Abstract
Enzyme-catalyzed degradation compromises the dental restoration-tooth interface, increasing cariogenic bacterial infiltration. In addition to bacterial ingress inhibition, antimicrobial-releasing adhesives may exhibit direct interfacial biodegradation inhibition as an additional benefit.
Objective:
assess the ability of an antimicrobial drug-releasing resin adhesive, containing octenidine dihydrochloride (OCT)-silica co-assembled particles (DSPs), to enhance the biostability and preserve the interfacial fracture toughness (FT) of composite restorations bonded to dentin.
Methods
Mini short-rod restoration bonding specimens with total-etch adhesive with/without 10% wt. DSPs were made. Interfacial fracture toughness (FT) was measured as-manufactured or post-incubation in simulated human salivary esterase (SHSE) for up to 6-months. Effect of OCT on SHSE and whole saliva/bacterial enzyme activity was assessed. Release of OCT outside the restoration interface was assessed.
Results
No deleterious effect of DSPs on initial bonding capacity was observed. Aging specimens in SHSE reduced FT of control but not DSP-adhesive-bonded specimens. OCT inhibited SHSE degradation of adhesive monomer and may inhibit endogenous proteases. OCT inhibited bacterial esterase and collagenase. No endogenous collagen breakdown was detected in the present study. OCT increased human saliva degradative esterase activity below its minimum inhibitory concentration (MIC) towards S. mutans, but inhibited degradation above MIC. OCT release outside restoration margins was below detection.
Significance
DSP-adhesive preserves the restoration bond through a secondary enzyme-inhibitory effect of released OCT, which is virtually confined to the restoration interface microgap. Enzyme activity modulation map produce a positive-to-negative feedback switch, by increasing OCT concentration via biodegradation-triggered release to an effective dose, then subsequently slowing degradation and degradation-triggered release.
Keywords: biodegradation, dental caries, esterase, enzyme inhibition, interfacial fracture toughness
Graphical Abstract

(For clarity, drawing of silica precursor, drug, particles, and schematic diagram of the restoration - tooth interface are not to scale)
1. Introduction
Resin composite restorative materials face several host and bacterial challenges to performance, compromising their efficacy as a long-term restorative material. Although aesthetic and mechanical performance of these materials immediately after restoration is quite high, failure due to recurrent caries caused in part by cariogenic bacteria at the restoration margins, or restoration bond fracture, significantly reduces the service life of resin composite restorations [1–6]. Salivary and bacterial esterase activity degrades the monomers and resin polymer matrix of these materials [7–11]. Low levels of endogenous protease activity from dentinal cathepsins and matrix metalloproteinases (MMPs) may also contribute to the degradation of dentin collagen fibrils [12, 13]. The effects of this biodegradation are most acute at the restoration margin where a resin adhesive is used to bond the restoration to exposed type I collagen within the demineralized dentin, producing an interlocking network of collagen and polymer, known as the hybrid layer [14–16]. The hydrolysis and resultant degradation and destruction of the hybrid layer results in the reduction of interfacial fracture toughness [17, 18]. This degradation also increases microleakage via marginal gap expansion, allowing cariogenic bacteria to penetrate, proliferate and further degrade the interface [10, 11]. Degradation by-products increase virulence of cariogenic bacteria [19, 20], and human neutrophils contribute to the degradation via their degradative enzymes [21].
There is a growing body of literature seeking to improve resin-based dental restorative materials through the inclusion of antimicrobial capabilities to combat cariogenic bacteria at the restoration margins [22]. This may include direct mixing of antimicrobial, mixing antimicrobial-carrying and releasing vehicles, or direct grafting of antimicrobials for surface killing effect or future hydrolytic release [23–31]. However the interactions between antimicrobial moieties and the biodegradation of resin materials is rarely investigated, despite evidence that these drugs usually affect esterase and/or proteases activity, and may even prevent biodegradation in a dual-action manner [31, 32].
In vivo biodegradation of dental materials can be mimicked using cholesterol esterase (CE) and pseudocholinesterase (PCE) to produce a simulated human salivary esterase (SHSE) media, allowing for more accurate long-term incubation of material and model restoration specimens in vitro in a biochemically dynamic environment [9, 17]. Previous studies have elucidated the effect of saliva on restoration bond fracture toughness, mode of failure, and bacterial microleakage at the restoration margin using in vitro studies with SHSE as a valid equivalent to human salivary derived esterases (HSDE) [15–18].
We previously developed resin adhesives utilizing antimicrobial drug-silica co-assembled particles (DSPs), which demonstrated high loading of the antimicrobial octenidine dihydrochloride (OCT) and greatly extended drug release period. DSPs were subsequently mixed with a commercial total-etch adhesive at 10% wt. and studied under biochemically realistic conditions via SHSE [34]. In this study, release of OCT reached a steady state within 15 days via diffusion from resin-embedded DSPs, while the DSPs remain intact to prevent the formation of voids within the resin. In addition to a steady state effective release, OCT release increased following exposure to SHSE. Modeled release dynamics predicted that an effective antibacterial concentration of OCT will be maintained in the restoration marginal gap under worst-case conditions for the patient lifetime (~30 years), while release of OCT to the broader oral cavity should be minimal due to the low surface area of adhesive exposed at the restoration margin periphery. Furthermore, during steady-state release and significant dilution into a large volume of media, S. mutans biofilm inhibition was maintained at the material surface, demonstrating that an inhibitory concentration of OCT (2 µg mL−1) at the resin surface was maintained throughout diffusive release regardless of the sub-inhibitory total incubation media concentration.
The current study aims to assess and compare the interfacial toughness and biochemical stability of resin-composites bonded to human dentin using either DSP-containing, drug free calcined DSP-containing (cDSPs), or stock adhesives. It also assesses the enzyme-modulating capabilities of OCT with respect to the breakdown of the restoration margin, and tests whether released OCT is contained within the interface and not released outside the margins of a realistic restoration geometry. It is hypothesized that the presence of DSPs in the adhesive will enable esterase-modulated release of antimicrobial drug that will preserve the strength of the restoration-tooth bond through drug-enzyme interactions, with no detectable release of the drug outside the interface.
2. Methods
A detailed description of all methods used is included in the supplementary information. Statistical analysis methods are described in each section.
Human teeth and saliva used in this study were obtained according to University of Toronto Human Ethics Protocol #25793. Teeth (3rd molars) were extracted and were handled as before [35], stored in tap water at −20°C until use. Cutting of tooth structure was performed with a water-cooled low-speed diamond saw (Buehler Ltd., Lake Bluff, IL). Enamel coronal edges were removed, and coronal dentin slabs were taken from within 2 mm of the cervical line plane before using for various experiments below. Photopolymerization of resins was carried out with a plasma arc device (Sapphire Plus Plasma Arc Curing System, Dent Mat, Santa Maria, CA) for 10 s (adhesive layer) or 20 s (per 1 mm layer of resin composite) at a distance of approximately 1 mm and a minimum intensity of 1730 mW cm−2 as verified by the device’s internal radiometer [17].
2.1. DSP-adhesive preparation
Spherical OCT-DSPs were prepared as described previously, containing 34% wt./50% vol. antimicrobial drug, with an average particle diameter of 424 ± 75 nm [33]. DSPs were calcined to remove OCT by heating the particles to 600°C over 2 hours followed by a 4 hour hold at 600°C to produce cDSPs with completely open porosity free of OCT. DSP- and cDSP-containing adhesives were prepared to match previous studies and allow direct comparison [34]: DSPs were added at 10% wt. to the adhesive component of a total-etch adhesive system (Adper Scotchbond Multi-Purpose Adhesive [SBMP], 3M Canada, London, ON), and mixed at 275 RPM for 24 hours in darkness. Loss of OCT mass was accounted for when producing cDSP-SBMP utilizing the above procedure to ensure approximately the same volume density of particles within resin (6.6% wt. cDSPs added to SBMP). Adhesives were shielded from light and stored at 4°C until warming to room temperature before use.
2.2. Analysis of restoration-tooth bond fracture toughness
Miniature short-rod (mini-SR) fracture toughness specimens were prepared as described and validated previously to reliably and relevantly assess ex vivo restoration bonding efficacy and degradation (schematic of mini-SR specimen dimensions used in Fig. 1 A) [17, 18, 36]. Details of specimen fabrication are provided in the supplementary information section, using the adhesives prepared in section 2.1 and a commercial resin composite (Filtek Z250 Universal Restorative, shade A1, 3M Canada, London, ON). Specimen bonding to human dentin was preformed using DSP-SBMP, cDSP-SBMP, or SBMP systems to analyze fracture toughness.
