Abstract
Purpose
Cancer as one of the major diseases with high mortality rates threats human life in the world. Subsequently, the design new potent anticancer agents has attracted much attention in the area of synthetic and medicinal chemistry. In this study, new triazol-linked spiroindolinequinazolinone, thiazol-oxindole and oxindole-thiosemicarbazone conjugates were synthesized and evaluated for their in vitro cytotoxic activity toward different cancer lines.
Methods
Some new triazol-linked oxindoles and spirooxindoles conjugates were synthesized. The synthesized compounds were tested for their in vitro cytotoxic activity toward cancer lines including A375, PC3, LNCaP, MDA MB231 and normal cells HDF (human dermal fibroblast).
Results
Among all synthesized compounds, the triazol-linked oxindol-thiosemicarbazone conjugate 10b showed the highest cytotoxic activity against different cancer cells. By using quantitative real time PCR (qRT-PCR), it was found that 10b is able to induce apoptosis by alteration of Bax, Bcl2 balance (i.e. by up regulation of Bax and down regulation of Bcl-2 mRNA expression levels). The DAPI staining was used to show the death of cancer cells in the presence of 10b. Interestingly, 10b suppressed the migration of LNCaP cancer cells by up-regulation of epithelial markers (E-cadherin) and down-regulation of mesenchymal markers (vimentin).
Conclusion
Our findings revealed that the compound 10b may be a new potent candidate with multiple biological activities to design therapeutic agents against different cancers.
Graphical abstract
Synthesis and evaluation of in vitro cytotoxic effects of triazol/spiroindolinequinazolinedione, triazol/indolin-3-thiosemicarbazone and triazol/thiazol-indolin-2-one conjugates.
Electronic supplementary material
The online version of this article (10.1007/s40199-020-00364-7) contains supplementary material, which is available to authorized users.
Keywords: Spirooxindole containing triazole, Spiroindolinequinazoline-dione, Oxindole containing thiazole, Isatin, Apoptosis, Anticancer activity
Introduction
Cancer as an uncontrollable growth of abnormal cells is considered as one of the major diseases with high mortality rates which threats human life in the world [1]. Most of the clinically available anticancer chemotherapeutic agents cannot distinguish between the normal and cancerous cells. Moreover, they have unwanted side effects and drug resistance [2, 3]. Subsequently, the design, development and synthesize new potent anticancer agents has attracted much attention in the area of synthetic and medicinal chemistry.
Isatin is endogenously found in human and other mammalian tissues and has been observed as a privileged scaffold in many natural products and alkaloids [4]. Isatin has become known as an important moiety that is endowed with many promising biological properties [4–8], mainly anticancer activity [9]. Spirotryprostatin A (I), Spirotryprostatin B (II) and Strychnofoline are natural spirooxindoles possessing anticancer activities (Fig. 1) [10]. The FDA has been approved Sunitinib (Sutent®) (III), a 5-fluoro-3-substituted oxindole derivative, for treating advanced renal cell carcinoma (RCC) and gastrointestinal stromal tumors (GIST) [11]. Nintedanib (Ofev®) (IV) in combination with docetaxel for the patients with non-small cell lung cancer is in first-line chemotherapy [12]. Nintedanib was tested against colorectal cancer [13] and breast cancer [14].
Fig. 1.
Design of target molecules based on reported isatin-based molecules with cytotoxic potential
Recently, molecular conjugation and hybridization have reported as efficient and applicable tools for development of new potentially active molecules [15]. The strategy is extremely interesting and can minimize the drug resistance and risk of drug-drug interactions [16]. Therefore, a lot of efforts have been focused on anticancer potential of isatin-based hybrid or conjugate molecules such as isatin-thiazol hybrids (V) [17], isatin–benzothiazole analogs (VI) [18], isatin-thiazolidinone (VII) [19], isatin-benzimidazole (VIII) [20] and N-indolylmethyl spiroindoline-quinazolines (IX) [21]. The outstanding structural properties of 1,2,3-triazoles like hydrogen bonding ability, moderate dipole character, and stability and rigidity under in vivo conditions make their enhanced biological activities [22]. Likewise, thiosemicarbazones have significant antitumor activities [23]. Their antitumor activity is because of an inhibition of DNA formation produced by the moderation in the reductive reaction of ribonucleotides to deoxyribonucleotides [23]. In the recent years the attention of organic and medicinal chemists has attracted to design the 1,2,3-triazoles or thiosemicarbazone conjugate or hybrid with isatin derivatives as new anticancer candidate drugs [24–30]. For example, the 1,2,3-triazole tethered nitroimidazole-isatin conjugates [25], and spirooxindole-derived morpholine-fused-1,2,3-triazoles [26] displayed a good anti-proliferative activity against selected human cancers. The potential anticancer activity of triazole-linked indole and oxindole glycoconjugates was evaluated by Babu’s research group [27]. The oxindole-thiosemicarbazones (X) with anti-cancer activity was synthesized and the effectiveness of aromatic/hydrophobic properties at the N4 position of the thiosemicarbazone was investigated [28]. The 4-thiazolidinone-indoles showed anti-proliferative activities against human tumor cells [29]. Inspired by the above reports and as a continuation of our previous work on the development of new methods for oxindole and spirooxindole synthesis [31–33], we report herein the synthesis and in vitro cytotoxic evaluation of 1,2,3-triazol/spiroindolinequinazoline-dione, 1,2,3-triazol/thiazol-indolin-2-one and 1,2,3-triazol/indolin-3-thiosemicarbazone conjugates (Fig. 1).
