Abstract
Newcastle disease (ND) is one of the most important avian diseases that seriously threaten the poultry industry worldwide. Sometimes vaccination might not effectively reduce ND occurrence in some poultry farms for unclear reasons. Infectious bursal disease (IBD) is one of the most important immunosuppressive diseases, and the novel variant IBD virus (IBDV) is threatening chicken farms in China. This study evaluated the influence of the novel variant IBDV (SHG19 strain) on immunization of ND vaccine (LaSota strain) in broiler and layer chickens. In commercial broilers, the hemagglutination inhibition antibody titers against LaSota strain were decreased by the prior infection of the novel variant IBDV. Pathological examination revealed that the novel variant IBDV severely damaged the key immune organs of the bursa and spleen, and the B lymphocytes in the bursa were severely destroyed, which was the primary reason involved in the immunosuppression on ND vaccination. Moreover, the novel variant IBDV dramatically reduced the BW of infected broilers by about 16% compared to that of control, which indicated huge economic losses. Furthermore, this study confirmed the immunosuppression induced by the novel variant IBDV in specific pathogen–free layer chickens. In this study, it was discovered that the novel variant IBDV could interfere with ND vaccination in both broilers and layers, which was one important factor involved in immune failure in poultry farms. This study therefore suggests the urgency to control the novel variant IBDV infection for healthy breeding.
Key words: novel variant infectious bursal disease virus, Immunosuppression, Newcastle disease
Introduction
Newcastle disease (ND) is one of the most important avian diseases, which severely affects commercial production and international trade in poultry industry (Miller et al., 2010). It is caused by ND virus (NDV), which belongs to avian avulavirus. According to the pathogenicity to chickens, NDV can be distinguished into 4 pathotypes named velogenic, mesogenic, lentogenic, and avirulent types, respectively. The F and HN proteins are mainly associated with the pathogenic process of NDV infection (Teng et al., 2019). Newcastle disease has caused tremendous economic losses through numerous epidemics associated with high mortality and morbidity (Rahman et al., 2018). To prevent production losses, even with a good biosecurity practice, vaccination has become an increasingly important routine in the poultry industry (Bello et al., 2018). However, sometimes, vaccination might not effectively reduce virus transmission and disease occurrence (Le et al., 2018; Gowthaman et al., 2019). There are many factors that play vital roles including immunosuppressive agents, virus variation, biosecurity breaks, inadequate management practices, and harsh environments, among which immunosuppression induced by other viruses could be a possible reason (Hoerr, 2010; Perozo et al., 2012; El-Aried et al., 2019).
Infectious bursal disease (IBD) is one of the most important immunosuppressive diseases that seriously threaten the poultry industry worldwide (Müller et al., 2003). It is caused by IBD virus (IBDV), which can destroy the bursa and kill B lymphocytes, resulting in severe immunosuppression of the humoral immune system (Dey et al., 2019). Immunosuppression promotes the susceptibility of chickens to other infections and interferes with vaccination against other diseases (Dey et al., 2019). Infectious bursal disease virus includes 2 serotypes: serotype I viruses are pathogenic to chickens and are classified into 4 subtypes, classic IBDV (Cosgrove, 1962), variant IBDV (Jackwood and Saif, 1987), and very virulent IBDV (vvIBDV) (Chettle et al., 1989), and serotype II viruses are nonpathogenic to chickens.
Since 2019, it was reported that the novel variant IBDV is now prevalent in China, which is seriously associated with the destruction of the central immune organ and B lymphocytes (Fan et al., 2019, 2020; Xu et al., 2019; Li et al., 2020). It was speculated that the novel variant IBDV might be involved in the immune failure of ND vaccination, which has not been clearly verified. Hence, the objective of this study is to determine whether the novel variant IBDV could suppress ND vaccination.
Materials and methods
Virus and Vaccine
The novel variant IBDV representative strain SHG19 (Fan et al., 2019, 2020) was previously isolated and identified in the Avian Immunosuppressive Disease Laboratory, Harbin Veterinary Research Institute (HVRI), Chinese Academy of Agricultural Sciences (CAAS) (hereinafter refer to as “our laboratory”). SHG19 was renamed 1/chicken/China/SHG19/17(G2) according to the new nomenclature scheme for IBDV (Jackwood et al., 2018). Newcastle disease vaccine (LaSota strain) was purchased from Ceva Animal Health Service Company (Beijing, China).
