Abstract
The present study examined associations between parents’ and their children’s ages of onset of cannabis use using a data synthesis methodology to pool data from three similarly designed intergenerational studies. Regarding age of first use of cannabis, prospective data were collected at one or more assessments from early to late adolescence in each generation. The extent to which parent and offspring gender separately or jointly moderated intergenerational effects was examined. Data were harmonized from studies originating in the states of Washington (Bailey, Hill, Epstein, Steeger, & Hawkins, 2018), New York (Thornberry, Henry, Krohn, Lizotte, & Nadel, 2018), and Oregon (Capaldi, Kerr, & Tiberio, 2018) when the parents were in late childhood to early adolescence; analyses concerned 1081 parents and their children from 971 unique families. Parents’ and their children’s age of cannabis use onset during adolescence were modeled using discrete-time survival analysis techniques. Although data were successfully synthesized across the studies, the primary hypothesis was not supported: parents’ earlier age of first cannabis use during adolescence was not significantly associated with earlier onset of cannabis use in the offspring generation. Rather, parents’ histories of any cannabis use in adolescence—regardless of timing—were linked with increased risk for early onset cannabis use by their children compared to parents with no history of use during adolescence. There were no significant parent, child, or parent-by-child gender moderation effects. Thus, prevention of adolescent onset of cannabis in one generation may have prevention benefits for the next.
Keywords: adolescence, discrete-time survival mixture analysis, intergenerational, marijuana, cannabis, onset
Parents’ cannabis use during their children’s lives often has been considered as a risk factor for child use of cannabis and other substances (Bailey, Hill, Epstein, Steeger, & Hawkins, 2018; Patrick, Maggs, Greene, Morgan, & Schulenberg 2014). However, few studies have examined direct or indirect associations of parents’ cannabis use during their own adolescence with their children’s use at the same stage (Henry & Augustyn, 2017). In particular, although studies of adolescents find that an earlier age of onset of substance use is an important indicator of their risk for later substance use problems (Moss, Chen, & Yi, 2014), the timing of parents’ cannabis use during adolescence has not been studied sufficiently as a risk factor for their children’s early cannabis use onset. Studies that prospectively assess individuals across adolescence in two generations are ideally suited to investigate such issues, as they avoid a number of biases that affect studies that rely on retrospective self-reports of risk behaviors (e.g., forward telescoping; Johnson & Schultz, 2005). Thus, the present study aggregates data from three intergenerational studies to answer novel yet fundamental questions about familial transmission of substance use risk—specifically, the extent to which parents’ and their children’s ages of first cannabis use are associated and whether the strength of the association depends on parent or child gender. The included studies are the Oregon Youth Study–Three Generational Study (OYS–3GS), the Rochester Youth Development Study and Rochester Intergenerational Study (RYDS–RIGS), and the Seattle Social Development Project–The Intergenerational Project (SSDP–TIP).
A better understanding of the magnitude of associations between key indicators of parents’ and children’s cannabis use risk, including age of onset, will inform prevention efforts. The strength of the intergenerational association indicates the net effects of all forms (i.e., genetic and environmental) of continuity from parent to child. Likewise, a robust estimate of the intergenerational association gives an indication of the extent to which there is discontinuity across generations in the early initiation of cannabis use, which could lead to the identification of factors that ameliorate or amplify the transmission of risk.
A Dynamic Developmental Systems (DDS) approach offers a theoretical framework for explaining the development of problem behaviors, which for the present study includes early cannabis use onset (Capaldi, Tiberio, & Kerr, 2018). DDS emphasizes the interplay among biologic systems (e.g., genetic), individual characteristics (e.g., temperament; Ganiban, Ulbricht, Saudino, Reiss, & Neiderhiser, 2011), contextual factors (e.g., neighborhood, socioeconomic status), and socialization experiences (Kerr & Capaldi, 2019). DDS builds on a lifespan perspective and highlights the interaction between the individual’s prior dispositions and learning and the environments into which they are placed or selected (e.g., Caspi & Elder, 1988). Regarding intergenerational risk, the premise is that there are intergenerational continuities in social risk contexts and that genetic and temperamental factors increase an individual’s risk when they are expressed at sensitive developmental periods (Witt, 2010). Thus, the timing of these manifestations (e.g., susceptibility to deviant peer influences) is hypothesized to be similar across generations, and intergenerational congruence—similarities between generations in patterns of substance use across development, including onset—is expected (Capaldi, Kerr, & Tiberio, 2017). It follows that, to identify intergenerational continuity and discontinuity, the behaviors of parents and offspring should be examined at similar developmental periods (Thornberry, 2016).
Prior Studies of Intergenerational Associations in Cannabis Use
Intergenerational similarities in the timing of adolescent cannabis use onset have not been examined previously, and associations between other aspects of parents’ and children’s cannabis use during adolescence have not been consistently supported. In terms of positive findings, Knight, Menard, and Simmons (2014) did not examine onset but reported a direct association between frequency of parental cannabis use during adolescence and young adulthood and offspring frequency of use in these same developmental periods.
Other studies have offered inconclusive or inconsistent evidence in terms of whether there may be intergenerational similarities in onset. First, using OYS–3GS data, Kerr, Tiberio, and Capaldi (2015) tested whether fathers’ cannabis use (frequency and quantity) during their own adolescence was associated with earlier onset of cannabis use among their children. With children’s prior alcohol use onset controlled, the direct effect was not significant; although there was an indirect effect of paternal use of cannabis in adolescence on children’s cannabis use onset through the social environment (e.g., liberal use norms, exposure to cannabis use, deviant and cannabis using peers, and less adult supervision). The young age of the offspring sample at the time of that study, the consideration of fathers only, and the adjustment for children’s prior onset of alcohol use limits comparisons with other studies.
Second, in Hill, Sternberg, Suk, Meier, and Chassin’s (2018) primary analyses, parental history of a cannabis use disorder (CUD) was prospectively related to their adolescent child’s cannabis use (ages 13–19 years), whereas a history of cannabis use by parents without CUD was not. However, the authors noted that assessments of parents’ histories were not confined to their adolescence and that, in a model that omitted parents’ lifetime alcohol use disorder diagnosis (through age 34 years) as a covariate, parents’ cannabis use histories did predict their adolescent children’s cannabis use.
Third, among SSDP–TIP families, past-month frequency of parents’ use of cannabis (ages 15 to 18 years) was not associated with use of cannabis by their children (varying ages 10–22 years) in the past year (Bailey et al., 2016). Notably, however, parents’ cannabis use in earlier adolescence was not examined, and some offspring were assessed after adolescence. Finally, Henry and Augustyn’s (2017) study of RYDS–RIGS data was similar to an examination of parent–child associations in onset, although the full range of adolescent onset ages was not considered. They found that 29% of parents and 14% of offspring had used cannabis by age 15 years, which was an a priori defined age category. They found an indirect association between fathers’ and children’s use by this age through fathers’ cannabis use disorder, but no intergenerational association between mothers’ and children’s use by age 15 years. In summary, intergenerational associations in adolescent cannabis use rarely have been examined directly, few studies have considered parents’ and their children’s identical behavior during the same developmental periods, and the timing of parents’ adolescent cannabis use onset has not previously been examined as a predictor of child onset.
Gender Differences in Intergenerational Transmission of Cannabis Onset
Moderation of intergenerational congruence by parent gender may be expected due to several factors, including gender differences in substance use prevalence, caregiving responsibilities, and parent–child contact. Given mothers’ primary role in caregiving (Raley, Bianchi, & Wang, 2012), their substance use may have a larger impact on parenting and their children’s risk behaviors than that of fathers (Capaldi, Tiberio, Kerr, & Pears, 2016). However, this might only be expected if parental use persists into adulthood and coincides with children’s development. Such persistence is less likely for mothers than fathers, given females’ lower rates of heavy cannabis and other drug use and stronger desistance over development. For fathers, substance use patterns during their own adolescence may contribute more to the formation of family risk than their use during adulthood, given the negative life-course consequences of boys’ early delinquency and substance use (Thornberry, Krohn, Freeman-Gallant, 2006) and boys’ higher rates of such behaviors (Baillargeon et al., 2007; Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2014; Loeber & Hay, 1994). However, fathers—and particularly heavier substance using fathers—more often show reduced contact with their children, significantly limiting potential direct influence (Kerr, Tiberio, Capaldi, & Owen, 2020).