Results and discussion
Chemistry
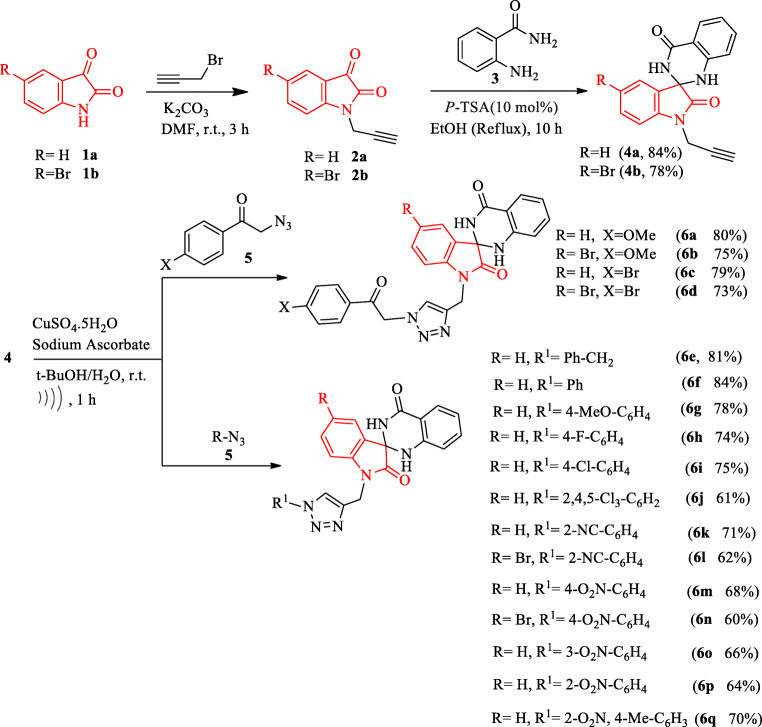
The synthesis pathway of 1,2,3-triazol/spiro[indoline-3,2′-quinazoline]-dione conjugate 6 has been shown in Scheme 1. The product 6 was synthesized by a sequential reaction starting from isatin 1. The cyclization and Cu-catalyzed click chemistry are the key steps for the operationally simple synthesis of 6. First, N-propargyl isatins 2 were synthesized by the nucleophilic reaction of isatins 1 and propargyl bromide in the presence of anhydrous potassium carbonate. The subsequent P-toluenesulfonic acid (p-TSA) catalyzed nucleophilic condensation reaction of 2 with 2-aminobenzamide 3 in EtOH under reflux conditions afforded 1-(prop-2-yn-1-yl)-1′H-spiro[indoline-3,2′-quinazoline]-2,4′(3′H)-diones 4 [31]. The target triazol-liked spiro[indoline-quinazoline]-diones 6 were obtained by the Cu-catalyzed click reaction of 4 and aryl or alkyl azids 5 under ultrasonic irradiation in t-BuOH-H2O for 1 h (Scheme 1). To delineate the role of ultrasound, the final step of 6a synthesis was done without ultrasonic irradiation at the same temperature in t-BuOH-H2O. The product 6a was obtained in 72% isolated yield after 5 h. It confirms the use of ultrasound irradiation leads to the higher yield at shorter reaction time (Scheme 1). Therefore, ultrasonic irradiation conditions was selected for the final step.
Scheme 1.
Synthesis of 1,2,3-triazol/spiro[indoline-3,2′-quinazoline]-diones 6
The potential anticancer activities of thiazole skeleton [34] encouraged us to synthesize a series of 1,2,3-triazol-linked thiazole-oxindole conjugates 9 (Scheme 2). The 1H-1,2,3-triazol-indoline-2,3-diones 7 were obtained by the Cu-catalyzed click reaction of 2 and phenacyl azides 5 under ultrasonic irradiation in t-BuOH-H2O at room temperature. Then, 1,2,3-triazol-linked thiazole-oxindole conjugates 9 were synthesized by a three-component reaction of 7, thiosemicarbazid and phenacyl bromides 8 in isopropyl alcohol under ultrasonic irradiation at 65 °C for 1 h.
Scheme 2.
Synthesis of triazol-linked thiazole-oxindole conjugates 9
So far, there is not any report to evaluate the possible cytotoxic effects of indolinone and thiosemicarbazone conjugate molecule. Therefore, the 1,2,3-triazol-linked oxindol-thiosemicarbazone conjugates 10 were synthesized by the reaction of 7 and thiosemicarbazid in isopropyl alcohol under ultrasonic irradiation at 65 °C for 1 h (Scheme 2). All compounds 6, 9 and 10 are stable solids whose structures were established by IR, Mass, and 1H, and 13C NMR spectroscopy.
In vitro cytotoxic evaluation
In an initial step, the MTT colorimetric assay was used to evaluate the cytotoxic potential of synthesized compounds 6a-q, 7a-c, 9a-i and 10a-c against cancer cell lines such as A375 cells, PC3 cells, LNCaP cells, MDA-MB-231 cells, and normal cell line HDF (human dermal fibroblast).
