Abstract
Introduction
Multicellular platforms and linked multi organ on chip devices are powerful tools for drug discovery, and basic mechanistic studies. Often, a critical constraint is defining a culture medium optimal for all cells present in the system. In this study, we focused on the key cells of the neuromuscular junction i.e., skeletal muscle and motor neurons.
Methods
Formulation of a chemically defined medium for the co-culture of C2C12 skeletal muscle cells and human induced pluripotent stem cell (hiPSC) derived spinal spheroids (SpS) was optimized. C2C12 cells in 10 experimental media conditions and 2 topographies were evaluated over a 14-day maturation period to determine the ideal medium formulation for skeletal muscle tissue development.
Results
During early maturation, overexpression of genes for myogenesis and myopathy was observed for several media conditions, corresponding to muscle delamination and death. Together, we identified 3 media formulations that allowed for more controlled differentiation, healthier muscle tissue, and long-term culture duration. This evidence was then used to select media formulations to culture SpS and subsequently assessed axonal growth. As axonal growth in SpS cultures was comparable in all selected media conditions, our data suggest that the neuronal basal medium with no added supplements is the ideal medium formulation for both cell types.
Conclusions
Optimization using both topographical cues and culture media formulations provides a comprehensive analyses of culture conditions that are vital to future applications for in vitro NMJ models.
Electronic supplementary material
The online version of this article (10.1007/s12195-020-00624-1) contains supplementary material, which is available to authorized users.
Keywords: Skeletal muscle, Organs on chips, Co-culture motor neurons, Neuromuscular junction, Chemically defined media
Introduction
Numerous debilitating diseases arise from pathophysiological dysfunction at the neuromuscular junction (NMJ) including myasthenia gravis, spinal muscular atrophy, amyotrophic lateral sclerosis, and Charcot-Marie-Tooth disease.29,38,39,50 To investigate the underlying mechanisms of the dysfunction, in vitro model systems are being developed as attractive testing platforms that focus on the major components of multicellular systems. An NMJ platform would require direct cell–cell contact between the 2 main cell types, motor neurons and skeletal muscle. Motor neurons extend axons to transmit electrochemical signals at the synapse of the skeletal muscle to transduce a signal into movement; this is the fundamental basis of the NMJ. Recapitulation of this basic physiologic function at the NMJ in vitro would require the two cell types to be cultured in proximity to one another, thus a culture medium that would support both the motor neurons and skeletal muscle cells.
C2C12 cells, a mouse skeletal muscle cell line, have been commonly used in NMJ platforms.58,70 When cultured alone, C2C12s have a 2-step culture process. First, cells are allowed to proliferate in growth medium containing high glucose DMEM and 10% fetal bovine serum (FBS). Second, a low serum differentiation muscle medium (MM) containing high glucose DMEM and 2% horse serum is added, inducing myoblast differentiation into myotubes.3 However, skeletal muscle is known to delaminate from surfaces easily, often after 7 or fewer days in culture. The occurrence of muscle delamination can be influenced by surface adhesions, over development, or strength of twitching.8,16,33,56 The delamination of muscle affects the ability to culture muscle tissue long term, therefore hindering the formation of functional NMJs. While C2C12 skeletal muscle cell culture is generally more permissive to changes in media formulations, stem cell derived human neurons are typically more sensitive to small changes in media composition. Human induced pluripotent stem cell (hiPSC) derived motor neurons require the addition of numerous supplements to the culture medium, although formulations vary.11,13,22,36,51
In this study, we report the optimization of cell culture medium for the culture of SpS and C2C12 mouse skeletal muscle cells. Based on the medium formulation reported by Maciel et al., a base neuronal medium containing DMEM/F12, N2-supplement, B27-supplement, d-glucose, and l-ascorbic acid supplemented with 7 additives: retinoic acid (RA), SB431542 (SB), smoothen agonist (SAG), dorsomorphin (D), brain-derived neurotrophic factor (B), ciliary neurotrophic factor (C), and glial-derived neurotrophic factor (G) was prepared.36 Although this medium formulation sustains the growth and culture of SpS, our preliminary data showed that this complete formulation was not conducive for C2C12 culture. In efforts to optimize a medium for the culture of both C2C12 cells and SpS, 7 experimental conditions were prepared containing C2C12 cells cultured in the complete formulation with one of the additives removed. Additionally, 3 control groups were included: traditional MM, complete formulation neuronal medium (NM), and basal neuronal medium with no additives (No Add). C2C12 cells were cultured on a gelatin-coated laminin (GEL-LN) hydrogel that was previously shown to promote long term culture.3 Two topographies were explored to determine ideal hydrogel architecture, alongside optimal media formulations. At 4 days of C2C12 cell maturation, gene expression profiles specific to myogenesis and myopathies were evaluated to determine the influence of each media formulation on the cells. Morphologic examination of the developed muscle tissue after 14 days of culture in each condition was correlated to the optimal culture medium formulation. Using this data, 4 media conditions were selected to test SpS axonal growth over 3 days. The deconstruction of complete neuronal medium to implement the culture of SpS with skeletal muscle cells implicates an optimal medium for future NMJ studies. Improving the culture conditions by providing an ideal medium formulation to both NMJ cell types is fundamental to the prospective investigations that will follow.
Materials and Methods
Skeletal Muscle Morphology Assessment
GEL-LN substrates were fabricated using previously established methods.3 Initially, 10% (w/v) type A porcine gelatin (Sigma, St. Louis, MO) and 4% (w/v) microbial transglutaminase (Ajinomoto, Tokyo, Japan), an enzymatic crosslinker, were mixed together, added to an 18-mm chemically activated glass coverslip, and micromolded using a 20 μm × 10 μm or 15 μm × 10 μm (grooves × ridges) polydimethylsiloxane (PDMS; Electron Microscope Sciences, Hatfield, PA) stamp32 The height of the ridge was 5 μm in both cases. The gelatin hydrogel cured overnight at room temperature and the stamp was removed. Subsequently, laminin (10 μg/mL; Gibco, Carlsbad, CA) and microbial transglutaminase (mTg; 4% w/v) were added to a non-adherent surface (i.e. parafilm) and the gelatin hydrogel was inverted onto the laminin-mTg solution and put in a 37 °C incubator for 1 h. After incubation GEL-LN hydrogels were stored in PBS (Gibco) at 4 °C until use. Commercially available C2C12 skeletal muscle cells (ATCC, Manassas, VA) were obtained and cultured in growth medium containing high glucose (4.5 g/L) DMEM (Gibco) with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (ThermoFisher Scientific, Waltham, MA). Once skeletal muscle cells were 80–90% confluent the cells were lifted and seeded onto GEL-LN substrates at a density of 400 cells/mm2. Cells were cultured on substrates in growth medium until 80–90% confluent, after which the medium was exchanged for one of the 10 types of media (Table 1). The three controls included MM, NM, and No Add, while the 7 experimental conditions included NM with one supplement removed denoted by that supplement with a –superscript. MM consisted of high glucose DMEM, 2% horse serum (ATCC), and 1% penicillin–streptomycin. NM contained DMEM/F12 GlutaMAX (ThermoFisher) medium containing 0.32% d-glucose (ThermoFisher), 0.8 mM L-Ascorbic Acid (Sigma), 2X N-2 supplement (ThermoFisher), 2X B27 supplement (ThermoFisher), 1% Penicillin/Streptomycin, 1.5 µM retinoic acid (Sigma), 10 µM SB431542 (Sigma), 1 µM dorsomorphin (Tocris, Bristol, UK), 200 nM SAG (Tocris), 2 ng/mL BDNF (ThermoFisher), 2 ng/mL GDNF (ThermoFisher), and 2 ng/mL CNTF (ThermoFisher). No Add was the neuronal basal medium consisting of DMEM/F12 GlutaMAX media, 0.32% d-glucose, 0.8 mM l-Ascorbic Acid, 2X N-2 supplement, 2X B27 supplement, and 1% Penicillin/Streptomycin.
