Abstract
Growth performance, nutrient digestibility, intestinal health, and endogenous enzyme secretion responses to dietary α-amylase supplementation during 4 growth phases of broiler chickens fed corn–soybean meal–based diets were evaluated in the present study. A total of 1,136 male broiler chicks were assigned at day 0 after hatching to 8 treatments in a 2 × 4 factorial arrangement. There were 2 dietary levels of α-amylase supplementation of 0 or 80 kilo-Novo alpha amylase units per kg diet and 4 posthatching growth phases of day 0 to 11, day 11 to 21, day 21 to 42, or day 42 to 56 in a randomized complete block design. Each treatment comprised 8 replicate pens, with either 25 (day 0–11), 20 (day 11–21), 16 (day 21–42), or 10 (day 42–56) birds per pen. Body weight gain and feed efficiency of birds improved (P < 0.01) with α-amylase supplementation. There were main effects of α-amylase, growth phase, and interaction (P < 0.01) on apparent ileal digestibility (AID) of starch. This ranged from 0.8% during day 11 to 21 to 2.8% during day 0 to 11 after hatching. The total tract retention of starch increased (P < 0.05) with amylase supplementation but was not different across growth phases. Amylase supplementation increased (P < 0.05) AID of gross energy, AME (kcal/kg), and AMEn (kcal/kg). Villus height in the jejunal tissue was increased (P < 0.01) by α-amylase supplementation. During day 11 to 21 after hatching, the viscosity of jejunal digesta and pancreatic amylase activity increased (P < 0.01) with amylase supplementation. In conclusion, dietary amylase supplementation improved growth performance, apparent nutrient digestibility, and digestive enzyme activity of broiler chickens fed a corn–soybean diet. The study indicates that the growth phase of birds may affect response to exogenous amylase.
Key words: amylase, broiler, digestibility, enzyme, starch
Introduction
The energy derived from the components of plants feedstuffs by broiler chickens is affected by enzyme access to substrates such as starch or protein (Theander et al., 1989; Slominski et al., 1993). Among the nutrients in poultry diets, starch is quantitatively the most important energy-yielding source. For instance, corn contains about 69% starch (Knudsen, 1997), which leads to its high content in corn-based diets. Although starch degradability is relatively high in broiler chickens, some proportion of the dietary starch may escape digestion in the small intestine (Englyst et al., 1982; Svihus, 2014). This varies among feed ingredients and in a complete diet, can significantly influence the metabolizable energy content for the birds (Tester et al., 2004). Therefore, there have been increased interests in the use of supplemental enzymes to improve the utilization of substrates that release energy for poultry.
Exogenous carbohydrases such as xylanases, amylases, and glucanases have been shown to improve energy utilization and the performance of broiler chickens (Olukosi and Adeola, 2008). In conventional diets formulated with corn and soybean meal (SBM), an estimated 450 kcal/kg of energy is available for utilization via exogenous enzymes, which may include up to 37% from undigested starch (Cowieson et al., 2010). One mode of action is by improving the access of endogenous enzymes to cell contents (Kocher et al., 2003; Meng et al., 2005). Another is by augmenting endogenous enzyme secretions (Gracia et al., 2003). Similarly, previous studies showed that α-amylase, supplemented alone, increased starch, and energy digestibility in broiler chickens (Cowieson et al., 2019; Stefanello et al., 2019; Woyengo et al., 2019) fed corn–SBM–based diets.
However, there are variations in nutrient utilization by birds and age is one of the explanatory variables (Noy and Sklan, 1995; Uni et al. 1995). It has been suggested that the immaturity of the digestive system of younger birds may result in the relatively poor utilization of dietary nutrients (Jin et al., 1998), and nutrient digestion rather than the ability to absorb nutrients may be a primary limiting factor (Parsons, 2004). This has led to findings that poultry develop an increased capacity to digest starch as the intestinal tract matures, and there is elevated pancreatic amylase production in older birds compared with their juvenile counterparts (Krogdahl and Sell, 1989). Therefore, the effect of animal age on nutrient digestibility may be relevant and the interaction between age and exogenous enzymes needs to be explored.
There are few reports in literature that evaluated the effect of dietary α-amylase supplementation in broiler chickens, as in most instances, amylase is added as part of a cocktail of carbohydrases. There are yet fewer data on the effect of α-amylase supplementation across different growth phases of broiler chickens. Therefore, the hypothesis for the present study is that responses to α-amylase supplementation would be affected by bird age. The present study was designed to evaluate the effects of α-amylase supplementation on growth performance, nutrient digestibility, and feedback enzyme secretion in broiler chickens fed a corn–SBM diet during 4 growth phases of day 0 to 11, 11 to 21, 21 to 42 or 42 to 56 after hatching.
Materials and methods
Protocols of animal experiments were reviewed and approved by the Purdue University Animal Care and Use Committee.
Experimental Birds and Diets
A total of 1,136 male 0-day-old broiler chicks (Cobb 500, Siloam Springs, AR) were purchased from a commercial hatchery. Birds were individually tagged, weighed, and raised in floor pens with temperature and lighting maintained as previously described by Park et al. (2017). The birds were assigned to 8 dietary treatments in a 2 × 4 factorial arrangement. There were 2 dietary levels of α-amylase (Ronozyme HiStarch, DSM Nutritional Products, Kaiseraugst, Switzerland); 0 or 80 kilo-Novo alpha amylase units (KNU) per kg of diet and 4 posthatching growth phases of day 0 to 11, day 11 to 21, day 21 to 42, or day 42 to 56 in a randomized complete block design. Each dietary treatment comprised 8 replicate pens, with either 25 (day 0–11), 20 (day 11–21), 16 (day 21–42), or 10 (day 42–56) birds per replicate. All diets were corn–SBM–based, formulated to meet breeder nutrient specifications and fed as mash (Table 1). The α-amylase was a granulated enzyme preparation produced by submerged fermentation of Bacillus amyloliquefaciens and contained 600 KNU/g. Birds on day 0 to 11 growth phase were fed experimental diets throughout. Birds on day 11 to 21 growth phase were fed the standard broiler starter diet until day 11, but the experimental diets from day 11 to 21. Birds on day 21 to 42 growth phase were fed the standard broiler starter diet until day 21 but the experimental diets from day 21 to 42 after hatching. Birds on day 42 to 56 growth phase were fed the standard broiler starter until day 21 and grower diets until day 42 after hatching but the experimental diets from day 42 to 56 after hatching. All diets contained phytase (Ronozyme HiPhos: DSM Nutritional Products, Kaiseraugst, Switzerland) at 1,000 phytase units/kg and titanium dioxide was added at 5 g/kg as an indigestible marker.
Table 1.