Figure 1:
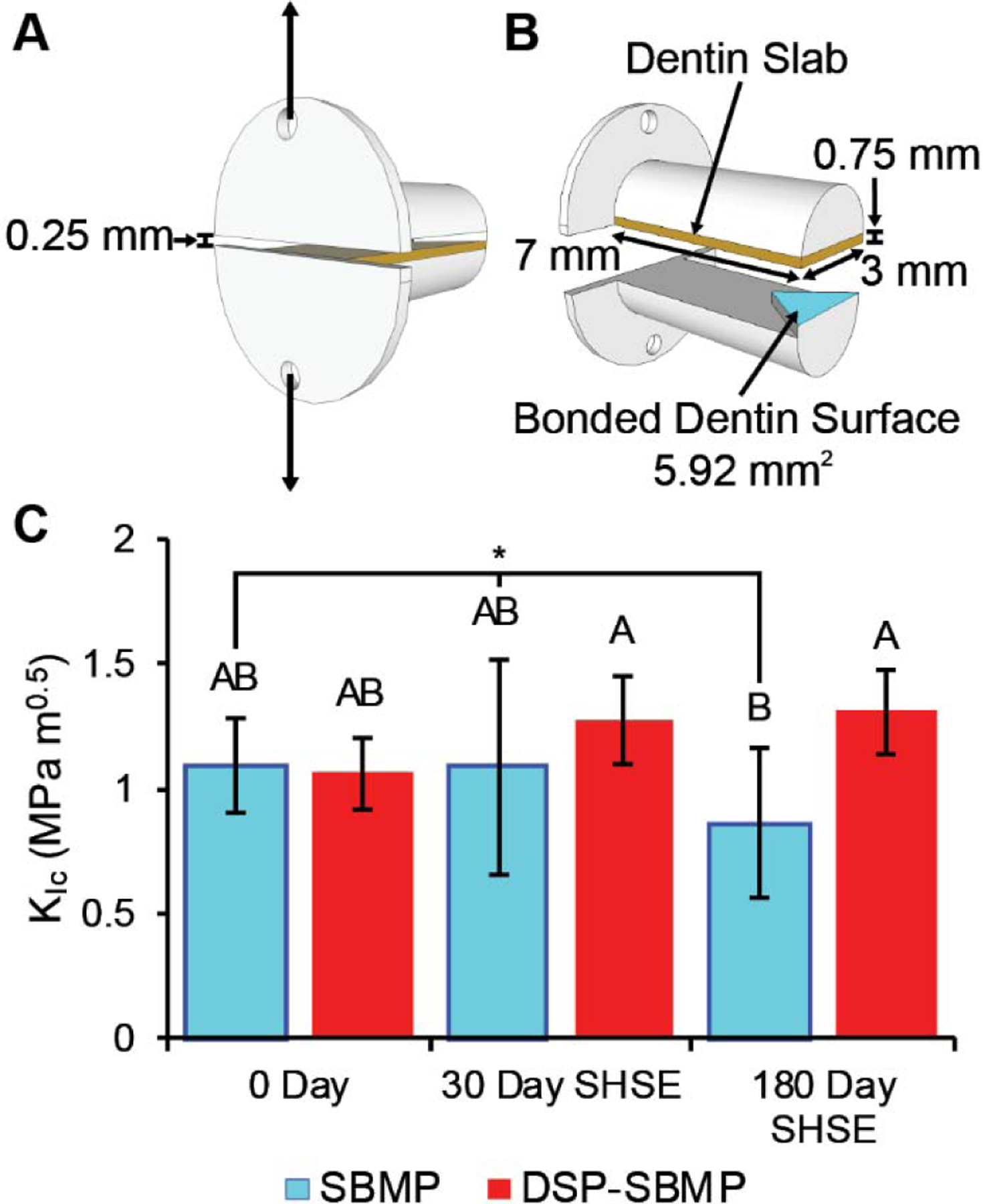
Mini-SR specimens were prepared, aged (0–180 days), and fractured; DSP-SBMP demonstrated improved dentin bonding of resin composite compared to control stock adhesive (SBMP) after incubation in simulated oral biodegradative environment (SHSE) for 180-days. A mini-SR specimen is shown in A with arrows indicating where force is applied, while an exploded view in B shows the chevron area where composite and dentin are bonded using a test of control adhesive system. 0, 30 and 180-day fracture toughness values are displayed in C with group letters for Tukey HSD test results (p<0.05). A Statistically significant decline in fracture toughness over time in SBMP-bonded specimens, but not DSP-SBMP-bonded specimens, was observed (group marked with *, least squares fit effect tests via ANOVA, p<0.05). No effect of drug-free cDSPs on bonding capability was observed. Results are presented as mean ± standard deviation.
DSP-SBMP and SBMP mini-SR fracture toughness specimens were tested immediately post-synthesis or after 30 or 180 days incubation in simulated human salivary esterase (SHSE) as previously described (N≥6 per experimental group per timepoint) [17, 18]. cDSP-SBMP specimens were tested immediately post-synthesis to isolate any potential physiomechanical effect of the novel filler on adhesive bonding performance. SHSE was prepared using cholesterol esterase (CE, Toyobo Co. Ltd., Osaka, Japan) and pseudocholine esterase (PCE, Sigma Aldrich, Mississauga, ON), with average esterase activity matched to that found in human saliva over the 5- to 10-day periods between refreshment or replacement [10, 15–17].
At the end of each respective incubation period, mini-SR specimens were attached to a microtensile tester (Bisco, Richmond, BC). Testing proceeded at 1 mm min−1 in the direction showed in Fig. 1 B. Force at failure (Pc) was recorded and used to calculate interfacial fracture toughness (KIc). The effect of friction within the horizontal testing apparatus was equal and negligible (< 0.1 N, or approximately 1% of total measured range of fracture toughness).
Statistical Analysis: was performed using ANOVA and least squares fit with a lack of fit test to assess effect significance, and Tukey’s HSD post-hoc analysis to compare groups, with incubation time and material independent variables, and fracture toughness dependant variable (p<0.05). T he O’Brien test was used to assess homogeneity of variance (p<0.05).
2.3. Analysis of OCT release from ex vivo restoration-tooth bonded interfaces
Ex vivo model simulating the gingival margins of proximal or cervical restorations [15, 16] were prepared to investigate OCT release outside the restoration margins using a 4 × 4 mm human dentin bonded to composite using DSP-SBMP. Specimens were then incubated at 37°C for 10 days in 3 mL of PBS or SHSE as in Section 2.2 (N=10). Incubation media were combined with equal parts methanol to halt esterase activity before analyses via high performance liquid chromatography (HPLC) in combination with UV spectroscopy (281 nm) (Waters, Mississauga, ON) to quantify the mass of OCT released [33, 34]. Further details are provided in the supplementary information section.
2.4. Effect of OCT on protein adsorption
Effect of OCT on enzyme and salivary protein adsorption to resin surfaces was assessed using a procedure adapted from Zhang et al. [37]. Disc-shaped photopolymerized SBMP specimens 8 × 0.5 mm were fabricated in a mould and cured 10s on each side as described above. Specimens were incubated in PBS with 0.2, 2, and 20 µg mL−1 of OCT for 2 hours at 37°C. Identical DSP-SBMP specimens were fabricated and incubated with PBS (0 µg mL−1 OCT) to analyse the effect of surface DSPs and released OCT into a non-OCT-containing buffer. OCT concentrations represent 0.1x, 1x, and 10x the MIC of OCT against S. mutans [33]. Specimens were then incubated for 2 hours in either 4.5 µg mL−1 CE or 1/10 diluted saliva pooled from 4 donors with OCT at 37°C. The dilution 1/10 dilution of saliva allowed for decreased viscosity and ease handling of whole saliva without further processing which may affect enzyme activity. Adhered protein was removed by sonicating 20 minutes in 1% sodium dodecyl sulfate (SDS, Sigma Aldrich, Mississauga, ON) in PBS and concentration determined using a bicinchoninic acid total protein assay kit (Sigma Aldrich, Mississauga, ON).
Statistical analyses:
Protein adsorption was compared to OCT-free control using Welch’s t-test, and all-group comparisons were made using ANOVA and Tukey’s HSD post-hoc analysis (N=4 for all groups).
2.5. OCT effect on margin degrading enzymes’ measured activity and biodegradation of dentin and resin
Statistical analyses:
All results in the below subsections were analysed using ANOVA and Tukey’s HSD multiple comparisons test (p<0.05, N=3 unless otherwise specified).
2.5.1. Preparation and incubation of human dentin specimens in OCT incubation media
The effect of OCT on endogenous protease activity and bacteria-derived collagenase was assessed. Coronal dentin from human teeth (N=3) was collected as described earlier and ground to an approximate particle size of 0.1 mm using an electric blade grinder (Black + Decker) for 20s. Powdered dentin was then demineralized in 10% phosphoric acid (Fisher Chemical, Ottawa, ON) under constant mixing for 24 hours [38–40]. Demineralized dentin was then washed 3 times in PBS and 25 mg wet aliquots were suspended in 1 mL of PBS with 0, 0.2, 2, or 20 µg mL−1 OCT and with or without 50 U mL−1 of bacterial collagenase type 1 (from Clostridium histolyticum, Sigma-Aldrich, Oakville, ON, Canada) as a positive control for collagen degradation. Specimens were incubated at 37°C.
2.5.2. OCT effect on endogenous dentinal MMP activity by fluorometric substrate
Vials were centrifuged for 5 minutes at 1250 RPM, and supernatant of 0, 0.2, 2, and 20 µg mL−1 OCT-dentin specimens (without bacterial collagenase type 1) were analyzed after 14-days incubation for generic and specific (MMP-1, −2, −8 and −9) MMP-like activity utilizing fluorometric substrates (Sensolyte 520 generic MMP, MMP1, −2, −8 and −9 assay kits, AnaSpec, Fremont, CA) [41]. 50 µL of supernatant was combined with 50 µL of fluorometric peptide substrate as per manufacturer instructions in a black 96-well plate, and fluorescence was monitored at Ex/Em = 490 nm/520 nm every minute for 1 hour (Cytation 5, Bio-Tek, Winooski, VT). Controls of OCT and PBS solutions with and without substrates were utilized for background correction. The resultant fluorescence reading slope was taken as relative MMP-like activity.