Table 1.
Media components for each culture condition.
| Condition | Media components |
|---|---|
|
Muscle Medium (MM) |
High glucose DMEM GlutaMAX, horse serum, penicillin/streptomycin |
| Neuronal medium (NM) | DMEM/F12 GlutaMAX, d-glucose, l-Ascorbic Acid, N-2 supplement, B27 supplement, penicillin/streptomycin, retinoic acid, SB431542, dorsomorphin, SAG, BDNF, GDNF, CNTF |
| SB- | NM without addition of SB431542 |
| RA- | NM without addition of retinoic acid |
| D- | NM without addition of dorsomorphin |
| SAG- | NM without addition of smoothened agonist SAG |
| B- | NM without addition of BDNF |
| G- | NM without addition of GDNF |
| C- | NM without addition of CNTF |
| Basal medium (No ADD) |
DMEM/F12 GlutaMAX, d-glucose, l-Ascorbic Acid, N-2 supplement, B27 supplement, penicillin/streptomycin |
Skeletal muscle cells were cultured in different media for 14 days and assessed for spontaneous twitching, and morphology. On day 14, skeletal muscle cultures were fixed for 15 min with a 4% paraformaldehyde (PFA) solution. Subsequently, cultures were permeabilized using a 0.05% Triton X-100 (Sigma) solution for 20 min. Cultures were incubated with primary antibodies for mouse monoclonal anti-α-sarcomeric actinin (n = 3; 1:200; Sigma, A7732) or mouse anti-myosin heavy chain (n = 2; MHC; 1:200; clone A4.1025; EMD Millipore, 05-716, Burlington, MA) for 1 h. Subsequently, cultures were incubated with DAPI DNA stain (1:200), Phalloidin Alexa Fluor 488 (Invitrogen, A12379, Carlsbad, CA), and Alexa Fluor 647-conjugated goat anti-mouse secondary antibodies (1:200; Invitrogen, A21237). Each condition (5 fields of view per coverslip) was imaged using a Nikon Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) Ti Analyzer software and assessed for total nuclei, myogenic index, percent positive area of F-actin, percent positive area of α-sarcomeric actinin, and percent positive area of myosin heavy chain. Myogenic index is calculated as the fraction of nuclei contained in myotubes compared to total nuclei in each field of view. Percent positive area was determined by assessing images for the number of pixels positive for a fluorescent signal over total number of pixels. A Leica SP5 inverted confocal microscope (Leica, Wetzlar, Germany) and ALS AF software were used for capture of immunofluorescent images, images were subsequently processed using ImageJ software (National Institute of Health, Bethesda, MA). To capture the images using the Leica SP5 the N.A. was 0.7 and the refractive index was 1.0. Three laser lines were used including 405, 488, and 633. Images captured using the Nikon Eclipse Ti were captured with an objective with an N.A value of 0.75 and refractive index 1. Three filters were used C-FL UV-2E/C DAPI, C-FL FITC HYQ, and AT-Cy5/AlexaFluor647/Draq5 with excitation/emission centered at 360/460, 480/535, and 620/699 respectively. The camera used to capture images on the Nikon Elipse Ti microscope was Andor Zyla camera. The camera used to capture images on the Nikon Elipse Ti microscope was Andor Zyla camera.
Gene Expression Analyses
C2C12 skeletal muscle cells were seeded onto GEL-LN hydrogels with 20 μm × 10 μm (10% gelatin w/v; 4% mTg; 10 μg/mL laminin; grooves X ridges) micromolded features and cultured until confluent. Once confluent cultures were switched to one of the 10 media conditions and cultured for 4 days. C2C12 cells were subsequently collected by exposure to 0.25% trypsin–EDTA (ThermoFisher Scientific) for 5 min and stored at − 80 °C as pellets in RNAlater™ Stabilization Solution (ThermoFisher Scientific). Upon isolation, pellets were thawed, and total RNA was isolated and purified using RNeasy Plus Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions. Total RNA (1 µg) was synthesized into cDNA using Superscript™ VILO™ cDNA synthesis kit (Invitrogen), diluted (10 ng/µL) with Ultrapure water (Invitrogen), and stored at − 20 °C until further use. Real time quantitative reverse transcriptase polymerase chain reaction (qPCR) was performed for the genes designated in the RT2 Profiler™ PCR Array selected for Mouse Skeletal Muscle: Myogenesis & Myopathy (Qiagen). According to Format A of the manufacturer’s instructions, component mixes for each sample was prepared fresh by combining RT2 SYBR Green qPCR Mastermix (Qiagen), 100 µL cDNA, and Ultrapure water prior to running each plate. Subsequently, 20 µL of component mix was added into each well of the array plate. Using a BIORAD CFX Connect thermocycler (Hercules, CA), plates were loaded, and qPCR was performed. Ct values were exported and properly formatted into Excel spreadsheets for importing into Qiagen’s Data Analysis Center online. Data were normalized using β-actin and GAPDH housekeeping genes, and unsupervised hierarchical clustering of each condition represented the relationships corresponding to gene expression activities.
Spinal Spheroids Differentiation and Growth Assessment
Human motor neurons were differentiated and purified according to previously published methods.36,51 Skin punch biopsies were obtained and fibroblasts were isolated from samples and subsequently induced into pluripotent stem cells. Briefly, fibroblast cultures were derived from skin punch biopsies obtained from patients with axonal forms of CMT and unaffected control individuals, as part of an initiative to establish a CMT iPSC collection at the University of Miami Department of Neurology. Fibroblasts were seeded on gelatin-coated 6-well plates and transduced with supernatant containing 4 retroviral vectors expressing SOX2, OCT3/4, KFL4 and C-MYC. After 7 to 10 days, iPSC clones could be identified and selectively propagated on Matrigel (BD Bioscience, San Diego, CA)-coated plates in mTeSR1 (Stem Cell Technologies) defined media. All study participants gave written informed consent before enrollment. IRB approval was granted by the Wayne State University Human Investigation Committee and University of Miami Institutional Review Board. hiPSCs were cultured until 90–95% confluency and then differentiated into motor neurons for 24 days. Motor neurons were subsequently purified using magnetic bead sorting based on L1CAM. The purified motor neurons were cultured in agitation until they formed SpS which was determined by an accumulation of multiple cells into a spheroid body. SpS were plated onto isotropic GEL-LN substrates (10% gelatin w/v; 4% mTg; 10 μg/mL laminin) and cultured for 4 days. The 3 optimal media conditions (No ADD, D−, and RA−) for skeletal muscle culture were chosen for SpS evaluation and full NM medium was used as a control (n = 3). Axonal growth was assessed from day 2 to day 4 of culture using a Nikon Eclipse Ti inverted microscope. On each of the 3 days, axon area was assessed, as well as the axon length and major and minor axis of the axon area. These measurements were then normalized to the spheroid area. Images captured using the Nikon Eclipse Ti were captured with an objective with a N.A value of 0.5 and refractive index 1. All images were taken using bright field and a Andor Zyla microscope.