Ingredient and calculated nutrient composition of experimental diets, as-fed basis.
| Growth phase day post hatching: |
Day 0 to 11 |
Day 11 to 21 |
Day 21 to 42 |
Day 42 to 56 |
||||
|---|---|---|---|---|---|---|---|---|
| Amylase, KNU/kg: | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 |
| Ingredients, g/kg | ||||||||
| Corn | 576.2 | 556.2 | 623.8 | 603.8 | 638.6 | 618.6 | 663.3 | 643.3 |
| Soybean meal | 340.0 | 340.0 | 291.0 | 291.0 | 271.0 | 271.0 | 245.0 | 245.0 |
| Soybean oil | 6.5 | 6.5 | 9.5 | 9.5 | 18.5 | 18.5 | 18.5 | 18.5 |
| Monocalcium phosphate1 | 10.2 | 10.2 | 9.0 | 9.0 | 8.0 | 8.0 | 8.5 | 8.5 |
| Limestone2 | 12.2 | 12.2 | 11.5 | 11.5 | 10.5 | 10.5 | 11.0 | 11.0 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Vitamin-mineral premix3 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| DL-Methionine | 2.0 | 2.0 | 1.7 | 1.7 | 1.5 | 1.5 | 1.5 | 1.5 |
| L-Lysine HCl | 1.9 | 1.9 | 2.0 | 2.0 | 0.9 | 0.9 | 1.2 | 1.2 |
| L-Threonine | 0.0 | 0.0 | 0.5 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| Amylase premix4 | 0.0 | 20.0 | 0.0 | 20.0 | 0.0 | 20.0 | 0.0 | 20.0 |
| Titanium dioxide premix5 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Phytase premix6 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Total | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 | 1,000.0 |
| Calculated composition | ||||||||
| Crude protein, g/kg | 220.2 | 220.2 | 200.8 | 200.8 | 190.8 | 190.8 | 180.6 | 180.6 |
| ME, kcal/kg | 3,036.6 | 3,036.6 | 3,108.2 | 3,108.2 | 3,180.1 | 3,180.1 | 3,203.5 | 3,203.5 |
| Ca, g/kg | 7.3 | 7.3 | 6.7 | 6.7 | 6.1 | 6.1 | 6.4 | 6.4 |
| P, g/kg | 6.0 | 6.0 | 5.6 | 5.6 | 5.3 | 5.3 | 5.3 | 5.3 |
| Nonphytate P, g/kg | 3.4 | 3.4 | 3.1 | 3.1 | 2.8 | 2.8 | 2.9 | 2.9 |
| Ca: total P | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Ca: nonphytate P | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 | 2.2 |
| Starch, g/kg | 452.8 | 452.8 | 483.0 | 483.0 | 492.1 | 492.1 | 507.7 | 507.7 |
| Total amino acids, g/kg | ||||||||
| Arg | 14.2 | 14.2 | 12.6 | 12.6 | 12.0 | 12.0 | 11.2 | 11.2 |
| His | 5.8 | 5.8 | 5.3 | 5.3 | 5.0 | 5.0 | 4.8 | 4.8 |
| Ile | 9.0 | 9.0 | 8.1 | 8.1 | 7.7 | 7.7 | 7.2 | 7.2 |
| Leu | 18.9 | 18.9 | 17.5 | 17.5 | 16.9 | 16.9 | 16.2 | 16.2 |
| Lys | 13.1 | 13.1 | 11.9 | 11.9 | 10.5 | 10.5 | 10.0 | 10.0 |
| Met | 5.4 | 5.4 | 4.8 | 4.8 | 4.5 | 4.5 | 4.4 | 4.4 |
| Cys | 3.6 | 3.6 | 3.3 | 3.3 | 3.2 | 3.2 | 3.0 | 3.0 |
| Phe | 10.3 | 10.3 | 9.3 | 9.3 | 8.9 | 8.9 | 8.4 | 8.4 |
| Tyr | 8.5 | 8.5 | 7.7 | 7.7 | 7.3 | 7.3 | 6.9 | 6.9 |
| Thr | 8.1 | 8.1 | 7.9 | 7.9 | 7.0 | 7.0 | 6.6 | 6.6 |
| Trp | 2.9 | 2.9 | 2.6 | 2.6 | 2.4 | 2.4 | 2.2 | 2.2 |
| Val | 10.0 | 10.0 | 9.1 | 9.1 | 8.7 | 8.7 | 8.3 | 8.3 |
| Met + Cys | 8.9 | 8.9 | 8.1 | 8.1 | 7.7 | 7.7 | 7.4 | 7.4 |
| Phe + Tyr | 18.8 | 18.8 | 17.0 | 17.0 | 16.2 | 16.2 | 15.3 | 15.3 |
| Analyzed composition | ||||||||
| Amylase (KNU/kg)7 | LOD | 84 | LOD | 89 | LOD | 81 | LOD | 83 |
Contained 16% Ca, 21% P.
Contained 38% Ca.
Supplied the following per kg diet: vitamin A, 5,484 IU; vitamin D3, 2,643 ICU; vitamin E, 11 IU; menadione sodium bisulfite, 4.38 mg; riboflavin, 5.49 mg; pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 ug; biotin, 55.2 ug; thiamine mononitrate, 2.2 mg; folic acid, 990 ug; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg; Mn, 66.06 mg; Cu, 4.44 mg; Fe, 44.1 mg; Zn, 44.1 mg; Se, 300 ug.
Provided 80 kilo-Novo alpha amylase units (KNU) per kg of diet (Ronozyme HiStarch; DSM Nutritional Products, Kaiseraugst, Switzerland).
1 g of Titanium dioxide added to 4 g corn.
Provided 1,000 FYT/kg of diet (Ronozyme HiStarch; DSM Nutritional Products, Kaiseraugst, Switzerland).
LOD = limit of detection.
Sampling Procedures
Feed and water were available ad libitum during the entire experimental period. Initial and final BW and average feed intake per pen were recorded within each growth phase. Mortality records were taken daily and were used to correct the calculated gain to feed ratio during the experimental period. Two days before the end of each growth phase, birds were randomly selected and transferred to metabolic cages for a 2-d excreta collection. Specifically, 5 birds per pen for during day 0 to 11, day 11 to 21; 3 birds per pen during day 21 to 42; and 2 birds per pen during day 42 to 56 growth phases. At the end of the trial for each of the growth phase, which corresponds to day 11, 21, 42, or 56 after hatching, the remaining birds in each pen were euthanized by CO2 asphyxiation. The pancreas was excised and weighed and digesta was collected from the distal two-thirds of the ileum (i.e., from the Meckel's diverticulum to approximately 2 cm cranial to the ileocecal junction), by flushing with distilled water into plastic containers and stored at −20°C before nutrient analyses. For viscosity measurement, the jejunal content was gently squeezed into plastic tubes and stored at −20°C before analysis.
