Abstract
Host defense peptides (HDP) are multifunctional effectors of the innate immune system, which has antimicrobial and pleiotropic immunomodulatory functions. Although there is a very sophisticated superposition of adaptive immune systems in vertebrates, this system is still essential. As an important family of HDP, cathelicidins are also known for their broad-spectrum antibacterial activity against bacteria, fungi, and enveloped viruses. It has been found in humans and other species, including cattle, pigs, sheep, goats, chickens, rabbits, and some kind of fish. Among them, cathelicidins in birds were described for the first time in 2005. This review focuses on the structure, biological activities, expression, and regulation of avian cathelicidin, especially main effects of host defense cathelicidin on potential therapeutic applications. According to the results obtained both in vitro and in vivo, good perspectives have been opened for cathelicidin. Nevertheless, further studies are needed to better characterize the mechanisms of action underlying the beneficial effects of cathelicidin as novel therapeutic alternatives to antibiotics.
Key words: cathelicidin, biological functions, expression, regulation, avian
Introduction
Traditionally, antibiotics are included in animal feed at subtherapeutic levels for promoting growth and preventing disease (Kelsy et al., 2018). However, in recent decades, the infection of multidrug-resistant bacteria caused by the abuse of antibiotics has become an urgent problem to be solved in contemporary clinical medicine (Van Boeckel et al., 2015; Wang et al., 2018). To this end, a ban of all antibiotics in livestock production by the European Union in 2006 and a removal of medically important antibiotics in animal feeds in the United States in January 2017 were put in force (Seal et al., 2013; Young-Speirs et al., 2018). According to the latest requirements of China's Ministry of Agriculture, pharmaceutical feed additives are also forbidden to be added in feed after 2020 and can also no longer be used in feed production. Therefore, there is an urgent need for antibiotic substitutes that can maintain animal health and productivity without triggering antimicrobial resistance (Young-Speirs et al., 2018).
The potential advantages of host defense peptides (HDP), also known as antimicrobial peptides (AMP), in the development of novel antimicrobials are beyond question (Hancock and Sahl, 2006). The HDP are a critical component of the animal innate immune system with direct antimicrobial and immunomodulatory activities (Kelsy et al., 2018; Chen et al., 2020). In addition, because the target of HDP are mainly located in the microbial cell membrane, it can induce microbial cell membrane depolarization, resulting in cell rupture and play a role which is not easy to cause microbial resistance (Moravej et al., 2018). Cathelicidin is a major family of HDP found in mammals, birds, reptiles, and fish (Van Dijk et al., 2005, 2011; Zanetti, 2005; Chang et al., 2006; Zhao et al., 2008; Moravej et al., 2018). It plays a critical role in animal innate immune system that can provide the first line of defense against a variety of microorganisms (Zanetti, 2005). Studies have found that that avian cathelicidins not only have a more broad spectrum and efficient antimicrobial activity but also have low hemolytic activity and cytotoxicity (Xiao et al., 2006; Wang et al., 2011; Zhang and Sunkara, 2014), which shows great potential in the field of new antimicrobial agents.
The aim of this article is to summarize the information about avian cathelicidins to provide an overview of the evolution, expression, regulation, biological activities, and potential application for human and veterinary medicine of these avian cathelicidin AMP.
General aspects of avian cathelicidins
Cathelicidin is a short peptide of less than 40 amino acids, whose name is derived from the similarity of the cathelicidin large middle domain to cathelin, a cathepsin L inhibitor originally isolated from porcine leukocytes (Ritonja et al., 1989; Cheng et al., 2015). Cathelicidins have been found in vertebrates, such as mammals (Zanetti, 2005), reptiles (Zhao et al., 2008), fish (Uzzell et al., 2003; Chang et al., 2006), and birds (Lynn et al., 2004; Van Dijk et al., 2005, 2011; Xiao et al., 2006).
Structure and Classes
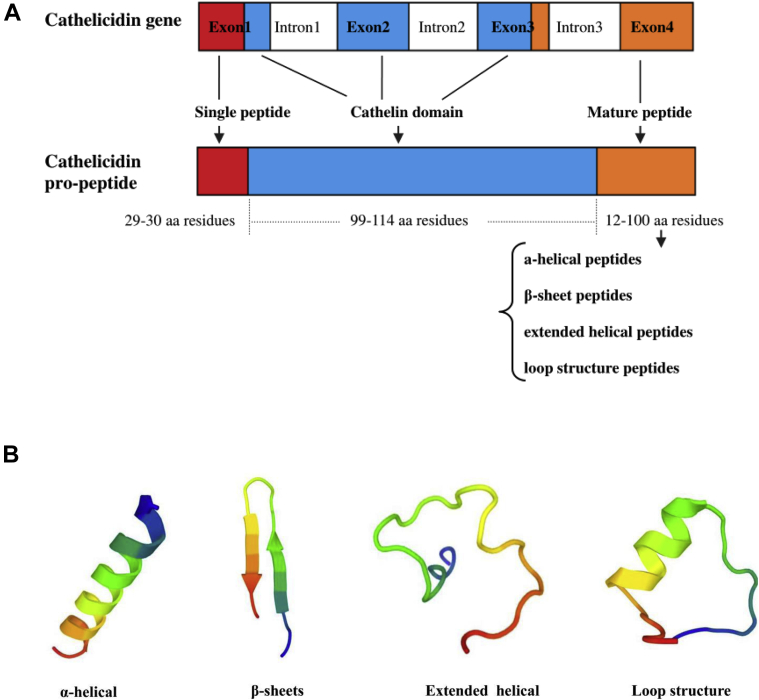
In chickens, the cathelicidin genes are densely clustered within the distance of 7.5 kb toward one end of chromosome 2 (Xiao et al., 2006; Kosciuczuk et al., 2012). Like the cathelicidin genes in mammal, all avian cathelicidin genes described so far consist of 4 exons and 3 introns (Figure 1A). The first exon encodes the part of the cathelin domain and signal peptide of 29–30 amino acid residues in size, whereas the second and third exon encode the major part of the cathelin domain of 99–114 amino acids. The fourth exon encodes the last few amino acid residues of the cathelin domain, and the mature peptide with the variable antimicrobial domain consisting of 12–100 amino acids (Zanetti et al., 1995; Zhang and Sunkara, 2014). Therefore, in brief, complete cathelicidins consist of inactive prepropeptides (include signal peptides and cathelin domain) and mature peptide. It has been reported that cathelicidin AMP are highly heterogeneous because their C-terminal mature peptides could be activated by proteolytic cleavage and exerts its antimicrobial and immunomodulatory activities after being released from the N-terminal cathelin portion of the holoprotein (Zanetti, 2004)
Figure 1.
Gene and peptide structure of cathelicidins. (A) Schematic representation of gene and propeptide of the cathelicidin in vertebrate. (Adapted from related references (Zanetti et al., 1995; Zanetti, 2004; van Dijk et al., 2011; Cuperus et al., 2013; Zhang and Sunkara, 2014; Young-Speirs et al., 2018)). Corresponding colors of exons to propeptide regions indicate that exon codes for that specific region. (B) The schematic diagram of secondary structures of cathelicidin include α-helical, β-sheet, extended helical, and loop structure, which were predicted online by the Ressource Parisienne en BioInformatique Structurale web portal (Alexis et al., 2006).
In addition, the mature peptides found after the protease cleaving steps are quite diverse. Based on amino acid sequences, mature cathelicidin peptides can be broadly categorized into peptides with α-helical, β-sheets, extended helical, and loop structure cathelicidins (Figure 1B): (1) α-helical cathelicidins—linear peptides with good amphiphilic properties, which usually have no intramolecular disulfide bridges; the N-terminal contains more hydrophilic amino acids, and the C-terminal is rich in hydrophobic amino acids (Wang et al., 2017); (2) β-sheet cathelicidins—short peptides formed by 2 intramolecular disulfide bridges, which is developed by 4 conserved cysteines (Tossi et al., 1995; Wang et al., 2017); (3) extended helical cathelicidins—usually contain a high amount of specific amino acids, such as indocyanine rich in tryptophan, and PR-39 rich in proline/arginine (Ando et al., 2010; Kosciuczuk et al., 2012); (4) loop structure cathelicidins—contain a disulfide bond in its molecule and show a chain structure as a whole, which is the structure of most amphibian frog–derived cathelicidins and sheep and bovine cyclic dodecapeptide (Storici et al., 1992; Zanetti, 2005). Among these structural peptides, the α-helical cathelicidin peptides are the most widely spread and found in all investigated mammalian species (Van Dijk et al., 2011; Cuperus et al., 2013), also including all avian cathelicidins.