The results is indicated in term of inhibitory concentration of 50% growth (IC50) at 48 h (Table 1) The Etoposide and DMSO (1%) were used as a positive and a negative control, respectively. As can be seen in Table 1, the most of compounds 6 showed inhibitory effects against A375 cancer cell line. The compounds 6f, 6i and 6m showed cytotoxicity against two cancer cell lines. However, their IC50 values were higher than IC50 values of Etoposide. Notably, the compounds 6q was able to inhibit cell growth in A375 and LNCaP cancer cell lines with IC50 values that was close to the response rates for Etoposide. The results revealed that the type of substituent on isatin and phenyl ring of triazol moiety considerably influences cytotoxic potential of conjugate molecules 6. As evident from the activity results, on in vitro evaluation of the intermediates 1H-1,2,3-triazol-indoline-2,3-diones 7, compounds 7a and 7c having 4-methoxy or nitro group in the phenyl ring of triazol moiety showed moderate to good activity against tested cancer cell lines. The cytotoxicity comparison of the triazol-linked thiazole-oxindoles 9 with triazol-indoline-2,3-dione intermediate 7 showed that the conjugation of hydrazino thiazole moiety to triazol-indoline-2,3-dione 7 did not improve the cytotoxicity effect in the most of compounds 9 and they were inactive against cancer cell lines. Compared to compounds 7, the compounds 9a and 9d showed better activity against LNCaP and PC3, respectively. The cytotoxicity evaluation of triazol-linked oxindol-thiosemicarbazone conjugates 10a-c suggested that the compounds activity is strongly influenced by the nature of the substituents in the phenyl ring of triazole moiety. The compound 10a with methoxy group was inactive while 10c with nitro group showed good activity against MDA-MB231 and PC3. Interestingly, compound 10b having Br demonstrated the highest cytotoxic activity against A375 cell line (IC50 = 25.91 μM), MDA-MB-231 cell line (IC50 = 18.42 μM), PC3 cell line (IC50 = 15.32 μM) and LNCaP cell line (IC50 = 29.23 μM). Therefore, 10b was selected for further study.
Table 1.
In vitro cytotoxic activities of 6, 7, 9, and 10 against A375, MDA-MB-231, LNCaP, PC3 cells and normal cell HDF. Data represent mean ± SD of three independent experiments
| Compounds | IC50 (μM) | ||||
|---|---|---|---|---|---|
| A375 | MDA-MB231 | PC3 | LNCaP | HDF | |
| 6a | 42.40 + 0.002 | >100 | >100 | >100 | >100 |
| 6b | 50.26 + 0.008 | >100 | >100 | >100 | >100 |
| 6c | 50.11 + 0.005 | >100 | >100 | >100 | >100 |
| 6d | >100 | >100 | >100 | >100 | >100 |
| 6e | >100 | >100 | >100 | >100 | >100 |
| 6f | 50.01 + 0.006 | >100 | >100 | 47.50 + 0.007 | >100 |
| 6g | >100 | >100 | >100 | >100 | >100 |
| 6h | >100 | >100 | >100 | >100 | >100 |
| 6i | 37.95 + 0.005 | >100 | 52.20 + 0.008 | >100 | >100 |
| 6j | 56.54 + 0.005 | >100 | >100 | >100 | >100 |
| 6k | 50.78 + 0.006 | >100 | >100 | >100 | >100 |
| 6l | 50.27 + 0.001 | >100 | >100 | >100 | >100 |
| 6m | 57.32 + 0.001 | 58.45 + 0.004 | >100 | >100 | >100 |
| 6n | >100 | >100 | >100 | >100 | >100 |
| 6o | 50.05 + 0.001 | >100 | >100 | >100 | >100 |
| 6p | 56.80 + 0.008 | >100 | >100 | >100 | >100 |
| 6q | 27.74 + 0.001 | >100 | >100 | 28.07 + 0.004 | >100 |
| 7a | 35.86 + 0.002 | 51.76 + 0.001 | >100 | 59.42 + 0.001 | >100 |
| 7b | >100 | >100 | >100 | >100 | >100 |
