Abstract
The present study aimed to evaluate the effects of medium-chain α-monoglycerides (MG) on productive performance, egg quality, serum biochemical indices, and gut microbiota in laying hens. A total of 252 40-wk-old Hy-Line Brown laying hens were randomly allotted into two groups (21 hens per replicate, 6 replicates per group) and fed with a basal diet (CON group) or a basal diet containing 300 mg/kg of MG (MG300 group). The eggs laid were recorded daily on a replicate basis, and egg quality was measured at 48, 56, and 64 wk of age. At the end of this trial, three randomly selected hens from each replicate were slaughtered, and the serum and cecal digesta were collected for analysis of serum biochemical indices and sex hormones and gut microbiota composition determination. The results revealed that the laying rate was significantly (P < 0.05) increased in the MG300 group, and the feed conversion ratio was decreased (P < 0.01) during 40–64 wk of age. The eggshell strength at 56 wk of age and eggshell thickness at 56 and 64 wk of age were significantly (P < 0.05) increased in the MG300 group. In addition, dietary MG significantly (P < 0.05) increased levels of serum follicle-stimulating hormone, luteinizing hormone, estradiol, glucose, Ca, serum total cholesterol, triglycerides, and high-density lipoprotein cholesterol, but decreased the lipopolysaccharide level. Notably, MG supplementation increased (P < 0.05) the relative abundance of genera Lachnospiraceae_NK4A136_group, Romboutsia, Syntrophomonas, Victivallis, Ruminiclostridium_6, and Family_XIII_UCG_001 (P < 0.01) and simultaneously decreased the abundances of Proteobacteria, Faecalibacterium, Alistipes, Cerasicoccus, Schlegelella, and Treponema_2. Spearman's correlation analysis indicated that the differentiated genera were significantly associated with the serum biochemical indices and sex hormone. In summary, the present study revealed that dietary supplementation with MG can improve productive performance and egg quality by modulating gut microbiota, suggested that MG may act as an efficient feed supplement in aged hens.
Key words: α-monoglyceride, sex hormone, reproductive performance, egg quality, gut microbiota
Introduction
The productive performance and egg quality of late laying period hens (older than 40–48 wk of age) are gradually decreased with increasingly increased age (Bain et al., 2016). As hens aged, the reproductive performance is largely declined owing to the reduction of yolk synthesis and accumulation of lack of sex hormones (Liu et al., 2001). Moreover, the egg quality is also rapidly decreased with enlarged egg size, thinner eggshell, higher broken rate, decreased albumen height, shortened shelf time, and poor flavor associated with poor feed nutrient utilization and health status of aged hens (Kim et al., 2014; Liu et al., 2018). Recently, many research studies have documented that some feed supplements such as rubber seed oil (Wen et al., 2019), peppermint (Abdel-Wareth and Lohakare, 2014), tea polyphenol (Wang et al., 2018), flaxseed oil (Lee et al., 2015), and glycerol monolaurate (GML) (Liu et al., 2020) are able to enhance feed efficiency, egg production, and egg quality in hens, which provide us a novel and effective approach to this problem.
Medium-chain fatty acids (MCFA) are saturated and unbranched with 8–12 carbons and naturally occur as medium-chain triglycerides (TG) in milk, coconut, and palm kernel oil. It has been reported that MCFA and corresponding glycerides are a group of safe and effective feed supplements for farm animal production (Lamot et al., 2016; Hanczakowska, 2017; Rimoldi et al., 2018). Among them, medium-chain α-monoglycerides (MG), including GML (C12:0), dietary glycerol monodecanoate (GMD; C10:0), and glycerol monocaprylin (GMC; C8:0), are regarded as promising feed supplements for poultry production (Van der Aar et al., 2017; Mustafa, 2018; Fortuoso et al., 2019). Recently published studies document that dietary GML improves laying rate, feed efficiency, fresh egg quality, and nutritive values in aged hens (Zhao et al., 2019b; Liu et al., 2020). However, little is known about the effect of dietary GMD and GMC in the diet of hens until now.
The cecum of chickens harbors a complex microbiota, which closely and intensively interact with the host and ingested feed (Pan and Yu, 2014). There are growing evidences revealing that the gut microbiota community and function are related to weight gain, feed nutrient utilization, and health of chickens (Pourabedin and Zhao, 2015; Angelakis, 2017). The gut microbiota can be modulated by the addition of feed supplements such as α-linolenic acid–rich flaxseed oil (Lee et al., 2015), probiotics (Kurtoglu et al., 2004), and Flos lonicerae extracts (Wang et al., 2019), and this resulted in positive effect on egg composition, yield performance, fresh egg quality, and gut health in laying hens. Likewise, dietary GML improves reproductive performance, feed efficiency, and egg quality in aged hens associated with gut microbiota alteration (Liu et al., 2020). Moreover, similar results are found in the application of GML in mice wherein both gut microbiota and serum lipid metabolism are affected by GML supplementation (Jiang et al., 2018; Mo et al., 2019).
Considering the intense goat-like odor of GMC and GMD (Van der Aar et al., 2017), synergistic bacteriostatic effect of GMD and GML in combination contributes to gut microbiota modulation (Batovska et al., 2009), and the current research progress of MG in hens; the present study aims to evaluate the effect of mixed MG rich in GML and GMD on productive performance, egg quality, serum biochemical indices and sex hormones, and gut microbiota in laying hens. Moreover, the present study intends to describe the links between gut microbiota and productive performance in aged hens.