Intestinal Morphological Analysis
On day 11, 21, 42, and 56 after hatching, mid-jejunal segments were collected from 1 bird per replicate with median BW, flushed with ice-cold 10% phosphate-buffered saline (VWR International, Radnor, PA) and fixed in 10% neutral buffered formalin (VWR International, Radnor, PA) for approximately 30 d. Fixed samples were subsequently dehydrated with ethanol (VWR International, Radnor, PA), cleared with Sub-X (Polysciences, Inc., Warrington, PA) and placed in paraffin (Polyfin paraffin, Sigma Polysciences, St. Louis, MO). Segments (5 μm) were stained with hematoxylin and eosin at the Purdue Histology and Phenotyping Laboratory (Purdue University, West Lafayette, IN). Villus height and crypt depth were measured from 4 complete, vertically oriented villi per slide and subsequently, the villus height to crypt depth ratio was calculated. Villus length is defined as the length from the villus tip to the valley between each villus, whereas crypt depth is defined as the length between the crypt opening and base. The histological sections were evaluated using a binocular light microscope (National Optical and Scientific Instruments, Inc., Schertz, TX). Quantitative measurements were performed with a computerized image analyzer software (AmScope version 3.7, Irvine, CA).
Viscosity Measurements
Approximately, 10 g of jejunal digesta sample were placed in a 50 mL plastic centrifuge tube, vortexed for 10 s and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was transferred into a 2-mL sample cup. The cup containing the supernatant was placed in a water bath (Precision, GCA Corp., College Park, MD) that had been preheated to 40°C until the temperature of the sample equilibrated with that of the water in the water bath (approximately 15 min). The viscosity, in centipoise (cP), of these samples was determined using a viscometer (Vibro viscometer, model SV-1A, A&D Instruments Ltd., Oxfordshire, United Kingdom).
Digestive Enzyme Assay
Duodenal digesta and the pancreas was collected from 1 bird per replicate with median BW, except for group day 0 to 11 where 2 birds per pen with median BW was selected to obtain sufficient samples for analysis. The digesta and pancreas were frozen in liquid nitrogen and stored at −80°C until required for assay. Enzymes activities were determined using a commercially available assay kit (Sigma Chemical Co., St. Louis, MO). The absorbance of the colorimetric final product was measured in a UV/visible spectrophotometer, and the concentration of the respective enzymes was calculated accordingly. For duodenal digesta, the samples were centrifuged at 13,000 rpm at 4°C for 10 min, and aliquots of the supernatant were used for enzyme assay. The activity of the pancreatic enzymes was determined after the whole organ was homogenized in appropriate buffers and centrifuged at 13,000 rpm at 4°C for 10 min, to get a clear supernatant. Amylase activity (EC 3.2.1.1) was determined using a coupled enzyme assay and absorbance of ethylidene-pNP-G7 cleaved by the amylase was measured at 405 nm. One unit is the amount of amylase that cleaves ethylidene-pNP-G7 to generate 1.0 μmol of p-nitrophenol per minute at 25°C.
Total RNA Extraction, Reverse Transcription, and Real-time PCR Analysis
A section of the jejunum was removed from 1 bird per replicate with median BW and flushed with ice-cold PBS (VWR International, Radnor, PA), cut longitudinally in half exposing the lumen, and mucosal contents were scraped with a metal spatula. Mucosal contents were immediately placed in 2 mL of Trizol reagent (Invitrogen, Grand Island, NY) and stored at −80°C before RNA isolation. Total RNA was extracted from the tissues using Trizol reagent (Invitrogen) following the manufacturer's protocol. RNA concentrations were determined by NanoDrop 1000 (Thermo Scientific), and RNA integrity was verified by 1% agarose gel electrophoresis. Extracted RNA was purified with DNA-free DNase Treatment and Removal Kit (Ambion). Afterward, 2 mg of total RNA from each sample were reverse transcribed into cDNA product using the MMLV reverse transcription system (Promega). The cDNA was then diluted 1:10 with nuclease-free water (Ambion) and stored at −20°C until use. Real-time PCR was performed with Bio-Rad iCycler with the FastStart SYBR green-based mix (Life Technologies). PCR programs for all genes were designed as follows: 10 min at 95°C; 40 cycles of 95°C for 30 s, primer-specific annealing temperature for 30 s, and 72°C for 30 s; followed by melting curve analysis. The primer sequences used in the present study are listed in Table 2. Primer specificity and efficiency were verified, subsequently the samples were analyzed in duplicate, and a difference lesser than or equal to 5% was considered acceptable. Relative gene expression was subsequently calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001) with normalization against glyceraldehyde-3-phosphate dehydrogenase, as the housekeeping gene (Tan et al., 2014).
Table 2.
Primers used in real-time quantitative PCR.
| Genes | Primer sequence (5′–3′) | Gene Bank ID | Reference |
|---|---|---|---|
| Housekeeping gene | |||
| GAPDH | F: TCCTAGGATACACAGAGGACCA | ENSGALG000000144421 | Grenier et al., 2015 |
| R: CGGTTGCTATATCCAAACTCA | |||
| Markers of glucose transport | |||
| SGLT-1 | F: GATGTGCGGATACCTGAAGC | AJ236903 | Hu et al., 2010 |
| R: AGGGATGCCAACATGACTG | |||
| GLUT-1 | F: GGCTTTGTCCTTTGAGATGC | L07300 | Humphrey et al. (2004) |
| R: CGCTTTGTTCTCCTCATTGC | |||
| GLUT-2 | F: TGTTCAGCTCCTCCAAGTACC | Z22932 | Humphrey et al. (2004) |
| R: ACAACGAACACATACGGTCC | |||
Abbreviations: F, forward primer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT, glucose transporter; R, reverse primer; SGLT-1, sodium-dependent glucose cotransporter 1.
Sequence obtained from Ensembl chicken genome data resources.
Chemical Analyses and Calculations
The ileal digesta and excreta samples were freeze-dried for 96 h and subsequently ground to pass through a 0.5 mm screen (Retsch ZM 100, GmbH, Haan, Germany). A portion of the samples were analyzed for DM by drying overnight at 105°C (Precision Scientific Co., Chicago, IL; method 934.01; AOAC, 2006) and the nitrogen content of the samples was subsequently determined by combustion method (Leco model TruMac N analyzer, Leco Corp., St. Joseph, MI; AOAC, 2000; Method 990.03) with EDTA as a calibration standard. Starch was determined using a Megazyme total starch determination kit (Method 996.11; AOAC, 2000). The absorbance of the colorimetric final product was measured in a UV/visible spectrophotometer at 510 nm and converted to the amount of glucose released by comparison with a standard curve. Gross energy (GE) concentration in diets, ileal digesta, and excreta samples was determined by isoperibol bomb calorimeter (Parr 1261; Parr 105 Instrument Co., Moline, IL). Titanium concentration was measured on a UV spectrophotometer following the method of Short et al. (1996).
The index method was used to calculate the apparent ileal digestibility (AID) or total tract retention (TTR) of nutrients, in accordance with the following equation:
where TiI is titanium concentration in diets, TiO is titanium concentration in output (ileal digesta or excreta), PI is nutrient concentration in diets, and PO is nutrient concentration in output (ileal digesta or excreta).
