Abstract
Introduction
Sirtuin1 (SIRT1), one of NAD+-dependent protein deacetylases, is proved to be neuroprotective in aging diseases, but its effect on neuronal apoptosis has not been clarified. To investigate the role of SIRT1 in inhibiting neuronal apoptosis, SIRT1 was interfered or overexpressed in cortical neurons.
Methods
We exerted overloading laminar shear stress with 10 dyn/cm2 for 4, 8, and 12 h on neurons to cause cortical neuronal apoptosis, and the apoptosis percentage was tested by TUNEL assay. The adenovirus plasmids containing SIRT1 RNA interference or SIRT1 wild type gene were transfected into neurons before shear stress loading. SIRT1 mRNA and protein level were tested by Real-time PCR, immunofluorescence and western blots assay.
Results
SIRT1 was primarily expressed in nucleus of cortical neurons, and its mRNA level was significantly increased after 4 h stimulation. SIRT1 RNAi cortical neurons had higher TUNEL positive cells, while SIRT1 overexpression significantly decreased the percentage of died cells induced by shear stress compared to control group.
Conclusions
SIRT1 plays a neuroprotective role in shear stress induced apoptosis and could be as potential pharmacological targets against neuronal degeneration in future.
Keywords: SIRT1, Cortical neurons, Apoptosis, Shear stress, Neuroprotective effect
Introduction
Sirtuins, a family of nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, are involved in energy metabolism and life span.15,18,35 Among the known Sirt isoforms (SIRT1-7), SIRT1, the mammalian homolog of yeast silent information regulator-2 (Sir-2), has attracted much attention for its potential role in regulating cellular functions, such as transcriptional silencing, apoptosis, aging, stress tolerance, and metabolism.14,34,42,45 In the process of SIRT1-induced deacetylation, NAD+ is not only an activator, but also the recipient of the acetyl. One molecule of NAD+ and one molecule of acetyl-lysine are readily catalyzed to one molecule of deacetylated lysine, nicotinamide, and 1-O-acetyl-ADP-ribose.47 SIRT1 was expressed at high levels in the heart, brain, spinal cord, and dorsal root ganglia in mouse embryos.40,41 High SIRT1 levels in the embryonic brain suggest that it might have a role in neuronal/brain development. Recent studies showed that SIRT1 is a NAD+-dependent class III protein deacetylase that can participate in multiple deacetylation reactions in cells, thereby inducing a variety of cellular effects. In the field of induced pluripotent stem cells, Sirt1 can deacetylate Sox2 and increase the efficiency of cell reprogramming.37 In cardiomyocytes, it can deacetylate Nkx2.5 and inhibit the transcriptional activity of Nkx2.5, thereby affecting the size and shape of the heart during development.46 Abdominal aortic aneurysms (AAA) are often accompanied by a decrease in SIRT1 and an increase in angiotensin II. Studies have shown that SIRT1 can affect the secretion of inflammatory molecules by controlling the polarization of macrophages (up-regulate M2 and down-regulate M1) and inhibit blood vessels Angiotensin II-induced AAA formation.5,56 In addition, caloric restriction (CR) can prevent the formation of AAA in mice, and the lack of SIRT1 will cause this effect to disappear.30 In pulmonary artery smooth muscle cells (PASMC), platelet-derived growth factor-BB (PDGF-BB) can inhibit pulmonary arterial hypertension (PAH) caused by excessive proliferation of PASMC. During this process, increased expression of SIRT1 and changes in cyclin was detected.57
High SIRT1 levels in the embryonic brain suggest that it might have a role in neuronal/brain development. Previous studies indicated that SIRT1 and NRF2 (Nuclear factor (erythroid-derived 2)-like 2) stimulate anti-inflammatory mechanisms in preclinical models of neurodegenerative disease, but SIRT1 and NRF2-mediated neuroprotection was reported in the context of multiple sclerosis pathogenesis and optic neuropathies.33,39 SIRT1 regulated the proliferation and differentiation of neural precursor cells through Notch1signaling pathway.11,15,17 Recent studies implied that SIRT1 exerts a role in neuroprotection. Upregulation of SIRT1 can protect neurons against neurodegeneration and neurotoxic insults in Alzheimer’s disease and amyotrophic lateral sclerosis.18,24 SIRT1 has been reported in many neurodegenerative diseases, it promotes non amyloidogenic APP-processing pathway to prevent Alzheimer disease, it also induces transcription of molecular chaperones to prevent Parkinson’s disease.16 Julien et al.21 declared that SIRT1 may regulate the aging and metabolic processed in Alzheimer’s disease and the loss of SIRT1 is closely associated with the Aβaccumulation and disease progression. Resveratrol, a known SIRT1 activator, which induced SIRT1 expression rescues the neuronal dysfunction against polyglutamines (polyQ) toxicity in Huntington’s disease.1,43 More recently, in a model of Wallerian degeneration, resveratrol protects neurons against degeneration by activating SIRT1.3 Thus, increased nuclear NAD+ biosynthesis and SIRT1 activation are important to prevent against neuronal degeneration, but the molecular mechanism of SIRT1 role on in mechanical-induced nerve injury is rarely reported.