Animals
The 21-day-old specific pathogen–free (SPF) layer chickens were purchased from the Experimental Animal Center of the HVRI of the CAAS (Harbin, China). The chick embryos of commercial white feather broiler chickens were purchased from a chicken farm in Harbin and hatched in our laboratory. These chickens were fed in negative pressure–filtered air isolators. All the animal experiments were approved by the HVRI of the CAAS and were performed in accordance with the animal ethics guidelines and approved protocols.
Animal Experiment of Commercial Broilers
To evaluate the influence of the novel variant IBDV on ND vaccine, an animal experiment of commercial broiler chickens was performed. Forty 1-day-old broilers were fed in negative pressure–filtered air isolators. At 12 and 21 days of age, 20 broilers were randomly chosen for sera collection, and the maternal antibodies were detected using the Infectious Bursal Disease Virus Antibody Test Kit (IDEXX, 99-09260, Portland) according to the manufacturer's instructions. Then, the 21-day-old broilers were randomly divided into 3 groups, and the BW of all the chickens was determined. The first group (n = 15) was infected with 8 × 106 viral RNA copies (200 μL) of SHG19 per chicken via the ocular and intranasal routes, whereas the second group (n = 10) and the third group (n = 15) received 200-μL PBS per chicken. To detect the pathogenicity induced by SHG19 strain, at 4 d post-infection (d p.i.), 5 chickens of the first and third groups were randomly euthanized for necropsy and examination of pathological changes. At 4 d p.i., the first and second groups were immunized with ND vaccine (LaSota strain, 200 μL per chicken) by the ocular and intranasal routes in accordance with the instructions. The third group, without infection and vaccination, was used as the control. At 0, 10, and 17 d post-vaccination (d p.v.), the sera of all chickens were collected, and the sera antibodies against NDV were detected by hemagglutination inhibition (HI) assays. At 4 and 21 d p.i., the BW, bursa:BW index (BBIX), spleen/BW ratio, and pathological changes of each group were examined. The spleen/BW ratio = (spleen weight × 1,000)/BW.
Animal Experiment of Layer Chickens
To further evaluate the influence of the novel variant IBDV on ND vaccine in layer chickens, thirty 21-day-old SPF layers were randomly divided into 3 groups (n = 10/group). The first group was infected with 8 × 106 viral RNA copies (200 μL) of SHG19 per chicken via the ocular and intranasal routes, and the other 2 groups received 200-μL PBS. At 4 d p.i., the first and second groups were immunized with ND vaccine (LaSota strain, 200 μL per chicken) by the ocular and intranasal routes according to the instructions. The third group was used as the control without infection and vaccination. At 0, 10, and 17 d p.v., the sera antibodies against NDV was detected by HI assays. At 21 d p.i., the BW, BBIX, and spleen/BW ratios of each group were examined.
BBIX Value
The BBIX value was calculated along with the SD [BBIX= (bursa:BW ratios in the infected group)/(bursa:BW ratios in the control group)]. The mean values and SD of the data obtained from multiple independent chicken samples were calculated. The bursa with a BBIX value less than 0.70 was considered as atrophy (Lucio and Hitchner, 1979).
Histopathology
Tissue samples (bursae and spleens) of chickens were fixed immediately in 10% neutral buffered formalin (Solarbio, G2160, Beijing, China) and were stained with hematoxylin and eosin for further histopathological examination. Tissue sections were prepared and observed for microscopic changes under light microscope.
Detection of Hemagglutination Inhibition Antibodies of NDV
The NDV-specific antibodies in broiler and layer chicken animal experiments were detected by the HI assays. Briefly, 25-μL PBS was added into each well of a V-shaped microtiter plate. Meanwhile, 25 μL of sera was pipetted into a well in the first column and was diluted serially with 2-fold in wells of the remaining columns, respectively, whereas the last 2 wells were not added. Then 25-μL 4 hemagglutination units (HAU) of antigen was then added to each well except the last well, and the mixture was incubated for 25 min at room temperature. Subsequently, 25 μL of 1% (v/v) chicken red blood cells in PBS were added to each well and mixed gently and then incubated for 40 min at room temperature. The HI titer was defined as the highest dilution of serum causing complete inhibition of 4 HAU of antigen. The mean values and SD of the data obtained from multiple independent chicken samples were calculated.
