24]. Therefore it is of great importance to characterize the differential effects of GC4419 on cancer cells and normal cells as well as its effects in selective sensitization of cancer cells to IR and the underlying mechanisms of this process.
In our studies we utilized three cell lines, including p53 positive (A549) and p53 null (H1299) lung cancer cells, and normal immortalized lung cells (Beas 2b). A549 cells are an adenocarcinomic human alveolar basal epithelial cell line with wild type p53, while H1299 cells are a human non-small cell lung carcinoma cell line derived from lymph node metastasis of the lung with a homozygous partial deletion of the p53 protein (p53 null). The lung epithelial line Beas 2b was isolated from normal human bronchial epithelium. Overall we observed similar sensitization to IR by GC4419 in both cancer cell lines, while in the normal cells protection was seen.
It has been suggested that manganese SOD (MnSOD) can function as a tumor suppressor. Many types of cultured tumor cells have low MnSOD activity compared to their normal counterparts [39–41]. Mutations in the MnSOD gene and its regulatory sequence have been reported in several types of human cancer [42, 43]. It has been shown that overexpression of MnSOD reduces tumorigenicity and metastatic ability in a large number of experimental tumors in vitro and in vivo [44–46]. In addition, it has been reported that overexpression of MnSOD increases cytotoxic activity of IR against squamous cell carcinomas with subsequent protection of normal tissue [47–50]. MnSOD plasmid/liposome pulmonary gene therapy prior to radiation protects normal lung and esophagus from IR-induced damage in mice [51–55] and increased mouse survival [50]. Previous studies have also reported that a manganese based SOD mimetic (MnTnHex-2PyP) synergizes IR cytotoxic effect [56] and protects normal tissues from IR-mediated side effects [57–63]. Thus, we propose that the new high potency SOD mimetic GC4419 could enhance IR-induced cancer cell killing while protecting normal cells.
O2•− production has been reported to be increased in cancer cells and has been proposed to be associated with and have a key role in the enhanced uncontrolled mitosis that occurs in cancer cells [64, 65]. Enhanced dismutation of O2•− with its scavenging and conversion to hydrogen peroxide (H2O2) could thus impair the process of mitosis in cancer cells. Furthermore, the H2O2 produced could be cytotoxic to the tumor cells, as it has also been reported that cancer cells have increased susceptibly to H2O2–mediated injury [66, 67]. This could be explained by a reduction in catalase or other peroxidase levels and activity in lung cancer cells compared to normal cells. It has been previously shown that glutathione peroxidase overexpression reduces tumor growth inhibitory effects of MnSOD [66–68]. Thus, the combination of MnSOD, or a similarly effective SOD mimetic, along with the deceased ability to metabolize and detoxify H2O2 in lung cancer cells, would enhance tumor cell death [69–71].
Ionizing radiation induces tumor cell killing by damaging the DNA of cancer cells. This damage is due to either direct or indirect ionization of the atoms which make up the DNA. Indirect ionization occurs due to ionization of water, forming free radicals, notably hydroxyl radicals (·OH), which then damage the DNA, triggering single or double strand breaks. We performed initial studies to determine if the SOD mimetic GC4419 would alter the process of IR-induced radical generation. EPR spin trapping studies were performed in the presence and absence of GC4419 in doses up to 20 μM to see if the drug altered the process of IR-induced radical generation. We observed that IR resulted in the generation of ·OH and ·H formed from the splitting of H2O (Fig. 4). No O2•− was detected and GC4419 did not significantly alter the acute process or magnitude of radical generation. There was no quenching of ·OH or of secondary radicals derived from it, as seen by identical levels of CH3C·HOH radicals formed from ethanol in the presence or absence of GC4419 (Fig. 5).