During head injury, high intracranial pressure associated with impact has been predicted to lead to the generation of regions of high shear stress (up to 140 dyn/cm2) and tissue strain.26,27 Previous study described a newly-established micromechanical system, which represents a novel means to evaluate the effects of compression and shear stress on different subcellular compartments of neurons, different from other in vitro systems that mainly focus on the effect of stretching.10,12,44 Diffuse axonal injury (DAI) is suggested to result from the initial increase in the membrane permeability caused by the mechanical forces acting on the axons. Permeability increases disturb ion balance and leads to cytoskeletal disruption resulting in the impairment of axonal transport. Kilinc’s study induced fluid shear stress injury (FSSI) on cultured primary chick forebrain neurons and characterized the resulting structural and morphological changes.23,31 The previous studies suggested that fluid shear stress could be used to model neural injury in vitro.2,9,22,23 Mechanical strain magnitude affects the time of neuronal death, strain rate influences the path morphology and extent of population injury.10 Cellular injury is not initiated through localized deformation of the cytoskeleton but rather driven by excess strain on the entire cell.2,23,25 In previous report, shear stress at low magnitude 5–40 dyn/cm2 for 1–24 h can well be used to establish the nerve injury model.31 And our own previous studies showed 10 dyn/cm2 shear stress could cause cortical neuron injury and result in apoptosis.29
Currently, due to the cellular metabolic networks after nerve injury are not known very well, less neuronal protection of nerve injury are used in clinic. So it is important to find a key molecular, which can activate endogenous survival signaling and repair mechanism, to develop a critical strategy for nerve injury therapy. Since the previous studies showed that SIRT1 was involved in regulating neuronal disease49 and it could response to shear stress.4,51,54 Thus, we hypothesized that SIRT1 could protect against shear stress induced apoptosis in primary cultured rat cortical neurons. To test this hypothesis, we employ 10 dyn/cm2 shear stress to mimic nerve injury. Based on this model, we analyzed the relationship of SIRT1 and neuron apoptosis to explore SIRT1’s neuroprotective effect. If SIRT1 can be shown to inhibit shear stress-induced apoptosis, it may be useful in the development of a new treatment strategy for nerve injury.
Materials and Methods
Isolation and Cell Culture of Rat Cortical Neurons
The rat cortical neurons were cultured according to our previous methods.29 Primary cultures of rat cortical neurons were prepared from 1 day-old newborn SD rats. Isolated rat cortical tissue were treated with 0.25% trypsin at 37 °C for 20 min, dispersed cells were diluted to a concentration of 5 × 105 cells/ml on poly-l-lysine-precoated glass slides. Cultures were maintained in modified Eagles’ medium, 10% newborn bovine serum, 5% d(+)-glucose, 50 IU/mL penicillin and 0.05 mg/mL streptomycin at 37 °C with 95% air and 5% CO2 humid incubator. On the second or third day, 4 μg/mL cytosine arabinoside was added to the medium to suppress the proliferation of glia cells. The medium was renewed every 3 days during the culturing period. The glass slides were used for experiment 5–7 days after the primary culture.
Shear Stress Stimulation
We used a parallel-plate flow chamber to exert the shear stress on cultured cortical neurons as described in our previous study.19 After 5–7 days culture, cells were exposed to a steady shear flow that was introduced by a parallel plate flow chamber. The pH was kept by the flow system with 95% air and 5% CO2, and the temperature was maintained at 37 °C. Cells were monitored during and after the stimulation with 10 dyn/cm2 for 4, 8, and 12 h. Control groups (marked with 0 h) with static flow were concurrently performed. Cells were cultured on glass slides which were placed in a dish, and the glass slides were placed in the flow chamber during the experiment. When cultured in the dish and placed in the chamber, all the cells were immersed in DMEM so that a standard culture condition was guaranteed.
MTT Assay and TUNEL Assay
After shear stress stimulation, the viability of rat cortical neurons was measured using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-[2H]-tetrazolium bromide (MTT) assay. The cortical neurons death was determined by TUNEL assay. TUNEL staining was performed using the in situ cell death detection kit II as described by the manufacturer (Roche, Canada), and 4,6-diamidino-2-phenylindole dihydrochloride (DAPI, 1:5000) was used to stain nuclei of the cultured cortical neurons. Fluorescence signal of TUNEL assay was recorded by a fluorescence microscope (IX71, Olympus, Japan). The percentage of cell death was determined by the ratio of the number of TUNEL-positive cells over the total of 100 cells in one count. The average of five counts was calculated as the percentage of neuronal cell death in a certain treatment.
Immunofluorescence
Rat cortical neurons were laid on the cover slips, and then fixed with 4% paraformaldehyde at room temperature for 15 min. Subsequently, cells were washed three times in phosphate buffered saline (PBS) and then permeabilized in PBS containing 0.1% Triton X-100 for 5 min. After that, cells were blocked with 3% albumin from bovine serum (BSA)/PBS followed by primary antibody incubation at 4 °C overnight. The cover slips were washed three times in PBS, and then incubated in the presence of FITC-conjugated secondary antibody for a further 60 min. Cover slips were washed again in PBS, incubated in 1 mg/mL DAPI for 5 min and then mounted on slides with a 90% glycerol-PBS (pH 7.5)-based medium containing 1 mg/mL paraphenylenediamine and sealed with nail polish. Immunostaining signal of cell preparations was recorded by a fluorescence microscope (IX71, Olympus, Japan).
Plasmids and Adenovirus
The adenovirus plasmids containing SIRT1 wild type gene or SIRT1 RNAi (5′-GATGAAGTTGACCTCCTCA-3′) were preserved in our lab. Adenovirus plasmids were transfected into HEK293 cells to generate infectious adenovirus. Amplification was conducted for three times to obtain enough active adenovirus, which was then used to transfect rat cortical neurons at MOI 100. To evaluate the potential neuroprotective effects of SIRT1, rat cortical neurons were transfected with adenovirus for 48 h and then exposed to shear stress for 4, 8, and 12 h respectively. The empty vector was fused with GFP to determine if the vector could be transferred into neurons successfully.
RNA Extraction and Semi-quantitative RT-PCR
The RNA extraction and semi-quantitative reverse transcription (RT)-PCR protocol were performed according to our previous study. Sequences of primers for the experiments were shown in Table 1. The program showed in Table 2 was completed in Eppendorf Mastercycler (Eppendorf, Hamburg, Germany)
Table 1.
Lists of primer sequences.
| Primer name | Sequence (5′–3′) |
|---|---|
| Rat SIRT1 |
Sence-CAGAGCATCACACGCAAGC Antisence-CAGGAAACAGAAACCCCAGC |
| Rat GAPDH |
Sence-TGTTCCTACCCCCAATGTATCCG Antisence-TGCTTCACCACCTTCTTGATGTCAT |
Table 2.