Despite the theoretical justification for examining differences in intergenerational continuity in substance use onset by parent gender, and the potential implications for tailored prevention, there has been little empirical work regarding such differences. Existing studies do not offer a clear picture of whether mothers’ or fathers’ substance use is linked more strongly with children’s substance use. For example, in an OYS–3GS study, children’s earlier age of alcohol use onset was associated with mothers’ adult alcohol and tobacco use and the interaction of fathers’ cannabis and alcohol use (Capaldi et al., 2016). In another OYS–3GS study, Kerr, Tiberio, Capaldi, and Owen (in press) reported that children more often showed early onset polysubstance use (including cannabis) if their father showed this pattern. Of note, fathers’ early polysubstance use was less strongly associated with child substance use compared to mothers’ use of more types of substances (alcohol, tobacco, and/or cannabis) by age 19 years, but maternal reports were retrospective in adulthood. In a study based on RYDS–RIGS data, children’s early substance use (any alcohol or cannabis use combined) was predicted by maternal but not paternal substance use (cannabis use and binge drinking) during adolescence and young adulthood (Thornberry et al., 2006). However, at that time only 20% of the offspring sample was aged 13 years or older. In a more recent RYDS–RIGS study, Henry and Augustyn (2017) found that 25.6% of fathers had used cannabis by age 15 years, versus 33.3% of mothers, but more fathers than mothers later met criteria for a cannabis use disorder in their late 20s or early 30s (6.8% vs. 1.9%). Regarding intergenerational associations, children were about twice as likely to show onset of cannabis use by age 15 years if their father had onset by that age (20.6%) than if he had not (11.1%). However, there were no associations between parents’ and children’s onset once background variables were controlled.
In summary, although there are theoretical and practical reasons to consider moderation by parent gender, there is not a clear basis for predicting which parents’ early cannabis use onset may be more strongly associated with risk for their children’s onset. Though evidence from OYS–3GS and RYDS–RIGS seems to suggest that mothers’ adolescent onset may be the more salient predictor, the original studies either exclusively or more heavily enrolled boys (who were later the parents of the third generation). Therefore, further research with larger samples—particularly of mothers during their adolescence —is needed to quantify whether parent gender moderates intergenerational similarities in cannabis use onset.
Regarding moderation of intergenerational congruence in timing of cannabis use onset by child gender, there is less evidence than compared to parent gender. Due in part to statistical power limitations, intergenerational studies often have considered child gender as a control variable, but not as a moderator (e.g., Kerr et al., 2015). In one exception, RYDS–RIGS data indicated parents’ (primarily mothers’) substance use predicted their children’s substance use in three different developmental periods (adolescence, emerging adulthood, and adulthood), but only for daughters (Thornberry et al., 2006). Thus, the larger sample size enabled by a data synthesis approach may help answer this question regarding child gender differences.
Hypotheses and Research Questions
Data were pooled from three studies to test hypotheses that no single study could adequately address alone. A synthesis approach increases power due to increased sample size, which is particularly valuable when testing moderation for a binary variable (e.g., gender). Furthermore, data synthesis heightens confidence in generalizability beyond individual study designs and sample characteristics (e.g., ethnicity and U.S. region) and contributes to a more cumulative science (see Hussong, Curran, & Bauer, 2013).
Our first hypothesis (H1) was that parents’ earlier age of onset of cannabis use during adolescence would predict their children’s earlier age of onset of use of cannabis. Again, this has not been established using prospective methods. The remaining research questions concerned differences in H1 by parent and child gender. We hypothesized (hypothesis 2; H2) that—compared to fathers’ age at onset of cannabis use—mothers’ onset age would be more strongly related to that of offspring (i.e., parent onset by parent-gender interaction). Next (hypothesis 3; H3) we predicted that—compared to sons’—daughters’ onset age would be more strongly related to that of their parents (i.e., parent onset by child gender interaction).
Finally, we examined moderation by both parent and child gender. Higher concordance for mothers and daughters than for other parent–child pairings has been supported in some research on cannabis use disorder (Kosty et al., 2015) and substance use more generally, perhaps reflecting girls’ sensitivity to modeling of mothers’ substance use behaviors (Thornberry et al., 2006). Thus, we hypothesized (hypothesis 4; H4) that mother–daughter dyads would show stronger intergenerational associations in cannabis use onset than other parent–child gender pairings.
Methods
Selection of Studies for Synthesis
The three studies considered presently have been underway for 30–35 years and have core features in common that enable and justify synthesis. Each study originally recruited a focal participant in late childhood or adolescence (Generation 2; G2) and their parents (Generation 1; G1) and the studies assessed G2 youth repeatedly to adulthood. Each project later initiated a second prospective study by recruiting and following offspring (Generation 3; G3) of the G2 focal participants over time. Although sampling strategies differed, there also were important areas of overlap. G2 focal participants were all from a similar age cohort, entering adolescence in the late 1980s and, thus, also began forming families at a similar time. Furthermore, all of the studies sampled youth at risk for crime due to neighborhood (but not individual) factors. Another critical design similarity was that, in all three studies, adolescents in both generations were asked about their age of first use of cannabis at one or more assessments from early to late adolescence.
Some features of the studies do not overlap but instead complement each other when data are pooled. The studies took different approaches to sampling boys (SSDP sampled boys and girls equally, RYDS oversampled boys, and OYS sampled boys only). Thus, the pooled sample more strongly represents fathers’ influences than is typical of studies of adolescents (Phares, Fields, Kamboukos, & Lopez, 2005) and permits examination of parent gender differences that the individual samples do not. Again, given the regional and ethnic compositions of the samples, pooling was expected to increase confidence in generalizability of findings. Thus, there was a strong a priori rationale in terms of overlap and was complementarity for selecting the three studies for potential synthesis. Formal testing and adjustment for study differences also follow below. The three studies are described next, and sample characteristics are summarized in Table 1.
Table 1.
Demographic Descriptions of G2 Focal Samples.
| SSDP–The Intergenerational Project | RYDS—Rochester Intergenerational Study | Oregon Youth Study–Three Generational Study | |
|---|---|---|---|
| State of G2 residence | Washington | New York | Oregon |
| Type of G2 sample | Urban, school based | Urban, school and community based | Medium-sized city, school based |
| Basis of G2 problem behavior risk | Schools that served higher and lower crime areas | Boys and residents of higher crime areas of city oversampled | Schools from neighborhoods with higher juvenile delinquency; boys only |
| Period | 1985–present | 1987–2018 | 1984–present |
| G2 focal sample size and gender | N = 808 (51% boys) | N = 1000 (75% boys) | N = 206 boys |
| Average year of birth of G2 | G2 men and women: 1975 | G2 men and women: 1974 | G2 men: 1974 |
| G2 Ethnicity (N, %): | |||
| European American | (381, 47.2%) | (150, 15.0%) | (185, 89.9%) |
| African American Hispanic or Latino | (207, 25.6%) * | (680, 68.0%) (170, 17.0%) | (5, 2.4%) (3, 1.5%) |
| Other (Asian or Native American) | (220, 27.2%) | (0) | (13, 6.3%) |
Note.
Of the SSDP parents, 5% were Hispanic or Latino; G2 = Generation 2.
Oregon Youth Study–Three Generational Study (OYS–3GS)
G2 boys and their families were recruited into the OYS by inviting the entire fourth grade (ages 9–10 years) of boys from schools in neighborhoods with a higher-than-usual incidence of delinquency within a medium-sized city in Oregon to participate. Neighborhood delinquency was assessed by analyzing the home addresses of youth committing delinquent acts compared with the school-boundary areas. Thus, the boys were at elevated risk for delinquency but were not necessarily showing conduct problems at the time of recruitment (Capaldi, Kerr, & Tiberio, 2018). Face-to-face home visits for recruitment were key to attaining strong participation rates (Capaldi, Chamberlain, Fetrow, & Wilson, 1997; Capaldi & Patterson, 1987). The OYS family recruitment rate was 74.4%, and retention rates of G2 men were 98% through high school, 97% through the mid-20s, and 93% through the mid-30s. Data collection for OYS began in 1984 and was yearly from ages 9–10 through 31–32 years, with additional assessments at ages 35–36 and 37–38 years. These assessments involved data on the G2 boys/men, and data on the G1 parents also were collected through their son’s age 17–18-year assessment.
Risk for externalizing behaviors was elevated for the OYS boys compared with a comparable national sample (NLSY–Child; Bureau of Labor Statistics, 2010) at ages 9–10 and 10–11 years (the first two waves of OYS) but not at later adolescent ages. The variance did not differ; thus, range was not restricted (Capaldi, Kerr, & Tiberio, 2018).
The OYS–3GS was initiated in 1995 to examine the intergenerational transmission of substance use and related problem behaviors using a fully prospective design. Data collection for G3 is on a developmental schedule (e.g., an assessment at age 2 years, the next assessment at age 3 years). The study included up to two children from each of the G2 men’s female partners who were mothers of his biological children (recruitment of stepchildren was dropped early in the study). The majority of OYS men who became fathers (n =154 of 161; 95.6%) participated in 3GS at one or of the child assessments. To be included in the present analyses, children had to have participated in at least one assessment between ages 11 and 19 years, yielding data on N = 113 fathers (70.2% of eligible OYS men who became fathers) and their N = 223 adolescent-aged children (n = 124 girls). OYS fathers included in the current analyses did not differ from nonparticipating fathers on age-of-onset categories (χ2(2) = .52, p = .77; with 40.7%, 29.2%, and 30.1% of participating fathers and 45.8%, 29.2%, and 25.0% of nonparticipating fathers having abstained, or onset in mid to late adolescence [ages 15–18 years] or early adolescence [age 14 years or younger], respectively).