Statistical Analysis
All statistical analysis was performed on Prism v8 software (GraphPad, San Diego, CA). Student t-tests and one-way analysis of variance followed by Tukey’s post hoc were used for statistical comparisons. All values were reported as the mean ± standard error of the mean unless reported otherwise, and p < 0.05 was considered statistically significant.
Results
Skeletal Muscle Morphology
Skeletal muscle cells were cultured on GEL-LN hydrogels with 2 different topographies, 20 μm × 10 μm × 1.5 μm or 15 μm × 10 μm × 1.5 μm (grooves × ridges × height) for 14 days. Each day, muscles tissue was evaluated for spontaneous twitching, delamination, confluency, and overall morphology including differentiation efficiency and area of mature muscle tissue. Differentiation efficiency was determined by increases in myogenic index. All cultures contained spontaneous twitching except for the MM condition. Spontaneous twitching occurred the earliest in RA− and D− conditions (day 3) and the latest in the SAG− condition (day 6). All the C2C12 cell cultures were still contracting on day 14 except for MM, NM, C−, and G−. The cultures with the least overall delamination over the 14-day period for both topographies were MM and No Add. For both topographies, NM, C−, and G− had the highest levels of myotube delamination over the 14 days. For a table of all visual observations refer to Supplementary Table 1.
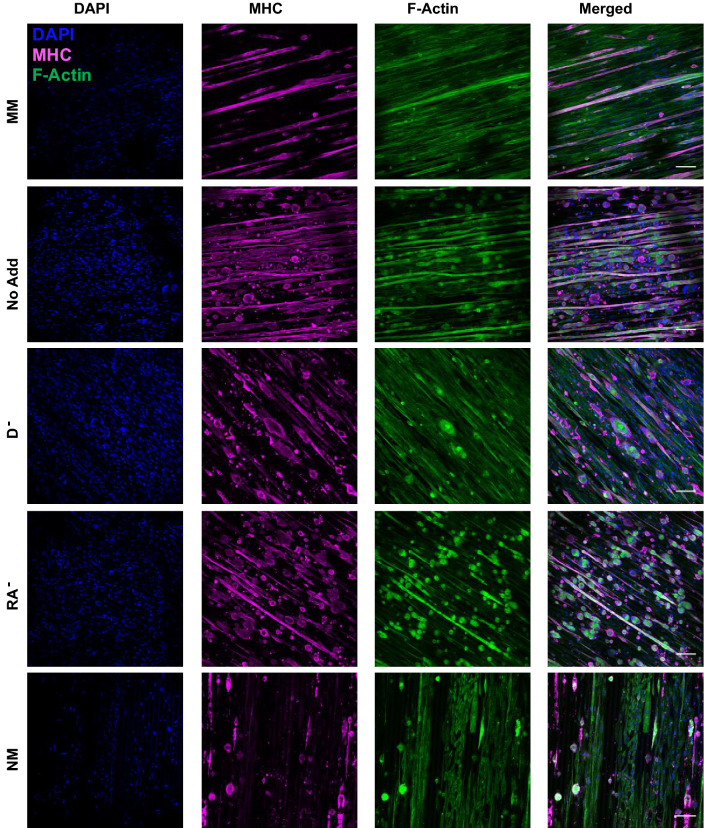
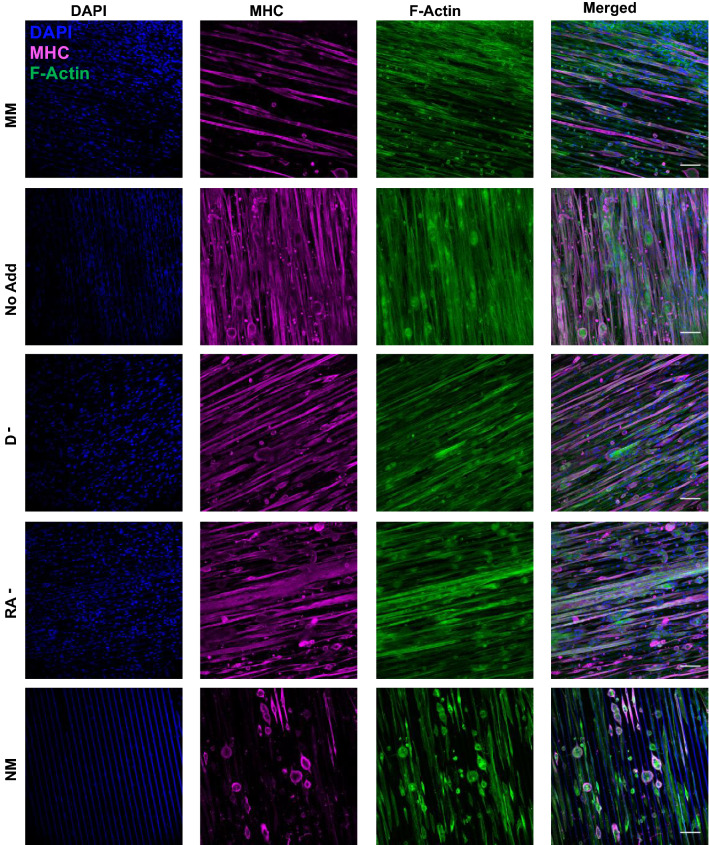
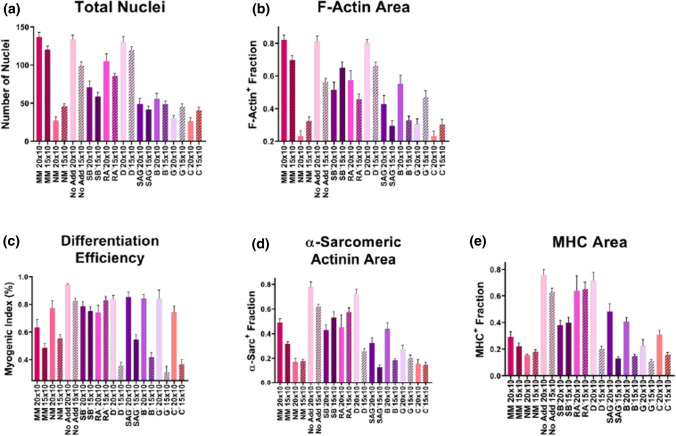
After 14-days, cultures were fixed and immunostained for DNA, F-actin, and MHC or α-sarcomeric actinin. C2C12 cells cultured on 20 μm × 10 μm generally showed better overall morphology across all conditions over the 14-day period (Figs. 1 and 2). Within the 20 μm × 10 μm hydrogels, the conditions with the most cells present at the end of the 14 days were MM, No Add, RA−, and D− (Figs. 3a and 3b). The group with the highest differentiation efficiency was the No Add condition, while most other groups showed similar differentiation, however the values were still higher than the MM control group (Fig. 3c). The conditions with the least amount of myotube delamination were No Add, RA−, and D− as indicated by the α-sarcomeric actinin positive area and MHC positive area on day 14 (Figs. 3d and 3e).