While the acute process of IR-triggered radical generation stops with cessation of IR, in the presence of cells there is a basal process of radical generation, and following radiation this cellular radical generation further increases. These radicals and secondary oxidants damage biological macromolecules and trigger the cellular injury induced by IR. The levels and activity of antioxidants and antioxidant enzymes in the cells have a key role in protecting against the cytotoxic effects of IR [34] [34]. In the current study, both using the fluorescence O2•− detecting probe DHE and EPR spin trapping, we detected low levels of O2•− generation in the two lung cancer cell lines studied at baseline, while in the normal lung cell line the levels were much lower, 2 - 3 fold or more below those in the cancer cells lines (Fig. 6 and 7). Following IR after 1 or 2 hours, EPR spin trapping demonstrated that the levels of O2•− trapped were increased by ~2-fold. The observed spin adduct signal was quenched by the SOD mimetic GC4419 confirming that it was O2•− -derived and that GC4419 at a 10 μM concentration was sufficient to near totally quench this cellular radical generation. In the control Beas 2b cells, IR also stimulated an increase in the measured radical signal; however, this only reached about half of the levels seen in the A549 cancer cells. Following IR, GC4419 quenched the observed spin adduct signals in both these cell lines, confirming that the detected radical adducts were O2•−-derived, and that GC4419 effectively dismutates this IR-stimulated O2•−.
It has been previously reported that GC4419 enhances tumor response to radiation treatment in a xenograph model of lung cancer [72]. Consistent with this, we observed that GC4419 enhanced IR-induced cytotoxicity and inhibited cell growth. With both the A549 and H1299 lung cancer cells, concentrations of GC4419 from 5 to 20 μM induced dose-dependent growth inhibition (Fig. 8); however, in the control Beas2b cells, no effect was seen. At 48 hours, with lower dose IR of 2 Gy, growth inhibition was ~10% higher in the normal control cells than the cancer cells. With higher dose IR of 8 Gy, ~35 to 50% growth inhibition was seen in the cancer cells line and ~65 % in the normal control cells. Thus, the cancer cells were generally more radiation resistant that the control cells. Interestingly with combination treatment with GC4419 and IR at either the lower dose of 2 Gy or the higher dose of 8 Gy, increased cancer cell cytotoxicity in both cell lines. This enhanced cytotoxicity was dependent on the GC4419 concentration with significant effect at doses as low as 5 μM, and maximum at the highest dose tested of 20 μM. In striking contrast to this, the normal Beas 2b cells exhibited GC4419-mediated protection at both 2 Gy at 8 Gy. With 2 Gy IR, 20 μM GC4419 rendered the cancer cells more susceptible to injury with ~35% inhibition of cell growth compared to only ~15% inhibition of the control cells. At the 8 Gy IR dose, much higher cytotoxicity was seen; however, relative protection of the control cells was still present with growth inhibition ~15% higher in the A549 cells and ~10% higher in the H1299 cells than in the Beas 2b cells. Thus, GC4419 was seen to be effective in enhancing cancer cell cytotoxicity and growth inhibition, while conferring protection to normal cells.
The overall mechanism of SOD mimetic induced cytotoxicity in cancer cells has been ascribed to their ability to rapidly convert O2•− to hydrogen peroxide (H2O2) that cannot be tolerated or neutralized by cancer cells [66, 67]. This could be explained by the reduction in catalase level and activity in lung cancer cells compared to normal cells which causes DNA damage and/or cell death. Prior studies have demonstrated the ability of glutathione peroxidase overexpression to reduce tumor growth inhibitory effects of MnSOD [66–68]. In addition, overexpression of catalase in cancer cells inhibited the cytotoxic effect of GC4419/AscH–, providing further evidence that H2O2 plays an important role in GC4419-mediated cancer cytotoxicity [73]. Thus, the combination of GC4419 and decreased catalase or other peroxidases in lung tumors may result in increased production and decreased detoxification of H2O2 that impairs tumor growth and development [69–71].
In an effort to further characterize the effects on GC4419 on longer term cancer cell survival and growth, clonogenic assays were performed with colony formation measured after 14 days. With IR doses of 2 Gy, 10 μM GC4419 decreased the survival fraction significantly below values seen with the IR alone. In contrast, in the normal cells, GC4419 increased the survival fraction, consistent with prior studies showing radiation protection in human fibroblasts [74]. With combination treatment with the cancer cell lines, ~50-60% decline in survival fraction was seen, while in the normal cells only about a 10% decline occurred. Thus, GC4419 was highly effective in decreasing cancer cell survival and growth, while protecting the normal cells.
We performed further studies to investigate and characterize the process by which GC4419 enhanced cancer cell death. IR-mediated tumor growth inhibition is often linked to its ability to cleave DNA and induce apoptosis. At 48 hours post IR, it was observed that GC4419 (10 μM) increased DNA fragmentation in both the cancer cells lines by 20 – 40 % compared to IR alone, while decreasing this in the control cells by over 50%. The increased DNA fragmentation is consistent with apoptotic cell death.
