Abstract
The present study aimed to research the effects of cyclic heat environment on the microbial diversity and structure of respiratory tract and cecum of chicken. A total of 360 layer-type pullets at 11 wk of age were subjected to different temperature treatments for 10 wk: constant 22°C; cyclic temperature 22°C to 24°C, 22°C to 26°C, 22°C to 28°C, 22°C to 30°C; the ambient temperature increased from 10:00, reached the set point within 1 h, and maintained until 18:00, thereafter the temperature was restored to 22°C; and the relative humidity was maintained at 60%. The result showed that feed intake of the chickens on ambient temperature 30°C group was significantly lower than that of the chickens on ambient temperature 24°C. The white blood cell, red blood cell, lymphocyte, hemoglobin, and pecked-cell volume content were highest at 24°C on 14, 16, and 18 wk. The ratio of CD3+CD4+/CD3+CD8+ T cells was lowest at 30°C. Meanwhile, the abundance of cecum bacteria in chickens at 30°C was lower than that at 24°C. Cyclic heat environment temperature treatment did not significantly affect the concentration of secretory immunoglobulin A in chicken bronchoalveolar lavage fluid (BALF) levels during 10 wk of trial. The diversity index analysis showed that the effect of 24°C on the cecum flora of chickens was optimal. Abundance of Firmicutes bacteria in the lung flora and cecum flora was lower at 30°C than at 24°C group. Similarly, the microorganism, Brevibacillus in the BALF was also significantly lower at 24°C. In conclusion, cyclic 24°C treatment was beneficial for the feed intake, blood routine indexes, microflora structure of the cecum, and respiratory tract in laying pullets.
Key words: mild heat stress, cecum microbiota, respiratory microbiota, layer-type pullet
Introduction
It is well known that environmental temperature influences the growth and health of animals (Wang et al., 2018). Although the technology for temperature and humidity control in chicken houses is developing constantly, the temperature ranges of 26°C to 32°C still occurs frequently in intensive chicken housing. The environmental temperature of 32°C or higher has been researched thoroughly (Sohail et al., 2015; Hartanto et al., 2019). However, the impact of environmental temperatures between 26°C and 31°C has not been sufficiently studied. A previous study has reported that at temperature ranges between 26°C and 32°C in chicken pens, the chickens are not very comfortable but can maintain basic physiological functions (May et al., 1998). Many studies have been conducted to elucidate how heat stress affects the immune response in chickens (Niu et al., 2009; Effati et al., 2014; Monson et al., 2018). Heat stress can suppress broiler chickens' immunity by elevating heterophils while reducing lymphocytes (Cheng et al., 2001). In addition, it has been reported that broilers subjected to heat stress had lower levels of secretory immunoglobulin A (SIgA), IgM, and IgG (IgY) in the small intestine (Song et al., 2018). Modulation of the immune response by the central nervous system is mediated by a complex network that operates bidirectionally between the nervous, endocrine, and immune systems (Lara and Rostagno, 2013). Under heat stress conditions, feed conversion ratios were lower, and the rate of skeletal muscle protein turnover was significantly increased (Yunianto et al., 1997). Continuous partial heat treatment (26°C, 31°C) affects glycolipid metabolism and avian uncoupling protein mRNA expression in broilers, and different degrees of heat have discrepant effects (Lei et al., 2009). Meanwhile, heat stress could be responsible for intestinal oxidative stress, respiratory alkalosis, and thus overproduction of free radicals in the body (Ramnath et al., 2008). The investigation on heat stress has been reported frequently.
Microbiotas colonizing in respiratory and digestive tracts are involved in the development of tissue and against pathogens (Sohail et al., 2015). Digestive tract microbiota plays a key role in the absorption of nutrients and enteric development (Sohail et al., 2015). Environmental temperature is one of the factors influencing gut microbiota in poultry. As the environment temperature changes, gastrointestinal tract flora was affected (Sohail et al., 2015). The gastrointestinal tract could be considered the body's largest immune organ. Changes in the composition and function of digestive tract flora affect the respiratory tract through mucosal immune system (Gray et al., 2017). Similarly, disturbed respiratory flora affects immune system of digestive tracts by immunoregulation, and this interaction between the gut and lungs is called gut-lung axis (Tamburini and Clemente, 2017). However, the effect of cyclic heat treatment on the interaction between lung microbiota and cecum microbiota has not been reported.
In this study, the effects of cyclic heat environment on the microbial diversity and immune function of lungs, cecum, and blood biochemical indexes of laying pullets were studied. Finally, this study was performed with a design aimed at identifying bacterial taxa associated with pullet performance and immune function in a cyclic heat exposure setting.
Materials and methods
Animal Ethics
The study was approved by the Ethics Committee of the Shandong Agricultural University and conducted in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China).
Experimental Animals
A total of 360 eleven-week-old healthy Isa brown layer-type pullets (Aote Layer Breeding Ltd., Qingdao, China) were randomly divided into 5 groups and reared in separate environmental chambers. Each group had 6 replicates of 12 birds. A set of temperatures with a maximum temperature of 30°C were set to mimic the common ambient temperature in a layer house (Ross and Herrick, 1983; Ugurlu and Karo, 2003). All 5 groups of experimental layer pullets were randomly subjected to one of the following treatments: rearing at constant 22°C temperature (T22C), cyclic 22°C to 24°C (T24C), cyclic 22°C to 26°C (T26C), cyclic 22°C to 28°C (T28C), and cyclic 22°C to 30°C (T30C). The temperature rose from 10:00 am and reached the set point with 1 h and maintained the set point for 8 h until 18:00 pm. The layer pullets were fed a standard commercial layer pullet diet providing 2,700 kcal of ME/kg and 16.50% crude protein (Changhao 635, 655; Shandong Zhongcheng Feed Technology Co., Ltd., Tai'an, China; Table 1). The pullets were kept under a lighting regime of 16 h of light (from 6:30 am to 22:30 pm) and 8 h of dark. The relative humidity was controlled at 60%. During the 10-wk experimental period, all the experimental pullets had free access to feed and water. Food intake was recorded weekly.
Table 1.
Composition and nutrient contents.
| Ingredients | 10–12 wk of age | 13–18 wk of age | 19–20 wk of age |
|---|---|---|---|
| Corn (7.5% crude protein) | 69 | 69 | 65 |
| Soybean meal (44% crude protein) | 26 | 25 | 25 |
| Limestone | 0 | 1 | 4 |
| Soy oil | 0 | 0 | 1 |
| Premix1 | 5 | 5 | 5 |
| Calculated nutrient values | |||
| Metabolizable energy, Mcal/kg | 2.910 | 2.887 | 2.841 |
| Crude protein, % | 17.18 | 16.74 | 16.29 |
| Lysine, % | 0.685 | 0.685 | 0.95 |
| Methionine, % | 0.280 | 0.280 | 0.41 |
| Calcium, % | 1.00 | 1.00 | 3.50 |
| Effective phosphorus, % | 0.40 | 0.40 | 0.41 |
The premix provides the follow quantities per kilogram of diet: salt, 32%; choline chloride (50%), 18%; vitamin A, 8,800 IU; vitamin D3, 3,300 IU; vitamin K, 2.2 mg; vitamin E, 16.5 IU; cholecalciferol, 2,800 IU; riboflavin, 18.0 mg; niacin, 50 mg; pantothenic acid, 28 mg; biotin, 0.1 mg; folic acid, 0.6 mg; iron (FeSo4 H2o), 55 mg; selenium (NaSeO3), 0.3 mg; copper (CuSo45H20), 5.5 mg; zinc (ZnO), 88 mg; I (KI), 1.7 mg; manganese (MnO), 88 mg.