Figure 1.
Presence of myosin heavy chain and F-actin at day 14 of culture. C2C12 cells were seeded on GEL-LN hydrogels micromolded with 15 µm × 10 µm patterning. Day 14 cells were fixed and immunostained for DAPI (blue), MHC (magenta), and F-Actin (green). MM, No Add, D−, RA−, and NM are shown here. Scale bars represent 100 µm.
Figure 2.
Presence of myosin heavy chain and F-actin at day 14 of culture. C2C12 cells were seeded on GEL-LN hydrogels micromolded with 20 µm × 10 µm patterning. Day 14 cells were fixed and immunostained for DAPI (blue), MHC (magenta), and F-Actin (green). MM, No Add, D−, RA−, and NM are shown here. Scale bars represent 100 µm.
Figure 3.
Quantification of 15 µm × 10 µm and 20 µm × 10 µm conditions on day 14 of culture. (a) Images were assessed for total nuclei. (b) To determine positive area fraction for F-Actin images were analyzed for number of pixels positive for a fluorescent signal and divided by total number of pixels. (c) The differentiation efficiency was calculated by determining the number of nuclei in positively stained myotubes and dividing by the number of total nuclei. To determine positive area fraction for (d) alpha-sarcomeric actinin and (e) MHC images were analyzed for number of pixels positive for a fluorescent signal and divided by total number of pixels.
Skeletal muscle cells cultured on hydrogels with 15 μm × 10 μm topography had decreased total nuclei compared to 20 μm × 10 μm conditions except for NM, G−, and C− conditions, which resulted in slightly increased total nuclei (Fig. 3a). The differentiation efficiency was decreased for the 15 μm × 10 μm conditions except C2C12 cells cultured in RA− medium (Fig. 3c). The percent positive area for α-sarcomeric actinin was decreased or equivalent for 15 μm × 10 μm conditions except for C2C12 cells cultured in SB− and RA− cultures which were increased compared to 20 μm × 10 μm conditions (Fig. 3d). When considering percent positive area for MHC, the 15 μm × 10 μm conditions that were increased compared to 20 μm × 10 μm topography were NM, SB−, and RA−, however the three increases were insignificant (Fig. 3e). Finally, the percent positive area for F-actin showed an increase for NM, SB−, G−, and C− 15 μm × 10 μm conditions (Fig. 3b). Overall, C2C12 cells cultured on 20 μm × 10 μm topography resulted in improved morphological outcomes with the exception of a few instances as described above.
The conditions with the best differentiation efficiency on 15 μm × 10 μm surfaces were No add, RA−, and SB− (Fig. 3c). However, the condition with the highest differentiation efficiency was seen in the 20 μm × 10 μm No Add medium. C2C12 cells cultured on 15 μm × 10 μm had the lowest levels of myotube delamination in No Add and RA− media. Interestingly, C2C12 cells maintained in D− medium on 15 μm × 10 μm surfaces had significantly higher levels of delamination compared to culture in D− media on 20 μm × 10 μm surfaces. For tables of significance for Fig. 3 refer to Supplementary Tables 2 to 6. For magnified images of Figs. 1 and 2 refer to Supplementary Figs. 1 and 2.
Myogenesis and Myopathy qPCR
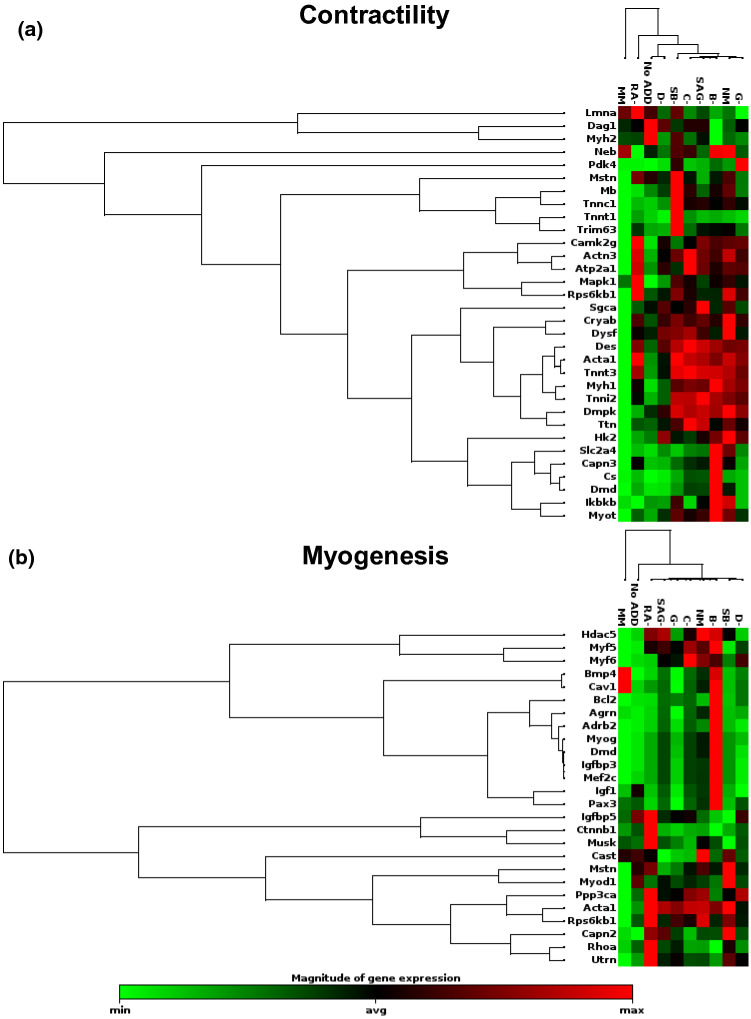
qPCR was performed using a RT2 Profiler™ PCR Array selected for Mouse Skeletal Muscle: Myogenesis & Myopathy. The mRNA for day 4 C2C12 cells cultured on 20 μm × 10 μm hydrogels was assessed for each of the 10 media conditions. After data was obtained the genes were grouped into categories and the results were organized using unsupervised hierarchal clustering. Genes associated with contractility were significantly downregulated for C2C12 cells cultured in MM (Fig. 4a). Cells maintained in B− and SB−, overall had the highest expression of genes associated with contractility. The expression of C2C12s cultured in No Add, RA−, and D− were the most comparable to MM. This downregulation of contractility genes is likely related to the observed low levels of delamination in these conditions. Similarly, the conditions MM, No Add, and D− had the lowest expression of genes associated with myogenesis, while RA− and B− had the greatest upregulation of these genes (Fig. 4b). This could indicate that MM, No Add, and D− have more controlled and delayed myogenesis processes.
Figure 4.