Statistical Analysis
A one-way ANOVA was used to evaluate the significance of the differences among the different groups. Significant treatment means were separated using Tukey's honestly significant difference (Tukey's HSD) at P = 0.05.
Results
Novel Variant IBDV Induced Immunosuppression and Weight Loss of Commercial Broilers
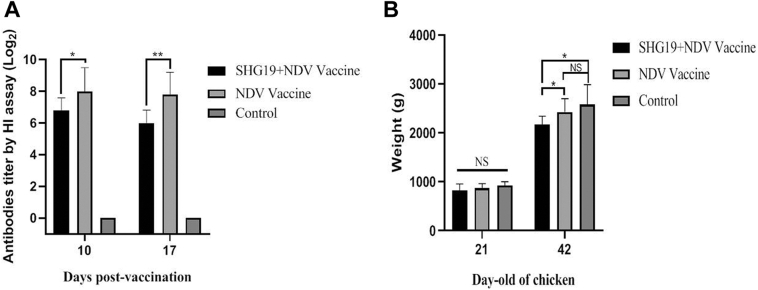
To evaluate the influence of the novel variant IBDV on ND vaccination, the 21-day-old commercial broilers were infected with SHG19 strain of IBDV followed by vaccination with ND vaccine 4 d later. At 10 d p.v., compared with noninfected chickens in the second group (NDV vaccine group), the mean HI titer of the NDV antibodies in the first group (SHG19 + NDV vaccine group) was suppressed by SHG19 infection (P < 0.05) (Figure 1). At 17 d p.v, the mean HI titer of NDV antibodies of the SHG19 + NDV vaccine group decreased by 23% compared with the NDV vaccine group (P < 0.01) (Figure 1A). Besides, compared with the control group (2,580 ± 405 g), the mean BW of the SHG19 + NDV vaccine group (2,171 ± 170 g) of 42-day-old chickens decreased by 409 g (P < 0.05), whereas no significant difference was observed between the NDV vaccine group and the control group (Figure 1B).
Figure 1.
The HI titer of NDV antibodies and BW of the commercial broilers. (A) Antibodies titer to the NDV vaccine of commercial broilers determined by homologous HI assay. (B) The BW of 21- and 42-day-old commercial broilers. The average weight and SD (error bars) from 10 independent samples are shown (∗P < 0.05, ∗∗P < 0.01; NS, not significant). Abbreviations: HI, hemagglutination inhibition; NDV, Newcastle disease virus.
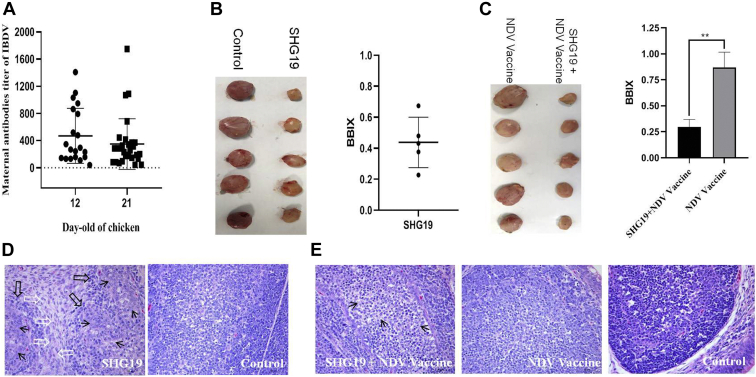
Novel Variant IBDV Was Pathogenic to Commercial Broilers
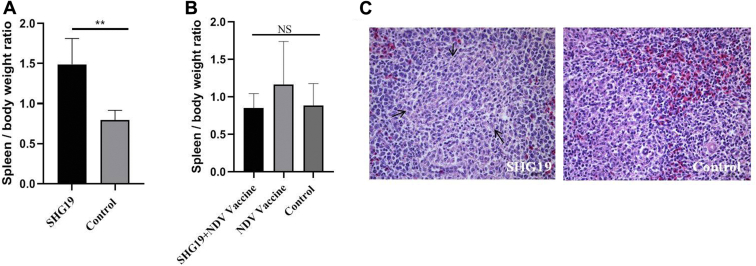
To analyze the factors involved in the immunosuppression mentioned above, the pathogenicity of SHG19 strain to commercial broilers was detected. The commercial broilers showed 27 to 40% positive for maternal antibodies against IBDV before infection with SHG19 strain (Figure 2A). At 4 d p.i., SHG19 strain induced severe atrophy of the bursa with a BBIX of 0.44 ± 0.16 (Figure 2B). The histopathological results showed that follicle atrophy, interstitial hyperplasia, and lymphopenia occurred in the bursa of the SHG19-infected group, whereas no pathological changes were found in the control group (Figure 2D). Besides, the spleen/BW ratio of the SHG19-infected group was significantly higher than that of the control group (Figure 3A), and white pulp lymphocytes decreased in comparison with the control group (Figure 3C).