The ileal digestible energy (IDE; kcal/kg DM) and AME (kcal/kg DM) of the diet was calculated as the product of the coefficient and GE concentrations (kcal/kg DM) in the diet. The AMEn was calculated by correcting for 0 N retention using a factor of 8.22 kcal/g (Hill and Anderson, 1958):
where Nret is N retention in g/kg of DM intake. The Nret was calculated as follows:
where Ni and No are the N concentrations (g/kg DM) in the diet and excreta, respectively.
Statistical Analyses
The data obtained were analyzed as a randomized complete block design using the GLM procedures of SAS (SAS Inst. Inc., Cary, NC). Initial body weight was used as the blocking factor. The pen of birds was used as the experimental unit for all analyses. The main effects of dietary α-amylase supplementation and growth phase, and the interaction were tested accordingly. Statistical significance was declared at P ≤ 0.05, with 0.05 < P ≤ 0.10 considered as a tendency.
Results
There were few recorded mortalities throughout the trial and were not directly related to the dietary treatments. Overall, there were 4, 4, 6, and 2 mortalities during day 0 to 11, day 11 to 21, day 21 to 42, and day 42 to 56 after hatching, respectively. The performance parameters of the broiler chickens in response to α-amylase supplementation are shown in Table 3. There was no interaction between α-amylase supplementation and growth phase for any of the growth performance indices. However, the final BW and BW gain increased (P < 0.01) with α-amylase supplementation and growth phase, whereas G:F increased (P < 0.01) with α-amylase supplementation but decreased (P < 0.01) as birds grew older. Numerical improvements in BW gain were lower during day 0 to 11 (0.8%), but relatively higher during day 42 to 56 (5.7%) resulting in a tendency (P = 0.08) for an interaction between dietary α-amylase supplementation and growth phase.
Table 3.
Effect of amylase supplementation on growth performance of broiler chickens in different growth phases.1
| Growth phase day after hatching: |
Day 0 to 11 |
Day 11 to 21 |
Day 21 to 42 |
Day 42 to 56 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase, KNU/kg: | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | Amylase | Phase | A × P | |
| Initial BW, g | 52 | 52 | 403 | 403 | 1,145 | 1,145 | 3,113 | 3,112 | 0.6 | 0.689 | <0.001 | 0.907 |
| Final BW, g | 295 | 298 | 1,072 | 1,089 | 3,254 | 3,313 | 4,601 | 4,685 | 17.5 | 0.003 | <0.001 | 0.105 |
| BW gain, g/bird | 243 | 245 | 668 | 686 | 2,109 | 2,167 | 1,488 | 1,573 | 17.0 | 0.002 | <0.001 | 0.081 |
| Feed intake, g/bird | 369 | 365 | 1,112 | 1,146 | 3,934 | 3,870 | 4,540 | 4,527 | 46.8 | 0.726 | <0.001 | 0.774 |
| G: F, g/kg | 658 | 672 | 601 | 598 | 536 | 560 | 328 | 348 | 6.0 | 0.003 | <0.001 | 0.147 |
Data are least square means of 8 replicates cages; A, amylase; P, phase.
Amylase supplementation improved (P < 0.01) the AID of DM, starch, and GE (Table 4). There was an interaction (P < 0.01) between α-amylase supplementation and growth phase on AID of starch. Amylase supplementation improved (P < 0.01) the AID of starch in all growth phases, and ranged from 0.8% during day 11 to 21 to 2.8% during day 0 to 11 after hatching. Furthermore, amylase supplementation improved (P < 0.01) the TTR of DM, starch, and GE (Table 4). There was no interaction between α-amylase supplementation and growth phase on TTR (P < 0.05) of starch. There were no interactions between α-amylase supplementation and growth phase on IDE, AME, and AMEn (kcal/kg DM).
Table 4.
Effect of amylase supplementation and growth phase on nutrient digestibility and retention responses of broiler chickens.1
| Growth phase, day after hatching: |
Day 0 to 11 |
Day 11 to 21 |
Day 21 to 42 |
Day 42 to 56 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase, KNU/kg: | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | Amylase | Phase | A × P | |
| Ileal digestibility | ||||||||||||
| DM, % | 73.6 | 76.4 | 72.6 | 75.4 | 71.5 | 73.4 | 70.6 | 75.7 | 0.75 | <0.001 | 0.014 | 0.213 |
| Starch, % | 95.5 | 98.2 | 96.5 | 97.3 | 96.0 | 98.2 | 96.6 | 98.7 | 0.24 | <0.001 | 0.007 | 0.003 |
| Energy, % | 70.6 | 75.1 | 73.5 | 75.9 | 71.8 | 74.3 | 71.3 | 76.5 | 0.75 | <0.001 | 0.015 | 0.286 |
| IDE, kcal/kg DM | 3,184 | 3,289 | 3,397 | 3,432 | 3,266 | 3,411 | 3,321 | 3,478 | 34.1 | <0.001 | 0.289 | 0.715 |
| Total tract retention | ||||||||||||
| DM, % | 74.7 | 79.0 | 73.5 | 77.3 | 71.7 | 75.0 | 74.8 | 76.4 | 0.46 | <0.001 | <0.001 | 0.041 |
| Starch, % | 98.0 | 98.2 | 97.7 | 98.1 | 97.6 | 98.3 | 98.1 | 98.3 | 0.15 | 0.010 | 0.087 | 0.576 |
| AME, % | 76.9 | 80.3 | 76.3 | 78.9 | 75.6 | 78.1 | 76.0 | 79.5 | 0.50 | <0.001 | 0.022 | 0.661 |
| AME, kcal/kg DM | 3,466 | 3,514 | 3,523 | 3,566 | 3,512 | 3,586 | 3,539 | 3,612 | 23.2 | 0.001 | 0.008 | 0.867 |
| Nitrogen | 71.7 | 76.0 | 71.1 | 74.7 | 70.4 | 72.8 | 72.3 | 73.8 | 0.61 | <0.001 | 0.009 | 0.120 |
| AMEn, % | 72.1 | 75.0 | 71.4 | 73.5 | 70.9 | 73.1 | 71.0 | 74.2 | 0.48 | <0.001 | 0.024 | 0.650 |
| AMEn, kcal/kg DM | 3,252 | 3,284 | 3,297 | 3,324 | 3,291 | 3,357 | 3,310 | 3,375 | 22.1 | 0.005 | 0.016 | 0.722 |
Data are least square means of 8 replicates cages; A, amylase; P, phase; IDE, ileal digestible energy.