PCR program.
| Step | Condition |
|---|---|
| 1 | 94 °C, 5 min |
| 2 | 94 °C, 30 s |
| 3 | 58 °C, 30 s |
| 4 | 72 °C, 10 s |
| 5 | Back to step 2 for 35 cycles |
| 6 | 72 °C, 10 min |
Protein Extraction and Western Blotting Analysis
Protein samples were collected from the cultured neurons after treatment and quantified the concentrations by bicinchoninic acid (BCA) kit (Pierce, Rockford, IL). Then all samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and western blots assay according to our previous methods. Transferred Poly(vinylidene fluoride) (PVDF) membranes were incubated with rabbit anti-SIRT1 antibody (1:500, Abcam, USA), and rabbit anti-GAPDH antibody (1:1000, Santa Cruz, CA) at 4 °C overnight. Horseradish peroxidase-conjugated secondary antibodies (1:3000) were incubated for 1.5 h. Finally, captured band images were detected under Universal Hood II (Bio-Rad, USA) system. The results were normalized to corresponding GAPDH bands.
Statistical Analysis
All data were expressed as means ± standard deviation (SD). A one-way analysis of variance (ANOVA) and L.S.D. test were performed with SPSS software (Version 11.5). Difference was considered to be statistically significant different when p < 0.05.
Results
Effects of Fluid Shear Stress (10 dyn/cm2,4, 8, 12 h) on Neuronal Apoptosis
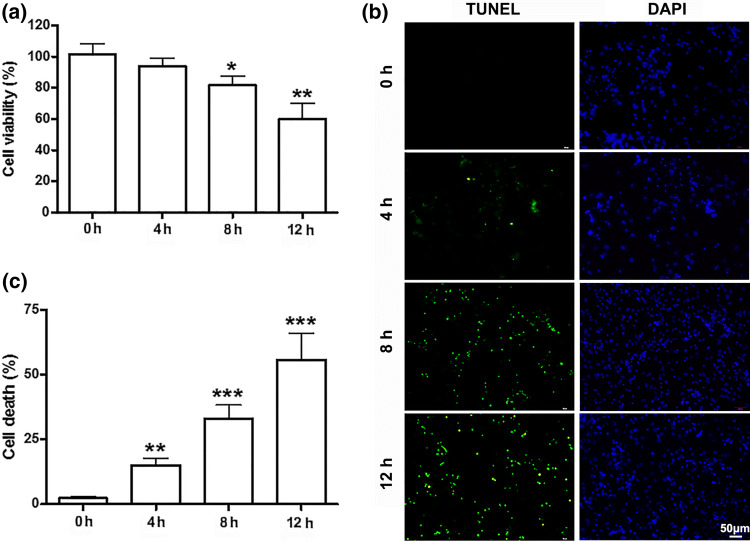
To explore the effect of fluid shear stress on cell viability, the primary cultured cortical neurons were exposed to 10 dyn/cm2 of fluid shear stress for 4, 8 and 12 h, respectively. Cortical neurons cultured in static conditions were used as control. Figure 1a showed the relative cell viability after being exposed to fluid shear stress. Compared to control group, the viability of cortical neurons decreased significantly (n = 6, p < 0.05) as fluid shear stress time increased (Fig. 1a).
Figure 1.
Effects of fluid shear stress on viability of cortical neurons. (a) The viability of cortical neurons decreased significantly after shear stress of 10 dyn/cm2 for 4, 8 and 12 h. (b) Photomicrographs and (c) histograms show that as shear stress time increased (from 4, 8, to 12 h) TUNEL positive cells increased significantly by TUNEL assay.* p < 0.05, **p < 0.01 and ***p < 0.001 were considered to be statistically significant.
To further confirm the mechanical damage induced by fluid shear stress in primary cultured cortical neurons, TUNEL assay was used. It was found that the number of died cells increased significantly after treatment of 10 dyn/cm2 fluid shear stress compared to control group (Figs. 1b and 1c). These results are consistent with our previous study19 and demonstrated that 10 dyn/cm2 fluid shear stress may induce neuronal damage in primary cultured cortical neurons. Although a large number of cells died, the culture was still about 60% viable after exposed to 10 dyn/cm2 fluid shear stress for 12 h (n = 6, t = 5.15, p < 0.001). Therefore, it can be considered a good research model of mechanical damage under this condition.
Effects of Fluid Shear Stress (10 dyn/cm2, 4, 8, 12 h) on SIRT1 mRNA Expression in Cortical Neurons
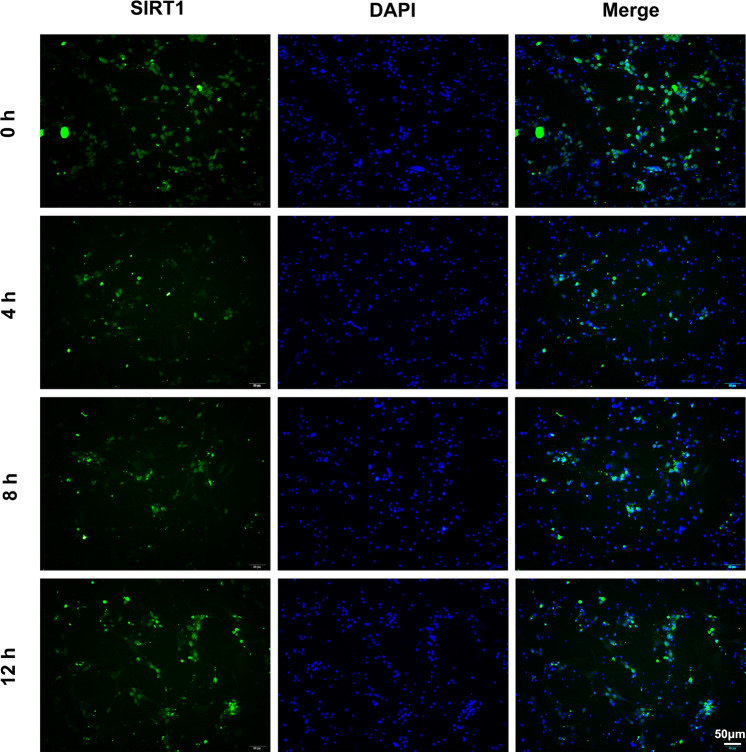
To investigate whether SIRT1 is involved in fluid shear stress induced mechanical damage of primary cultured cortical neurons, in this study, first we determined the presence of SIRT1 in the primary cultured cortical neurons of rats through immunofluorescence assay. As Fig. 2 showed, SIRT1 was found both in cell cytoplasm and cell nucleus of the primary cultured cortical neurons and, but the cell nuclei was the main subcellular localization of SIRT1. Additionally, we checked SIRT1’s subcellular localization through the same method in shear stress stimulated cortical neurons, but we did not find any change (Fig. 2).