Materials and methods
Birds, Diets, and Experimental Design
A total of 252 (40-wk-old) Hy-Line Brown laying hens were randomly allotted into two groups (21 hens per replicate, 6 replicates per group) and fed with a basal diet (CON group) or a basal diet containing 300 mg/kg of MG (MG300 group). The basal diet was formulated to meet nutrient requirements of the National Research Council (NRC, 1994). Medium-chain α-monoglycerides containing GML (CAS no. 142-18-7) and GMC (CAS no. 26402-22-2) with 95% purity was acquired from Hangzhou Kangyuan Food Technology Co., Ltd. (Hangzhou, Zhejiang, China) and was added into the diet by replacing the same energy amount of rapeseed oil. The feeding trial lasted for 24 wk. Birds were housed in cages with a floor slope of 12° and shared a room maintained at 25 ± 2 °C and 60–65% humidity with a 16-h photoperiod. Feed (115 g per hen per day) was provided at 5:00 am and 1:00 pm, and water was provided ad libitum. Feeding and egg collection were conducted daily. The birds were handled in accordance with the guidelines of the Animal Care and Use Committee of Zhejiang University (no. ZJU-BEFS-2016004), Hangzhou, China.
Productive Performance and Sample Collection
During 40–64 wk of age, eggs laid were recorded daily on a replicate basis, including egg number, egg weight, shell-less eggs, and cracked eggs. The laying rate, average egg weight, and feed conversion ratio (FCR) were calculated per week, and the statistical analysis was conducted at the period of 40–44, 45–48,49–52, 53–56, 57–60, and 61–64 wk of age.
Three randomly selected chickens from each replicate were weighed and slaughtered after feed deprivation for 12 h at the end of the experiment. Cecal digesta was sampled and rapidly frozen in liquid nitrogen, transported to the laboratory in a dry ice pack, and stored at – 80°C. Serum was obtained by centrifugation (2,000× g for 15 min at 4 °C) and stored at – 80 °C for further analysis.
Egg Quality Measurement
At the end of 48, 56, and 64 wk of age, five eggs from each replicate were sampled, and the Haugh units (HU), albumen height, eggshell strength, and eggshell thickness were measured immediately using a digital egg tester (DET 6000; NABEL Co., Ltd., Kyoto, Japan).
Serum Biochemical Indices and Sex Hormones
Lipopolysaccharide (LPS), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) levels were measured using the commercial ELISA kit (catalog number: JYM0109Ch [LPS], JYM0055Ch [FSH], JYM0032Ch [LH], and JYM0049Ch [E2]; Wuhan Colorful Gene Biological Technology Co., Ltd., Wuhan, China). Serum total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, alkaline phosphatase, glucose, calcium (Ca), total protein, and total antioxidant capacity levels were determined using kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) by following the manufacturer's instructions.
16S rRNA Sequencing and Analysis
Ten cecal digesta samples randomly selected from18 hens of each treatment were used for 16S rRNA sequencing. Bacterial DNA was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Venlo, The Netherlands) according to the manufacturer's instructions. The V4 region of the 16s DNA gene was amplified by PCR with primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT) for the microbial community structure analysis. The amplicons were purified using a Qiagen Gel Extraction Kit (Qiagen, Venlo, The Netherlands), quantified using a Qubit 2.0 Fluorometer (Thermo Fisher, Foster City, CA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA), and sequenced using an Illumina HiSeq 2500 platform (Illumina, San Diego, CA). The gene sequence analysis was the similar to that of our previous work (Zhao et al., 2019a). Silva128/16s_bacteria database (www.arb-silva.de) based on the RDP classifier version 2.2 (sourceforge.net/projects/rdp-classifier) was used for taxonomic classification. The linear discriminant analysis effect size algorithm was applied to identify specific taxa.
Statistical Analysis
The data of results were collected and calculated individually for each treatment and checked for normality test first. Statistical analysis of the results was performed using SPSS 22.0 software (SPSS, Inc., Chicago, IL) or R software (version 2.15.0) and presented using GraphPad Prism version 8 (GraphPad Software, La Jolla, CA). Statistical differences were determined using the unpaired two-tailed t-test or Wilcoxon rank-sum test. Spearman's correlations between bacterial abundance and serum biochemical indices and sex hormones were determined and performed using a heatmap with R software version 2.15.0 (https://www.R-project.org). A P-value <0.05 was considered significant (P < 0.05, P < 0.01, P < 0.001), and 0.05 < P-value < 0.10 was discussed as tendencies.
Results
Dynamic Changes of Productive Performance During the Whole Experiment
During 40–64 wk of age, the laying rate (Figure 1A) of the CON group gradually decreased from 96.83% to 84.42%, and egg weight (Figure 1B) increased with increasing weeks of age. Moreover, the FCR (Figure 1C) of the CON group tended to increase, and the broken egg rate (Figure 1D) increased at the end of this experiment. These changes were in line with the productive performance of aged hens. Moreover, Figure 1 visually documented that MG supplementation significantly increased the laying rate and reduced the FCR in hens during 40–64 wk of age.
Figure 1.
Dynamic changes of (A) laying rate, (B) egg weight, (C) feed conversion ratio (FCR), and (D) broken rate for the CON group and MG300 group during the whole experiment (n = 6). Abbreviations: CON, basal diet; MG, medium-chain α-monoglycerides; MG300, basal diet +300 mg/kg of MG.
It was worth noting that obvious increase in the laying rate was observed after 52 wk of age, which was in contradiction with productive performance in aged hens. This could be explained by the extreme weather outside the farmhouse during the experimental time. It was summertime during the time of 44–52 wk of age. The continuous high temperature at this time led to a little increase in the temperature of the experimental room than that designed, which accelerated the reduction of laying rate. However, the environmental temperature gradually fell after 52 wk of age as autumn commenced, consequently resulting in slightly sustained increase of laying rate until the end of the trial.
Effect of Dietary MG on Productive Performance
Productive performance for the CON and MG300 group during the whole experiment was statistically analyzed at an interval of 4 wk and is presented in Figure 2. The laying rate of the MG300 group at 45–48, 49–52, 53–56, and 61–64 wk of age increased by 1.71%, 1.12%, 3.66%, (P < 0.05) and 2.01% (Figure 2A), respectively, and the egg mass increased by 1.82%, 4.32% (P < 0.05), 5.30% (P < 0.05), and 4.35% (P = 0.086) (Figure 2B), respectively. Moreover, dietary MG reduced the FCR by 4.83% (P < 0.05), 3.45% (P < 0.05), 5.02% (P < 0.05), and 3.78% at 45–48, 49–52, 53–56, and 61–64 wk of age (Figure 2C), respectively, and increased the egg weight by 2.32%, 2.98% (P < 0.05), 2.113% (P < 0.05), and 1.28% (Figure 2D), respectively. These results indicated that dietary MG can improve egg production and feed efficiency in the late laying period.