Caspase-3 is a key molecule in the apoptotic signaling pathway as activation of caspase-3 plays a central role in induction of apoptosis [75]. Therefore, additional studies to assess the process of apoptosis were performed with assays of the caspase cascade. While 2Gy of IR induced an increase in caspase-3 activity in both lung cancer cell lines of 30 to 70% and co-treatment with GC4419 increased the caspase-3 activity by 60 to 130%, in the control cells co-treatment with GC4419 decreased the caspase-3 activity to 58% of the levels in untreated cells. Thus, GC4419 was observed to stimulate apoptotic signalling in the cancer cells while supressing it in the normal cells.
We also examined the effects of GC4419 and IR on the expression of pro-apoptotic and anti-apoptotic proteins, Bax and Bcl-2. With 2 Gy IR, the expression of Bax was increased by ~60% in the cancer cell lines and by ~50% in the control cells. In contrast, GC4419 decreased the expression of Bax in the control Beas 2b cells by > 60% (Fig. 12). GC4419 in combination with IR further increased the expression of Bax in both cancer cell lines with 90 to 120% increases seen. In contrast, combination treatment of the control cells decreased Bax expression by ~25% compared to IR alone. For the anti-apoptotic protein Bcl-2, after treatment with IR the expression was decreased by 40 to 50% in both the cancer and control cells. GC4419 decreased the expression of Bcl-2 in the cancer cells by 24 to 42 %, with no change of Bcl-2 expression in the control cells. With combination of IR and GC4419, in the cancer cells Bcl-2 expression was similarly decreased as with IR alone. However, combination treatment enhanced the expression of Bcl-2 by over 2-fold compared to IR alone. Overall, with GC4419 treatment alone, the ratio of Bax to Bcl-2 was seen to be increased in the cancer cell lines but decreased in the control cells, and upon combined treatment with IR this ratio was further increased in the cancer cells but decreased in the control cells. This data indicates that combination treatment exerts its cytotoxicity through induction of increased apoptosis in cancer cells while protecting normal cells from IR-induced apoptosis.
Our results are consistent with previous studies that have reported the ability of another SOD mimetic to enhance IR-induced apoptosis [56]. In that study, this effect was accompanied by an increase in the expression of Bax and procaspase 7, 8 in A549 and colorectal cancer cells [76, 77]. Cleaved caspase-3, with imbalance between Bcl2 and Bax, was also reported after IR of A549 and H1299 cells using 125I seeds or 60Co γ-rays, with the reduction of the survival fraction [78]. In line, heavy ion irradiation induced apoptosis in H1299 cells with increased expression of caspase-3 [75]. In view of the similar pro-apoptotic effects of these other forms of radiation, one can hypothesize that GC4419 may also be beneficial in enhancing the process of cancer cell apoptosis with these modalities of cancer therapy.
The regulation of the cellular redox state is of high importance for cell viability and growth. TrxR is a critical regulator of cellular redox state and its expression and function is critical for cancer cell growth [38]. Higher expression and activity of TrxR is correlated with prevention of apoptosis, acceleration of tumor growth and resistance of cancer cells to chemotherapy. We observed that IR significantly increased the activities of TrxR in the cancer cell lines, while in normal cells IR decreased TrxR activity to 41% of values in untreated controls (Fig. 13). Co-treatment of the cells with GC4419 and IR totally or partially reversed the increases in TrxR activity seen with IR alone in the cancer cell lines; however, in the control cells with GC4419 co-treatment, over a 4-fold increase was seen compared to IR alone. Thus, GC4419 may increase the sensitivity of the cancer cells to IR by decreasing TrxR activity with resultant oxidative shift in cellular redox state, while increased resistance is conferred in the normal cells by an increase in TrxR activity.