Figure 2.
Subcellular localization of SIRT1 in primary cultured cortical neurons after 10 dyn/cm2 shear stress of for 4, 8 and 12 h. Cortical neurons cultured in static conditions were used as control.
Since SIRT1 is an important factor that protects neuronal injury, we examined SIRT1 expression at mRNA level in fluid shear stress stimulated primary cultured cortical neurons. As shown in Fig. 3, the mRNA level was first significantly increased after shear stress stimulated for 4 h (n = 3, t = 3.51, p < 0.05) and then decreased sharply even less than normal level from 8 to 12 h (n = 3, t = 2.86, p < 0.05) in shear stress stimulated cortical neurons, with the peak at 4 h treatment. The fluctuant expression of SIRT1 suggested that an unknown relationship between SIRT1 and shear stress induced neuronal injury might be existed.
Figure 3.
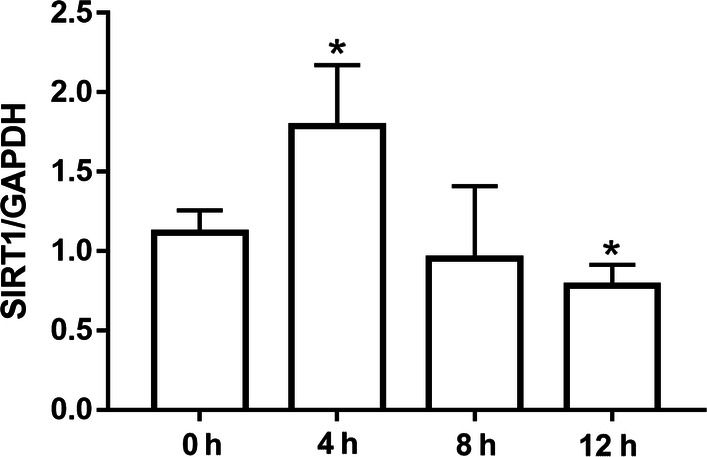
The expression of SIRT1 after shear stress of 10 dyn/cm2 for 4, 8 and 12 h.*p < 0.05, **p < 0.01 and ***p < 0.001 were considered to be statistically significant.
Sub-physiological Amounts of SIRT1 Cannot Protect Against Fluid Shear Stress Mechanical Damages
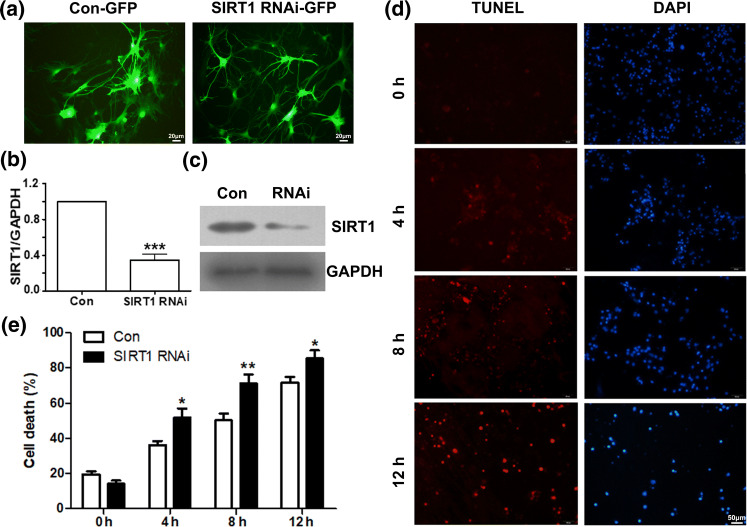
In order to investigate the possible effect of SIRT1 on fluid shear stress induced mechanical damage of the primary cultured cortical neurons, we first used the RNA interference (RNAi) approach to assess the effects of SIRT1 knock-down in the primary cultured cortical neurons. The efficiency of RNAi in transient transfected cortical neurons was assessed by fluorescence, RT-PCR and Western Blotting analysis (Figs. 4a, 4b and 4c). The efficiency of SIRT1 RNAi was around 60–70%. Compared to control group, SIRT1 expressed decreased significantly (n = 3, t = 10.22, p < 0.01) in the RNAi treated cortical neurons.
Figure 4.
SIRT1 interference accelerates fluid shear stress induced mechanical damage. (a) Detection of SIRT1 RNAi-GFP efficiency in transient transfected cortical neurons by fluorescence assay. (b) Detection of SIRT1 RNAi–efficiency in transient transfected cortical neurons by RT-PCR. (c) Detection of SIRT1 RNAi efficiency in transient transfected cortical neurons by Western blotting. (d) Photomicrographs and (e) histograms show that as shear stress time increased (from 4, 8, to 12 h) TUNEL positive cells in SIRT1 interference cortical neurons increased more significantly than control by TUNEL assay. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered to be statistically significant.
