Abstract
Background
Acetylcholine deficiencies in hippocampus and cortex, aggregation of β-amyloid, and β-secretase over activity have been introduced as main reasons in pathogenesis of Alzheimer’s disease.
Methods
Colorimetric Ellman’s method was used for determination of IC50 value in AChE and BChE inhibitory activity. The kinetic studies, neuroprotective and β-secretase inhibitory activities, evaluation of inhibitory potency on β-amyloid (Aβ) aggregations induced by AChE, and docking study were performed for prediction of the mechanism of action.
Result and discussion
A new series of cinnamic acids-tryptamine hybrid was designed, synthesized, and evaluated as dual cholinesterase inhibitors. These compounds demonstrated in-vitro inhibitory activities against acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE). Among of these synthesized compounds, (E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3,4-dimethoxyphenyl)acrylamide (5q) demonstrated the most potent AChE inhibitory activity (IC50 = 11.51 μM) and (E)-N-(2-(1H-indol-3-yl)ethyl)-3-(2-chlorophenyl)acrylamide (5b) were the best anti-BChE (IC50 = 1.95 μM) compounds. In addition, the molecular modeling and kinetic studies depicted 5q and 5b were mixed type inhibitor and bound with both the peripheral anionic site (PAS) and catalytic sites (CAS) of AChE and BChE. Moreover, compound 5q showed mild neuroprotective in PC12 cell line and weak β-secretase inhibitory activities. This compound also inhibited aggregation of β-amyloid (Aβ) in self-induced peptide aggregation test at concentration of 10 μM.
Conclusion
It is worth noting that both the kinetic study and the molecular modeling of 5q and 5b depicted that these compounds simultaneously interacted with both the catalytic active site and the peripheral anionic site of AChE and BChE. These findings match with those resulted data from the enzyme inhibition assay.
Graphical abstract.
A new series of cinnamic-derived acids-tryptamine hybrid derivatives were designed, synthesized and evaluated as butyrylcholinesterase (BChE) and acetylcholinesterase (AChE) inhibitors and neuroprotective agents. Compound 5b and 5q, as the more potent compounds, interacted with both the peripheral site and the choline binding site having mixed type inhibition. Results suggested that derivatives have a therapeutic potential for the treatment of AD.
Electronic supplementary material
The online version of this article (10.1007/s40199-020-00346-9) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Butyrylcholinesterase, Docking study, Cinnamic acid derivatives, In-vitro assay
Introduction
Alzheimer’s disease (AD) is the most common form of progressive dementia in the elderly people, that is cause loss of cognitive abilities such as memory, language skills, attention, disorientation, and depression [1, 2]. The etiology of AD is still not known and many factors such as reduced level of cholinergic transmitters, accumulation of beta-amyloid peptide (Aβ) plaque, oxidative stress, and hyper-phosphorylation of microtubule-associated tau protein involved in the progression of the disease [3]. The most common and important hypothesis about AD has been attributed to the levels of acetylcholine [4, 5]. There is two important types of catalytic enzyme, namely acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) which are able to hydrolyzed cholinergic neurotransmitters. According to a recent report on Alzheimer’s patient, AChE activity in a specific region of the brain dramatically reduced, while BChE activity increase to compensate for the lack of AChE. Based on the important role of BChE in the hydrolysis of acetylcholine, simultaneous inhibition of both enzymes can be effective to the improvement of the symptoms in Alzheimer’s patients [6]. The X-ray crystallography studies have shown that AChE has two binding sites containing the catalytic active site (CAS) and the peripheral anionic site (PAS) [7, 8]. AChE inhibitors act through the interaction with amino acid residues in the CAS and PAS and consequently inhibition of this two site increases the inhibitory activity.
Another most important cause of AD is the accumulation of β-amyloid (Aβ) plaques in the brain. One of the ways to prevent the accumulation of Aβ plaques is prevention the hydrolysis of amyloid precursor protein (APP) by inhibition of β-secretase (BACE1). There are also reports that show accumulation of amyloid plaques and neurofibrillary tangles in the brain increased by oxidative damage. Therefore, preventing the formation of free radicals by antioxidant agents can be effective for treating AD [9–11].
Even though researches were conducted to develop inhibitors of AChE, there aren’t many drugs on the market due to the adverse effects of therapeutic drugs [12]. In the recent years, four AChE-inhibitors contains donepezil, tacrine, rivastigmine, and galantamine have been approved by FDA, However, these drugs only improve the symptoms in the short term, but not treating. Tacrine was withdrawn from clinical usage due to sever its hepatotoxicity by FDA at 2012. The efficacy and safety of donepezil encourages researches for the development of donepezil-like compounds for the treatment of AD. In the literature, various compounds are presented in the article as inhibitors of acetyl cholinesterase such as carbamates, organophosphates, coumarin and cinnamic acids [11–14].
The aim of this study was to synthesize and investigate cinnamic acids-tryptamine scaffold hybrids as anticholinesterase agents.
Cinnamic acid derivatives has been recognized as a core pharmacophor to develop for AD [10, 15]. There are several reports that introduced different hybrid between cinnamic acid derivative and other chemical groups including carbazole, tacrin, rivasetigmine and tryptamine. A new series of coupled cinnamic acid and donepezil has been introduced as ChE inhibitors (Fig. 1, B). Some of them showed low activity in AChE inhibition and the more activity for inhibition of BChE. On the other hands, tryptamine has been introduced as a suitable scaffold for preparation of ChE inhibitors [3]. A coumarin-amide conjugated tryptamine (Fig. 1, A) showed inhibition of AChE and BChE with IC50 of 5.9 and 50.5 μM respectively.
Fig. 1.
Structure of ferulic acid, cinnamic acid and coumarine-triptamine based anti-AChE agents as a lead compounds for a newly designed AChE inhibitors
In the current work we prepared a new hybrid of cinnamic acid-tryptamine in order to study their biological activity. However cinnamic acid-tryptamine did not demonstrated good activity for inhibition of AChE, our results showed that this hybrid increased activity for inhibition of BChE in comparison to the coumarin-trypatime hybrid and could be considered for design more new active AChE and BChE inhibitors.
Experimental
Chemistry
Melting points was taken on a Kofler hot stage apparatus and are uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker FT-500, using TMS as an internal standard at 500 and 125 MHz respectively. The mass spectra were recorded on an Agilent 5975C spectrometer. Elemental analysis was carried out by a Perkin Elmer 2400 CHN Elemental Analyzer using a tin capsule including 2–3 mg of sample. The analysis was performed under excess oxygen exposure in the reactor chamber, 990 °C and tungsten trioxide catalyst for complete oxidation. Elemental analyses were within ±0.4% of theoretical values for C, H and N. IR spectra were obtained on a Nicolet Magna FTIR 550 spectrophotometer (KBr disks). All the commercially available reagents were purchased from Merck or Sigma-Aldrich and used without further purification. Electriceel (Torpedo California) AChE (type VI-S), BuChE (E.C.3.1.1.8, from equine serum), acetylthiocholine iodide, butyrylthiocholine iodide, 5,5′-dithiobis[2-nitrobenzoic acid] (DTNB) and Donepezil hydrochloride was obtained from Sigma-Aldrich (Steinheim, Germany).
General procedure for the synthesis of compound 3
A solution of benzaldehyde derivatives 1 (5 mmol), malonic acid 2 (5.5 mmol), in 1.25 mL of pyridine, 0.5 mL of piperidine, and 5 mL of ethanol 95% was heated at 100 °C for 24 h. After completion of the reaction, monitored by TLC, diluted HCl solution (0.1 M) was added to the reaction mixture in an ice bath. The resulting solid was filtered and dried. To obtain pure product 3, the dried solid crystalized in ethanol.
General procedure for the synthesis of compounds 5
A mixture of cinnamic-derived acid 3 (2 mmol), N-hydroxy benzotriazole (HOBT) (2.4 mmol) and 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) (2.4 mmol) in 10 mL of dry acetonitrile was stirred for 1 h at ambient temperature and then tryptamine (2 mmol) was added. The reaction mixture was stirred at ambient temperature for 48 h and completion of the reaction monitored by TLC. The crude product was extracted using CH2Cl2 (50 mL) and NaCl 10% (10 mL), NaHCO3 10% (10 mL) and citric acid 10% (10 mL). The organic phase was dried (Na2SO4) and the solvent was evaporated under vacuum. All the compounds were purified by flash chromatography on silica gel using petroleum ether/ ethyl acetate (6:1) to obtain products 5, and then were recrystallized from ethyl acetate and hexane (1:1).