At 14, 16, and 18 wk of age, 2 chickens from each replicate and 12 chickens in every treatment were randomly selected. After overnight feed withdrawal, a blood sample was collected from a brachial vein using heparinized syringes (367861, Becton; Dickinson and Company, New York City, NY). The whole blood samples were then used for the measurement of blood cell profiles and immune cell populations. At 20 wk of age, 2 chickens from each replicate and 12 chickens in every treatment were randomly selected for blood sampling. The blood was centrifuged at 500 × g for 10 min, and serum was obtained and stored at −20°C for further analysis. Immediately after the blood sample was obtained, the pullets were sacrificed by exsanguination after cervical dislocation. The trachea, duodenum, jejunum, and ileum were harvested for the collection of lavage fluid, and the cecum was sampled for the collection of cecum content. All the samples were snap-frozen in liquid nitrogen and stored at −80°C before further analysis.
The Preparation of the Bronchoalveolar Lavage Fluid (BALF) and Lavage Fluids from Duodenum, Jejunum, and Ileum
The BALF was collected according to the description by Tanaka et al. (2018) with modifications. The sterile tube, which measures between 2.5 cm and 3.0 cm in length, was inserted into the trachea at a distance of 3 cm from the bronchia of chickens, 5 mL of sterile phosphate buffer solution (PBS; P1020; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) was pushed, and the lungs were flushed twice. The pooled bronchoalveolar lavage fluid was pooled and centrifuged at 3,000 × g for 10 min under 4°C to obtain the BALF samples without cells.
About 5 cm of gut segment was respectively obtained at the middle part of duodenum, jejunum, and ileum after one side of the gut was ligated (Fan et al., 2015). The enteric cavity was washed with 5 mL of sterile PBS, and the wash solution was obtained and centrifuged (5702R; Eppendorf China Ltd., Jinan, China) at 3,000 × g for 10 min under 4°C. The supernatant lavage solution was obtained and snap-frozen in liquid nitrogen and used for the measurement of SIgA.
Blood Cell Profiles and T-Cell Populations Analysis
The whole blood samples were used to the measurement of white blood cell (WBC), red blood cell (RBC), lymphocyte, hemoglobin (Hb), and pecked-cell volume (PCV) by using a hematology analyzer (Sysmex Corporation, Japan). For the measurement of CD3+CD4+ and CD3+CD8+ T-cell populations, 3 mL of whole blood was mixed with 1 mL of PBS to obtain homogeneous liquid for isolating T cells. The mixture was carefully added to 4 mL of lymphocyte separation solution (LTS1077; Tianjin Hao Yang Biological Manufacture Co., Ltd., Tianjin, China), then centrifuged at 300 × g for 20 min at 4°C. Two milliliters of milky white misty lymphocytes was pipetted (Eppendorf China Ltd., Jinan, China) into 2 new Eppendorf tubes, and 1 mL of PBS was added and centrifuged at 10°C with 400 × g for 15 min. The obtained lymphocytes were washed 3 times with 500 μL of PBS washing. Thereafter, 20 μL of anti-Chicken CD3-FITC (8200-02; Southern Biotechnology Associates, Inc., Birmingham, AL), anti-Chicken CD4-FITC (8210-02; Southern Biotechnology Associates, Inc.), and anti-Chicken CD8α-FITC (8220-02; Southern Biotechnology Associates, Inc.) antibody were added to 50 μL of suspension and incubated at 37°C for 20 min, then 800 μL of PBS was added before centrifugation at 4°C with 200 × g for 10 min. Seven hundred seventy microliters of supernatant was discarded, and cells were resuspended with the remaining supernatant. Finally, the CD3+CD4+ and CD3+CD8+ T-cell populations were analyzed using a FACSCalibur flow cytometer (Becton; Dickinson and company, Birmingham, AL).
SIgA Measurement
The SIgA content was measured by using the commercial ELISA kit (H108-2; Jiancheng Biology Engineering Institute, Nanjing, China).
Lung and Cecum Microbiota
The 16S rRNA genes of distinct regions (16SV3-V4) were amplified using a specific primer (338F, 5′-ACTCCTACGGGAGGCAGCAG-3'; 806R, 5′-GGACTACHVGGGTWTCTAAT-3′) with the barcode. All PCR reactions were carried out with Phusion High-Fidelity PCR Master Mix (M0541L; New England Biolabs, Suzhou, China). Same volume of 1× loading buffer (containing SYB green) with PCR products was mixed and operated by 2% agarose gel for detection. Samples with a bright main strip between 400 and 450 bp were chosen for further sequence libraries. Mixture was purified with a Qiagen Gel Extraction Kit (208706; Qiagen, Dusseldorf, Germany). Sequencing samples were generated by a TruSeq DNA PCR-Free Sample Preparation Kit (FC-121-3001; Illumina, San Diego, CA) according to the manufacturer's recommendations. The library quality was assessed on the Qubit 2.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA) and Agilent Bioanalyzer 2100 system (Agilent Technologies, Palo Alto, CA). At last, the library was sequenced on an Illumina HiSeq 2500 platform, and 250-bp paired-end reads were generated. Alpha diversity is applied in analyzing the complexity of species diversity per sample. Beta diversity analysis was used to evaluate differences between samples in species complexity. The average number of observed operational taxonomic units (OTUs) at 97% identity, the portion of the diversity covered by our subsampling, the diversity of the OTUs found in the samples, and their evenness were measured. All these indices in our samples were calculated with QIIME (version 1.7.0, New York, NY) and displayed by using R software (version 2.15.3). Cluster analysis was preceded by a principal component analysis, which was applied to reduce the dimension of the original variables using the FactoMineR package and ggplot2 package in R software (version 2.15.3). Principal coordinate analysis was performed to get principal coordinates and to visualize from complex, multidimensional data. A distance matrix of weighted or unweighted unifrac among samples obtained before was transformed to a new set of orthogonal axes, by which the maximum variation factor is demonstrated by the first principal coordinate, and the second maximum one by the second principal coordinate, and so on. Principal coordinate analysis was displayed by the WGCNA package, stat packages, and ggplot2 package in R software (version 2.15.3).
Statistical Analysis
Before analysis, all data were examined for the homogeneity and normal distribution plots of variances among the treatments by using the UNIVARIATE procedure. For the variables feed intake and blood cell profiles, a 2-way ANOVA model (version 8e; SAS Institute, Cary, NC) was used to estimate the main effect of temperature and time as well as their interaction. For the variables T-cell population and SIgA, the main effect of temperature treatment was estimated with a one-way ANOVA model. As lack of normality, the PCV and T-cell data were first subjected to square root arcsine transformation before analysis. When the main effect of temperature treatment was significant, the differences between means were assessed by Duncan's multiple range analysis. P < 0.05 was considered significantly different.
Microbiota data split paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Sequence assembly paired-end reads were merged using Fast Length Adjustment of Short reads (FLASH) (Magoč and Salzberg, 2011), a very fast and accurate analysis tool, which was designed to merge paired-end reads when at least some of the reads overlap the read generated from the opposite end of the same DNA fragment, and the splicing sequences were called raw tags. Data filtration quality filtering on the raw tags was performed under specific filtering conditions to obtain the high-quality clean tags (Bokulich et al., 2013) according to the QIIME quality-controlled process. Chimera removal of the tags was compared with the reference database using the UCHIME algorithm (Edgar et al., 2011) to detect chimera sequences, and then the chimera sequences were removed (Haas et al., 2011). OTU production sequences analysis was performed by using the UPARSE software (UPARSE, version 7.0.1001; Edgar, 2013). Sequences with ≥97% similarity were assigned to the same OTUs. Representative sequence for each OTU was screened for further annotation. The SILVA Database (DeSantis et al., 2006) was used based on RDP classifier (version 2.2) algorithm to annotate taxonomic information for each representative sequence. In order to study phylogenetic relationship of different OTUs, and the difference between the dominant species in different groups, a multiple-sequence alignment was conducted using the MUSCLE software (Edgar, 2004). Data normalization OTU abundance information was normalized using a standard of sequence number corresponding to the sample with the least sequences. Subsequent analyses of alpha diversity and beta diversity were all performed based on this output normalized data.
