Abstract
Phosphorus (P) is an integral part of diet formulation for broiler chickens as P is required for various biochemical processes essential to life. A study was designed to examine the additivity of apparent ileal digestibility (AID) and standardized ileal digestibility (SID) of P in mixed diets containing corn and soybean meal (SBM) with or without phytase supplementation. Birds were fed a commercial starter diet from day 0 to 21 after hatching and then allotted to 7 dietary treatments in a randomized complete block design with the BW as a blocking factor. Four semipurified diets were prepared to contain corn or SBM as the sole source of P with or without the addition of phytase at 1,000 phytase units/kg of diet. Two mixed diets were also prepared to contain corn and SBM with or without the addition of phytase at 1,000 phytase units/kg diet. A P-free diet (PFD) was formulated to determine the basal ileal endogenous loss of P. There were 16 replicate cages of the PFD and 8 replicate cages of the 6 experimental diets, with 8 birds per replicate cage for a total of 512 birds. Diets were fed for 3 d. The ileal digesta of birds were collected from the distal two-thirds of the ileum on day 24 after hatching. The SID of P in corn and SBM were 52.2 and 65.4%, respectively (SEM = 1.37). The addition of phytase improved (P < 0.05) both the AID and SID of P in the corn, SBM, and mixed diets. The determined AID or SID in the corn and SBM with or without phytase was used to predict the AID or SID in the mixed diets. There were no differences between the predicted and determined digestibility values in the mixed diets for either AID or SID of P and thus additive. Phytase supplementation of the mixed diet did not influence the additivity of AID or SID. In conclusion, the AID or SID of P in the corn and SBM was additive in the mixed diets containing corn and SBM with or without the addition of phytase.
Key words: additivity, corn, digestibility, phosphorus, soybean meal
Introduction
Phosphorus (P) is one of the important nutrients, which should be considered when formulating diets for broiler chickens. The concentration of nonphytate P (nPP) has been used to describe the availability of P in feed ingredients and the P requirements of broiler chickens (NRC, 1994). However, a portion of the nPP may be unavailable to birds because of the intrinsic characteristics of various feed ingredients such as their phytate content or other antinutritional components (Liu et al., 2012). Similarly, a portion of P in phytate may become partially available to broiler chickens because of the limited action of endogenous phytase enzymes. Thus, to prevent the consequent loss of P into the environment due to the overfeeding of P to broiler chickens, it is necessary to accurately determine the digestible P in feed ingredients for broiler chickens.
The basal endogenous loss (BEL) of P is important in measuring the actual digestibility of P in diets either by using the regression method to determine the true ileal digestibility of P as described by Dilger and Adeola (2006) or by feeding a P-free diet (PFD) to determine the standardized P digestibility. Although some studies have reported the BEL of P using the regression method (Dilger and Adeola, 2006; Mutucumarana et al., 2014), there are limited studies estimating the BEL of P in broiler chickens using a PFD. The use of the regression method in determining endogenous losses may be prone to errors because of the specificity to a feed ingredient and the dependence on the extrapolation to zero on the regression curve. Several points on the regression line may be required, thus increasing the treatments required and the cost of running experimental trials. Thus, nitrogen-free diets are more common in determining the BEL of amino acids in broiler chickens (Kong and Adeola, 2013; Osho et al., 2019) than the regression method. The ileal BEL of P is important in calculating the standardized ileal digestibility (SID) of P. Ileal digestibility of nutrients may show a more accurate picture of nutrient utilization from broiler diets than the total tract utilization because of the action of microbes in the large intestine and the presence of urine in the excreta (Ravindran et al., 1999). Thus, estimating the SID of P instead of standardized total tract retention may be more useful in formulating diets for broiler chickens.
It is also of importance to verify the assumption of additivity of the apparent ileal digestibility (AID) and SID of P in mixed diets for broiler chickens. In the formulation of diets, it is assumed that the amount of available nutrients in a mix diet is equal to the sum of available nutrients originating from each of the individual feed ingredients (Fang et al., 2007). However, there is a lack of information that the AID and SID of P measured in feed ingredients are additive in mixed diets for broiler chickens, although previous studies have been reported on the additivity of P digestibility in pigs (Fang et al., 2007; She et al., 2018).