2.5.3. OCT Effect on the degradation of human dentinal collagen
At 24 hours supernatant from collagenase type 1-containing OCT-dentin specimens were analysed for hydroxyproline (Hyp) content as a marker of bacterial collagenase mediated collagen degradation [21, 42] via a commercial amino acid derivatization product and protocol (AccQ-Tag, Waters, Mississauga, ON) and ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS, Acquity H-class with C18 column, coupled to a Xevo G2-XS Q-ToF MS with electro-spray ionization source, and quantified with QuanLynx analysis software, all from Waters, Mississauga, ON).
After 1, 14, and 36 days of incubation supernatant of specimens without added collagenase type 1 were analysed for Hyp content as before. The supernatant of these 36-day incubated specimens without added bacterial collagenase were also analysed for collagen fragment content as a marker of partial collagen degradation; supernatant was digested via trypsin (Trypsin Gold, Promega, Madison, WI), and analysed using UPLC-MS/MS as before in positive MSE. Results were quantified relatively using Progenesis QI for Proteomics (v4.2, Nonlinear Dynamics, Durham, NC) and searched against the UniProt database [43].
Two complementary methods to detect release of collagen degradation by-products by endogenous proteases were used to increase the sensitivity of the measurement. Dentinal collagen that has undergone complete digestion and solubilization by protease will release hydroxyproline [39, 40]. The method above used can detect quantities of Hyp released as low as 1 ng mL−1. Collagen fragments in the form of peptides may also be released [44] either as identifiable by-products of specific protease cleavages, or as detached and solubilized collagen fragments, and are quantifiable by label free analysis via MS/MS. Although the sensitivity of this method is not as high as Hyp derivatization, incomplete collagen digestion not resulting in significant Hyp release could be detected using the latter method.
2.5.4. OCT effect on model and salivary esterase activity
The effect of OCT on stock CE and salivary CE-like esterase activity was assessed. OCT was prepared at 0.2, 2, and 20 µg mL−1 in PBS with either 16 U mL−1 CE, 1/10 diluted whole unstimulated human saliva in PBS pooled from 3 donors as before to analyse human salivary derived esterases (HSDE), or S. mutans bacterial esterase (SMU_118c) in PBS at 0.05 mg mL−1 [11]. Samples were incubated 24 hours at 37°C. CE-like activity was measured using para-nitrophenyl butyrate (p-NPB) as a colorimetric substrate.
Biodegradation of bisphenol A-glycidyl methacrylate (bisGMA), a universal monomer of adhesive and resin composite, and the primary monomer of SBMP [45], by CE, HSDE, or SMU_118c was measured with and without OCT. Solutions were prepared and incubated as above, replacing p-NPB with 100 µM bisGMA [46]. BisGMA-derived biodegradation by-product 2,2-bis[4(2,3-hydroxypropoxy)phenyl]propane (bisHPPP) was quantified via UPLC-MS/MS and photodiode array (as in Section 2.5.3) as a marker of biodegradative activity.
3. Results
3.1. Analysis of restoration-tooth bond fracture toughness
Fracture toughness results of mini-SR specimens of composite bonded to human dentin using SBMP or DSP-SBMP are shown in Fig. 1 C. Initial post-fabrication non-aged interfacial fracture toughness values were not statistically different between stock SBMP, DSP-SBMP (Fig. 1 C). C-DSP-SBMP specimens had an immediate fracture toughness of 0.80 ± 0.35 MPa m−1 and was not statistically different from either stock SBMP or DSP-SBMP (Tukey’s HSD, p>0.05). Incubation time had a statistically significant negative effect on fracture toughness in control SBMP specimens (ANOVA and least squares fit, p < 0.05). Specimens made using DSP-SBMP show no statistically significant change in their fracture toughness values over time (ANOVA and least squares fit, p>0.05). At 180-days incubation DSP-SBMP specimens had significantly higher fracture toughness values than stock SBMP ( Tukey’s HSD, p<0.05). For the complete ANOVA model, the achieved p-values correspond to an observed power of 0.847.
3.2. Analysis of OCT release from ex vivo restoration-tooth bonded interfaces
OCT was not detected by HPLC (detection limit of 4.34 ng mL−1, corresponding to 26 ng OCT per day) in either PBS or SHSE during the first 10 days of incubation with restoration-dentin specimens utilizing DSP-SBMP adhesive, when release rate of OCT into the interface, and subsequently outside the interface is expected to be the highest [34].
3.3. Effect of OCT on protein adsorption
Protein adsorption results are presented in Fig. 2. At high 20 µg mL−1 of OCT, CE adsorption on SBMP was significantly inhibited by 28 ± 14% (Fig. 2 A, Welch’s t-test p=0.047). DSP-SBMP also showed a statistically significant inhibition of CE adsorption compared to SBMP + 0 µg mL−1 control with a reduction of 35 ± 25% (Welch’s t-test p=0.040). However no overall effect of OCT or DSPs were seen comparing all groups simultaneously (ANOVA p>0.05, Tukey’s HSD analysis p>0.05) No effect on total protein adsorption from saliva is seen at tested concentrations of OCT (Fig. 2 B, ANOVA p>0.05, Tukey’s HSD analysis p>0.05, Welch’s t-test p’s > 0.05).
Figure 2:
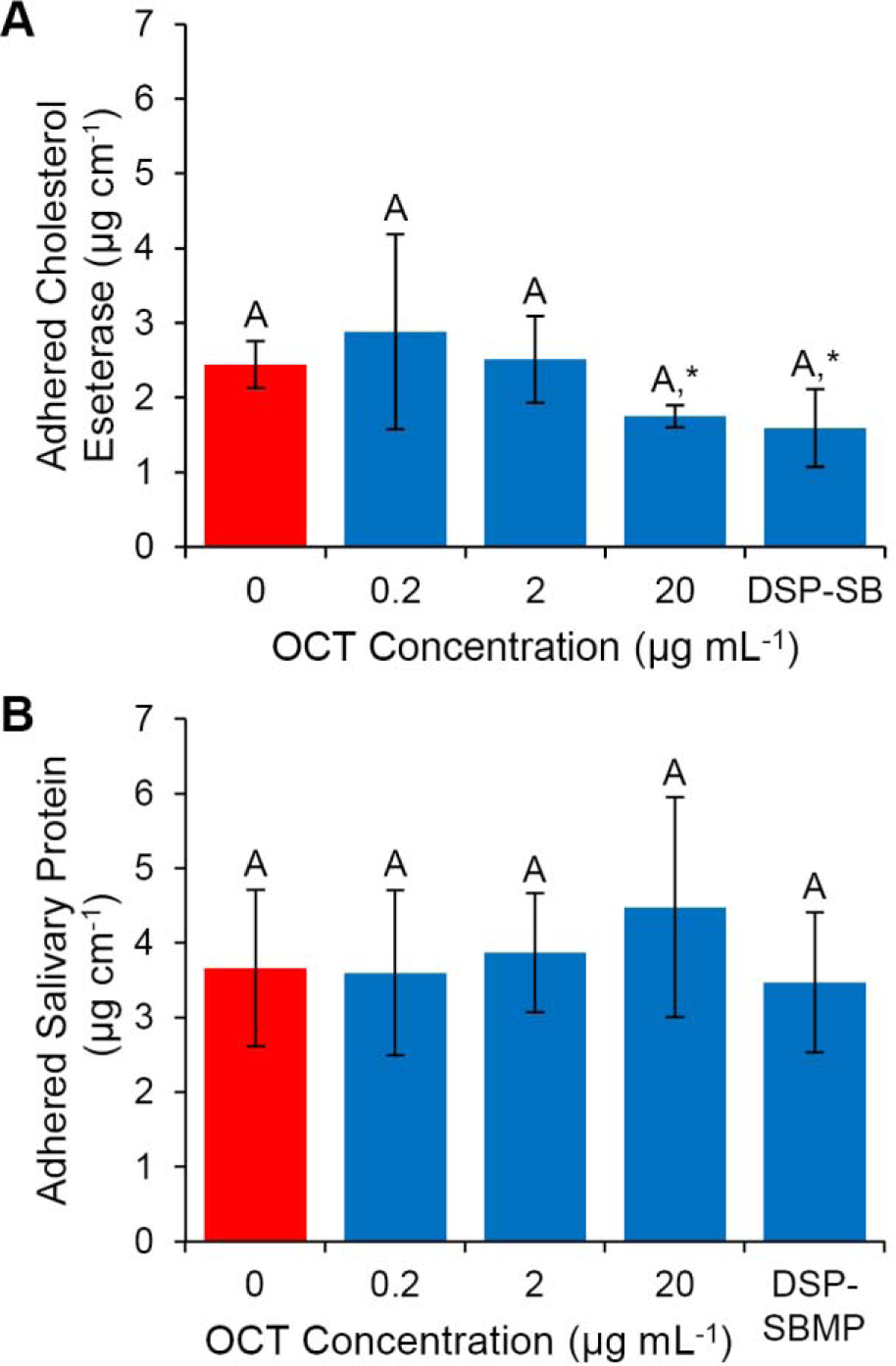
Protein adsorption of CE and salivary proteins on polymerized SBMP with/without OCT and on DSP-SBMP. No significant differences between CE groups (A) were found in whole-experiment comparisons (ANOVA p>0.05, Tukey’s HSD analysis p>0.05, groups denoted by letters). Comparing individual data points to SBMP without OCT, 20 µg mL−1 OCT (p=0.047) and DSP-SBMP (p=0.04) significantly inhibited CE adsorption (A), however no other inhibition was observed (marked with an asterisk, Welch’s t-test). OCT and DSP-SBMP had no effect on the adsorption of salivary proteins to resin adhesive (ANOVA p>0.05, Tukey’s HSD analysis p>0.05, groups denoted by letters, Welch’s t-tests with control p>0.05). Results are presented as mean ± standard deviation.
