Abstract
A study was conducted to evaluate the effect of dietary 25-hydroxyvitamin D3 (25OHD) on pullet and egg-laying hen growth performance, egg production, and egg quality. Three hundred and ninety 1-day-old Hy-Line W36 pullets were randomly allocated to 3 treatments with 10 replicated cages and 13 birds per cage. Dietary treatments were vitamin D3 at 2,760 IU/kg (D); vitamin D3 at 5,520 IU/kg (DD), and vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg (25D). Body weight and feed intake were recorded at the end of each stage: starter 1 (0–3 wk), starter 2 (4–6 wk), grower (7–12 wk), developer (13–15 wk), prelay (15–17 wk), peaking (18–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk). Egg production was recorded daily. Egg quality was evaluated every 8 wk starting from 25 wk. There was no difference in growth performance during the rearing period (0–17 wk). In the laying period (18–95 wk), DD showed lower feed intake at layer 2, but higher intake at layer 3 along with lower hen day production (HDP) from 22 to 48 wk compared to the other treatments. During the same period, the DD group laid smaller eggs with higher specific gravity and shell thickness compared with the other treatments or D alone at 40 wk, which may be partly due to the lower body weight. In contrast, 25D had better feed conversion ratio (feed intake per dozen of eggs) at layer 2, and higher overall (22–60 wk) HDP compared with DD. For the egg quality analysis, at 25 and 33 wk, both DD and 25D had higher Haugh unit compared with D. However, 25OHD has no effects on eggshell quality during the entire production period and no beneficial effects on egg production during the later laying period (after 60 wk). In summary, long-term and early supplementation of 25OHD has positive effects on egg production and egg quality, and the beneficial effects were mainly observed during the early laying stage.
Key words: 25-hydroxyvitamin D3, laying hen, growth performance, egg production, egg quality
Introduction
Vitamin D3 can be obtained from the conversion of 7-dehydrocholesterol in the skin under 290 to 315 nm UV light (Holick et al., 1980). However, modern layer flocks are mostly kept entirely indoors (without direct sunlight). Their primary source of vitamin D3 is from their diet (Świątkiewicz et al., 2017). Vitamin D3 requires 2 biological conversions to become an active form (Christakos et al., 2010). First, vitamin D3 is converted in the liver to its primary circulating form, 25-hydroxyvitamin D3 (25OHD). Then, mainly in the kidney, 25OHD is converted to its bioactive form, 1, 25-dihydroxyvitamin D3 (1,25OHD). Vitamin D3 is associated with mineral hemostasis (Chang et al., 2005), direct or indirect regulation of bone development, egg production, and eggshell quality in chicken (Rodriguez-Lecompte et al., 2016; Świątkiewicz et al., 2017).
Feeding laying hen with higher vitamin D3 than their requirement showed no beneficial effects on laying performance and bone quality (Keshavarz, 2003; Mattila et al., 2004; Persia et al., 2013); however, supplementation of 1,25OHD could increase bone mineralization and integrity in poultry (Frost et al., 1990). These observations suggest that the conversion of vitamin D3 to its bioactive form is most likely insufficient (Koreleski and Świątkiewicz, 2005). Particularly in young pullets and old laying hens, the functional defect of the liver and kidney results in decreased production of active vitamin D3 metabolites (Bar et al., 1988; Świątkiewicz et al., 2017). An alternative strategy to optimize vitamin D3 effect in laying hens is supplementation of the active forms of vitamin D3 metabolites in the diets.
25-hydroxyvitamin D3, a metabolite of vitamin D3, is commercially available in the market. It is 2- to 4-fold more active compared with vitamin D3 in chicken diets (Soares Jr. et al., 1995; Atencio et al., 2005). However, previous studies of 25OHD on laying hens have shown either no effects (Roland and Harms, 1976; Keshavarz, 2003; Käppeli et al., 2011; Mattila et al., 2011; Nascimento et al., 2014; Adhikari et al., 2020) or beneficial effects (Koreleski and Świątkiewicz, 2005; Torres et al., 2009; Silva, 2017) on eggshell quality and/or egg production. Inconsistent results among these studies may be due to differences in 25OHD treatment duration, treatment timing, and laying stage. None of the studies showing no effects included 25OHD in the diets during the rearing period or covered the entire laying period, especially the early laying period. The use of 25OHD during the rearing period and early laying phases could promote bone development and protect bone loss during peak production, which may benefit the birds for egg production during the later laying period (Silva, 2017). However, to our knowledge, limited studies have focused on this approach of using 25OHD to target early and long-term supplementation.
It was hypothesized that early and long-term supplementation of 25OHD in layer diets could enhance egg production and egg quality. This study was performed to evaluate the effects of long-term supplementation of 25OHD in pullets and laying hen diets from 1-day-old to 95 wk on Hy-Line W36 laying hen egg production and egg quality.