Figure 2.
Effect of dietary MG on productive performance during the whole experimental period. (A) Laying rate, (B) egg mass, (C) feed conversion ratio (FCR), and (D) egg weight. Data are expressed as means ± SD (n = 6). Asterisks indicate significant differences as per the unpaired two-tailed t-test (∗P < 0.05, ∗∗P < 0.01). Abbreviations: CON, basal diet; MG, medium-chain α-monoglycerides; MG300, basal diet +300 mg/kg of MG.
Effect of Dietary MG on Egg Quality
The albumen height (Figure 3A) and HU (Figure 3B) of the CON group were gradually reduced during 48–64 wk of age, indicating the decline of egg quality as hens aged. Dietary MG had no significant impact on egg quality during 40–48 wk of age. However, the albumen height, HU, shell strength, and shell thickness at 56 wk of age increased by 4.32% (8.56 mm vs 8.93 mm), 3.38% (91.36 vs 94.45, P < 0.05), 12.56% (3.98 kgf/m2 vs 4.48 kgf/m2, P < 0.05), and 5.72% (0.385 mm vs 0.407 mm, P < 0.05), respectively, compared with the CON group. At the end of this trial, the eggshell thickness (P < 0.001) in the MG300 group was still significantly higher than that of the CON group.
Figure 3.
Effect of dietary MG on egg quality during 40–64 wk of age. (A) Albumen height, (B) Haugh units, (C) Shell strength, (D) Shell thickness. Data are expressed as means ± SD (n = 6). Asterisks indicate significant differences as per the unpaired two-tailed t-test (∗P < 0.05, ∗∗∗P < 0.001). Abbreviations: CON, basal diet; MG, medium-chain α-monoglycerides; MG300, basal diet +300 mg/kg of MG.
Effect of MG on Serum Biochemical Indices and Sex Hormones
Compared with the CON group, the values of the serum lipid profile, including TG (P < 0.05), TC (P < 0.05), and HDL-C (P < 0.05), in the MG300 group tended to increase (Table 1), suggesting that blood lipid metabolism was affected by MG supplementation. The serum glucose level of the MG300 group increased significantly (P < 0.05), while the LPS level decreased sharply (P < 0.05). Besides, the serum sex hormone levels of FSH, LH, and E2 in the MG300 group were 1.87 (P < 0.01), 2.24 (P < 0.05), and 1.39 (P < 0.05) times higher than those of the CON group, respectively, suggesting that dietary MG may improve productive performance (Figure 2) by inducing the secretion of egg production–related hormones.
Table 1.
Effect of dietary MG on serum biochemical indices and sex hormones in aged hens.
| Item | CON | MG300 |
|---|---|---|
| TG (mmol) | 10.57 ± 2.17 | 15.71 ± 6.091 |
| TC (mmol) | 4.96 ± 0.75 | 6.03 ± 1.591 |
| HDL-C (mmol) | 2.69 ± 0.68 | 3.42 ± 1.121 |
| LDL-C (mmol) | 0.74 ± 0.08 | 0.79 ± 0.15 |
| Ca (mmol) | 1.32 ± 0.03 | 1.38 ± 0.061 |
| AKP (mmol) | 26.20 ± 8.07 | 32.37 ± 17.55 |
| Glucose (mmol) | 16.55 ± 2.91 | 19.96 ± 4.271 |
| Total protein (gprot/L) | 28.64 ± 1.86 | 29.60 ± 2.88 |
| T-AOC (U/mL) | 364.83 ± 38.44 | 399.83 ± 97.78 |
| FSH (ng/mL) | 32.32 ± 10.78 | 60.34 ± 19.162 |
| LH (pg/mL) | 487.95 ± 124.54 | 1,093.01 ± 581.402 |
| E2 (pg/mL) | 120.39 ± 19.71 | 167.13 ± 54.731 |
| LPS (ng/mL) | 61.12 ± 18.34 | 43.44 ± 12.501 |
All data were expressed as means ± SD (n = 18). Means in the same row with the asterisk indicate significant differences as per the unpaired two-tailed t-test.
Abbreviations: AKP, alkaline phosphatase; Ca, calcium; CON, basal diet; E2, estradiol; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LH, luteinizing hormone; LPS, lipopolysaccharide; MG, medium-chain α-monoglycerides; MG300, basal diet +300 mg/kg of MG; T-AOC, total antioxidant capacity; TC, total cholesterol; TG, triglycerides.
P < 0.05.
P < 0.01.
Dietary MG Altered Gut Microbiota
To profile the effects of MG on gut microbiota composition, we performed 16S rRNA sequencing on the Illumina HiSeq 2500 platform. A total of 4,365 operational taxonomic units (OTUs) were obtained, and 1,420 and 1,015 OTUs were found only in the CON and MG300 group, respectively (Figure 4A). The effects of MG supplementation on the α-diversity of gut microbiota were presented in Figure 4B. No significant differences were observed for Chao 1 and ACE (bacterial richness) and Shannon and Simpson indexes (bacterial diversity and evenness) between the MG300 and CON groups. To reveal the alteration of gut microbiota between the MG300 and CON group, β-diversity was assessed by plotting a square matrix of visualized ‘distance’ for the evaluation of the dissimilarity and the community composition between samples based on unweighted Unifrac distances (principal coordinates analysis, Figure 4C). The principal coordinates analysis plot showed that the samples in the MG300 group were clustering intensively and shifted away from the CON group. Moreover, there existed significant differences in PC1 (9.1%, P < 0.01), suggesting that MG altered the communities of gut microbiota in a characteristic direction (Figure 4C). Taxon-based analysis revealed structural changes after MG treatment. At the phylum level, significant decreases in Proteobacteria (P < 0.05) were observed in the MG300 group, and the relative abundances of Verrucomicrobia (P = 0.076) and Cyanobacteria (P = 0.086) also showed significant decreased tendencies compared with the CON group (Figures 4D and 4E).