In summary, treatment with GC4419 does not alter the cytotoxic radical generation which occurs during IR, which is primarily ·OH; however, it quenches the increased levels of O2•− formed in the cancer cells following IR. GC4419 increases cancer cell death and inhibits cancer cell growth and proliferation with no adverse effect on normal cells. Combination of GC4419 with IR augments the cytotoxic effects of IR on cancer cells, while protecting normal cells. Increased DNA fragmentation, caspase-3 activity, and increased Bax levels with decreased Bcl-2 and elevated Bax/Bcl-2 ratio all indicated that combination of GC4419 with IR enhances cancer cell apoptosis. GC4419 also increased TrxR activity in the normal cells but decreased activity in cancer cells, conferring increased cancer cell sensitivity to oxidative stress. In conclusion, GC4419 increases the cytotoxic and pro-apoptotic activity of IR in lung cancer cells while decreasing injury in normal cells. Thus, GC4419 isa promising therapeutic for cancer treatment by enhancing the efficacy of IR in cancer cell killing while decreasing IR-induced toxicity to normal tissues.
The effect of SOD mimetic GC4419 on cancer killing and radiosensitization is studied.
GC4419 quenches O2•− levels in cancer cells before and after irradiation.
However, it does not alter the ·OH radical generation triggered by irradiation.
GC4419 triggers apoptotic cancer cell death and inhibits cancer cell proliferation.
GC4419 selectively enhanced cancer cell killing while protecting normal cells.
Acknowledgments
We thank Dr. Dennis Riley and Dr. Jeffery L. Keene of Galera Therapeutics for their helpful scientific guidance and advice on these studies and their comments on this manuscript. We acknowledge Galera Therapeutics for providing the GC4419 compound used in these studies. This work was supported by Galera Therapeutics and NIH/NHLBI grants R01HL131941 and R01HL135648.
Abbreviations:
- NSCLC
Non-small-cell lung cancer
- IR
Ionizing Radiation
- SOD
Superoxide dismutase
- SODm
SOD mimetics
- DMPO
5,5-Dimethyl-1-pyrroline N-oxide
- DTPA
diethylenetriaminepentaacetic acid
- EPR
Electron paramagnetic resonance
- DHE
dihydroethidium
- DEPMPO
5-diethoxyphosphoryl-5-methyl-1-pyrroline-n-oxide
- DIPPMPO
5-diisopropoxyphosphoryl-5-methyl-1-pyrroline N-oxide
- 4-oxo-TEMPO
4-oxo-2,2,6,6-tetramethyl-1-piperidinyloxy
- XTT
2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
- TrxR
Thioredoxin Reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer Statistics, 2017, CA Cancer J Clin 67(1) (2017) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Hirsch FR, Franklin WA, Gazdar AF, Bunn PA Jr., Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology, Clin Cancer Res 7(1) (2001) 5–22. [PubMed] [Google Scholar]
- [3].Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr., Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR, American O Society of Clinical, American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003, J Clin Oncol 22(2) (2004) 330–53. [DOI] [PubMed] [Google Scholar]
- [4].O’Sullivan B, Griffin AM, Dickie CI, Sharpe MB, Chung PW, Catton CN, Ferguson PC, Wunder JS, Deheshi BM, White LM, Kandel RA, Jaffray DA, Bell RS, Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma, Cancer 119(10) (2013) 1878–84. [DOI] [PubMed] [Google Scholar]
- [5].Ricardi U, Badellino S, Filippi AR, Stereotactic body radiotherapy for early stage lung cancer: History and updated role, Lung Cancer 90(3) (2015) 388–96. [DOI] [PubMed] [Google Scholar]
- [6].Spasova I, [Treatment of the unresectable non small cell lung carcinoma], Cas Lek Cesk 144(9) (2005) 602–12; discussion 612-3. [PubMed] [Google Scholar]
- [7].Ryu MR, Paik SY, Chung SM, Combined effect of heptaplatin and ionizing radiation on human squamous carcinoma cell lines, Mol Cells 19(1) (2005) 143–8. [PubMed] [Google Scholar]
- [8].Field LS, Furukawa Y, O’Halloran TV, Culotta VC, Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria, J Biol Chem 278(30) (2003) 28052–9. [DOI] [PubMed] [Google Scholar]