Discovery of Avian Cathelicidins (Evolution)
About a dozen of cathelicidin family members (Table 1) have been isolated in avian species. Four cathelicidins were identified in chickens (Gallus gallus cathelicidin CATH1, 2/CMAP27, 3, and CATH-B1) (Lynn et al., 2004; Van Dijk et al., 2005; Xiao et al., 2006), of which CATH1, 2, and 3 have also been independently reported as fowlicidin 1-3 (Xiao et al., 2006). Furthermore, peptides similar to cathelicidins were described in quail (Coturnix coturnix cathelicidin Cc-CATH1, 2, and 3) (Feng et al., 2011), pheasant (Phasianus colchicus cathelicidin Pc-CATH1, 2, and 3) (Wang et al., 2011), duck (Anas platyrhynchos cathelicidin dCATH) (Gao et al., 2015), turkey (Meleagris gallopavo cathelicidin CATH2 and 3) (Yacoub et al., 2016; Hamad et al., 2017; Ishige et al., 2017), rock pigeon (Columba livia cathelicidin Cl-CATH2 and 3) (Yu et al., 2015), and Japanese quail (Coturnix japonica cathelicidin Cj-CATH-1, -2, -3, and -B1) (Ishige et al., 2017). In addition to the completion of the cathelicidin amino acid sequencing of these avian species in Table 1, currently the cathelicidin sequences of some other avian species, such as budgerigar (Melopsittacus undulatus), peregrine falcon (Falco peregrinus), ground tit (Pseudopodoces humilis), and emperor penguin (Aptenodytes forsteri), have been predicted and obtained in GenBank.
Table 1.
The gene name, amino acid sequences, and length of avian cathelicidins.
| Source | Gene name | Peptide name | AA sequence | Mature peptide length | GenBank no. | Reference |
|---|---|---|---|---|---|---|
| Chicken (Gallus gallus) | CATH1 | Cathelicidin1/fowlicidin-1 | RVKRVWPLVIRTVIAGYNLYRAIKKK | 26 | HQ640431 |
Lynn et al., 2004; Xiao et al., 2006 |
| CMAP27/CATH2 | Cathelicidin2/fowlicidin-2 | LVQRGRFGRFLRKIRRFRPKVTITIQGSARFG | 32 | HQ640432 | Van Dijk et al., 2005; Xiao et al., 2006 | |
| CATH3 | Cathelicidin3/fowlicidin-3 | RVKRFWPLVPVAINTVAAGINLYKAIRRK | 29 | HQ640433 |
Bommineni et al., 2007; Veldhuizen et al., 2013 |
|
| CATHB1 | Cathelicidin-B1 | PIRNWWIRIWEWLNGIRKRVRQRSPFYVRGHLNVTSTPQP | 40 | AB915170 | Goitsuka et al., 2007 | |
| Quail (Coturnix coturnix) | Cc-CATH1 | Cathelicidin | RVKRVLPLVIRTVIAGYNLYRAIKRK | 26 | GU232858 | Feng et al., 2011 |
| Cc-CATH2 | Cathelicidin CATH2 | LVQRGRFGRFLKKVRRFIPKVIIAAQIGSRFG | 32 | GU171373 | ||
| Cc-CATH3 | Cathelicidin CATH3 | RVRRFWPLVPVAINTVAAGINLYKAIRRK | 29 | GU171374 | ||
| Pheasant (Phasianus colchicus) | Pc-CATH1 | Cathelicidin CATH1 | RIKRFWPLVPVAINTVAAGINLYKAIKRK | 29 | GU143407 | Wang et al., 2011 |
| Pc-CATH2 | cathelicidins | LVQRGRFGRFLSKIRRFRPKFTITIQGSGRFG | 32 | GU143408 | ||
| Pc-CATH3 | Cathelicidin CATH3 | RIKRFWPVVIRTVVAGYNLYRAIKKK | 26 | GU171372 | ||
| Duck (Anas platyrhynchos) | dCATH | Cathelicidin | KRFWQLVPLAIKIYRAWKRR | 20 | KT230679 | Gao et al., 2015 |
| Turkey (Meleagris gallopavo) | CATH2 | Cathelicidin-2 | LVQRGRFGRFLSKFRRFRPRVTITIQGSARFG | 32 | XM003206909 | Hamad et al., 2017; Ishige et al., 2017 |
| CATH3 | Cathelicidin-3 | RVKRFWPLVPVAINTVAAGINLYKAIKRK | 29 | XM010712309 | ||
| Rock pigeon (Columba livia) | Cl-CATH2 | Cathelicidin 2 | LIQRGRFGRFLGRIRRFRPRINFDIRARGSIRLG | 34 | KP645199 | Yu et al., 2015 |
| Cl-CATH3 | Cathelicidin 3 | RVKRFWPLVPVAINTVAAGINLYKAIKRK | 29 | KP645200 | ||
| Japanese quail (Coturnix japonica) | Cj-CATH1 | Cathelicidin | RVKRVLPLVIRTVIAGYNLYRAIKRK | 26 | LC136907 | Ishige et al., 2017 |
| Cj-CATH2 | Cathelicidin CATH2 | LVQRGRFGRFLKKVRRFIPKVIIAAQIGSRFG | 32 | LC136907 | ||
| Cj-CATH3 | Cathelicidin CATH3 | RVKRFWPLVPVAINTVAAGINLYKAIRRK | 29 | LC136907 | ||
| Cj-CATB1 | Cathelicidin-B1 precursor | PIRNWWTRIREWWDGIRRRLRQRSPFHVRGRLNISSTAQP | 40 | LC136907 |
Abbreviation: AA, amino acid.
Phylogenetic analysis demonstrated that cathelicidins of mammals, avian species, and fish were classified into distinctly separated clusters (Goitsuka et al., 2007), and when comparing avian cathelicidins with mammalian proteins, the highest sequence similarity was found with neutrophilic granule proteins (NGP)-like cathelicidin, such as rabbit, mouse, and bovine NGP, suggesting that they may share a common ancestor. This hypothesis was confirmed in subsequent studies, that is, NGP and avian cathelicidin gene clusters were located in close proximity to the Kelch-like 18 gene in both types of animals, with synteny (Xiao et al., 2006; Cuperus et al., 2013). Although they have evolved from a single, remotely related gene, the difference is that the C-terminal region of cathelicidins is highly variable across species and could cleave from the cathelicidin-like domain to become bioactive by proteolysis; the NGPs are conserved and functionally active in the whole sequence, and there is no proteolysis (Kosciuczuk et al., 2012; Zhang and Sunkara, 2014). In addition, it is worth noting that the chicken CATHB1 seems to be an outlier, located between the fish sequence and the group containing the other avian cathelicidins, not only a great distance from NGPs but also only 40% identity with CATH1, and sharing only 40% identity with CATH1 (Goitsuka et al., 2007; Zhang and Sunkara, 2014).
Currently, among the 4 identified chicken cathelicidins, CATH1 and CATH3 are most closely related with a >90% identity throughout the entire peptide sequence (Zhang and Sunkara, 2014). At the level of amino acid, the signal peptides of CATH1 and CATH2 show a high similarity of 94%, whereas the cathelin domains of the 2 genes share only 56% homology (Van Dijk et al., 2005; Xiao et al., 2006). Conversely, the mature peptides are highly differentiated, such as the CATH2 mature peptide have been found to have less than 10% homology with other chicken cathelicidins (Van Dijk et al., 2005). The orthologs of chicken CATH1-3 has also been reported in other avian species such as the C. coturnix and P. colchicus (Feng et al., 2011; Wang et al., 2011). The comparison of 3 Cc-CATHs from C. coturnix showed that Cc-CATH1 and Cc-CATH3 were more closely related, with 93% homology throughout the entire sequence, suggesting that the 2 genes were the result of gene duplication (Feng et al., 2011). Two cathelicidin genes, Cl-CATH-2 and -3, have been described in C. livia, and these 2 genes share a high degree of similarity with previously characterized CATH-2 and -3 from chicken (Yu et al., 2015). Furthermore, the identification and initial characterization of the C. japonica CATH genes (Cj-CATH-1, -2, -3, and -B1) were recently reported by Ishige et al. (2017). The percent identities between the coding sequences of Cj-CATHs and chicken cathelicidins is more than 85.3%, and the predicted amino acid sequences of Cj-CATHs exhibited >75.4% identity to chicken cathelicidin orthologs (Ishige et al., 2017).