Results
Effects of Cyclic Temperature Treatment on Feed Intake and Immune Function
Cyclic temperature treatment had a significant influence (P < 0.05) on average daily feed intake from weeks 11 to 20 (Table 2). Feed intake of birds that suffered from cyclic high temperatures (the T26C, T28C, and T30C groups) was lower than that of the T24C group at 16, 17, 18, 19, and 20 wk of age (P < 0.05, Table 2).
Table 2.
Effect of cyclic temperature treatment on feed intake of layer-type pullets.
| Weeks | T22C | T24C | T26C | T28C | T30C | P value |
|---|---|---|---|---|---|---|
| Week 1 | 78.2 ± 1.2 | 76.9 ± 0.8 | 74.2 ± 2.7 | 77.1 ± 2.3 | 74.7 ± 1.6 | 0.509 |
| Week 2 | 86.5 ± 1.6 | 80.2 ± 0.9 | 81.7 ± 2.0 | 81.9 ± 2.1 | 82.7 ± 1.0 | 0.108 |
| Week 3 | 83.0 ± 1.9 | 80.8 ± 3.2 | 79.9 ± 3.0 | 75.6 ± 2.2 | 79.3 ± 0.7 | 0.281 |
| Week 4 | 90.0 ± 2.5a | 88.1 ± 1.2a | 80.7 ± 1.2b | 81.0 ± 1.5b | 85.9 ± 1.0a | 0.000 |
| Week 5 | 87.5 ± 2.1 | 84.8 ± 1.5 | 80.0 ± 1.4 | 79.4 ± 3.5 | 83.4 ± 1.7 | 0.079 |
| Week 6 | 85.7 ± 1.7a | 82.3 ± 3.0a | 82.0 ± 1.5a | 71.6 ± 2.9b | 80.9 ± 1.2a | 0.002 |
| Week 7 | 74.0 ± 4.5 | 94.3 ± 4.1 | 83.0 ± 5.8 | 80.3 ± 8.8 | 74.5 ± 4.5 | 0.130 |
| Week 8 | 84.5 ± 2.3a,b | 89.5 ± 2.0a | 78.5 ± 3.6b | 88.2 ± 5.0a | 76.7 ± 1.7b | 0.028 |
| Average | 83.7 ± 1.2a | 84.6 ± 0.6a | 76.9 ± 1.3b | 76.9 ± 1.3b | 79.6 ± 1.0b | 0.000 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SE (n = 6).
Effects of Cyclic Temperature Treatment on Blood Cell Profiles and T-Cell Populations
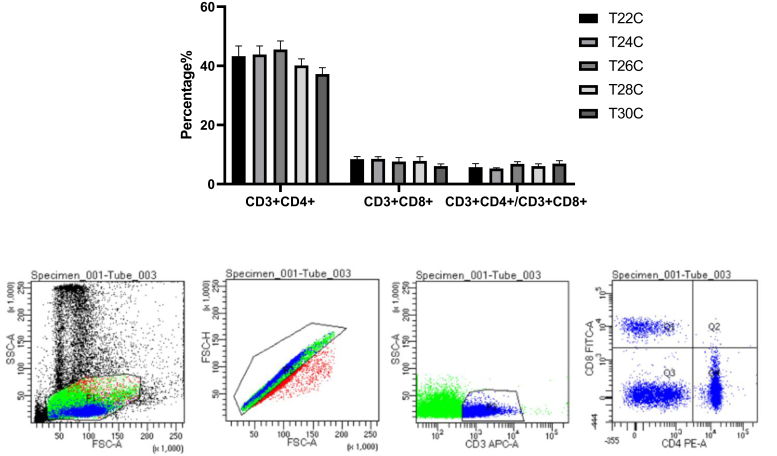
The WBC level of the T24C group was the highest at 16 wk. The WBC level was decreased in the T22C, T26C, T28C, and T30C groups compared with that in the T24C group, but only the T26C group showed statistical significance (P < 0.05, Table 3). The lymphocyte level was significantly downregulated in comparison to the T24C group on 14, 16, and 18 wk (P < 0.05, Table 3). Meanwhile, the RBC, Hb, and PCV levels were also increased by cyclic temperature 22°C to 24°C treatment in layer-type pullets (P < 0.05, Table 3). The contents of blood CD3+CD4+ and CD3+CD8+ T cells were examined using flow cytometry, and then the ratio of CD3+CD4+/CD3+CD8+ was calculated. The CD3+CD4+ and CD3+CD8+ percentages in blood of the T30C group were the lowest, but the populations of CD3+CD4+ and CD3+CD8+ in blood were not significantly different (P > 0.05, Figure 1). And the ratio of CD3+CD4+/CD3+CD8+ was higher in the T30C group than that in the T24C group at 20 wk (P > 0.05, Figure 1).
Table 3.
Effect of cyclic temperature treatment on blood cell profiles at 14 wk.
| Item | T22C | T24C | T26C | T28C | T30C | P value |
|---|---|---|---|---|---|---|
| Week 4 | ||||||
| WBC, 109/L | 180 ± 3 | 195 ± 11 | 180 ± 3 | 183 ± 4 | 178 ± 8 | 0.420 |
| Lymphocyte, 109/L | 146 ± 2 | 170 ± 4 | 148 ± 2 | 147 ± 3 | 146 ± 7 | 0.120 |
| RBC, 1012/L | 2.63 ± 0.05b | 3.06 ± 0.13a | 2.66 ± 0.06b | 2.58 ± 0.06b | 2.42 ± 0.18b | 0.009 |
| Hb, g/L | 142 ± 3b | 166 ± 7a | 144 ± 3b | 146 ± 3b | 143 ± 9b | 0.043 |
| PCV, % | 30.2 ± 1.0b | 35.9 ± 1.6a | 31.1 ± 0.6b | 30.3 ± 0.6b | 28.4 ± 2.4b | 0.020 |
| Week 6 | ||||||
| WBC, 109/L | 190 ± 3 | 201 ± 9 | 184 ± 3 | 187 ± 6 | 193 ± 4 | 0.102 |
| Lymphocyte, 109/L | 151 ± 3 | 161 ± 2 | 149 ± 2 | 152 ± 5 | 155 ± 4 | 0.153 |
| RBC, 1012/L | 2.87 ± 0.10 | 3.15 ± 0.08 | 2.82 ± 0.07 | 3.18 ± 0.17 | 2.87 ± 0.11 | 0.074 |
| Hb, g/L | 148 ± 6 | 163 ± 5 | 145 ± 4 | 160 ± 8 | 154 ± 6 | 0.208 |
| PCV, % | 33.0 ± 1.1 | 36.1 ± 1.6 | 31.9 ± 0.8 | 35.5 ± 1.7 | 33.4 ± 1.1 | 0.091 |
| Week 8 | ||||||
| WBC, 109/L | 182 ± 5 | 203 ± 4 | 200 ± 12 | 199 ± 4 | 194 ± 1 | 0.605 |
| Lymphocyte, 109/L | 145 ± 4b | 161 ± 4a | 154 ± 3a,b | 157 ± 3a,b | 148 ± 5b | 0.034 |
| RBC, 1012/L | 3.12 ± 0.17a | 3.12 ± 0.18a | 2.76 ± 0.10a,b | 3.08 ± 0.10a | 2.78 ± 0.13a,b | 0.022 |
| Hb, g/L | 147 ± 3 | 166 ± 5 | 150 ± 6 | 163 ± 5 | 150 ± 7 | 0.064 |
| PCV, % | 31.2 ± 2.4b | 36.8 ± 1.2a | 33.0 ± 1.2a,b | 36.7 ± 1.1a | 33.0 ± 1.5a,b | 0.034 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SD (n = 12).