| 7c | 37.69 + 0.002 | 51.05 + 0.002 | 72.01 + 0.003 | >100 | >100 |
| 9a | 46.33 + 0.001 | >100 | >100 | 45.01 + 0.012 | >100 |
| 9b | >100 | 55.10 + 0.005 | >100 | >100 | >100 |
| 9c | >100 | >100 | >100 | >100 | >100 |
| 9d | >100 | >100 | 62.95 + 0.010 | >100 | >100 |
| 9e | >100 | >100 | >100 | >100 | >100 |
| 9f | >100 | >100 | >100 | >100 | >100 |
| 9g | >100 | >100 | >100 | >100 | >100 |
| 9h | >100 | >100 | >100 | >100 | >100 |
| 9i | >100 | >100 | >100 | 51.34 + 0.006 | >100 |
| 10a | >100 | >100 | >100 | >100 | >100 |
| 10b | 25.91 + 0.005 | 18.42 + 0.002 | 15.32 + 0.002 | 29.23 + 0.003 | >100 |
| 10c | >100 | 32.74 + 0.003 | 57.98 + 0.012 | >100 | >100 |
| Etoposide | 24.46 + 0.019 | 31.02 + 0.051 | 30 + 0.037 | 31.21 + 0.005 | >100 |
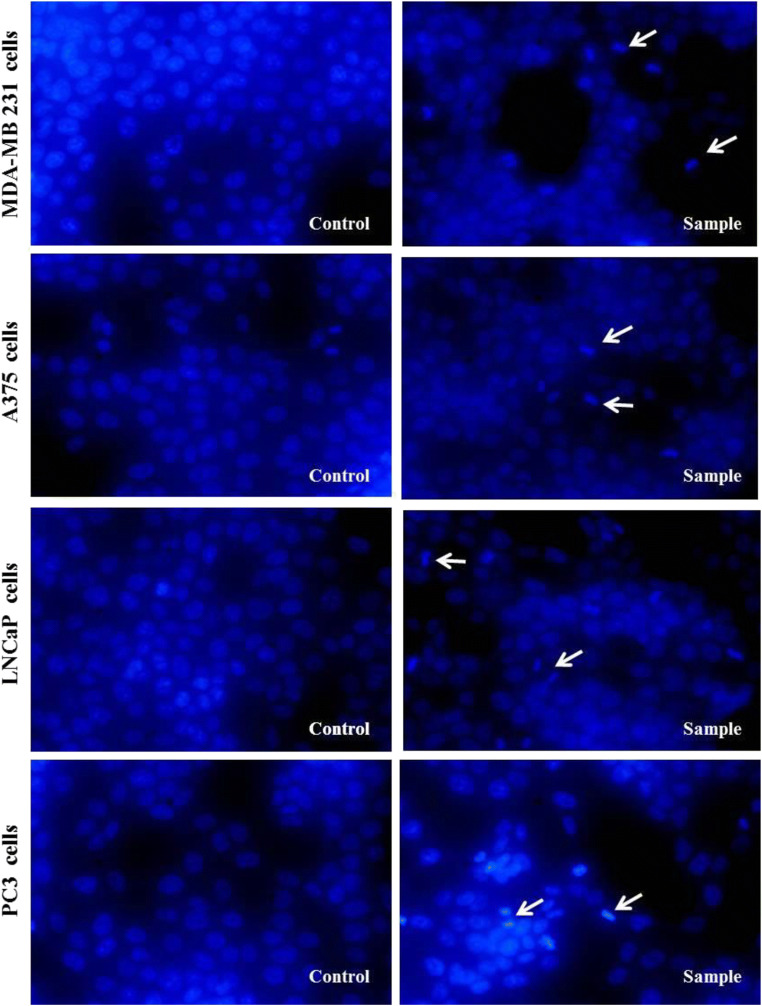
Then, DAPI staining was used to show clear morphological changes and chromatin fragmentation within the nucleus of A375, MDA-MB-231, PC3 and LNCaP treated cells with 10b. It was found, their morphology is not altered in untreated cells (or controls). The images of inverted fluorescence microscopy reveal that compound 10b led to cell death of cancer cells (Fig. 2).
Fig. 2.
Inverted fluorescent microscopy images of chromatin damages occurrence in the nucleus of treated cells with 10b compound and DMSO (1%), which have been stained with DAPI in MDA-MB 231, A375, LNCaP, and PC3 cancer cell lines. The experiments were performed three times (original microscope magnification, 40X, Scale bar, 10 μm)
To prove the cancer cell death, we used the qRT-PCR method to test the expression levels of Bax and Bcl-2 mRNAs. The balance of Bcl-2 protein and pro-apoptotic Bax prevents cytochrome c translocation from the mitochondria to cytoplasm and represents the apoptosis resistance. This balance is compromised by down-regulation of Bcl-2 and/or up-regulation of Bax and contributes to apoptosis. The results showed the apoptosis of LNCaP cancer cells (Fig. 3a and b).
Fig. 3.
(a), (b): In treated LNCaP cells with 10b compound (as sample) and no treated cells (as control), relative expression of Bax mRNA and relative expression of Bcl-2 mRNA were shown. Data represent mean ± SD of three independent experiments. p < 0.05 was considered to be statistically significant
We used wound healing assay to find out the effect of 10b on the cancer cells migration. In this regard, the LNCaP cells were scratched with a pipette tip and then treated with 10b. The cells migration into wound was assessed at 0 and 24 h after treatment. It was found, 10b has potential inhibitory activity on LNCaP cells migration (compared to untreated cells as control) (Fig. 4).
Fig. 4.
Effects of compounds 10b on the migration of LNCaP cells in different time (0 and 24 h after treatment; concentration of 10b compound was 29.23 μM). Images were obtained using phase-contrast microscopy. Scale bars represent 50 μm
Eventually, we examined the expression rate for mRNAs of two genes as potent markers of EMT (E-cadherin, vimentin) to validate the impact of 10b on cancer cell migration [35, 36] (Fig. 5a). The expression levels of E-cadherin and vimentin mRNA were evaluated by qRT-PCR method. As can be seen in Fig. 5b and c, the E-cadherin expression was significantly increased, while vimentin expression was significantly reduced in 10b-treated LNCaP cells. This indicates that 10b is able to inhibit EMT.
Fig. 5.