3.4. OCT effect on margin degrading enzymes and biodegradation of dentin and resin
OCT effect on endogenous MMP-like activity, and bacterial collagenase type-1 degradative activity are summarized in Fig. 3. MMP-like activity towards specific and generic peptide substrates was measured from human dentin incubation media. OCT significantly reduced endogenous dentinal MMP-1, −2, and - 9-like activity towards specific peptide substrates at all measured OCT concentrations by up-to 75%, but had no statistically significant reduction on activity towards MMP-8 specific peptide substrate (p=0.1) (Fig. 3 A–D). OCT significantly reduced endogenous dentinal non-specific MMP-like activity towards peptide substrate at 20 µg mL−1 OCT by 83% (Fig. 3 E).
Figure 3:
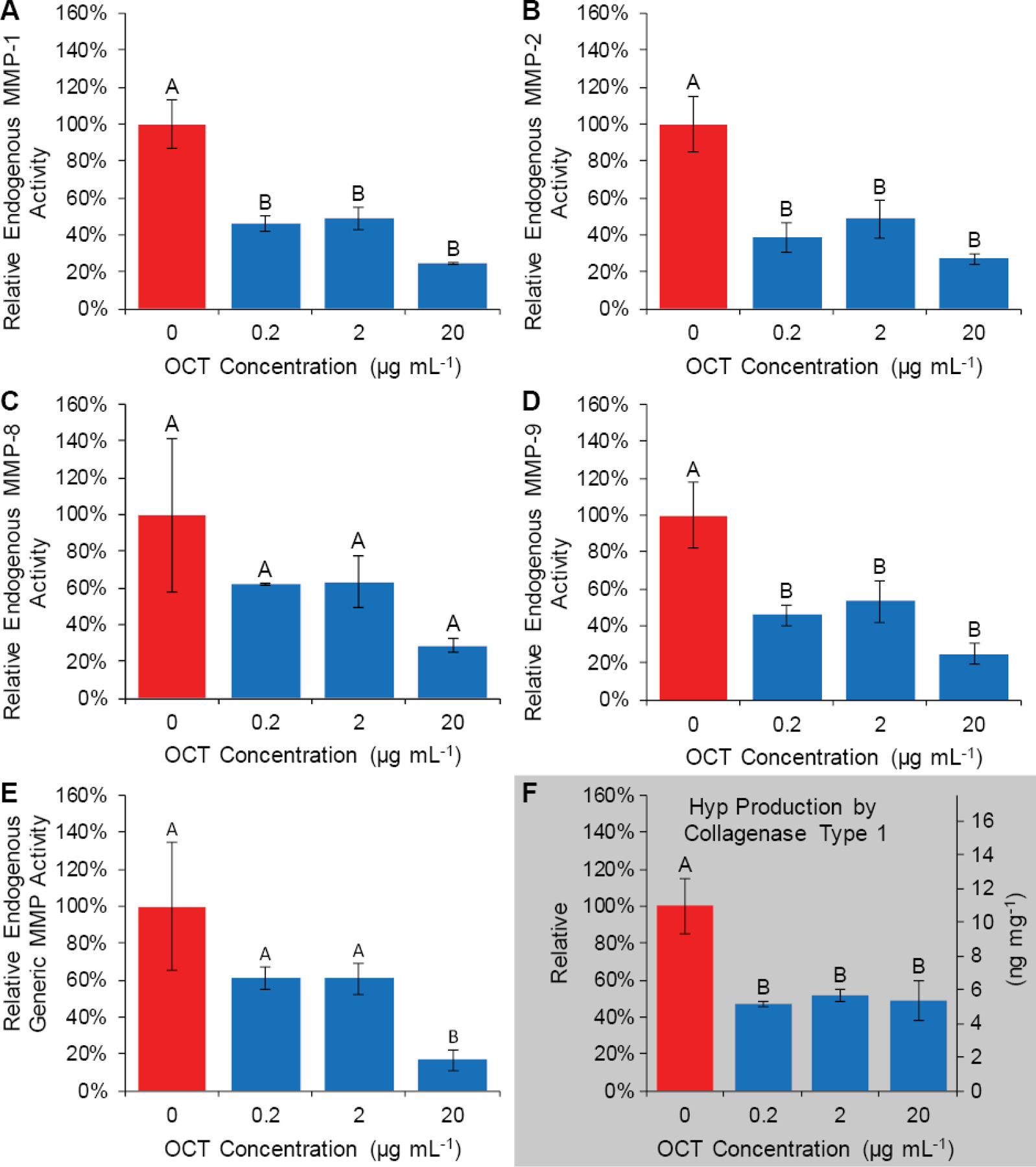
OCT may decrease endogenous MMP-like protease activity and decreases bacterial collagenolytic activity towards human dentin. OCT decreases endogenous dentinal MMP-1-, 2-, and 9-like activity when measured via MMP-specific fluorometric substrate, as well as activity towards generic fluorometric MMP substrate (A, B, D, E), however, no detectable endogenous breakdown of collagen was observed from demineralized dentin specimens at any OCT concentration. OCT decreases the ability of bacterial type-1 collagenase to degrade human dentinal collagen as measured by Hyp production (F). Group letters indicate Tukey’s HSD analysis (p<0.05). Data are presented as mean relative activity ± standard deviation.
No Hyp was detected from powdered human dentin specimens incubated in PBS with or without OCT and without collagenase type 1 at 1, 14 or 36 days, and thus Hyp content is assumed to be below the limit of detection (1 ng mL−1 or 40 pg mg−1 demineralized dentin). For comparison, a relatively large amount of Hyp was detected from specimens incubated with 50 U mL−1 bacterial collagenase type 1 (approximately 10 ng mg−1 demineralized dentin). Furthermore, Hyp production by type-1 collagenase was significantly reduced at all concentrations of OCT tested by up to 53% (Fig. 3 F).
All supernatant from demineralized dentin incubation contained type-1 collagen (Homo sapiens collagen alpha-2(I) chain, confidence score of 25.3 with 3 to 5 unique peptides observed in each sample), however no statistical difference in the relative quantity of collagen was observed between samples with varying OCT concentrations (ANOVA p=0.752). A detailed results report is included in the supplementary information.
Displayed in Fig. 4 A, OCT increases the colorimetric assay-measured hydrolytic activity of CE toward p-NPB at 2 and 20 µg mL−1 by 66%, and 74%, respectively, compared with no OCT. In contrast, OCT strongly inhibits the hydrolytic activity of CE towards bisGMA at all concentrations studied (Fig. 4 B).