Abbreviations: Hb, hemoglobin; PCV, pecked-cell volume; RBC, red blood cell; WBC, white blood cell.
Figure 1.
Effect of cyclic temperature treatment on T-cell populations of CD3+CD4+ and CD3+CD8+. Data are presented as mean ± SD (n = 12).
Effects of Cyclic Temperature Treatment on SIgA Level
The effect of cyclic 22°C to 30°C was observed after 10 wk of treatment in the SIgA of BALF, and the SIgA content in BALF of the T28C group remained at a lower level than that of the T22C, T24C, T26C, and T30C groups (P < 0.05, Table 4). In contrast, the SIgA contents in lavage fluids from duodenum, jejunum, and ileum had no significant difference between different cyclic temperature groups (P > 0.05; Table 4).
Table 4.
Effect of cyclic temperature treatment on the SIgA concentration (mg/mL).
| Item | T22C | T24C | T26C | T28C | T30C | P value |
|---|---|---|---|---|---|---|
| BALF | 1,173 ± 58a,b | 1,168 ± 35a,b | 1,251 ± 39a | 1,062 ± 24b | 1,159 ± 54a,b | 0.025 |
| DLF | 1,334 ± 204 | 1,251 ± 238 | 1,728 ± 143 | 1,681 ± 211 | 1,494 ± 273 | 0.117 |
| JLF | 957 ± 137 | 1,045 ± 102 | 1,082 ± 128 | 1,085 ± 44 | 1,301 ± 142 | 0.454 |
| ILF | 1,669 ± 167 | 1,732 ± 159 | 1,843 ± 91 | 1,922 ± 70 | 1,635 ± 141 | 0.214 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SD (n = 12).
Abbreviations: BALF, bronchoalveolar lavage fluid; DLF, lavage fluids from duodenum; ILF, lavage fluids from ileum; JLF, lavage fluids from jejunum; SIgA, secretory immunoglobulin A.
Effects of Cyclic Temperature Treatment on the Microbiota Diversity in Respiratory Tract
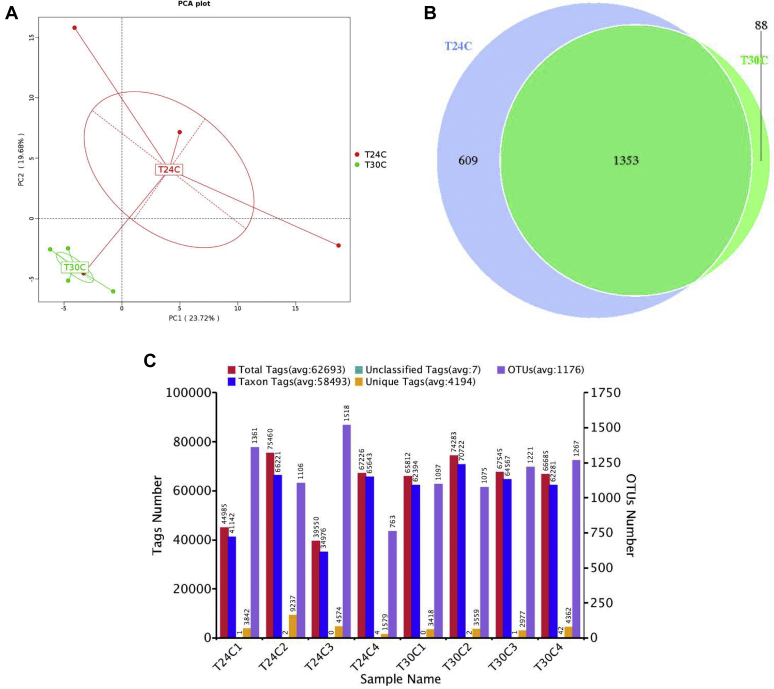
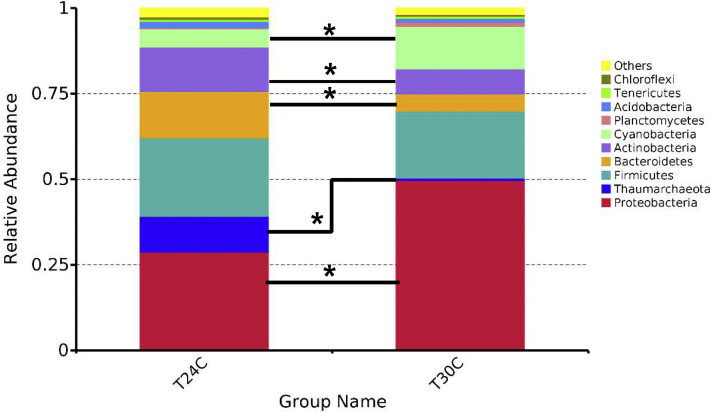
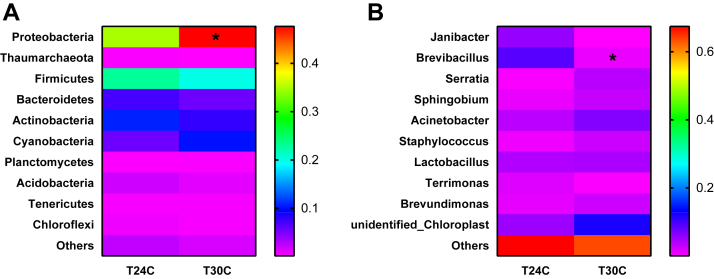
According to the aforementioned results, the microbiota diversity in T24C and T30C groups was further determined. The microbiota in respiratory tract was analyzed by sequencing the bacterial 16S rRNA V3+V4 region. On the base of principal component analysis of BALF bacterial community of T24C samples and T30C samples, the microbial composition of 2 groups were apparently clustered separately (Figure 2A). High-throughput pyrosequencing of the samples produced 62,693 total tags (Figure 2C). Clean tags were subjected to the following analysis after weeding out the lower quality reads. Based on 97% similarity level, all the effective sequences were clustered into OTUs. To better understand the differences in richness between the T30C and T24C groups, the overlap OUTs between the groups were illustrated using a Venn diagram (Figure 2B). This result showed that the BALF microbial population of chickens in the T30C and T24C groups shared 1,353 OTUs. Numbers of individual OUTs of microbiota in 24°C and 30°C were 609 and 88, respectively (Figure 2B). The most common bacterial OTUs found on average in BALF bacterial phyla were Proteobacteria (28.7%), Firmicutes (22.9%), Bacteroidetes (13.7%), and Actinobacteria (12.9%) in the T24C group, accounting for more than 78% of the total bacterial community (Figure 3). However, in the T30C group, the most common bacterial OTUs found in lung bacterial phyla were Proteobacteria (49.8%), Firmicutes (19.6%), Cyanobacteria (12.4%), and Actinobacteria (7.3%), accounting for 89% of the total bacterial community (Figure 3). Specifically, the T30C group microbiota showed a relative increase in bacteria belonging to the Proteobacteria phyla and Gamma-Proteobacteria class, meanwhile there was lower abundance of bacteria from the Paenibacillaceae family and Brevibacillus genus (Figures 4A, 4B). Upon analyzing the total reads, we found that Proteobacteria in the T30C group was significantly higher than those in the T24C group (P < 0.05) on phylum level (Figure 4A), and Brevibacillus in the T24C group was significantly higher than those in the T30C group (P = 0.01) on genus level (Figure 4B).
Figure 2.
Effect of cyclic temperature treatment on the microbiota diversity in respiratory tract. (A) The lung microbiota analyzed using principal component analysis (PCA); (B) Venn diagram of the operational taxonomic units (OTUs) in different treatments; (C) statistics of OTUs clustering and annotation of each sample; data are presented as mean ± SD (n = 4).