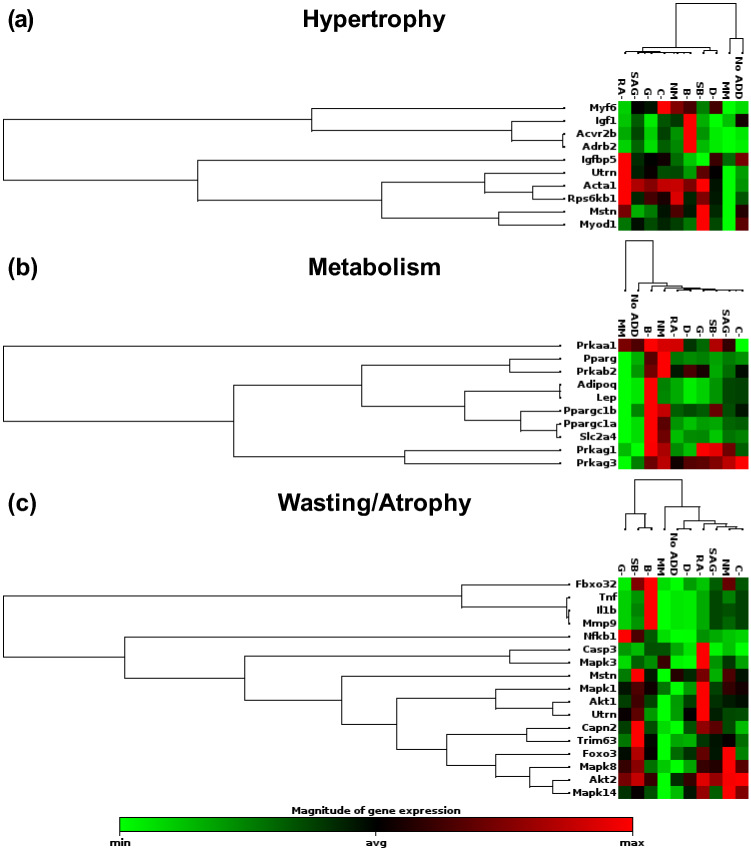
Unsupervised hierarchical clustering of genes associated with contractility and myogenesis for day 4 C2C12 cells cultured on GEL-LN patterned with 20 µm × 10 µm grooves and ridges. Genes are listed to the left for (a) contractility and (b) Myogenesis. Media conditions are represented on the top of each cluster. Green represents downregulation, black is the average gene expression, and red represents upregulation of a gene.
The gene that encodes for α-actin (Acta1) was of particular interest when assessing hypertrophy (Fig. 5a). This gene, which is associated with congenital myopathies, was upregulated in almost every condition except for MM and No Add in which it was downregulated.18 The upregulation of α-actin could indicate unhealthy formation of muscle. Metabolism was evaluated and both NM and B− conditions had multiple genes which were upregulated compared to all other conditions (Fig. 5b). Again, MM and No Add had the lowest expression overall for genes associated with metabolism. Lastly, genes associated with skeletal muscle wasting and atrophy were assessed, which revealed several changes between conditions (Fig. 5c). Overall, SB−, B−, RA−, and NM had highest levels of expression in this category, while MM, No Add, and D− had the lowest levels. When evaluating the entire genetic panel for myogenesis and myopathy, a consistent pattern emerged where MM, No Add, and D− had downregulation of most genes that were analyzed, while SB−, NM and B− were upregulated for many of the genes. The overexpression of key genes could lead to delamination and deformation of the skeletal muscle tissue.
Figure 5.
Unsupervised hierarchical clustering of genes associated with wasting/atrophy, hypertrophy, and metabolic syndrome for day 4 C2C12 cells cultured on GEL-LN patterned with 20 µm × 10 µm grooves and ridges. Genes are listed to the left for (a) wasting/atrophy, (b) hypertrophy, and (c) metabolic syndrome. Media conditions are represented on the top of each cluster. Green represents downregulation, black is the average gene expression, and red represents upregulation of a gene.
SpS Axonal Growth
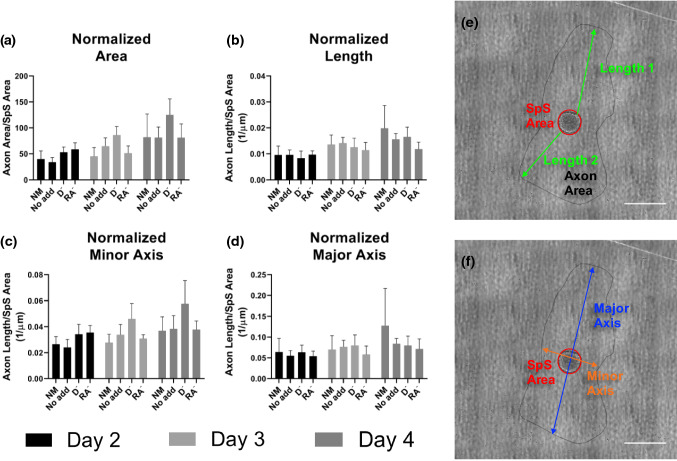
SpS were seeded onto isotropic GEL-LN hydrogels and cultured for 7 days in the 3 optimal media conditions for C2C12 cell culture, No Add, RA−, and D−, additionally NM was used as a control. On days 2, 3, and 4 of culture, tile images were taken of each SpS and the entire axonal area. ImageJ software was used to draw a perimeter around the axon area. Using this perimeter, the axon area, axon length, and major and minor axis were determined (Figs. 6e and 6f). Additionally, all measurements were normalized to the area of the SpS body and graphed (Figs. 6a to 6d). There was no significant difference between any of the media conditions on any of the times points. Axons grew at a steady and expected rate over the culture period in all conditions. On day 7, SpS were fixed and immunostained for neuronal markers beta tubulin III (BTIII) and neurofilament light (Supplemental Fig. 3).
Figure 6.
SpS axonal growth over 3 days of culture in 4 different media conditions. SpS were cultured on isotropic GEL-LN hydrogels for 4 days and images were taken on days 2, 3, and 4. (a) The area of axonal coverage was measured for each condition and normalized to the area of the SpS cell body. The ratio of these two areas is reported. (b) Each image was assessed for 2 axon lengths within the axon coverage area. Axon length was then normalized to SpS cell body area. Lines were drawn around the area of axonal coverage and the (c) minor and (d) major axis were determined for each day and media condition. The values were then normalized to SpS cell body area.
Discussion
There are several NMJ platforms reported in the literature with various muscle and neuron sources.11,21–23,55 Guo et al. first reported the formation of a cross species NMJ using C2C12s and human stem cell derived motor neurons.22 The study used a cell culture medium amendable to both cell types however the medium formulation included numerous components implemented over multiple days and the human neuronal source used was spinal cord stem cells instead of hiPSC-derived motor neurons and rat embryonic skeletal muscle.
In this study, the ideal culture conditions for both C2C12 cells and SpS were explored. C2C12 cells have been widely used to create in vitro NMJ platforms.58,70 These cells are easy to maintain in culture, proliferative, and well characterized, however differentiation in traditional media is suboptimal and they readily delaminate from most culture surfaces, limiting their use for functional NMJ formation and long-term culture. Gelatin and GEL-LN surfaces have been shown to promote maturation and prolonged culture times for C2C12 cells.3,4 Additionally, GEL-LN is a substrate that enhances neuron attachment and maturation.3 To optimize the culture of C2C12 cells and SpS, both medium formulation and GEL-LN hydrogel topography were assessed. Initially, C2C12 cells were cultured in 10 different media conditions outlined in Table 1 and on 15 μm × 10 μm or 20 μm × 10 μm GEL-LN hydrogels. Proper muscle maturation is observed by uniform alignment with highly organized sarcomeric structures and longer myotube formation. This study determined that GEL-LN hydrogels with 20 μm × 10 μm features were ideal across most media conditions compared to 15 μm × 10 μm. Therefore, our discussion will focus on C2C12 cells cultured on hydrogels with 20 μm × 10 μm features.