Figure 4.
Medium-chain α-monoglycerides (MG) supplementation altered the composition of gut microbiota in laying hens. (A) Venn diagram between treatments. (B) α-diversity: Chao 1, ACE, Shannon and Simpson. (C) β-diversity of gut microbiota. (D) Relative abundance of gut microbiota at the phylum level. (E) Relative abundance of Proteobacteria, Verrucomicrobia, and Cyanobacteria. Data are expressed as means ± SD (n = 10). Asterisks indicate significant differences as per the Wilcoxon rank-sum test (∗P < 0.05, ∗∗P < 0.01). Abbreviations: CON, basal diet; MG300, basal diet +300 mg/kg of MG; PCoA, principal coordinates analysis.
The core gut microbiota at the genus level identified in the two groups were similar and comprised 25 genera (the relative abundance of OTUs > 0.01%, Figure 5A). Bacteroidetes were dominated by the genera Bacteroides, Alloprevotella, Prevotellaceae_Ga6A1_group, Prevotellaceae_UCG-001, Alistipes, Rikenellaceae_RC9_gut_group, and Parabacteroides. The Firmicutes were dominated by the genera Christensenellaceae_R-7_group, Ruminococcus_torques_group, Butyricicoccus, Faecalibacterium, Fournierella, Ruminiclostridium_9, Ruminococcaceae_UCG-005, Ruminococcaceae_UCG-010, Ruminococcaceae_UCG-014, Erysipelotrichaceae_UCG-004, Phascolarctobacterium, and Megamonas. The phyla Fusobacteria, Proteobacteria, Actinobacteria, Synergistetes, Verrucomicrobia, and Epsilonbacteraeota were dominated by the genera Fusobacterium, Desulfovibrio, Olsenella, Synergistes, Akkermansia, and Campylobacter, respectively. The genera Faecalibacterium (P < 0.05) and Alistipes (P < 0.05) in the MG300 group were notably decreased compared with the CON group; however, no other significant differences were observed in core gut microbiota. A linear discriminant analysis effect size analysis and cladogram (P < 0.05, linear discriminant analysis > 2.0) were performed to discriminate the differences in the community composition between the two groups (Figure 5B). A total of 17 significant differential bacterial genere were identified in the CON group and the MG300 group. Syntrophomonas, Romboutsia, Odoribacter, Lachnospiraceae_NK4A136_group, Family_XIII_UCG_001, Ruminiclostridium_6, and Victivallis were found to be enriched in MG300 group, whereas the microbiota from the CON group were differentially enriched with Faecalibacterium, Alistipes, Cerasicoccus, Eubacterium, Schlegelella, and Treponema_2. The Wilcoxon rank-sum test was further used to explore the differences in the microbial composition (Figure 5C). Dietary MG significantly increased the abundances of Romboutsia, Odoribacter (P = 0.053), Lachnospiraceae_NK4A136_group, Family_XIII_UCG_001, and Victivallis and simultaneously decreased the abundances of Alistipes (P < 0.05) and Faecalibacterium (P < 0.05). Syntrophomonas and Ruminiclostridium_6 were only found in the MG300 group, whereas Cerasicoccus, Eubacterium, Schlegelella, and Treponema_2 were only detected in the CON group.
Figure 5.
Medium-chain α-monoglyceride (MG) supplementation altered the composition of gut microbiota in laying hens. (A) Relative abundance of gut microbiota at the genus level (those with >0.1% are represented). (B) Cladogram derived from LEfSe analysis of metagenomic sequences from the CON group and the MG300 group (linear discriminant analysis > 2.0). The prefixes ‘o,’ ‘f,’ and ‘g’ represent the annotated level of the order, family, and genus. (C) Relative abundance of differential gut microbiota between the treatments identified by LEfSe analysis at the genus level. Data are expressed as means ± SD (n = 10). Asterisks indicate significant differences as per the Wilcoxon rank-sum test (∗P < 0.05, ∗∗P < 0.01). Abbreviations: CON, basal diet; LEfSe, linear discriminant analysis effect size; MG300, basal diet +300 mg/kg of MG.