N-(2-(1H-indol-3-yl)ethyl)cinnamamide (5a)
Yield: 73%, mp: 141–143 °C. IR (KBr) cm−1 υ: 3440, 3312, 2928, 2831, 1653, 1600, 1513 1. 1HNMR (DMSO-d6, 500 MHz): δ 2.91 (t, J = 7.0 Hz, 2H, CH2), 3.50 (q, J = 7.0 Hz, 2H, NH-CH2), 6.66 (d, J = 15.5 Hz, 1H, CHα),
), 7.07 (t, J = 7.0 Hz, 1H, H6), 7.18 (s, 1H, H2), 7.35–7.42 (m, 4H, H3’, 4’, 5’, 7), 7.42 (d, J = 15.5 Hz, 1H, CHβ), 7.55–7.58 (m, 3H, H2’,6’, 4), 8.21 (t, J = 5.5 Hz, 1H, NH-CH2), 10.82 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.2, 39.6, 111.3, 111.76, 118.18, 11.63, 120.87, 122.4, 122.59, 127.2, 127.4, 128.8, 129.27, 134.9, 136.2, 138.4, 164.87 ppm. Mass, m/z (%): 290(33), 143(100), 130(95), 103(58), 77(45). Anal. Calcd for C19H18N2O: C, 78.59%; H, 6.25%; N, 9.65%. Found: C, 78.77%; H, 6.35%; N, 9.93%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(2-chlorophenyl)acrylamide (5b)
Yield: 75%, mp: 140–142 °C. IR (KBr) cm−1 υ: 3444, 3295, 3053, 2921, 1650, 1603, 1547. 1HNMR (DMSO-d6, 500 MHz): δ = 2.91 (t, J = 7.5 Hz, 2H, CH2), 3.50 (q, J = 6.5 Hz, 2H, NH-CH2), 6.69 (d, J = 16.0 Hz, 1H, CHα), 6.98 (t, J = 6.5 Hz, 1H, H5), 7.07 (t, J = 7 Hz, 1H, H6), 7.17 (s, 1H, H2), 7.34 (d, J = 7.5 Hz, 1H, H7), 7.38–7.40 (m, 2H, H3’, 5’), 7.52 (d, J = 5 Hz, 1H, H6’), 7.56 (d, J = 7.5 Hz, 1H, H4), 7.70 (t, J = 5.0 Hz, 1H, H4’), 7.74 (d, J = 16.0 Hz, 1H, CHβ), 8.28 (t, J = 5.5 Hz, 1H, NH-CH2), 10.78 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.1, 39.6, 111.2, 111.6, 118.1, 118.8, 120.8, 121.1, 122.5, 125.4, 127.1, 127.5, 129.8, 130.02, 130.6, 132.7, 133.1, 138.1, 164.3 ppm. Mass, m/z (%): 324(3), 165(5), 143(50), 130(36), 101(35), 86(100), 58(34). Anal. Calcd for C19H17ClN2O: C, 70.26%; H, 5.28%; N, 8.62%. Found: C, 70.17%; H, 5.35%; N, 8.44%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3-chlorophenyl)acrylamide (5c)
Yield: 68%, mp: 153–156 °C. IR (KBr) cm−1 υ: 3444, 3244, 3052, 2923, 1647, 1601, 1512. 1H NMR (DMSO-d6, 500 MHz): δ = 2.92 (t, J = 7.5 Hz, 2H, CH2), 3.51 (q, J = 6.5 Hz, 2H, NH-CH2), 6.72 (d, J = 16.0 Hz, 1H, CHα), 6.98 (t, J = 7.5 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.18 (s, 1H, H2), 7.35 (d, J = 8.5 Hz, 1H, H7), 7.40–7.45 (m, 3H, CHβ, H5’, 6’), 7.51 (d, J = 7.0 Hz, 1H, H4’), 7.57 (d, J = 7.5 Hz, 1H, H4), 7.62 (s, 1H, H2’), 8.21 (t, J = 5.5 Hz, 1H, NH-CH2), 10.80 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.1, 39.7, 111.1, 111.7, 118.2, 119.0, 120.9, 122.6, 124.1, 126.0, 127.1, 129.0, 130.6, 132.4, 133.6, 136.12, 136.95, 137.29, 164.6 ppm. Mass, m/z (%): 324(2), 237(3), 182(5), 165(10), 143(40), 130(35), 101(29), 86(100), 58(33). Anal. Calcd for C19H17ClN2O: C, 70.26%; H, 5.28%; N, 8.62%. Found: C, 70.33%; H, 5.39%; N, 8.59%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-chlorophenyl)acrylamide (5d)
Yield: 71%, mp: 165–169 °C. IR (KBr) cm−1 υ: 3408, 3287, 3072, 2918, 651, 1619, 1552. 1HNMR (DMSO-d6, 500 MHz): δ = 2.90 (t, J = 7.0 Hz, 2H, CH2), 3.49 (q, J = 7.0 Hz, 2H, NH-CH2), 6.65 (d, J = 16.0 Hz, 1H, CHα), 6.98 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.16 (s, 1H, H2), 7.34 (d, J = 8.0 Hz, 1H, H7), 7.42 (d, J = 16.0 Hz, 1H, CHβ), 7.46 (d, J = 8.0 Hz, 2H, H3’, 5’), 7.55–7.58 (m, 3H, H2’, 6’, 4), 8.17 (t, J = 5.5 Hz, 1H, NH-CH2), 10.77 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.5, 39.6, 111.2, 111.7, 118.1, 118.2, 120.8, 122.5, 123.2, 127.1, 128.9, 129.1, 133.7, 133.9, 136.2, 137.0, 164.6 ppm. Mass, m/z (%): 324(12), 165(9), 143(100), 130(82), 102(16), 77(8), 58(5). Anal. Calcd for C19H17ClN2O: C, 70.26%; H, 5.28%; N, 8.62%. Found: C, 70.11%; H, 5.05%; N, 8.50%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(2-fluorophenyl)acrylamide (5e)
Yield: 72%, mp: 137–140 °C. IR (KBr) cm−1 υ: 3451, 3228, 3052, 2928, 1667, 1628, 1560. 1HNMR (DMSO-d6, 500 MHz): δ = 2.92 (m, 2H, CH2), 3.51 (m, 2H, NH-CH2), 6.33 (d, J = 16.0 Hz, 1H, CHα), 6.52 (t, J = 7.0 Hz, 1H, H5), 6.61(t, J = 7.0 Hz, 1H, H6), 6.71–6.75 (m, 3H, H4, 4’, 5’), 6.90–6.92 (m, 2H, H7,3’), 7.11–7.15 (m, 3H, CHβ, H2, 6’), 7.88 (m, 1H, NH-CH2), 10.35 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.5, 39.8, 111.3, 111.5, 111.8, 115.9 (JC-F = 21.25 Hz), 118.3, 118.37, 120.9, 121.89, 122.6 (JC-F = 11.25 Hz), 124.8, 125.2, 127.3, 128.9 (JC-F = 21.25), 130.9, 136.4, 137.16, 161.5 (JC-F = 248.75 Hz), 164.8 ppm. Mass, m/z (%): 308(10), 160(11), 143(96), 130(100), 101(28), 86(99), 58(26). Anal. Calcd for C19H17FN2O: C, 74.01%; H, 5.56%; N, 9.08%. Found: C, 74.19%; H, 5.39%; N, 8.84%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3-fluorophenyl)acrylamide (5f)
Yield: 67%, mp: 149–151 °C. IR (KBr) cm−1 υ: 3276, 3066, 2933, 1663, 1619, 1586. 1HNMR (DMSO-d6, 500 MHz): δ = 2.90 (m, 2H, CH2), 3.49 (m, 2H, NH-CH2), 6.69 (d, J = 16.0 Hz, 1H, CHα), 6.99 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.0 Hz, 1H, H6), 7.18–7.22 (m, 2H, H2, 5’), 7.35 (d, J = 7.5 Hz, 1H, H7), 7.39–7.46 (m, 5H, CHβ, H2’, 4’, 5’, 6’), 7.57 (d, J = 7.5 Hz, 1H, H4), 8.24 (m, 1H, NH-CH2), 10.83 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.2, 39.8, 111.4, 111.7, 113.76, 115.9, 118.3, 119.09, 120.9, 121.8, 122.6, 123.4, 127.2, 130.7, 130.91, 136.2, 137.2, 161.4 (JC-F = 242.5 Hz), 164.6 ppm. Mass, m/z (%): 308(8), 160(5), 143(100), 130(99), 101(25), 86(55), 58(22). Anal. Calcd for C19H17FN2O: C, 74.01% H, 5.56%; N, 9.08%. Found: C, 74.24%; H, 5.69%; N, 9.22%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-fluorophenyl)acrylamide (5g)