- [9].Holley AK, Miao L, St Clair DK, St Clair WH, Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases, Antioxid Redox Signal 20(10) (2014) 1567–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC, Greenberger J, Blumberg D, Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury, J Gastrointest Surg 7(2) (2003) 229–35; discussion 235-6. [DOI] [PubMed] [Google Scholar]
- [11].Kam WW, Banati RB, Effects of ionizing radiation on mitochondria, Free Radic Biol Med 65 (2013) 607–619. [DOI] [PubMed] [Google Scholar]
- [12].Oberley LW, Oberley TD, The role of superoxide dismutase and gene amplification in carcinogenesis, J Theor Biol 106(3) (1984) 403–22. [DOI] [PubMed] [Google Scholar]
- [13].Behrend L, Mohr A, Dick T, Zwacka RM, Manganese superoxide dismutase induces p53-dependent senescence in colorectal cancer cells, Mol Cell Biol 25(17) (2005) 7758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weydert C, Roling B, Liu J, Hinkhouse MM, Ritchie JM, Oberley LW, Cullen JJ, Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase, Mol Cancer Ther 2(4) (2003) 361–9. [PubMed] [Google Scholar]
- [15].Duan H, Zhang HJ, Yang JQ, Oberley LW, Futscher BW, Domann FE, MnSOD up-regulates maspin tumor suppressor gene expression in human breast and prostate cancer cells, Antioxid Redox Signal 5(5) (2003) 677–88. [DOI] [PubMed] [Google Scholar]
- [16].Salvemini D, Riley DP, Cuzzocrea S, SOD mimetics are coming of age, Nat Rev Drug Discov 1(5) (2002) 367–74. [DOI] [PubMed] [Google Scholar]
- [17].Batinic-Haberle I, Reboucas JS, Spasojevic I, Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential, Antioxid Redox Signal 13(6) (2010) 877–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hume PS, Anseth KS, Polymerizable superoxide dismutase mimetic protects cells encapsulated in poly(ethylene glycol) hydrogels from reactive oxygen species-mediated damage, J Biomed Mater Res A 99(1) (2011) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Riley DP, Schall OF, Structure-activity studies and the design of synthetic superoxide dismutase (SOD) mimetics as therapeutics, Advances in Inorganic Chemistry: Including Bioinorganic Studies, Vol 59 59 (2007) 233–263. [Google Scholar]
- [20].Salvemini D, Riley DP, M40403 - Superoxide dismutase mimetic, Drugs of the Future 25(10) (2000) 1027–1033. [Google Scholar]
- [21].Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP, A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats, Science 286(5438) (1999) 304–6. [DOI] [PubMed] [Google Scholar]
- [22].Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D, On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies, Br J Pharmacol 140(3) (2003) 445–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Anderson CM, Sonis ST, Lee CM, Adkins D, Allen BG, Sun W, Agarwala SS, Venigalla ML, Chen Y, Zhen W, Mould DR, Holmlund JT, Brill JM, Buatti JM, Phase 1b/2a Trial of the Superoxide Dismutase Mimetic GC4419 to Reduce Chemoradiotherapy-Induced Oral Mucositis in Patients With Oral Cavity or Oropharyngeal Carcinoma, Int J Radiat Oncol Biol Phys 100(2) (2018) 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anderson CM, Lee CM, Saunders DP, Curtis A, Dunlap N, Nangia C, Lee AS, Gordon SM, Kovoor P, Arevalo-Araujo R, Bar-Ad V, Peddada A, Colvett K, Miller D, Jain AK, Wheeler J, Blakaj D, Bonomi M, Agarwala SS, Garg M, Worden F, Holmlund J, Brill JM, Downs M, Sonis ST, Katz S, Buatti JM, Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer, Journal of Clinical Oncology 37(34) (2019) 3256-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuppusamy P, Zweier JL, Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation, J Biol Chem 264(17) (1989) 9880–4. [PubMed] [Google Scholar]
- [26].Villamena FA, Zweier JL, Detection of reactive oxygen and nitrogen species by EPR spin trapping, Antioxid Redox Signal 6(3) (2004) 619–29. [DOI] [PubMed] [Google Scholar]
- [27].Kuppusamy P, Zweier JL, Identification and Quantitation of Free-Radicals and Paramagnetic Centers from Complex Multicomponent Epr-Spectra, Applied Radiation and Isotopes 44(1-2) (1993) 367–372. [Google Scholar]