The consequences of SIRT1 down-regulation by RNAi on fluid shear stress induced mechanical damage of the primary cultured cortical neurons were analyzed using the TUNEL assay. Due to the fusion expression of Green fluorescent protein (GFP), we changed the dye of TUNEL assay from green to red. The result showed that SIRT1 down-regulated cortical neurons had higher TUNEL positive cells compare to control group (transfected with irrelevant siRNA) (Figs. 4d and 4e). There is significant increase of TUNEL positive cells after RNAi treatment (shear stress for 4 h, n = 5, t = 2.44, p < 0.05; 8 h, n = 5, t = 3.16, p < 0.01; 12 h, n = 5, t = 1.99, p < 0.05). It seems that SIRT1 has potential protective effects on neuronal injury.
SIRT1 Overexpression Could Suppress Fluid Shear Stress Induced Neuronal Apoptosis
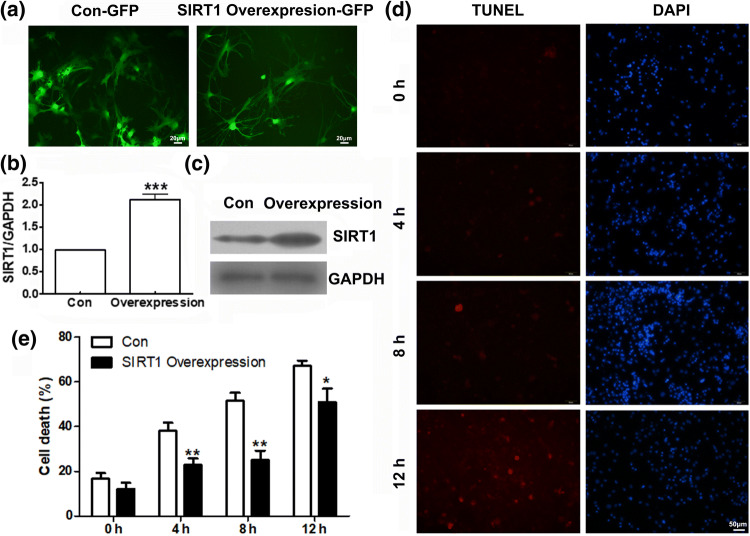
In order to investigate the protective effects of Sirt1 on neuronal injury, we employed an adenovirus-mediated gene transfer to establish strains of cortical neurons carrying GFP (used as control) or wild type gene of SIRT1.The efficiency of SIRT1 overexpression in transient transfected cortical neurons was assessed by fluorescence, RT-PCR and Western Blotting analysis. As shown in Figs. 5a, 5b and 5c, the efficiency of SIRT1 overexpression was around 2-fold.
Figure 5.
SIRT1 overexpression suppress fluid shear stress induced mechanical damage. (a) Detection of SIRT1 Overexpression-GFP efficiency in transient transfected cortical neurons by fluorescence assay. (b) Detection of SIRT1 overexpression efficiency in transient transfected cortical neurons by RT-PCR. (c) Detection of SIRT1 overexpression efficiency in transient transfected cortical neurons by Western blotting. (d) Photomicrographs and (e) histograms show that as shear stress time increased (from 4, 8, to 12 h) TUNEL positive cells in SIRT1 overexpressed cortical neurons were less than control group by TUNEL assay. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered to be statistically significant.
Both SIRT1 overexpression cortical neurons and their control (transfected with empty vector carrying GFP) were exposed to 10 dyn/cm2 shear stress for 4, 8, and 12 h. The consequences of Sirt1 overexpression were analyzed using TUNEL assay with red dye. As shown in Figs. 5d and 5e, SIRT1 overexpression significantly decreased the percentage of died cells compared with relative control cells (shear stress for 4 h, n = 5, t = 3.70, p < 0.01; for 8 h, n = 5, t = 7.47, p < 0.01; 12 h, n = 5, t = 2.21, p < 0.05). The significant decrease indicates that SIRT1 plays potential protective effects in shear stress induced neuronal injury.
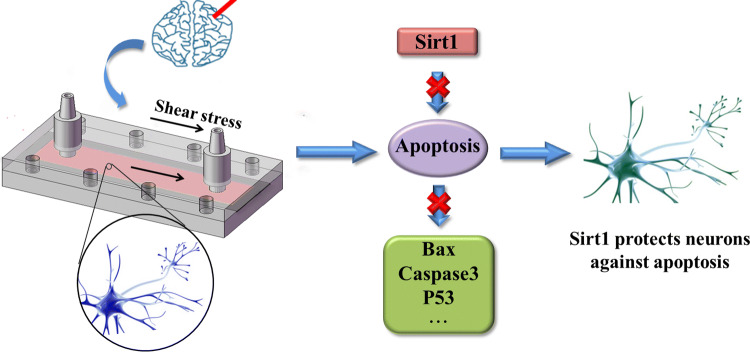
Schematic drawing of SIRT1 protection pathway was showed in Fig. 6. Two approaches have been used for confirming neuroprotective effect of SIRT1 during shear stress induced nerve injury: RNAi and gene overexpression of SIRT1. High shear stress induced nerve injury, which caused significant cell apoptosis, but SIRT1 protect against neuronal injury (Fig. 6). In this complex regulation mechanism, the downstream target molecules of SIRT1 may contain some protein related to apoptosis such as Bax, caspase and P53.29
Figure 6.
Schematic drawing of SIRT1 protection pathway. High shear stress induced nerve injury, which caused significant cell apoptosis. SIRT1 protect against neuronal apoptosis by inhibiting the Bax-Caspase 3 pathway.
Discussion
In this study, we explored the neuroprotective effect of SIRT1 during shear stress induced nerve injury. SIRT1 is the best-characterized sirtuin among the seven family members and expressed at a high level in the brain compared to other organs.34,36 And the postnatal survival of SIRT1 knockout mice is infrequent, with developmental defects such as exencephaly and retinal anomaly.6,32,40 Zakhary et al.53 provided the information on the anatomical distribution of SIRT1 in rodent and human nervous system, and showed that SIRT1 pathways are broadly distributed in neurons most susceptible to senescence injury. These studies suggest important roles of SIRT1 in neurons, further understanding of the relationship between SIRT1 and neuronal diseases may promise novel strategies in clinical intervention.