Materials and methods
Housing, Birds, and Treatments
The study was conducted at the research facility of the Department of Poultry Science at the University of Georgia and approved by the Institutional Animal Care and Use Committee at the University of Georgia (A2016 11-003). Three hundred and ninety 1-day-old Hy-Line W36 pullets (3 treatments × 10 repetitions × 13 birds per cage) were housed in wire cages and allocated to 3 treatment groups: control vitamin D3 (D; 2,760 IU/kg); double dosage vitamin D3 (DD; 5,520 IU/kg); and control vitamin D3 + 69 μg/kg 25OHD (25D; equivalent from DD; HyD, DSM, Pasippany, NJ). The diets were formulated based on the Hy-Line W36 guide (2015) (Tables 1 and 2). The pullets were housed in colony cages, 90 cm (L) × 46 cm (W) × 38 cm (H), until 17 wk, which resulted in 318 cm2/bird. Before the birds started laying eggs, they were transferred to a laying hen house and kept in an individual cage: 41 cm (L) × 26 cm (W) × 46 cm (H), which resulted in 1,066 cm2/bird. Due to the bird loss of sampling during the pullet period, during the laying period, 8 birds were used for each replication.
Table 1.
Diet formulation and calculated nutrient composition for rearing period (0–17 wk).1
| Ingredients, % (unit %) | Starter 12 1–3 wk |
Starter 2 4–6 wk |
Grower 49–60 wk |
Developer | Prelay |
|---|---|---|---|---|---|
| Corn | 67.11 | 62.19 | 65.93 | 69.43 | 62.17 |
| Soybean meal -48% | 28.08 | 27.34 | 24.00 | 20.00 | 23.10 |
| Soybean oil | 1.00 | 3.00 | 2.54 | 2.57 | 3.02 |
| Limestone | 0.68 | 0.71 | 0.8 | 1.95 | 4.68 |
| Defluorinated phosphate | 2.03 | 2.01 | 1.92 | 1.85 | 2.01 |
| Common salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lysine HCl | 0.19 | 0.13 | 0.11 | 0.08 | 0.01 |
| DL-Methionine | 0.21 | 0.23 | 0.18 | 0.14 | 0.20 |
| Threonine | 0.23 | 0.08 | 0.06 | 0.05 | 0.03 |
| Vitamin premix3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix4 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Amprolium | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Sand | 0 | 3.85 | 3.99 | 3.47 | 4.33 |
| ME (kcal/kg) | 3,030 | 3,030 | 3,030 | 3,050 | 2,920 |
| CP% | 20.00 | 18.25 | 17.50 | 16.00 | 16.50 |
| Ca% | 1.00 | 1.00 | 1.00 | 1.40 | 2.50 |
| Available P (%) | 0.50 | 0.49 | 0.47 | 0.45 | 0.48 |
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
Treatments were added as a form of vitamin premix in the diet: D treatment: vitamin D3 at 2,760 IU/kg; DD treatment: vitamin D3 at 5,220 IU/kg; 25D treatment: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Starter 1 (0–3 wk), starter 2 (4–6 wk), grower (7–12 wk), developer (13–15 wk), prelay (15–17 wk).
Supplied per kilogram of diet: vitamin A, 9,900 IU; vitamin E, 22.10 IU; vitamin B12, 0.02 mg; biotin, 0.06 mg; menadione, 3.3 mg; thiamine, 2.20 mg; riboflavin, 6.60 mg; pantothenic acid, 11.00 mg; vitamin B6, 4.40 mg; niacin, 33.00 mg; folic acid, 0.90 mg; choline, 191.36 mg.
Supplied per kilogram of diet: Mn, 80.4 mg; Zn, 64.2 mg; Mg, 16.08 mg; Fe, 15.78 mg; Cu, 2.4 mg; I, 0.6 mg; Se, 0.24 mg.
Table 2.
Diet formulation and calculated nutrient composition for laying period (18–95 wk).1
| Ingredients, % | Peaking2 | Layer 2 | Layer 3 | Layer 4 | Layer 5 |
|---|---|---|---|---|---|
| Corn | 53.61 | 62.99 | 61.54 | 64.18 | 62.57 |
| Soybean meal -48% | 28.10 | 21.35 | 19.99 | 17.77 | 17.90 |
| Soybean oil | 3.75 | 2.90 | 3.00 | 2.87 | 3.21 |
| Limestone | 7.44 | 6.89 | 6.87 | 7.13 | 7.33 |
| Oyster shell | 3.19 | 2.95 | 2.94 | 3.06 | 3.14 |
| Defluorinated phosphate | 2.55 | 2.09 | 1.89 | 1.52 | 1.47 |
| Common salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lysine HCl | 0.46 | 0.09 | 0.04 | 0.05 | 0.04 |
| DL-Methionine | 0.33 | 0.22 | 0.17 | 0.14 | 0.14 |
| Threonine | 0.11 | 0.06 | 0.03 | 0.03 | 0.02 |
| Vitamin premix3 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix4 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Sand | 0.05 | 0.05 | 3.11 | 2.84 | 3.78 |
| ME (kcal/kg) | 2,840 | 2,900 | 2,820 | 2,840 | 2,820 |
| CP% | 19.05 | 16.15 | 15.27 | 14.42 | 14.32 |
| Ca% | 4.94 | 4.48 | 4.40 | 4.42 | 4.51 |
| Available P (%) | 0.58 | 0.49 | 0.45 | 0.38 | 0.37 |
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
Treatments were added as a form of vitamin premix in the diet: D treatment: vitamin D3 at 2,760 IU/kg; DD treatment: vitamin D3 at 5,220 IU/kg; 25D treatment: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Peaking (18–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk).