Figure 2.
The pathogenicity to the bursa of commercial broilers induced by the IBDV novel variant strain SHG19. (A) The maternal antibody titer of IBDV at 12 and 21 d of age. (B) The bursae image and the BBIX value of infection and control groups at 4 d post-infection (day p.i.). (C) The bursae image and the BBIX value of infection and control groups of vaccinated broilers at 21 d p.i. (D) The histopathological appearance of the bursal sections (hematoxylin and eosin staining) of infected and control groups at 4 d p.i. The follicle atrophy (black hollow arrow), interstitial hyperplasia (black thin arrow), and lymphopenia (white hollow arrow) that occurred in the bursa of the infected group are shown. (E) The histopathological appearance of the bursal sections of the infected and control groups of vaccinated broilers at 21 d p.i. Lymphocyte decreasing (black thin arrow) was observed in the infected group. The mean value from 20 (A), 10 (C), or 5 (B) independent samples are shown (∗∗P < 0.01). Abbreviations: BBIX, bursa:BW index; d p.i, days post-infection; IBDV, infectious bursal disease virus.
Figure 3.
The pathogenicity to the spleen of commercial broilers induced by the IBDV novel variant strain SHG19. (A) The spleen/BW ratio at 4 d p.i. (B) The spleen/BW ratio at 21 d p.i. (C) The histopathological appearance of the spleen sections of infected and control groups at 4 d p.i. The decrease in white pulp lymphocytes (black thin arrow) was observed in the infected group. The mean value and SD (error bars) from 5 (A) or 10 (B) independent samples are shown (∗∗P < 0.01; NS, not significant). Abbreviations: d p.i, days post-infection; IBDV, infectious bursal disease virus.
At 4 d p.i., 10 infected and another 10 noninfected broilers were vaccinated with ND vaccine. The results showed that the bursa of the infected group (SHG19 + NDV vaccine group) was still atrophic with inflammatory exudation and yellow staining at 21 d p.i., and the BBIX value was 0.30 ± 0.07 (Figure 2C). Lymphocyte decreasing was observed in the SHG19 + NDV vaccine group, whereas there were no pathological changes in the NDV vaccine group and control group (Figure 2E). No obvious difference was observed in spleen/BW ratio of the SHG19 + NDV vaccine group, the NDV vaccine group, and the control group (Figure 3B).
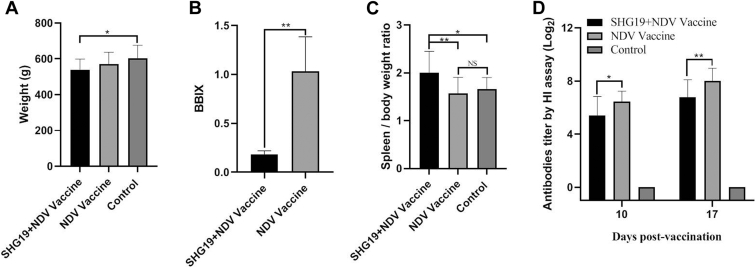
Novel Variant IBDV Induced Pathological Damages and Immunosuppression on ND Vaccination of Layer Chickens
The influence of the novel variant IBDV on ND vaccine was further evaluated in SPF layer chickens. Similarly, SHG19 strain induced significant BW loss in layers aged 42 d (P < 0.05) (Figure 4A). The bursa of the infected group was atrophic with a BBIX of 0.18 ± 0.04 (Figure 4B). Swelling of the spleen was observed in the infected group with a higher spleen/BW ratio than in the control group (Figure 4C). As a result, the vaccination efficiency of ND vaccine in layers was negatively affected. In addition, compared with the NDV vaccine group, the HI titer of the NDV antibodies was suppressed in the SHG19 + NDV vaccine group at 10 d p.v. (P < 0.05) and 17 d p.v. (P < 0.01) (Figure 4D).