Previous studies have reported that neurons could be damaged by fluid shear stress.2,22,23,31 Kilinc’s study indicated that 45 dyn/cm2 with 20 ms onset time can induce FSSI in cultured primary chick forebrain neurons.22 During head injury, high intracranial pressure associated with impact has been predicted to lead to the generation of regions of high shear stress (up to 140 dyn/cm2) and tissue strain.26 And our previous study showed that low shear stress of 5 dyn/cm2 did not cause nerve injury, while 10 dyn/cm2 shear stress caused obvious neuronal damage.29 Despite the obvious importance of mechanical damage in neuronal injury, the associated cellular pathways are not clearly understood.
Emerging evidence suggest that SIRT1 could response to mechanic shear stress stimulation. Laminar flow might increase NO bioavailability through regulating SIRT1-Endothelial nitric oxide synthase (eNOS) association and eNOS deacetylation.4 SIRT1 regulated histone H3 acetylation is crucial in the progress of shear stress-induced endothelial progenitor cells differentiation into endothelial cells.7 Laminar shear stress inhibits the proliferation of Endothelial cells (ECs) via SIRT1 and Connexin4 (Cx40) in the presence or absence of vascular smooth muscle cells.52
There are growing evidence suggesting that SIRT1 plays a neuroprotective effect in a variety of neuronal systems.38 Resveratrol induced SIRT1 was found to protect neurons against polyglutamine toxicity and in Wallerian degeneration slow mice.1,13 Overexpression of SIRT1 and the addition of SIRT1 activator resveratrol reduced polyglutamine toxicity.15,28 Overexpression of SIRT1 or Resveratrol increase survival in SH-SY5Y neurons during NO exposure.8
Thus, we hypothesized that SIRT1 could protect against shear stress induced nerve injury in primary cultured rat cortical neurons. To test this hypothesis, we detected SIRT1’s localization in primary cultured rat cortical neurons by immunofluorescence and the results showed that SIRT1 was expressed both in cytoplasm and nucleus, but nucleus was the main subcellular localization. As a protein deacetylase, SIRT1 was found to be localized in the nucleus and play diverse functions in numerous studies.18,36 However, recent studies found SIRT1 lies in both cytoplasm and nucleus with the preference of cytoplasm in postnatal day 3 brain while predominantly localized to cytoplasm in adult mouse brain.50 Some studies suggest that cytoplasm-localized SIRT1 promote apoptosis,20,50,55 while the nucleus- localized SIRT1 anti-apoptosis.48 It seems that the subcellular localization of SIRT1 could play a role in its regulation of cell death.
In the further study, we employed 10 dyn/cm2 shear stress to mimic nerve injury. Based on this model, we analyzed the expression of SIRT1. Our results showed that the mRNA level of SIRT1 was first significantly increased at 4 h treatment and then decreased sharply even less than normal level from 8 to 12 h in shear stress stimulated cortical neurons. This demonstrated that SIRT1 played important roles in shear stress induced neuronal injury. The change of SIRT1 expression is in line with its nucleus localization and regulation the process of the apoptosis, which may inhibit neuronal programmed cell death, indicating the neuroprotective effect of SIRT1.
Two approaches have been used for confirming neuroprotective effect of SIRT1 during shear stress induced nerve injury: RNAi and gene overexpression of SIRT1. Our result showed that SIRT1 RNA interference cortical neurons had higher TUNEL positive cells compared with control group, while SIRT1 overexpression significantly decreased the percentage of died cells compared with control cells.
Conclusion
In this study, shear stress with 10 dyn/cm2 could cause marked apoptosis of cortical neurons. However, SIRT1 can reverse this apoptosis process, which was mainly expressed in nucleus before and after shear stress stimulation. SIRT1 RNAi increased shear stress-induced apoptosis, while overexpression of SIRT1 inhibits this process. Our data strongly suggests that activating endogenous SIRT1 may offer a new therapeutic approach to inhibit apoptosis induced by shear stress.
Acknowledgments
This study was supported by funds from National Natural Science Foundation of China (NSFC) Research Grant (31971238, 61871014, 51574246, 31771019, 11472032, 11120101001), and National Basic Research Program of China (973 Program, 2011CB710901), the 111 Project (B13003).
Author Contributions
PL and Y-BF conceived and designed the experiments. WS, M-LL, Z-JZ, C-QH and A-QW performed the experiments. WS, M-LL, J-WX and PL analyzed the data. M-LL contributed reagents/materials/analysis tools. M-LL, PL and Y-BF wrote the paper. All authors read and approved the final manuscript.
Data Availability
The data used to support the findings of this study are included within the article.
Conflict of interest
Wei Song, Mei-Li Liu, Zhi-Jun Zhao, Chong-Quan Huang, Jun-Wei Xu, An-Qing Wang, Ping Li and Yu-Bo Fan declare that they have no conflicts of interest.
Ethical Approval
No human studies were carried out by the authors for this article. All experiments involving the use of animals were in compliance with Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation and were approved by Beijing Municipal Science & Technology Commission (Permit Number: SCXK (Beijing) 2006-0008 and SYXK (Beijing) 2006-0025).
Abbreviations
- SIRT1
Sirtuin1
- NAD+
Nicotinamide adenine dinucleotide
- SIRT1-7
Sirtuin family isoforms
- Sir-2
Silent information regulator-2
- Nkx2.5
NK2 Homeobox 5
- AAA
Abdominal aortic aneurysms
- CR
Caloric restriction
- PASMC
Pulmonary artery smooth muscle cells
- PAH
Pulmonary arterial hypertension
- PDGF-BB
Platelet-derived growth factor-BB
- NRF2
Nuclear factor (erythroid-derived 2)-like 2
- DAI
Diffuse axonal injury
- FSSI
Fluid shear stress injury
- MTT
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-[2H]-tetrazolium bromide
- TUNEL
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling
- DAPI
4,6-Diamidino-2-phenylindole dihydrochloride
- BSA
Albumin from bovine serum
- PBS
Phosphate buffered saline
- RT-PCR
Reverse transcription-polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- PAGE
Polyacrylamide gel electrophoresis
- RNAi
RNA interference
- PVDF
Poly(vinylidene fluoride)
- BCA
Bicinchoninic acid
- GFP
Green fluorescent protein
- eNOS
Endothelial nitric oxide synthase
- ECs
Endothelial cells
- Cx40
Connexin40
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wei Song and Mei-Li Liu have contributed equally to this work.