Supplied per kilogram of diet: vitamin A, 9,900 IU; vitamin E, 22.10 IU; vitamin B12, 0.02 mg; biotin, 0.06 mg; menadione, 3.3 mg; thiamine, 2.20 mg; riboflavin, 6.60 mg; pantothenic acid, 11.00 mg; vitamin B6, 4.40 mg; niacin, 33.00 mg; folic acid, 0.90 mg; choline, 191.36 mg.
Supplied per kilogram of diet: Mn, 80.4 mg; Zn, 64.2 mg; Mg, 16.08 mg; Fe, 15.78 mg; Cu, 2.4 mg; I, 0.6 mg; Se, 0.24 mg.
Water and experimental diet were provided ad libitum from 0 to 95 wk. The pullets were subjected to an intermittent lighting program during the first 7 d with 4 h of light followed by 2 h of dark period. The lighting management was customized by the Hy-Line North America lighting program from 2 to 17 wk (http://sales.hyline.com/NALighting/WebLighting.aspx). In brief, it is a slow step down of light hours from week 2 to 12 (19–12 h). During week 13 to 17, 12 h of light was set for the trial. Starting from week 18, the layers received 15.5 h of light and 8.5 h of darkness.
Performance Data Collection
Bird BW and feed consumption were recorded at the conclusion of each feeding stage: starter 1 (0–3 wk), starter 2 (4–6 wk), grower (7–12 wk), developer (13–15 wk), prelay (15–17 wk), peaking (18–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk). Egg production was recorded daily. Hen day production (HDP) was calculated at the end of each feeding stage starting from 22 wk when the layers reached peak production (more than 90%). FCR (feed intake/BW gain from 0 to 17 wk, and feed intake/dozens of eggs from 22 to 95 wk) was calculated by feeding phases.
Egg Quality
Starting from 25 wk, egg quality was evaluated every 8 wk throughout the laying period. Thirty eggs were collected from each treatment each time (3 eggs per replication). Collected eggs were stored at 4°C overnight before analysis. At measurement, egg specific gravity was determined according to the method of Holder and Bradford using different levels of salt solutions (0.065, 0.070, 0.075, 0.080, 0.085, 0.090, 0.095, and 0.100) (Holder and Bradford, 1979) Then the eggs were broken onto a flat surface where the height of the thick inner albumen was measured with a Haugh meter (Model S8400, AMES, Melrose, MA) (Um and Paik, 1999). The yolk was separated from the albumen and weighed. The shells were washed, dried in a dryer at 50°C for 2 d, and then weighed. The shell thickness measurement was noted 3 times per sample at the equator of the egg using a gauge (Model 25M-5, AMES); then, the average value was recorded. The weight of the albumen was calculated as the difference between the weight of the egg and the weight of the yolk plus shell.
Statistical Analysis
All experimental data were analyzed statistically by one-way ANOVA, using GLM procedure, with feed treatment as the main effect. All the data were analyzed by SAS software version 9.3 (SAS Institute, Cary, NC). The data were compared to each other at each sampling time point. Differences between means were determined using Duncan's Multiple Range Test. The level of significance was assessed at P ≤ 0.05.
Results
Growth Performance
No significant difference was found in growth performance during the rearing period (0–17 wk; data not shown). During the egg-laying period (18–95 wk), DD treatment had the lowest BW at 40 wk (P < 0.0001; Table 3), and the lowest feed consumption during layer 2 (38–48 wk; P < 0.0001; Table 4) among all treatments. At the layer 3 phase (49–60 wk), DD treatment had the highest feed consumption (P = 0.0005; Table 4) and similar BW compared to the other treatments (P > 0.05; Table 3). For the later period, D treatment was found to have the highest BW at 75 wk among all the treatments (P = 0.0388; Table 3).
Table 3.
Effect of 25OHD on laying hen BW during the laying period.
| Week | Guide1 (g/bird) | D (g/bird) | DD (g/bird) | 25D (g/bird) | SEM | P-value |
|---|---|---|---|---|---|---|
| 17 | 1,280 | 1,261.9 | 1,245.6 | 1,236.3 | 10.1 | 0.218 |
| 40 | 1,540 | 1,615.8A | 1,418.7B | 1,579.0A | 22.1 | <0.0001 |
| 60 | 1,560 | 1,691.8 | 1,636.8 | 1,637.2 | 21.2 | 0.129 |
| 75 | 1,560 | 1,766.1A | 1,702.9B | 1,690.9B | 20.3 | 0.039 |
| 95 | N/A | 1,755.7 | 1,699.7 | 1,661.4 | 27.7 | 0.083 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
Guide: referenced BW (average) according to Hy-Line W36 guide (2015); D: vitamin D3 at 2,760 IU/kg; DD: vitamin D3 at 5,520 IU/kg; 25D: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Table 4.