Figure 4:
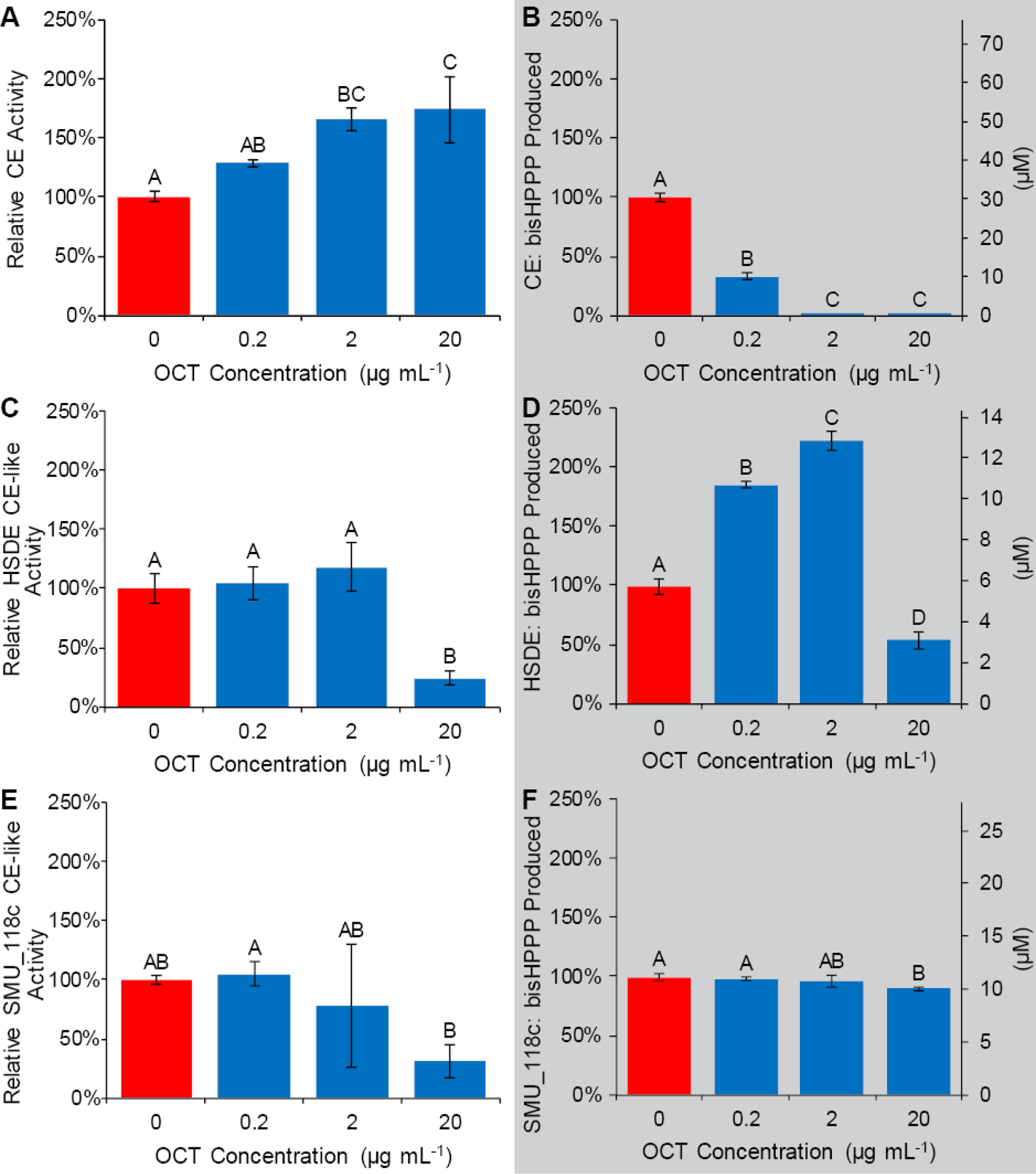
OCT modulates esterase activity in several ways, depending on enzyme, substrate, and OCT concentration. OCT effects on enzyme CE-like activity assay were measured via either colorimetric assay using p-NPB as a substrate (A, C, E) or enzyme hydrolytic activity towards the adhesive’s universal monomer bisGMA via bisHPPP production (B, D, F) is shown. In A, OCT appears to increase the hydrolytic activity of CE towards the p-NPB substrate with increasing concentration. In contrast, OCT decreases the hydrolytic activity of CE towards adhesive monomer of interest bisGMA (B). Activity of HSDE (C, D) is decreased at 20 µg mL−1 toward both p-NPB and bisGMA, but is increased toward bisGMA at lower concentrations (D). OCT reduced the activity of SMU_118c toward both p-NPB and bisGMA only at 20 µg mL−1, and the inhibitory effect was higher toward p-NPB degradation (E, F). Group letters indicate Tukey’s HSD analysis (p<0.05). Data are presented as mean relative activity ± standard deviation.
At 20 µg mL−1 only, OCT reduced HSDE’s CE-like activity towards p-NPB to 24 ± 13 %. HSDE hydrolytic activity towards bisGMA was significantly inhibited by 20 µg mL−1 to 54 ± 8 % (Fig. 4 D). However, at lower concentrations of OCT (0.2 and 2 µg mL−1), HSDE’s bisGMA hydrolytic activity increased (187 ± 4 % and 224 ± 8 %, respectively). OCT reduced the activity of SMU_118c toward both p-NPB and bisGMA only at 20 µg mL−1, and the inhibitory effect was higher toward p-NPB degradation (Fig. 4 E, F).
4. Discussion
The current study assessed the effects of incorporating antimicrobial-releasing drug-silica co-assembled particles (DSPs) into a total-etch resin adhesive’s bonding ability and biostability of the material and the restoration-tooth interface under relevant biodegradative conditions, mimicking the intra-oral pathogenic environment. To summarize, DSP-SBMP successfully bonded resin composite to dentin, preserved bond strength after considerable in vitro biodegradative challenge, and showed no release outside the restoration tooth margins, indicating no systemic exposure. Preservation of bond strength for the DSP-SBMP was likely due to the inhibitory effects of the released antimicrobial drug, OCT, on the hydrolyzing activity of the model esterase CE, and a potential contributory effect of its inhibition of endogenous proteases. Furthermore, OCT inhibited the resin-hydrolyzing activity of salivary esterases, cariogenic bacteria-derived esterase SMU_118c when OCT was above its bacterial minimum inhibitory concentration, suggesting its efficacy in in vivo conditions [33, 34]. Below this concentration OCT may increase resin biodegradation, increasing its own release from DSP-SBMP [34], thereby introducing a positive-to-negative feedback switch holding drug concentration within the restoration margin at a bactericidal level.
4.1. Long term maintenance of the restoration-tooth bond strength by DSP-SBMP
The miniature short-rod specimens used to measure interfacial fracture toughness of a resin restoration bond were previously validated to reliably assess resin-tooth bond strength [17, 18, 36]. Interfacial fracture toughness is a more appropriate indication of the performance of a brittle bonded interface than microtensile strength as it accounts for both bonding material strength and interfacial defects [36], while providing a measured value independent of the exact specimen geometry and therefore is a good measure of interface biodegradation [17, 18]. Furthermore, fracture toughness specimens generally are far more likely to fail as intended at the actual bonded interface than microtensile beam specimens, reducing the number of specimens needed to accurately assess the material and more accurately mimicking the mode of failure observed clinically [47].
Resin composites require bonding to tooth structure to remain attached, maintain interfacial seal and prevent microbial ingress into the interface [15–17, 22]. With the addition of DSPs to the adhesive, it was important to confirm that the bonding capacity of the adhesive to primed dentin and composite restorative material was not initially compromised and remained stable over time [17, 18]. Due to the geometry of the applied adhesive in a restoration, as well as it’s important function as a bonding agent, fracture toughness (KIc) is regarded as a more appropriate measure of physical capability than bulk flexural, tensile, or compressive properties [17, 18, 36].
Initial fracture toughness values show no difference between stock SMBP, drug-free cDSP-SBMP and DSP-SBMP mini-SR specimens, indicating no deleterious physiomechanical or chemical effect of the DSP addition on the quality of the bond between the restoration and the tooth. No difference in degree of conversion between SBMP and DSP-SBMP after curing was observed, further confirming effective photopolymerization (see Supplementary Material). Immediate fracture toughness values were similar to previous reports for these materials [17, 18]. Furthermore, this suggests that the addition of mechanically stable cDSPs did not alter the fracture toughness of the resin dentin bond. It is unexpected that the addition of chemically inert silica alone at the concentration used in the current study would have an effect on the long-term biodegradation of the interface, and thus this was not examined in the study. Although there is evidence that mesoporous silica particles with open porosity (not drug-filled pores) may increase the mechanical properties of dental resin composites, these effects may not translate to thin resin layers in an adhesive bond or with low loads of particles as in the current study [48]. This mechanical impact is dependant on percolation between particles and occurs primarily well above the 10% wt. filler content used in this study (20–60% wt. depending on particle size, passing to the threshold of what would typically be considered a dental “composite” as opposed to a dental adhesive) [49–51]. Investigation of the potential mechanical effects of DSPs under cyclical loading conditions may be an interesting avenue in the future. Finally, although cDSPs and DSPs-post-OCT-release are structurally and mechanically identical, this is not the case when they are first mixed into SBMP. cDSPs contain open pores 1–2 nm in diameter, which may allow for resin monomer infiltration and micromechanical locking [48]. The pores of DSPs are filled with antimicrobial drug and thus we hypothesize that no resin infiltration takes place, making the two groups not entirely comparable. Thus, conclusions about the effects of DSP-addition on the adhesive should be drawn primarily from DSP-SBMP and SBMP groups.
Previous work has shown that fracture toughness of bonded restorations with the stock total-etch adhesive used in the current study demonstrate a reduction in fracture toughness when incubated in SHSE over time, with a statistically significant reduction in the fracture toughness values after 180-days [17, 18]. These results are corroborated in the present study, with the fracture toughness of stock SBMP specimens showing decline after 180-days in SHSE. In contrast, DSP-SBMP specimens’ fracture toughness did not significantly change with time and had significantly higher fracture toughness than SBMP alone at 180 days. At high concentrations non-covalently-bound silica filler within resin may increase its biodegradation through filler release and increased surface area according to percolation theory [52]. The maintenance of bond strength found in the current investigation in opposition to percolation theory further suggests a biochemical effect preserving bond strength, and not a mechanical one.
These results demonstrate DSP-SBMP adhesive’s inhibition of interface-degrading factors may begin to improve bond stability well before the clinical 5–10-year timeframe of recurrent caries formation and the reported life span of resin composite restorations [3, 53]. This significant improvement in performance is in addition to the antimicrobial properties of the DSP-SBMP over the control reported previously [34], suggesting additional improvement to the biostability of the restoration-tooth interface as a result of incorporating DSPs in the adhesive. Since OCT release was previously calculated to be above MIC for the restoration service life [34], this protective effect is expected to last for the optimal life of the restoration (~30 years). Possible mechanisms for bond preservation were subsequently explored further.