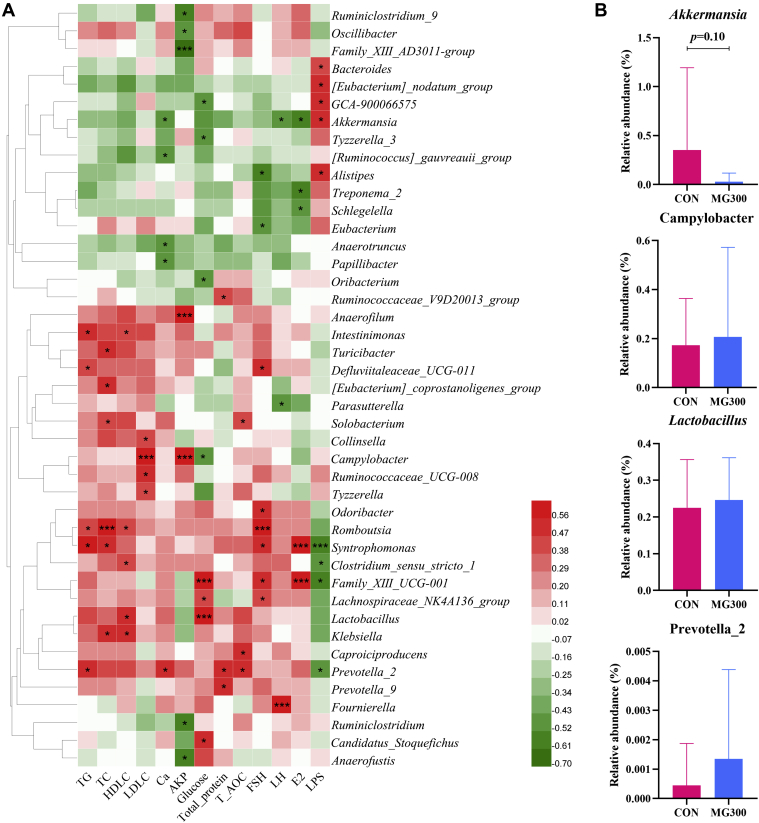
Correlation of Gut Microbiota Composition With Serum Biochemical Indices and Sex Hormones
Spearman's correlation analysis was performed to investigate the associations between the changes in gut microbiota at the genus level and serum biochemical indices and sex hormones in all the groups (Figure 6A). Seven genera were positively (P < 0.05) associated with serum sex hormones (FSH, LH, and E2), implying a positive correlation with productive performance. Conversely, six genera were correlated (P < 0.05) with decreased serum FSH, LH, and E2 levels, suggesting that these genera were negatively correlated with egg production. However, fifteen genera positively (P < 0.05) were associated with increased serum lipid content, and five genera were positively (P < 0.05) associated with serum LPS concentration. Notably, genera abundant in the MG-treated groups included Family_XIII_UCG-001, Romboutsia, Lachnospiraceae_NK4A136_group, Syntrophomonas, and Odoribacter, which were significantly, positively (P < 0.05) correlated with increased serum FSH, LH, and E2 levels (Figure 5C). The abundance of Family_XIII_UCG_001 and Syntrophomonas also showed a significant (P < 0.05), negative correlation with serum LPS concentration, whereas the abundance of Romboutsia and Syntrophomonas was significantly (P < 0.05), positively associated with increased blood lipid content. The abundance of Akkermansia (Figure 6B) was significantly (P < 0.05), positively associated with the increased serum LPS content, but was negatively (P < 0.05) associated with increased laying-related hormones and serum Ca content. Alistipes, which was decreased after MG supplementation, was significantly (P < 0.05) positively correlated with increased serum LPS levels. Schlegelella and Treponema_2 also displayed significantly (P < 0.05) positive correlations with decreased serum E2 concentration.
Figure 6.
The correlation between gut microbiota and serum biochemical indices and sex hormones in aged hens. (A) Heatmap of Spearman's correlation between gut microbiota (all core and differential gut microbiota at the genus level) and serum biochemical indices and sex hormones in all laying hens. (B) Relative abundance of Akkermansia, Campylobacter, Lactobacillus, and Prevotella_2. Asterisks indicate significant differences as per the Wilcoxon rank-sum test (∗P < 0.05, ∗∗∗P < 0.001). Abbreviations: AKP, alkaline phosphatase; Ca, calcium; CON, basal diet; E2, estradiol; FSH, follicle-stimulating hormone; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LH, luteinizing hormone; MG, medium-chain α-monoglycerides; MG300, basal diet +300 mg/kg of MG; T-AOC, total antioxidant capacity; TC, total cholesterol; TG, triglycerides.
Discussions
In the present study, we demonstrated that GML and GMC used in combination notably increased productive performance, improved egg quality, and affected the serum biochemical indices and sex hormones, especially in the serum sex hormones in laying hens. The gut microbiota community composition was also altered by MG supplementation. Moreover, the observed serum biochemical indices and sex hormones were closely associated with the alternation of gut microbiota composition as per Spearman's correlation analysis, indicating that the improvement of productive performance was related to the modulation of gut microbiota induced by MG supplementation.
Hy-Line Brown laying hens commonly begin producing eggs at 18 wk of age and last for over a year. After the onset of laying, egg production generally increases for about 6 wk to reach a maximum and remains for around 20 wk, which is described as peak egg-laying period (Joyner et al., 1987). Thereafter, the laying rate decreases gradually with the increasing weeks of age, and the eggs tend to increase in size along with thinner eggshell and increased broken egg rate (Kim et al., 2014; Liu et al., 2018). The dynamic changes of egg production in the CON group (Figure 1) were in line with the productive performance description of hens after the peak egg-laying period. Statistical analysis revealed that MG supplementation exerted a positive effect on the productive performance during 40–64 wk of age. Although the laying rate, egg mass, FCR, and egg weight did not always show significant differences, an obvious positive upward trend of productive performance in the whole trial was observed. This was in consistent with our previous study, wherein dietary GML significantly increased the laying rate and decreased the FCR in Hy-Line Brown hens (Liu et al., 2020). Similar results were reported by Fortuoso et al. (2019), who demonstrated that dietary GML notably increased average body weight, feed consumption, and carcass yield in male Cobb 500 broilers. Likewise, Mustafa (2018) reported that inclusion of GML in the diet of Ross 308 broilers significantly improved body weight gain, reduced FCR, and enhanced the immunological and nutritional status. These studies documented that the improvements of egg production in the present study were attributed to MG supplementation owing to its unique physiological and biological properties. First of all, MG could be transported directly into the liver via the portal vein and cross the double mitochondrial membrane rapidly without the presence of carnitine (Sidossis et al., 1996; Wang et al., 2015); then, it could be rapidly metabolized and provide energy sources for extrahepatic organs in the body (Dayrit, 2015). Medium-chain α-monoglyceride supplementation provided an immediate, extra source of energy for energy supply and decreased the expenditure of nutrients such as protein as a source of energy, consequently resulting in increased protein synthesis in return (Mabayo et al., 1993). Besides, MG could be used directly by the enterocytes for energy production and thereby help to support the integrity of the intestinal tissue in poultry, which was also beneficial for nutrient utilization (Van der Aar et al., 2017). In addition, MG have strong antibacterial, anticoccidial, and antiviral effects, and they could act synergistically when they were used together with organic acids, essential oils, or probiotics, which exerted positive effects on health, production, and feed digestibility (Baltić et al., 2017).