The supplementation of phytase in diets has been known to improve the digestibility of P in broiler chickens (Babatunde et al., 2019a,b) and is now commonly included in diets for broiler chickens. For the appropriate use of phytase in mixed diets, matrix values of phytase have been routinely used when formulating diets (Adeola and Cowieson, 2011), and therefore, a certain value of the available P concentration has been given to phytase although phytase per se does not contain P at all. Moreover, efficacy of phytase may vary among feed ingredients depending on the concentration of phytate, leading to the changes in matrix values of phytase added into diets consisting of various feed ingredients. Therefore, using digestibility of P elevated by phytase in each feed ingredient when formulating diets may provide the accurate concentration of available P in mixed diets. However, information for the additivity of P digestibility elevated by phytase in mixed diets is scarce. Therefore, the objective of this study was to examine the additivity of AID and SID of P in mixed diets containing corn and soybean meal (SBM) with or without phytase supplementation. The study was designed to test the null hypothesis that the determined AID or SID of P in mixed diets containing corn and SBM was not different from the predicted values calculated from the AID or SID of P in each feed ingredient regardless of the addition of phytase.
Materials and methods
All protocols of animal experiments were reviewed and approved by the Purdue University Animal Care and Use Committee.
Birds, Management, and Experimental Design
Male broiler chicks (Cobb 500, Siloam Springs, AR) were donated by a commercial hatchery at hatch. Birds were tagged with identification numbers and housed inside battery cages equipped with thermostatically controlled heaters (model SB 4T, Alternative Design Manufacturing, Siloam Springs, AR) and located in an environmentally controlled room. Birds had free access to water via water nipples and were fed a commercial starter diet prepared to meet or exceed the nutrient requirements of broiler chicks from day 0 to 21 after hatching (NRC, 1994). On day 21 after hatching, each bird was weighed individually and allotted to experimental diets. The experiment was conducted as a randomized complete block design with birds assigned into 6 experimental diets with 8 replicate cages and a PFD with 16 replicate cages. Each replicate cage contained 8 birds, and the BW was used as a blocking factor. Birds were fed experimental diets for 3 d.
Experimental Diets
A PFD was prepared with dextrose and gelatin to estimate the BEL of P in broiler chickens. Four diets were formulated to contain corn or SBM as the sole source of P with or without the inclusion of phytase at 1,000 phytase units (FYT)/kg diet (Table 1). Two mixed diets containing corn and SBM with or without the inclusion of phytase at 1,000 FYT/kg were formulated. Phytase (RONOZYME HiPhos, DSM Nutritional Products, Kaiseraugst, Switzerland) premix was prepared with dextrose to contain 50 FYT/g and added at 20 g/kg diets in the mash form. The calculated P levels were 0, 26, 30, and 39 g/kg for the PFD, corn, SBM, and corn–SBM mixed diets, respectively. The ratio of the total calcium (Ca) to nPP was maintained at 2:1. All other mineral contents met or exceeded the requirement estimates of broiler chickens (NRC, 1994). Chromic oxide was incorporated into the diets at 5 g/kg as an indigestible marker.
Table 1.
Ingredient composition of experimental diets, g/kg as-fed basis.
| Ingredients | Diet1 |
||||||
|---|---|---|---|---|---|---|---|
| 0 FYT/kg phytase |
1,000 FYT/kg phytase |
PFD | |||||
| Corn | SBM | C + SBM | Corn | SBM | C + SBM | ||
| Ground corn | 912.4 | - | 526.1 | 912.4 | - | 526.1 | - |
| Soybean meal | 0.0 | 482.0 | 386.0 | 0.0 | 482.0 | 386.0 | - |
| Dextrose | 20.0 | 451.7 | 20.0 | - | 431.7 | - | 460.0 |
| Corn starch | - | - | - | - | - | - | 145.0 |
| Gelatin2 | - | - | - | - | - | - | 262.0 |
| Soybean oil | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Cellulose3 | - | - | - | - | - | - | 50.0 |
| Ground limestone | 3.6 | 2.3 | 3.9 | 3.6 | 2.3 | 3.9 | 5.5 |
| Salt | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | - |
| Potassium carbonate | - | - | - | - | - | - | 2.6 |
| Magnesium oxide | - | - | - | - | - | - | 2.0 |
| Sodium bicarbonate | - | - | - | - | - | - | 7.5 |
| Choline chloride | - | - | - | - | - | - | 2.5 |
| Potassium chloride | - | - | - | - | - | - | 2.9 |
| Vitamin-mineral premix4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Phytase premix5 | - | - | - | 20.0 | 20.0 | 20.0 | - |
| Chromic oxide premix6 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Total | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
FYT = phytase units; SBM = soybean meal; C + SBM = corn + soybean meal; PFD = phosphorus-free diet.