Initially, when C2C12 cells were cultured in NM, the differentiation efficiency was robust, and spontaneous muscle twitching was present early in the culture period. However, rapid development of skeletal muscle led to delamination resulting in low cell attachment on day 14 of culture. To investigate this phenomenon and enhance long term culture potential, each of the 7 medium components including retinoic acid (RA), SB431542 (SB), smoothen agonist (SAG), dorsomorphin (D), brain-derived neurotrophic factor (B), ciliary neurotrophic factor (C), and glial-derived neurotrophic factor (G) were considered.35,36 A compilation of the effects of each component on skeletal muscle and motor neurons is summarized in Supplementary Table 7. RA induces the activation of Smad 1/5 pathway.8 The activation of this pathways promotes improved glucose tolerance and insulin sensitivity in C2C12 cells resulting in changes in cellular metabolism.2,34 Additionally, the mRNA transcription of genes encoding RA receptors is upregulated during differentiation of myotubes, indicating an important role in myogenic differentiation of C2C12s.2,14,73 Interestingly, SB431542 has been reported to activate Smad 1/5 as well as enhance the effects of RA, while D counteracts RA, thus inhibiting the Smad 1/5 pathway. In this study, C2C12 cells cultured in the RA− group had high myogenic index and low rates of delamination. The RA− medium was determined to be one of the top 3 conditions in this study, indicating that the inclusion of RA for C2C12 culture can have a strong effect on muscle during differentiation when combined with the other supplements, leading to delamination of muscle tissue. When assessing the mRNA transcription of the RA− group, the genes associated with skeletal muscle contractility overall had lower expression compared to many of the other experimental groups. This low expression suggests less spontaneous contraction during culture and reducing the incidence of delamination.
C2C12 cells that have been cultured with SB showed accelerated and enhanced myotube formation in vitro.20,62SB has been shown to inhibit ALK5, myostatin and block TGF-β1 induced Smad2 phosphorylation resulting in the formation of enlarged myotubes.20,25,37,62 Additionally, SB has been shown to reduce the amount of MHC protein production but increase expression of other myogenic factors and muscle hypertrophy.20,64 The myogenic index of C2C12 cells cultured in SB− medium was comparable to NM medium, however the level of delamination of SB− cultures was lower than NM. Consistent with these findings, qPCR analysis of genes associated with contractility showed overall lower expression in SB− medium compared to NM cultures, however higher expression when compared to MM cultures. The expression levels of genes related to myogenesis were similar in NM and SB− cultures but higher than C2C12 cells cultured in MM. Interestingly, MM, No Add, and SB−, (i.e. all deficient in SB), had similar levels of expression for genes associated with hypertrophy. Conditions containing SB had higher levels of expression of Acta1, Rps6kb1, and Myf6, indicating potentially more hypertrophy in these conditions.10,53,72
D had the greatest effect on the C2C12 culture regarding morphology and delamination over the 14-day period. Dorsomorphin is a BMP type 1 and Smad 1/5 inhibitor, as well as an inhibitor of p38 and Akt both of which are associated with the BMP pathway.5,48 The BMP type 1 pathway is an important hypertrophic and anti-atrophic pathway in muscle.61 When the BMP receptor is active it can lead to substantial hypertrophy in the muscle. Additionally, treatment of muscle cultures with D has been shown to promote myogenesis and contractile activity in vitro.24 Conversely, Furutani et al. found that treatment with BMP type 1 inhibitors down regulated myogenic regulatory factors, resulting in impaired muscle formation.20 With D removed from C2C12 muscle culture conditions, the myogenic index remained high but the rate of delamination was significantly lower than C2C12 cells cultured in NM. Removing D from the culture medium resulted in an overall gene expression profile that is comparable to MM and No Add conditions. Genes associated with contractility are reduced compared with NM indicating lower rates of spontaneous contraction and therefore delamination in D− conditions. When assessing myogenesis, the expression is intermediary, exhibiting higher levels of genes associated with myogenesis than MM but lower levels when compared to NM. C2C12 cells cultured in D− medium displayed improved morphology and gene expression profile compared to NM.
Similar to RA, SAG affects cell metabolism including that of C2C12 cells. Teperino et al. reported the culture of C2C12 cells with the inclusion of SAG resulted in increased glucose uptake and lactose production.57 Additionally, SAG resulted in increased extracellular acidification and reduced oxygen consumption rates in C2C12 cultures, indicating a metabolic shift.57 Further, SAG activates the AMPK pathway in muscle satellite cells, influencing muscle differentiation and regeneration.19 The removal of SAG from C2C12 culture medium had little effect on the myogenic index or the rate of delamination, indicating no negative effect on the culture of C2C12 cells. Likewise, genes associated with contractility and myogenesis had similar expression for NM and SAG− media conditions, making it likely that SAG does not have a significant effect on levels of muscle contraction or myogenesis in vitro.
The final 3 additives to NM are neurotrophic factors BDNF, CNTF, and GDNF, which are all known to increase neuron survival and promote neurite extension.42,46B, G, and C are expressed in skeletal muscle during muscle differentiation and stimulate NMJ formation.27,31,41,65C is present in differentiating muscle, however exogenous C has been shown to promote proliferation and inhibit differentiation.63 Another report specified that the addition of C to skeletal muscle culture can cause dedifferentiation of myotubes and down-regulate myogenic regulatory factors.7 However, when C was removed from culture in the current study, there was no upregulation of MyoD or Myf5. Furthermore, the removal of C had minimal effect on the overall gene expression profile compared to the NM medium condition. This could be due to the low concentration of C used in NM. One report indicated that C inhibits myoblast differentiation when present at a concentration of 10 ng/mL, while the concentration in this study is 2 ng/mL.60 The C− medium condition had similar myogenic index and rates of delamination compared to NM cultures. The production of G by skeletal muscle is known, however the mechanisms behind this production are largely unknown.65G is important in the formation of NMJs and overexpression of G leads to hyperinnervation.44,69,74 Interestingly, Vianney et al. reported that the presence of short term electrical stimulation and acetylcholine inhibits skeletal muscle G production.59 The role of G in NMJ formation will be important for future co-culture studies with skeletal muscle and neurons, however it did not have a significant effect in this study. The results when G was removed from NM were comparable to both NM and C− conditions. High levels of delamination were still present in the cultures, indicating the presence of G does not have a significant effect on maintaining long term culture of C2C12 cells. C2C12 cells secrete B, specifically during the proliferation stage after which it is downregulated during differentiation. Therefore, unlike C and G, myoblasts and not myotubes are responsible for the bulk of B production and secretion. B plays a significant role in myogenic differentiation and repair by modulating the development and differentiation of myoblasts, as well as maintaining a population of healthy muscle progenitors.43 However, contraction stimulates B production in mature myofibrils.9,41 In the present study, the removal of B from NM did not lead to an observable morphological effect; the myogenic index and level of delamination was comparable to NM, as well as C− and G−. Surprisingly, the B− medium induced a significant amount of changes in the overall gene expression profile. For contractility and myogenesis almost all the associated genes were highly upregulated. Genes specifically indicating differentiation, such as MyoD and Myf5, were not upregulated but genes corresponding to satellite cell development, such as Pax3 and Pax7, revealed relatively high expressions. These results indicate the inclusion of B plays some role in overall downregulation of detected transcripts of C2C12 cells cultured in NM.