- [28].Duling DR, Simulation of multiple isotropic spin-trap EPR spectra, J Magn Reson B 104(2) (1994) 105–10. [DOI] [PubMed] [Google Scholar]
- [29].Lee MC, Velayutham M, Komatsu T, Hille R, Zweier JL, Measurement and characterization of superoxide generation from xanthine dehydrogenase: a redox-regulated pathway of radical generation in ischemic tissues, Biochemistry 53(41) (2014) 6615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C, Clonogenic assay of cells in vitro, Nat Protoc 1(5) (2006) 2315–9. [DOI] [PubMed] [Google Scholar]
- [31].Liu KJ, Miyake M, Panz T, Swartz H, Evaluation of DEPMPO as a spin trapping agent in biological systems, Free Radical Bio Med 26(5-6) (1999) 714–721. [DOI] [PubMed] [Google Scholar]
- [32].Chalier F, Tordo P, 5-Diisopropoxyphosphoryl-5-methyl-1-pyrroline N-oxide, DIPPMPO, a crystalline analog of the nitrone DEPMPO: synthesis and spin trapping properties, J Chem Soc Perk T 2 (12) (2002) 2110–2117. [Google Scholar]
- [33].Roubaud V, Sankarapandi S, Kuppusamy P, Tordo P, Zweier JL, Quantitative measurement of superoxide generation using the spin trap 5-(diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide, Anal Biochem 247(2) (1997) 404–11. [DOI] [PubMed] [Google Scholar]
- [34].Riley PA, Free radicals in biology: oxidative stress and the effects of ionizing radiation, Int J Radiat Biol 65(1) (1994) 27–33. [DOI] [PubMed] [Google Scholar]
- [35].Benov L, Sztejnberg L, Fridovich I, Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical, Free Radic Biol Med 25(7) (1998) 826–31. [DOI] [PubMed] [Google Scholar]
- [36].Lindsay J, Esposti MD, Gilmore AP, Bcl-2 proteins and mitochondria--specificity in membrane targeting for death, Biochim Biophys Acta 1813(4) (2011) 532–9. [DOI] [PubMed] [Google Scholar]
- [37].Renault TT, Dejean LM, Manon S, A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2, Mech Ageing Dev 161 (Pt B) (2017) 201–210. [DOI] [PubMed] [Google Scholar]
- [38].Myers CR, Myers JM, The effects of acrolein on peroxiredoxins, thioredoxins, and thioredoxin reductase in human bronchial epithelial cells, Toxicology 257(1-2) (2009) 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oberley LW, Buettner GR, Role of superoxide dismutase in cancer: a review, Cancer Res 39(4) (1979) 1141–9. [PubMed] [Google Scholar]
- [40].St Clair DK, Oberley LW, Manganese superoxide dismutase expression in human cancer cells: a possible role of mRNA processing, Free Radic Res Commun 12-13 Pt 2 (1991) 771–8. [DOI] [PubMed] [Google Scholar]
- [41].Sun Y, Oberley LW, Oberley TD, Elwell JH, Sierra-Rivera E, Lowered antioxidant enzymes in spontaneously transformed embryonic mouse liver cells in culture, Carcinogenesis 14(7) (1993) 1457–63. [DOI] [PubMed] [Google Scholar]
- [42].Xu Y, Krishnan A, Wan XS, Majima H, Yeh CC, Ludewig G, Kasarskis EJ, St Clair DK, Mutations in the promoter reveal a cause for the reduced expression of the human manganese superoxide dismutase gene in cancer cells, Oncogene 18(1) (1999) 93–102. [DOI] [PubMed] [Google Scholar]
- [43].Zhang HJ, Yan T, Oberley TD, Oberley LW, Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype, Cancer Res 59(24) (1999) 6276–83. [PubMed] [Google Scholar]
- [44].Zhong W, Oberley LW, Oberley TD, St Clair DK, Suppression of the malignant phenotype of human glioma cells by overexpression of manganese superoxide dismutase, Oncogene 14(4) (1997) 481–90. [DOI] [PubMed] [Google Scholar]
- [45].Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS, Trent JM, Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells, Proc Natl Acad Sci U S A 90(7) (1993) 3113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Urano M, Kuroda M, Reynolds R, Oberley TD, St Clair DK, Expression of manganese superoxide dismutase reduces tumor control radiation dose: gene-radiotherapy, Cancer Res 55(12) (1995) 2490–3. [PubMed] [Google Scholar]
- [47].Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, Wang Y, Qian Q, Qian C, Wu J, Liu XY, Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy, Cancer Res 66(8) (2006) 4291–8. [DOI] [PubMed] [Google Scholar]