In the present study, dietary MG notably improved eggshell strength and thickness at the end of the trial, indicating that MG supplementation could enhance egg quality in laying hens. These findings were also supported by the changes in serum biochemical indices, in which the serum Ca level and alkaline phosphatase activity in the MG300 group increased by 4.55% (P < 0.05) and 23.55%, respectively, compared with the CON group. Similar results were reported by a previous study, in which dietary MCFA exerted positive effects on eggshell density and eggshell breaking strength (Świątkiewicz et al., 2010). Medium-chain fatty acids can participate in Ca metabolism in direct or indirect ways, and this might be the reason for the increased egg quality by MG supplementation (Wauquier et al., 2015). In addition, eggshell strength is a key attribute for consumable eggs. Low eggshell strength is associated with high fragility and decreased viability for carriage and storage. Fragile eggs cause economic loss and food safety concerns. Even the occurrence of hair cracks raises the risk of bacterial contamination of the broken egg and of other eggs when leaking, creating problems with internal and external quality and food safety (Mertens et al., 2006). Thus, the improvements of eggshell thickness and strength caused by MG supplementation in the late laying period were of great importance for egg production.
FSH and LH play a particularly important role in the course of follicular development and ovulation. Follicule-stimulating hormone is the main hormone responsible for the development and maturation of small follicles, and LH mainly promotes the secretion of progesterone. Estradiol can also promote follicular development via feedback effects on the hypothalamus and pituitary (Liu et al., 2001). The serum hormone levels have been considered a sensitive indicator of productive performance. The main reason for the decline of egg production in the late laying period was the decrease in yolk synthesis and accumulation due to decreased hormone levels in aged hens (Liu et al., 2001; Xie et al., 2019). Therefore, the increased levels of serum FSH, LH, and E2 by MG supplementation may be the main reason for the improvement of productive performance in the present study. However, MG supplementation also affected layers' blood metabolism by increasing serum TG, TC, and HDL-C levels. These results were in line with a previous study, in which a high dose of GML increased serum TC, TG, and HDL concentration in broilers (Mustafa, 2018). Conversely, Fortuoso et al. (2019) reported that dietary GML in broilers had no adverse effects on serum TC and TG content at the levels of 100–300 mg/kg. Moreover, several recent studies documented that inclusion of MCFA reduced the serum TC, TG, and low-density lipoprotein cholesterol concentration in broilers (Shokrollahi et al., 2014; Wang et al., 2015; Saeidi et al., 2016). These inconsistencies in the effects of MG or MCFA on blood lipid metabolism may be related to differences in their composition and addition levels, diet type, environmental conditions, animal age, breed, and hygienic conditions between studies. The effect of MG on serum lipid metabolism required further analysis.
There are growing evidences indicating that animal performance and production were closely associated with the modulation of gut microbiota by feed supplements (Pan and Yu, 2014). In the present study, the beneficial effects of MG supplementation on productive performance were concomitant with the modulation of gut microbiota composition. Consistent with previous studies (Stanley et al., 2014; Zhou et al., 2020), the gut microbiota between the MG300 and CON group were dominated by five phyla: Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, and Actinobacteria. Medium-chain α-monoglyceride supplementation significantly decreased the phylum Proteobacteria (approximately 5.86%), which was a minor constituent within a balanced gut-associated microbial community and was generally recognized as a microbial signature of dysbiosis in gut microbiota (Litvak et al., 2017). A sustained increase in the abundance of the phylum Proteobacteria was a characteristic of imbalanced gut microbes, which led to host nutritional and metabolic disorders (Shin et al., 2015). Moreover, the relative abundance of Proteobacteria in gut microbiota showed a negative relationship with gut health because it contained a wide variety of remarkable conditional pathogens, such as Escherichia–Shigella (Ma et al., 2017). Therefore, the significant reduction in the relative abundance of Proteobacteria may bring about the promotion of nutrient utilization and gut health in MG-treated hens. Obviously, it was worth noting that the significant low level of serum LPS in the MG300 group was associated with the significant reduction in the LPS-producing phylum Proteobacteria, although the LPS-suppressing phylum Verrucomicrobia also showed a decreased trend. These results were in line with those of our previous study, in which 300 mg/kg of GML supplementation significantly reduced both the serum LPS level and the abundance of Verrucomicrobia in mice fed with high-fat diet (Zhao et al., 2019a). Lipopolysaccharide was an important link in the cross talk between the gut microbiota and host inflammation (Jiang et al., 2018), and the significantly decreased LPS load by MG supplementation indicated ameliorated systematic inflammation in aged hens. Kono et al. (2003) demonstrated that rats fed with medium-chain TG showed a significant improvement in intestinal permeability and prevented LPS-mediated endotoxemia by remodeling gut microbiota based on their antimicrobial properties. Thus, these findings revealed that MG may improve productive performance and egg quality by enhancing nutrient utilization and gut health via modulating gut microbiota.
At the genus level, MG supplementation significantly increased the abundance of Lachnospiraceae_NK4A136_group, Romboutsia, Odoribacter (P = 0.053), Syntrophomonas, Victivallis, Ruminiclostridium_6, and Family_XIII_UCG_001 (P < 0.01) and simultaneously decreased the abundance of Faecalibacterium, Alistipes (P < 0.05), Cerasicoccus, Schlegelella, and Treponema_2. Among them, some bacteria were reported to be beneficial for egg production and quality. Lachnospiraceae_NK4A136_group were a group of Lachnospiraceae, known for their ability to promote health, including the production of host nutrients and energy supply to the colonic epithelium, as well as the maintenance of host immune homeostasis (Shi et al., 2020). Odoribacter were short-chain fatty acid producers in broilers, which were important for both microbial and host epithelial cell growth (Li et al., 2016). Moreover, Spearman's correlation analysis revealed that the increased abundance of genera was closely associated with the increase in serum sex hormones and the reduction in the LPS level, suggesting that the serum biochemical indices and sex hormone differences were related to alteration of gut microbiota modulated by MG supplementation. On the contrary, the decreased abundance of genera showed negative correlation with the serum sex hormone content and positive association with the LPS load. Therefore, these findings demonstrated that dietary MG may improve the productive performance and gut health by selectively increasing the abundance of some beneficial bacteria.