G2 fathers’ reports of adolescent cannabis use were collected annually from ages 11–12 to 17–18 years. Assessments of G3 children’s cannabis use started in late childhood (age 9 years) and four biannual assessments occurred across adolescence. Mothers, fathers, and children were interviewed separately. The N available for each wave is determined by the ages of the maturing G3 children (see Table 2); for adolescent-aged G3 children, 95.1% participated at an assessment of cannabis use prior to age 13 years, whereas 46.6% of the sample was old enough to have participated at ages 17–18 years. All data collection procedures were approved by the Oregon Social Learning Center Institutional Review Board (IRB).
Table 2.
Descriptive Statistics for Current Sample
| Overall | SSDP–TIP | RYDS–RIGS | OYS–3GS | |
|---|---|---|---|---|
| Total sample size (N, % male) | G2: 1081 (63.1%) | G2: 342 (36.5%) | G2: 516 (64.7%) | G2: 113 unique fathers (100%) |
| G3: 1017 (49.0%) | G3: 340 (51.0%) | G3: 454 (49.7%) | G3: 223 (44.4%) | |
| Ethnicity (N, %): | ||||
| European American | G2: (426, 39.4%) | G2: (161, 47.1%) | G2: (64, 12.4%) | G2*: (201, 90.1%) |
| G3: (318, 32.3%) | G3: (103, 30.4%) | G3: (45, 10.6%) | G3: (170, 76.2%) | |
| African American | G2: (477, 44.1%) | G2: (96, 28.1%) | G2: (376, 72.9%) | G2*: (5, 2.2%) |
| G3: (364, 36.8%) | G3: (57, 16.7%) | G3: (297, 70.0%) | G3: (10, 4.5%) | |
| Hispanic or Latino | G2: (81, 7.5%) | G2: (0) | G2: (76, 14.7%) | G2*: (5, 2.2%) |
| G3: (99, 10.0%) | G3: (41, 12.0%) | G3: (48, 11.3%) | G3: (10, 4.5%) | |
| Multiracial/ethnic | G2: NA | G2: NA | G2: NA | G2: NA |
| G3: (148, 15.0%) | G3: (101, 29.8%) | G3: (34, 8.0%) | 3: (13, 5.8%) | |
| Other (Asian or Native American) | G2: (97, 9.0%) | G2: (85, 24.9%) | G2: (0) | G2*: (12, 5.4%) |
| G3: (59, 6.0%) | G3: (38, 11.1%) | G3: (1, .1%) | G3: (20, 9.0%) | |
| Birth cohort year (Mean, SD) | G2: 1974 (.79) | G2: 1975 (.50) | G2: 1974 (.79) | G2: 1974 (.72) |
| G3: 1997 (4.80) | G3: 1998 (3.99) | G3: 1995 (4.48) | G3: 1988 (4.96) | |
| Maximum age of participation (Mean, SD) | G2: 18.05 (.68) | G2: 18.13 (.43) | G2: 17.86 (.70) | G2: 18.40 (.78) |
| G3: 16.56 (2.23) | G3: 15.39 (2.43) | G3: 17.76 (.77) | G3: 15.91 (2.64) | |
| Sample size by age (N) | ||||
| G2 parents: | ||||
| Ages < 13 years | 517 | 341 | 63 | 113 |
| Ages 13–14 years | 902 | 339 | 451 | 112 |
| Ages 15–16 years | 966 | 340 | 516 | 110 |
| Ages 17–18 years | 885 | 326 | 448 | 111 |
| G3 offspring: | ||||
| Ages < 13 years | 875 | 251 | 412 | 212 |
| Ages 13–14 years | 767 | 162 | 430 | 175 |
| Ages 15–16 years | 707 | 153 | 414 | 140 |
| Ages 17–18 years | 630 | 147 | 379 | 104 |
| Cannabis use onset: n who onset / n at risk to onset, (% who onset) | ||||
| G2 parents: | ||||
| Prior to age 13 years | 113/990 (11%) | 40/341 (12%) | 24/426 (6%) | 49/223 (22%) |
| Ages 13–14 years | 154/946 (16%) | 53/301 (18%) | 74/473 (16%) | 27/172 (16%) |
| Ages 15–16 years | 171/809 (21%) | 65/247 (26%) | 72/418 (17%) | 34/144 (24%) |
| Ages 17–18 years | 112/582 (19%) | 26/176 (15%) | 54/296 (18%) | 32/110 (29%) |
| G3 offspring: | ||||
| Prior to age 13 years | 24/991 (2%) | 7/320 (2%) | 3/449 (1%) | 14/222 (6%) |
| Ages 13–14 years | 101/852 (12%) | 39/251 (16%) | 30/434 (7%) | 32/167 (19%) |
| Ages 15–16 years | 112/666 (17%) | 29/178 (16%) | 50/386 (13%) | 33/102 (32%) |
| Ages 17–18 years | 86/444 (19%) | 7/93 (8%) | 65/301 (22%) | 14/50 (28%) |
Note:
Ethnicity for OYS fathers presented at the G3 child level, originating from N = 113 unique fathers; G3 ethnicity was only available for 93.6% (n = 425) of the RYDS–RIGS G3 offspring. G2 = Generation 2, G3 = Generation 3; SD = Standard deviation.
Measures: cannabis use onset in adolescence.
Beginning at age 9 years, G3 children were asked at each assessment if they had ever tried marijuana1 (yes or no) and, if so, their age at first use. Lifetime-use questions and self-reported age of first use from the first assessment in which children reported having ever used marijuana were used to create four binary variables for cannabis use onset at ages < 13, 13–14, 15–16, and 17–18 years. Once a child onset, all subsequent scores were set to missing values as s/he was no longer at risk for onset at those ages. Right censoring of onset due to age (e.g., if a child was too young to have participated yet at the age 17–18-year assessment) also was represented with missing data codes.
Fathers’ cannabis use was measured annually across adolescence starting at ages 11–12. Specifically, at that first assessment, boys were asked if they had ever used marijuana and, if so, their age of first use. At all subsequent annual assessments to ages 17–18 years, boys were asked about use in the past year. New reports of past-year use at the later assessments were used to define onset for each subsequent period.
Rochester Youth Development Study and Rochester Intergenerational Study (RYDS–RIGS)
RYDS began in 1988; the intergenerational extension, RIGS, began in 1999. Detailed information about the designs of these studies is presented elsewhere (Thornberry, Henry, Krohn, Lizotte, & Nadel, 2018). The original RYDS sample of 1,000 G2 adolescents is representative of the seventh- and eighth-grade public school population of Rochester, NY in 1988. RYDS was initially funded by the Office of Juvenile Justice and Delinquency Prevention to study delinquency during adolescence. As such, youth at high risk for antisocial behavior were overrepresented by oversampling males (75% males/25% females) and residents in high-crime areas of the city. Some 80% of the initial families invited to participate in the study agreed, families that refused were replaced by another family from the same stratum (sex, school grade, and census tract). The RYDS families are predominantly African American (see Table 1). Although the sample represented the full socioeconomic spectrum of the city of Rochester at the inception of the study (Farnworth, Thornberry, Krohn, & Lizotte, 1994), many of the families were relatively poor. For example, at the start of RYDS, some 50% of the families received public assistance and, on average, G1 completed 11 years of education.
RYDS participants completed regular interviews in school or home every 6 months from 1988–1992 (Phase 1), annually from 1994–1996 (Phase 2), and biannually from 2003–2006 (Phase 3). In general, sample retention was high (88% at Phase 1, 83% at Phase 2, and 80% at Phase 3), and analyses reveal attrition did not bias the sample (Thornberry, 2013).
Beginning in 1999, RIGS selected G2’s oldest biological child and added new firstborns to this G3 sample in each subsequent year. G2 in RYDS–RIGS did not differ from the full sample of RYDS participants who became parents on rates of cannabis use disorder or early onset cannabis use (Henry & Augustyn, 2017). Both G2 and G3’s other primary caregiver completed annual in-home interviews (with phone interviews administered when necessary) since the inception of RIGS (continuing until G3 turns/turned age 18 years), and G3 completed annual interviews once they turned age 8 years. There are prospective, longitudinal data on 539 parent–child dyads. The present analysis utilizes data from 516 parent–child dyads (334 father–child, 182 mother–child); this includes all dyads in which G3 was of early childhood or adolescent age (when substance use was assessed). The children were evenly split by sex. All data collection procedures were approved by the University at Albany IRB.
Measures: cannabis use onset in adolescence.
At the first interview (Phase 1 of RYDS) for G2, and during the age 8-year RIGS interview for G3, respondents reported if they ever used cannabis and, if they had, at what age they started using. For both G2 and G3 in RYDS and RIGS, respectively, in subsequent interviews, respondents reported if they used cannabis since the last interview. Using this information, a binary indicator of age of onset cannabis use was created for each discrete-time age category.