Contributor Information
Ping Li, Email: liping@buaa.edu.cn.
Yu-Bo Fan, Email: yubofan@buaa.edu.cn.
References
- 1.Anekonda TS. Resveratrol–a boon for treating Alzheimer’s disease? Brain Res. Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Kochba E, Scimone MT, Estrada JB, Franck C. Strain and rate-dependent neuronal injury in a 3D in vitro compression model of traumatic brain injury. Sci. Rep. 2016;6:30550. doi: 10.1038/srep30550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calliari A, Bobba N, Escande C, Chini EN. Resveratrol delays Wallerian degeneration in a NAD(+) and DBC1 dependent manner. Exp. Neurol. 2014;251:91–100. doi: 10.1016/j.expneurol.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc. Natl. Acad. Sci. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen HZ, Wang F, Gap P, Pei JF, Liu Y, Xu TT, Tang X, Fu WY, Lu J, Yan YF, Wang XM, Han L, Zhang ZQ, Zhang R, Zou MH, Liu DP. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ. Res. 2016;119:1076–1088. doi: 10.1161/CIRCRESAHA.116.308895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng BB, Yan ZQ, Yao QP, Shen BR, Wang JY, Gao LZ, Li YQ, Yuan HT, Qi YX, Jiang ZL. Association of SIRT1 expression with shear stress induced endothelial progenitor cell differentiation. J. Cell Biochem. 2012;113:3663–3671. doi: 10.1002/jcb.24239. [DOI] [PubMed] [Google Scholar]
- 8.Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr. Neurovasc. Res. 2008;5:159–170. doi: 10.2174/156720208785425666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullen DK, LaPlaca MC. Neuronal response to high rate shear deformation depends on heterogeneity of the local strain field. J. Neurotraum. 2006;23:1304–1319. doi: 10.1089/neu.2006.23.1304. [DOI] [PubMed] [Google Scholar]
- 10.Cullen DK, Vernekar VN, LaPlaca MC. Trauma-induced plasmalemma disruptions in three-dimensional neural cultures are dependent on strain modality and rate. J. Neurotrauma. 2011;28:2219–2233. doi: 10.1089/neu.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai M, Li T, Han G, Ross MG. Programmed hyperphagia secondary to increased hypothalamic SIRT1. Brain Res. 2014;1589:26–36. doi: 10.1016/j.brainres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolle JP, Morrison B, Schloss RS, Yarmush ML. An organotypic uniaxial strain model using microfluidics. Lab Chip. 2013;13:432–442. doi: 10.1039/c2lc41063j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, Cai Z, Cai M, Liu K, Liu D, Zhang Q, Tan J, Ma Q. AMPK/SIRT1/p38 MAPK signaling pathway regulates alcohol induced neurodegeneration by resveratrol. Mol. Med. Rep. 2018;17:5402–5408. doi: 10.3892/mmr.2018.8482. [DOI] [PubMed] [Google Scholar]
- 14.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 15.Harrison IF, Powell NM, Dexter DT. The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson’s disease. J. Neurosci. 2018;48:136–153. doi: 10.1111/jnc.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hisahara S, Chiba S, Matsumoto H, Tanno M, Yagi H, Shimoham S, Sato M, Horio Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc. Natl. Acad. Sci. 2008;105:15599–15604. doi: 10.1073/pnas.0800612105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou Y, Lautrup SCS, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. 2018;115:1876–1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Jia XL, Bai K, Gong XH, Fan YB. Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch. Med. Res. 2010;41:497–505. doi: 10.1016/j.arcmed.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Jin Q, Yan T, Ge X, Sun C, Shi X, Zhai Q. Cytoplasm-localized SIRT1 enhances apoptosis. J. Cell Physiol. 2007;213:88–97. doi: 10.1002/jcp.21091. [DOI] [PubMed] [Google Scholar]
- 21.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Bennett DA, Calon F. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropath. Exp. Neur. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilinc D, Gallo G, Barbee K. Poloxamer 188 reduces axonal beading following mechanical trauma to cultured neurons. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007;2007:5395–5398. doi: 10.1109/IEMBS.2007.4353562. [DOI] [PubMed] [Google Scholar]
- 23.Kilinc D, Gallo G, Barbee KA. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp. Neurol. 2008;212:422–430. doi: 10.1016/j.expneurol.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaPlaca MC, Cullen DK, McLoughlin JJ, Cargill RS. High rate shear strain of three-dimensional neural cell cultures: a new in vitro traumatic brain injury model. J. Biomech. 2005;38:1093–1105. doi: 10.1016/j.jbiomech.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Laplaca MC, Prado GR. Neural mechanobiology and neuronal vulnerability to traumatic loading. J. Biomech. 2010;43:71–78. doi: 10.1016/j.jbiomech.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 27.LaPlaca MC, Prado GR, Cullen DK, Irons HR. High rate shear insult delivered to cortical neurons produces heterogeneous membrane permeability alterations. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006;1:2384–2387. doi: 10.1109/IEMBS.2006.260633. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Yokota T, Gama V, Yoshida T, Gomez JA, Ishikawa K, Sasaguri H, Cohen HY, Sinclair DA, Mizusawa H, Matsuyama S. Bax-inhibiting peptide protects cells from polyglutamine toxicity caused by Ku70 acetylation. Cell Death Differ. 2007;14:2058–2067. doi: 10.1038/sj.cdd.4402219. [DOI] [PubMed] [Google Scholar]