Effect of 25OHD on laying hen feed consumption during laying period.
| Feeding stages | Guide1 (g/bird/day) | D (g/bird/day) | DD (g/bird/day) | 25D (g/bird/day) | SEM | P-value |
|---|---|---|---|---|---|---|
| Peaking2 | 84 | 105.8 | 103.7 | 105.4 | 1.4 | 0.542 |
| Layer 2 | 96 | 106.0A | 95.9B | 104.9A | 1.4 | <0.0001 |
| Layer 3 | 100 | 108.6B | 113.2A | 107.9B | 0.9 | 0.001 |
| Layer 4 | 94 | 108.7 | 110.5 | 108.3 | 0.9 | 0.312 |
| Layer 5 | 93 | 106.7 | 106.7 | 106.0 | 0.9 | 0.843 |
| 22–60 wk3 | 94 | 106.6 | 104.3 | 106.0 | 1.1 | 0.346 |
| 61–90 wk | 96 | 107.6 | 108.5 | 107.1 | 0.9 | 0.519 |
| 22–95 wk | 95 | 107.0 | 105.9 | 106.4 | 0.9 | 0.696 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
Guide: calculated feed consumption (average) from Hy-Line W36 guide (2015); D: vitamin D3 at 2,760 IU/kg; DD: vitamin D3 at 5,520 IU/kg; 25D: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Peaking (22–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk).
Accumulated feed consumption during the period indicated in the column.
Egg-Laying Performance
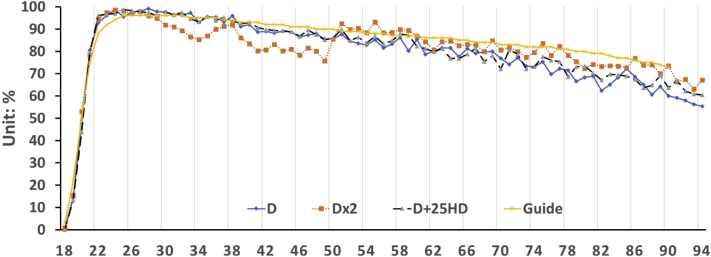
The flock reached the peak production (more than 90%) at 22 wk. The treatments did not affect either the time of initial laying or the HDP before the peaking (17–22 wk; data not shown). After the peak production, the HDP gradually declined throughout the production period (Figure 1). 25D treatment showed higher egg production during the peak (22–38 wk) and layer 2 (39–48 wk) periods compared with DD treatment (P < 0.0001; Table 5), and the highest overall production throughout the first 60 wk of age (22–60 wk; P = 0.0149; Table 5). The calculated feed conversion ratio (FCR) (feed intake/dozens of eggs) suggested that 25D treatment had the lowest FCR compared with DD in layer 2 (P = 0.0405; Table 6). However, no significant difference in overall egg production, feed intake, and FCR was observed (22–95 wk; P > 0.05).
Figure 1.
Effects of dietary supplementation of 25OHD on hen day production from 18 to 90 wk. From the optimal production data from Hy-Line W36 guide (2015). Abbreviations: D, vitamin D3 at 2,760 IU/kg; DD, vitamin D3 at 5,220 IU/kg; 25HD, vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg; 25OHD, 25-hydroxyvitamin D3.
Table 5.
Effect of 25OHD on laying hen egg production during the laying period.
| Stages | D1 (%) | DD (%) | 25D (%) | SEM | P-value |
|---|---|---|---|---|---|
| Peaking2 | 96.28A | 92.85B | 96.36A | 0.49 | <0.0001 |
| Layer 2 | 89.49A | 82.40B | 90.06A | 0.79 | <0.0001 |
| Layer 3 | 84.28 | 88.27 | 85.65 | 1.24 | 0.099 |
| Layer 4 | 77.95 | 82 | 77.61 | 1.67 | 0.156 |
| Layer 5 | 64.58 | 70.83 | 66.82 | 2.76 | 0.304 |
| 22–60 wk3 | 90.67A,B | 88.53B | 91.29A | 0.64 | 0.015 |
| 61–90 wk | 70.31 | 75.62 | 71.44 | 2.22 | 0.240 |
| 22–95 wk | 81.04 | 82.41 | 81.9 | 1.22 | 0.740 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
D: vitamin D3 at 2,760 IU/kg; DD: vitamin D3 at 5,520 IU/kg; 25D: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Peaking (22–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk).
Accumulated egg production during the period indicated in the columns.
Table 6.
Effect of 25OHD on laying hen feed conversion ratio during the laying period.
| Stages | D1 (g/dozen eggs) | DD (g/dozen eggs) | 25D (g/dozen eggs) | SEM | P-value |
|---|---|---|---|---|---|
| Peaking3 | 1.32 | 1.35 | 1.34 | 0.02 | 0.676 |
| Layer 2 | 1.34A,B | 1.40A | 1.33B | 0.02 | 0.041 |
| Layer 3 | 1.60 | 1.61 | 1.56 | 0.03 | 0.310 |
| Layer 4 | 1.69 | 1.6 | 1.66 | 0.04 | 0.276 |
| Layer 5 | 2.01 | 1.79 | 1.82 | 0.12 | 0.426 |
| 22–60 wk2 | 1.41 | 1.43 | 1.39 | 0.02 | 0.359 |
| 61–90 wk | 1.83 | 1.67 | 1.75 | 0.06 | 0.226 |
| 22–95 wk | 1.54 | 1.51 | 1.51 | 0.03 | 0.648 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviation: 25OHD, 25-hydroxyvitamin D3.
D: vitamin D3 at 2,760 IU/kg; DD: vitamin D3 at 5,220 IU/kg; 25D: vitamin D3 at 2,760 IU/kg plus 25OHD at 2,760 IU (69 μg)/kg.