Seattle Social Development Project–The Intergenerational Project (SSDP–TIP)
SSDP G2 participants (N = 808; 77% of those eligible) were recruited in 1985 from fifth-grade classrooms in 18 schools in an urban school district in Washington State. G2 were surveyed annually from ages 10–16 years and at ages 18, 21, 24, 27, 30, 33, 35, and 39 years (in 2014). A G1 parent and G2’s teachers were surveyed annually when the G2 students were ages 10–16 and 10–14 years, respectively. Ethnicity for the focal G2 participants is provided in Table 1, who were approximately one half European American, and one quarter African American and Asian American. About 52% of G2 students were eligible for the U.S. National Free and Reduced-Price Lunch Program in Grades 5, 6, or 7.
In 2000, TIP was initiated and G3 children were recruited. The TIP sample families include: the G2 parent who participated in SSDP (all SSDP participants with biological children were screened for eligibility), the eldest biological child with whom they had regular contact (G3), and an other caregiver (usually the G2 participant’s spouse) identified by the G2 parent as sharing the greatest responsibility for raising the child.
To date, the TIP sample includes 423 families. About 48% of the G3 children and 60% of the SSDP G2 parents were female. The G3 sample was racially/ethnically diverse: 31% European American, 20% African American, and 35% multiethnic, with the remainder being primarily Asian American/Pacific Islander or Native American; 12% of G3s were Latinx. The median family income in 2015 was U.S. $65,001–$70,000. Recruitment of eligible SSDP parents and their children has averaged 82% across waves. Retention from wave to wave averaged 90%. Across waves, SSDP G2 mothers and G2s who were married were consistently more likely to meet eligibility criteria (regular, face-to-face contact with the child) than SSDP fathers and unmarried SSDP participants. Once eligible, families were slightly less likely to be recruited if the SSDP parent was Asian American or had been eligible for free lunch in Grades 5–7. G2 parents’ eligibility for and recruitment to TIP were not related to their rates of cannabis use in high school or at age 27 years (Bailey et al., 2018).
In 2002 when the first wave of TIP data collection occurred, G3 children ranged in age from 1 to 13 years. New families were recruited as SSDP respondents became biological parents for the first time. Families were eligible to participate in TIP if SSDP G2 parents had face-to-face contact with the target child at least once a month. Data were collected annually around the child’s birthday (+/− 6 weeks). To date, 10 waves of annual data have been collected between 2002 and 2018. Data were collected via face-to-face and phone interviews, web surveys, and observations. Reporters included the SSDP G2 parent, the other caregiver, and G3 children age 6 years or older; younger children were not interviewed directly, but their SSDP parent and other caregiver were interviewed. SSDP parents, other caregivers, and children age 18 years or older provided informed consent at each data collection. Parents provided permission for children under age 18 years, and children assented. Parents provided permission for teacher contact, and teachers were consented at each data collection. All procedures were approved by the University of Washington IRB.
Measures: cannabis use onset in adolescence.
G2 adolescents were asked at the age 11-year assessment of SSDP whether they had ever used marijuana and, if yes, the age at which they first used. At the subsequent annual assessments, adolescents were asked about use in the past year. Similarly, G3 were asked at the age 10-year assessment whether they had ever used marijuana and, if yes, the age at which they first used. At subsequent annual assessments, G3 adolescents were asked about use in the past year. For G2 and G3, new reports of use at the later assessments were used to define onset for each subsequent period.
Analysis Plan
There were three steps to the present analyses. First, ages of cannabis use onset were specified for adolescents in each generation using survival analysis techniques, and models were run to account for any study differences and person-level differences in age of onset. Second, heterogeneity in G2 adolescent cannabis use onset was characterized by specifying latent age of onset classes. Third, intergenerational hypotheses H1–H4 were tested relating G2 to G3 ages of onset and evaluating gender moderation.
G2 and G3 ages of cannabis use onset were modeled using DTSA techniques (Muthén & Masyn, 2005). Four discrete-time age categories were included: prior to age 13 years, 13–14 years, 15–16 years, and 17–18 years. Once an adolescent showed onset, all of her or his scores at older ages were set to missing values. Given dependence among OYS–3GS siblings’ scores for OYS fathers who had multiple participating children, a sandwich estimator was used to adjust the standard errors in all models (Muthén, & Muthén, 1998–2017).
Accounting for study and person-level differences in age of onset.
Prior to examining hypotheses, DTSA models were estimated separately for G2 and G3 to examine differences in age of onset attributable to study membership and person-level covariates (i.e., gender, child maximum age of participation, birth cohort year), and their two-way interactions. Models involving two-way interactions between covariates on age of onset were estimated first. In addition, interactive effects were estimated separately for each of the four DSTA age categories to allow effects to differ across age (i.e., person-level covariate). (All model results are available from authors by request.) Given that all OYS G2 parents were men and there was not sufficient variance to examine differences by G2 birth cohort year, only two types of interactions were tested for G2. Results indicated no significant parent gender by SSDP versus RYDS interaction, nor were the interactions between parent gender and maximum age of participation significant, at any of the age categories. For G3, all two-way interactions between study and person-level covariates were nonsignificant at all ages of adolescence or could not be tested.2
After determining there were no significant interactions between study membership and person-level covariates, DTSA models were estimated separately for each generation accounting for main effects of the aforementioned covariates as follows. Rates of adolescent cannabis onset were allowed to vary across studies by including study indicator variables, which denoted differences in all pairwise study comparisons (i.e., SSDP–TIP vs. RYDS–RIGS, OYS–3GS vs. RYDS–RIGS, SSDP–TIP vs. OYS–3GS). Furthermore, the study indicator variables predicted each of the binary discrete-time age-of-onset categories (i.e., proportional odds in study differences in rates of onset across adolescence were not assumed). Whereas for model identification, the effects of the remaining control variables were constrained to be equal across adolescence. First, G2 and G3 gender was included as a predictor of age of onset (coded as male = 1, female = 0). Second, to account for study design differences related to right censoring, child age at her or his last completed assessment was included as a predictor of onset for both generations.3 Third, birth cohort year was included as a predictor of G3 age of onset (M[SD] = 1997[4.8], range 1986–2010). Note that similarities in when the initial G2 studies began and the ages of the G2 focal children recruited resulted in little variance in G2 birth cohort year (M[SD] = 1974[.8], range: 1972 – 1976); thus, it was not included as a predictor of G2 age of onset. Results for the study and person-level covariates are discussed below and were included in all subsequent models involving hypothesis tests.
Identifying heterogeneity in G2 adolescent cannabis use onset.
In DTSA, only the mean rates of onset at each age category are estimated (i.e., hazard rates); as such, there is no variance in age of onset between persons (Muthén & Masyn, 2005). Thus, in order to predict between-subjects’ variability in age of onset in one process (i.e., G3 onset) from between-subjects’ variability in another age of onset process (i.e., G2 onset), variance into G2 age of onset must be introduced. This can be achieved either by fitting a frailty model, which allows for age of onset—specifically the hazard rate—to be modeled as a random effect that varies across persons (e.g., akin to random effects in a latent growth curve model). Alternatively, mixture modeling can be used to identify latent classes defined by different age-of-onset patterns (e.g., early and late adolescent onset classes). “Another way to conceptualize mixture modeling is as a nonparametric approach to estimating an underlying continuous frailty distribution; that is, a random effect for the outcome that represents differences in individuals with respect to the survival process (p. 37; Muthén & Masyn, 2005).” In the present study, a mixture modeling approach was utilized to allow for greater interpretability of the results (compared to a random effects frailty model) by direct comparisons of G3 ages of onset across different types of G2 parental adolescent onset patterns (i.e., parents who never used vs. those who used in early vs. late adolescence).
While accounting for study and person-level covariates, heterogeneity in G2 mothers’ and fathers’ ages of onset was examined using discrete-time survival mixture analyses (Muthén & Masyn, 2005). To differentiate among parents who had abstained from cannabis use as adolescents versus those who onset in early or late adolescent, one of the classes was defined by long-term survivors (Muthén & Masyn, 2005); parameters for other latent classes were freely estimated. The number of classes necessary to summarize the variance in G2 adolescent cannabis use onset rates most adequately was based on information criteria and the Lo-Mendell-Rubin likelihood ratio test (Lo, Mendell, & Rubin, 2001), as well as on the parsimony and interpretability of the classes.
Models testing H1–H4 regarding intergenerational transmission of cannabis use onset.
To test H1 regarding intergenerational transmission of adolescent cannabis use onset, G2 mothers’ and fathers’ most likely class memberships then served as the predictors of G3 offspring age of onset in a DTSA. Note, given that moderation of intergenerational transmission was examined by gender, these analyses used the observed most likely class memberships and thus did not correct for uncertainty in class assignment (Asparouhov & Muthén, 2013). Next, we tested separately whether intergenerational transmission was moderated by parent (H2) or child (H3) gender by creating two two-way interaction terms between G2 onset class membership and parent or child gender. Lastly, to evaluate H4, a three-way interaction among G2 onset class membership, child gender, and parent gender was examined. Effects coding was used to test all interaction terms by assigning values of 0 and 1 to the dummy coded predictor variables and then creating the interaction terms.