4.2. Drug release outside the resin-dentin interface and confinement within interface
Ex vivo model restorations, simulating the gingival margins of proximal or cervical restorations [15, 16] were prepared using OCT-containing DSP-SBMP, and OCT release was monitored to examine confinement of drug within the restoration margin and the potential of undesirable systemic exposure. Handling of DSP-SBMP during restoration preparation was similar to the stock adhesive, resulted with similar initial FT values (see above in 4.1) and therefore required no change of technique. No OCT was detected in the incubation media during the first 10 days of incubation in both media conditions, when theoretical release from DSP-SBMP is highest [34]. If the thickness and exposed edge of the 4 × 4 mm DSP-SBMP layer is estimated at 20 µm [15, 16], the theoretical maximum release of drug to the oral cavity as determined using the model in SHSE is 5.8 ng day−1, well below the limit of detection for OCT in the current experiment (26 ng). Therefore, it is not surprising that OCT was not detected in the incubation media by the HPLC system during the first 10-days of incubation from model restorations. Even at a low salivary flow rate of 0.5 mL min−1 (720 mL day−1) [54], average peak oral concentration of OCT would be 8.1 pg mL−1, well below a level expected to have any systemic biological effect [55]. These results corroborate previously predicted marginal OCT release levels and indicate that DSPs are largely stabilised and confined within the restoration margin [34].
These results should not be interpreted as a lack of OCT interfacial antimicrobial or anti-degradative efficacy. OCT maintains an inhibitory concentration (minimum 2 µg mL−1) at the material surface as OCT releases from the resin when DSPs are exposed to moisture and before diffusion from the material surface during steady state release, as demonstrated previously by biofilm inhibition [34]. Furthermore, interfacial OCT concentration adjacent to exposed adhesive within the interfacial microgap will be higher due to the confined nature, small volume, and therefore slowed diffusion from this region into the oral cavity [34]. Confined interfacial marginal gap OCT concentration at 5 years reaches 10.6 µg mL−1 after 24 hours, as modeled previously [34]. These two factors provide the rationale for the selection of 0.2, 2 and 20 µg mL−1 (x0.1, x1 and x10 the MIC [33]) of OCT in the current investigation for enzyme inhibition, representing realistic OCT concentrations at the adhesive surface and within the interfacial microgap. The critical regions of the restoration that should be protected are not in the open, immediately diluted environment of the oral cavity, but rather are in direct contact with the adhesive. Thus, the concentration of antimicrobial at the adhesive surface is the most important determinant of its efficacy.
4.3. Localized OCT preserves restoration-tooth interfacial integrity by inhibition of simulated and in vivo degradative activities
4.3.1. OCT effect on protein adsorption
Protein adsorption in the presence of OCT and on the surface of DSP-SBMP was investigated to determine if accumulated drug concentration or drug release affect the ability of CE and salivary proteins to adhere to, and therefore potentially hydrolyze, dental resins. The bacterial esterase SMU_118c was not tested for protein adsorption due to the high concentration that is required for this test and the limited availability of the SMU_118c protein [11]. At 20 µg mL−1 OCT decreased the amount of CE adsorbed to resin SBMP surfaces, but only by a small amount and only when compared to OCT-free control. DSP-SBMP had a similarly limited effect, possibly due to locally high OCT concentration at the material surface as it diffuses away to the incubation media. The pI of CE is approximately 4.3, and thus at the neutral pH that this experiment was carried out the enzyme was negatively charged [56]. The positively charged OCT could have bound to cholesterol esterase via charge attraction, and subsequently interfered with its adsorption on the resin surface [55]. Limiting the ability of CE to accumulate on the interfacial surface and hydrolyze the polymerized resin within the adhesive and hybrid layer could have contributed to the maintenance of the fracture toughness values of restoration specimens containing DSPs, but based on the limited effect on adsorption found in the current investigation this was likely a secondary cause of bond preservation. In contrast, no overall effect was seen on salivary protein adsorption. It is important to keep in mind that saliva is a complex mixture of biological materials including many proteins not directly involved in the hydrolysis of dental materials, and that these proteins may be affected differently by OCT through various interactions.
4.3.2. OCT effect on protease activity
Proteases such as MMPs and cathepsins within dentin degrade dentin collagen [57–59], but their effect on fracture toughness and bond integrity may have been minimal in the current study as demonstrated by the measurement of dentinal collagen biodegradation by-products, despite some inhibitory effect when measuring using fluorometric substrates. The antimicrobial chlorhexidine (established to inhibit MMPs) showed very limited preservation of bond strength in previous studies, while the targeted MMP inhibitor galardin only modulated fracture mode in specimens incubated in a more realistic degradative environment containing esterases [17, 60, 61]. Incubating bonded specimens in a protease-inhibiting medium versus buffer alone similarly showed no change to bond strength degradation rates in a previous study [61]. Esterase activity within human saliva or produced by bacteria degrades the restoration margin more quickly and at higher activity levels than endogenous proteases, however these were not present in the current fracture toughness study [10, 15–18]. None the less, inhibition of collagenolytic activity indicated by the fluorometric substrates by the novel material studied here may limit marginal microgap formation and contribute to the long-term integrity of the resin-dentin bond in vivo and remains a useful property.
The ability of OCT to inhibit endogenous proteases was assessed using a kit test method with these past results in mind. OCT did inhibit MMP-1, −2, and −9-like activities towards these sensitive fluorometric peptide substrates over short periods of time, which based on previous studies indicates an ability to modulate fracture mode. The lack of clear inhibition of endogenous MMP-8-like collagenolytic activity may explain why inhibition of activity towards a generic MMP-substrate was only seen at the highest OCT concentration. MMP-8 is a major source of dentinal endogenous collagenolytic activity and therefore would significantly affect the breakdown of the generic MMP peptide substrate in testing [62]. OCTs mechanism of inhibition may be similar to that seen for other quaternary ammonium-containing compounds and act through zinc ion chelation [60, 63, 64]. The surfactant properties of OCT may further interfere with enzyme function [65, 66]. Ideally the inhibitory effects of OCT on endogenous collagenolytic activities would be observed with the most relevant substrate; dentinal collagen. However, endogenous collagenolytic activity is too low to observe degradation by by-product concentration through the methods and timeframe utilized. These results match previous observations from our group that have failed to find significant dentinal collagen degradation by endogenous activity through Hyp production [16, 21, 42]. The release of larger collagen fragments used in the current study as a supplement and more sensitive means of measurement of collagen degradation could mark the degradation of the dentin structure without complete decomposition of collagen into its base amino acid constituents, but results were very limited and only quantifiable in relative terms, with no inter-group differences observed. Therefore, we cannot conclude over the 6-month study timeframe that OCT inhibition of endogenous collagenolytic activity directly contributed to bond strength preservation, especially in the presence of much faster-acting resin-degrading enzymes.
Although bacterial collagenase type 1 is a mixture of proteases with the ability to decompose human dentinal collagen [42], OCT inhibition of its activity against dentinal collagen observed via reduction in Hyp production (Fig. 3 F) still suggests broad inhibitory action against these proteases. This collagen-preservative property may persist in vivo and may prevent bacteria and host neutrophils from degrading the hybrid layer dentin, as this proteolytic activity is present at much higher rates [21, 42]. Since no additional collagenase was present in the current fracture toughness study it does not explain the current results. Additionally, evidence of OCT inhibitory capability towards specific collagenases relevant to secondary caries would still need to be collected to draw definitive conclusions.
4.3.3. OCT effect on esterase activity
To predict the effect of released OCT from DSP-SBMP on potential resin degrading factors’ hydrolytic capability in vivo, the esterase activity of several relevant enzyme solutions was measured via a standard colorimetric assay using p-NPB as a substrate [10, 46], as well as through the production of bisHPPP, a biodegradation by-product of the universal monomer bisGMA [17, 67], in the presence of several relevant concentrations of OCT.
OCT appears to enhance the activity of CE towards p-NPB substrate at concentration of 2 and 20 µg mL−1 (Fig. 4 A). This enhancement is not surprising since OCT is a surfactant, and surfactants have been shown to increase the activity of enzymes towards water soluble substrates like p-NPB under very specific conditions (dependant on enzyme, substrate, surfactant structure, and the concentration of surfactant) by modulating water-enzyme interactions or selectively denaturing the enzyme to increase exposure to a catalytic site [8, 65]. However, these results cannot explain the preservation of bond strength and the lack of reduction in interfacial fracture toughness in SHSE (mainly CE activity) for DSP-SBMP mini-SR specimens versus stock SBMP groups observed in the current study. For this reason, the effect of OCT on CE’s ability to hydrolyze the main component of the adhesive, composite and the hybrid layer, bisGMA, was also analysed.