Accumulated egg production during the period indicated in the columns.
Peaking (22–38 wk), layer 2 (39–48 wk), layer 3 (49–60 wk), layer 4 (61–75 wk), and layer 5 (76–95 wk).
Egg Quality
At 25 and 33 wk, both DD and 25D treatments had higher Haugh unit compared with D (P < 0.05; Tables 7 and 8). At 41 wk, the DD group produced smaller eggs (P = 0.009), with a lower yolk weight (P < 0.0001) but higher specific gravity (P = 0.007) and shell thickness (P = 0.042) compared with the other treatments or D alone (Table 9). After 41 wk, no difference in egg quality was observed until the end of the study (95 wk; data not shown).
Table 7.
Effect of 25OHD on laying hen egg quality at 25 wk.
| Treatment | EW (g) | YW (g) | SW (g) | AW (g) | HU (n/a) | SG (n/a) | ST (0.01 mm) |
|---|---|---|---|---|---|---|---|
| T1 | 54.0 | 13.1 | 5.2 | 35.7 | 100.7B | 1.09 | 37.3 |
| T2 | 53.6 | 13.0 | 5.1 | 35.4 | 102.7A | 1.09 | 37.1 |
| T3 | 54.1 | 13.1 | 5.2 | 35.7 | 102.5A | 1.09 | 37.3 |
| SEM | 0.6 | 0.2 | 0.1 | 0.4 | 0.5 | 0.01 | 0.3 |
| P-value | 0.793 | 0.888 | 0.526 | 0.860 | 0.014 | 0.722 | 0.925 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviations: AW, albumen weight; EW, egg weight; HU, Haugh unit; 25OHD, 25-hydroxyvitamin D3; n/a, no unit; SG, specific gravity; ST, shell thickness; SW, shell weight; YW, yolk weight.
Table 8.
Effect of 25OHD on laying hen egg quality at 33 wk.
| Treatment | EW (g) | YW (g) | SW (g) | AW (g) | HU (n/a) | SG (n/a) | ST (0.01 mm) |
|---|---|---|---|---|---|---|---|
| T1 | 57.4 | 14.7 | 5.4 | 37.3 | 95.4B | 1.09 | 37.9 |
| T2 | 55.8 | 14.0 | 5.4 | 36.4 | 98.7A | 1.09 | 38.7 |
| T3 | 56.4 | 14.6 | 5.3 | 36.5 | 98.2A | 1.09 | 37.7 |
| SEM | 0.8 | 0.2 | 0.1 | 0.6 | 0.7 | 0.01 | 0.3 |
| P-value | 0.348 | 0.116 | 0.540 | 0.459 | 0.005 | 0.054 | 0.134 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviations: AW, albumen weight; EW, egg weight; HU, Haugh unit; 25OHD, 25-hydroxyvitamin D3; n/a, no unit; SG, specific gravity; ST, shell thickness; SW, shell weight; YW, yolk weight.
Table 9.
Effect of 25OHD on laying hen egg quality at 41 wk.
| Treatment | EW (g) | YW (g) | SW (g) | AW (g) | HU (n/a) | SG (n/a) | ST (0.01 mm) |
|---|---|---|---|---|---|---|---|
| T1 | 59.3A | 16.6A | 5.5 | 37.2 | 92.2 | 1.08B | 37.5B |
| T2 | 56.3B | 14.7B | 5.4 | 36.3 | 95.7 | 1.09A | 39.3A |
| T3 | 60.2A | 16.6A | 5.5 | 38.0 | 94.0 | 1.08B | 37.9A,B |
| SEM | 0.7 | 0.2 | 0.1 | 0.5 | 1.0 | 0.01 | 0.5 |
| P value | 0.001 | <0.0001 | 0.745 | 0.084 | 0.050 | 0.001 | 0.042 |
A,BMeans within a column with different superscripts are significantly different (P < 0.05).
Abbreviations: AW, albumen weight; EW, egg weight; HU, Haugh unit; 25OHD, 25-hydroxyvitamin D3; n/a, no unit; SG, specific gravity; ST, shell thickness; SW, shell weight; YW, yolk weight.