Yield: 69%, mp: 152–154 °C. IR (KBr) cm−1 υ: 3403, 3300, 3074, 2932, 1652, 1601, 1551. 1HNMR (DMSO, 500 MHz): δ = 2.90 (t, J = 7.0 Hz, 2H, CH2), 3.49 (q, J = 7.0 Hz, 2H, NH-CH2), 6.58 (d, J = 15.5 Hz, 1H, CHα), 6.99 (t, J = 7.5 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.17 (s, 1H, H2), 7.24 (t, J = 7.5 Hz, 2H, H3’, 5’), 7.35 (d, J = 8.0 Hz, 1H, H7), 7.44 (d, J = 16.0 Hz, 1H, CHβ), 7.56 (d, J = 8.0 Hz, H4), 7.60–7.63 (m, 2H, H2’, 6’), 8.17 (t, J = 5.5 Hz, 1H, NH-CH2), 10.80 (s, 1H, NH) ppm. 13CNMR (DMSO, 125 MHz): δ = 25.1, 39.5, 111.3, 111.7, 115.7 (JC-F = 21.5 Hz), 118.1, 118.2, 120.8, 122.3, 122.5, 127.2, 129.4 (JC-F = 8.7 Hz), 131.5, 136.2, 137.1, 161.5 (JC-F = 246 Hz), 164.7 ppm. Mass, m/z (%): 308(25), 143(100), 130(88), 121(20), 101(24), 77(15). Anal. Calcd for C19H17FN2O: C, 74.01%; H, 5.56%; N, 9.08%. Found: C, 74.19%; H, 5.39%; N, 8.89%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(o-tolyl)acrylamide (5h)
Yield: 68%, mp: 159–161 °C. IR (KBr) cm−1 υ: 3449, 3317, 3047, 2920, 1694, 1603, 1546. 1HNMR (DMSO-d6, 500 MHz): δ = δ = 2.37 (s, 3H, CH3), 2.89 (t, J = 7.5 Hz, 2H, CH2), 3.48 (q, J = 6.5 Hz, 2H, NH-CH2), 6.58 (d, J = 16.0 Hz, 1H, CHα), 6.99 (t, J = 7.5 Hz, 1H, H4’), 7.07 (t, J = 8 .0 Hz, 1H, H5), 7.17 (s, 1H, H2), 7.213–7.26 (m, 3H, H3’, H6, H5’), 7.34 (d, J = 8.0 Hz, 1H, H6’), 7.51 (d, J = 7.5 Hz, 1H, H7), 7.56 (d, J = 8.0 Hz, 1H, H4), 7.66 (d, J = 15.5 Hz, 1H, Hβ), 8.213 (m, 1H, NH-CH2), 10.79 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 19.27, 25.15, 39.76, 111.21, 112.12, 118.12, 120.09, 122.58, 123.59, 125.72, 126.29, 127.16, 128.94, 129.80, 130.53, 133.76, 135.88, 136.51, 137.01, 164.2 ppm. Mass, m/z (%): 304(2), 230(11), 217(14), 162(13), 143(50), 115(57), 101(25), 86(100), 58(29). Anal. Calcd for C20H20N2O: C, 78.92%; H, 6.62%; N, 9.20%. Found: C, 78.88%; H, 6.41%; N, 9.01%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(m-tolyl)acrylamide (5i)
Yield: 64%, mp: 122–124 °C. IR (KBr) cm−1 υ: 3312, 3284, 3051, 2922, 1653, 1607, 1547. 1HNMR (DMSO-d6, 500 MHz): δ = 2.32 (s, 3H, CH3), 2.93 (t, J = 7.0 Hz, 2H, CH2), 3.53 (q, J = 7.0 Hz, 2H, NH-CH2), 6.66 (d, J = 16.0 Hz, 1H, CHα), 6.99 (t, J = 7.0 Hz, 1H, H5), 7.08 (t, J = 7.5 Hz, 1H, H6), 7.17 (s, 1H, H2’), 7.18 (s, 1H, H2), 7.29 (t, J = 7.5 Hz, 1H, H5’), 7.34–7.37 (m, 3H, H7, 4, 4’), 7.43 (d, J = 15.5 Hz, 1H, CHβ), 7.51 (d, J = 8.0 Hz, 1H, H6’), 8.16 (t, J = 5.5 Hz, 1H, NH-CH2), 10.80 (s, 1H, NH) ppm. 13CNMR (DMSO, 125 MHz): δ = 20.7, 25.1, 39.4, 111.2, 111.7, 118.1, 118.2, 120.8, 122.2, 122.5, 124.5, 127.1, 127.8, 128.6, 129.8, 134.8, 136.2, 137.9, 138.4, 164.8 ppm. Mass, m/z (%): 304(8), 143(100), 130(45), 115(14), 58(10). Anal. Calcd for C20H20N2O: C, 78.92%; H, 6.62%; N, 9.20%. Found: C, 78.71%; H, 6.53%; N, 9.41%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(p-tolyl)acrylamide (5j)
Yield: 72%, mp: 171–173 °C. IR (KBr) cm−1 υ: 3447, 3229, 2919, 2723, 1651, 1605, 1543. 1HNMR (DMSO-d6, 500 MHz): δ = 2.32 (s, 3H, CH3), 2.90 (t, J = 7.0 Hz, 2H, CH2), 3.49 (q, J = 7.0 Hz, 2H, NH-CH2), 6.58 (d, J = 15.5 Hz, 1H, CHα), 6.98 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.0 Hz, 1H, H6), 7.17 (s, 1H, H2), 7.21 (d, J = 7.5 Hz, 2H, H3’, 5’), 7.34 (d, J = 7.5 Hz, 1H, H7), 7.42 (d, J = 15.5 Hz, 1H, CHβ), 7.44 (d, J = 7.5 Hz, 2H, H2’, 6’), 7.56 (d, J = 7.5 Hz, 1H, H4), 8.14 (t, J = 5.5 Hz, 1H, NH-CH2), 10.78 (s, 1H, NH) ppm. 13C NMR (DMSO, 125 MHz): δ = 20.8, 25.2, 39.6, 111.2, 111.7, 118.1, 118.2, 120.8, 121.4, 122.5, 127.2, 127.4, 129.4, 132.2, 136.2, 138.3, 139.0, 138.9, 164.9 ppm. Mass, m/z (%): 304(24), 143(100), 130(83), 115(30), 103(14), 91(15), 77(13). Anal. Calcd for C20H20N2O: C, 78.92%; H, 6.62%; N, 9.20%. Found: C, 78.59%; H, 6.75%; N, 9.33%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(2-nitrophenyl)acrylamide (5k)
Yield: 79%, mp: 153–155 °C. IR (KBr) cm−1 υ: 3452, 3308, 3054, 2938, 1663, 1614, 15291. 1HNMR (DMSO-d6, 500 MHz): δ = 2.91 (t, J = 7.5 Hz, 2H, CH2), 3.50 (q, J = 7.0 Hz, 2H, NH-CH2), 6.64 (d, J = 15.5 Hz, 1H, CHα), 6.99 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.0 Hz, 1H, H6), 7.18 (s, 1H, H2), 7.34 (d, J = 8.0 Hz, 1H, H7), 7.56 (d, J = 7.5 Hz, 1H, H4), 7.63 (t, J = 7.5 Hz, 1H, H4’), 7.70 (d, J = 15.5 Hz, 1H, CHβ), 7.74–7.77 (m, 2H, H5’, 6’), 8.03 (d, J = 8.0 Hz, 1H, H3’), 8.3 (t, J = 5.5 Hz, 1H, NH-CH2), 10.78 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.0, 39.6, 111.3, 111.6, 118.1, 118.22, 120.8, 122.48, 122.6, 124.5, 127.1, 128.5, 129.9, 133.1, 133.2, 133.6136.2, 148.3, 164.0 ppm. Mass, m/z (%): 335(7), 193(10), 176(15), 143(100), 132(69), 102(11), 86(56). Anal. Calcd for C19H17N3O3: C, 68.05%; H, 5.11%; N, 12.53%. Found: C, 68.28%; H, 5.31%; N, 12.21%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3-nitrophenyl)acrylamide (5l)