PB Leiner, Vilvoorde, Belgium.
Solka-floc 40 FCC, International Fiber Corporation, North Tonawanda, NY.
Supplied the following quantities per kg of the diet: vitamin A, 5,484 IU; vitamin D3, 2,643 ICU; vitamin E, 11 IU; menadione sodium bisulfite, 4.38 mg; riboflavin, 5.49 mg; D-pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 μg; biotin, 55.2 μg; thiamine mononitrate, 2.2 mg; folic acid, 990 μg; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg; Mn, 66.06 mg; Cu, 4.44 mg; Fe, 44.1 mg; Zn, 44.1 mg; Se, 300 μg.
Phytase product contains 2500 FYT (RONOZYME HiPhos, DSM Nutritional Products, Switzerland). The phytase premix was prepared with dextrose to provide 1,000 FYT/kg of diet.
Prepared as 1-g chromic oxide added to 4-g dextrose.
Sampling Procedures and Chemical Analysis
On day 24 after hatching, all birds were euthanized by CO2 asphyxiation. Ileal digesta were flushed from the distal two-thirds of the ileum with distilled water into plastic containers, pooled by cage, and stored in −20°C. Frozen ileal digesta were dried in a forced-air drying oven at 56°C until constant weight.
Diets and dried ileal digesta were finely ground using an electric coffee grinder and analyzed for DM, CP, gross energy, chromium, P, and Ca concentrations. The DM was determined by placing samples in a drying oven for 24 h at 105°C (Precision Scientific Co., Chicago, IL; method 934.01; AOAC, 2000). Nitrogen contents in ingredients, diets, and ileal digesta samples were determined by the combustion method (TruMac N; LECO Corp., St. Joseph, MI; method 990.03; AOAC, 2000), and the CP content was calculated by multiplying the content of nitrogen by 6.25. The gross energy was determined in diet samples using the isoperibol bomb calorimeter (model 1261; Parr Instrument Co., Moline, IL). Chromium concentrations in the diets and ileal digesta were determined after a wet-ash digestion of samples as previously described by Fenton and Fenton (1979). Phosphorus concentration was determined from digested samples by spectrophotometry (Spark 10 M; Tecan Group Ltd., Männedorf, Switzerland), with absorbance read at 630 nm, while Ca concentrations in samples were determined by flame atomic absorption spectrometry using a Varian SpectrAA 220FS (Varian Australia Pty Ltd., Victoria, Australia; Babatunde et al., 2019a).
Calculations and Statistical Analysis
The AID (%), BEL (g/kg DM intake), and SID (%) were calculated using the equations described by Adeola (2001):
where CRI and CRO are the concentration of chromium (g/kg DM) in diets and ileal digesta, respectively; PI and PO are the concentration of P (g/kg DM) in diets and ileal digesta, respectively; and the BEL of P was calculated with birds fed the PFD. Data for the AID and SID in ingredients and mixed diets were analyzed using the mixed procedure of SAS (SAS Inst. Inc., Cary, NC) with diet, phytase, and their interaction as fixed variables and block as a random variable. Statistical significance was set at a P < 0.05. Cage served as the experimental unit.
The AID or SID of P in corn and SBM determined in semipurified diets were used for calculating the predicted AID (%) or SID (%) of P in the mixed diets according to the following equation (Xue et al., 2014);
in which AIDP (%) is the predicted AID of P in the mixed diet; PCorn and PSBM are the concentrations (g/kg) of P contributed by corn and SBM calculated by multiplying the concentration of P (%) in the ingredient by the proportion of the ingredient in the mixed diet; AIDCorn and AIDSBM are the determined AID of P (%) in the ingredient. The predicted SID of P (%) in mixed diet was calculated using the same equation except that the SID replaced AID.
Differences between determined and predicted AID or SID in the mixed diets were analyzed using a one-sample two-tailed t-test by the TTEST procedure of SAS to test the difference from zero as follows:
With the predicted being one mean value for each of the AID or SID, the difference between the determined and predicted AID or SID for each of 8 replicates was calculated as follows:
The difference for each replicate was output into SAS and analyzed using the following Proc TTEST code: Proc ttest h0 = 0 plots(showh0) sides = u alpha = 0.05; var Difference;.