Figure 4.
The pathogenicity and immunosuppression to layer chickens induced by the IBDV novel variant strain SHG19. (A) The BW of 42-day-old SPF layer chickens. (B) The BBIX values of the infected and control groups of vaccinated SPF layer chickens at 21 d p.i.. (C) The spleen/BW ratio at 21 d p.i.. (D) Antibodies titer to the ND vaccine of layer chickens determined by homologous HI assay. The mean value and SD (error bars) from 10 independent samples are shown (∗P < 0.05, ∗∗P < 0.01; NS, not significant). Abbreviations: BBIX, bursa:BW index; d p.i, days post-infection; HI, hemagglutination inhibition; IBDV, infectious bursal disease virus; ND, Newcastle disease; SPF, specific pathogen free.
Discussion
It is well known that vaccination plays a significant role in the control of ND, especially in large-scale intensive chicken farms. However, it has been observed that ND sometimes occurred in some vaccinated poultry farms in recent years (Mariappan et al., 2018; Almubarak, 2019). On the one hand, a few NDV strains were reported as novel subgroups and genotype mismatches (Le et al., 2018; Gowthaman et al., 2019; Liu et al., 2019). On the other hand, the immune failure associated with immunosuppression caused by other viruses cannot be ignored (Perozo et al., 2012; El-Aried et al., 2019). In recent years, as a new threaten of poultry farm, novel variant IBDV associated with immune organ and B lymphocyte destruction is now prevalent in China (Fan et al., 2019, 2020; Xu et al., 2019). Thereafter, it was speculated that the novel variant IBDV might be responsible for the immune failure of ND vaccination.
To prove the speculation, the commercial white feather broilers were hatched in our laboratory and then fed in negative pressure–filtered air isolators, which could minimize other interfering factors. Newcastle disease vaccine (LaSota strain), the most widely used ND vaccine in China, was used in this experiment. Our results clearly showed that the novel variant IBDV SHG19 strain had seriously destroyed the bursa, which is important for the induction of humoral immune responses in chickens. In the infected broilers, the B lymphocytes in the bursa were severely destroyed so that the HI antibodies titer against ND vaccine decreased compared with the control group. It is well known that high levels of systemic antibody have always been associated with protection against ND (Van Boven et al., 2008). This decreased HI antibodies showed in this study might be not too low to interfere with the immune protection against NDV. However, in chicken farms, the NDV HI antibodies titer might be suppressed more significantly with more early infection of SHG19 because field IBDV can infect chickens at any time. It was reported that an early infection of one-day-old chickens with variant IBDV might induce more severe suppression of antibodies against avian influenza virus (AIV) vaccine (Spackman et al., 2018). Moreover, coinfection of the variant IBDV and other immunosuppressive pathogens might further aggravate the interference to the immune protection effect of ND vaccine. In addition, SHG19 induced swelling of the spleen, which is another key immune organ. The data obtained using SPF layers further confirmed the immunosuppression induced by SHG19 strain. To our knowledge, it is the first time to prove that the novel variant IBDV interfered with the immune effect of ND vaccine in both commercial broilers and layers. In addition, it has been reported that the variant IBDV could also dramatically induce immunosuppression of AIV vaccination, which might result in the occasional poor effect of AIV vaccines in some farms despite good experimental efficacy in the laboratory (Spackman et al., 2018; Fan et al., 2019). Thereafter, the variant IBDV is one important factor involved in the immune failure in chicken farms. Although this study supports that the novel variant IBDV significantly interferes with the humoral immunity responses to ND vaccine, it is valuable in our further studies to strengthen the monitoring of the coinfection of the novel variant IBDV and other viruses and further evaluate the immunosuppression effect of the novel variant IBDV using the animal challenge protection experiment, and the immunosuppression mechanism will be systematically studied from the aspects of both humoral and cellular immunity.