Yield: 73%, mp: 168–171 °C. IR (KBr) cm−1 υ: 3395, 3249, 3080, 2926, 1660, 1621, 1546. 1HNMR (DMSO-d6, 500 MHz): δ = 2.92 (t, J = 7.0 Hz, 2H, CH2), 3.51 (q, J = 6.5 Hz, 2H, NH-CH2),6.84 (d, J = 16.0 Hz, 1H, CHα), 6.99 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.18 (s, 1H, H2), 7.35 (d, J = 7.5 Hz, 1H, H7), 7.54–7.57 (m, 2H, CHβ, H4), 7.70 (t, J = 7.5 Hz, 1H, H5’), 7.99 (d, J = 7.5 Hz, 1H, H6’), 8.19 (d, J = 8.0 Hz, 1H, H4’), 8.23 (m, 1H, NH-CH2), 8.38 (s, 1H, H2’), 10.78 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.0, 39.6, 111.2, 111.6, 118.1, 120.8, 121.5, 122.5, 123.4, 123.6, 125.3, 127.1, 130.4, 133.8, 136.1, 136.2, 1336.8, 148.2, 164.2 ppm. Mass, m/z (%): 335(8), 176(3), 143(100), 132(64), 102(7), 77(5). Anal. Calcd for C19H17N3O3: C, 68.05%; H, 5.11%; N, 12.53%. Found: C, 68.18%; H, 5.27%; N, 12.87%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-nitrophenyl)acrylamide (5 m)
Yield: 81%, mp: 173–175 °C. IR (KBr) cm−1 υ: 3382, 3268, 3052, 2931, 1666, 1625, 15441. 1HNMR (DMSO-d6, 500 MHz): δ = 2.91 (t, J = 7.0 Hz, 2H, CH2), 3.52 (q, J = 7.0 Hz, 2H, NH-CH2), 6.83 (d, J = 15.5 Hz, 1H, CHα), 6.98 (t, J = 7.0 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.17 (s, 1H, H2), 7.34 (d, J = 8.0 Hz, 1H, H7), 7.53–7.57 (m, 2H, CHβ, H4), 7.82 (d, J = 8.5 Hz, 2H, H2’,6’), 8.25 (d, J = 8.5 Hz, 2H, H3’, 5’), 8.32 (t, J = 5.5 Hz, 1H, NH-CH2), 10.78 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.0, 39.5, 111.2, 111.6, 118.1, 120.8, 122.4, 123.8, 124.1, 126.7, 127.1, 128.4, 136.1, 136.2, 141.6, 147.4, 164.1 ppm. Mass, m/z (%): 335(14), 176(4), 143(100), 132(72), 102(11), 77(7). Anal. Calcd for C19H17N3O3: C, 68.05%; H, 5.11%; N, 12.53%. Found: C, 68.12%; H, 5.28%; N, 12.49%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(2-methoxyphenyl)acrylamide (5n)
Yield: 71%, mp: 152–155 °C. IR (KBr) cm−1 υ: 3397, 3260, 3063, 2934, 1657, 1616, 1540. 1HNMR (DMSO-d6, 500 MHz): δ = 284 (t, J = 7.0 Hz, 2H, CH2), 3.10 (q, J = 7.0 Hz, 2H, NH-CH2), 3.78 (s, 3H, OCH3), 6.29 (d, J = 16.0 Hz, 1H, CHα), 6.49 (t, J = 7.0 Hz, 1H, H5), 6.53–6.56 (m, 2H, H3’,5’), 6.63 (t, J = 7.0 Hz, 1H, H6), 6.74 (s, 1H, H2), 6.86 (t, J = 7.5 Hz, 1H, H4’), 6.93 (d, J = 7.5 Hz, 1H, H7), 7.07 (d, J = 7.5 Hz, 1H, H6’), 7.14 (d, J = 7.5 Hz, 1H, H4), 7.33 (d, J = 16.0 Hz, 1H, CHβ), 7.81 (t, J = 5.5 Hz, 1H, NH-CH2), 10.40 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.3, 39.6, 55.5, 111.6, 111.9, 112.2, 118.2, 118.3, 120.7, 120.9, 122.6, 122.9, 123.4, 127.3, 127.6, 130.5, 133.8, 136.3, 157.5, 165.5 ppm. Mass, m/z (%): 320(8), 161(31), 143(100), 130(38), 118(6), 103(6), 77(7). Anal. Calcd for C20H20N2O2: C, 74.98%; H, 6.29%; N, 8.74%. Found: C, 74.88%; H, 6.31%; N, 8.98%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3-methoxyphenyl)acrylamide (5o)
Yield: 69%, mp: 169–170 °C. IR (KBr) cm−1 υ: 3442, 3232, 3053, 2921, 1655, 1613, 1551. 1HNMR (DMSOd-6, 500 MHz): δ = 2.81 (t, J = 7.5 Hz, 2H, CH2), 3.39 (q, J = 7.5 Hz, 2H, NH-CH2), 3.70 (s, 3H, CH3), 6.54 (d, J = 15.5 Hz, 1H, CHα), 7.00 (t, J = 7.0 Hz, 1H, H5), 7.12 (t, J = 7.5 Hz, 1H, H6), 7.19 (s, 1H, H2’), 7.25 (s, 1H, H2), 7.23 (t, J = 7.5 Hz, 1H, H5’), 7.40–7.45 (m, 3H, H4,4’,7), 7.41 (d, J = 15.5 Hz, 1H, CHβ), 7.62 (d, J = 7.0 Hz, 1H, H6´), 8.20 (t, J = 5.5 Hz, 1H, NH-CH2), 10.78 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.01, 39.6, 55.2, 111.3, 111.6, 115.1, 118.1, 120.8, 122.5, 124.0, 125.5, 127.1, 127.6, 128.2, 130.9, 132.9, 133.1, 134.5, 158.2, 164.2 ppm. Mass, m/z (%): 320(6), 279(7), 174(6), 161(97), 143(100), 130(39), 118(28), 105(20), 77(22). Anal. Calcd for C20H20N2O2: C, 74.98%; H, 6.29%; N, 8.74%. Found: C, 74.69%; H, 6.48%; N, 8.73%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-methoxyphenyl)acrylamide (5p)
Yield: 65%, mp: 177–179 °C. IR (KBr) cm−1 υ: 3450, 3231, 3049, 2929, 1648, 1600, 1539. 1HNMR (DMSO-d6, 500 MHz): δ = 2.89 (t, J = 7.0 Hz, 2H, CH2), 3.47 (q, J = 7.0 Hz, 2H, NH-CH2), 3.78 (s, 3H, OCH3), 6.48 (d, J = 15.5 Hz, 1H, CHα), 6.98 (d, J = 8.5 Hz, 2H, H3’,5’), 6.99 (t, J = 7.5 Hz, 1H, H5), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.17 (s, 1H, H2), 7.34 (d, J = 8.0 Hz, 1H, H7), 7.38 (d, J = 16.0 Hz, 1H, CHβ), 7.50 (d, J = 8.5 Hz, 2H, H2’, 6’), 7.56 (d, J = 8.0 Hz, 1H, H4), 8.10 (t, J = 5.5 Hz, 1H, NH-CH2), 10.80 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.2, 39.5, 55.1, 111.2, 111.8, 114.3, 118.1, 118.2, 119.9, 120.8, 122.5, 127.2, 127.5, 128.9, 136.2, 138.0, 160.2, 165.1 ppm. Mass, m/z (%): 320(18), 161(41), 143(100), 130(51), 118(12), 103(13), 77(15). Anal. Calcd for C20H20N2O2: C, 74.98%; H, 6.29%; N, 8.74%. Found: C, 74.74%; H, 6.52%; N, 9.04%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(3,4-dimethoxyphenyl)acrylamide (5q)
Yield: 76%, mp: 151–153 °C. IR 1 (KBr) cm−1 υ: 3376, 3299, 3054, 2939, 1648, 1609, 1545. 1HNMR (DMSO-d6, 500 MHz): δ = 2.89 (t, J = 7.5 Hz, 2H, CH2), 3.47 (q, J = 7.0 Hz, 2H, NH-CH2), 3.78 (s, 6H, 2-OCH3), 6.51 (d, J = 16.0 Hz, 1H, CHα), 6.96–6.99 (m, 2H, H5, 5’), 7.07 (t, J = 7.0 Hz, 1H, H6), 7.10 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, H6’), 7.15 (dd, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H, H2’), 7.33–7.38 (m, 3H, H2, CHβ-H7), 7.55 (d, J = 8.0 Hz, 1H, H4), 8.06 (t, J = 5.5 Hz, 1H, NH-CH2), 10.79 (s, 1H, NH) ppm. 13CNMR (DMSO-d6, 125 MHz): δ = 25.2, 39.5, 55.4, 55.5, 110.1, 111.3, 111.8, 112.0, 118.1, 118.2, 120.2, 120.8, 121.2, 122.5, 127.2, 127.8, 136.2, 138.4, 149.8, 150.0, 165.1 ppm. Mass, m/z (%): 350(3), 272(3), 255(7), 225(5), 191(6), 143(100), 130(60), 103(7), 77(8). Anal. Calcd for C21H22N2O3: C, 71.98%; H, 6.33%; N, 7.99%. Found: C, 71.74%; H, 6.22%; N, 7.69%.