- [48].Guo H, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS, Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic Lewis lung carcinomas, In Vivo 17(1) (2003) 13–21. [PubMed] [Google Scholar]
- [49].Guo H, Seixas-Silva JA Jr., Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, Archer H, Greenberger JS, Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene, Radiat Res 159(3) (2003) 361–70. [DOI] [PubMed] [Google Scholar]
- [50].Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger JS, Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation, Gene Ther 7(12) (2000) 1011–8. [DOI] [PubMed] [Google Scholar]
- [51].Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger J, Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy, Gene Ther 5(2) (1998) 196–208. [DOI] [PubMed] [Google Scholar]
- [52].Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, Zwacka R, Travis EL, Greenberger JS, Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis, Int J Radiat Oncol Biol Phys 43(1) (1999) 169–81. [DOI] [PubMed] [Google Scholar]
- [53].Epperly MW, Travis EL, Sikora C, Greenberger JS, Manganese [correction of Magnesium] superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: modulation of irradiation-induced mRNA for IL-I, TNF-alpha, and TGF-beta correlates with delay of organizing alveolitis/fibrosis, Biol Blood Marrow Transplant 5(4) (1999) 204–14. [DOI] [PubMed] [Google Scholar]
- [54].Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS, Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase transgene, Radiat Oncol Investig 7(4) (1999) 204–17. [DOI] [PubMed] [Google Scholar]
- [55].Epperly MW, Sikora C, Defilippi S, Bray J, Koe G, Liggitt D, Luketich JD, Greenberger JS, Plasmid/liposome transfer of the human manganese superoxide dismutase transgene prevents ionizing irradiation-induced apoptosis in human esophagus organ explant culture, Int J Cancer 90(3) (2000) 128–37. [DOI] [PubMed] [Google Scholar]
- [56].Shin SW, Choi C, Lee GH, Son A, Kim SH, Park HC, Batinic-Haberle I, Park W, Mechanism of the Antitumor and Radiosensitizing Effects of a Manganese Porphyrin, MnHex-2-PyP, Antioxid Redox Signal 27(14) (2017) 1067–1082. [DOI] [PubMed] [Google Scholar]
- [57].Ashcraft KA, Boss MK, Tovmasyan A, Roy Choudhury K, Fontanella AN, Young KH, Palmer GM, Birer SR, Landon CD, Park W, Das SK, Weitner T, Sheng H, Warner DS, Brizel DM, Spasojevic I, Batinic-Haberle I, Dewhirst MW, Novel Manganese-Porphyrin Superoxide Dismutase-Mimetic Widens the Therapeutic Margin in a Preclinical Head and Neck Cancer Model, Int J Radiat Oncol Biol Phys 93(4) (2015) 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas JS, Ye X, Leong KW, Dewhirst MW, Vujaskovic Z, Benov L, Spasojevic I, Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics, Free Radic Biol Med 51(5) (2011) 1035–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, Spasojevic I, SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways, Antioxid Redox Signal 20(15) (2014) 2372–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Batinic-Haberle I, Tovmasyan A, Spasojevic I, An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins--From superoxide dismutation to H2O2-driven pathways, Redox Biol 5 (2015) 43–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hosseinimehr SJ, The protective effects of trace elements against side effects induced by ionizing radiation, Radiat Oncol J 33(2) (2015) 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Weitzel DH, Tovmasyan A, Ashcraft KA, Boico A, Birer SR, Roy Choudhury K, Herndon J 2nd, Rodriguiz RM, Wetsel WC, Peters KB, Spasojevic I, Batinic-Haberle I, Dewhirst MW, Neurobehavioral radiation mitigation to standard brain cancer therapy regimens by Mn(III) n-butoxyethylpyridylporphyrin-based redox modifier, Environ Mol Mutagen 57(5) (2016) 372–81. [DOI] [PubMed] [Google Scholar]