Biological activities of avian cathelicidins
Since the first identification of cathelicidin propeptides (Zanetti et al., 1993), many cathelicidins from different species have been studied. Avian cathelicidins not only have direct antibacterial activity but also can selectively enhance the host immune response by regulating the production of cytokines and the recruitment of immune cells (Cuperus et al., 2013; Zhang and Sunkara, 2014). Herein, we reviewed the biological activities and some internal mechanisms of avian cathelicidins.
Antimicrobial Activities
Avian cathelicidins have been demonstrated to exhibit active antimicrobial activity toward both gram-positive and gram-negative bacteria such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Pseudomonas aeruginosa, and so forth (Zhang and Sunkara, 2014; Ishige et al., 2017). The antibacterial spectrum and minimum inhibitory concentration (MIC) of some major avian cathelicidins AMP are summarized in Table 2. The MIC of mature chicken fowlicidins (CATH1-3) and CATH-B1 to most strains tested in a salt-independent manner are in the range of 0.4–2.5 μM (Xiao et al., 2006; Bommineni et al., 2007). Quail CATH-2 and -3, pheasant CATH-1, and duck CATH show MIC values in the range of 0.3–2.5, 0.1–2.95, and 2.0–4.0 μM for most gram-positive and gram-negative bacteria, respectively (Feng et al., 2011; Wang et al., 2011; Gao et al., 2015). Pigeon Cl-CATH2 exerted broad-spectrum but moderate antimicrobial abilities with most MIC ranging from 9.38 to 37.5 μg/mL, which is higher than CATH1, Cc-CATH3, and Pc-CATH1 (Yu et al., 2015). In addition, fungi such as Candida albicans, Candida glabrata, and slime mold are susceptible to avian cathelicidins, showing MIC values in the range of about 1–10 μΜ (Feng et al., 2011; Wang et al., 2011; Cuperus et al., 2013; Yu et al., 2015).
Table 2.
Activity spectrum of avian cathelicidin peptides in a salt-independent manner described in literature.
| Gene name | Gram positive1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. epidermidis | S. haemolyticus | N. asteroides | P. acnes | L. monocytogenes | E. faecium | B. cereus | B. subtilis | Reference | |
| CATH1 | 0.4-1.0 | + | + | 0.8-2.0 | + |
Bommineni et al., 2007; Xiao et al., 2006 |
||||
| CATH2 | 0.33-1.25 | + | + | 1.33 | + | Veldhuizen et al., 2013; Xiao et al., 2006 | ||||
| CATH3 | 1.0-1.25 | + | + | 2.0 | + | Bommineni et al., 2007; Veldhuizen et al., 2013 | ||||
| CATH-B1 | 1.25 | Goitsuka et al., 2007 | ||||||||
| Cc-CATH2 | 0.3-1.3 | 2.5 | + | 1.3 | 1.3 | + | Feng et al., 2011 | |||
| Cc-CATH3 | 0.2-0.7 | − | + | 0.7 | 1.4 | + | Feng et al., 2011 | |||
| Pc-CATH1 | 0.18-0.74 | 2.95 | + | 0.74 | 0.74 | + | Wang et al., 2011 | |||
| dCATH | 4.0 | 4.0 | 4.0 | + | Gao et al., 2015 | |||||
| Cl-CATH2 | 2.27 | + | Yu et al., 2015 | |||||||
| Gene name | Gram negative2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. sobria | A. baumannii | E. coli | P. aeruginosa | P. vulgaris | P. mirabilis | K. pneumoniae | K. oxytoca | S. typhimurium | S. enteritidis | S. maltophilia | Reference | |
| CATH1 | 0.8-2.0 | 3.18 | 0.4-1.0 | 0.4-2.0 | 2.0 | + |
Bommineni et al., 2007; Xiao et al., 2006 |
|||||
| CATH2 | 0.66-2.66 | 5.32 | 0.66-1.25 | 0.66-1.33 | + |
Veldhuizen et al., 2013; Xiao et al., 2006 |
||||||
| CATH3 | 2 | 1.25-2.5 | 0.6-1.0 | 2.0 | 2.0 | + |
Bommineni et al., 2007; Veldhuizen et al., 2013 |
|||||
| CATH-B1 | 2.5 | 0.63 | Goitsuka et al., 2007 | |||||||||
| Cc-CATH2 | 1.3 | + | 2.5-5.1 | 10.1 | 10.1 | 5.1 | + | 2.5 | + | 1.3 | Feng et al., 2011 | |
| Cc-CATH3 | 1.4 | + | − | 5.6 | >29.6 | 1.4 | − | 22.2 | − | 1.4 | Feng et al., 2011 | |
| Pc-CATH1 | 1.48 | + | 1.48-2.95 | 5.90 | 23.62 | 1.48 | + | 11.81 | + | 0.74 | Wang et al., 2011 | |
| dCATH | 2.0 | 4.0 | Gao et al., 2015 | |||||||||
| Cl-CATH2 | 4.54 | 4.54 | Yu et al., 2015 | |||||||||
The minimum inhibitory concentrations (MIC) of peptides are indicated in μM. The presence of inhibition is denoted by “+”. The lack of inhibition is denoted by “−”.
S. aureus, Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; S. haemolyticus, Staphylococcus haemolyticus; N. asteroids, Nocardia asteroids; P. acnes, Propionibacterium acnes; L. monocytogenes, Listeria monocytogenes; E. faecium, Enterococcus faecium; B. cereus, Bacillus cereus; B. subtilis, Bacillus subtilis.
A. sobria, Aeromonas sobria; A. baumannii, Acinetobacter baumannii; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; P. vulgaris, Proteus vulgaris; P. mirabilis, Proteus mirabilis; K. pneumoniae, Klebsiella pneumoniae; K. oxytoca, Klebsiella oxytoca; S. typhimurium, Salmonella typhimurium; S. enteritidis, Salmonella enteritidis; S. maltophilia, Stenotrophomonas maltophilia.
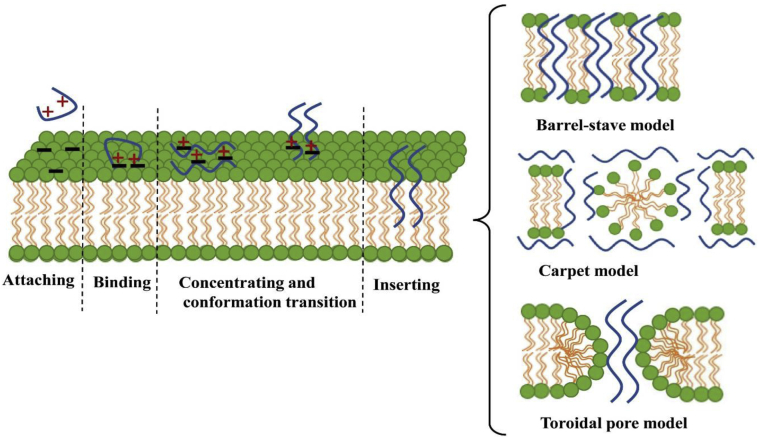
Avian cathelicidins are cationic polypeptides that play an important role in the initial response of invasive pathogens. The major bactericidal mechanism is killing microbes via disruption and lysis of their membrane integrity (Xiao et al., 2009; Derache et al., 2012). Currently, 3 main mechanisms for cationic peptides to penetrate microbial membranes have been proposed, that is, the barrel-stave, carpet, and toroidal pore model (Van Dijk et al., 2011), as shown in Figure 2. However, the above 3 mechanisms do not exist independently but are inter-related. It has been reported that the barrel-stave, carpet, and toroidal pore models are actually several continuous stages based on the effect of peptides on the cell membrane (Dathe and Wieprecht, 1999). In addition, the antibacterial activity of avian cathelicidins is closely related to its structural characteristics. The kink or hinge region of the peptide center and amphiphilic structure in the α-helical cathelicidins molecule plays a key role in antimicrobial activities (Oh et al., 2000; Tossi et al., 2000; Cuperus et al., 2013). The cationic side interacts with the cell membrane of bacteria and fungi with anions, the central hinge region (including proline or glycine, etc.) induces flexibility and inserts into the bacterial membrane resulting in pore formation, and the hydrophobic side also causes stomata in the cell membrane. Eventually, the phospholipid bilayer of the cell membrane was destroyed. A peptide can use different mechanisms depending on its concentration (Brogden, 2005; Nicolas, 2009). Generally, the mode of action of AMP in vitro depends on the high multiples of the MIC of peptides and (or) high peptide-to-lipid ratios. At low peptide-to-lipid ratios, peptides are bound parallel to a lipid bilayer. At a high value of peptide-to-lipid ratios, peptide molecules are orientated perpendicular to the bilayer, forming transmembrane pores that are lethal to a cell (Huang, 2000; Yang et al., 2001; Brogden, 2005). This is consistent with the 2 general mechanisms of AMP entering microbial cells proposed by Nicolas (2009), which are spontaneous lipid-assisted translocation and stereospecific receptor–mediated membrane translocation, respectively. The results reported by Podda et al. (2006) suggest that proline-rich cathelicidin Bac7 may inactivate bacteria through 2 different modes of action, depending on its concentration: (1) a mechanism of stereospecific-dependent uptake and then binding to intracellular targets when close to MIC concentrations and (2) nonstereospecific membrane dissolution mechanism when concentrations are higher than the MIC value. Other microbial killing mechanisms have also been proposed, for example, some cathelicidins could bind to negatively charged biological macromolecules such as DNA, RNA, or protein, thus inhibiting DNA replication and impairing protein synthesis and function (Zasloff, 2002).