(E)-N-(2-(1H-indol-3-yl)ethyl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide (5r)
Yield: 58%, mp: 168–170 °C. IR (KBr) cm−1 υ: 3359, 3294, 3084, 2931, 1649, 1575, 1420. 1HNMR (DMSO-d6, 500 MHz): δ = 2.89 (t, J = 7.5 Hz, 2H, CH2), 3.47 (q, J = 7.0 Hz, 2H, NH-CH2), 3.80 (s, 3H, OCH3), 6.46 (d, J = 15.5 Hz, 1H, CHα), 6.79 (d, J = 8.0 Hz, 1H, H5’), 6.96–6.99 (m, 2H, H5, 6’), 7.07 (t, J = 7.5 Hz, 1H, H6), 7.12 (s, 1H, H2’), 7.17 (s, 1H, H2), 7.32–7.35 (m, 2H, CHβ-H7), 7.56 (d, J = 7.5 Hz, 1H, H4), 8.02 (t, J = 5.5 Hz, 1H, NH-CH2), 9.36 (s, 1H, OH), 10.80 (s, 1H, NH) ppm. 13CNMR (DMSO, 125 MHz): δ = 25.2, 39.5, 55.5, 110.8, 111.3, 111.8, 115.6, 118.1, 118.2, 119.2, 120.8, 121.4, 122.6, 126.4, 127.2, 136.2, 138.8, 147.8, 148.1, 165.3 ppm. Mass, m/z (%): 336(18), 221(4), 194(6), 177(52), 143(100), 130(98), 117(15), 103(16), 77(20). Anal. Calcd for C20H20N2O3: C, 71.41%; H, 5.99%; N, 8.33%. Found: C, 71.64%; H, 6.19%; N, 8.60%.
AChE and BChE inhibition assay
To evaluate the potential inhibition of this series of compounds, Ellmanʾs method was used on AChE and BChE inhibitory test. Acetylcholinesterase (AChE, E.C. 3.1.1.7, Type V-S, lyophilized powder, from electric eel 1000 units), butyrylcholinesterase (BChE, E.C. 3.1.1.8, from equine serum) and butyrylthiocholine iodide were obtained from Sigma-Aldrich. 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), potassium dihydrogen phosphate, dipotassium hydrogen phosphate, potassium hydroxide, sodium hydrogen carbonate, acetylthiocholine iodine and butyrylthiocholine iodine were purchased from Fluka. Donepezil hydrochloride was obtained from Merck, Darmstadt, Germany. To prepare the stock solution of testing compounds, 10 mg of testing compounds were dissolved in DMSO (10 μL) and ethanol 99% (400 μL). To determine IC50 values, four different concentrations of each compound were examined in the range of 20–80% for AChE and BChE. All experiments were performed at 25 °C. The assay was performed as the literature reported [9]. The IC50 values were determined [3, 16] graphically from inhibition curve (log inhibitor concentration vs. percent of inhibition).
Kinetic studies of BChE and AChE inhibition
For estimation of inhibition model and inhibition constant (Ki), cal plots of 1/V versus 1/[S] was used. Three concentrations of the inhibitor (compound 5b and 5q) were selected for the analysis of BChE and AChE inhibition. The rate of enzymatic reaction was obtained with three different concentrations of the inhibitor and in the absence of inhibitor. For each of them, the initial velocity (V) of substrate hydrolysis was measured at the different concentration of substrate (S) in the range of 0.14–0.69 μM [6] and with the obtained data; double reciprocal plots (1/V vs. 1/[S]) were made. By increasing the inhibitor concentration, the resulting Lineweaver-Burk plot showed increasing slopes and intercepts which are characterized by mixed-type inhibition. Slops of these reciprocal plots were then plotted against the concentration of compound 5b and 5q in a weighted analysis, and Ki was determined as the intercept on the negative x-axis. All rate measurements were performed in triplicate and data analysis was performed with Microsoft Excel 2013 [17].
Neuroprotective assay
The neuroprotective activity of the synthesized compounds was evaluated according to the literature [1]. Before treatment with H2O2 (300 mM), differentiated PC12 cells were incubated with different concentrations of the compound 5q for 3 h. The assay was performed as the literature reported [9, 11, 18]. Results were adjusted considering OD measured in the blank.
BACE1 enzymatic assay
By using fluorescence resonance energy transfer (FRET), BACE1 enzyme assay kit was used (Invitrogen, former Pan Vera Corporation, Madison, WI) and the assay was accomplished according to the manufacturer’s instructions (Invitrogen. http://tools.com/content/sfs/manuals/L0724.pdf). The sample preparation was performed as the literature reported [9]. Fluorescence measurements were performed with a multimode microplate reader (BMG Labtech) at excitation and emission wavelength of 545 nm and 585 nm, respectively. This test was repeated for 3 to 5 times eventually average percent of enzyme inhibitory activities at 10 and 50 μM concentration of test compounds were calculated [9, 17, 19–21].
Determination of the inhibitory potency on Aβ1–42 self-aggregation induced
Inhibition of self-induced Ab (1–42) aggregation was measured using a Thioflavin T(ThT)-binding assay [22–24]. The sample preparation and the assay were performed as the literature reported [5]. Each measurement was run in triplicate. Fluorescence was measured on a Synergy HTX Multi-Mode reader from BioTek instruments with excitation and emission wavelengths at 440 nm and 485 nm, respectively. The percent inhibition of aggregation was calculated by the expression (1- IFi/IFO) × 100% in which IFi and IFO are the fluorescence intensities obtained for Aβ in the presence and absence of inhibitors, respectively.
Molecular docking study
Based on the X-ray crystal structures of AChE and BChE docking studies for compounds 5q and 5b were performed using Autodock 4.2 program. The PDB structures of 1EVE (for compound 5q) and 4BDS (for compound 5b) [6, 14, 17] were retrieved from the Brookhaven protein database (http://www.rcsb.org). Then the water molecules and the original inhibitors were removed and the amino acid chain was kept. Subsequently, the 3D structure of the compounds 5q and 5b were prepared by using Marvin Sketch 5.8.3, 2012, ChemAxon (http://chemaxon.com) and using Autodock 4.2 program converted to pdbqt format. Also, the pdbqt of protein was provided by using the same software. It should be noted that polar hydrogen atoms were added to the receptor and resulting protein by using Autodock tools (ADT; version1.5.6), Kullman charges were appointed to all atoms of an enzyme. The resulting structure of enzyme was used as an input format of the AUTOGRID software which is performed a pre-calculated atomic affinity grid map. For the docking calculation, the dimensions of grid box size 60 × 60 × 60 Ao was set at the geometrical center of co-crystalized ligand. The other parameters were placed by default. At the end, the conformation which has the lowest free energy of binding was selected for analyzing the interactions between AChE and the inhibitor. The results were visualized using Discovery studio 4.0 Client [25]. The same method was utilized in the case of BChE.
Computational methods
The log P values, tPSA, HBD, and HBA were calculated by the means of MarvineSketch 5.8.3. RBC was calculated using Autodock tools (ver.1.5.6).
Result and discussion
Chemistry
The cinnamic-derived acids 5a-r were synthesized through the routes depicted in Scheme 1. The related benzaldehyde (1) were reacted with malonic acid (2) in a mixture of pyridine, piperidine and ethanol under refluxing condition. The product was crystallized from ethanol to product cinnamic-derived acids (3) in a good to excellent yield. The reaction of compounds 3 and tryptamine (4) in the presence of N-hydroxy benzotriazole (HOBT), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC) gave 5a-54 in dry acetonitrile at room temperature. The purification of the product was carried out using flash chromatography using hexane and ethyl acetate (8:1). The final compounds were crystallized in ethyl acetate and hexane (1:1). These structures were characterized by IR, 1H-NMR, 13C-NMR and elemental analysis.
Scheme 1.
