Abstract
The aim of the study was to compare production results and quality of meat, as well as histological features of the jejunum in broiler chickens administered feed with 1% addition of zeolite or halloysite, with the addition of aluminosilicates to litter (4.50 kg/m2) throughout the rearing. In the experiment, 300 male broilers were used for 6 wk. They were divided into 3 groups, each of 10 repetitions (10 birds each). Group 1 was a control, halloysite was added to feed and litter in group 2, while zeolite was applied instead of halloysite in group 3. After rearing, 10 selected birds from each group were slaughtered. Selected production properties and degree of footpad dermatitis were examined, and histomorphometric examination of the jejunum was performed. The lowest yield and the highest proportion of neck with skin in the carcass were demonstrated in group 2 (P < 0.05). The lowest percentage of skin with subcutaneous fat was found in group 3 (P < 0.05). A decrease in lightness (L∗) and yellowness (b∗) was demonstrated in group 2, while redness (a∗) was the lowest in group 3 (P < 0.05). Group 2 was characterized by the lowest water-holding capacity in breast muscles, and in group 3, in leg muscles (P < 0.05). In group 3, the highest fat content and the lowest water content in the breast muscles (P < 0.05) were found. The leg muscles in groups 2 and 3 were characterized by the highest a∗, and in group 2, by b∗. The control group had the lowest protein and the highest fat content in leg muscles. In the intestine from group 2, a higher height (P < 0.05) and surface area (P < 0.01) of intestinal villi were found, in comparison to group 3. The width of intestinal villi was higher in groups 2 and 1 than in group 3 (P < 0.05), similarly the depth of intestinal crypts. The addition of zeolite could be proposed because of the obtained production results, while the halloysite had a positive effect on the histomorphometric features of the jejunum.
Key words: broiler chicken, zeolite, halloysite, footpad dermatitis, intestinal villi
Introduction
The production of broiler chickens is the most dynamically developing part of the poultry farming sector because of the short chicken rearing time, while maintaining good quality meat and low costs associated with the production (Vissers et al., 2019). The quality of production is influenced by numerous factors that may affect production results, the proper functioning of the digestive system of chickens, the general health status of birds, and the quality of meat. All these factors are related to bird well-being (Kuźniacka et al., 2014; Kers et al., 2019; Özlem et al., 2019). Rearing conditions consist of many elements, where the important factor is the level of ammonia in the litter, which comes from bird droppings, especially with a large amount of protein in the diet, and can have a large impact on production parameters and the health of birds and people working on the farm (Mahardihka et al., 2019). The broiler production is carried out in an intensive system on litter, which causes intensive ammonia accumulation (Proch et al., 2019). In addition to ammonia, another threat is the presence of pathogenic microorganisms in the litter, where the development conditions are good because of the high temperature and adequate moisture (Altınçekiç and Sözcü, 2016). In addition to reduced production results, ammonia and poor litter condition result in the occurrence of footpad dermatitis in chickens, which not only affects the quality of the product but also, above all, constitutes a threat to the well-being of chickens. The rearing system, stocking density in the hen house, environmental conditions, and litter hygiene, which are directly related to the presence of ammonia, have a great impact on the incidence of this disease (Bilgili et al., 2009). Therefore, it can be concluded that the presence of ammonia can significantly reduce the value of broiler production. Thus, a solution should be sought to reduce ammonia in production. It is necessary to find an agent that is able to bind ammonia molecules, which will result in the decrease of its content in the hen house. One of the solutions available in Poland may be the addition of zeolite and halloysite, which are characterised by the ability to absorb ammonia and the potential to produce high-quality compound feed, to feed (Korniewicz et al., 2006; Pilarska et al., 2013). Cohuo-Colli et al. (2018) undertook research, in which organic minerals and plant extract were used in broiler rearing. The authors showed that these additives reduced ammonia emissions from litter by 36%. In turn, Zarnab et al. (2019) applied potassium aluminium sulphate, aluminosilicates, and plant extract from Yucca schidigera in chicken rearing. It was concluded that all solutions reduce ammonia emissions, with aluminosilicates having the most favorable effect on the obtained results. Similar conclusions were drawn in a previous study by Cabuk et al. (2004). In the study of Zhou et al. (2014), 2% of zeolite and attapulgite (1:1) was added to chicken feed. It was concluded that it may partially improve production results, that is, cause an increase in weight gain and feed intake (FI). Zeolite and other silicate minerals may be a nontoxic material with sorption and ion exchange properties, at low financial outlays (Cabuk et al., 2004).