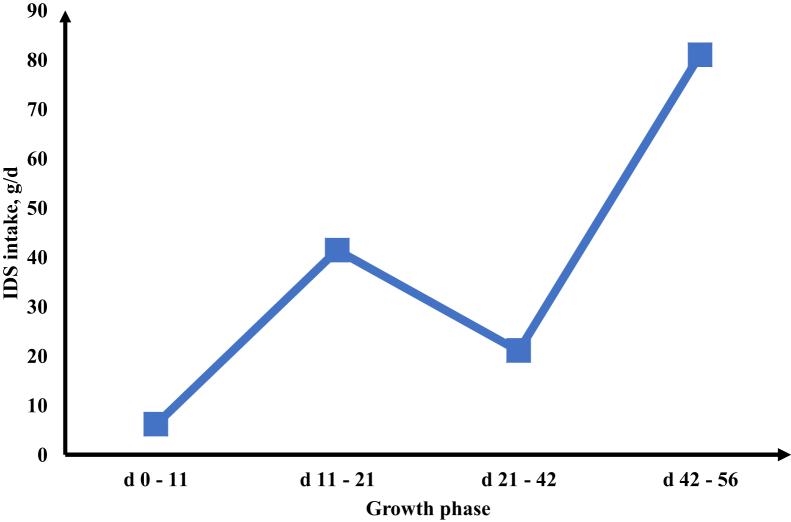
The effect of amylase supplementation on ileal digestible starch (IDS) intake is presented in Figure 1. The mean improvement in IDS intake due to amylase supplementation are 6.1, 41.4, 21.0, and 81.0 g/d during day 0 to 11, 11 to 21, 21 to 42, and 42 to 56 growth phases, respectively.
Figure 1.
Changes in ileal digestible starch (IDS) intake of broiler chickens in the 4 growth phases as a result of α-amylase supplementation. Square data points represent the mean α-amylase effect on IDS intake, relative to the control diet, in the 4 growth phases.
As shown in Table 5, there were increases in villus height (P < 0.01) and crypt depth (P < 0.05) of the jejunal tissue due to dietary α-amylase supplementation. However, there was a tendency for an interaction (P = 0.058) between α-amylase and growth phase for villus height. The improvements in villus height due to α-amylase supplementation were 2.4% (day 0–11), 7.9% (day 11–21), 38.8% (day 21–42), and 23.1% (day 42–56).
Table 5.
Effect of amylase supplementation and growth phase on pancreas weight, gut morphology, and viscosity of jejunal digesta of broiler chickens.1
| Growth phase day after hatching: |
Day 0 to 11 |
Day 11 to 21 |
Day 21 to 42 |
Day 42 to 56 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase, KNU/kg: | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | Amylase | Phase | A × P | |
| Villus height, μm | 959.8 | 982.6 | 1,154.7 | 1,246.6 | 1,067.3 | 1,481.7 | 1,427.8 | 1,757.3 | 79.13 | 0.001 | <0.001 | 0.058 |
| Crypt depth, μm | 124.0 | 147.2 | 124.3 | 141.4 | 164.5 | 180.9 | 148.5 | 173.1 | 13.06 | 0.036 | 0.012 | 0.985 |
| Villus: crypt ratio | 7.9 | 7.0 | 9.3 | 9.1 | 6.6 | 8.3 | 10.7 | 10.3 | 0.65 | 0.937 | <0.001 | 0.217 |
| Pancreas, g | 1.11 | 1.06 | 2.36 | 2.35 | 3.94 | 4.09 | 4.73 | 4.54 | 0.079 | 0.685 | <0.001 | 0.224 |
| Pancreas, g/kg BW | 3.14 | 3.06 | 2.03 | 2.02 | 1.14 | 1.19 | 0.97 | 0.92 | 0.061 | 0.609 | <0.001 | 0.774 |
| Viscosity, mPas | 3.30 | 3.04 | 2.78 | 2.82 | 2.82 | 1.94 | 3.04 | 2.98 | 0.066 | <0.001 | <0.001 | <0.001 |
Data are least square means of 8 replicates cages; A, amylase; P, phase.
Although affected by growth phase (P < 0.01), the absolute and relative pancreas weight was not affected by α-amylase supplementation. There was an effect of α-amylase supplementation and growth phase and an interaction (P < 0.01) on viscosity of jejunal digesta. Amylase supplementation reduced the viscosity (P < 0.01) of the jejunal digesta during day 0 to 11, day 21 to 42, and day 42 to 56 after hatching. However, during day 11 to 21 after hatching, α-amylase supplementation increased (P < 0.01) the viscosity of jejunal digesta.
The amylase activities in the duodenal digesta and pancreas and gene expression of glucose transporters of broiler chickens in response to α-amylase supplementation are shown in Table 6. There were effects of α-amylase supplementation and growth phase and an interaction (P < 0.01) on amylase activities in the duodenal digesta and pancreas. In all growth phases, duodenal amylase activity increased (P < 0.01) with amylase supplementation. Amylase supplementation decreased (P < 0.01) the pancreatic amylase activity in all phases, except during day 11 to 21 after hatching. There was no effect of α-amylase supplementation or growth phase on the mRNA expression of markers of glucose transport.
Table 6.
Effect of amylase supplementation and growth phase on amylase activity and mRNA expression of glucose transporters in the jejunal tissue of broiler chickens.1
| Growth phase day after hatching: |
day 0 to 11 |
day 11 to 21 |
day 21 to 42 |
day 42 to 56 |
SEM |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase, KNU/kg: | 0 | 80 | 0 | 80 | 0 | 80 | 0 | 80 | Amylase | Phase | A × P | |
| Amylase activity | ||||||||||||
| Duodenum, u/mL | 174.20 | 258.80 | 126.30 | 131.50 | 91.90 | 122.80 | 143.90 | 178.60 | 5.081 | <0.001 | <0.001 | <0.001 |
| Pancreas, u/mg | 33.60 | 17.54 | 17.80 | 19.18 | 28.60 | 16.43 | 17.70 | 11.30 | 1.821 | <0.001 | <0.001 | <0.001 |
| Glucose markers | ||||||||||||
| GLUT-1 | 0.83 | 0.72 | 0.60 | 1.20 | 1.35 | 1.07 | 0.99 | 1.01 | 0.157 | 0.623 | 0.096 | 0.058 |
| GLUT-2 | 1.13 | 1.35 | 1.12 | 0.87 | 1.06 | 0.67 | 1.27 | 0.93 | 0.269 | 0.336 | 0.555 | 0.713 |
| SGLT-1 | 0.64 | 1.08 | 1.14 | 1.02 | 0.80 | 0.86 | 0.98 | 0.88 | 0.325 | 0.768 | 0.873 | 0.772 |
Data are least square means of 8 replicates cages; GLUT, glucose transporter; SGLT-1, sodium-dependent glucose cotransporter 1.