Figure 2.
Three main mechanisms for cationic peptides to penetrate microbial membranes, including the barrel-stave model, carpet model, and toroidal pore model.
Adapted with permission from Wang et al. (2018).
Immunomodulatory Activities
In addition to directly killing bacteria, the immunomodulatory activity is the main biological function of HDP, which has been proven by more studies (Afacan et al., 2012; Wang, 2014). More recently, it has become evident that avian cathelicidins have a diverse range of functions in modulating immunity. The immunomodulatory function of avian cathelicidins mainly includes the following 2 aspects: (1) enhance anti-inflammatory immune modulation. It has been reported that chicken CATH2 and CATH3 can prevent toll-like receptor signaling by directly blinding to endotoxins lipopolysaccharide (LPS), which can in turn inhibit LPS-induced tumor necrosis factor-α production in macrophages, monocytes, or dendritic cells (Van Dijk et al., 2009a; Coorens et al., 2017a). Chicken CATH1-3 can inhibit the expression of cytokines IL-1β, IL-6, and nitric oxide production induced by LPS in both chicken peripheral blood mononuclear cells and mouse RAW264.7 cells (Coorens et al., 2017b; Van Harten et al., 2018). Besides inhibiting the production of proinflammatory cytokine-mediated by endotoxin, avian cathelicidins could also stimulate the production of anti-inflammatory cytokines (Van Harten et al., 2018). For example, Kraaij et al. reported that chicken CATH2 upregulates IL-10 mRNA levels in chicken peripheral blood mononuclear cells (Kraaij et al., 2017). (2) Modulation of the expression of chemokines—on the one hand, cathelicidins could directly bind to chemokine receptors on immune cells and induce migration. Chicken CATH1 was found to have specific chemotaxis to neutrophils after injection into the mouse peritoneum (Bommineni et al., 2014). Cathelicidins, released from infected sites where neutrophils gather mainly, will attract other immune cells. These immune cells may then also secrete cathelicidins, which can lead to a local high concentration of cathelicidin peptides. It was reported that this cumulative local concentration, even in the presence of salt, might be high enough to make cathelicidins bactericidal (Van Harten et al., 2018). On the other hand, in addition to direct chemotactic function, cathelicidins can also indirectly induce influx of a variety of innate and adaptive immune cells to gather to inflammatory sites by stimulating the expression of chemokines and chemokine receptors. For example, chicken CATH1 can activate the ability of RAW264.7 mouse macrophages by inducing the expression of inflammatory mediators including IL-1β, CCL2, and CCL3 (Bommineni et al., 2014).
Expression and regulation of avian cathelicidins
Tissue Expression Pattern
Most of the avian cathelicidins are derived from the various epithelial cells or bone marrow, with expression in most tissues except for the breast muscle (Selsted and Ouellette, 2005; Achanta et al., 2012). In chickens, CATH-1, -2, and -3 mRNAs are primarily of the myeloid origin and are expressed in a wide range of tissues, including the respiratory tract, gastrointestinal tract, and multiple lymphoid organs (Van Dijk et al., 2005; Lynn et al., 2007; Achanta et al., 2012). Van Dijk et al. (2005) and Achanta et al. (2012) have found that the high expression of CATH1-3 in the bone marrow, bursa of Fabricius, and cecal tonsils. Interestingly, although no CATH-1 has not been detected in skin tissue, there is a certain amount of CATH-2 expression in normal intact skin, which can assist in wound repair when the injury is induced (Dorschner et al., 2001; Heilborn et al., 2003; Lynn et al., 2004; Van Dijk et al., 2005). Immunohistochemical staining showed CATH-2 protein in heterophilic granulocytes, whereas not in other blood cells, such as monocytes, thrombocytes, or lymphocytes (Van Dijk et al., 2009a,b). Van et al. (Van Dijk et al., 2009b) observed the sections of gastrointestinal tissues and found that there was no expression of CATH-2 protein either in the control group or in the infected intestinal tissues, and the expression was equally restricted to heterotrophic cells.
In contrast, CATH-B1, as a distant member of avian cathelicidins, is derived from epithelial cells. It was reported that CATH-B1 mRNA displays a more restricted expression pattern, preferentially expressed in the secretory epithelial cells of the bursa of Fabricius (Goitsuka et al., 2007). Mature CATH-B1 peptide in the chicken bursa is secreted from the epithelial cells that are in close to proximity of M cells (Goitsuka et al., 2007; Achanta et al., 2012). In addition to selective expression in the bursa of Fabricius, studies have also shown that there are certain transcriptional levels of CATH-B1 in other organs, including the thymus, jejunum, colon, and peripheral blood leukocyte (Meade et al., 2009a; Van Dijk et al., 2011; Cuperus et al., 2013).
For pheasant, the expression level of Pc-CATH-1 mRNA is the highest in the bone marrow and bursa of Fabricius, moderate to high in the brain, heart, lung, spleen, and testis, and lower in the liver and thymus (Wang et al., 2011). In addition, the Pc-CATH-2 (-3), Cc-CATH, and d-CATH genes were cloned from pheasant and Coturnix spleen and duck bone marrow cDNA library, respectively (Feng et al., 2011; Gao et al., 2015; Wang et al., 2018). However, their expression levels in the spleen, bone marrow, and other tissues have not been widely investigated. Our preliminary study found that the expression of duck HDPs d-CATH was higher in the bone marrow, liver, and brain but lower in the bursa of Fabricius (unpublished).
Developmental Regulation
It was reported that the mRNA expression levels of chicken cathelicidin were closely related to their phylogenetic relationship during the embryonic development (Meade et al., 2009b). Through the whole embryo gene expression profile analysis of 4 known avian cathelicidin genes (including CATH1-3 and CATH-B1) across the development process, it was found that most cathelicidin mRNAs were expressed at day 3 of embryonic stage, expect for CATH-B1, which was not expressed until day 9, and increased at day 12 (Meade et al., 2009b; Zhang and Sunkara, 2014). In contrast to 3 d after laying, expression of CATH-1, CATH-2, and CATH-3 was increased by between 6- and 9-folds at day 6, reduced at day 9, and was also subsequently increased again at day 12 (Meade et al., 2009b). All 4 chicken cathelicidins mRNA expression was generally increased as embryos developed (Meade et al., 2009b). There was no significant evidence of preferential expression in either the head or the abdomen of the embryo. CATH1-3 and CATH-B1 gene expression in the head of the embryo increased by 12-, 6-, 6-, and 3-fold, respectively, and the expression of these genes in the abdomen increased by 21-, 8-, 5-, and 3-fold, respectively (Meade et al., 2009b; Van Dijk et al., 2011).
Chicken cathelicidins are also developmentally regulated in both gene and tissue-specific patterns after hatching. During the first 28 d, CATH1-3 in the cecal tonsil and lung increased in an age-dependent manner, whereas all 4 cathelicidins peaked in the bursa on day 4 after hatching and gradually declined on the 28th d (Achanta et al., 2012). Moreover, the peak expression of CATH1-3 appeared in the cecum on day 28, whereas CATH-B1 showed the highest expression in the lung and cecum tonsil on day 14 (Achanta et al., 2012). In summary, although the 4 cathelicidins are widely expressed in a variety of tissues in chicken, the bursa and bone marrow are the primary sites for synthesis of CATH-B1 and CATH1-3, respectively, suggesting their important innate defense role.