All the supplements in the NM conditions have beneficial effects on motor neurons, specifically for differentiation and maintenance. RA induces neural phenotypes in stem cells and is involved in the switch between proliferation and differentiation.15 During development, the number of neurons depends on the levels and distribution of RA.26 Additionally, RA is necessary for motor neuron specification.45,54SB aids in the differentiation of stem cells into neurons by inhibition of the TGF-β pathway which in turn blocks endodermal and mesodermal cell fates.6,49 Patani et al. demonstrated that the combination of RA, SB, and a sonic hedgehog agonist treatment induces a motor neuron fate.47 Similarly, RA, SB, and SAG induced high specificity for motor neurons from a stem cell population and allows for accelerated differentiation.1 Additionally, SAG has been shown to play a role in myelination in the peripheral nervous system, which could be important in future co-culture NMJ studies.71D inhibits the BMP pathway, which further enhances neural induction and stimulates the outgrowth of neurites.17,30 Neurotrophic factors play a role in motor neuron survival, maintenance, and neurite outgrowth.42B helps prevent motor neuron death, promotes differentiation, stimulates a cholinergic phenotype in developing neurons, and induces axonal growth.28,66,67G mostly plays a role in motor neuron survival in vivo, while C promotes survival and differentiation of motor neurons.12,42,46,68 Additionally, mice lacking C exhibit severe motor neuron degeneration, indicating C has an important role in maintenance as well.40,52
In this study, C2C12 cultures underwent morphological and transcriptional assessments that led to 3 potentially optimal media formulations including D−, RA−, and No Add. These 3 formulations, along with positive control NM, were used to culture SpS and their axonal growth over 3 days was assessed. RA, SAG, D, and SB each contribute to the differentiation of stem cells inducing enriched motor neuron populations. After axonal growth assessments, it was determined these supplements can be included in the differentiation process and subsequently removed during the co-culture period as there was no significant difference in the D− and RA− conditions compared to NM. The No Add condition, which contained none of the supplements including the neurotrophic factors that are known to be essential for axonal growth, had slightly less axonal growth, however it was not significant. Therefore, it is possible that muscle cells can produce and secrete neurotrophic factors that will help regulate and promote axonal growth from the SpS. The No Add medium formulation results in high myogenic index and low levels of delamination of C2C12 cells over a 14-day period. Additionally, culture of C2C12 cells in the No Add medium leads to a transcriptional profile that is most comparable to the standard medium used to culture muscle, with one notable exception. MyoD is upregulated in No Add compared to MM indicating greater differentiation potential, which is confirmed by an increased myogenic index. In conclusion, the ideal medium for use in a culture system that includes C2C12 skeletal muscle cells and SpS contains DMEM/F12, N2 supplement, B27 supplement, d-glucose, and L-ascorbic acid.
Conclusion
In this study, an optimal medium for the culture of C2C12 skeletal muscle cells and SpS was explored. Analysis of C2C12 cell morphology was performed over a 14-day period, in addition to day 4 qPCR to evaluate gene expressions for myogenesis and myopathy. After C2C12 assessment, No Add, D−, RA−, and control MM were used to analyze axonal growth of SpS. There was no measureable difference between conditions and SpS axons grew at a steady rate. Our study suggests that for future studies, No Add medium is optimal to create a co-culture platform consisting of C2C12 skeletal muscle and SpS for in vitro NMJ formation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Wallace H. Coulter Center Translational Research Commercialization Grant (AA), the NIH (1UC4DK104208, 1U01CA233363, UG3DK122638 to AA), the Mentored Translational Research Scholars Program Award (KL2TR002737) by Miami CTSI (MS), and a Charcot-Marie-Tooth Association research grant (MS).
Conflict of interest
Rachel R. Besser, Annie C. Bowles, Ahmad Alassaf, Daniel Carbonero, Renata Maciel, Mario Saporta, and Ashutosh Agarwal declare that they have no conflicts of interest.
Ethical Approval
Approval for human subject research was Granted by the Wayne State University Human Investigation Committee and University of Miami Institutional Review Board. All study participants gave written informed consent before enrollment. No animal studies were carried out by the authors for this article.
Footnotes
The original online version of this article was revised: This Erratum is to add the 2nd affiliation for Ahmad Alassaf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/30/2020
A Correction to this paper has been published: 10.1007/s12195-020-00662-9
Contributor Information
Mario Saporta, Email: mas638@miami.edu.
Ashutosh Agarwal, Email: A.agarwal2@miami.edu.
References
- 1.Amoroso MW, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 2013;33:574–586. doi: 10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry EM, Odermatt AJE. Retinoic acid reduces glucocorticoid sensitivity in C2C12 myotubes by decreasing 11β-hydroxysteroid dehydrogenase type 1 and glucocorticoid receptor activities. Endocrincology. 2009;150:2700–2708. doi: 10.1210/en.2008-1618. [DOI] [PubMed] [Google Scholar]
- 3.Besser RR, et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020;8:591–606. doi: 10.1039/c9bm01430f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettadapur A, et al. Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels. Sci. Rep. 2016;6:28855. doi: 10.1038/srep28855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boergermann J, Kopf J, Yu P, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 2010;42:1802–1807. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, et al. Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol. Biol. Cell. 2005;16:3140–3151. doi: 10.1091/mbc.E05-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, et al. Retinoic acid regulates germ cell differentiation in mouse embryonic stem cells through a Smad-dependent pathway. Biochem. Biophys. Res. Commun. 2012;418:571–577. doi: 10.1016/j.bbrc.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Chevrel G, Hohlfeld R, Sendtner M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerv. 2006;33:462–476. doi: 10.1002/mus.20444. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico A, et al. Fatal hypertrophic cardiomyopathy and nemaline myopathy associated with ACTA1 K336E mutation. Neuromusc. Disord. 2006;16:548–552. doi: 10.1016/j.nmd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31:4880–4888. doi: 10.1016/j.biomaterials.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeChiara TM, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 13.Demestre M, et al. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 2015;15:328–336. doi: 10.1016/j.scr.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Downes M, Mynett-Johnson L, Muscat G. The retinoic acid and retinoid X receptors are differentially expressed during myoblast differentiation. Endocrinology. 1994;134:2658–2661. doi: 10.1210/endo.134.6.8194491. [DOI] [PubMed] [Google Scholar]
- 15.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy RM, Sun Y, Feinberg AW. Understanding the role of ECM protein composition and geometric micropatterning for engineering human skeletal muscle. Ann. Biomed. Eng. 2016;44:2076–2089. doi: 10.1007/s10439-016-1592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faravelli I, et al. Motor neuron derivation from human embryonic and induced pluripotent stem cells: experimental approaches and clinical perspectives. Stem Cell Res. Ther. 2014;5:87. doi: 10.1186/scrt476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J-J, Marston S. Genotype–phenotype correlations in ACTA1 mutations that cause congenital myopathies. Neuromusc. Disord. 2009;19:6–16. doi: 10.1016/j.nmd.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Fu X, Zhu M-J, Dodson MV, Du M. AMP-activated protein kinase stimulates Warburg-like glycolysis and activation of satellite cells during muscle regeneration. J. Biol. Chem. 2015;290:26445–26456. doi: 10.1074/jbc.M115.665232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furutani Y, Umemoto T, Murakami M, Matsui T, Funaba M. Role of endogenous TGF-β family in myogenic differentiation of C2C12 cells. J. Cell. Biochem. 2011;112:614–624. doi: 10.1002/jcb.22953. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32:9602–9611. doi: 10.1016/j.biomaterials.