Synthesis pathway for preparation of compounds 5. Reagents and conditions: I: pyridine, piperidine, ethanol, 100 °C, reflux, 24 h; II: HOBT, EDCI, dray acetonitrile, r.t, 48 h
In the 1HNMR spectra of compounds 5, there are several specified signals that could be referred to the structure. For example hydrogen of vinyl group, indol ring, methylen groups in aliphatic bridge and also aromatic hydrogen of indol between amid group and indol ring are identified. In the more derivatives, H2 indol appeared at about 7.2 ppm as singlet with integration value of one proton. However, in some derivatives this peak was overlapped with other aromatic hydrogens. Two separated peaks at about 7 and 7.1 ppm appeared as triplet that could be attributed to 5-H and 6-H of indol ring respectively. The two triplet signals at 2.9 and 3.5 ppm could be referred to CH2 group of aliphatic side chain. In α, β-unsaturated carbonyl group, two 15 Hz-spilited signals appeared about 6.6 and 7.4 ppm. Other aromatic protons are observable at a broad range (6.7–8 ppm) related to the substituted groups on the cinnamic acid moiety.
Pharmacology
In vitro inhibition of AChE, BChE
The in vitro AChE and BChE inhibitory activity of new cinnamic acid-tryptamine hybrids (5a-5r) were studied using the Ellmanʾs method and donepezil as a reference drug. Inhibition potency of the synthesized compounds under the title of IC50 is presented in Table 1. Among these compounds, 5q (IC50 = 11.51 μM) showed the best AChE inhibitory activity. The simplest compound 5a, with no substitution on the aryl groups, showed moderated activity against AChE (IC50 = 53.21 μM) and BChE (IC50 = 25.27 μM). Replacement of hydrogen atom on the cinnamic acid moiety with chlorine atom on every third position of ortho, meta and para improved AChE inhibitory activity (IC50 = 21.11, 32.63 and 27.2 μM), while the insertion of fluorine atom instead of the chlorine atom decreased anticholinesterase inhibitory activity (IC50 = 33.57, 31.89 and 48.14 μM). The methyl group at every third position showed a mild increase in the AChE inhibitory activity (IC50 = 23.77, 31.34 and 39.22 μM). The presence of electron-donating methoxy groups in every third position of the aryl group of cinnamic-derived acids resulted from increasing the AChE inhibitory activities (IC50 = 19.34, 27.85 and 14.36 μM). It should be noted that replacement of Hydrogen in position R2 and R3 by methoxy in the aryl group of cinnamic-derived acids (5q) showed the highest activity in this group (IC50 = 11.51 μM). It was 5 times more potent than parent compound 5a, while in compound 5r, that has a methoxy group in position R2 and a hydroxyl group in position R3, resulted in the reduction of anti-AChE activity with IC50 = 21.92 μM compared with compound 5q. Insertion of a nitro substituent on the ring compared with the parent compound 5a led to the significant reduction of anti-AChE activity (IC50 = 95.85, >100 and 65.36 μM). Another instructive point that is a replacement of hydrogen by fluorine, methyl, chlorine and methoxy group in the aryl group of cinnamic-derived acids moieties respectively led to increasing of anti-AChE activity. It seems that the AChEI activities of these synthesized compounds were under the influenced of electronic effect of substituent groups on the aromatic ring of cinnamic acids moieties.
Table 1.
AChE and BChE inhibition IC50 (μM) of compounds, Selectivity index IC50 (BChE)/IC50 (AChE)
Based on our data, compound 5b depicted the best BChEI activity (IC50 = 1.95 μM) which has chlorine in position R1, It was 25 folds more potent than parent compound 5a. The observed IC50 values of these designed compounds (5a-5r) against BChE demonstrated that these compounds had mild to good activity against BChE and Compound 5q with two methoxy group in position R2 and R3, showed no inhibitory effect against BChE. It should be noted that compound 5q acted as a selective AChE inhibitor. Replacement of hydrogen in position R2 with the nitro group (IC50 = 50.46 μM) or methoxy group (IC50 = 41.25 μM) led to decrease of inhibitory activity against BChE significantly, and showed less anti-BChE activity compared with parent compound 5a and other derivatives. Compounds 5n (IC50 = 18.25 μM), 5 k (IC50 = 18.55 μM) and 5r (IC50 = 17.81 μM) which respectively had methoxy group or nitro group in position R1 or methoxy group in position R2 and hydroxyl group in position R3 instead of hydrogen depicted better inhibitory activity against BChE with negligible difference in IC50 values. Also, compounds 5f with fluorine atoms in position R2 and 5i with the methyl group in position R2 showed good inhibitory activity toward BChE as IC50 = 15.77 μM and 16.44 μM respectively. In compounds 5 h which had the methyl group in position R1 and 5e which had fluorine atom in position R1, we have seen an increase in inhibitory activity of BChE as IC50 = 11.8 μM and 6.67 μM respectively. However, the best inhibitory activity was obtained by compound 5b with IC5 0 = 1.95 μM which had chlorine in position R1.
With information obtained from IC50 values, the process of anti-BChE activity did not follow anti-AChE activity as compound 5q with the strongest inhibitory effect toward AChE, showed no inhibitory effect on BChE and it was exclusively served for the inhibition of AChE and compounds 5 k and 5 l were exclusive for BChE.
Parallel to the progression of AD, there is a significant increase in BChE level in the most parts of the brain, therefore inhibition of BChE could be important as well as AChE. Recent studies shows selective BChE inhibitors enhance learning in animal models [26].
Kinetic studies
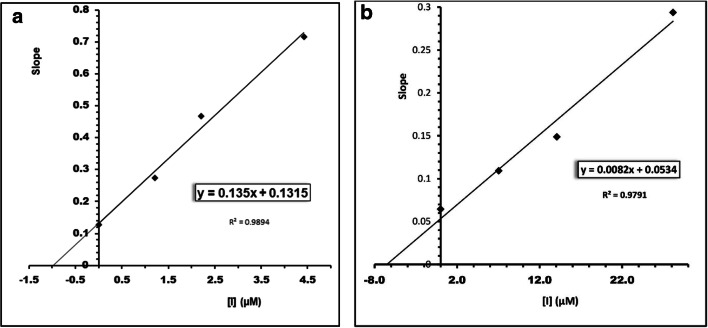
In order to obtain the kinetic model of BChE and AChE inhibition by the most active compounds 5b and 5q, Ellmanʾs method has been used to achieve this aim. The graphical analysis of Lineweaver-Burk reciprocal plot showed in Fig. 2. This graph depicted a mixed type of inhibition for BChE and AChE and suggesting that these compounds have the ability to bind both the peripheral anionic site (PAS) and the catalytic anionic site (CAS) of BChE and AChE. As can be seen in Fig. 3, for compound 5b the inhibition constant Ki (Ki = 0.97 μM) and for compound 5q the inhibition constant Ki (Ki = 6.53 μM) were calculated using the graph of the slope versus concentration of synthesis inhibitors (Table 2).
Fig. 2.
The Lineweaver-Burk secondary plots of compounds 5b (A) and 5q (B)
Fig. 3.
Steady state inhibition of BChE and AChE by compound 5b (A) and 5q (B)
Table 2.
Ki value for compounds 5b and 5q
| Compounds | Ki (μM, BChE) | Ki (μM, AChE) |
|---|---|---|
| 5b | 0.97 | – |
| 5q | – | 6.53 |
Neuroprotective study
A useful model to assess the neuronal differentiation is the PC12 cell line. For this aim, differentiated PC12 used as in vitro model and hydrogen peroxide (H2O2) used as an oxidative agent. Therefore subjecting PC12 to H2O2-induced damage depicted the neuroprotective activity of the most potent compound 5q toward AChE. The percent of cell viability in comparison to control group (Quercetin) showed in Fig. 4 [1, 25]. On the basis of the result, compound 5q showed mild neuroprotective activity at 10 μM (P < 0.05 vs. H2O2 alone) but in concentration 1 and 100 μM showed no significant neuroprotective activity.
Fig. 4.
Neuroprotective effect of compound 5q on cell viability of PC12 cells in H2O2-induced damage. Data are expressed as mean ± SD (n = 8) and one-way analysis of variance (ANOVA) followed by Tukeys multiple comparision test was carried out to determine the level of significance. ####P < 0.0001 vs. control, ****P < 0.0001 vs. H2O2 and *P < 0.05 vs. H2O2
BACE1 inhibitory acivity
To investigate the BACE-1 inhibitory activity of the most potent compound toward AChE (5q) through the BACE-1 kit that includes of BACE-1 enzyme and specific APP based peptide substrate and on the basis of fluorescence resonance energy transfer (FRET) was done. All the experiments were repeated three times and were compared with the reference compound OM99–2, a hexapeptid derivative (CAS Number 314266–76-7). The results in Table 3 show that compound 5q demonstrated BACE1 inhibitory activity weaker than OM99–2 with IC50 = 0.014 ± 0.001 μM.