Results and discussion
All birds were healthy during the experimental period and readily consumed all the experimental diets except the PFD. No mortality or leg problems were recorded during this period. The average initial BW of birds at day 21 was 789 g. However, after consuming the experimental diets for 3 d, the average BW of birds fed the corn, SBM, and mixed diets were 951, 961, and 998 g (with phytase), and 944, 951, and 973 g (without phytase), respectively, whereas birds fed the PFD lost weight and had an average BW of 740 g. Similarly, the feed intake of birds fed the corn, SBM, and mixed diets for 3 d were 331, 271, and 302 g/bird (with phytase) and 318, 281, and 289 g/bird (without phytase), respectively, while the birds fed the PFD had a feed intake of 76 g/bird. This indicated the significant role of P in the metabolism of broiler chickens and negative impacts of a deficiency of P on the growth and development of broiler chickens (Adeola and Walk, 2013). Analyzed composition of the DM, CP, P, and Ca in the feed ingredients is similar to concentrations reported by the NRC (1994) and Mutucamarana et al. (2015). The concentration of P in the PFD was −0.13 g/kg, whereas the concentration of P and other nutrients in the ingredients and mixed diets were similar to the calculated values (Table 2).
Table 2.
Analyzed concentration of DM, gross energy, CP, P, and Ca in experimental diets, g/kg as-fed basis.
| Item | Diet1 |
||||||
|---|---|---|---|---|---|---|---|
| 0 FYT/kg phytase |
1,000 FYT/kg phytase |
PFD | |||||
| Corn | SBM | C + SBM | Corn | SBM | C + SBM | ||
| DM | 877 | 799 | 869 | 876 | 787 | 865 | 834 |
| CP | 75 | 228 | 230 | 76 | 229 | 228 | 229 |
| GE, kcal/kg | 3,946 | 3,759 | 4,047 | 3,924 | 3,783 | 4,011 | 3,811 |
| P | 2.67 | 2.93 | 3.85 | 2.62 | 3.01 | 3.83 | −0.13 |
| Ca | 1.96 | 2.75 | 3.25 | 1.98 | 2.71 | 3.28 | 2.68 |
Abbreviations: Ca, calcium; GE, gross energy; P, phosphorus.
FYT = phytase units; SBM = soybean meal; C + SBM = corn + soybean meal; PFD = P-free diet.
There are limited studies that use PFD to determine the BEL of P in broiler chickens. However, the composition of the PFD used in this trial was similar to that fed to broiler chickens (Liu et al., 2012, 2013) and pigs (Petersen and Stein, 2006), with the exception that crystalline amino acids were not included in the PFD used in the present study. The source of CP (i.e., gelatin) in the PFD used in this trial was also different from that used in the study by Mutucumarana and Ravindran (2016) where dried egg albumen served as the main source of CP. The estimated BEL of P in broiler chickens after feeding the PFD for 72 h was 0.166 g/kg DM intake. From previous work in our laboratory, it was reported that feeding P-deficient diets to broiler chickens over a long period of time could influence the P digestibility as birds are able to adapt to the deficiency by secreting P-containing compounds into the gut (Babatunde et al., 2019a, Babatunde et al., 2019b). Thus, ileal digesta were collected after 72 h of feeding to estimate the endogenous flow of P in the gut. Although ileal P endogenous loss values are scarce in literature, the value estimated in this trial was lower than that reported by Rutherfurd et al. (2002, 2004), where a range of 260 to 450 mg/kg DM intake was estimated. The greater BEL of P reported in these trials may be a result of the high levels of synthetic amino acids and the absence of Ca in the PFD. The total absence of Ca in their PFD may result in the increased resorption of minerals from the skeleton, thus affecting the metabolism of the birds (Mahan, 1982; Plumstead et al., 2008). Another method that has been used in previous studies to estimate the endogenous loss of P in broilers is the regression method. However, values from this method are highly variable and could be prone to errors because they are ingredient-specific and involve the extrapolation to zero P intake. Nonetheless, the BEL of P from this trial was similar to values from Dilger and Adeola (2006), where the prececal endogenous P loss was estimated at 176.5 mg/kg DM intake by regression analysis. The BEL of P from the current trial is also slightly greater than the 123 and 109 mg/kg DM intake reported in Liu et al., 2012, Liu et al., 2013. An important difference is that the BEL of P was estimated in the excreta after feeding the PFD to broiler chickens for 4 h and collecting the excreta for 52 h. Estimating the BEL of P from the excreta may not give a true picture of the endogenous flow of P because of the activities of P-utilizing bacteria that may be present in the ceca and the presence of urine in the excreta. A study by Borda-Molina et al. (2016) reported the presence of microbes (Clostridium spp) in the cecum of broilers with the capacity to produce phytases that could further increase the hydrolysis of phytate for P release. Because there could be reverse peristalsis in the gut of broiler chickens, released P in the ceca may be absorbed by epithelial cells in the small intestine, thus misrepresenting the actual P utilization of the birds. Therefore, it may be pertinent to estimate the endogenous flow of P from the ileum as have been established with amino acids in several studies to prevent the influence of microbes on BEL values (Ravindran et al., 1999; Osho et al., 2019).