(a): Schematic model of epithelial-mesenchymal transition (EMT). (b, c): Relative expression of E-cadherin and Vimentin mRNAs as two potential markers of EMT in treated LNCaP cells with 10b compound (compared to control) were shown. Data represent mean ± SD of three independent experiments. p < 0.05 was considered to be statistically significant
Previously, the anti-tyrosine kinase activity of indolinones [37, 38] and inhibitory effects of thiosemicarbazones on DNA replication by suppression of ribonucleotide reductase activity were reported [23, 39, 40]. Notably, it seems that 10b may involve to inhibit tyrosine kinases activity or/and DNA synthesis process. Moreover, we showed inhibition effects of 10b on cell migration. Thus, it supports this idea that 10b may have anti tyrosine activity. Most of tyrosine kinases regulate the cell motility. There are some potent candidates as mitogen-activated protein kinase (MAPK) cascade activated by epidermal growth factor receptor (EGFR). Proliferation and migration are outcomes of EGFR activity that are tightly regulated by MAPKs. Thus, the exploring of the possible effects of 10b on related tyrosine kinases in MAPK cascade was suggested. Also, in vivo experiments will be required to find the side effects of 10b.
Conclusion
In conclusion, in vitro cytotoxic activity of some new synthesized triazol-linked spiroindolinequinazolinone 6, thiazol-oxindole 7 and oxindole-thiosemicarbazone conjugates 9 were evaluated toward different cancer lines. The synthesized compounds 6, 7, and 9 showed IC50 values higher than IC50 value of Etoposide as positive control. Among three synthesized triazol-linked oxindol-thiosemicarbazone 10a-c, compound 10b displayed promising antitumor activity against all tested cancer cell lines (IC50 = 15.32–29.23 μ) compared to the Etoposide. It showed the importance of bromophenyl group substitution in triazol/indolin-3-thiosemicarbazone series 10 in inducing more cytotoxic effects on cancer cells. The results revealed that the compound 10b induces apoptosis and has EMT inhibition activity. Therefore, the compound 10b as an indolinone and thiosemicarbazone conjugate molecule could be a novel active agent with multiple activities to design anticancer therapeutic strategies. Our current study reports that conjugation of indolinone, thiosemicarbazone and triazole moieties can be considered as a new therapeutic agent against different cancer cells.
Experimental
General information
Melting points were determined on a melting point apparatus and are uncorrected. IR spectra were taken with a Bomem FT-IR MB spectrometer. The NMR spectra were recorded on a BRUKERDRX-300AVANCE spectrometer. Mass spectra were recorded on an Agilent 5975C VL MSD with Tripe-Axis Detector operating at an ionization potential of 70 eV. Elemental analyses were performed using a Heraeus CHN–O–Rapid analyzer. All chemicals were purchased from Merck or Aldrich and were used without further purification.
Synthesis of N-propargyl isatins 2
A mixture of isatins (1 mmol), propargyl bromide (1 mmol) and Na2CO3 (2 mmol) in DMF (3 mL) was stirred at room temperature for 3 h. Then, water (5 mL) was added and stirred for 15 min. Then, the solid residue was filtered and crystallized with EtOH to afford the pure product.
Synthesis of spiro[indoline-3,2′-quinazoline]-diones 4
A mixture of N-propargyl isatins 2 (1 mmol),2-aminobenzamide 3 (1 mmol) and P-TSA (10 mol%) in EtOH (5 mL) was stirred at reflux conditions for 10 h. Then, water (15 mL) was added and stirred for 15 min. The solid residue was filtered and washed with water. Then, the solid was crystallized with EtOH to afford the pure product.
Synthesis of compound 6
A mixture of spiro[indoline-3,2′-quinazoline]-diones 4 (1 mmol), azide 5 (1 mmol), CuSO4.5H2O (15 mol%) and sodium ascorbate (30 mol%) in t-BOH/H2O (2 mL, 1:1) was sonicated at room temperature for 1 h. Then, water (5 mL) was added and stirred for 15 min. The solid residue was filtered and washed with water. Then, the solid was crystallized with DMF/H2O to afford the pure product.
Synthesis 1H-1,2,3-triazol-indoline-diones 7
A mixture of N-propargyl isatins 2 (1 mmol), azide 5 (1 mmol), CuSO4.5H2O (15 mol%) and sodium ascorbate (30 mol%) in t-BOH/H2O (2 mL, 1:1) was sonicated at room temperature for 1 h. Then, water (3 mL) and EtOAc (3 mL) was added and stirred for 15 min. The aqueous layer was extracted with EtOAc for three times. The combined organic layers were washed with brine, dried over MgSO4 and evaporated to give the pure product.
Synthesis of triazol/thiazol-indolinones 9
A mixture of 7 (1 mmol), thiosemicarbazid (1 mmol) and phenacyl bromides (1 mmol) in iso-PrOH (2 mL) was sonicated at 65 °C for 1 h. Then, water (5 mL) was added and stirred for 15 min. The solid residue was filtered and washed with water and EtOH to afford the pure product.
Synthesis of triazol/indolin-thiosemicarbazones 10
A mixture of 7 (1 mmol) and thiosemicarbazid (1 mmol) in iso-PrOH (2 mL) was sonicated at 65 °C for 1 h. Then, water (5 mL) was added and stirred for 15 min. The solid residue was filtered and washed with water and EtOH to afford the pure product.
Cell lines and cell culture
Human malignant melanoma cells (A375), human prostate cancer cells (PC3 cells, LNCaP cells), human breast cancer cells (MDA-MB-231) and normal cells HDF (human dermal fibroblast) were received from Pasture Institute, Tehran, Iran. A375, PC3, LNCaP and HDF cell lines were grown in DMEM medium and MDA-MB-231 cell line was grown in RPMI 1640 medium. All media contains 10% fetal bovine serum (FBS; Bioidea BI 201, Iran), penicillin G, streptomycin 100 μg/mL and 1% L-Glutamine. The cells were cultured and incubated under humidified 5% CO2 atmosphere at 37 °C.