Table 3.
Inhibitory activity of compound 5q against BACE1 comparing with OM99–2
| Entry | Compound | Inhibition at 50 μMa (%) | Inhibition at 10 μMa (%) | IC50 (μM) |
|---|---|---|---|---|
| 1 | 5q | 43.73 | 27.81 | – |
| 2 | OM99–2b | – | – | 0.014 ± 0.001 |
aValues represent means ± standard error (S.E.) of three independent experiments. bOM99–2 was tested at 10, 1 and 0.1 nM
Inhibition of self-induced Aβ aggregation
The most potent compound of this series of synthesized compound (5q) was selected to investigate its ability to inhibit Aβ1–42 peptide self-aggregation utilizing the thioflavin T (ThT) fluorescence method and compared with donepezil and curcumin as reference compounds. The result of the inhibition for compound 5q at 50 μM concentration was 10.12 ± 1.23% that could be comparable with donepezil (14.70 ± 2.35%).
Docking studies
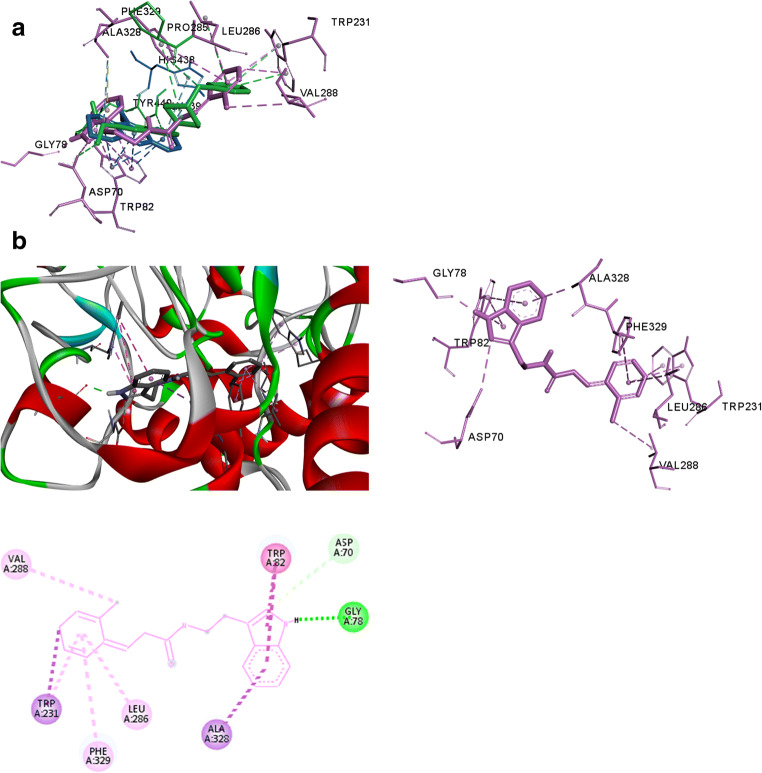
In order to better understand the inhibition of AChE, BChE and possible interaction between compounds 5q and 5b with active site of the enzyme, the molecular modeling study was carried out. It was done using the docking program, auto duck 4.2 and discovery studio 4.0 client. The results in Figs. 5a, b and 6a, b depicted that compounds 5q and 5b with a proper orientation located in the binding site of AChE and BChE. These figure showed that compounds 5q and 5b are long enough to accommodate in the peripheral anionic site (PAS) and the catalytic site (CAS) of AChE. As Fig. 5b, tryptamine moiety occupied the CAS with two П-П stacking interaction between Phe330 and Trp 84 residues respectively. There is an extra polar interaction with Glu 199 residues in CAS and cinnamic acid moiety that was located the PAS and there is hydrophobic interaction between methoxy group and Tyr 121 and Tyr 70 residues which they are two key residues in PAS. In addition, there is another hydrophobic interaction between the methoxy group and Asn 85 and a hydrogen bonding between the oxygen of carboxyl group and Tyr130 led to stabilization of resulting complex between compound 5q and the active site of the enzyme.
Fig. 5.
a Superimosition of the most potent compound 5q (purple) and donepezil (green) in the active site of AChE. b The binding mode of the most active compound 5q in the active site of AChE
Fig. 6.
a Superimposition of the most potent compound 5b (purple), donepezil (green) and tacrinne (blue) in the active site of BChE. b The binding mode of the most active compound 5b in the active site of BChE
On the other hand, docking result of compound 5b in the active site of BChE was illustrated in Fig. 6b. As this figure, cinnamic acid moiety of compound 5b formed П-alkyl stacking interaction between Leu 286 and Val 288 residues. Furthermore, П-П stacking interaction between Triptamine moiety and Trp 82 residue and a hydrogen bonding with Asp 70 residue (in the PAS) could be observed. Moreover, two П-sigma interactions with Trp 231 and Ala 328, one П-alkyl interaction with Phe 329 and one conventional hydrogen bonding with Gly 78 residues led to well fitted of compound 5b in the active site of BChE.
These figures clear that compounds 5q and 5b were a dual binding site inhibitors which could be simultaneously interacted with both CAS and PAS of AChE and BChE.
Virtual calculation of pharmacokinetic properties
In order to evaluate the competence of compound 5q as a drug candidate, it is necessary some pharmacokinetic properties such as octanol-water partition coefficient (ClogP), number of H-bond donors (HBD), number of H-bond acceptor (HBA), rotatable bonds count (RBC) and topological polar surface area (tPSA) calculated and the outcome of the assessment for compound 5q shown in Table 4. Based on Lipinski’s rule-of-five including MW ≤ 500, HBD ≤ 5, HBA ≤ 10, ClogP≤5 and polar surface area ≤ 140Ao2, it seems that these synthesized compound are the good candidate to orally active drug in human.
Table 4.
Molecular descriptors of the compound 5q
| Entry | Compound | HBD | HBA | Clog P | tPSA (Ao2) | MW | RBC |
|---|---|---|---|---|---|---|---|
| 1 | 5q | 2 | 3 | 3.55 | 59.59 | 350.42 | 7 |
ClogP: Calculated octaol-water partition coefficient, HBD: number of H-bond donors, HBA: number of H-bond acceptor, RBC: rotatable bonds count, tPSA: topological polar surface area
Conclusion
In summary, we reported design and synthesis of a new series of cinnamic acids-tryptamine hybrids as the new anti-AD agent. The potency of these compounds for inhibition of AChE was moderate to good. In this series, compound 5q with IC50 = 11.51 μM was the most potent compound with the inhibitory effect against AChE. The synthesized compounds contained triptamine moiety and the cinnamic acid group occupied the CAS and the PAS that was confirmed by the kinetic and molecular modeling study. Furthermore, these compounds was examined for anti-cholinesterase, neuroprotective, BACE1 inhibitory activity. It should be noted that the cholinesterase inhibitory and neuro-protective effect of 5q was acceptable; however it did not show potent BACE1 inhibitory activity. Docking study showed tryptamine group of 5b captures the peripheral anionic site of BChE. This interaction is reinforced by cinnamic acid moiety that interacts with catalytic site of enzyme. These findings shows that hybrid between cinnamic acid and tryptamine increased the potency of compounds 5 series via formation of a dual binding interaction with CAS and PAS. Based on Lipinski’s rule this compound is the good candidate to orally active drug in human. The results showed that this series with dual binding site for interaction with AChE and BChE have a potential for the treatment of AD.