Discussion
The hen's BW and feed consumption were affected by the treatments in the present study. DD treatment had lower feed consumption during the layer 2 phase (28–48 wk), which was associated with a lower BW at 40 wk. Consequently, lower egg production during this phase was observed compared with the other treatments. As a result of the lower BW, DD treatment also led to smaller eggs, lower egg parts weight, and denser eggshell (higher thickness). This is in agreement with previous research that the BW of hens significantly affects egg quality including egg weight (Summers and Leeson, 1983). However, during the layer 3 phase (49–60 wk), the feed consumption improved by DD treatment. At the same time, the BW decreased (P > 0.05). Subsequently, a trend of higher HDP compared with the other 2 treatments was observed during this period (P < 0.1; data not shown). The changes of feed intake in DD treatment also impacted the actual vitamin D3 intake in the current experiment. The vitamin D3 intake of DD treatment was modified by −55.72 IU/bird (−1%) and +25.4 IU/bird (+0.5%) compared to D treatment, respectively. However, this subtle change may not be the primary factor that affected BW and egg production. The reasons for such a fluctuation in BW, feed intake, and egg production were unclear. One study showed that birds fed vitamin D3 up to 15,000 IU/kg had no adverse effects on growth performance or egg production in 20 to 68 wk Lohmann LSL white laying hens (Mattila et al., 2004). Similarly, a short-period (30 d) dietary treatment of up to 20,000 IU on 87-week-old ISA brown molted laying hens had no effect on laying and growth performance (Park et al., 2005). A more recent study raised the non-toxic level of vitamin D3 to 102,200 IU/kg in 19- to 58-week-old Hy-Line W36 laying hens (Persia et al., 2013). However, it was reported that long-term supplementation of vitamin D3 at 68,348 IU (0–68 wk) had negative effects on growth performance and egg production (Wen et al., 2019). Increasing vitamin D3 dosage to 200,000 IU/kg, even for a shorter period (16 wk), showed a decrease of egg weight, eggshell quality, and feed consumption (Ameenuddin et al., 1986). However, based on the current knowledge, the modern laying hen has considerable tolerance on a high level of dietary vitamin D3, and the layer performance should not be affected by the dosage we applied in this study (5,220 IU/kg). Other factors such as environmental management, treatment distribution, and outbreak of diseases were also carefully examined, but none of the factors were associated with the current results.
Furthermore, D treatment had the highest BW at 75 wk. Previous studies showed that vitamin D3 influences fat and muscle development (Ceglia, 2009; Ding et al., 2012). Moreover, during the entire laying period, the overall growth performance showed no difference among the treatments by the end of the study.
In respect to egg production, no difference was observed around the onset of laying (18 wk) and the time of reaching peak production (22 wk). However, supplementation of 25OHD improved the overall HDP and FCR (feed intake/dozen of eggs) from 22 to 60 wk in the current study.
No effect was observed during the later period (60–95 wk), which concurs with the findings of Silva (2017). The laying hens were fed with 69 μg/kg of 25OHD + 3,000 IU/kg of vitamin D3 from 0 to 50 wk (25OHD was removed from the diet after 50 wk). Increases in FCR during the period of 18 to 34 wk and egg production during the period of 18 to 50 wk were observed. However, no difference in cumulative egg production was observed overall (18–87 wk) (Silva, 2017).
The lack of the effects of 25OHD on egg production in older hens may be due to the conversion efficiency in the kidney, which was inadequate to convert 25OHD to 1,25OHD during the late laying stage (Abe et al., 1982). In contrast to our findings, a number of other studies failed to find any beneficial effects on egg production in laying hens or broiler breeders (Koreleski and Świątkiewicz, 2005; Torres et al., 2009; Käppeli et al., 2011; Mattila et al., 2011; Adhikari et al., 2020). However, in these studies, 25OHD was not included in the diets during the rearing period or was supplemented during certain laying periods, instead of the entire laying period. On the contrary, other studies showed beneficial effects on egg production/quality with long-term (at least 30 wk feeding) or early (rearing or early laying stage) supplementation of 25OHD (Koreleski and Świątkiewicz, 2005; Silva, 2017), indicating that the duration and timing of supplementation of 25OHD in layer diets are critical.
For egg quality in the present study, the beneficial effects were mainly observed during the early stage. Results showed that both DD and 25D increased the Haugh unit at 25 and 33 wk compared with D treatment. However, the eggs from all the treatments fell in the category of AA eggs (100-72) according to the United States egg grades. The reason why the Haugh unit was increased by 25D is unclear and needs further investigation. A murine study indicated that vitamin D3 could enhance protein synthesis rates (Salles et al., 2013). Such a function may contribute to albumen formation in hens and lead to a higher Haugh unit. However, this hypothesis needs further study. It has been reported that the proportion of yolk tended to be smaller in small eggs (Şekeroǧlu and Altuntaş, 2009). This agrees with our observation of a lower yolk weight observed in the present study.
In agreement with our findings, a number of previous studies have reported that dietary 25OHD had no effects on eggshell quality (Keshavarz, 2003; Käppeli et al., 2011; Mattila et al., 2011; Nascimento et al., 2014). In contrast to our findings, Torres et al. (2009) concluded that the supplementation of 25OHD (35 or 69 μg/kg, 32–67 wk) resulted in better eggshells evaluated by specific gravity at 60 wk of age. Furthermore, replacing 25% of vitamin D3 with 25OHD (9.35 μg/kg, 26–70 wk) was reported to improve shell quality (Koreleski and Świątkiewicz, 2005). Long-term supplementation of 25OHD (69 μg/kg, 0–50 wk) during the rearing and early laying period increased the shell thickness (Silva, 2017). In these studies, the beneficial effects mainly occurred with long-term (at least 30 wk feeding) or early (rearing or early laying stage) supplementation of 25OHD. However, in the current study, we failed to detect the effects of long-term supplementation of 25OHD on eggshell quality. The role of vitamin D3 on shell formation has not been fully understood. Lack of vitamin D3 effects on eggshell quality may be due to eggshell gland Ca2+ transportation-related protein such as calbindin D28k and carbonic anhydrases, which are probably not vitamin D3 dependent (Bar, 2008). Furthermore, the reason behind our results may be attributed to the calcium level in the control diet, adequate to maintain egg quality; thus, additional vitamin D3 did not further improve eggshell quality as shown in earlier literature (Bar, 2008; Plaimast et al., 2015).