Conclusions
The present study demonstrated that the combination of GML and GMC at a supplementation level of 300 mg/kg improved productive performance, egg quality, and gut health in late laying period hens by inducing laying-related sex hormone secretion and modulating gut microbial composition. The improvements of productive performance and health by MG supplementation were associated with the increased abundance of some beneficial genera, such as Lachnospiraceae_NK4A136_group, Romboutsia, Odoribacter, Syntrophomonas, Victivallis, Ruminiclostridium_6, and Family_XIII_UCG_001 (P < 0.01). These findings offered new perspectives for the use of MG as a functional ingredient to promote egg production and health in aged hens. However, the mechanism and optimum ratio of MG need to be further explored.
Acknowledgments
This work was supported by the Technology and Achievement Transformation Project of Hangzhou, China (grant no. 20161631E01), Zhejiang University New Rural Development Research Institute Agricultural Technology Promotion Fund (grant no. 2017ZDNT006), Key Project of Natural Science Foundation of Zhejiang Province (grant no. LD19C200001), and Natural Science Foundation of China (no. 31601561). The authors are especially grateful to Yanyun Ying, Bo Peng, Tingting Bu, Jianli Wang, Hangzhou Guo, and all people who participated in the preparation and analysis of this study. The use of all the birds and experimental protocols in this study were approved by the Animal Care and Use Committee of Zhejiang University.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Abdel-Wareth A.A.A., Lohakare J.D. Effect of dietary supplementation of peppermint on performance, egg quality, and serum metabolic profile of Hy-Line Brown hens during the late laying period. Anim. Feed Sci. Technol. 2014;197:114–120. [Google Scholar]
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltić B., Starčević M., Đorđević J., Mrdović B., Marković R. Importance of medium chain fatty acids in animal nutrition. IOP Conf. Ser. Earth Environ. Sci. 2017;85:012048. [Google Scholar]
- Batovska D.I., Todorova T., Tsvetkova V., Najdenski H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol. J. Microbiol. 2009;58:43–47. [PubMed] [Google Scholar]
- Dayrit F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015;92:1–15. [Google Scholar]
- Fortuoso B.F., Dos Reis J.H., Gebert R.R., Barreta M., Griss L.G., Casagrande R.A., de Cristo T.G., Santiani F., Campigotto G., Rampazzo L. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb. Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Hanczakowska E. The use of medium-chain fatty acids in piglet feeding–a review. Ann. Anim. Sci. 2017;17:967–977. [Google Scholar]
- Jiang Z., Zhao M., Zhang H., Li Y., Liu M., Feng F. Antimicrobial emulsifier–glycerol monolaurate induces metabolic syndrome, gut microbiota dysbiosis, and systemic low-grade inflammation in low-fat diet fed mice. Mol. Nutr. Food Res. 2018;62:1700547. doi: 10.1002/mnfr.201700547. [DOI] [PubMed] [Google Scholar]
- Joyner C.J., Peddie M.J., Taylor T.G. The effect of age on egg production in the domestic hen. Gen. Comp. Endocrinol. 1987;65:331–336. doi: 10.1016/0016-6480(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Song J.H., Lee J.C., Lee K.W. Age-related changes in egg quality of Hy-Line brown hens. Int. J. Poult. Sci. 2014;13:510–514. [Google Scholar]
- Kono H., Fujii H., Asakawa M., Yamamoto M., Matsuda M., Maki A., Matsumoto Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 2003;237:246–255. doi: 10.1097/01.SLA.0000048450.44868.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtoglu V., Kurtoglu F., Seker E., Coskun B., Balevi T., Polat E. Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol. Food Addit. Contam. 2004;21:817–823. doi: 10.1080/02652030310001639530. [DOI] [PubMed] [Google Scholar]
- Lamot D., Van der Klein S., van de Linde I., Wijtten P., Kemp B., Van Den Brand H., Lammers A. Effects of feed access after hatch and inclusion of fish oil and medium chain fatty acids in a pre-starter diet on broiler chicken growth performance and humoral immunity. Animal. 2016;10:1409–1416. doi: 10.1017/S1751731116000288. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Kang S.K., Heo Y.J., Shin D.W., Choi Y.J. Influence of flaxseed oil on fecal microbiota, egg quality and fatty acid composition of egg yolks in laying hens. Curr. Microbiol. 2015;72:259–266. doi: 10.1007/s00284-015-0946-z. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Litvak Y., Byndloss M.X., Tsolis R.M., Bäumler A.J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017;39:1–6. doi: 10.1016/j.mib.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Liu H.-K., Long D., Bacon W. Preovulatory luteinizing hormone surge interval in old and young laying Turkey hens early in the egg production period. Poult. Sci. 2001;80:1364–1370. doi: 10.1093/ps/80.9.1364. [DOI] [PubMed] [Google Scholar]
- Liu T., Li C., Li Y., Feng F. Glycerol monolaurate enhances reproductive performance,egg quality and albumen amino acids composition in aged hens with gut microbiota alternation. Agriculture. 2020;10:250. [Google Scholar]
- Liu Z., Sun C.J., Yan Y.Y., Li G.Q., Shi F.Y., Wu G.Q., Liu A.Q., Yang N. Genetic variations for egg quality of chickens at late laying period revealed by genome-wide association study. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-29162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Wang Q., Li H., Xu C., Cui N., Zhao X. 16S rRNA genes Illumina sequencing revealed differential cecal microbiome in specific pathogen free chickens infected with different subgroup of avian leukosis viruses. Vet. Microbiol. 2017;207:195–204. doi: 10.1016/j.vetmic.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Mabayo R., Furuse M., Kita K., Okumura J. Improvement of dietary protein utilisation in chicks by medium chain triglyceride. Br. Poult. Sci. 1993;34:121–130. doi: 10.1080/00071669308417568. [DOI] [PubMed] [Google Scholar]