Electronic supplementary material
(PDF 108 kb)
Acknowledgments
This work was supported by grants (9211302002) from the Research Council of Tehran University of Medical Sciences.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Najafi Z, Mahdavi M, Saeedi M, Karimpour-Razkenari E, Asatouri R, Vafadarnejad F, Homayouni Moghadam F, Khanavi M, Sharifzadeh M, Akbarzadeh T. Novel tacrine-1,2,3-triazole hybrids: in vitro, in vivo biological evaluation and docking study of cholinesterase inhibitors. Eur J Med Chem. 2017;125:1200–1212. doi: 10.1016/j.ejmech.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Ting Luo X, Wang C, Liu Y, Huang ZG. New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant and neuroprotective properties as innovative drugs for Alzheimer’s disease. Eur J Med Chem. 2015;103:302–311. doi: 10.1016/j.ejmech.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 3.Ghanei-Nasab S, Khoobi M, Hadizadeh F, Marjani A, Moradi A, Nadri H, Emami S, Foroumadi A, Shafiee A. Synthesis and anticholinesterase activity of coumarin-3-carboxamides bearing tryptamine moiety. Eur J Med Chem. 2016;121:40–46. doi: 10.1016/j.ejmech.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Mostofi M, Mohammadi Ziarani G, Mahdavi M, Moradi A, Nadri H, Emami S, Alinezhad H, Foroumadi A, Shafiee A. Synthesis and structure-activity relationship study of benzofuran-based chalconoids bearing benzylpyridinium moiety as potent acetylcholinesterase inhibitors. Eur J Med Chem. 2015;103:361–369. doi: 10.1016/j.ejmech.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 5.Mollazadeh M, Mohammadi-Khanaposhtani M, Zonouzi A, Nadri H, Najafi Z, Larijani B, Mahdavi M. New benzyl pyridinium derivatives bearing 2,4-dioxochroman moiety as potent agents for treatment of Alzheimer’s disease: design, synthesis, biological evaluation, and docking study. Bioorg Chem. 2019;87:506–515. doi: 10.1016/j.bioorg.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Khoobi M, Alipour M, Saakhteman A, Nadri H, Moradi A, Ghanadi M, Emami S, Foroumadi A, Shafiee A. Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur J Med Chem. 2013;68:260–269. doi: 10.1016/j.ejmech.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 7.Pb W, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimerʼs disease. JAMA. 1994;271:992–998. doi: 10.1001/jama.1994.03510370044030. [DOI] [PubMed] [Google Scholar]
- 8.Baharloo F, Moslemin MH, Nadri H, Asadipour A, Mahdavi M, Firoozpour L, Mohebat R, Shafiee A, Foroumadi A. Benzofuran-derived benzylpyridinium bromides as potent acetylcholinesterase inhibitors. Eur J Med Chem. 2015;93:196–201. doi: 10.1016/j.ejmech.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Najafi Z, Saeedi M, Mahdavi M, Sabourian R, Khanavi M, Tehrani MB, Moghadam FH, Edraki N, Karimpor-Razenari E, Sharifzadeh M, Foroumadi A, Shafiee A, Akbarzadeh T. Design and synthesis of novel anti-Alzheimerʼs agents: Acridine-chroomenone and quinolone-chromenone hybrids. Bioorg Chem. 2016;67:84–94. doi: 10.1016/j.bioorg.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Estrada M, Herrera-Arozamena C, Perez C, Vina D, Romero A, Morales-Garcia JA, Perez-Castillo A. New cinnamic-N-benzylpiperidine and cinnamic-N,N-dibenzyl (N-methyl) amine hybrids as Alzheimer-directed multitarget drugs with antioxidant, cholinergic, neuroprotective and neurogenic properties. Eur J Med Chem. 2016;121:376–386. doi: 10.1016/j.ejmech.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Arab S, Sadat-Ebrahimi S, Mohammady-Khanaposhtani M, Moradi A, Nadri H, Mahdavi M, Firoozpoor L, Pirali-Hamedani M, Shafiee A, Foroumadi A. Synthesis and evaluation of chroman-4-one linked to N-benzyl pyridinium derivatives as new acethylcholinesterase inhibitors. Arch Pharm Chem. 2015;384:1–7. doi: 10.1002/ardp.201500149. [DOI] [PubMed] [Google Scholar]
- 12.Saeed A, Mahesar PA, Zaib S, Siraj Khan M, Matin A, Shahid M, Iqbal J. Synthesis, cytotoxicity and molecular modeling studies of new phenylcinnamide derivatives as potent inhibitors of cholinesterase. Eur J Med Chem. 2014;78:43–53. doi: 10.1016/j.ejmech.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Hariri R, Afshar Z, Mahdavi M, Safavi M, Saeedi M, Najafi Z, Sabourian R, Karimpour-Razknari E, Edraki N, Homayouni Moghadam F, Shafiee A, Khanavi M, Akbarzadeh T. Novel Tacrine-based Pyranol [3′,4′:5,6]pyrano[2,3-b]quinolinones: synthesis and cholinesterase inhibitory activity. Arch Pharm Chem. 2016;349:1–10. doi: 10.1002/ardp.201500337. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Bing Wang X, Min Wang Z, Wu JJ, Li F, Wang J, Kong LY. Synthesis and evaluation of donepezil-ferulic acid hybrids as multi-targeted ligands against Alzheimerʼs disease. Med Chem Commun. 2016;7:990–998. doi: 10.1039/C6MD00053C. [DOI] [Google Scholar]
- 15.Zhang X, He X, Chen Q, Lu J, Rapposelli S, Pi R. A review on the hybrids of hydroxycinnamic acid as multi-targetdirected ligands against Alzheimer’s disease. Bioorg Med Chem. 2016;26:543–550. doi: 10.1016/j.bmc.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Khoobi M, Ghanoni F, Nadri H, Moradi A, Pirali Hamedani M, Hmayouni Moghadam F, Emami S, Vosoghi M, Zadmard R, Foroumadi A, Shafiee A. New tetracyclic tacrine analogs containing pyranol [2,3-c] pyrazole: efficient synthesis, biological assessment and docking simulation study. Eur J Med Chem. 2015;89:296–303. doi: 10.1016/j.ejmech.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi-Khanaposhtani M, Mahdavi M, Saeedi M, Sabourian R, Safavi S, Khanavi M, Foroumadi A, Shafiee A, Akbarzadeh T. Design, synthesis, biological evaluation, and doking study of acetylcholinesterase inhibitors: new acridone-1,2,4-oxadiazole-1,2,3-triazole hybrids. Chem Biol Drug Des. 2015;86:1425–1432. doi: 10.1111/cbdd.12609. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Cai P, Yang X, Li F, Wu JJ, Kong LY, Wang X. Novel cinnamide-dibenzyl hybrids: potent neurogenic agents with antioxidant, cholinergic, and neuroprotective properties as innovative drugs for Alzheimerʼs disease. Eur J Med Chem. 2017;139:68–83. doi: 10.1016/j.ejmech.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 19.Bhakta HK, Park CH, Yokozowa T, Tanaka T, Jung AH, Choi JS. Potent anti-cholinesterase and β-amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from cornus officinalis fruits. Arch Pharm Res. 2017;40:836–53. [DOI] [PubMed]
- 20.Pietrzik C, Behl C. Concepts for the treatment of Alzheimerʼs disease: molecular mechanisms and clinical application. Int J Exp Pathol. 2005;86:173–185. doi: 10.1111/j.0959-9673.2005.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeedi M, Safavi M, Karimpour-Razkenari E, Mahdavi M, Edraki N, Homayouni Moghadam F, Khanavi M, Akbarzadeh T. Synthesis of novel chromenons linked to 1,2,3-triazole ring system: investigation of biological activities against Alzheimerʼs disease. Bioorg Chem. 2017;70:86–93. doi: 10.1016/j.bioorg.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Bartolini M, Bertucci C, Bolognesi ML, Cavalli A, Melchiorre C, Andrisano V. Insight into the kinetic of amyloid beta (1-42) peptide self-aggregation: elucidation of inhibitors’ mechanism of action. ChemBioChem. 2007;8:2152–2161. doi: 10.1002/cbic.200700427. [DOI] [PubMed] [Google Scholar]
- 23.Zha GF, Zhang CP, Qin HL, Jantan L, Sher M, Amjad MW, Hussain MA, Hussain Z, Bukhari SNA. Biological evaluation of synthetic α,β-unsaturated carbonyl based cyclohexanone derivatives as neuroprotective novel inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-β aggregation. Bioorg Med Chem. 2016;24:2352–2359. doi: 10.1016/j.bmc.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Reinke AA, Gestwicki JE. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: influence of linker length and flexibility. Chem Biol Drug Des. 2007;70:206–215. doi: 10.1111/j.1747-0285.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 25.Gordone J, Amini S, White MK. General overview of neuronal cell culture. Methods Mol Biol. 2013;1078:1–8. doi: 10.1007/978-1-62703-640-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghobadian R, Mahdavi M, Nadri H, Moradi A, Edraki N, Akbarzadeh T, Sharifzadeh M, Bukhari SNA, Amini M. Novel tetrahydrocarbazole benzyl pyridine hybrids as potent and selective butryl cholinesterase inhibitors with neuroprotective and b-secretase inhibition activities. Eur J Med Chem. 2018;155:49–60. doi: 10.1016/j.ejmech.2018.05.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 108 kb)