Discussion
The present study showed that exogenous amylase supplementation of diets improved the growth performance response of broiler chickens. This observation is similar to previous reports (Onderci et al., 2006; Vieira et al., 2015; Stefanello et al., 2019) for broilers fed amylase-supplemented, corn–SBM–based diets. Likewise, Ritz et al. (1995) showed 3% improvements in BW gain for 21-day-old poults fed a corn–SBM diet supplemented with an enzyme complex containing predominantly amylase. Although improvements were observed relative to the control, the present study showed that the effect of the exogenous amylase on BW gain and feed efficiency was not different across the 4 growth phases. This might be due to the lack of change in feed intake response of the birds as a result of the enzyme supplementation. Although birds eat more as they grow older, it is possible that this lack of effect of amylase supplementation on feed intake could be a limiting factor to substrate availability for the enzyme. This might partly explain the observed similarity in amylase effect on bird performance responses across the 4 growth phases. However, Svihus and Hetland (2001) previously indicated that increases in feed intake in birds reduces the digesta transit time and is inversely correlated with starch digestibility. There are other previous reports that show this lack of effect of exogenous amylase on feed intake (Kaczmarek et al. 2014); however Gracia et al. (2003) reported increased feed intake due to exogenous amylase with increasing age of birds. Similarly, Jiang et al. (2008) showed a linear increase in feed intake and BW gain but observed no effect on feed efficiency with birds fed diets supplemented with amylase. These inconsistencies in the effect of amylase supplementation on growth performance could be due to discrepancies in the source, composition, and concentration of the enzyme preparation or age of the birds used in the various studies.
Furthermore, the present study showed significant improvements in the AID and TTR of starch and GE as a result of dietary amylase supplementation. This observation is similar to a previous report by Stefanello et al. (2019), who observed an increase in energy utilization in broiler chickens fed corn–SBM diet supplemented with amylase. Zanella et al. (1999) found that the respective AID and TTR of starch in 37-day-old broilers increased from 91.2 to 93.0% and from 98.2 to 98.5% when fed a corn–SBM diet supplemented with an enzyme complex containing amylase. It is presumed that while chickens readily adapt well to starch-based diets (Svihus, 2011), the very high feed intake of the modern fast-growing broiler chickens may present some physiological limitations for starch digestion and absorption. These limitations include factors such as the nature of the starch crystals, inadequacies in endogenous amylases, and issues around extraction of glucose from the intestinal lumen via Na-dependent transport systems. This could leave significant portions of the dietary starch undigested and available to react with the supplemental amylase. Furthermore, the improvements in apparent ileal starch and energy digestibility was also observed across all 4 growth phases, with the greatest impact during day 0 to 11 after hatching, and suggests an efficacy of the enzyme irrespective of the stage of digestive system development of broilers.
Starch is an extremely heterogeneous structure (Tester et al., 2004), and inherent properties such as its crystallinity (Bjorck et al., 2000) and the ratio between the amylose and the waxier amylopectin fractions would play a major role in its rate of digestion by digestive amylases (Zhang et al., 2006). Compared with other species, the increased capacity to digest native starch by chickens may be due to the high pancreatic secretion of amylolytic juice (Lehrner and Malacinski, 1975). However, previous work by Croom et al. (1999) noted that as birds grow older, the intestinal mass and pancreatic tissue become an increasingly diminished proportion of the metabolic weight of the bird which may limit the overall effectiveness of the enzyme secreted. This has led to the assumption that birds may be responsive to exogenous amylases due to a limiting supply of endogenous amylase to cater for the changes in body weight and physiological needs. Conversely, Gracia et al. (2003) observed a significant increase in starch and energy digestibility when exogenous amylase was added to corn–based diets, thus indicating that α-amylase secretion may be a limiting factor. In the present study, the improvements in starch digestibility in older birds could also be due to an amylase-induced increase in the digestible starch intake. It is therefore possible that the newly hatched chicks require assistance to augment pancreatic amylase production due to their relatively immature gut, whereas the older birds would require exogenous amylase to augment pancreatic output only at a time of very high starch intake.
An elevation of duodenal amylase activity in all growth phases, especially during day 0 to 11 after hatching, with an associated feedback inhibition of pancreatic amylase secretion was seen in the present study, which is similar to observations by Gracia et al. (2003) and Onderci et al. (2006). However, during day 11 to 21 after hatching, an increase in duodenal amylase activity as a result of the amylase supplementation did not result in sparing of pancreatic amylase secretion. Instead, there was an increase and the reason for this observation is not clear but may be related to the degree of homology between exogenous and endogenous amylases. In addition, it may be that compared with other growth phases, there was a relatively low change in duodenal amylase activity due to the exogenous amylase and could suggest a compensatory action by the pancreas. In previous work and largely consistent with the present study, Cowieson et al. (2019) suggested that birds may have 2 windows of exogenous amylase sensitivity, which is immediately after hatch, and in the grower–finisher phase. Furthermore, there were inconsistencies in the intestinal and pancreatic amylase activity and this difference in response, also observed in previous data in literature, may be due to age of birds. For example, Zhu et al. (2014) reported inconsistent pancreatic amylase activities on day 7, 14, and 21 after hatching in birds fed diets supplemented with an enzyme cocktail containing 800 U/g of amylase. Yuan et al. (2008) reported increased amylase activities in both pancreas and duodenal digesta, as a result of an enzyme cocktail supplementation containing predominantly amylase. Inborr (1990) and Ritz et al. (1995) opined that the inconsistencies in literature may also be due to differences that exists between the chemical characteristics of endogenous amylase and that of bacterial or plant origin which may not always result in feedback inhibition of pancreatic amylase production.
In the present study, exogenous amylase altered the morphology of the gut. This was observed as increases in the length of the villi and crypt depth within the jejunal tissue, which may have enhanced nutrient absorption (Caspary, 1992). This improvement by the exogenous amylase increases with the age of bird. This is similar to a previous report by Onderci et al. (2006) who observed increased villi length in broilers fed diets supplemented with 2 strains of amylase-producing bacteria. Therefore, it is possible that the observed improvements in growth performance of the birds may not only be due to increased release of simple sugars from starch digestion but rather to the changes in the morphology of the small intestine which would have favored nutrient absorption. Similarly, Ritz et al. (1995) reported that α-amylase supplementation increases the length of the villi within the jejunal and ileal sections of 21-day-old turkey poults fed corn–SBM diets. Although there were changes in gut morphology and increases in starch degradability, it is pertinent to note that exogenous amylase did not affect the expression of glucose transporters in the jejunum in any of the growth phases. While this observation is not clear, it was reported that the rate of digestion of starch differs along the length of the chicken intestine (Weurding et al., 2001). This would lead to variation in the amount of glucose available for absorption at each different intestinal site and could have resulted in the lack of change in the glucose transporter expressions. In the present study, only the mid-jejunal section was assayed for glucose transporters.