The mean values for the ileal DM digestibility in corn and SBM diets were 70.3 and 73.2%, respectively (Table 3). Birds fed the mixed diets had 62.0 and 63.0% ileal DM digestibility with and without the inclusion of phytase, respectively. The AID of P in corn, SBM, and mixed diets were 46.7, 60.9, and 55.8%, respectively, when phytase was not added to the diets. Corn and SBM have low concentrations of P, most of which are bound as phytate complexes (NRC, 2012). Thus, it is not surprising that birds were not able to fully use the P present in these feed ingredients. The AID of P in corn and SBM in this trial were lower than values reported by Wu et al. (2004) and Dilger and Adeola (2006), which may be due to cultivar differences in the phytate concentrations. However, the AID values in the present study were close to the values reported by Mutucumarana et al., 2014, Mutucumarana et al., 2015. Phytase increases the utilization of P by the hydrolysis of phytate–P complexes present in cereals and oil grains (Dilger et al., 2004; Olukosi et al., 2013). As expected, the addition of phytase in the corn, SBM, and mixed diets improved (P < 0.01) the AID of P in birds as compared with the birds fed diets without phytase. A similar trend was observed with Ca digestibility. Birds fed diets without phytase had a lower (P < 0.01) apparent digestibility of Ca in corn, SBM, and mixed diets than birds fed diets containing phytase.
Table 3.
| Item3 | 0 FYT/kg phytase |
1,000 FYT/kg phytase |
SEM |
P Value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Corn | SBM | C + SBM | Corn | SBM | C + SBM | Diet | Phytase | Diet × phytase | ||
| AID DM | 68.9 | 71.2 | 63.0 | 71.7 | 75.1 | 62.0 | 1.46 | <0.01 | 0.12 | 0.22 |
| AID Ca | 41.0 | 30.3 | 42.6 | 52.8 | 56.8 | 58.7 | 3.15 | 0.10 | <0.01 | 0.07 |
| AID P | 46.7 | 60.9 | 55.8 | 64.3 | 78.4 | 73.5 | 1.38 | <0.01 | <0.01 | 1.00 |
| SID P4 | 52.2 | 65.4 | 59.6 | 69.9 | 82.8 | 77.3 | 1.38 | <0.01 | <0.01 | 1.00 |
Abbreviations: Ca, calcium; P, phosphorus.
Data are least squares mean of 8 observations.
FYT = phytase units; SBM = soybean meal; C + SBM = corn + soybean meal.
AID = apparent ileal digestibility; SID = standardized ileal digestibility.
For the calculation of SID, values of AID were corrected for the basal endogenous loss of P at 0.166 g/kg.
The SID of P has not been widely used in the formulation of diets for broiler chickens as compared with the SID of amino acids because of challenges in estimating the BEL of P. More commonly found in literature are values for the true ileal digestibility of P in feed ingredients from regression analysis. However, with increasing concerns for the environmental impact of P excretion and the efforts going toward reducing the environmental footprint of animal husbandry, it may be relevant to use SID values for ingredients when formulating diets for broiler chickens. Similarly, using the SID of P - instead of the nPP concentrations in ingredients may also be advantageous because some portion of the nPP may be unavailable to broiler chickens, leading to wastage of dietary P. In pigs, the standardized total tract digestibility of P in feed ingredients has been commonly used for diet formulation (Almeida and Stein, 2010). For an accurate use of SID of P in diet formulation for broiler chickens, further research may be needed to investigate the standardized ileal digestible P requirement of broiler chickens and the appropriate methodology to determine the BEL of P in broiler chickens. In the present study, the SID of P in corn, SBM, and mixed diets were determined at 52.2, 65.4, and 59.6%, respectively, without the addition of phytase. Because of the dearth of the SID values in literature, values from the present study could not be compared with values from other studies. However, the SID of P in the current study was similar to the standardized P retention in corn, SBM, and corn-SBM–based mixed diets (45, 70, and 53%, respectively), reported by Liu et al. (2012), and greater than the true ileal digestibility of P in corn and SBM (34 and 42%, respectively), reported by Trairatapiwan et al. (2018). The differences in the method of estimating the BEL of P and the collection site may explain the disparities in P digestibility values between the present study and other studies. The inclusion of phytase to the corn, SBM, and mixed diets improved (P < 0.01) the SID of P by 26, 20, and 23%, respectively.