Figure 3.
Distribution of lung bacteria at phylum level. Data are presented as mean ± SD (n = 4). ∗P < 0.05.
Figure 4.
Effect of cyclic temperature treatment on the bacterial community structure in respiratory tract. (A) Distribution of lung bacteria at phylum level; (B) distribution of lung bacteria at genus level. Data are presented as mean ± SD (n = 4). ∗P < 0.05.
Effects of Cyclic Temperature Treatment on Microbiota Diversity in Cecum
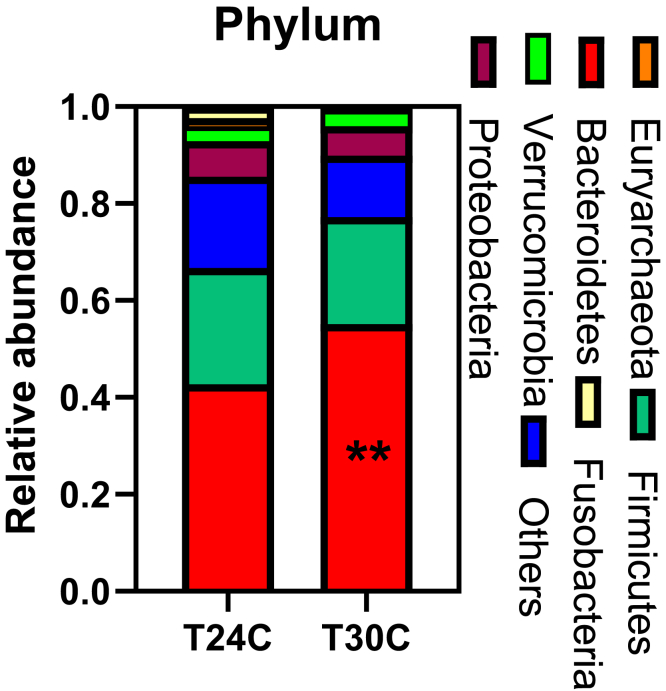
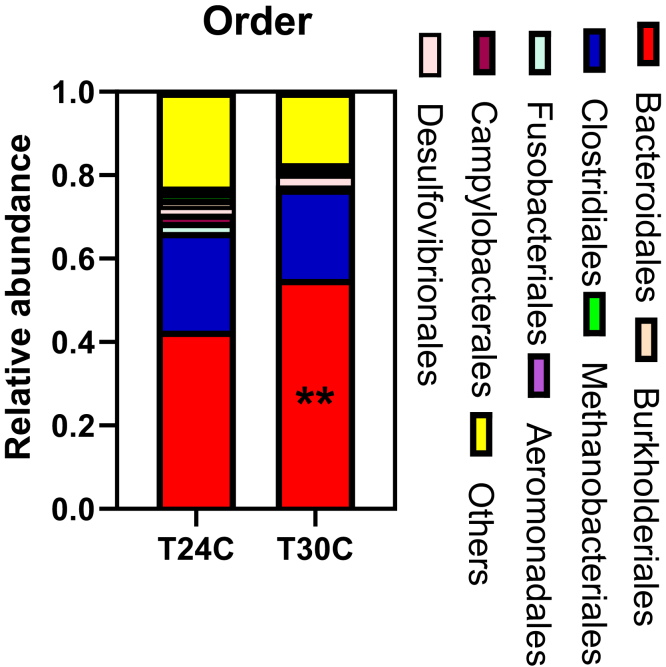
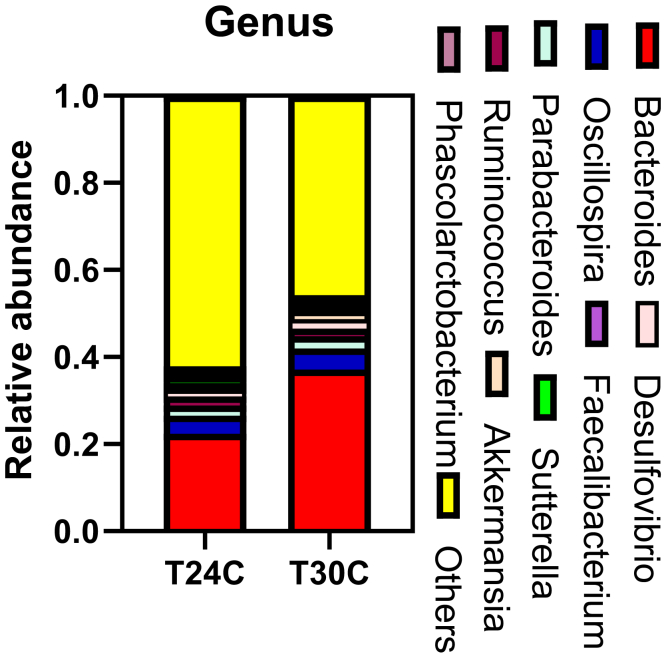
The cecum microbiota was analyzed by sequencing the bacterial 16S rRNA V3+V4 region. High-throughput pyrosequencing of samples produced the raw reads. After removing low-quality sequences, 355,477 clean tags were subjected to the following analysis. Based on 97% similarity level, all the effective reads were clustered into OTUs. Bacteroidetes, Firmicutes, and Proteobacteria are dominant phyla in the cecum community of ISA brown bred chicken at 20 wk of age, accounting for about 75% of the total cecum bacterial community (Figure 5). At the same time, the main composition of Bacteroidia, at the phylum and order levels, and the abundance of Bacteroidetes and Bacteroidales were increased in the T30C group (P < 0.05; Tables 5 and 6). The samples of the T24C group had a higher relative abundance of Campylobacterales (P < 0.05; Table 6, Figure 6). Euryarchaeota in cecum were mainly composed of Methanobacteriales; Methanobacteriales in the T24C group were higher than those in the T30C group (P = 0.0653; Table 6). At the genus level, the relative abundance of Faecalibacterium was decreased in the T30C group (P = 0.0752, Table 7; Figure 7), meanwhile the relative percentages of Desulfovibrio and Akkermansia increased in the T30C group (0.05 < P < 0.1; Table 7). The relative percentage of Faecalibacterium, Desulfovibrio, and Akkermansia are subject to that of Proteobacteria.
Figure 5.
Effect of cyclic temperature treatment on the cecum bacterial community structure at the phylum level. Data are presented as mean ± SD (n = 5). ∗∗P < 0.01.
Table 5.
Effect of cyclic temperature treatment on the cecum microbiota compositions at the phylum level.
| Taxonomy | T24C | T30C | P value |
|---|---|---|---|
| Bacteroidetes | 0.42 ± 0.02b | 0.55 ± 0.02a | 0.002 |
| Firmicutes | 0.25 ± 0.04 | 0.22 ± 0.01 | 0.437 |
| Proteobacteria | 0.07 ± 0.01 | 0.06 ± 0.008 | 0.616 |
| Verrucomicrobia | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.776 |
| Euryarchaeota | 0.01 ± 0.003 | 0.001 ± 0.0002 | 0.057 |
| Fusobacteria | 0.03 ± 0.01 | 0.0004 ± 0.0001 | 0.112 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SD (n = 5).
Table 6.
Effect of cyclic temperature treatment on the cecum microbiota compositions at the order level.
| Taxonomy | T24C | T30C | P value |
|---|---|---|---|
| Bacteroidales | 0.42 ± 0.02b | 0.55 ± 0.02a | 0.002 |
| Clostridiales | 0.25 ± 0.04 | 0.22 ± 0.01 | 0.423 |
| Fusobacteriales | 0.03 ± 0.006 | 0.0004 ± 0.0002 | 0.112 |
| Campylobacterales | 0.02 ± 0.004a | 0.007 ± 0.001b | 0.029 |
| Desulfovibrionales | 0.02 ± 0.002 | 0.03 ± 0.003 | 0.102 |
| Burkholderiales | 0.02 ± 0.003 | 0.013 ± 0.001 | 0.489 |
| Methanobacteriales | 0.01 ± 0.003 | 0.006 ± 0.002 | 0.065 |
| Aeromonadales | 0.01 ± 0.005 | 0.009 ± 0.001 | 0.688 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SD (n = 5).