Previous research has shown that the circulating 25OHD level was lower in laying hens at 60 wk than 50 wk, which indicated the liver's functional defect due to age resulting in a limited production of active vitamin D3 metabolites (Harms et al., 1985). Supplementation of 25OHD could be an alternative strategy to avoid vitamin D shortage in old laying hens. Seldom research has been done regarding the effects of 25OHD on extending the laying period. However, improvement of bone health in laying hens by dietary supplementation of 25OHD may contribute to the prolonged laying period (Silva, 2017). This is due to the close relationship between bone and egg in egg-laying birds (Bar, 2008). However, the application of 25OHD in laying hens for increasing the egg producing period needs further research.
In summary, the long-term supplementation of 25OHD increased egg production during the early stage (22–60 wk). However, no beneficial effect was observed during the late laying stage. This may be because the calcium in this study was adequate for maintaining laying performance. The BW and feed consumption fluctuation may be not a result from the effects of vitamin D3 in layer diets directly. However, it showed an interesting relationship with egg production and quality. As 25OHD becomes commercially available in the industry at a cost-effective price, long-term and early supplementation of 25OHD in pullets and laying hens could be a potential strategy to improve egg-laying performance in laying hens.
Acknowledgments
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Abe E., Horikawa H., Masumura T., Sugahara M., Kubota M., Suda T. Disorders of cholecalciferol metabolism in old egg-laying hens. J. Nutr. 1982;112:436–446. doi: 10.1093/jn/112.3.436. [DOI] [PubMed] [Google Scholar]
- Adhikari R., White D., House J., Kim W. Effects of additional dosage of vitamin D3, vitamin D2, and 25-hydroxyvitamin D3 on calcium and phosphorus utilization, egg quality and bone mineralization in laying hens. Poult. Sci. 2020;99:364–373. doi: 10.3382/ps/pez502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameenuddin S., Sunde M., DeLuca H., Cook M. Excessive cholecalciferol in a layers diet: Decline in some aspects of reproductive performance and increased bone mineralisation of progeny. Br. Poult. Sci. 1986;27:671–677. doi: 10.1080/00071668608416926. [DOI] [PubMed] [Google Scholar]
- Atencio A., Edwards H.M., Jr., Pesti G.M. Effect of the level of cholecalciferol supplementation of broiler breeder hen diets on the performance and bone abnormalities of the progeny fed diets containing various levels of calcium or 25-hydroxycholecalciferol. Poult. Sci. 2005;84:1593–1603. doi: 10.1093/ps/84.10.1593. [DOI] [PubMed] [Google Scholar]
- Bar A. Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2008;151:477–490. doi: 10.1016/j.cbpa.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Bar A., Striem S., Rosenberg J., Hurwitz S. Egg shell quality and cholecalciferol metabolism in aged laying hens. J. Nutr. 1988;118:1018–1023. doi: 10.1093/jn/118.8.1018. [DOI] [PubMed] [Google Scholar]
- Ceglia L. Vitamin D and its role in skeletal muscle. Curr. Opinion Clinical Nutrition Metabolic Care. 2009;12:628. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Hoefs S., Van Der Kemp A., Topala C., Bindels R., Hoenderop J. The ß-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science (New York, N.Y.) 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Christakos S., Ajibade D.V., Dhawan P., Fechner A.J., Mady L.J. Vitamin D: metabolism. Endocrinol. Metab. Clin. North. Am. 2010;39:243–253. doi: 10.1016/j.ecl.2010.02.002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C., Gao D., Wilding J., Trayhurn P., Bing C. Vitamin D signalling in adipose tissue. Br. J. Nutr. 2012;108:1915–1923. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- Frost T., Roland Sr D., Untawale G. Influence of vitamin D3, 1α-Hydroxyvitamin D3, and 1, 25-Dihydroxyvitamin D3 on eggshell quality, tibia strength, and various production parameters in commercial laying hens. Poult. Sci. 1990;69:2008–2016. doi: 10.3382/ps.0692008. [DOI] [PubMed] [Google Scholar]
- Harms R.H., Junqueira O.M., Miles R.D. Plasma calcium, phosphorus, 25-dihydroxyvitamin D3, and 1-25-dihydroxyvitamin D3 of hens with fatty liver syndrome. Poult. Sci. 1985;64:768–770. doi: 10.3382/ps.0640768. [DOI] [PubMed] [Google Scholar]
- Holder D.P., Bradford M.V. Relationship of specific gravity of chicken eggs to number of Cracked eggs observed and Percent Shell1. Poult. Sci. 1979;58:250–251. [Google Scholar]