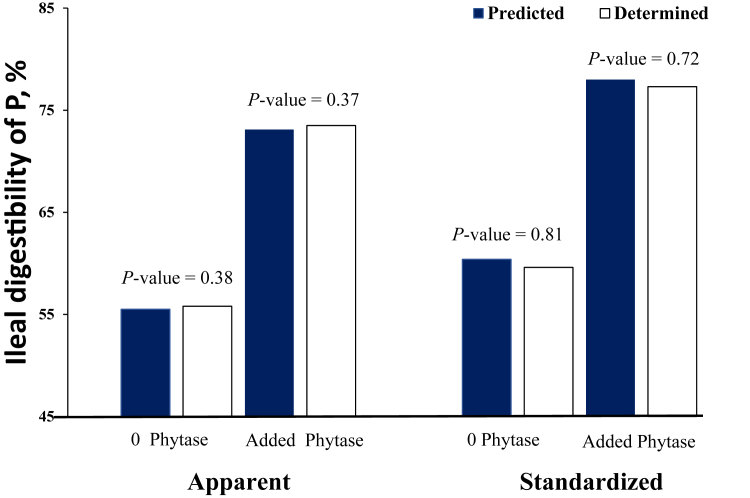
In the present study, there was no difference between the predicted and determined AID or SID of P in the corn-SBM–based mixed diet (Figure 1), which indicated that digestibility values in the mixed diets for either the AID or SID are additive. To the best of our knowledge, this is the first to examine the additivity of the AID or SID of P in diets for broiler chickens. However, the additivity of P digestibility in feed ingredients has been examined with pigs. In contrast with our study, Fang et al. (2007) observed that the AID of P was not additive but the true ileal P digestibility values in feed ingredients containing low concentrations of phytate P and antinutritional factors such as corn and SBM were additive in mixed diets. Differences in the physiology and metabolism of P between pigs and chickens may explain why the differences in findings were observed. In agreement with the present study, She et al. (2018) reported that standardized total tract digestibility of P were additive in mixed diets containing corn, SBM, and canola meal fed to pigs. Zhai and Adeola (2013) also reported additivity for true total tract digestibility of P in mixed diets containing corn and SBM fed to pigs. From these studies, there were indications that P digestibility values that accounted for endogenous losses of P in the gastrointestinal tract were additive in pigs. This may therefore explain why the SID values determined in the present study was additive.
Figure 1.
Predicted (solid bar) and determined (open bar) apparent and standardized ileal digestibility of phosphorus (P) in mixed diets containing corn and soybean meal with or without phytase supplementation. Predicted values in mixed diets were calculated from determined values in corn and soybean meal. Each bar represents the least square means of 8 observations. Predicted and determined apparent and standardized digestibility values for 0 phytase units/kg (0 phytase) were 55.5 and 55.8, and 60.4 and 59.6%, respectively, with a standard error of difference of 1.28. Corresponding values for 1,000 phytase units/kg (added phytase) were 73.1 and 73.5, and 78.0 and 77.3%, respectively, with a standard error of difference of 1.60.
The AID and SID values in the mixed diets containing corn, SBM, and phytase were additive. This study is the first to determine additivity of the AID or SID in mixed diets supplemented with phytase and fed to broiler chickens or pigs. Phytase is known to improve the digestibility and utilization of P in most feed ingredients fed to broiler chickens. However, because of differences in the composition and structures of phytate between corn and SBM (Selle and Ravindran, 2007), it was not known if the AID or SID of P in mixed diets containing these ingredients and phytase would be additive. Results from this study revealed that the inclusion of phytase did not affect the additivity of the AID or SID of P in mixed diets containing corn and SBM. Whether this holds for mixed diets containing phytate-rich ingredients such as canola meal or rice bran, even in the presence of phytase, requires further investigation. Because phytase is more commonly included in commercial broiler diets, care should be taken to not use the AID or SID values in feed ingredients determined without phytase for formulating diets containing phytase as the AID or SID would be underestimated. A more appropriate method is to determine the SID in commonly used feed ingredients with phytase included and use these determined values in the formulation of mixed diets containing phytase for broilers chickens. The knowledge of the P utilization of feed ingredients in the presence of phytase is fundamental and will succor in formulating diets with adequate P levels for broiler chickens while reducing wastage and loss of P to the environment. In conclusion, the AID or SID of P in corn and SBM was improved in the presence of phytase. The expected P digestibility in their mixture was not different from the determined digestibility with or without the presence of phytase, indicating additivity of the AID or SID of P in mixed diets containing corn and SBM fed to broiler chickens.