MTT assay
The effect of compounds treatment on the viability of cancer cell lines was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide or MTT assay (MTT assay kit, Bio IDEA, CatNo:BI1017, Iran) based on the ability of live cells to cleave the tetrazolium ring to a molecule that absorb at 490 nm as per the manufacturer’s instructions [41]. Etoposide (was kindly provided by Dr. A. Foroumadi, Tehran medical Science University, Iran) and DMSO was used as positive and negative controls, respectively. Briefly, cells were plated in 96-well culture plates (5 × 103 cells/well). After 24 h incubation, the cells were treated with different concentrations of the compounds. After 48 h at 37 °C, the medium was removed and 100 μL of MTT reagent (1 mg/mL) was added to each well, and cells were further incubated at 37 °C for 4 h. The MTT solution was removed, 50 μL of DMSO was added to each well to dissolve formazan crystals, and the plates were gently shaken for 10 min, followed by reading with an ELISA plate reader (BiotekELx 800, USA). The 50% inhibition concentration (IC50) was defined as the concentration that inhibited cell proliferation by 50% when compared to DMSO treated cells (as negative control).
DAPI staining assay
DAPI staining assay was used to determine chromatin changes. Related cancer cell lines were seeded in six well plates (5 × 104 cells/well) containing 12 mm cover-slips and subsequently treated for 10b compound (Sample or treated cells) and DMSO (Control or untreated cells) for 24 h. Cells then were fixed with 3.7% paraformaldehyde, permeabilized in 0.5% (w/v) Triton X-100, 1% BSA (w/v) for 5 min, washed in PBS, and stained with DAPI (Sigma-Aldrich, USA). All images were taken by an inverted fluorescent microscope (Nikon Eclipse Ti-E).
Wound-healing migration assay
The LNCaP cells were seeded in culture medium onto 6-well plates at a density of 4 × 105 cells per well. The confluent monolayer of cells was scratched with a fine pipette tip, and cell migration into the wound was visualized and scored by measuring the size of the initial wound and comparing it to the size of the wound after 24 h by microscopy.
RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR)
For quantitative real-time RT-PCR analysis, after 48 h of treatment with 29.23 μM of related drug (10b), LNCaP cells was lysed and the total RNA was extracted using 500 μL of Trizol® reagent according to the protocol provided by the manufacturer (Invitrogen Life Technologies, Carlsbad, CA, USA) followed by reverse transcription into cDNA according to manufactures protocol (ReveretAid M-Mulv reverse transcriptase kit, Thermo Fisher Scientific, MA, USA). Real-time RT-PCR was then performed to amplify cDNA using SYBR Green dye universal master mix (Bioron GmbH, Germany), on a Light Cycler 480 (Roche) using the primers for GAPDH-F: 5′-CAA GGT CAT CCA TGA CAA CTTTG-3′, R:5′-GTCCACCACCCTGTTGCTGTAG-3′; Bax-F:5′-GTCGCCCTTTTCTACTTTGCC-3′, R: 5′-CTCCCGCCACAAAGATGGTCA-3′and Bcl-2-F: 5′-CCCCTCGTCCAAGAATGCAA-3′, R: 5′- TCTCCCGGTTATCGTACCCTG-3′ for 40 cycles. Data represent averaged copy number normalized to the GAPDH housekeeping gene. Primer synthesis was done by Pishgam Biotech Co. Tehran, Iran. The negative control reaction was set as a reaction similar to the above but with deionized water instead of cDNA. Thermal conditions of the PCR consisted of primary denaturation at 94 °C for 2 min, 45 cycles of denaturation at 94 °C for 30 s, annealing at 59 °C for 30 s, amplification at 72 °C for 30 s. Primers were used for E-cadherin–F: 5′-GCCGAGAGCTACACGTTCAC-3′, R: 5′- CAGGCGTAGACCAAGAAATG-3′and for vimentin-F: 5′-CTACGTCCACCCGCACCTAC-3′; R: 5′- CCAGCGAGAAGTCCACCGAG-3′ with the following conditions: 95 °C for 90 s; followed by 45 cycles of 95 °C for 30 s, 60 °C for 30 s (E-cadhein) and 57 °C for 30 s (vimentin), and 72 °C for 30 s. All reactions were triplicated.
Electronic supplementary material
(DOCX 9.96 mb)
Acknowledgments
Financial support of this research from Shahid Beheshti University, University of Guilan and the Iran National Science Foundation (INSF) is gratefully acknowledged.
Authors’ contributions
SN and MBM synthesis of the title compounds. FS supervision of the pharmacological part and collaboration in manuscript preparations. AB supervision of the chemistry part, designing of title compounds and manuscript preparation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Safari, Email: fsafari@guilan.ac.ir.
Ayoob Bazgir, Email: a_bazgir@sbu.ac.ir.