Nutritional Manipulation
The expression of cathelicidins is inducible and regulated by microbial infection, inflammatory stimulation, and nutritive active substances. Studies have confirmed that there are multiple regulatory elements in the 5′ upstream regions of the cathelicidins gene, such as nuclear factor-κB, NF-IL-6, bacterial LPS binding site, IL-6, and γ-interferon response factor (Wang et al., 2004a). Exogenous trace components could act on these sites directly or indirectly to regulate the expression of cathelicidins. It has been found that exogenous pathogenic factors can affect the expression level of cathelicidins. The expression levels of CATH1 in chick cecal tonsils were significantly increased in response to Salmonella typhimurium infection on day 3 and 5 (Akbari et al., 2008). However, parasitic poultry pathogen Eimeria praecox downregulated CATH3 expression in the jejunum of infected chickens on day 3 after infection (Sumners et al., 2011). Avian cathelicidin levels are also affected by Campylobacter jejuni. Chicken CATH2 and CATH3 gene expression at 6 h post-infection in peripheral blood leukocytes significantly reduced in response to C. jejuni infection with oral challenge (Meade et al., 2009a). A decrease in CATH2 mRNA expression levels was observed in the small intestine of C. jejuni–challenged broiler chicks at 48 h p.i (Van Dijk et al., 2012). Based on the different results observed in these experiments, it could be inferred that the expression of cathelicidins is related to the infection load and the inhibition of the cathelicidin level may be part of the immune escape strategy of pathogenic microorganisms.
It is an effective method to regulate the expression of endogenous HDP by nutritional means. The expression of avian cathelicidins is regulated by butyrate, vitamin D3, and other substances. Butyrate, a major type of short-chain fatty acids produced by bacterial fermentation of undigested dietary fiber, not only plays a positive role in energy supply, inflammation, barrier integrity, and gut health but also regulates intestinal immunity and enhances disease resistance by inducing endogenous HDP in chickens (Sunkara et al., 2011; Chen et al., 2020). Sunkara et al. (2011) found that butyrate could significantly increase the expression of CATH-B1 in chicken immune cells (HD11 macrophage cells and primary monocytes) and the small intestine (the jejunum and ceca), whereas CATH1-3 were essentially not modulated by butyrate in either cell type. This indicates that butyrate has interspecific differences in regulating the expression and mechanism of cathelicidins, and it is also related to the types of AMP. Notably, although butyrate at the concentration used had no direct antibacterial activity, butyrate treatment enhanced the antibacterial activity of chicken monocytes (Kelsy et al., 2018). Feed with 0.1% butyrate reduced the bacterial titer in the chicken cecum by a nearly 10-fold after experimental infections with Salmonella enteritidis (Sunkara et al., 2011).
As a secosteroid molecule, vitamin D3 not only plays the important role in regulating calcium homeostasis but also is thought to participate in the regulation of innate and adaptive immune responses by enhancing HDP expression (Adams and Hewison, 2008; Chen et al., 2020). A study of Zhang et al. (2010) showed that vitamin D3 could significantly promote the mRNA expression of CATH1 in the bursa of Fabricius and thymus of chickens in a dose-dependent manner in the range of 800–3,200 IU/kg, which is the potential to improve the innate ability to resist diseases. Consistently, CATH1 and CATH-B1 levels were dose-dependently increased by dietary vitamin D in the spleen of broiler chickens and that induction was further enhanced by calcium- and phosphorus-deficient diet, although CATH3 was downregulated (Rodriguez-Lecompte et al., 2016). Such studies have been described in mammals, and the vitamin D receptor elements in cathelicidin promoter were shown to be involved (Wang et al., 2004a; Cuperus et al., 2013). However, it is uncertain whether vitamin D receptor element is the only recognition and regulation site of avian cathelicidins. There are other mechanisms in the process of vitamin D3 regulating the expression of avian HDP, which are also worthy of further study.
In addition to butyric acid and vitamin D3 described above, amino acid, microelement, and some kind of polysaccharide could also induce the expression of endogenous cathelicidins. As a nutritional strategy, dietary amino acid supplementation has been proved to be effective in regulating intestinal immune function and controlling intestinal diseases (Lallès et al., 2007). Some amino acids could affect the intestinal AMP expression by regulating the activity of key proteins in the signaling pathway of intestinal epithelial cells. Hashimoto et al. (2012) found that increasing the level of dietary tryptophan could activate the mammalian target of rapamycin signal pathway, thus increasing the mRNA expression of intestinal α-defensin genes (Defa1 and Defa5). Similarly, branched-chain amino acids including isoleucine, valine, and leucine in vivo and in vitro have been demonstrated to augment the expression of porcine intestinal β-defensins through activation of the Sirt1/ERK/90RSK signaling pathway (Ren et al., 2016). Based on these, we can find that some studies were focused on the effect of amino acids as immune-enhancing formulas to promote the expression of defensins, whereas little is known about their impact on avian cathelicidins. Therefore, more experiments are needed to investigate whether amino acids such as tryptophan, lysine, and branched-chain amino acids could alleviate intestinal inflammation in avian species through cathelicidin expression.
It was found that microelements play a major role in regulating HDP expression, especially zinc. The mechanism of zinc protecting the intestinal mucosa barrier and reducing the diarrhea ratio is mediated in part by promoting the expression of HDP (Wu et al., 2019). In the Caco-2 intestinal epithelial cell line, zinc was found to induce phosphorylation of ERK and p38MAP kinases and regulate LL-37 secretion through these MAP kinases (Talukder et al., 2011). A high level of ZnO supplementation (3,000 mg/kg) in the diet significantly increased cathelicidin PR-39 peptide mRNA expression (Wang et al., 2004b). For broilers, increasing the dietary zinc and manganese content or feeding zinc and manganese in the OHCl form synergistically increased the amounts of IL-1 and cathelicidin mRNA in immune cells (Perez et al., 2007).
Some active polysaccharides in plants have broad areas of bioactivities including immune adjunction and antibacterial and antiviral activities, and so on. For example, astragalus polysaccharides have been found to induce the expression of cathelicidin LL-37 through p38MAPK/JNK and nuclear factor-κB signaling pathways in human respiratory epithelial cells, thus participating in its mediated antibacterial action (Zhao et al., 2018). To some extent, polysaccharides from microorganisms also have beneficial bioactivity in modulating pathogen-induced inflammatory responses. Shao et al. (2016) reported that the addition of 200 mg/kg yeast-β-glucans to the diet of broilers can enhance the defensins and cathelicidins expression and reduced the higher level of S. enteritidis colonization and internal organs invasion in the S. enteritidis infected birds. In addition, it is worth noting that some studies have found that exopolysaccharides from bacteria could improve the immune function (Jones et al., 2014; Wu et al., 2019). The Se-enriched exopolysaccharides produced by Enterobacter cloacae Z0206 significantly increased the serum antibody titers against Newcastle disease virus and enhance the immunity of broilers (Lu et al., 2013). However, whether cathelicidin expression is one of the functions of extracellular polysaccharides is still unknown and worthy of attention.
Furthermore, some studies have shown that although probiotics could not increase the transcription of HDP genes, probiotic treatment prevents the increase of CATH1 expression induced by S. typhimurium, and the combination of probiotics and organic acids can increase the CATH-B1 expression of in the bursa of young broiler chickens (Akbari et al., 2008; Rodríguez-Lecompte et al., 2012).
Perspective and conclusion
Host defense peptides are important effectors of animal immune function, which can activate T cells through chemotaxis induction or direct activation of full-time antigen presenting cells and participate in the regulation of animal-specific immune response. In recent years, HDPs have become a research hotspot in many fields. On the one hand, researchers study the biological function, expression, and distribution characteristics and regulation mechanism of AMP in animals at the molecular level, and on the other hand, it is actively developing the application of HDP in the fields of medical treatment, food and health care, animal husbandry, and veterinary medicine.