Results
Descriptive Statistics
G2 and G3 sample sizes, maximum ages of participation, and cannabis use onset rates at each of the four age categories are presented in Table 2 for all participants and separately by study. Sample size by age generally reflects differences in generations and study designs. G2 parents from all studies had, on average, participated at the end of adolescence (ages 17–18 years). This also was true for RIGS G3 offspring; although fewer G3 TIP and OYS–3GS adolescents participated at the older compared with the younger ages, with children being on average in midadolescence (ages 15–16 years) at their last assessment.
In general, rates of cannabis use onset increased across adolescence for both generations. For G2, OYS fathers were more likely to have used prior to age 13 years than SSDP and RYDS parents, but few other study differences were apparent. For G3, OYS–3GS adolescents had the highest rates of onset at all ages compared to other studies. Prevalence of G2 parents’ adolescent cannabis use was 50.7% (548/1081) across all studies, with prevalence for SSDP, RYDS, and OYS parents equaling 53.2% (182/342), 43.4% (224/516), and 63.7% (142/223), respectively. For G3, prevalence of adolescent cannabis use was 29.0% (314/1081) across all studies, equaling 24.0% (82/342) for SSDP–TIP, 28.3% (146/516) for RYDS–RIGS, and 38.6% (86/223) for OYS–3GS. Detailed information regarding the length of time between age of onset and when onset was assessed is given in the Supplemental Table. Among those adolescents who had used cannabis, for 89.2% of G2 parents and 82.8% of G3 offspring no more than 2 years had passed between their age of onset and when onset was assessed.
Again, the three steps to the present analyses involved: (a) modeling ages of onset for G2 and G3 adolescents, accounting for any study and person-level difference; (b) characterizing heterogeneity in G2 adolescent cannabis use onset in latent classes; and (c) testing hypotheses regarding intergenerational associations and moderation by parent and child gender.
Study and Person-Level Differences in Age of Onset of Cannabis Use
DTSA results for both generations denoting differences in rates of cannabis use onset across the studies are shown in Table 3. Estimated survival curves by study are depicted in Figure 1. In general, rates of cannabis use onset significantly differed across the studies, although not at all of the adolescence assessments. For G2 (Table 3, column 1), OYS–3GS fathers were significantly more likely to have used cannabis prior to age 13 years compared to RYDS–RIGS and SSDP–TIP parents, and greater rates of use prior to age 13 years were also observed for SSDP–TIP than for RYDS–RIGS parents. The only other significant study differences were that SSDP–TIP parents were significantly more likely to have onset on cannabis use at ages 15–16 years, compared to RYDS–RIGS parents, but less likely to have onset at ages 17–18 years compared to OYS–3GS parents. In sum, OYS fathers showed the greatest risk for pre- and early adolescent onset, followed by SSDP–TIP and then RYDS–RIGS parents, but few study differences were found later in adolescence.
Table 3.
Discrete-Time Survival Analysis Results: Differences in Adolescent Cannabis Use Onset Rates by Study and Person-Level Covariates
| G2 parents | G3 offspring | |
|---|---|---|
| Ages < 13 years: | ||
| Threshold for RYDS–RIGS | 2.68 (.23)*** | 5.49 (.61)*** |
| SSDP–TIP vs. RYDS–RIGS | .67 (.27)** | 1.78 (.72)* |
| OYS–3GS vs. RYDS–RIGS | 1.39 (.34)*** | 2.72 (.66)*** |
| SSDP–TIP vs. OYS–3GS | –.72 (.33)* | –.94 (.49) |
| Ages 13–14 years: | ||
| Threshold for RYDS–RIGS | 1.55 (.15)*** | 3.10 (.23)*** |
| SSDP–TIP vs. RYDS–RIGS | .02 (.20) | 1.43 (.28)*** |
| OYS–3GS vs. RYDS–RIGS | –.16 (.35) | 1.47 (.29)*** |
| SSDP–TIP vs. OYS–3GS | .18 (.36) | –.04 (.29) |
| Ages 15–16 years: | ||
| Threshold for RYDS–RIGS | 1.44 (.15)*** | 2.43 (.20)*** |
| SSDP–TIP vs. RYDS–RIGS | .43 (.20)* | .63 (.27)* |
| OYS–3GS vs. RYDS–RIGS | .24 (.34) | 1.30 (.27)*** |
| SSDP–TIP vs. OYS–3GS | .19 (.35) | –.67 (.31)* |
| Ages 17–18 years: | ||
| Threshold for RYDS–RIGS | 1.42 (.17)*** | 1.84 (.19)*** |
| SSDP–TIP vs. RYDS–RIGS | –.32 (.27) | –1.17 (.45)** |
| OYS–3GS vs. RYDS–RIGS | .49 (.35) | .39 (.32) |
| SSDP–TIP vs. OYS–3GS | –.81 (.39)* | –1.56 (.51)** |
| Ages < 13 thru 18 years: | ||
| Maximum age of participation | .35 (.10)*** | .22 (.07)** |
| Adolescent is male | –.11 (.12) | .15 (.12) |
| Birth cohort year | NA | –.05 (.02)* |
Note.
p < .05.
p < .01.
p < .001;
NA = not applicable. G2 = Generation 2, G3 = Generation 3.
Figure 1.
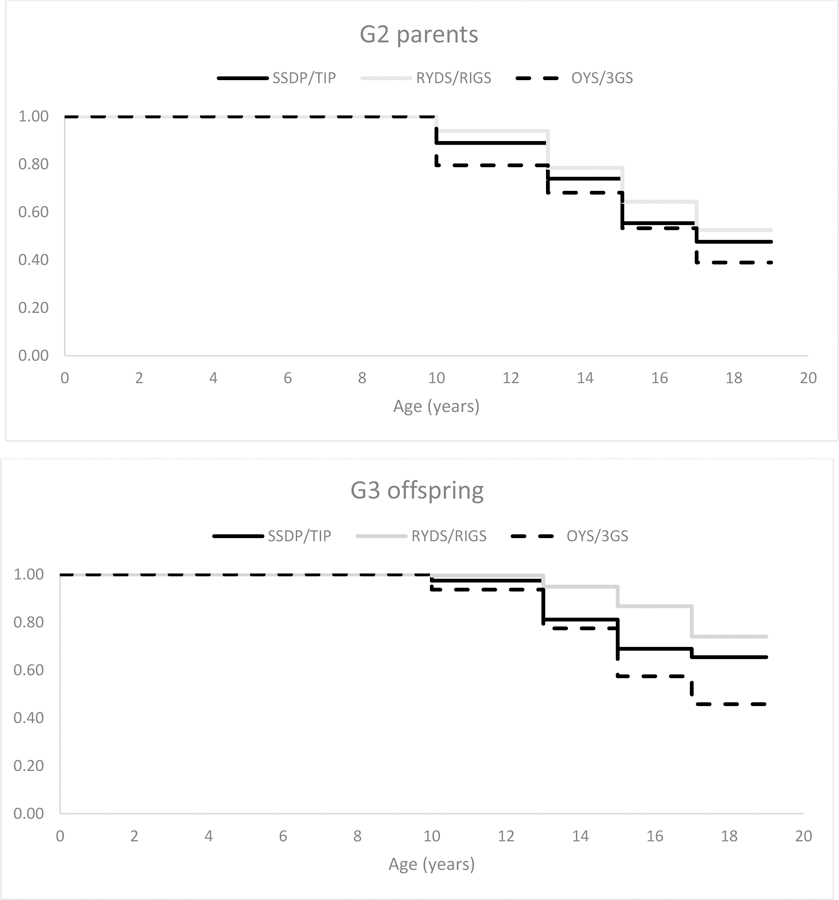
Estimated survival curves for Generation 2 parents and Generation 3 offspring adolescent cannabis use onset by study; onset prior to age 13 years is depicted across adolescent ages 10 to 12.99 years; note, however, that n = 31 of N = 113 (27.4%) Generation 2 parents and n = 3 of N = 24 (12.5%) Generation 3 offspring reported onset prior to age 10 years.
For the G3 offspring (Table 3, column 2), RYDS–RIGS adolescents were significantly less likely to have onset on cannabis use at all ages except 17–18 years, compared to SSDP–TIP and OYS–3GS adolescents. Whereas in late adolescence (ages 17–18 years), SSDP–TIP adolescents were significantly less likely to have onset on cannabis use than RYDS–RIGS and OYS–3GS adolescents. Finally, in midadolescence (ages 15–16 years), SSDP–TIP adolescents were less likely to have onset then OYS–3GS adolescents. Thus, RYDS–RIGS adolescents showed the lowest rates of cannabis use onset in early and midadolescence relative to G3 adolescents in the other two studies; whereas in mid and late adolescence, SSDP–TIP adolescents showed the lowest risk. Differences in ages of onset across studies are accounted for by including study membership as covariates when performing hypothesis tests.