The viscosity of the jejunal digesta was significantly reduced by amylase supplementation in all phases, except during day 11 to 21. This reduction in viscosity is however in dissonance to previous reports (Zanella et al., 1999; Gracia et al., 2003) for corn–SBM–based diets. Corn and soybeans, compared with barley or wheat, are relatively are low in nonstarch polysaccharides and therefore should not present problems of viscosity. Given they make the bulk of the experimental diets for chickens, it is curious that amylase supplementation alone, and not as part of a carbohydrase cocktail, affected the viscosity of the digesta. However, owing to the interfering effects of the branched amylopectin α-1,6 bonds on crystal formation, waxy starches with a high proportion of amylopectin relative to amylose tend to be more amorphous and soluble. This could create viscous gels in the intestine of the birds and interfere in the digestion and absorption of nutrients (Gohl and Gohl, 1977; van der Klis et al., 1993). Hence, the improvements observed in nutrient digestibility by exogenous amylase may also have been partially due to a reduction in the viscosity of the digesta and a greater access to digestive enzymes. Again, it is not clear why the viscosity of the jejunal digesta was increased by amylase supplementation during day 11 to 21 compared with other growth phases.
Anatomically, the relative pancreas weight decreased with age of birds and is consistent with the reports by Nitsan et al. (1991a,b). However, there was no effect of α-amylase supplementation on the relative pancreas weight, for all growth phases. This response is similar to previous report by Onderci et al. (2006). However, it is in dissonance to the study by Gracia et al. (2003) that reported a reduction in relative pancreas weight at day 7 and day 28 after hatching due to amylase supplementation. The pancreas produces and secretes digestive enzymes which are consequently affected by the concentration of enzymes and substrates or products of their hydrolysis in the lumen of the small intestine (Moran, 1985). Therefore, a reduction in pancreas weight has been related to less secretion of endogenous enzymes, which is partly due to the presence of exogenous enzyme in the intestine.
In conclusion, the data showed that exogenous amylase improves growth performance and apparent nutrient digestibility of broiler chickens fed diets containing mostly corn and SBM. In addition, the study showed that the apparent ileal digestibility of starch, viscosity of the jejunal digesta, and intestinal amylase activity is age-of-bird dependent. However, there were marked deviations in the overall responses of birds during day 11 to 21 after hatching compared with other growth phases and this observation warrants further investigations.
Acknowledgments
This research was supported by funding from DSM Nutritional Products, Kaiseraugst, Switzerland. The authors thank Cobb-Vantress, Monticello, KY, for donating the chicks and Pat Jaynes for her technical assistance.
Conflict of Interest Statement: The authors declare that there is no conflict of interest.
References
- AOAC. 17th ed. Assoc. Off. Anal. Chem.; Arlington, VA: 2000. Official Methods of Analysis. [Google Scholar]
- AOAC. 18th ed. Assoc. Off. Anal. Chem.; Gaithersburg, MD: 2006. Official Methods of Analysis. [Google Scholar]
- Björck I., Liljeberg H., Östman E. Low glycaemic-index foods. Br. J. Nutri. 2000;83:149–155. doi: 10.1017/s0007114500001094. [DOI] [PubMed] [Google Scholar]
- Caspary W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992;55:299S–308S. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Bedford M.R., Ravindran V. Interactions between xylanase and glucanase in maize-soy-based diets for broilers. Br. Poult. Sci. 2010;51:246–257. doi: 10.1080/00071661003789347. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Vieira S.L., Stefanello C. Exogenous microbial amylase in the diets of poultry: what do we know? J. Appl. Poult. Res. 2019;28:556–565. [Google Scholar]
- Croom W.J., Brake J., Coles B.A., Havenstein G.B., Christensen V.L., McBride B.W., Peebles E.D., Taylor I.R. Is intestinal absorption capacity rate-limiting for performance in poultry? J. Appl. Poult. Res. 1999;8:242–252. [Google Scholar]
- Englyst H.N., Wiggins H.S., Cummings J.H. Determination of the non-starch polysaccharides in plant foods by gas liquid chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Gohl B., Gohl I. The effect of viscous substances on the transit time of barley digesta in rats. J. Sci. Food Agric. 1977;28:911–915. doi: 10.1002/jsfa.2740281008. [DOI] [PubMed] [Google Scholar]
- Gracia M.I., Araníbar M.J., Lázaro R., Medel P., Mateos G.G. Alpha-amylase supplementation of broiler diets based on corn. Poult. Sci. 2003;82:436–442. doi: 10.1093/ps/82.3.436. [DOI] [PubMed] [Google Scholar]
- Grenier B., Schwartz-Zimmermann H.E., Caha S., Moll W.D., Schatzmayr G., Applegate T.J. Dose-dependent effects on sphingoid bases and cytokines in chickens fed diets prepared with Fusarium verticillioides culture material containing fumonisins. Toxins. 2015;7:1253–1272. doi: 10.3390/toxins7041253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Hu X.F., Guo Y.M., Huang B.Y., Bun S., Zhang L.B., Li J.H., Liu D., Long F.Y., Yang X., Jiao P. The effect of glucagon-like peptide 2 injection on performance, small intestinal morphology, and nutrient transporter expression of stressed broiler chickens. Poult. Sci. 2010;9:1967–1974. doi: 10.3382/ps.2009-00547. [DOI] [PubMed] [Google Scholar]
- Humphrey B.D., Stephensen C.B., Calvert C.C., Klasing K.C. Glucose and cationic amino acid transporter expression in growing chickens (Gallus gallus domesticus) Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2004;138:515–525. doi: 10.1016/j.cbpb.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Inborr J. Enzymes in animal feeding. Milling. 1990;2:28–34. [Google Scholar]
- Jiang Z., Zhou Y., Lu F., Han Z., Wang T. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian-Australas. J. Anim. Sci. 2008;21:97–102. [Google Scholar]
- Jin S.H., Corless A., Sell J.L. Digestive system development in post-hatch poultry. World’s Poult. Sci. J. 1998;54:335–345. [Google Scholar]
- Kaczmarek S.A., Rogiewicz A., Mogielnicka M., Rutkowski A., Jones R.O., Slominski B.A. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult. Sci. 2014;93:1745–1753. doi: 10.3382/ps.2013-03739. [DOI] [PubMed] [Google Scholar]
- Knudsen K.E.B. Carbohydrate and lignin content of plant materials used in animal feeding. Anim. Feed. Sci. Tech. 1997;67:319–338. [Google Scholar]