Acknowledgments
The authors appreciate Cobb-Vantress, Monticello, KY, for donating the chicks, Pat Jaynes for her technical assistance, and Ayodeji Aderibigbe for his help with the farm work.
Disclosures
There are no conflicts of interest in this article.
References
- Adeola O. Digestion and balance techniques in pigs. In: Lewis A.J., Southern L.L., editors. Swine Nutrition. 2nd ed. CRC Press; Washington, DC: 2001. pp. 903–916. [Google Scholar]
- Adeola O., Cowieson A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011;89:3189–3218. doi: 10.2527/jas.2010-3715. [DOI] [PubMed] [Google Scholar]
- Adeola O., Walk C.L. Linking ileal digestible phosphorus and bone mineralization in broiler chickens fed diets supplemented with phytase and highly soluble calcium. Poult. Sci. 2013;92:2109–2117. doi: 10.3382/ps.2013-03068. [DOI] [PubMed] [Google Scholar]
- Almeida F.N., Stein H.H. Performance and phosphorus balance of pigs fed diets formulated on the basis of values for standardized total tract digestibility of phosphorus. J. Anim. Sci. 2010;88:2968–2977. doi: 10.2527/jas.2009-2285. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists International . 17th ed. AOAC Int.; Gaithersburg, MD: 2000. Official Methods of Analysis. [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. The impact of age and feeding length on phytase efficacy during the starter phase of broiler chickens. Poult. Sci. 2019;98:6742–6750. doi: 10.3382/ps/pez390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda-Molina D., Vital M., Sommerfeld V., Rodehutscord M., Camarinha-Silva A. Insights into broilers' gut microbiota fed with phosphorus, calcium, and phytase supplemented diets. Front Microbiol. 2016;7:2033. doi: 10.3389/fmicb.2016.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger R.N., Adeola O. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing chicks fed conventional and low-phytate soybean meals. Poult. Sci. 2006;85:661–668. doi: 10.1093/ps/85.4.661. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Onyango E.M., Sands J.S., Adeola O. Evaluation of microbial phytase in broiler diets. Poult. Sci. 2004;83:962–970. doi: 10.1093/ps/83.6.962. [DOI] [PubMed] [Google Scholar]
- Fang R.J., Li1 T.J., Yin F.G., Yin Y.L., Kong X.F., Wang K.N., Yuan Z., Wu G.Y., He J.H., Deng Z.Y., Fan M.Z. The additivity of true or apparent phosphorus digestibility values in some feed ingredients for growing pigs. Asian-aust. J. Anim. Sci. 2007;20:1092–1099. [Google Scholar]
- Fenton T.W., Fenton M. An improved procedure for the determination of dietary chromic oxide in feed and feces. Can. J. Anim. Sci. 1979;59:631–634. [Google Scholar]
- Kong C., Adeola O. Additivity of amino acid digestibility in corn and soybean meal for broiler chickens and White Pekin ducks. Poult. Sci. 2013;92:2381–2388. doi: 10.3382/ps.2013-03179. [DOI] [PubMed] [Google Scholar]
- Liu S.B., Li S.F., Lu L., Xie J.J., Zhang L.Y., Luo X.G. Estimation of standardized phosphorus retention for corn, soybean meal, and corn-soybean meal diet in broilers. Poult. Sci. 2012;91:1879–1885. doi: 10.3382/ps.2011-02061. [DOI] [PubMed] [Google Scholar]
- Liu S.B., Xie J.J., Lu L., Li S.F., Zhang L.Y., Jiang Y., Luo X.G. Estimation of standardized phosphorus retention for inorganic phosphate sources in broilers. J. Anim. Sci. 2013;91:3766–3771. doi: 10.2527/jas.2012-5729. [DOI] [PubMed] [Google Scholar]
- Mahan D.C. Dietary calcium and phosphorus levels for weanling swine. J. Anim. Sci. 1982;54:559–564. doi: 10.2527/jas1982.543559x. [DOI] [PubMed] [Google Scholar]
- Mutucumarana R.K., Ravindran V. Measurement of true ileal phosphorus digestibility in meat and bone meal for broiler chickens using the direct method. Anim. Feed Sci. Tech. 2016;219:249–256. doi: 10.3382/ps/pev132. [DOI] [PubMed] [Google Scholar]