Figure 6.
Effect of cyclic temperature treatment on the cecum bacterial community structure at the order level. Data are presented as mean ± SD (n = 5). ∗∗P < 0.01.
Table 7.
Effect of cyclic temperature treatment on the cecum microbiota compositions at the genus level.
| Taxonomy | T24C | T30C | P value |
|---|---|---|---|
| Methanobrevibacter | 0.01 ± 0.005 | 0.009 ± 0.002 | 0.688 |
| Bacteroides | 0.01 ± 0.004 | 0.008 ± 0.002 | 0.688 |
| Parabacteroides | 0.02 ± 0.002 | 0.03 ± 0.002 | 0.202 |
| Ruminococcus | 0.02 ± 0.004 | 0.02 ± 0.004 | 0.677 |
| Faecalibacterium | 0.01 ± 0.002 | 0.007 ± 0.001 | 0.075 |
| Oscillospira | 0.04 ± 0.008 | 0.05 ± 0.001 | 0.500 |
| Phascolarctobacterium | 0.01 ± 0.001 | 0.0061 ± 0.002 | 0.543 |
| Fusobacterium | 0.03 ± 0.01 | 0.0004 ± 0.0001 | 0.112 |
| Sutterella | 0.02 ± 0.003 | 0.01 ± 0.001 | 0.473 |
| Desulfovibrio | 0.016 ± 0.002 | 0.02 ± 0.002 | 0.079 |
| Akkermansia | 0.0002 ± 0.00002 | 0.03 ± 0.008 | 0.070 |
Means with different superscript letters within the same line differ significantly, P < 0.05; data are presented as mean ± SD (n = 5).
Figure 7.
Effect of cyclic temperature treatment on the cecum bacterial community structure at the genus level. Data are presented as mean ± SD (n = 5).
Discussion
The present study was conducted to evaluate the effect of cyclic heat exposure on the immunity and the microbiota diversity in the respiratory tract and the hinder gut of layer-type pullets. This was performed in a manner to better define the layer-type pullets microbiome and to define what temperature has the greatest impact on the pullets. The result suggests that layer-type pullets had better adaptation ability to cyclic heat exposure of 22°C to 24°C than other groups.
Mild Heat Stress Has No Disadvantage Influence on the Blood Cell Profiles
Results of a study with heat stress indicated that WBC and antibody production were significantly inhibited (Mashaly et al., 2004). However, Price et al. (2018) found that heat stress did not significantly change total WBC count. In this study, feed intake, blood cell profiles, and SIgA content are presented. Daily cyclic high temperature (T26C, T28C, and T30C groups) showed significant effects on feed intake of pullets from 16 to 20 wk of age. We found that the WBC, lymphocyte, RBC, Hb, and PCV levels were highest in the T24C group, compared with the T22C, T26C, T28C, and T30C groups of birds. These results could indicate that intermittent and mild heat treatment can affect the blood routine examination index slightly and damage health status of the body. Organisms maintain immune functions by mediating between CD4+ T cells and CD8+ T cells. CD4+ T cells, which can recognize antigens presented by major histocompatibility complex Ⅱ, participate in an assistant role and in delayed-type hypersensitivity. CD8+ T cells recognize antigen peptides presented by major histocompatibility complex I and can directly kill specific target cells, thus playing roles in cell toxicity and immune suppression (Liu et al., 2012). To further define the relationship between lymphocyte subsets and ambient temperature, multiparameter flow cytometry was used to determine the distribution of the CD3+, CD4+, and CD8+ T cells on pullet blood. Here, there are no significantly differences on pullet blood in 20 wk. SIgA is the predominant IgA on mucosal surface, which resists pathogens by combining with pathogen antigen to block adhesion of bacteria and virus on the intestinal mucosa (Li et al., 2017, 2018). Ten weeks of cyclic heat treatment resulted in a slightly decreased SIgA content of lavage fluids from ileum. This reduction could be an indirect effect because of an increase in inflammatory cytokines.
Mild Heat Stress Changes the Cecum Microbiota Diversity
The gut microbiome is involved in regulating host metabolism via establishing microbial interactions with the host, including the modulation of nutritional balance, the maturation and activation of immune system, and mediation of brain activity (Nicholson et al., 2012). Exposure to a high temperature influences the bacterial composition and community structure of the ileal microbiota of broilers, specifically by decreasing the species richness (Wang et al., 2018). In the present study, the effect of mild heat stress was evaluated. The higher the ambient temperature is, the less the microbiota richness held in the cecum, suggesting that the cyclic 30°C treatment may inhibit the growth and disrupt the homeostasis of bacteria in gut. The heat stress–induced impairment of intestinal morphology and permeability may contribute to the disadvantageous changes in intestinal microbiota floras (Zhang et al., 2017). In the present study, although the cecum microbiota diversity was slightly affected, the samples from the T24C and T30C groups were separately clustered, suggesting that the ambient temperature has an influence on the cecum microbiota diversity. In the cecum of broiler chickens, the Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Tenericutes are the predominant phyla and play an important role in the gut homeostasis (Johnson et al., 2018). In line with the result, the present study indicated that the Bacteroidetes, Firmicutes, and Proteobacteria were the predominant phyla in the cecum of layer-type pullets in the T24C group. The Bacteroidetes proportion increased to 55.1% in 30°C, which contributes to the change of microflora (Wu et al., 2018). Our results also show a marked reduction of relative abundance of Euryarchaeota and an increase in Bacteroidetes in the T30C group at the phylum level. Bacteroidetes encoded genes for the biosynthesis of a gram-negative cell wall; gram-positive anaerobes encoded genes for the biosynthesis of a gram-positive cell wall (Matej et al., 2018). Bacteroidetes were reported to increase in gut microbiota of birds with high fiber content in their diet (Ivarsson et al., 2014; Kubasova et al., 2017) and to forage on host-derived polysaccharides in the absence of fiber (Martens et al., 2008; Benjdia et al., 2011; Wu et al., 2015). The Bacteroidetes were found increased in the fecal microbiota under heat stress (Zhu et al., 2019). Our results also show a marked increment of relative abundance of Bacteroidetes. Collectively, the result suggests that mild heat stress could change the microbiota diversity in the cecum of chicken via increasing the proportion of Bacteroidetes and decreasing the proportion of Euryarchaeota.
Mild Heat Stress Changes the Microbiota Diversity in Respiratory Tract
The respiratory tract is a complex organ system responsible for the exchange of oxygen and carbon dioxide. Historically, the lungs were considered sterile because of several misconceptions and data misinterpretations that gave rise to false dogma in the field (Huffnagle et al., 2017). Actually, from the nostrils to the alveoli, the respiratory tract colonizes bacterial communities at specific locations (Anand and Mande, 2018). Respiratory flora plays a role as a gatekeeper against colonization of pathogens and may also be involved in respiratory physiology, maturation of immune function, and maintenance of steady state (Budden et al., 2017). In healthy lungs, the microbial biomass is low (Tulic et al., 2016). The source and maintenance of the airway microbiota are thought to be determined by the balance between microbial immigration from the upper respiratory tract and microbial elimination by host defense mechanisms, with relatively little contribution from the local reproduction of the microbes themselves. The immigration of microbes into the lungs occurs mainly through microaspiration (Wypych et al., 2019).