In addition to the death induced directly by IBDV, the loss of production performance is an important economic threat for farms. In this animal experiment, the mean weight of the 42-day-old commercial broilers was 2,580 g. Newcastle disease vaccination did not obviously interfere with the chicken growth. However, the infection of SHG19 strain reduced the BW by 409 g, which was 16% of the weight of control. In SPF layers, the growth was also negatively influenced by SHG19 infection. It has been reported that there had been tremendous economic losses as a result of loss of weight in broilers due to infection of variant IBDV strains in Canada (Zachar et al., 2016).
The novel variant IBDV is now prevalent even in chicken farms immunized with vvIBDV vaccines in China (Fan et al., 2019; Xu et al., 2019). It was further confirmed that these variant IBDV could break through the immunoprotection induced by some vvIBDV vaccines because of the antigen mismatch (Fan et al., 2020). In this study, the novel variant IBDV could acutely damage the key immune organs of the commercial broilers in the presence of some maternal antibodies and then interfere with ND vaccination. The novel variant IBDV, therefore, pose new challenges to the healthy breeding.
Conclusion
This study discovered that the novel variant IBDV could interfere with ND vaccination in both broilers and layers, which is one important factor involved in immune failure in poultry farms. Our study further reminded us of the importance and urgency of control of the novel variant IBDV for healthy breeding. Conflict of Interest: The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (No. 2016YFE0203200, No. 2017YFD0500704), the Heilongjiang Province Foundation for the National Key Research and Development Program of China (GX18B011), the Major Project of National Natural Science Foundation of China (No. 31430087), and the Modern Agro-industry Technology Research System (No. CARS-41-G15).
Contributor Information
Xiaomei Wang, Email: wangxiaomei@caas.cn.
Xiaole Qi, Email: qixiaole@caas.cn.
References
- Almubarak A.I.A. Molecular and biological characterization of some circulating strains of Newcastle disease virus in broiler chickens from Eastern Saudi Arabia in 2012-2014. Vet. World. 2019;12:1668–1676. doi: 10.14202/vetworld.2019.1668-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello M.B., Yusoff K., Ideris A., Hair-Bejo M., Peeters B.P.H., Omar A.R. Diagnostic and vaccination approaches for Newcastle disease virus in poultry: the current and emerging perspectives. Biomed. Res. Int. 2018;2018:7278459. doi: 10.1155/2018/7278459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettle N., Stuart J.C., Wyeth P.J. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989;125:271–272. doi: 10.1136/vr.125.10.271. [DOI] [PubMed] [Google Scholar]
- Cosgrove A.S. An apparently new disease of chickens: avian nephrosis. Avian Dis. 1962;6:385–389. [Google Scholar]
- Dey S., Pathak D.C., Ramamurthy N., Maity H.K., Chellappa M.M. Infectious bursal disease virus in chickens: prevalence, impact, and management strategies. Vet. Med. (Auckl.) 2019;10:85–97. doi: 10.2147/VMRR.S185159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Aried T.A., Mansour S.M.G., ElBakrey R.M., AE N.I., Eid A.A.M. Infectious bursal disease virus: molecular epidemiologic perspectives and impact on vaccine efficacy against avian influenza and Newcastle disease viruses. Avian Dis. 2019;63:606–618. doi: 10.1637/aviandiseases-D-19-00086. [DOI] [PubMed] [Google Scholar]
- Fan L., Wu T., Hussain A., Gao Y., Zeng X., Wang Y., Gao L., Li K., Wang Y., Liu C., Cui H., Pan Q., Zhang Y., Liu Y., He H., Wang X., Qi X. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbio. 2019;230:212–220. doi: 10.1016/j.vetmic.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Fan L., Wu T., Wang Y., Hussain A., Jiang N., Gao L., Li K., Gao Y., Liu C., Cui H., Pan Q., Zhang Y., Wang X., Qi X. Novel variants of infectious bursal disease virus can severely damage the bursa of fabricius of immunized chickens. Vet. Microbio. 2020;240:108507. doi: 10.1016/j.vetmic.2019.108507. [DOI] [PubMed] [Google Scholar]