OCT significantly inhibited CE’s hydrolytic activity towards bisGMA at relevant OCT concentrations. This suggests that OCT present in the restoration interface inhibits the CE catalyzed biodegradation of mini-SR specimens’ DSP-SBMP adhesive restoration-tooth interface, providing a possible explanation for the preserved bond strength as evidenced by the fracture toughness values measured in the current study. Effect of surfactants on enzyme activity is highly dependent on the species and concentrations involved, and thus this inhibition may be due to factors not present in p-NPB activity assays such as a decrease in CE specificity towards bisGMA through CE denaturation or OCT-bisGMA interactions [68]. Activity assays such as the colorimetric one used here are still useful for calibrating levels of CE across experimental groups [9, 15–18, 67, 69], but do not necessarily correlate in a 1-to-1 manner with activity towards substrates of interest under all circumstances [11].
Since under oral condition the restoration-tooth interface will be exposed to human saliva, with its human salivary derived esterase (HSDE) activities [9], and bacterial esterase [10, 11], the effect of OCT on HSDE and S. mutans SMU_118c activities was assessed as well. At 20 µg mL−1 both enzymes’ activity towards p-NPB and bisGMA were decreased, indicating a strong ability to preserve the bond in vivo. However, at lower concentrations, OCT appears to enhance the hydrolytic activity in human saliva towards bisGMA. As with CE, OCT could affect HSDE activity in several ways depending on the conditions tested [65, 66, 68]. The near total insolubility of some methacrylate monomers such as bisGMA in water has been strongly correlated with their decreased degradation by esterase [70]: the surfactant effects of OCT could have increased solubility of bisGMA in water, increasing the monomer’s surface area and availability to esterase attack [8]. Conversely, these effects may lead to the deactivation of enzymes by OCT micelles at higher concentrations due to the locally high charge concentration at the micelle-water interface (as opposed to a single or double charge on a lone OCT molecule) [65]. Although an increase in HSDE activity towards bisGMA occurs at low OCT concentrations, at higher concentrations (such as the marginal gap OCT concentration of 10.6 µg mL−1 [34]) an overall inhibitory effect of OCT on the biodegradation is expected based on these results, even after several years of service, suggesting long-term inhibition and preservation of the margin.
4.4. Utilizing OCT enzyme modulation as a positive-to-negative feedback switch
Overall, the effect of OCT on biodegradation is a balance of its activities as an enzyme inhibitor from one side, and as a surfactant on the other. The results of the current study suggest that within the complex oral environment, the breakdown of polymer resin matrix in DSP-SBMP restorations, and subsequent esterase-modulated release of OCT [34], will result in a positive-to-negative feedback effect system on the biodegradation process by the released OCT; higher concentrations of OCT will inhibit esterase activity toward the restoration and restoration-tooth interface [18, 46], while lower OCT concentrations may result in transient enhancement of the degradative activity against the adhesive that in turn will increase the level of OCT within the interface [34], and as a result induce greater antimicrobial and antidegradative effects. Since these inhibitory concentrations of OCT towards in vivo degradative enzymes are above the MIC of OCT towards S. mutans, increased esterase-modulated OCT release into the restoration margin will continue well above the MIC of the drug, and esterase inhibition by OCT should not interfere with the antimicrobial capabilities of DSP-SBMP. This positive-to-negative feedback switch should effectively hold the steady-state concentration of OCT within the margin at the surface of the adhesive at a bactericidal concentration. Therefore, it can be concluded that the modulation of esterase activity in the restoration margin by OCT has secondary effects, enhancing the mechanical stability of the bond [18], as well as inhibiting the deterioration of the marginal gap that bacteria may colonize [10]. This inhibitory effect could also be beneficial since the destruction of bac terial cells such as S. mutans by OCT may cause the release of bacterial esterases and collagenases, such as SMU_118c [10, 11, 71]; inhibition of these enzymes will ensure that biodegradation of the restoration margin ceases with the bacteria’s death. In situ investigation of these effects will be an important avenue of study in the future.
4.5. The importance of relevant aging conditions to biomaterial evaluation
It is important at this point to reinforce one of the principles of this study’s analysis on enzymatic degradation of dentin and dental materials: the importance of analyzing biodegradation and inhibition using the most realistic and biologically relevant conditions possible. In this discussion frequent use is made of the term enzyme-like to describe enzymatic activity that behaves like a known enzyme but cannot be confirmed as such. An example is CE-like activity in HSDE; HSDE behaves like CE when cleaving the colorimetric substrate p-NPB and shows similar affinity towards common dental resins. However, there is no evidence that human saliva contains CE specifically, and the reaction of HSDE and CE to concentrations of the drug OCT are quite different. Similarly, although OCT may inhibit endogenous MMP-like activity towards a substrate, this activity was not high enough to elicit a measurable change in the biodegradation of dentin. These substrate- and enzyme-specific effects need to be carefully considered in future experimental designs seeking to prevent the biodegradation of dental materials, lest researchers be led astray by spurious results not indicative of real-world performance.
5. Conclusions
The presence of DSPs in an adhesive enabled esterase-modulated release of antimicrobial drug and preserved the strength of the restoration-tooth bond through drug-enzyme interactions, with no detectable release outside the interface. Antimicrobial and antidegradative effects in concert could enhance the service life of the restoration and deterioration of the margins by human and bacterial degradative activities. These results highlight the importance of testing new materials in physiologically and pathogenically relevant conditions, as some beneficial effects will only be observed when materials are challenged under such conditions. Biodegradation remains an important consideration in dental and medical material design and increasing the stability of materials will enhance their longevity and improve patient outcomes.
The novel material evaluated in this investigation shows promising mechanical and biological effects. Future work will evaluate the material under ex vivo and in vivo conditions over extended timeframes to assess its ultimate caries-inhibiting properties.
Supplementary Material
Highlights.
Octenidine dihydrochloride (OCT) antimicrobial preserves the resin-dentin interface
OCT provides long-term inhibition of enzymatic degradation
OCT provides long-term antimicrobial activity
Interface OCT level is modulated by a feedback loop mechanism
Biomaterial testing should use enzymes relevant to pathogenic oral conditions
7. Acknowledgements
This work was supported by the National Institutes of Health [R01DE021385–0]; the Canadian Institutes of Health Research [MOP 115113, PJT-165957]; Canada Foundation for Innovation John R. Evans Leaders Fund (CFI_JELF) [project #35378], and Ministry of Research and Innovation (MRI), Ontario Research Fund (ORF) [ORF-35378].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure statement
Authors C. Stewart, B. Hatton and Y. Finer have filed a technology disclosure to the University of Toronto’s Research and Innovation Office, and a PCT application has been submitted (PCT/CA2017/050586 (P1869)) based on the technology and material presented in the manuscript. Author C. Stewart is involved in the commercialization of the technology presented in the manuscript.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
9 References
- [1].Kopperud SE, Tveit AB, Gaarden T, Sandvik L, Espelid I. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci. 2012;120:539–48. [DOI] [PubMed] [Google Scholar]
- [2].Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28:87–101. [DOI] [PubMed] [Google Scholar]
- [3].Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. Journal of the American Dental Association (1939). 2007;138:775–83. [DOI] [PubMed] [Google Scholar]
- [4].Murray PE, Hafez AA, Smith AJ, Cox CF. Bacterial microleakage and pulp inflammation associated with various restorative materials. Dent Mater. 2002;18:470–8. [DOI] [PubMed] [Google Scholar]
- [5].Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: findings From the New England Children’s Amalgam Trial. J Am Dent Assoc. 2007;138:763–72. [DOI] [PubMed] [Google Scholar]
- [6].Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27:29–38. [DOI] [PubMed] [Google Scholar]
- [7].Chauncey HH. Salivary enzymes. Journal of the American Dental Association (1939). 1961;63:360–8. [DOI] [PubMed] [Google Scholar]
- [8].Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol M. 2001;12:136–51. [DOI] [PubMed] [Google Scholar]
- [9].Finer Y, Jaffer F, Santerre JP. Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 2004;25:1787–93. [DOI] [PubMed] [Google Scholar]
- [10].Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang B, Siqueira WL, Cvitkovitch DG, Finer Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018;71:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. Cysteine cathepsins in human carious dentin. J Dent Res. 2011;90:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].van Strijp AJ, Jansen DC, DeGroot J, ten Cate JM, Everts V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003;37:58–65. [DOI] [PubMed] [Google Scholar]