It has been found that compared with other families, such as defensins, insect-derived and frog skin–derived HDP, cathelicidin families have stronger antibacterial activity and lower MIC values (Feng et al., 2011; Wang et al., 2011). Besides these in vitro tests, among the avian species, only the antibacterial efficacy of chicken CATH1 in vivo was evaluated. Intraperitoneal injection of 10 mg/kg of CATH1 analog (fowlicidin-1 (6–26)) increased the survival rate of mice with a lethal dose of methicillin-resistant S. aureus–induced neutropenia by 50%, while reducing bacterial titers in the peritoneal fluid and spleen of mice (Bommineni et al., 2010). In addition, Bommineni et al. (2010) further found that due to the ability of fowlicidin-1(6–26) to induce neutrophil chemotaxis and macrophage activation, 50% of mice could be protected by taking fowlicidin-1(6–26) 4 d before S. aureus infection, and all mice could survive if the peptide was received 1–2 d before infection (Bommineni et al., 2014). Taken together, as a novel antimicrobial, fowlicidin-1(6–26) has a good application prospect in treatment and prevention. Furthermore, the bactericidal effect of cathelicidin family HDPs was rapid, and both chicken fowlicidins (CATH1 and 2) showed rapid killing of E. coli with the maximum killing occurring at 30 min at MIC90 concentrations (Xiao et al., 2006). More importantly, some cathelicidins have very strong activity against amounts of clinically isolated drug-resistant strains, even super drug–resistant bacteria (Guang et al., 2012). Quail Cc-CATH2 and 3 displayed broad and potent antimicrobial activity against most of the 41 strains of bacteria and fungi tested, especially the clinically isolated drug-resistant strains, such as S. aureus and P. aeruginosa (Feng et al., 2011). At the same time, Cc-CATH2 and 3 showed considerable reduction of cytotoxic activity and hemolytic activity compared with other avian cathelicidins (Feng et al., 2011). Therefore, they are expected to become a class of important new antimicrobial agents, providing new means and ways to solve the increasingly serious problem of drug resistance of strains.
Host defense peptides can affect the development of adaptive immune response by regulating the migration, maturation, and activation of dendritic cells and T and B lymphocytes (Yang et al., 2004; Nicholls et al., 2010; Hancock et al., 2012; Zhang and Sunkara, 2014). As an important member of HDPs, cathelicidins can stimulate Th1 immunoreaction without LPS, thus they could be used as immunomodulators, vaccine adjuvants, or additives to strengthen the adaptive immune response (Van Harten et al., 2018). The adjuvanticity of chicken CATH1 has been experimentally verified. When mice were immunized with ovalbumin and chicken CATH1, the mice challenged with ovalbumin produced higher titers of IgG1 and IgG2a (Bommineni et al., 2014). The reason for this phenomenon may be that CATH1 might induce the expression of costimulatory molecule CD86 on the surface of macrophages more effectively than LL-37; CATH1 may be more effective in facilitating antigen presentation and adaptive immunity, so CATH1 could be a very useful adjuvant or a component of adjuvant complexes (Kindrachuk et al., 2009; Zhang and Sunkara. 2014; Bommineni et al., 2014).
Cathelicidin-related HDP have become effective substances in the innate immune system. Inferred from some other important biological functions of cathelicidins, avian cathelicidins should also have biological functions including wound repair, antitumor property, proangiogenesis, and so on. Therefore, to study the specific application of avian cathelicidin, several important topics will have to be addressed in the future. The basic identification and relevant function analysis of cathelicidins from different birds were carried out, and the role and mechanism of avian cathelicidins in disease were evaluated by in vivo or in vitro experiments, followed by clinical trial evaluation and targeted development of new antimicrobial drugs.
Conclusion
In the present review, we have described recent work on the structure, evolution, biological activity mechanisms, and expression regulation of avian cathelicidins. All these characteristics of avian cathelicidins show their potential as a substitute for antibiotics. However, various studies have shown that although these small bioactive peptides have health effects, they are still limited in animal trials and human clinical evidence. Therefore, we hope that this review could provide a reference for the health benefit assessment and further application of avian cathelicidins.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31772638) and the Natural Science Foundation of Heilongjiang Province (C2016022).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.09.030.
Supplementary data
References
- Achanta M., Sunkara L.T., Dai G., Bommineni Y.R., Jiang W., Zhang G. Tissue expression and developmental regulation of chicken cathelicidin antimicrobial peptides. J. Anim. Sci. Biotechnol. 2012;3:15. doi: 10.1186/2049-1891-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.S., Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afacan N.J., Yeung A.T.Y., Pena O.M., Hancock R.E.W. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012;18:807–819. doi: 10.2174/138161212799277617. [DOI] [PubMed] [Google Scholar]
- Akbari M.R., Haghighi H.R., Chambers J.R., Brisbin J., Read L.R., Sharif S. Expression of antimicrobial peptides in cecal tonsils of chickens treated with probiotics and infected with Salmonella enterica serovar typhimurium. Clin. Vaccine Immunol. 2008;15:1689–1693. doi: 10.1128/CVI.00242-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexis L., Thévenet P., Julien R., Marek V., Philippe D., Pierre T. PEPp-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016;44:W449–W454. doi: 10.1093/nar/gkw329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S., Mitsuyasu K., Soeda Y., Hidaka M., Ito Y., Matsubara K., Shindo M., Uchida Y., Aoyagi H. Structure-activity relationship of indolicidin, a Trp-rich antibacterial peptide. J. Pept. Sci. 2010;16:171–177. doi: 10.1002/psc.1217. [DOI] [PubMed] [Google Scholar]
- Bommineni Y.R., Pham G.H., Sunkara L.T., Achanta M., Zhang G. Immune regulatory activities of fowlicidin-1, a cathelicidin host defense peptide. Mol. Immunol. 2014;59:55–63. doi: 10.1016/j.molimm.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Bommineni Y.R., Dai H., Gong Y.X., Soulages J.L., Fernando S.C., DeSilva U., Prakash O., Zhang G. Fowlicidin-3 is an α-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities. FEBS. J. 2007;274:418–428. doi: 10.1111/j.1742-4658.2006.05589.x. [DOI] [PubMed] [Google Scholar]
- Bommineni Y.R., Achanta M., Alexander J., Sunkara L.T., Ritchey J.W., Zhang G. A fowlicidin-1 analog protects mice from lethal infections induced by methicillin-resistant Staphylococcus aureus. Peptides. 2010;31:1225–1230. doi: 10.1016/j.peptides.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Chang C.I., Zhang Y.A., Zou J., Nie P., Secombes C.J. Two cathelicidin genes are present in both rainbow trout (Oncorhynchus mykiss) and atlantic salmon (Salmo salar) Antimicrob. Agents Chemother. 2006;50:185–195. doi: 10.1128/AAC.50.1.185-195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Zhai Z.Y., Long H.R., Yang G.M., Deng B.C., Deng J.P. Inducible expression of defensins and cathelicidins by nutrients and associated regulatory mechanisms. Peptides. 2020;123:170–177. doi: 10.1016/j.peptides.2019.170177. [DOI] [PubMed] [Google Scholar]