- Kocher A., Choct M., Ross G., Broz J., Chung T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003;12:275–283. [Google Scholar]
- Krogdahl A., Sell J.L. Influence of age on lipase, amylase, and protease activities in pancreatic tissue and intestinal contents of young turkeys. Poult. Sci. 1989;68:1561–1568. doi: 10.3382/ps.0681561. [DOI] [PubMed] [Google Scholar]
- Lehrner L.M., Malacinski G.M. Biochemical genetics of α-amylase isoenzymes of the chicken pancreas. Biochem. Genet. 1975;13:145–173. doi: 10.1007/BF00486012. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Meng X., Slominski B.A., Nyachoti C.M., Campbell L.D., Guenter W. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nu-trient utilization and broiler chicken performance. Poult. Sci. 2005;84:37–47. doi: 10.1093/ps/84.1.37. [DOI] [PubMed] [Google Scholar]
- Moran E.T. Digestion and absorption of carbohydrates in fowl and events through perinatal development. J. Nutr. 1985;115:665–674. doi: 10.1093/jn/115.5.665. [DOI] [PubMed] [Google Scholar]
- Nitsan Z., Ben-Avraham G., Zoref Z., Nir I. Growth and development of the digestive organs and some enzymes in broiler chicks after hatching. Br. Poult. Sci. 1991;32:515–523. doi: 10.1080/00071669108417376. [DOI] [PubMed] [Google Scholar]
- Nitsan Z., Dunnington E., Siegel P.B. Organ growth and digestive enzyme levels to fifteen days of age in lines of chickens differing in body weight. Poult. Sci. 1991;70:2040–2048. doi: 10.3382/ps.0702040. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Adeola O. Whole body nutrient accretion, growth performance and total tract nutrient retention responses of broilers to supplementation of xylanase and phytase individually or in combination in wheat-soybean meal-based diets. J. Poult. Sci. 2008;45:192–198. [Google Scholar]
- Onderci M., Sahin N., Sahin K., Cikim G., Aydín A., Ozercan I., Aydín S. Efficacy of supplementation of α-amylase-producing bacterial culture on the performance, nutrient use, and gut morphology of broiler chicken fed a corn-based diet. Poult. Sci. 2006;85:505–510. doi: 10.1093/ps/85.3.505. [DOI] [PubMed] [Google Scholar]
- Park C.S., Helmbrecht A., Htoo J.K., Adeola O. Comparison of amino acid digestibility in full-fat soybean, two soybean meals, and peanut flour between broiler chickens and growing pigs. J. Anim. Sci. 2017;95:3110–3119. doi: 10.2527/jas.2017.1404. [DOI] [PubMed] [Google Scholar]
- Parsons C.M. Proceedings of the 25th West-Ern Nutrition Conference, Saskatoon, Canada. University of Saskatchewan; Saskatoon, Canada: 2004. Gastrointestinal development and nutrient di-gestion in chicks; pp. 169–176. [Google Scholar]
- Ritz C.W., Hulet R.M., Self B.B., Denbow D.M. Growth and intestinal morphology of male turkeys as influenced by dietary supplementation of amylase and xylanase. Poult. Sci. 1995;74:1329–1334. doi: 10.3382/ps.0741329. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Slominski B.A., Guenter W., Campbell L.D. New approach to water-soluble carbohydrate determination as a tool for evaluation of plant cell wall degrading enzymes. J. Agric. Food Chem. 1993;41:2304–2308. [Google Scholar]
- Stefanello C., Vieira S.L., Soster P., Santos B.D., Dalmoro Y.K., Favero A., Cowieson A.J. Utilization of corn-based diets supplemented with an exogenous α-amylase for broilers. Poult. Sci. 2019;98:5862–5869. doi: 10.3382/ps/pez290. [DOI] [PubMed] [Google Scholar]
- Svihus B. Limitations to wheat starch digestion in growing broiler chickens: a brief review. Anim. Prod. Sci. 2011;51:583–589. [Google Scholar]
- Svihus B. Starch digestion capacity of poultry. Poult. Sci. 2014;93:2394–2399. doi: 10.3382/ps.2014-03905. [DOI] [PubMed] [Google Scholar]
- Svihus B., Hetland H. Ileal starch digestibility in growing broiler chickens fed on a wheat-based diet is improved by mash feeding, dilution with cellulose or whole wheat inclusion. Br. Poult. Sci. 2001;42:633–637. doi: 10.1080/00071660120088461. [DOI] [PubMed] [Google Scholar]
- Tan J.Z., Applegate T.J., Liu S., Guo Y., Eicher S.D. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014;112:1098–1109. doi: 10.1017/S0007114514001846. [DOI] [PubMed] [Google Scholar]
- Tester R.F., Karkalas J., Qi X. Starch–Composition, fine structure and architecture. J. Cereal Sci. 2004;39:151–165. [Google Scholar]
- Theander O., Westerlund E., Aman P., Graham H. Plant cell walls and monogastric diets. Anim. Feed Sci. Technol. 1989;23:205–225. [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch changes in morphology and function of the small intestines in heavy-and light-strain chicks. Poult. Sci. 1995;74:1622–1629. doi: 10.3382/ps.0741622. [DOI] [PubMed] [Google Scholar]
- van der Klis J.D., Verstegen M.W.A., van Voorst A. Effect of a soluble polysaccharide (carboxy methyl cellulose) on the absorption of minerals from the gastrointestinal tract of broilers. Br. Poult. Sci. 1993;34:985–997. doi: 10.1080/00071669308417658. [DOI] [PubMed] [Google Scholar]
- Vieira S.L., Stefanello C., Rios H.V., Serafini N.C., Hermes R.G., Sorbara J.O.B. Efficacy and metabolizable energy equivalence of an α-amylase-β-glucanase complex for broilers. Rev. Bras. Cienc. Avic. 2015;17:227–235. [Google Scholar]
- Weurding R.E., Veldman A., Veen W.A.G., van der Aar P.J., Verstegen M.W.A. Starch digestion rate in the small intestine of broiler chickens differs among feedstuffs. J. Nutr. 2001;131:2329–2335. doi: 10.1093/jn/131.9.2329. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Bogota K.J., Noll S.L., Wilson J. Enhancing nutrient utilization of broiler chickens through supplemental enzymes. Poult. Sci. 2019;98:1302–1309. doi: 10.3382/ps/pey452. [DOI] [PubMed] [Google Scholar]
- Yuan J.J., Yang Y.F., Xiaodan Y., Wan X., Han J., Wang Y. Effects of supplementing different levels of a commercial enzyme complex on performance, nutrient availability, enzyme activity and gut morphology of broilers. Asian-Australas. J. Anim. Sci. 2008;21:692–700. [Google Scholar]
- Zanella I., Sakomura N.K., Silversides F.G., Fiqueirdo A., Pack M. Effect of enzyme supplementation of broiler diets based on corn and soybeans. Poult. Sci. 1999;78:561–568. doi: 10.1093/ps/78.4.561. [DOI] [PubMed] [Google Scholar]
- Zhang G., Ao Z., Hamaker B.R. Slow digestion property of native cereal starches. Biomacromolecules. 2006;7:3252–3258. doi: 10.1021/bm060342i. [DOI] [PubMed] [Google Scholar]
- Zhu H.L., Hu L.L., Hou Y.Q., Zhang J., Ding B.Y. The effects of enzyme supplementation on performance and digestive parameters of broilers fed corn-soybean diets. Poult. Sci. 2014;93:1704–1712. doi: 10.3382/ps.2013-03626. [DOI] [PubMed] [Google Scholar]