The main effects of the person-level covariates (i.e., gender, maximum age, and for G3 birth cohort year) on age of onset were examined assuming proportional odds, thus effects did not differ across adolescence. Earlier ages of cannabis use onset were not observed for adolescent boys versus girls in either the G2 parent generation or in the G3 offspring generation. Maximum age of participation was a significant predictor of onset for both generations, indicating that adolescents who participated through only the younger ages were estimated to have onset at older ages than those youth who participated through the end of adolescence (which would be consistent with a right-censoring design effect). Finally, on average, G3 born in more recent cohort years had onset at significantly older ages compared to youth born in prior cohort years. In sum, although there were many differences in age of onset due to study and person-level factors, all of these variables are included as covariates when performing hypothesis tests, thus ensuring that results are not an artifact of differences in studies or persons.
Heterogeneity in G2 Adolescent Cannabis Use Onset
Class enumeration results for the discrete-time survival mixture analyses are presented in Table 4. Three latent classes (defined by one abstainer class and two freely estimated classes) were needed to adequately summarize the heterogeneity in G2 age of cannabis use onset. Estimated survival curves for the G2 early and late cannabis use classes are depicted by study in Figure 2. In the early onset class (N = 237, 21.9%; n = 49, 71, and 117 for OYS, SSDP, and RYDS, respectively), all G2 parents were estimated to have onset prior to age 13 years. In the late onset class (N = 281, 26.0%; n = 61, 113, and 107 for OYS, SSDP, and RYDS, respectively), with the exception of a few OYS fathers who had onset at before age 13 years, G2 parents had onset by ages 13–14 years or older. OYS fathers showed the earliest onset with essentially all fathers having used by ages 15–16 years; followed by SSDP parents who had all onset by the end of adolescence at ages 17–18 years; and RYDS parents, 71.8% of whom had onset by ages 17–18 years. The abstainer class (N = 563, 52.1%; n = 113, 158, and 292 for OYS, SSDP, and RYDS, respectively) indicated no cannabis use onset by age 17–18 years and is not depicted in Figure 2.
Table 4.
G2 Parent Class Enumeration Results for Discrete-Time Survival Mixture Analyses with a Predefined Abstainer Class.
| Number of classes | Number of parameters | Log likelihood | AIC | BIC | Sample size adjusted BIC | Entropy | Class sizes | LMR k versus k + 1 classes |
|---|---|---|---|---|---|---|---|---|
| 1 class | 14 | –1438.74 | 2905.48 | 2975.28 | 2930.82 | NA | 1081 | NA |
| 2 classes | 15 | –1431.27 | 2892.53 | 2967.32 | 2919.68 | .82 | 562/519 | 13.08, p = .081 |
| 3 classes | 20 | –1383.84 | 2807.67 | 2907.39 | 2843.86 | .80 | 563/237/281 | 92.22, p = .004 |
| 4 classes | 25 | –1378.55 | 2807.09 | 2931.74 | 2852.33 | .79 | 563/100/164/254 | 10.39, p = .208 |
Note. LMR = Lo-Mendell-Rubin adjusted likelihood ratio test. AIC = Akaike information criterion. BIC = Bayesian information criterion. NA = Not applicable. G2 = Generation 2.
Figure 2.
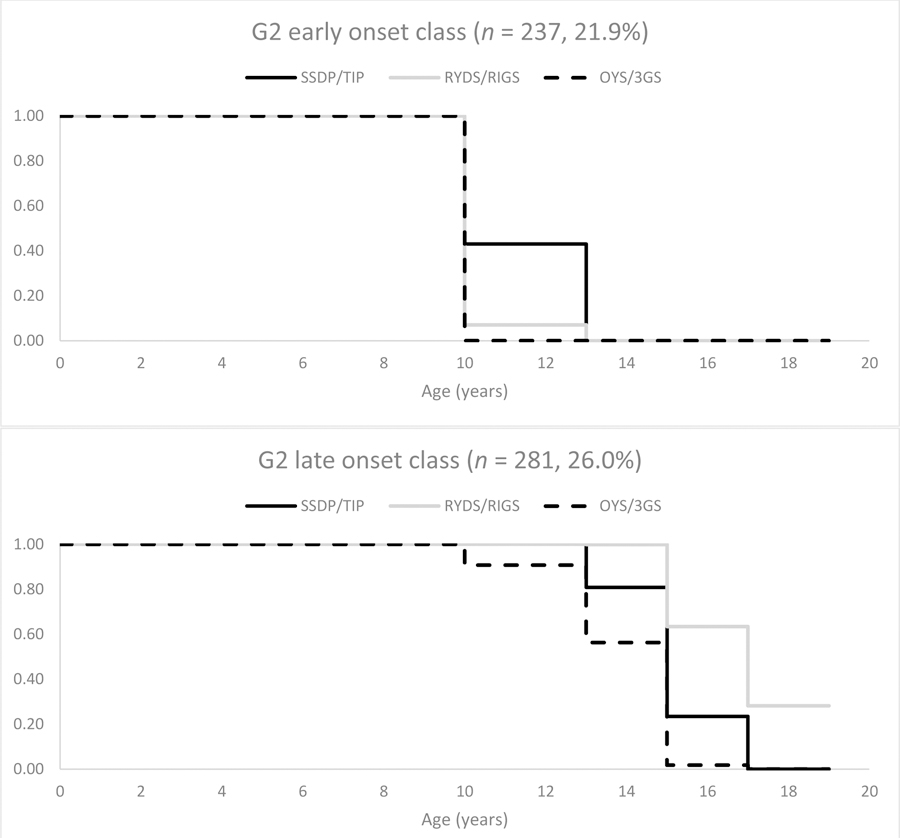
Estimated survival curves for Generation 2 early and late cannabis use onset classes by study; abstainer class (n = 563, 52.1%) not shown. Onset prior to age 13 years is depicted across adolescent ages 10 to 12.99 years; note, however, that n = 31 of N = 113 (27.4%) Generation 2 parents reported younger ages of onset.
Intergenerational Transmission of Cannabis Use Onset
Prediction of G3 cannabis onset from G2 onset (H1).
Contrary to our primary prediction (H1), age of onset did not significantly differ for G3 adolescents whose G2 parent was assigned to the early versus late cannabis use onset class (b[se] = .07[.18], p = .678). However, G3 adolescents were estimated to have onset on cannabis use later in adolescence if G2 parents had abstained compared to offspring of parents who had used early (b[se] = .47[.17], p = .005) or late (b[se] = .54[.15], p < .001). Thus, having a parent who used versus did not use cannabis as an adolescent was associated with earlier cannabis use onset in G3 offspring, but there was no differentiation between G3 children whose parents had onset earlier or later in adolescence.
Moderation of intergenerational transmission by parent (H2) and child (H3) gender.
Next, we examined the hypotheses that intergenerational transmission of risk for earlier onset of cannabis use would be stronger: (H2) from G2 mothers than from fathers, (H3) for G3 girls than G3 boys, and (H4) that mother–daughter dyads relative to other G2–G3 gender pairs. Regarding H2, the results indicated that intergenerational transmission did not vary by parent gender for any of the G2 parent onset class comparisons: early versus late onset (b[se] = .47[.38], p = .220), early onset versus abstainer (b[se] = .15[.37], p = .685), and late onset versus abstainer (b[se] = −.32[.34], p = .345). Next, contrary to H3, the magnitude of the association between G2 adolescent cannabis use onset class and G3 cannabis onset did not differ significantly by G3 gender for any of the comparisons (b[se] = −.35[.37], p = .336 for early vs. late onset; b[se] = −.04[.34], p = .916 for early onset versus abstainer; and b[se] = .32[.32], p = .327 for late onset versus abstainer). There also was no support for H4, as the three-way interactions of G2 adolescent cannabis use onset class by G2 parent gender and G3 child gender were nonsignificant for all comparisons (b[se] = .43[.47], p = .364 for early vs. late onset; b[se] = .06 [.56], p = .913 for early onset vs. abstainer; and b[se] = −.39[.47], p = .403 for late onset vs. abstainer).
Discussion
The goal of the present study was to examine associations between parents’ and their children’s age of onset of cannabis use in adolescence, and whether the strength of intergenerational associations would depend on parent gender, child gender, or the intersection of parent and child gender. These questions were examined using a data harmonization and synthesis approach to combine three prospective studies of 1081 G2 parents and their G3 children (originating from N = 971 unique families) while accounting for variations in onset ages attributable to studies and person-level factors. Prior studies have considered risk factors for adolescents’ cannabis use onset, including parents’ concurrent cannabis use. Few studies, however, have examined effects from parents’ histories of cannabis use during adolescence, and associations between parents and children in terms of age of adolescent cannabis use onset have not been tested previously. Compared to other research designs, prospective studies offer at least three advantages to answering these intergenerational questions: (a) avoidance of recall bias in parents’ retrospective reporting across development periods, (b) consideration of parents and offspring across nearly identical developmental periods, and (c) stronger representation of fathers—and therefore of the risks fathers may confer—than is typically achieved (Phares et al., 2005). Furthermore, although prospective intergenerational studies previously have considered questions about parents’ early cannabis use (e.g., Henry & Augustyn, 2017; Kerr et al., 2015), this is the first study to examine onset processes (i.e., with survival analysis techniques rather than a priori onset categories) across generations and to attempt a direct replication across studies.