- Gowthaman V., Singh S.D., Dhama K., Ramakrishnan M.A., Malik Y.P.S., Gopala Krishna Murthy T.R., Chitra R., Munir M. Co-infection of Newcastle disease virus genotype XIII with low pathogenic avian influenza exacerbates clinical outcome of Newcastle disease in vaccinated layer poultry flocks. Virusdisease. 2019;30:441–452. doi: 10.1007/s13337-019-00533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Jackwood D.H., Saif Y.M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987;31:766–770. [PubMed] [Google Scholar]
- Jackwood D.J., Schat K.A., Michel L.O., de Wit S. A proposed nomenclature for infectious bursal disease virus isolates. Avian Pathol. 2018;47:576–584. doi: 10.1080/03079457.2018.1506092. [DOI] [PubMed] [Google Scholar]
- Le X.T.K., Doan H.T.T., Le T.H. Molecular analysis of Newcastle disease virus isolates reveals a novel XIId subgenotype in Vietnam. Arch. Virol. 2018;163:3125–3130. doi: 10.1007/s00705-018-3961-0. [DOI] [PubMed] [Google Scholar]
- Li G., Kuang H., Guo H., Cai L., Chu D., Wang X., Hu J., Rong J. Development of a recombinant VP2 vaccine for the prevention of novel variant strains of infectious bursal disease virus. Avian Pathol. 2020:1–49. doi: 10.1080/03079457.2020.1791314. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang J., Ge S., Lv Y., Li Y., Zheng D., Zhao Y., Castellan D., Wang Z. Molecular characterization of new emerging sub-genotype VIIh Newcastle disease viruses in China. Virus Genes. 2019;55:314–321. doi: 10.1007/s11262-019-01651-5. [DOI] [PubMed] [Google Scholar]
- Lucio B., Hitchner S.B. Response of susceptible versus immune chicks to killed, live-modified, and wild infectious bursal disease virus vaccines. Avian Dis. 1979;23:1037–1050. [PubMed] [Google Scholar]
- Mariappan A.K., Munusamy P., Kumar D., Latheef S.K., Singh S.D., Singh R., Dhama K. Pathological and molecular investigation of velogenic viscerotropic Newcastle disease outbreak in a vaccinated chicken flocks. Virusdisease. 2018;29:180–191. doi: 10.1007/s13337-018-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.J., Decanini E.L., Afonso C.L. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 2010;10:26–35. doi: 10.1016/j.meegid.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Müller H., Islam M.R., Raue R. Research on infectious bursal disease—the past, the present and the future. Vet. Microbio. 2003;97:153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Perozo F., Marcano R., Afonso C.L. Biological and phylogenetic characterization of a genotype VII Newcastle disease virus from Venezuela: efficacy of field vaccination. J. Clin. Microbiol. 2012;50:1204–1208. doi: 10.1128/JCM.06506-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A.U., Habib M., Shabbir M.Z. Adaptation of Newcastle disease virus (NDV) in feral birds and their potential role in interspecies transmission. Open Virol. J. 2018;12:52–68. doi: 10.2174/1874357901812010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E., Stephens C.B., Pantin-Jackwood M.J. The effect of infectious bursal disease virus-induced immunosuppression on vaccination against highly pathogenic avian influenza virus. Avian Dis. 2018;62:36–44. doi: 10.1637/11769-110717-Reg.1. [DOI] [PubMed] [Google Scholar]
- Teng J.L.L., Wernery U., Lee H.H., Joseph S., Fung J., Elizabeth S.K., Yeong K.Y., Kinne J., Chan K.H., Lau S.K.P., Woo P.C.Y. First isolation and rapid identification of Newcastle disease virus from aborted fetus of dromedary camel using next-generation sequencing. Viruses. 2019;11:E810. doi: 10.3390/v11090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boven M., Bouma A., Fabri T.H.F., Katsma E., Hartog L., Koch G. Herd immunity to Newcastle disease virus in poultry by vaccination. Avian Path. 2008;37:1–5. doi: 10.1080/03079450701772391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A., Pei Y., Zhang K., Xue J., Ruan S., Zhang G. Phylogenetic analyses and pathogenicity of a variant infectious bursal disease virus strain isolated in China. Virus Res. 2019;276:197833. doi: 10.1016/j.virusres.2019.197833. [DOI] [PubMed] [Google Scholar]
- Zachar T., Popowich S., Goodhope B., Knezacek T., Ojkic D., Willson P., Ahmed K.A., Gomis S. A 5-year study of the incidence and economic impact of variant infectious bursal disease viruses on broiler production in Saskatchewan, Canada. Can. J. Vet. Res. 2016;80:255–261. [PMC free article] [PubMed] [Google Scholar]