During heat exposure, the respiration rate will be increased in chicken (Yakubu et al., 2018; Shakeri et al., 2019). However, whether the microbiota in the respiratory tract is influenced by heat stress remains to be elucidated. The core microbiota defined here was in line with the previous work in broilers (Sohail et al., 2015). The bacterial community of the T24C group had much more OUT number and Chao estimate and higher Shannon index than the T30C group, suggesting that the chickens reared at 20°C to 24°C have high bacterial richness and bacterial diversity than the birds at 20°C to 30°C. The R value of ANOSIM based on UniFrac also indicated that bacteria structure in the T24C group was significantly different from that in the T30C pullets. We used LEfSe to identify the specific taxa in the respiratory microbiome, and 4 discriminative features were identified in the T30C group, in which the microbiota was enriched with Proteobacteria and Gamma-Proteobacteria at phyla and class level, respectively. In contrast, at family and class level, the Paenibacillaceae and genus Brevibacillus were found enriched in the T24C group, compared with their levels in the T30C group. However, a lower level of the genus Brevibacillus was observed in the T30C group, indicating the altered microbiota diversity. Many Brevibacillus species are commonly used as probiotics because they secrete antibacterial substances such as antimicrobial peptides, inhibit the growth of pathogenic microorganisms, and have excellent stress resistance (Yang and Yousef, 2018). It is speculated that Brevibacillus may have a protective effect against the damage of pathogenic microorganisms in the poultry respiratory tract. Hence, the physiological significance of the deprived colonization of Brevibacillus in the respiratory health of chicken under heat stress needs to be investigated further. The altered bacterial populations may represent new biomarkers of environment stress on respiratory tract.
Perturbation of the Gut-Lung Axis During Cyclic Mild Heat Stress
It is well known that both the gastrointestinal tract and respiratory tract are parts of the mucosal immune system. From embryonic perspective, the lungs and intestines are differentiated from the same stem cells, and the lungs and trachea develop from the foregut of the intestine, while the respiratory epithelium and glands are differentiated from the archenteron endoderm (Budden et al., 2017). The gut microbiota could produce signaling molecules that interact with the host, triggering reactions locally and remotely in several tissues such as the lungs, liver, and brain (Wang et al., 2018). Furthermore, changes in the composition and function of the digestive flora affect the respiratory tract through the mucosal immune system, and the respiratory flora disturbance also affects the digestive tract through immune regulation; this intestinal and pulmonary interaction affects the gut-lung axis (Anand and Mande, 2018; Mjösberg and Rao, 2018). In humans, a gut-lung axis of communication, exemplified by gut microbiota's impact on respiratory diseases and infections, up to 50% of adults with inflammatory bowel disease and 33% of patients with irritable bowel syndrome have pulmonary involvement, such as inflammation or impaired lung function, although many patients have no history of acute or chronic respiratory disease (Budden et al., 2017). People with colitis, Crohn's disease, or other intestinal disease are more likely to have asthma, allergies, or other respiratory problems (Schuijt et al., 2016; Bradley et al., 2017) and have indicated high probabilities for alterations in intestinal microbiota composition and abundance which may affect the lungs' immune status, but these mechanisms remain to be further explored (Trompette et al., 2014; Marsland et al., 2015; Li et al., 2018). The core pullet microbiota defined here is similar to that of previous studies, including those that have defined the core microbiota of the respiratory system and cecum (Johnson et al., 2018). Core microbiota analysis suggests that the respiratory microbiota is distinct from cecum samples but seems to be partially reflective of both. This differs from what one would expect from mammals and likely reflects the barn environment, where birds are constantly exposed to litter dust and the microbes contained within. Of course, it is also dependent on what bacteria will actually colonize in the trachea. The date from this study indicates that the tracheal microbiota is not simply a reflection of exposure to the environment, rather it is a combination of exposure on the cecum environment and preferential selection for microbes from that environment with the capacity to colonize. This is perhaps best exemplified by Brevibacillus, which is abundant in the BALF samples and significantly less abundant in the cecum. Brevibacillus have been found in almost all environmental habitats, including plants, animal intestinal tract, seawater, soil, and many food products (Yang and Yousef, 2018); members of the genus have been applied as probiotics for a long time. This also enables the possible discernment between colonizing and transient tracheal bacteria based on relative abundance. As we find lower levels of Brevibacillus in the trachea, this represents what is very likely to be transient levels of the bacteria and could also represent a potential cut-off between transient and colonizing species.
The present result indicated that both the microbiota diversity and microbial community structure in the cecum and respiratory tract were changed by mild heat stress, which is a result of disturbed gut-lung axis and contributes to susceptibility of chickens to ambient environment. A strong interest has emerged in characterizing the linkage of microbiota in the gut and respiratory tract.
Acknowledgments
This research was supported by grants from the National Key Research Program of China (2016YFD0500510), Modern Agro-industry Technology Research System (CARS-40), and the Taishan Scholars Program (No. 201511023). The authors thank the reviewers for their valuable comments and suggestions on the article.
Conflict of Interest Statement: The authors declare that they have no conflicts of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.09.024.
Contributor Information
Shuhong Sun, Email: jqybfkyjs@163.com.
Hai Lin, Email: hailin@sdau.edu.cn.
Supplementary data
References
- Anand S., Mande S.S. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjdia A., Martens E.C., Gordon J.I., Berteau O. Sulfatases and a radical S-Adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J. Biol. Chem. 2011;286:25973–25982. doi: 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C.P., Teng F., Felix K.M., Sano T., Naskar D., Block K.E., Huang H., Knox K.S., Littman D.R., Wu H.J. Segmented filamentous bacteria provoke lung autoimmunity by inducing Gut-Lung Axis Th17 cells expressing dual TCRs. Cell Host Microbe. 2017;22:697–704. doi: 10.1016/j.chom.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budden K.F., Gellatly S.L., Wood D.L.A., Cooper M.A., Morrison M., Hugenholtz P., Hansbro P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- Cheng H.W., Dillworth G., Singleton P., Chen Y., Muirt W.M. Effects of group selection for productivity and longevity on blood concentrations of serotonin, catecholamines, and corticosterone of laying hens. Poult. Sci. 2001;80:1278–1285. doi: 10.1093/ps/80.9.1278. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effati M., Samadi F., Dastar B., Azari M.A., Hashemi S.R. Effects of different levels of artichoke (cynara scolymus) on growth performance and immune responses of broilers underheat stress. Iranian J. Appl. Anim. Sci. 2014;4:399–403. [Google Scholar]
- Fan X., Liu S., Liu G., Zhao J., Jiao H., Wang X., Song Z., Lin H. Vitamin a deficiency impairs mucin expression and suppresses the mucosal immune function of the respiratory tract in chicks. PLoS One. 2015;10:e0139131. doi: 10.1371/journal.pone.0139131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J., Oehrle K., Worthen G., Alenghat T., Whitsett J., Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci. Transl. Med. 2017;9:eaaf9412. doi: 10.1126/scitranslmed.aaf9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E., Methé B., DeSantis T.Z., Petrosino J.F., Knight R., Birren B.W. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartanto S., Ko H.S., Jee S.H., Kang J.U., Seo J.S., Kang Y.H., Kim H.N., Ohh S.J. Effect of dietary nutmeg oil on heat-stress tolerance-related parameters in Korean native chicken reared under hot temperature. J. Anim. Physiol. Anim. Nutr. (Berf) 2019;103:1160–1167. doi: 10.1111/jpn.13113. [DOI] [PubMed] [Google Scholar]