- Mertens K., Bamelis F., Kemps B., Kamers B., Verhoelst E., De Ketelaere B., Bain M., Decuypere E., De Baerdemaeker J. Monitoring of eggshell breakage and eggshell strength in different production chains of consumption eggs. Poult. Sci. 2006;85:1670–1677. doi: 10.1093/ps/85.9.1670. [DOI] [PubMed] [Google Scholar]
- Mo Q., Fu A., Deng L., Zhao M., Li Y., Zhang H., Feng F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: new insights into its antimicrobial potential. Nutrients. 2019;11:1981. doi: 10.3390/nu11091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa N.G. Biochemical trails associated with different doses of alpha- monolaurin in chicks. Adv. Anim. Vet. Sci. 2018;7:187–192. [Google Scholar]
- NRC. 9th rev. ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. National Research Council. [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourabedin M., Zhao X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015;362:fnv122. doi: 10.1093/femsle/fnv122. [DOI] [PubMed] [Google Scholar]
- Rimoldi S., Gliozheni E., Ascione C., Gini E., Terova G. Effect of a specific composition of short-and medium-chain fatty acid 1-Monoglycerides on growth performances and gut microbiota of gilthead sea bream (Sparus aurata) PeerJ. 2018;6:e5355. doi: 10.7717/peerj.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi E., Shokrollahi B., Karimi K., Amiri-Andi M. Effects of medium-chain fatty acids on performance, carcass characteristics, blood biochemical parameters and immune response in Japanese quail. Br. Poult. Sci. 2016;57:358–363. doi: 10.1080/00071668.2016.1169508. [DOI] [PubMed] [Google Scholar]
- Shi S., Qi Z., Jiang W., Quan S., Sheng T., Tu J., Shao Y., Qi K. Effects of probiotics on cecal microbiome profile altered by duck Escherichia coli 17 infection in Cherry Valley ducks. Microb. Pathog. 2020;138:103849. doi: 10.1016/j.micpath.2019.103849. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae J.-W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Shokrollahi B., Yavari Z., Kordestani A. Effects of dietary medium-chain fatty acids on performance, carcass characteristics, and some serum parameters of broiler chickens. Br. Poult. Sci. 2014;55:662–667. doi: 10.1080/00071668.2014.955836. [DOI] [PubMed] [Google Scholar]
- Sidossis L.S., Stuart C.A., Shulman G.I., Lopaschuk G.D., Wolfe R.R. Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. J. Clin. Invest. 1996;98:2244–2250. doi: 10.1172/JCI119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Świątkiewicz S., Koreleski J., Arczewska A. Laying performance and eggshell quality in laying hens fed diets supplemented with prebiotics and organic acids. Czech J. Anim. Sci. 2010;55:294–306. [Google Scholar]
- Van der Aar P., Molist F., Van Der Klis J. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed Sci. Technol. 2017;233:64–75. [Google Scholar]
- Wang J., Wang X., Li J., Chen Y., Yang W., Zhang L. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. Asian Australas. J. Anim. Sci. 2015;28:223–230. doi: 10.5713/ajas.14.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.-w., Jia H.-j., Zhang H.-j., Wang J., Lv H.-y., Wu S.-g., Qi G.-h. Supplemental plant extracts from Flos Ionicerae in combination with Baikal Skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by salmonella pullorum. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.C., Wang X.H., Wang J., Wang H., Zhang H.J., Wu S.G., Qi G.H. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period. J. Anim. Sci. 2018;96:225–235. doi: 10.1093/jas/skx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauquier F., Leotoing L., Philippe C., Spilmont M., Coxam V., Wittrant Y. Pros and cons of fatty acids in bone biology. Prog. Lipid Res. 2015;58:121–145. doi: 10.1016/j.plipres.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Wen Z., Wu Y., Qi Z., Li X., Li F., Wu X., Yang P. Rubber seed oil supplementation enriches n-3 polyunsaturated fatty acids and reduces cholesterol contents of egg yolks in laying hens. Food Chem. 2019;301:125198. doi: 10.1016/j.foodchem.2019.125198. [DOI] [PubMed] [Google Scholar]
- Xie T., Bai S.P., Zhang K.Y., Ding X.M., Wang J.P., Zeng Q.F., Peng H.W., Lu H.Y., Bai J., Xuan Y., Su Z.W. Effects of Lonicera confusa and Astragali Radix extracts supplementation on egg production performance, egg quality, sensory evaluation, and antioxidative parameters of laying hens during the late laying period. Poult. Sci. 2019;98:4838–4847. doi: 10.3382/ps/pez219. [DOI] [PubMed] [Google Scholar]
- Zhao M., Cai H., Jiang Z., Li Y., Zhong H., Zhang H., Feng F. Glycerol-monolaurate-mediated attenuation of metabolic syndrome is associated with the modulation of gut microbiota in high-fat-diet-fed mice. Mol. Nutr. Food Res. 2019;63:1801417. doi: 10.1002/mnfr.201801417. [DOI] [PubMed] [Google Scholar]
- Zhao M.J., Cai H.Y., Liu M.Y., Deng L.L., Li Y., Zhang H., Feng F.Q. Dietary glycerol monolaurate supplementation for the modification of functional properties of egg white protein. J. Sci. Food Agric. 2019;99:3852–3859. doi: 10.1002/jsfa.9607. [DOI] [PubMed] [Google Scholar]
- Zhou B.-h., Jia L.-s., Wei S.-s., Ding H.-y., Yang J.-y., Wang H.-w. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult. Sci. 2020;99:1297–1305. doi: 10.1016/j.psj.2019.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]