- [14].Spencer P, Jonggu Park QY, Misra A, Bohaty BS, Singh V, Parthasarathy R, et al. Durable bonds at the adhesive/dentin interface: an impossible mission or simply a moving target? Braz Dent Sci. 2012;15:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y. Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res. 2010;89:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang B, Cvitkovitch DG, Santerre JP, Finer Y. Biodegradation of resin-dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition. Dent Mater. 2018;34:1253–62. [DOI] [PubMed] [Google Scholar]
- [17].Serkies KB, Garcha R, Tam LE, De Souza GM, Finer Y. Matrix metalloproteinase inhibitor modulates esterase-catalyzed degradation of resin-dentin interfaces. Dent Mater. 2016;32:1513–23. [DOI] [PubMed] [Google Scholar]
- [18].Shokati B, Tam LE, Santerre JP, Finer Y. Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater. 2010;94:230–7. [DOI] [PubMed] [Google Scholar]
- [19].Sadeghinejad L, Cvitkovitch DG, Siqueira WL, Merritt J, Santerre JP, Finer Y. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent Mater. 2017;33:175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Huang B, Sadeghinejad L, Adebayo OIA, D M, Xiao Y, Siqueira WL, et al. Gene Expression and Protein Synthesis of Esterase from Streptococcus mutans are affected by Biodegradation By-product from Methacrylate Resin Composites and Adhesives. Acta Biomater. 2018. [DOI] [PMC free article] [PubMed]
- [21].Gitalis R, Zhou L, Marashdeh MQ, Sun C, Glogauer M, Finer Y. Human neutrophils degrade methacrylate resin composites and tooth dentin. Acta Biomater. 2019;88:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. Adhesive/Dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Timofeeva L, Kleshcheva N. Antimicrobial polymers: mechanism of action, factors of activity, and applications. Appl Microbiol Biotechnol. 2011;89:475–92. [DOI] [PubMed] [Google Scholar]
- [24].Wicht MJ, Haak R, Kneist S, Noack MJ. A triclosan-containing compomer reduces Lactobacillus spp. predominant in advanced carious lesions. Dent Mater. 2005;21:831–6. [DOI] [PubMed] [Google Scholar]
- [25].Beyth N, Farah S, Domb AJ, Weiss EI. Antibacterial dental resin composites. Reactive & Functional Polymers. 2014;75:81–8. [Google Scholar]
- [26].Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Padois K, Bertholle V, Pirot F, Hyunh TT, Rossi A, Colombo P, et al. Chlorhexidine salt-loaded polyurethane orthodontic chains: in vitro release and antibacterial activity studies. AAPS PharmSciTech. 2012;13:1446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rupf S, Balkenhol M, Sahrhage TO, Baum A, Chromik JN, Ruppert K, et al. Biofilm inhibition by an experimental dental resin composite containing octenidine dihydrochloride. Dent Mater. 2012;28:974–84. [DOI] [PubMed] [Google Scholar]
- [29].Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–62. [DOI] [PubMed] [Google Scholar]
- [30].Zhang JF, Wu R, Fan Y, Liao S, Wang Y, Wen ZT, et al. Antibacterial dental composites with chlorhexidine and mesoporous silica. J Dent Res. 2014;93:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Delaviz Y, Nascimento MA, Laschuk MW, Liu TW, Yang M, Santerre JP. Synthesis and characterization of Ciprofloxacin-containing divinyl oligomers and assessment of their biodegradation in simulated salivary esterase. Dent Mater. 2018;34:711–25. [DOI] [PubMed] [Google Scholar]
- [32].Renne WG, Lindner A, Mennito AS, Agee KA, Pashley DH, Willett D, et al. Antibacterial properties of copper iodide-doped glass ionomer-based materials and effect of copper iodide nanoparticles on collagen degradation. Clin Oral Investig. 2017;21:369–79. [DOI] [PubMed] [Google Scholar]
- [33].Stewart CA, Finer Y, Hatton BD. Drug self-assembly for synthesis of highly-loaded antimicrobial drug-silica particles. Sci Rep. 2018;8:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stewart CA, Hong JH, Hatton BD, Finer Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018;76:283–94. [DOI] [PubMed] [Google Scholar]
- [35].Gitalis R, Bae JH, Preston M, Patel M, Liu Z, Sun C, et al. Human neutrophils compromise the restoration-tooth interface. Acta Biomater. 2020. [DOI] [PubMed]
- [36].Tam LE, Pilliar RM. Fracture toughness of dentin/resin-composite adhesive interfaces. J Dent Res. 1993;72:953–9. [DOI] [PubMed] [Google Scholar]
- [37].Zhang N, Weir MD, Romberg E, Bai Y, Xu HH. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent Mater. 2015;31:845–54. [DOI] [PubMed] [Google Scholar]
- [38].Seseogullari-Dirihan R, Apollonio F, Mazzoni A, Tjaderhane L, Pashley D, Breschi L, et al. Use of crosslinkers to inactivate dentin MMPs. Dental Materials. 2016;32:423–32. [DOI] [PubMed] [Google Scholar]
- [39].Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, et al. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dental Materials. 2010;26:1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li F, Majd H, Weir MD, Arola DD, Xu HHK. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dental Materials. 2015;31:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thompson JM, Agee K, Sidow SJ, McNally K, Lindsey K, Borke J, et al. Inhibition of Endogenous Dentin Matrix Metalloproteinases by Ethylenediaminetetraacetic Acid. Journal of Endodontics. 2012;38:62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marashdeh MQ, Gitalis R, Levesque C, Finer Y. Endodontic pathogens possess collagenolytic properties that degrade human dentine collagen matrix. Int Endod J. 2019;52:416–23. [DOI] [PubMed] [Google Scholar]
- [43].UniProt C. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, et al. The Type I Collagen Fragments ICTP and CTX Reveal Distinct Enzymatic Pathways of Bone Collagen Degradation. Journal of Bone and Mineral Research. 2003;18:859–67. [DOI] [PubMed] [Google Scholar]
- [45].Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. [DOI] [PubMed] [Google Scholar]
- [46].Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–19. [DOI] [PubMed] [Google Scholar]
- [47].De Munck J, Luehrs A-K, Poitevin A, Van Ende A, Van Meerbeek B. Fracture toughness versus microtensile bond strength testing of adhesive–dentin interfaces. Dental Materials. 2013;29:635–44. [DOI] [PubMed] [Google Scholar]
- [48].Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. [DOI] [PubMed] [Google Scholar]
- [49].Rodríguez HA, Kriven WM, Casanova H. Development of mechanical properties in dental resin composite: Effect of filler size and filler aggregation state. Materials Science and Engineering: C. 2019;101:274–82. [DOI] [PubMed] [Google Scholar]
- [50].Chen L, Yu Q, Wang Y, Li H. BisGMA/TEGDMA dental composite containing high aspect-ratio hydroxyapatite nanofibers. Dental Materials. 2011;27:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Giannini M, Liberti MS, Arrais CAG, Reis AF, Mettenburg D, Rueggeberg FA. Influence of filler addition, storage medium and evaluation time on biaxial flexure strength and modulus of adhesive systems. Acta odontologica Scandinavica. 2012;70:478–84. [DOI] [PubMed] [Google Scholar]
- [52].Shajii L, Santerre JP. Effect of filler content on the profile of released biodegradation products in micro-filled bis-GMA/TEGDMA dental composite resins. Biomaterials. 1999;20:1897–908. [DOI] [PubMed] [Google Scholar]
- [53].Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Rep. 2007;122:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lagerlof F, Dawes C. The volume of saliva in the mouth before and after swallowing. J Dent Res. 1984;63:618–21. [DOI] [PubMed] [Google Scholar]
- [55].Hubner NO, Siebert J, Kramer A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin pharmacology and physiology. 2010;23:244–58. [DOI] [PubMed] [Google Scholar]
- [56].Labow RS, Adams KA, Lynn KR. Porcine cholesterol esterase, a multiform enzyme. Biochim Biophys Acta. 1983;749:32–41. [DOI] [PubMed] [Google Scholar]
- [57].Palma‐ Dibb RG, de Moraes Rego Roselino L, Neto PT, Faraoni JJ. Strategies to Inactivate the Endogenous Dentin Proteases to Promote Resin‐ Dentin Bond Longevity in Adhesive Dentistry: A Critical Review. Progress in Adhesion and Adhesives. 2018;3:369–90. [Google Scholar]
- [58].Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. [DOI] [PubMed] [Google Scholar]
- [59].Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. [DOI] [PubMed] [Google Scholar]
- [60].Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. Chlorhexidine inhibits the activity of dental cysteine cathepsins. J Dent Res. 2012;91:420–5. [DOI] [PubMed] [Google Scholar]
- [61].Carrilho MR, Carvalho RM, de Goes MF, di Hipolito V, Geraldeli S, Tay FR, et al. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007;86:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. [DOI] [PubMed] [Google Scholar]
- [63].Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin Diagn Lab Immunol. 1999;6:437–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tezvergil-Mutluay A, Mutluay MM, Gu LS, Zhang K, Agee KA, Carvalho RM, et al. The anti-MMP activity of benzalkonium chloride. J Dent. 2011;39:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Goldfeder M, Fishman A. Modulating enzyme activity using ionic liquids or surfactants. Appl Microbiol Biotechnol. 2014;98:545–54. [DOI] [PubMed] [Google Scholar]
- [66].Rubingh DN. The influence of surfactants on enzyme activity. Curr Opin Colloid In. 1996;1:598–603. [Google Scholar]
- [67].Finer Y, Santerre JP. The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res A. 2004;69:233–46. [DOI] [PubMed] [Google Scholar]
- [68].Rouse JD, Sabatini DA, Suflita JM, Harwell JH. Influence of surfactants on microbial degradation of organic compounds. Critical Reviews in Environmental Science and Technology. 1994;24:325–70. [Google Scholar]
- [69].Finer Y, Santerre JP. Influence of silanated filler content on the biodegradation of bisGMA/TEGDMA dental composite resins. J Biomed Mater Res A. 2007;81:75–84. [DOI] [PubMed] [Google Scholar]
- [70].Yourtee DM, Smith RE, Russo KA, Burmaster S, Cannon JM, Eick JD, et al. The stability of methacrylate biomaterials when enzyme challenged: Kinetic and systematic evaluations. Journal of Biomedical Materials Research. 2001;57:522–31. [DOI] [PubMed] [Google Scholar]
- [71].Perry JA, Cvitkovitch DG, Levesque CM. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol Lett. 2009;299:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