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo X, et al. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng. Part C. 2010;16:1347–1355. doi: 10.1089/ten.tec.2010.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Happe CL, Tenerelli KP, Gromova AK, Kolb F, Engler AJ. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol. Biol. Cell. 2017;28:1950–1958. doi: 10.1091/mbc.E17-01-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horbelt D, et al. Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation. J. Biol. Chem. 2015;290:3390–3404. doi: 10.1074/jbc.M114.604397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 26.Janesick A, Wu SC, Blumberg B. Retinoic acid signaling and neuronal differentiation. Cell. Mol. Life Sci. 2015;72:1559–1576. doi: 10.1007/s00018-014-1815-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RW, White JD, Walker EC, Martin TJ, Sims NA. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone. 2014;64:47–56. doi: 10.1016/j.bone.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 28.Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp. Neurol. 1997;144:273–286. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- 29.Kong L, et al. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. 2009;29:842–851. doi: 10.1523/JNEUROSCI.4434-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo TA, et al. Dorsomorphin stimulates neurite outgrowth in PC12 cells via activation of a protein kinase A-dependent MEK-ERK1/2 signaling pathway. Genes Cells. 2011;16:1121–1132. doi: 10.1111/j.1365-2443.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Varendi K, Peränen J, Andressoo J-O. Tristetraprolin is a novel regulator of BDNF. SpringerPlus. 2014;3:502. doi: 10.1186/2193-1801-3-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo PL, et al. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 2012;181:2030–2037. doi: 10.1016/j.ajpath.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam MT, Sim S, Zhu X, Takayama S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials. 2006;27:4340–4347. doi: 10.1016/j.biomaterials.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Lee YM, et al. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J. Biol. Chem. 2008;283:33969–33974. doi: 10.1074/jbc.M804469200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maciel R, Correa R, Bosso Taniguchi J, Prufer Araujo I, Saporta MA. Human tridimensional neuronal cultures for phenotypic drug screening in inherited peripheral neuropathies. Clin. Pharmacol. Ther. 2019;107:1231–1239. doi: 10.1002/cpt.1718. [DOI] [PubMed] [Google Scholar]
- 36.Maciel R, et al. The human motor neuron axonal transcriptome is enriched for transcripts related to mitochondrial function and microtubule-based axonal transport. Exp. Neurol. 2018;307:155–163. doi: 10.1016/j.expneurol.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-β signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maselli RA, Richman DP, Wollmann RL. Inflammation at the neuromuscular junction in myasthenia gravis. Neurology. 1991;41:1497–1497. doi: 10.1212/wnl.41.9.1497. [DOI] [PubMed] [Google Scholar]
- 39.Maselli RA, et al. Neuromuscular transmission in amyotrophic lateral sclerosis. Muscle Nerv. 1993;16:1193–1203. doi: 10.1002/mus.880161109. [DOI] [PubMed] [Google Scholar]
- 40.Masu Y, et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 41.Matthews VB, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 42.Mitsumoto H, et al. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265:1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- 43.Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J. Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci. Res. 2003;45:391–399. doi: 10.1016/s0168-0102(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 45.Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Oppenheim RW, et al. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 47.Patani R, et al. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat. Commun. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul BY, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 2008;4:33. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sances S, et al. Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat. Neurosci. 2016;19:542. doi: 10.1038/nn.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saporta MA. Charcot-Marie-Tooth disease and other inherited neuropathies. CONTINUUM. 2014;20:1208–1225. doi: 10.1212/01.CON.0000455885.37169.4c. [DOI] [PubMed] [Google Scholar]
- 51.Saporta MA, et al. Axonal Charcot-Marie-Tooth disease patient-derived motor neurons demonstrate disease-specific phenotypes including abnormal electrophysiological properties. Exp. Neurol. 2015;263:190–199. doi: 10.1016/j.expneurol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sendtner M, et al. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992;358:502. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- 53.Smith LR, Meyer G, Lieber RL. Systems analysis of biological networks in skeletal muscle function. Wiley Interdiscip. Rev. 2013;5:55–71. doi: 10.1002/wsbm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sockanathan S, Jessell TM. Motor neuron–derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- 55.Southam KA, King AE, Blizzard CA, McCormack GH, Dickson TC. Microfluidic primary culture model of the lower motor neuron–neuromuscular junction circuit. J. Neurosci. Methods. 2013;218:164–169. doi: 10.1016/j.jneumeth.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y, Duffy R, Lee A, Feinberg AW. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013;9:7885–7894. doi: 10.1016/j.actbio.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 57.Teperino R, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Umbach JA, Adams KL, Gundersen CB, Novitch BG. Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS ONE. 2012;7:e36049. doi: 10.1371/journal.pone.0036049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vianney J-M, Miller DA, Spitsbergen JM. Effects of acetylcholine and electrical stimulation on glial cell line-derived neurotrophic factor production in skeletal muscle cells. Brain Res. 2014;1588:47–54. doi: 10.1016/j.brainres.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, et al. Effects of interleukin-6, leukemia inhibitory factor, and ciliary neurotrophic factor on the proliferation and differentiation of adult human myoblasts. Cell. Mol. Neurobiol. 2008;28:113–124. doi: 10.1007/s10571-007-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang RN, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, et al. Sirtuin 1 promotes the proliferation of C2C12 myoblast cells via the myostatin signaling pathway. Mol. Med. Rep. 2016;14:1309–1315. doi: 10.3892/mmr.2016.5346. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, et al. MiR-696 regulates C2C12 cell proliferation and differentiation by targeting CNTFRα. Int. J. Biol. Sci. 2017;13:413. doi: 10.7150/ijbs.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watt KI, et al. SB431542 treatment promotes the hypertrophy of skeletal muscle fibers but decreases specific force. Muscle Nerv. 2010;41:624–629. doi: 10.1002/mus.21573. [DOI] [PubMed] [Google Scholar]
- 65.Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerv. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- 66.Wong V, Arriaga R, Ip NY, Lindsay RM. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur. J. Neurosci. 1993;5:466–474. doi: 10.1111/j.1460-9568.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]
- 67.Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360:753. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 68.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 69.Yang L-X, Nelson P. Glia cell line-derived neurotrophic factorregulates the distribution of acetylcholine receptors in mouse primary skeletal muscle cells. Neuroscience. 2004;128:497–509. doi: 10.1016/j.neuroscience.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida M, et al. Modeling the early phenotype at the neuromuscular junction of spinal muscular atrophy using patient-derived iPSCs. Stem Cell Rep. 2015;4:561–568. doi: 10.1016/j.stemcr.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshimura K, Takeda S. Hedgehog signaling regulates myelination in the peripheral nervous system through primary cilia. Differentiation. 2012;83:S78–S85. doi: 10.1016/j.diff.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Zammit PS. SEMINARS in Cell & Developmental Biology. Amsterdam: Elsevier; 2017. pp. 19–32. [Google Scholar]
- 73.Zhu G-H, et al. Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation. 2009;78:195–204. doi: 10.1016/j.diff.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp. Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.