- Holick M.F., MacLaughlin J., Clark M., Holick S., Potts J., Anderson R., Blank I., Parrish J., Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- Käppeli S., Fröhlich E., Gebhardt-Henrich S.G., Pfulg A., Schäublin H., Zweifel R., Wiedmer H., Stoffel M.H. Effects of dietary supplementation with synthetic vitamin D3 and 25-hydroxycholecalciferol on blood calcium and phosphate levels and performance in laying hens. Archiv. Für. Geflügelkunde. 2011;75:179–184. [Google Scholar]
- Keshavarz K. A comparison between cholecalciferol and 25-OH-cholecalciferol on performance and eggshell quality of hens fed different levels of calcium and phosphorus. Poult. Sci. 2003;82:1415–1422. doi: 10.1093/ps/82.9.1415. [DOI] [PubMed] [Google Scholar]
- Koreleski J., Świątkiewicz S. Efficacy of different levels of a cholecalciferol 25-OH-derivative in diets with two limestone forms in laying hen nutrition. J. Anim. Feed Sci. 2005;14:305–315. [Google Scholar]
- Mattila P., Valaja J., Rossow L., Venäläinen E., Tupasela T. Effect of vitamin D2-and D3-enriched diets on egg vitamin D content, production, and bird condition during an entire production period. Poult. Sci. 2004;83:433–440. doi: 10.1093/ps/83.3.433. [DOI] [PubMed] [Google Scholar]
- Mattila P.H., Valkonen E., Valaja J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs and chicken meat. J. Agric. Food Chem. 2011;59:8298–8303. doi: 10.1021/jf2012634. [DOI] [PubMed] [Google Scholar]
- Nascimento G.R.d., Murakami A.E., Guerra A., Ospinas-Rojas I.C., Ferreira M.F.Z., Fanhani J.C. Effect of different vitamin D sources and calcium levels in the diet of layers in the second laying cycle. Rev. Bras. Cienc. Avic. 2014;16:37–42. [Google Scholar]
- Park S., Namkung H., Ahn H., Paik I. Enrichment of vitamins D3, K and iron in eggs of laying hens. Asian-aust. J. Anim. Sci. 2005;18:226–229. [Google Scholar]
- Persia M.E., Higgins M., Wang T., Trample D., Bobeck E.A. Effects of long-term supplementation of laying hens with high concentrations of cholecalciferol on performance and egg quality. Poult. Sci. 2013;92:2930–2937. doi: 10.3382/ps.2013-03243. [DOI] [PubMed] [Google Scholar]
- Plaimast H., Kijparkorn S., Ittitanawong P. Effects of vitamin D3 and calcium on productive performance, egg quality and vitamin D3 content in egg of second production cycle hens. Thai. J. Vet. Med. 2015;45:189–195. [Google Scholar]
- Rodriguez-Lecompte J.C., Yitbarek A., Cuperus T., Echeverry H., van Dijk A. The immunomodulatory effect of vitamin D in chickens is dose-dependent and influenced by calcium and phosphorus levels. Poult. Sci. 2016;95:2547–2556. doi: 10.3382/ps/pew186. [DOI] [PubMed] [Google Scholar]
- Roland S.D.A., Harms R.H. The lack of Response of 25-Hydroxy-vitamin D3 on egg shell quality or other Criteria in laying Hens1. Poult. Sci. 1976;55:1983–1985. [Google Scholar]
- Salles J., Chanet A., Giraudet C., Patrac V., Pierre P., Jourdan M., Luiking Y.C., Verlaan S., Migné C., Boirie Y. 1, 25 (OH) 2-vitamin D 3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through A kt/PKB and m TOR mediated pathways in murine C 2 C 12 skeletal myotubes. Mol. Nutr. Food Res. 2013;57:2137–2146. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- Şekeroǧlu A., Altuntaş E. Effects of egg weight on egg quality characteristics. J. Sci. Food Agric. 2009;89:379–383. [Google Scholar]
- Silva F.A. University of Alberta; Edmonton, Canada: 2017. Effects of Dietary 25-hydroxycholecalciferol on Growth, Production Performance, Eggshell Quality and Bone Traits of Brown Egg Layers Housed under Commercial Conditions. [Google Scholar]
- Soares J., Jr., Kerr J., Gray R. 25-hydroxycholecalciferol in poultry nutrition. Poult. Sci. 1995;74:1919–1934. doi: 10.3382/ps.0741919. [DOI] [PubMed] [Google Scholar]
- Summers J.D., Leeson S. Factors influencing early egg size. Poult. Sci. 1983;62:1155–1159. [Google Scholar]
- ŚWiĄTkiewicz S., Arczewska-WŁOsek A., Bederska-Lojewska D., JÓZefiak D. Efficacy of dietary vitamin D and its metabolites in poultry - review and implications of the recent studies. Worlds. Poult. Sci. 2017;73:57–68. [Google Scholar]
- Torres C.A., Vieira S.L., Reis R.N., Ferreira A.K., Silva P.X.d., Furtado F.V.F. Productive performance of broiler breeder hens fed 25-hydroxycholecalciferol. R. Bras. Zootec. 2009;38:1286–1290. [Google Scholar]
- Um J., Paik I. Effects of microbial phytase supplementation on egg production, eggshell quality, and mineral retention of laying hens fed different levels of phosphorus. Poult. Sci. 1999;78:75–79. doi: 10.1093/ps/78.1.75. [DOI] [PubMed] [Google Scholar]
- Wen J., Livingston K., Persia M. Effect of high concentrations of dietary vitamin D3 on pullet and laying hen performance, skeleton health, eggshell quality, and yolk vitamin D3 content when fed to W36 laying hens from day of hatch until 68 wk of age. Poult. Sci. 2019;98:6713–6720. doi: 10.3382/ps/pez386. [DOI] [PMC free article] [PubMed] [Google Scholar]