- Cheng Y.Y., Prickett M.D., Gutowska W., Kuo R., Belov K., Burt D.W. Evolution of the avian β-defensin and cathelicidin genes. BMC Evol. Biol. 2015;15:188. doi: 10.1186/s12862-015-0465-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorens M., Scheenstra M.R., Veldhuizen E.J., Haagsman H.P. Interspecies cathelicidin comparison reveals divergence in antimicrobial activity, TLR modulation, chemokine induction and regulation of phagocytosis. Sci. Rep. 2017;7:40874. doi: 10.1038/srep40874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorens M., Schneider V.A.F., Groot A.M.D., Dijk A.V., Haagsman H.P. Cathelicidins inhibit escherichia coli-induced TLR2 and TLR4 activation in a viability-dependent manner. J. Immunol. 2017;199:1418. doi: 10.4049/jimmunol.1602164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus T., Coorens M., van Dijk A., Haagsman H.P. Avian host defense peptides. Dev. Comp. Immunol. 2013;41:352–369. doi: 10.1016/j.dci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Dathe M., Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta. 1999;1462:71–87. doi: 10.1016/s0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- Derache C., Meudal H., Aucagne V., Mark K.J., Cadène M., Delmas A.F., Lalmanach A.C., Landon C. Initial insights into structure-activity relationships of avian defensins. J. Biol. Chem. 2012;287:7746–7755. doi: 10.1074/jbc.M111.312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner R.A., Pestonjamasp V.K., Tamakuwala S., Ohtake T., Rudisill J., Nizet V., Agerberth B., Gudmundsson G.H., Gallo R.L. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Feng F.F., Chen C., Zhu W.J., He W.Y., Guang H.J., Li Z., Wang D., Liu J.Z., Chen M., Wang Y.P., Yu H.N. Gene cloning, expression and characterization of avian cathelicidin orthologs, Cc-CATHs, from Coturnix coturnix. FEBS. J. 2011;278:1573–1584. doi: 10.1111/j.1742-4658.2011.08080.x. [DOI] [PubMed] [Google Scholar]
- Gao W., Xing L.W., Qu P., Tan T.T., Yang N., Li D., Chen H.X., Feng X.J. Identification of a novel cathelicidin antimicrobial peptide from ducks and determination of its functional activity and antibacterial mechanism. Sci. Rep. 2015;5:12. doi: 10.1038/srep17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitsuka R., Chen C.H., Benyon L., Asano Y., Kitamura D., Cooper M.D. Chicken cathelicidin-B1, an antimicrobial guardian at the mucosal M cell gateway. Proc. Natl. Acad. Sci. USA. 2007;104:15063–15068. doi: 10.1073/pnas.0707037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guang H.J., Li Z., Wang Y.P., Lai R., Yu H.N. Progress in cathelicidins antimicrobial peptides research. Zool. Res. 2012;33:523–526. doi: 10.3724/SP.J.1141.2012.05523. [DOI] [PubMed] [Google Scholar]
- Hamad S.K., Kim S., El-Kadi S.W., Wong E.A., Dalloul R.A. Comparative expression of host defense peptides in Turkey poults. Poult. Sci. 2017;96:2083–2090. doi: 10.3382/ps/pew500. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Sahl H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Hancock R.E., Nijnik A., Philpott D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012;10:243. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilborn J.D., Nilsson M.F., Kratz G., Weber G., Sørensen O., Borregaard N., Ståhle-Bäckdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Huang H.W. Action of antimicrobial peptides: two-state model. Biochemistry. 2000;39:8347–8352. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- Ishige T., Hara H., Hirano T., Kono T., Hanzawa K. Characterization of the cathelicidin cluster in the Japanese quail (Coturnix japonica) Anim. Sci. J. 2017;88:1249–1257. doi: 10.1111/asj.12752. [DOI] [PubMed] [Google Scholar]
- Jones S.E., Paynich M.L., Kearns D.B., Knight K.L. Protection from intestinal inflammation by bacterial exopolysaccharides. J. Immunol. 2014;192:4813–4820. doi: 10.4049/jimmunol.1303369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsy R., Ma X., Liu Y., Qian S.Y., Hou Y.Q., Zhang G.L. Dietary modulation of endogenous host defense peptide synthesis as an alternative approach to in-feed antibiotics. Anim. Nutr. 2018;4:160–169. doi: 10.1016/j.aninu.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J., Jenssen H., Elliott M., Townsend R., Nijnik A., Lee S.F., Gerdts V., Babiuk L.A., Halperin S.A., Hancock R.E.W. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine. 2009;27:4662–4671. doi: 10.1016/j.vaccine.2009.05.094. [DOI] [PubMed] [Google Scholar]
- Kosciuczuk E.M., Lisowski P., Jarczak J., Strzalkowska N., Jozwik A., Horbanczuk J., Krzyzewski J., Zwierzchowski L., Bagnicka E. Cathelicidins: family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012;39:10957–10970. doi: 10.1007/s11033-012-1997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij M.D., Van Dijk A., Haagsman H.P. CATH-2 and LL-37 increase mannose receptor expression, antigen presentation and the endocytic capacity of chicken mononuclear phagocytes. Mol. Immunol. 2017;90:118–125. doi: 10.1016/j.molimm.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Lallès J.P., Bosi P., Smidt H., Stokes C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007;66:260–268. doi: 10.1017/S0029665107005484. [DOI] [PubMed] [Google Scholar]
- Lu Z., Jin M., Huang M., Wang Y., Wang Y. Bioactivity of selenium-enriched exopolysaccharides produced by Enterobacter cloacae Z0206 in broilers. Carbohydr. Polym. 2013;96:131–136. doi: 10.1016/j.carbpol.2013.03.063. [DOI] [PubMed] [Google Scholar]
- Lynn D.J., Higgs R., Lloyd A.T., O'Farrelly C., Hervé-Grépinet V., Nys Y., Brinkman F.S., Yu P.L., Soulier A., Kaiser P., Zhang G., Lehrer R.I. Avian beta-defensin nomenclature: a community proposed update. Immunol. Lett. 2007;110:86–89. doi: 10.1016/j.imlet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lynn D.J., Higgs R., Gaines S., Tierney J., James T., Lloyd A.T., Fares M.A., Mulcahy G., O'Farrelly C. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics. 2004;56:170–177. doi: 10.1007/s00251-004-0675-0. [DOI] [PubMed] [Google Scholar]
- Meade K.G., Narciandi F., Cahalane S., Reiman C., Allan B., O’Farrelly C. Comparative in vivo infection models yield insights on early host immune response to Campylobacter in chickens. Immunogenetics. 2009;61:101–110. doi: 10.1007/s00251-008-0346-7. [DOI] [PubMed] [Google Scholar]
- Meade K.G., Higgs R., Lloyd A.T., Giles S., O'Farrelly C. Differential antimicrobial peptide gene expression patterns during early chicken embryological development. Dev. Comp. Immunol. 2009;33:516–524. doi: 10.1016/j.dci.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Moravej H., Moravej Z., Yazdanparast M., Heiat M., Mirhosseini A., Moosazadeh Moghaddam M., Mirnejad R. Antimicrobial peptides: features, action, and their resistance mechanisms in bacteria. Microb. Drug Resist. 2018;24:747–767. doi: 10.1089/mdr.2017.0392. [DOI] [PubMed] [Google Scholar]
- Nicholls E.F., Madera L., Hancock R.E.W. Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann. NY. Acad. Sci. 2010;1213:46–61. doi: 10.1111/j.1749-6632.2010.05787.x. [DOI] [PubMed] [Google Scholar]
- Nicolas P. Multifunctional host defense peptides: intracellular-targeting antimicrobial peptides. FEBS J. 2009;276:6483–6496. doi: 10.1111/j.1742-4658.2009.07359.x. [DOI] [PubMed] [Google Scholar]
- Oh D., Shin S.Y., Lee S., Kang J.H., Kim S.D., Ryu P.D., Hahm K.S., Kim Y. Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A(1-8)-magainin 2(1-12) and its analogues, on their antibiotic activities and structures. Biochemistry. 2000;39:11855–11864. doi: 10.1021/bi000453g. [DOI] [PubMed] [Google Scholar]
- Perez V., Shanmugasundaram R., Sifri M., Parr T.M., Selvaraj R.K. Effects of hydroxychloride and sulfate form of zinc and manganese supplementation on superoxide dismutase activity and immune responses post lipopolysaccharide challenge in poultry fed marginally lower doses of zinc and manganese. Poult. Sci. 2017;96:4200–4207. doi: 10.3382/ps/pex244. [DOI] [PubMed] [Google Scholar]
- Podda E., Benincasa M., Pacor S., Micali F., Mattiuzzo M., Gennaro R., Scocchi M. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta. 2006;1760:1732–1740. doi: 10.1016/j.bbagen.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Ren M., Zhang S., Liu X.T., Li S., Mao X., Zeng X., Qiao S. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous β-defensin expression through the Sirt1/ERK/90RSK pathway. J. Agr. Food Chem. 2016;64:3371–3379. doi: 10.1021/acs.jafc.6b00968. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Kopitar M., Jerala R., Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 1989;255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Lecompte J.C., Yitbarek A., Brady J., Sharif S., Cavanagh M.D., Crow G., Guenter W., House J.D., Camelo-Jaimes G. The effect of microbial-nutrient interaction on the immune system of young chicks after early probiotic and organic acid administration. J. Anim. Sci. 2012;90:2246–2254. doi: 10.2527/jas.2011-4184. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lecompte J.C., Yitbarek A., Cuperus T., Echeverry H., Van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult. Sci. 2016;95:2547–2556. doi: 10.3382/ps/pew186. [DOI] [PubMed] [Google Scholar]
- Seal B.S., Lillehoj H.S., Donovan D.M., Gay C.G. Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim. Health Res. Rev. 2013;14:78–87. doi: 10.1017/S1466252313000030. [DOI] [PubMed] [Google Scholar]
- Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- Shao Y.J., Wang Z., Tian X.Y., Guo Y.M., Zhang H.B. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016;85:573–584. doi: 10.1016/j.ijbiomac.2016.01.031. [DOI] [PubMed] [Google Scholar]
- Storici P., Sal G.D., Schneider C., Zanetti M. cDNA sequence analysis of an antibiotic dodecapeptide from neutrophils. FEBS Lett. 1992;314:187–190. doi: 10.1016/0014-5793(92)80971-i. [DOI] [PubMed] [Google Scholar]
- Sumners L.H., Miska K.B., Jenkins M.C., Fetterer R.H., Cox C.M., Kim S., Dalloul R.A. Expression of Toll-like receptors and antimicrobial peptides during Eimeria praecox infection in chickens. Exp. Parasitol. 2011;127:714–718. doi: 10.1016/j.exppara.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Sunkara L.T., Achanta M., Schreiber N.B., Bommineni Y.R., Dai G., Jiang W., Lamont S., Lillehoj H.S., Beker A., Teeter R.G., Zhang G. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 2011;6:e27225. doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder P., Satho T., Irie K., Sharmin T., Hamady D., Nakashima Y., Kashige N., Miake F. Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int. Immunopharmacol. 2011;11:141–144. doi: 10.1016/j.intimp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Tossi A., Sandri L., Giangaspero A. Amphipathic, α-helical antimicrobial peptides. Pept. Sci. 2000;55:4–30. doi: 10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Tossi A., Scocchi M., Zanetti M., Storici P., Gennaro R. PMAP-37, a novel antibacterial peptide from pig myeloid cells. cDNA cloning, chemical synthesis and activity. Eur. J. Biochem. 1995;228:941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]
- Uzzell T., Stolzenberg E.D., Shinnar A.E., Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. From the cover: Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk A., Herrebout M., Tersteeg-Zijderveld M.H., Tjeerdsma-van Bokhoven J.L., Bleumink-Pluym N., Jansman A.J., Veldhuizen E.J., Haagsman H.P. Campylobacter jejuni is highly susceptible to killing by chicken host defense peptide cathelicidin-2 and suppresses intestinal cathelicidin-2 expression in young broilers. Vet. Microbiol. 2012;160:347–354. doi: 10.1016/j.vetmic.2012.05.034. [DOI] [PubMed] [Google Scholar]
- Van Dijk A., Molhoek E.M., Bikker F.J., Yu P.L., Veldhuizen E.J.A., Haagsman H.P. Avian cathelicidins: Paradigms for the development of anti-infectives. Vet. Microbiol. 2011;153:27–36. doi: 10.1016/j.vetmic.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Van Dijk A., Molhoek E.M., Veldhuizen E.J., Bokhoven J.L., Wagendorp E., Bikker F., Haagsman H.P. Identification of chicken cathelicidin-2 core elements involved in antibacterial and immunomodulatory activities. Mol. Immunol. 2009;46:2465–2473. doi: 10.1016/j.molimm.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Van Dijk A., Tersteeg-Zijderveld M.H., Tjeerdsma-van Bokhoven J.L., Jansman A.J., Veldhuizen E.J., Haagsman H.P. Chicken heterophils are recruited to the site of salmonella infection and release antibacterial mature cathelicidin-2 upon stimulation with LPS. Mol. Immunol. 2009;46:1517–1526. doi: 10.1016/j.molimm.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Van Dijk A., Veldhuizen E.J.A., van Asten A.J.A.M., Haagsman H.P. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Vet. Immunol. Immunopathol. 2005;106:321–327. doi: 10.1016/j.vetimm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Van Harten R.M., Van Woudenbergh E., Van Dijk A., Haagsman H.P. Cathelicidins: immunomodulatory antimicrobials. Vaccines. 2018;6:63. doi: 10.3390/vaccines6030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen E.J., Brouwer E.C., Schneider V.A., Fluit A.C. Chicken cathelicidins display antimicrobial activity against multiresistant bacteria without inducing strong resistance. PLoS One. 2013;8:e61964. doi: 10.1371/journal.pone.0061964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.J., Dou X.J., Song J., Lyu Y.F., Zhu X., Xu L., Li W.Z., Shan A.S. Antimicrobial peptides: promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2018;39:831–859. doi: 10.1002/med.21542. [DOI] [PubMed] [Google Scholar]
- Wang C., Feng L., Yu H.N., Wang Y.P. Relationship between structure and function of cathelicidins and their molecular design: a review. Chin. J. Biotechnol. 2017;33:27–35. doi: 10.13345/j.cjb.160247. [DOI] [PubMed] [Google Scholar]
- Wang Y.P., Lu Z.K., Feng F.F., Zhu W., Guang H.J., Liu J.Z., He W.Y., Chi L.L., Li Z., Yu H.N. Molecular cloning and characterization of novel cathelicidin-derived myeloid antimicrobial peptide from Phasianus colchicus. Dev. Comp. Immunol. 2011;35:314–322. doi: 10.1016/j.dci.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J.W., Mader S., White J.H., Hanrahan J.H. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Wang Y.Z., Xu Z.R., Lin W.X., Huang H.Q., Wang Z.Q. Developmental gene expression of antimicrobial peptide PR-39 and effect of zinc oxide on gene regulation of PR-39 in piglets. Asian Austral. J. Anim. 2004;17:1635–1640. [Google Scholar]
- Wang Y.Z. Antimicrobial peptides of animal origin: current situation and prospect. Chin. J. Anim. Nutr. 2014;26:2934–2941. [Google Scholar]
- Wu J., Ma N., Johnston L.J., Ma X. Dietary nutrients mediate intestinal host defense peptide expression. Adv. Nutr. 2019;00:1–11. doi: 10.1093/advances/nmz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.J., Cai Y.B., Bommineni Y.R., Fernando S.C., Prakash O., Gilliland S.E., Zhang G.L. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 2006;281:2858–2867. doi: 10.1074/jbc.M507180200. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Herrera A.I., Bommineni Y.R., Soulages J.L., Prakash O., Zhang G. The central kink region of fowlicidin-2, an alpha-helical host defense peptide, is critically involved in bacterial killing and endotoxin neutralization. J. Innate. Immun. 2009;1:268–280. doi: 10.1159/000174822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub H.A., Elazzazy A.M., Mahmoud M.M., Baeshen M.N., Al-Maghrabi O.A., Alkarim S., Ahmed E.S., Almehdar H.A., Uversky V.N. Chicken cathelicidins as potent intrinsically disordered biocides with antimicrobial activity against infectious pathogens. Dev. Comp. Immunol. 2016;65:8–24. doi: 10.1016/j.dci.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Yang D., Biragyn A., Hoover D.M., Lubkowski J., Oppenheim J.J. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- Yang L., Harroun T.A., Weiss T.M., Ding L., Huang H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Speirs M., Drouin D., Cavalcante P.A., Barkema H.W., Cobo E.R. Host defense cathelicidins in cattle: types, production, bioactive functions and potential therapeutic and diagnostic applications. Int. J. Antimicrob. Agents. 2018;51:813–821. doi: 10.1016/j.ijantimicag.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Yu H.N., Lu Y.L., Qiao X., Wei L., Fu T.T., Cai S.S., Wang C., Liu X.L., Zhong S.J., Wang Y.P. Novel cathelicidins from pigeon highlights evolutionary convergence in avain cathelicidins and functions in modulation of innate immunity. Sci. Rep. 2015;5:13. doi: 10.1038/srep11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- Zanetti M., Del Sal G., Storici P., Schneider C., Romeo D. The cDNA of the neutrophil antibiotic Bac5 predicts a pro-sequence homologous to a cysteine proteinase inhibitor that is common to other neutrophil antibiotics. J. Biol. Chem. 1993;268:522–526. [PubMed] [Google Scholar]
- Zanetti M., Gennaro R., Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang G.W., Li D.B., Lai S.J., Chen S.Y., Lei R.P., Zhou D.G. Effects of dietary vitamin D3 supplementation on AvBD-1 and chCATH-1 genes expression in chicken. J. Poult. Sci. 2010;48:254–258. [Google Scholar]
- Zhang G.L., Sunkara L.T. Avian antimicrobial host defense peptides: from biology to therapeutic applications. Pharmaceuticals. 2014;7:220–247. doi: 10.3390/ph7030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Gan T.X., Liu X.D., Jin Y., Lee W.H., Shen J.H., Zhang Y. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29:1685–1691. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Zhao L., Tan S., Zhang H., Liu P., Tan Y.Z., Li J.C., Jia D., Shen X.F. Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother. Res. 2018;32:1521–1529. doi: 10.1002/ptr.6080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.