- Mutucumarana R.K., Ravindran V., Ravindran G., Cowieson A.J. Measurement of true ileal digestibility of phosphorus in some feed ingredients for broiler chickens. J. Anim. Sci. 2014;92:5520–5529. doi: 10.2527/jas.2014-7884. [DOI] [PubMed] [Google Scholar]
- Mutucumarana R.K., Ravindran V., Ravindran G., Cowieson A.J. Measurement of true ileal phosphorus digestibility in maize and soybean meal for broiler chickens: Comparison of two methodologies. Anim. Feed Sci. Tech. 2015;206:76–86. [Google Scholar]
- National Research Council (NRC) 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- National Research Council (NRC) 11th rev. ed. Natl. Acad. Press; Washington, DC: 2012. Nutrient Requirements of Swine. [Google Scholar]
- Olukosi O.A., Kong C., Fru-Nji F., Ajuwon K.M., Adeola O. Assessment of a bacterial 6-phytase in the diets of broiler chickens. Poult. Sci. 2013;92:2101–2108. doi: 10.3382/ps.2012-03005. [DOI] [PubMed] [Google Scholar]
- Osho S.O., Babatunde O.O., Adeola O. Additivity of apparent and standardized ileal digestibility of amino acids in wheat, canola meal, and sorghum distillers dried grains with solubles in mixed diets fed to broiler chickens. Poult. Sci. 2019;98:1333–1340. doi: 10.3382/ps/pey457. [DOI] [PubMed] [Google Scholar]
- Petersen G.I., Stein H.H. Novel procedure for estimating endogenous losses and measurement of apparent and true digestibility of phosphorus by growing pigs. J. Anim. Sci. 2006;84:2126–2132. doi: 10.2527/jas.2005-479. [DOI] [PubMed] [Google Scholar]
- Plumstead P., Leytem A., Maguire R., Spears J., Kwanyuen P., Brake J. Interaction of calcium and phytate in broiler diets. 1. Effects on apparent prececal digestibility and retention of phosphorus. Poult. Sci. 2008;87:449–458. doi: 10.3382/ps.2007-00231. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Bryden W.L. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 1999;78:699–706. doi: 10.1093/ps/78.5.699. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S.M., Chung T.K., Moughan P.J. The effect of microbial phytase on ileal phosphorus and amino acid digestibility in the broiler chicken. Br. Poult. Sci. 2002;43:598–606. doi: 10.1080/0007166022000004516. [DOI] [PubMed] [Google Scholar]
- Rutherfurd S.M., Chung T.K., Morel P.C.H., Moughan P.J. Effect of microbial phytase on ileal digestibility of phytate phosphorus, total phosphorus, and amino acids in a low-phosphorus diet for broilers. Poult. Sci. 2004;83:61–68. doi: 10.1093/ps/83.1.61. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Ravindran V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007;135:1–41. [Google Scholar]
- She Y., Wang Q., Stein H.H., Liu L., Li D., Zhang S. Additivity of values for phosphorus digestibility in corn, soybean meal, and canola meal in diets fed to growing pigs. Asian-Aust. J. Anim. Sci. 2018;31:1301–1307. doi: 10.5713/ajas.17.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trairatapiwan T., Ruangpanit Y., Songserm O., Attamangkune S. Determination of true ileal phosphorus digestibility of maize and soybean meal and true ileal calcium digestibility of soybean meal for broiler chickens. Anim. Prod. Sci. 2018;59:681–687. [Google Scholar]
- Wu Y.B., Ravindran V., Hendriks W.H. Influence of exogenous enzyme supplementation on energy utilization and nutrient digestibility of cereal for broilers. J. Sci. Food Agric. 2004;84:1817–1822. [Google Scholar]
- Xue P.C., Ragland D., Adeola O. Determination of additivity of apparent and standardized ileal digestibility of amino acids in diets containing multiple protein sources fed to growing pigs. J. Anim. Sci. 2014;92:3937–3944. doi: 10.2527/jas.2014-7815. [DOI] [PubMed] [Google Scholar]
- Zhai H., Adeola O. True total-tract digestibility of phosphorus in corn and soybean meal for fifteen-kilogram pigs are additive in corn–soybean meal diet. J. Anim. Sci. 2013;91:219–224. doi: 10.2527/jas.2012-5295. [DOI] [PubMed] [Google Scholar]