References
- 1.Shirinyan VZ, Markosyan AI, Baryshnikova MA, Yaminova LV, L’vov AG, Gabrielyan SA. Synthesis and antiproliferative activity evaluation of aryl(Hetaryl)cyclopentenone analogs of combretastatin A-4. Pharm Chem J. 2018;51:867–872. [Google Scholar]
- 2.Avendano C, Menendez JC. Medicinal chemistry of anticancer drugs. 1. Amsterdam: Elsevier; 2008. [Google Scholar]
- 3.Kathawala RJ, Gupta P, Ashby CR, Jr, Chen ZS., Jr The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Update. 2015;18:1–7. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.El-Naggar M, Eldehna WM, Almahli EA, Fares M, Elaasser MM, Abdel-Aziz HA. Novel thiazolidinone/thiazolo[3,2-a]benzimidazolone-isatin conjugates as apoptotic anti-proliferative agents towards breast cancer: one-pot synthesis and in vitro biological evaluation. Molecules. 2018;23:1420. doi: 10.3390/molecules23061420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sridhar SK, Ramesh A. Synthesis and pharmacological activities of hydrazones, Schiff and Mannich bases of isatin derivatives. Biol Pharm Bull. 2001;24:1149–1152. doi: 10.1248/bpb.24.1149. [DOI] [PubMed] [Google Scholar]
- 6.Al-Wabli RI, Zakaria AS, Attia MI. Synthesis, spectroscopic characterization and antimicrobial potential of certain new isatin-indole molecular hybrids. Molecules. 2017;22:1958. doi: 10.3390/molecules22111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang T, Kuhen KL, Wolff K, Yin H, Bieza K, Caldwell J, Bursulaya B, Tuntland T, Zhang K, Karanewsky D, He Y. Design, synthesis, and biological evaluations of novel oxindoles as HIV-1 non-nucleoside reverse transcriptase inhibitors. Part 2. Bioorg Med Chem Lett. 2006;16:2109–2112. doi: 10.1016/j.bmcl.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 8.Igosheva N, Lorz C, O’Connor E, Glover V, Mehmet H. Isatin, an endogenous monoamine oxidase inhibitor, triggers a dose- and time-dependent switch from apoptosis to necrosis in human neuroblastoma cells. Neurochem Int. 2005;47:216–224. doi: 10.1016/j.neuint.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Vine KL, Matesic L, Locke JM, Skropeta D. Recent highlights in the development of isatin-based anticancer agents. Adv Anticancer Agents Med Chem. 2013;2:254–312. [Google Scholar]
- 10.Al-Rashood ST, Hamed AR, Hassan GS, Alkahtani HM, Almehizia AA, Alharbi A, AlSanea MM, Eldehna WM. Antitumor properties of certain spirooxindoles towards hepatocellular carcinoma endowed with antioxidant activity. J Enz Inhibit Med Chem. 2020;35:831–839. doi: 10.1080/14756366.2020.1743281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JVS, Booth BP, Verbois SL, Morse DE, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 12.Dhillon S. Nintedanib: a review of its use as second-line treatment in adults with advanced non-small cell lung cancer of adenocarcinoma histology. Target Oncol. 2015;10:303–310. doi: 10.1007/s11523-015-0367-8. [DOI] [PubMed] [Google Scholar]
- 13.Rossi A, Latiano TP, Parente P, Chiarazzo C, Limosani F, Di Maggio G, Maiello E. The potential role of nintedanib in treating colorectal cancer. Expert Opin Pharmacother. 2017;18:1153–1162. doi: 10.1080/14656566.2017.1346086. [DOI] [PubMed] [Google Scholar]
- 14.Quintela-Fandino M, Urruticoechea A, Guerra J, Gil M, Gonzalez-Martin A, Marquez R, Hernandez-Agudo E, Rodriguez-Martin C, Gil-Martin M, Bratos R, Escudero MJ, Vlassak S, Hilberg F, Colomer R. Phase I clinical trial of nintedanib plus paclitaxel in early HER-2- negative breast cancer (CNIO-BR-01-2010/GEICAM-2010-10 study) Br J Cancer. 2014;111:1060–1064. doi: 10.1038/bjc.2014.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raj R, Gut J, Rosenthal PJ, Kumar V. 1H-1,2,3-Triazole-tethered isatin-7-chloroquinoline and 3-hydroxy-indole-7-chloroquinoline conjugates: synthesis and antimalarial evaluation. Bioorg Med Chem Lett. 2014;24:756–759. doi: 10.1016/j.bmcl.2013.12.109. [DOI] [PubMed] [Google Scholar]
- 16.Contelles JM, Soriano E. The medicinal chemistry of hybrid-based drugs targeting multiple sites of action. Curr Top Med Chem. 2011;11:2714–2715. doi: 10.2174/156802611798184382. [DOI] [PubMed] [Google Scholar]
- 17.Eldehna WM, Al-Wabli RI, Almutairi MS, Keeton AB, Piazza GA, Abdel-Aziz HA, Attia MI. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent antiproliferative agents. J Enz Inhib Med Chem. 2018;33:867–868. doi: 10.1080/14756366.2018.1462802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raja Solomona V, Hu C, Lee H. Hybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activity. Bioorg Med Chem Lett. 2010;17:1563–1572. doi: 10.1016/j.bmc.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 19.Ramshid PK, Jagadeeshan S, Krishnan A, Mathew M, Nair SA, Pillai MR. Synthesis and in vitro evaluation of some isatin-thiazolidinone hybrid analogues as anti-proliferative agents. Med Chem. 2010;6:306–312. doi: 10.2174/157340610793358909. [DOI] [PubMed] [Google Scholar]