The study was successful in synthesizing data across the three studies; however, the primary hypothesis (H1) was not supported. Parents’ earlier versus later onset of first cannabis use in adolescence was not significantly associated with earlier onset of cannabis use by their offspring. More specifically, children of parents who first used cannabis in pre- or early adolescence were at no higher risk than those of parents who showed onset later in adolescence. Rather, children’s earlier onset of use was predicted by having a parent who used cannabis at any point before the end of adolescence (to ages 17–18 years) compared to a parent who did not. The findings have implications for the DDS model in which the hypothesis was grounded. Namely, there was evidence for intergenerational congruence in cannabis use onset that was developmentally broad (parent–child similarities in adolescence) rather than more sensitive or specific (e.g., in early adolescence). The DDS model and the theory of congruence also may be more relevant to higher risk behavior patterns such as early polysubstance use onset (Kerr et al., 2020) than to cannabis use at any point in adolescence.
Additionally, whereas it was hypothesized (H2) that there would be stronger effects of maternal than paternal adolescent onset of cannabis use on age of onset of cannabis use in offspring, moderation of effects by parent gender was not supported. Likewise, moderation by child gender (H3) was not supported. Thus, both boys and girls may be at risk for using cannabis earlier in adolescence if either their mother or father used cannabis at any time during their own adolescence.
Ages of onset of cannabis use differed across studies. OYS–3GS fathers showed the highest risk for pre- and early adolescent onset, followed by SSDP–TIP, and then RYDS–RIGS parents; however, few other study differences in onset rates were found later in adolescence for parents. For the offspring generation, RYDS–RIGS adolescents showed the lowest rates of cannabis use onset in early and midadolescence relative to adolescents in the other two studies; whereas in mid and late adolescence, SSDP–TIP adolescents showed the lowest risk. These patterns may reflect regional and other site differences in cannabis use and related risks. Notably, the RYDS–RIGS sample was predominantly African American and from the Eastern United States, whereas the OYS–3GS and SSDP–TIP samples were from the Pacific Northwest, and the OYS-3GS in particular included a larger proportion of White participants. Of note, the synthesis methodology accounted for cross-site differences in onset, as well as other design and person-level factors related to gender, birth cohort year, and right censoring; therefore, these patterns do not account for the lack of support for the study hypotheses.
Again, although the primary hypothesis was not supported, parents’ earlier or later use versus nonuse of cannabis during adolescence (prior to the birth of the child in almost all cases) was associated with an earlier age of onset for use of cannabis by their child. This finding has the key implication that preventing cannabis use at any time during adolescence for one generation may have beneficial effects both for that individual and for their family of procreation, a notion that is consistent with recent models of development and risk behavior (see Cheng, Johnson, & Goodman, 2016; Patton et al., 2018). To our knowledge, however, this idea has not been communicated adequately to parents or adolescents. Effectively crafting this message for future mothers and fathers (see Garfield, 2015) would contribute to a longer view on the potential power of prevention.
Intergenerational transmission effects were not moderated by gender. Therefore, findings did not support that: (a) risks from mothers’ versus fathers’ use of cannabis during their own adolescence are stronger in relation to their offspring’s adolescent cannabis use onset, (b) boys and girls in the offspring generation are differentially vulnerable to such effects, or (c) mother–daughter (or any other parent–child dyad) continuities are particularly strong (e.g., if socialization via modeling was more salient in same—vs. different—gender dyads). These findings differ from those of studies reporting mothers’ substance use that occurs during the child’s lifetime is more strongly associated with child substance use than is fathers’ use (Capaldi et al., 2016). The prevention implications of the present findings are that there is a lack of evidence that focusing on the adolescent cannabis use histories of fathers over mothers (or vice versa) in prevention programs for children would be beneficial. Rather, the findings may reinforce the value of including both fathers and mothers in such programs. Although the advice that fathers’ behaviors should be considered relevant to prevention efforts may sound obvious, it is routinely ignored for reasons that are in many cases systemic to parenting interventions (see review and recommendations by Panter-Brick et al., 2014). Furthermore, the present findings may mean that both sons and daughters of parents who used cannabis in adolescence may benefit from additional efforts regarding prevention of early onset of cannabis use, perhaps attending to any gender differences in socialization experiences (e.g., parenting, parental-use beliefs and norms).
The present study did not test mechanisms whereby parental use of cannabis in adolescence was related to earlier onset of cannabis use in adolescence by their children. These mechanisms may include a number of factors, which may be transmitted from parent to child (e.g., underlying risks including poor inhibitory control), and also parent continued use of cannabis in adulthood (e.g., daily use and cannabis abuse and dependence). Parental use in adulthood is more likely among adolescents reporting onset by age 16 years (Bailey et al., 2016; Moss et al., 2014). Additionally, we have not yet conducted data syntheses to examine whether intergenerational congruence in cannabis use onset is explained by other substance use.
Despite considerable advantages of the approaches used here, the study also had a number of limitations. First, the offspring generation is still maturing through adolescence for two of the studies; therefore, not all G3 children have yet passed through the years of peak risk for onset of cannabis use. Second, G2 parents were recruited as youth on the basis of community risk for problem behavior, which may limit generalizability of the findings. Third, only one parent’s history was prospectively assessed per child, precluding the examination of the relative or joint influences from mothers and fathers in the same family. Finally, although there was considerable variation in G2 cannabis use by site, we cannot rule out cohort effects, given that the G2 samples were born in the mid-1970s and entered adolescence at a time of relatively lower national cannabis use rates (Miech et al., 2019).
In conclusion, it would seem to be common sense that parent cannabis use should predict children’s cannabis use and that factors associated with higher risk —such as earlier adolescent onset—would show stronger intergenerational continuity. Yet, in this multisite data synthesis study of the timing of first cannabis use during adolescence, this hypothesis was not supported, nor was risk ameliorated or amplified depending on parent or child gender. Models, however, did support that a parental history of cannabis use in adolescence (earlier and later onset) was linked with increased risk for earlier onset of cannabis use by their children compared to risk transmitted by parents who had abstained from cannabis use as adolescents. Thus, prevention of adolescent onset in one generation may have prevention benefits for the next.
Supplementary Material
Acknowledgments
Funding for this work was supported by the National Institutes of Health (NIH) from the National Institute of Drug Abuse (NIDA) grant number R01 DA015485 awarded to Drs. Capaldi and Kerr; grant number 5R01DA023089 awarded to Dr. Bailey; grant number 5R01DA020195 awarded to Dr. Henry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIDA. NIH or NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. These data and research findings have not been previously disseminated at any conferences or meetings, nor posted on a listserv or shared on any websites including academic social networks.
Footnotes
Assessment instruments used the word marijuana rather than cannabis.
Limited data for the cannabis use onset for children younger than age 13 years (only 2.4% [n = 24] G3 had onset during the first age category) and for the study membership by birth cohort year interactions precluded tests of these two-way interactions.
Note that right censoring occurred predominantly for TIP and OYS–3GS G3 participants due to ongoing maturation (n = 169 [49.4%] and n = 95 [42.6%], respectively—compared to only n = 26 [5.0%] of RIGS G3 participants). For G2, only n = 52 of all N = 971 participants (5.4%) were subject to right censoring (0% of SSDP, n = 50 [9.7%] of RYDS, and n = 2 [1.8%] of OYS participants).
Contributor Information
Stacey S. Tiberio, Oregon Social Learning Center
David C. R. Kerr, Oregon Social Learning Center
Jennifer A. Bailey, University of Washington Seattle, School of Social Work
Kimberly L. Henry, Colorado State University, Department of Psychology
Deborah M. Capaldi, Oregon Social Learning Center
References
- Asparouhov T, & Muthén BO (2013). Auxiliary variables in mixture modeling: A 3-step approach using Mplus (Mplus Web Notes: No. 15, Version 6). Retrieved from http://statmodel.com/examples/webnotes/AuxMixture_submitted_corrected_webnote.