- 29.Liu ML, Song W, Li P, Huang Y, Gong XH, Zhou G, Jia XL, Zheng LS, Fan YB. Galanin protects against nerve injury after shear stress in primary cultured rat cortical neurons. PLoS ONE. 2013;8:e63473. doi: 10.1371/journal.pone.0063473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Wang TT, Zhang R, Fu WY, Wang X, Wang F, Gao P, Ding YN, Xie Y, Hao DL, Chen HZ, Liu DP. Calorie restriction protects against experimental abdominal aortic aneurysms in mice. J. Exp. Med. 2016;213:2473–2488. doi: 10.1084/jem.20151794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maneshi MM, Sachs F, Hua SZ. A threshold shear force for calcium influx in an astrocyte model of traumatic brain injury. J. Neurotrauma. 2015;32:1020–1029. doi: 10.1089/neu.2014.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDougald DS, Dine KE, Zezulin AU, Bennett J, Shindler KS. SIRT1 and NRF2 gene transfer mediate distinct neuroprotective effects upon retinal ganglion cell survival and function in experimental optic neuritis. Investig. Ophthalmol. Vis. Sci. 2018;59:1212–1220. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging. 2013;5:144–150. doi: 10.18632/aging.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mu WL, Wang YJ, Xu P, Hao DL, Liu XZ, Wang TT, Chen F, Chen HZ, Lv X, Liu DP. Sox2 deacetylation by Sirt1 is involved in mouse somatic reprogramming. Stem Cells. 2015;33:2135–2147. doi: 10.1002/stem.2012. [DOI] [PubMed] [Google Scholar]
- 38.Munoz A, Correa CL, Lopez-Lopez A, Costa-Besada MA, Diaz-Ruiz C, Labandeira-Garcia JL. Physical exercise improves aging-related changes in angiotensin, IGF-1, SIRT 1, SIRT3 and VEGF in the Substantia Nigra. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:1594–1601. doi: 10.1093/gerona/gly072. [DOI] [PubMed] [Google Scholar]
- 39.Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NR, Trisler D, Judge SI, Royal W, Chandrasekaran K, Russell JW, Makar TK. Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J. Immunol. 2013;190:4595–4607. doi: 10.4049/jimmunol.1202584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimmagadda VK, Makar TK, Chandrasekaran K, Sagi AR, Ray J, Russell JW, Bever CT. SIRT1 and NAD+ precursors: therapeutic targets in multiple sclerosis a review. J. Neuroimmunol. 2017;304:29–34. doi: 10.1016/j.jneuroim.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa T, Wakai C, Saito T, Murayama A, Mimura Y, Youfu S, Nakamachi T, Kuwagata M, Satoh K, Shioda S. Distribution of the longevity gene product, SIRT1, in developing mouse organs. Congenit. Anom. 2013;51:70–79. doi: 10.1111/j.1741-4520.2010.00304.x. [DOI] [PubMed] [Google Scholar]
- 42.Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol. Cells. 2013;35:474–480. doi: 10.1007/s10059-013-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawda C, Moussa C, Turner RS. Resveratrol for Alzheimer’s disease. Ann. NY Acad. Sci. 2017;1403:142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Servello D, Gu Y, Gu C. A microbiomechanical system for studying varicosity formation and recovery in central neuron axons. J. Vis. Exp. 2018;134:e57202. doi: 10.3791/57202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14:511–523. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang X, Ma H, Han L, Zheng W, Lu YB, Chen XF, Liang ST, Wei GH, Zhang ZQ, Chen HZ, Liu DP. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Sci. Rep. 2016;6:36576. doi: 10.1038/srep36576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product,1-O-acetyl-ADP-ribose. P. Natl. Acad. Sci. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 49.Testa G, Staurenghi E, Giannelli S, Gargiulo S, Guglielmotto M, Tabaton M, Tamagno E, Gamba P, Leonarduzzi G. A silver lining for 24-hydroxycholesterol in Alzheimer’s disease: The involvement of the neuroprotective enzyme sirtuin 1. Redox Biol. 2018;17:423–431. doi: 10.1016/j.redox.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu W, Zhang Q, Liu Y, Han L, Wang Q, Chen P, Zhang S, Wang A, Zhou X. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharm. 2018;347:60–69. doi: 10.1016/j.taap.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Velagapudi R, Ajileye OO, Okorji U, Jain P, Aderogba MA, Olajide OA. Agathisflavone isolated from Anacardium occidentale suppresses SIRT1-mediated neuroinflammation in BV2 microglia and neurotoxicity in APPSwe-transfected SH-SY5Y cells. Phytother. Res. 2018;32:1957–1966. doi: 10.1002/ptr.6122. [DOI] [PubMed] [Google Scholar]
- 52.Yao QP, Qi YX, Zhang P, Cheng BB, Yan ZQ, Jiang ZL. SIRT1 and Connexin40 Mediate the normal shear stress-induced inhibition of the proliferation of endothelial cells co-cultured with vascular smooth muscle cells. Cell Physiol. Biochem. 2013;31:389–399. doi: 10.1159/000343376. [DOI] [PubMed] [Google Scholar]
- 53.Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, Torres G. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat. Rec. 2010;293:1024–1032. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zendedel E, Butler AE, Atkin SL, Sahebkar A. Impact of curcumin on sirtuins: a review. J. Cell Biochem. 2018;11:10291–10300. doi: 10.1002/jcb.27371. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J. Biol. Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Xu J, Liu Y, Wang T, Pei J, Cheng L, Hao D, Zhao X, Chen HZ, Liu DP. Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin II-induced abdominal aortic aneurysm formation. J. Genet. Genom. 2018;20:25–32. doi: 10.1016/j.jgg.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhou S, Liu MT, Jia YY, Liu JJ, Wang Q, Wang Z, Tian Z, Liu YT, Chen HZ, Liu DP, Zeng XF. Regulation of cell cycle regulators by SIRT1 contributes to resveratrol-mediated prevention of pulmonary arterial hypertension. Biomed. Res. Int. 2015;2015:762349. doi: 10.1155/2015/762349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.