- Huffnagle G.B., Dickson R.P., Lukacs N.M. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10:299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson E., Roos S., Liu H.Y., Lindberg J.E. Fermentable non-starch polysaccharides increases the abundance of bacteroides prevotella porphyromonas in ileal microbial community of growing pigs. Animal. 2014;8:1777–1787. doi: 10.1017/S1751731114001827. [DOI] [PubMed] [Google Scholar]
- Johnson T.J., Youmans B.P., Noll S., Cardona C., Evans N.P., Karnezos T.P., Ngunjiri J.M., Abundo M.C., Lee C.W. A consistent and predictable commercial broiler chicken bacterial microbiota in antibiotic-free production displays strong correlations with performance. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00362-18. e00362-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Davidova-Gerzova L., Merlot E., Medvecky M., Polansky O., Gardan-Salmon D., Quesnel H., Rychlik I. Housing systems influence gut microbiota composition of sows but not of their piglets. PLoS One. 2017;12:e0170051. doi: 10.1371/journal.pone.0170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Yu J., Bao E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br. Poult. Sci. 2009;50:504–511. doi: 10.1080/00071660903110851. [DOI] [PubMed] [Google Scholar]
- Li X., Leonardi I., Semon A., Doron I., Gao I.H., Putzel G.G., Kim Y., Kabata H., Artis D., Fiers W.D., Ramer A.E., Iliev I.D. Response to fungal dysbiosis by gut-resident CX3CR1+ mononuclear phagocytes aggravates allergic airway disease. Cell Host Microbe. 2018;24:847–856. doi: 10.1016/j.chom.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Li C., Sihai H. Effect of lactobacillus acidophilus 1.1878 on siga content in the intestinal mucous and antioxidant capacity of broiler chicks. Anim. Husbandry Feed Sci. 2017;4:21–23. [Google Scholar]
- Liu X., Li H., Lu A., Zhong Y., Hou X., Wang N., Jia D., Zan J., Zhao H., Xu J., Liu F. Reduction of intestinal mucosal immune function in heat-stressed rats and bacterial translocation. Int. J. Hyperthermia. 2012;28:756–765. doi: 10.3109/02656736.2012.729173. [DOI] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland B.J., Trompette A., Gollwitzer E.S. The gut-lung axis in respiratory disease. Ann. Am. Thorac. Soc. Suppl. 2015;2:S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- Martens E.C., Chiang H.C., Gordon J.I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaly M.M., Hendricks G.L., Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Matej M., Darina C., Ondrej P., Daniela K., Tereza K., Alois C., Ivan R. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genomics. 2018;19:561. doi: 10.1186/s12864-018-4959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J.D., Lott B.D., Simmons J.D. The effect of environmental temperature and body weight on growth rate and feed: gain of male broilers. Poult. Sci. 1998;77:499–501. doi: 10.1093/ps/77.4.499. [DOI] [PubMed] [Google Scholar]
- Mjösberg J., Rao A. Lung inflammation originating in the gut. Science. 2018;359:36–37. doi: 10.1126/science.aar4301. [DOI] [PubMed] [Google Scholar]
- Monson M.S., Goor A.G.V., Ashwell C.M., Persia M.E., Rothschild M.F., Schmidt C.J., Lamont S.J. Immunomodulatory effects of heat stress and lipopolysaccharide on the bursal transcriptome in two distinct chicken lines. BMC Genomics. 2018;19:643. doi: 10.1186/s12864-018-5033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J.K., Elaine H., James K., Remy B., Glenn G., Wei J., Sven P. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Price P.T., Byrd J.A., Alvarado C.Z., Pavlidis H.O., McIntyre D.R., Archer G.S. Utilizing original XPC™ in feed to reduce stress susceptibility of broilers. Poult. Sci. 2018;97:855–859. doi: 10.3382/ps/pex386. [DOI] [PubMed] [Google Scholar]
- Ramnath V., Rekha P.S., Sujatha K.S. Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by Brahma Rasayana. Evid. Based Complement. Alternat. Med. 2008;5:77–84. doi: 10.1093/ecam/nel116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E., Herrick R.B. Evaporative cooling of laying hens in Hawaii. Poult. Sci. 1983;62:741–745. doi: 10.3382/ps.0620741. [DOI] [PubMed] [Google Scholar]
- Schuijt T.J., Lankelma J.M., Scicluna B.P., Melo F.S., Roelofs J.J.T.H., Boer J.D., Hoogendijk A.J., Beer R., Vos A., Belzer C., Vos W.M., Poll T., Wiersinga W.J. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri M., Cottrell J.J., Wilkinson S., Le H.H., Suleria H.A.R., Warner R.D., Dunshea F.R. Growth performance and characterization of meat quality of broiler chickens supplemented with betaine and antioxidants under cyclic heat stress. Antioxidants (Basel) 2019;8:336. doi: 10.3390/antiox8090336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Shabbir M.Z., Ijaz A., Rehman H. Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian Pathol. 2015;44:67–74. doi: 10.1080/03079457.2015.1004622. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Tamburini S., Clemente J.C. Gut microbiota: neonatal gut microbiota induces lung immunity against pneumonia. Nat. Rev. Gastroenterol. Hepatol. 2017;14:263–264. doi: 10.1038/nrgastro.2017.34. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yanagihara T., Ikematsu Y., Inoue H., Ota K., Kashiwagi E., Suzuki K., Hamada N., Takeuchi A., Tatsugami K., Eto M., Ijichi K., Oda Y., Otsubo K., Yoneshima Y., Iwama E., Nakanishi Y., Okamoto I. Detection of identical T cell clones in peritumoral pleural effusion and pneumonitis lesions in a cancer patient during immune-checkpoint blockade. Oncotarget. 2018;9:30587–30593. doi: 10.18632/oncotarget.25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A., Gollwitzer E.S., Yadava K., Sichelstiel A.K., Sprenger N., Ngom-Bru C., Blanchard C., Junt T., Nicod L.P., Harris N.L., Marstand B.J. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- Tulic M.K., Piche T., Verhasselt V. Lung-gut cross-talk: evidence, mechanisms and implications for the mucosal inflammatory diseases. Clin. Exp. Allergy. 2016;46:519–528. doi: 10.1111/cea.12723. [DOI] [PubMed] [Google Scholar]
- Ugurlu N., Karo M. The effects of evaporative cooling on reduction of cage house temperature and production performance of the laying hens. Arch. Geflügelk. 2003;67:138–142. [Google Scholar]
- Wang X.J., Feng J.H., Zhang M.H., Li X.M., Ma D.D., Chang S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018;97:2153–2158. doi: 10.3382/ps/pey032. [DOI] [PubMed] [Google Scholar]
- Wu M., McNulty N.P., Rodionov D.A., Khoroshkin M.S., Griffin N.W., Cheng J., Latreille P., Kerstetter R.A., Terrapon N., Henrissat B., Osterman A.L., Gordon J.I. Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science. 2015;350:aac5992. doi: 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xiao Z., An W., Dong Y., Zhang B. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 2018;13:e0197762. doi: 10.1371/journal.pone.0197762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019;20:1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- Yakubu A., Oluremi O.I.A., Ekpo E.I. Predicting heat stress index in Sasso hens using automatic linear modeling and artificial neural network. Int. J. Biometeorol. 2018;62:1181–1186. doi: 10.1007/s00484-018-1521-7. [DOI] [PubMed] [Google Scholar]
- Yang X., Yousef A.E. Antimicrobial peptides produced by Brevibacillus spp.: structure, classification and bioactivity: a mini review. World J. Microbiol. Biotechnol. 2018;34:57. doi: 10.1007/s11274-018-2437-4. [DOI] [PubMed] [Google Scholar]
- Yunianto V.D., Hayashi K., Kaneda S., Ohtsuka A., Tomita Y. Effect of environmental temperature on muscle protein turnover and heat production in tube-fed broiler chickens. Br. J. Nutr. 1997;77:897–909. doi: 10.1079/bjn19970088. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhu L., Liao R., Wu N., Zhu G., Yang C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019;103:461–472. doi: 10.1007/s00253-018-9465-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.