- 20.Taher AT, Khalil NA, Ahmed EM. Synthesis of novel isatin-thiazoline and isatin-benzimidazole conjugates as anti-breast cancer agents. Arch Pharm Res. 2011;34:1615–1621. doi: 10.1007/s12272-011-1005-3. [DOI] [PubMed] [Google Scholar]
- 21.Rambabu D, Raja G, Sreenivas BY, Seerap GPK, Lalith Kumar K, Singh Deora G, Haldar D, Basaveswara Rao MV, Pal M. Spiro heterocycles as potential inhibitors of SIRT1: Pd/C-mediated synthesis of novel N-indolylmethyl spiroindoline-3, 2′-quinazolines. Bioorg Med Chem Lett. 2013;23:1351–1357. doi: 10.1016/j.bmcl.2012.12.089. [DOI] [PubMed] [Google Scholar]
- 22.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 23.Hu W-X, Zhou W, Xia C-N, Wen X. Synthesis and anticancer activity of thiosemicarbazones. Bioorg Med Chem Lett. 2006;16:2213–2218. doi: 10.1016/j.bmcl.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Sharma P, Anand A, Bedi PM, Kaur T, Saxena AK, Kumar V. Azide-alkyne cycloaddition en route to novel 1H-1,2,3-triazole tethered isatin conjugates with in vitro cytotoxic evaluation. Eur J Med Chem. 2012;55:455–461. doi: 10.1016/j.ejmech.2012.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Saha ST, Gu L, Palma G, Perumal S, Singh-Pillay A, Singh P, Anand A, Kaur M, Kumar V. 1H-1,2,3-triazole tethered nitroimidazole-isatin conjugates: synthesis, docking, and anti-proliferative evaluation against breast Cancer. ACS Omega. 2018;3:12106–12113. doi: 10.1021/acsomega.8b01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senwar KR, Sharma P, Reddy TS, Jeengar MK, Nayak VL, Naidu VG, Kamal A, Shankaraiah N. Spirooxindole-derived morpholine-fused-1,2,3-triazoles: design, synthesis, cytotoxicity and apoptosis inducing studies. Eur J Med Chem. 2015;102:413–424. doi: 10.1016/j.ejmech.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Nagarsenkar A, Prajapti SK, Guggilapu SD, Birineni S, Kotapalli SS, Ummanni R, Babu BN. Investigation of triazole-linked indole and oxindole glycoconjugates as potential anticancer agents: novel Akt/PKB signaling pathway inhibitors. Med Chem Commun. 2016;7:646–653. [Google Scholar]
- 28.Hall MD, Salam NK, Hellawell JL, Fales HM, Kensler CB, Ludwig JA, Szaka’cs G, Hibbs DE, Gottesman MM. Synthesis, activity, and pharmacophore development for isatin-β-thiosemicarbazones with selective activity toward multidrug-resistant cells. J Med Chem. 2009;52:3191–3204. doi: 10.1021/jm800861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Oliveira JF, Lima TS, Vendramini-Costa DB, de Lacerda Pedrosa SCB, Lafayette EA, da Silva RMF, de Almeida SMV, de Moura RO, Ruiz ALTG, de Carvalho JE, de Lima MCA. Thiosemicarbazones and 4-thiazolidinones indole-based derivatives: synthesis, evaluation of antiproliferative activity, cell death mechanisms and topoisomerase inhibition assay. Eur J Med Chem. 2017;136:305–314. doi: 10.1016/j.ejmech.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Meng M, Zhang Y, Hao X, Wang Y, Mu S. Synthesis, cytoprotective and anti-tumor activities of isatin Schiff bases. Lett Drug Des Discov. 2015;12:802–805. [Google Scholar]
- 31.Ahadi S, Khavasi HR, Bazgir A. Highly efficient construction of bisspirooxindoles containing vicinal spirocenters through an organocatalytic modified Feist-Bénary reaction. Chem Eur J. 2013;19:12553–12559. doi: 10.1002/chem.201301175. [DOI] [PubMed] [Google Scholar]
- 32.Imani Shakibaei G, Bazgir AA. Highly efficient one-pot synthesis of indenopyridine-fused spirocyclic systems. RSC Adv. 2016;6:22306–22311. [Google Scholar]
- 33.Ghahremanzadeh R, Fereshtehnejad F, Mirzaei P, Bazgir A. Ultrasound-assisted synthesis of 2,2′-(2-oxoindoline-3,3-diyl)bis(1H-indene-1,3(2H)-dione) derivatives. Ultrason Sonochem. 2011;18:415–418. doi: 10.1016/j.ultsonch.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Sarangi PKN, Sahoo J, Paidesetty SK, Mohanta GP. Thiazoles as potent anticancer agents: a review. Indian Drugs. 2016;53:5–11. [Google Scholar]
- 35.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 37.Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 38.Zhang C, Xu D, Wang J, Kang C. Efficient synthesis and biological activity of novel Indole derivatives as VEGFR-2 tyrosine kinase inhibitors. Russ J Gen Chem. 2017;87:3006–3016. [Google Scholar]
- 39.Moore EC, Zedeck MS, Agrawal KC, Sartorelli AC. Inhibition of ribonucleoside diphosphate reductase by 1-formylisoquinoline thiosemicarbazone and related compounds. Biochemistry. 1970;9:4492–4498. doi: 10.1021/bi00825a005. [DOI] [PubMed] [Google Scholar]
- 40.Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev Med Chem. 2004;4:31–39. doi: 10.2174/1389557043487484. [DOI] [PubMed] [Google Scholar]
- 41.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 9.96 mb)