- [63].Weitzel DH, Tovmasyan A, Ashcraft KA, Rajic Z, Weitner T, Liu C, Li W, Buckley AF, Prasad MR, Young KH, Rodriguiz RM, Wetsel WC, Peters KB, Spasojevic I, Herndon JE 2nd, Batinic-Haberle I, Dewhirst MW, Radioprotection of the brain white matter by Mn(III) n-Butoxyethylpyridylporphyrin-based superoxide dismutase mimic MnTnBuOE-2-PyP5+, Mol Cancer Ther 14(1) (2015) 70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR, Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation, Biochem J 418(1) (2009) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Liou GY, Storz P, Reactive oxygen species in cancer, Free Radic Res 44(5) (2010) 479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li S, Yan T, Yang JQ, Oberley TD, Oberley LW, The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase, Cancer Res 60(14) (2000) 3927–39. [PubMed] [Google Scholar]
- [67].Spitz DR, Elwell JH, Sun Y, Oberley LW, Oberley TD, Sullivan SJ, Roberts RJ, Oxygen toxicity in control and H2O2-resistant Chinese hamster fibroblast cell lines, Arch Biochem Biophys 279(2) (1990) 249–60. [DOI] [PubMed] [Google Scholar]
- [68].Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, Greenberger JS, Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors, Radiat Res 167(3) (2007) 289–97. [DOI] [PubMed] [Google Scholar]
- [69].Chung-man Ho J, Zheng S, Comhair SA, Farver C, Erzurum SC, Differential expression of manganese superoxide dismutase and catalase in lung cancer, Cancer Res 61(23) (2001) 8578–85. [PubMed] [Google Scholar]
- [70].Glorieux C, Calderon PB, Catalase down-regulation in cancer cells exposed to arsenic trioxide is involved in their increased sensitivity to a pro-oxidant treatment, Cancer Cell Int 18 (2018) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB, Regulation of catalase expression in healthy and cancerous cells, Free Radic Biol Med 87 (2015) 84–97. [DOI] [PubMed] [Google Scholar]
- [72].Story MD, Sishc BJ, Polsdofer EM, Bloom DA, Heer C, Spitz DR, Saha D, The Radioprotector GC4419 Ameliorates Radiation Induced Lung Fibrosis While Enhancing the Response of Non-Small Cell Lung Cancer Tumors to High Dose per Fraction Radiation Exposures, International Journal of Radiation Oncology Biology Physics 102(3) (2018) S187–S187. [Google Scholar]
- [73].Heer CD, Davis AB, Riffe DB, Wagner BA, Falls KC, Allen BG, Buettner GR, Beardsley RA, Riley DP, Spitz DR, Superoxide Dismutase Mimetic GC4419 Enhances the Oxidation of Pharmacological Ascorbate and Its Anticancer Effects in an H(2)O(2)-Dependent Manner, Antioxidants (Basel) 7(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mapuskar KA, Flippo KH, Schoenfeld JD, Riley DP, Strack S, Hejleh TA, Furqan M, Monga V, Domann FE, Buatti JM, Goswami PC, Spitz DR, Allen BG, Mitochondrial Superoxide Increases Age-Associated Susceptibility of Human Dermal Fibroblasts to Radiation and Chemotherapy, Cancer Res 77(18) (2017) 5054–5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xu H, Gao L, Che T, Du W, Li Q, Gao Q, Wang Z, The effects of 12C6+ irradiation on cell cycle, apoptosis, and expression of caspase-3 in the human lung cancer cell line h1299, Cancer Biother Radiopharm 27(2) (2012) 113–8. [DOI] [PubMed] [Google Scholar]
- [76].Shen J, Behrens C, Wistuba L II Feng JJ Lee WK Hong R Lotan, Identification and validation of differences in protein levels in normal, premalignant, and malignant lung cells and tissues using high-throughput Western Array and immunohistochemistry, Cancer Res 66(23) (2006) 11194–206. [DOI] [PubMed] [Google Scholar]
- [77].Wang S, Shu J, Chen L, Chen X, Zhao J, Li S, Mou X, Tong X, Synergistic suppression effect on tumor growth of ovarian cancer by combining cisplatin with a manganese superoxide dismutase-armed oncolytic adenovirus, Onco Targets Ther 9 (2016) 6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang Z, Zhao Z, Lu J, Chen Z, Mao A, Teng G, Liu F, A comparison of the biological effects of 125I seeds continuous low-dose-rate radiation and 60Co high-dose-rate gamma radiation on non-small cell lung cancer cells, PLoS One 10(8) (2015) e0133728. [DOI] [PMC free article] [PubMed] [Google Scholar]