- Bailey JA, Hill KG, Epstein M, Steeger CM, & Hawkins JD (2018). Seattle Social Development Project—The Intergenerational Project (SSDP-TIP) In Eichelsheim VI & van de Weijer SGA (Eds.), Intergenerational continuity of criminal and antisocial behaviour. An international overview of current studies (pp. 186–206). New York, NY: Routledge. [Google Scholar]
- Bailey JA, Hill KG, Guttmannova K, Epstein M, Abbott RD, Steeger CM, & Skinner ML (2016). Associations between parental and grandparental marijuana use and child substance use norms in a prospective, three-generation study. Journal of Adolescent Health, 59, 262–268. doi: 10.1016/j.jadohealth.2016.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon RH, Zoccolillo M, Keenan K, Côté S, Pérusse D, Wu H-X, … Tremblay RE (2007). Gender differences in physical aggression: A prospective population-based survey of children before and after 2 years of age. Developmental Psychology, 43, 13–26. doi: 10.1037/0012-1649.43.1.13 [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. (2010). Department of Labor, and National Institute for Child Health and Human Development. Children of the NLSY79, 1986–2010 [computer file]. Produced and distributed by the Center for Human Resource Research: Columbus, OH: The Ohio State University. [Google Scholar]
- Capaldi DM, Chamberlain P, Fetrow RA, & Wilson J (1997). Conducting ecologically valid prevention research: Recruiting and retaining a “whole village” in multimethod, multiagent studies. American Journal of Community Psychology, 25, 471–492. doi: 10.1023/A:1024607605690 [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Kerr DCR, & Tiberio SS (2017). Intergenerational transmission of risk for behavioral problems including substance use In Braddick O (Ed.), Oxford research encyclopedia of psychology (Online doi: 10.1093/acrefore/9780190236557.013.42). New York, NY: Oxford University Press. [DOI] [Google Scholar]
- Capaldi DM, Kerr DCR, & Tiberio SS (2018). The Oregon Youth Study—Three Generational Study: A review of design, theory, and findings In Eichelsheim VI & van de Weijer SGA (Eds.), Intergenerational continuity of criminal or antisocial behaviour: An international overview of studies (pp. 137–161). New York, NY: Routledge. [Google Scholar]
- Capaldi DM, & Patterson GR (1987). An approach to the problem of recruitment and retention rates for longitudinal research. Behavioral Assessment, 9, 169–177. [Google Scholar]
- Capaldi DM, Tiberio SS, & Kerr DCR (2018). Assessing associations in substance use across three generations: From grandparents to sons and from sons to their children. Contemporary Social Science, 13, 288–304. doi: 10.1080/21582041.2018.1433313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi DM, Tiberio SS, Kerr CR, & Pears KC (2016). The relationships of parental alcohol versus tobacco and marijuana use with early adolescent onset of alcohol use. Journal of Studies on Alcohol and Drugs, 77, 95–103. doi: 10.15288/jsad.2016.77.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, & Elder GH (1988). Childhood precursors of the life course: Early personality and life disorganization In Hetherington EM, Lerner RM, & Perlmutter M (Eds.), Child development in life-span perspective (pp. 115–142). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Cheng TL, Johnson SB, & Goodman E (2016). Breaking the intergenerational cycle of disadvantage: The three generation approach. Pediatrics, 137, e20152467. doi: 10.1542/peds.2015-246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnworth M, Thornberry TP, Krohn MD, & Lizotte AJ (1994). Measurement in the study of class and delinquency: Integrating theory and research. Journal of Research in Crime and Delinquency, 31, 32–61. [Google Scholar]
- Ganiban JM, Ulbricht J, Saudino KJ, Reiss D, & Neiderhiser JM (2011). Understanding child-based effects on parenting: Temperament as a moderator of genetic and environmental contributions to parenting. Developmental Psychology, 47, 676–692. doi: 10.1037/a0021812 [DOI] [PubMed] [Google Scholar]
- Garfield CF (2015). Supporting fatherhood before and after it happens. Pediatrics, 135, e528–e530. doi: 10.1542/peds.2014-3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KL, & Augustyn MB (2017). Intergenerational continuity in cannabis use: The role of parent’s early onset and lifetime disorder on child’s early onset. Journal of Adolescent Health, 60, 87–92. doi: 10.1016/j.jadohealth.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Sternberg A, Suk HW, Meier MH, & Chassin L (2018). The intergenerational transmission of cannabis use: Associations between parental history of cannabis use and cannabis use disorder, low positive parenting, and offspring cannabis use. Psychology of Addictive Behaviors, 32, 93–103. doi: 10.1037/adb0000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Curran PJ, & Bauer DJ (2013). Integrative data analysis in clinical psychology research. Annual Review of Clinical Psychology, 9, 61–89. doi: 10.1146/annurev-clinpsy-050212-185522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, & Schultz L (2005). Forward telescoping bias in reported age of onset: An example from cigarette smoking. International Journal of Methods in Psychiatric Research, 14, 119–129. doi: 10.1002/mpr.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, & Schulenberg JE (2014). Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. [Google Scholar]
- Kerr DCR, & Capaldi DM (2019). Intergenerational transmission of parenting In Bornstein MH (Ed.), Handbook of parenting: Being and becoming a parent (3rd ed., Vol. 3, pp. 443–481). New York, NY: Routledge. [Google Scholar]
- Kerr DCR, Tiberio SS, & Capaldi DM (2015). Contextual risks linking parents’ adolescent marijuana use to offspring onset. Drug and Alcohol Dependence, 154, 222–228. doi: 10.1016/j.drugalcdep.2015.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CR, Tiberio SS, Capaldi DM, & Owen LD (in press). Intergenerational congruence in adolescent onset of alcohol, tobacco, and marijuana use. Psychology of Addictive Behaviors. doi: 10.1037/adb0000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DCR, Tiberio SS, Capaldi DM, & Owen LD (2020). Paternal and maternal prescription opioid use and misuse: General and specific risks for early adolescents’ substance use. Addictive Behaviors, 103, 1–6. doi: 10.1016/j.addbeh.2019.106248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KE, Menard S, & Simmons SB (2014). Intergenerational continuity of substance use. Substance Use and Misuse, 49, 221–233. doi: 10.3109/10826084.2013.824478 [DOI] [PubMed] [Google Scholar]
- Kosty DB, Farmer RF, Seeley JR, Gau JM, Duncan SC, & Lewinsohn PM (2015). Parental transmission of risk for cannabis use disorders to offspring. Addiction, 1110–1117. doi: 10.1111/add.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Y, Mendell N, & Rubin D (2001). Testing the number of components in a normal mixture. Biometrika, 88, 767–778. doi: 10.1093/biomet/88.3.767 [DOI] [Google Scholar]
- Loeber R, & Hay DF (1994). Developmental approaches to aggression and conduct problems In Rutter M & Hay DF (Eds.), Development through life: A handbook for clinicians (pp. 488–515). Oxford, England: Blackwell Scientific Publications. [Google Scholar]
- Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2019). Monitoring the Future national survey results on drug use, 1975–2018: Secondary school students (Vol. I). Ann Arbor: Institute for Social Research, The University of Michigan. [Google Scholar]
- Moss HB, Chen CM, & Yi H-Y (2014). Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug and Alcohol Dependence, 134, 51–62. doi: 10.1016/j.drugalcdep.2013.12.011 [DOI] [PubMed] [Google Scholar]
- Muthén BO, & Masyn K (2005). Discrete-time survival mixture analysis. Journal of Educational and Behavioral Statistics, 30, 27–58. doi:10769986030001027 [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus User’s Guide (8th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Panter-Brick C, Burgess A, Eggerman M, McAllister F, Pruett K, & Leckman JF (2014). Practitioner review: Engaging fathers—recommendations for a game change in parenting interventions based on a systematic review of the global evidence. Journal of Child Psychology and Psychiatry, 55, 1187–1212. doi: 10.1111/jcpp.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Maggs JL, Greene KM, Morgan NR, & Schulenberg JE (2014). The link between mother and adolescent substance use: Intergenerational findings from the British Cohort Study. Longitudinal and Life Course Studies, 5, 56–63. doi: 10.14301/llcs.v5i1.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Olsson CA, Skirbekk V, Saffery R, Wlodek ME, Azzopardi PS, …Sawyer SM (2018). Adolescence and the next generation. Nature, 554, 458–466. doi: 10.1038/s41586-018-0069-3 [DOI] [PubMed] [Google Scholar]
- Phares V, Fields S, Kamboukos D, & Lopez E (2005). Still looking for poppa. American Psychologist, 60, 735–736. doi: 10.1037/0003-066X.60.7.735 [DOI] [PubMed] [Google Scholar]
- Raley S, Bianchi SM, & Wang W (2012). When do fathers care? Mothers’ economic contribution and fathers’ involvement in child care. American Journal of Sociology, 117, 1422–1459. doi: 10.1086/663354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry TP (2013). Life course continuity and change in antisocial behavior Final report (5R01 MH063386). Submitted to the National Institute of Mental Health. Bethesda, MD: U.S. Department of Health and Human Services. [Google Scholar]
- Thornberry TP (2016). Three generation studies: Methodological challenges and promise In Shanahan MJ, Mortimer JT, & Johnson MK (Eds.), Handbook of the life course (1st ed., Vol. II, pp. 571–598). New York, NY: Springer. [Google Scholar]
- Thornberry TP, Henry KL, Krohn MD, Lizotte AJ, & Nadel EL (2018). Key findings from the Rochester Intergenerational Study In Eichelsheim VI & van de Weijer SGA (Eds.), Intergenerational continuity of criminal and antisocial behaviour: An international overview of current studies (pp. 214–234). New York, NY: Routledge. [Google Scholar]
- Thornberry TP, Krohn MD, & Freeman-Gallant A (2006). Intergenerational roots of early onset substance use. Journal of Drug Issues, 36, 1–28. doi: 10.1002/cbm.709 [DOI] [Google Scholar]
- Witt ED (2010). Research on alcohol and adolescent brain development: Opportunities and future directions. Alcohol, 44, 119–124. doi: 10.1016/j.alcohol.2009.08.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