The aim of the study was to compare production results and quality of meat, as well as histological features of the jejunum in broiler chickens administered feed with 1% addition of zeolite or halloysite, as well as the addition of aluminosilicates to litter at an amount of 4.50 kg/m2 throughout the rearing period. In addition, in the study, the health status of foot pads of broiler chickens was assessed.
Material and methods
According to directive no. 2010/63/EU, the approval of the local ethics committee is not required in this type of research. The rearing of birds was conducted on a large-scale broiler chicken farm, and the material for laboratory tests was collected after slaughter, which was carried out under the supervision of people responsible for rearing.
Animals and Diets
The presented studies were pilot studies. In the experiment, 300 one-day-old male Ross 308 broiler chickens were used. The birds were divided into equal groups. Each group was divided into 10 repetitions, of 10 birds each. Group 1 (control) was kept on the litter without the addition of aluminosilicates and fed a compound feed with no additive. In group 2, the compound feed was supplemented with 1% halloysite, while in group 3, 1% zeolite was added to the feed. The rearing was carried out for a period of 42 d and divided into 3 feeding periods. From the day of placement until the 14th day of life, the birds were fed a starter-type complete feed mixture, then from the 15th to 28th day, a grower-type mixture, and from 29th day to the slaughter day (42th day), the birds were fed a finisher-type complete feed mixture. The compound feed was purchased in the form of granules. The content of nutrients and the energy value of the compound feeds were in line with the applicable standards for the feeding of broiler poultry (chickens) (Smulikowska and Rutkowski, 2018). They were standard commercial compound feeds. The conditions in the hen house were consistent with the recommendations described in the technological instructions for rearing the flock of Ross 308 broiler chickens (Aviagen). Aluminosilicates were added to litter at the time of supplementing litter, as shown in Table 1. In total, 4.50 kg of aluminosilicate addition was used for each group throughout the entire rearing period.
Table 1.
Addition of aluminosilicates to litter throughout the entire rearing period of broiler chickens.
| Item | Additive to litter |
|||
|---|---|---|---|---|
| Group 1 |
Group 2 |
Group 3 |
||
| Halloysite | Zeolite | Halloysite | Zeolite | |
| Under the litter [g/m2] | 0 | 0 | 300 | 300 |
| Under the litter before placement [g/m2] | 0 | 0 | 100 | 100 |
| After first supplementation of litter [g/m2] | 0 | 0 | 50 | 50 |
| After second supplementation of litter [g/m2] | 0 | 0 | 50 | 50 |
| After third supplementation of litter [g/m2] | 0 | 0 | 50 | 50 |
| After fourth supplementation of litter [g/m2] | 0 | 0 | 100 | 100 |
| After fifth supplementation of litter [g/m2] | 0 | 0 | 100 | 100 |
| Quantity [kg] for the whole group | 0.00 | 0.00 | 4.50 | 4.50 |
Growth Performance
Throughout the rearing period, in individual replications, the amount of administered feed and uneaten leftovers, as well as cases of death and euthanasia for health reasons, were monitored, which allowed the calculation of the following production indicators: FI per individual, feed conversion ratio (FCR), and European Production Efficiency Factor (EPEF). The EPEF was calculated based on the following formula: EPEF = [average body weight (kg) × survival rate (%) × 100]/[days of rearing × FI per kg body weight (FCR)]. A value above 220 indicates effective production. In order to determine the efficiency of rearing birds from groups, body weight was monitored on the first day of life, as well as at the end of each feeding period, or on the 14th, 28th, and 42nd day of rearing. In addition, at the end of the rearing, 20 broiler chickens were randomly selected from each group of birds, 2 from each repetition (60 birds from whole experiment).
Footpad Dermatitis Evaluation
In order to assess the degree of skin lesions on the surface of the foot pads of all birds, the evaluation using 2 methods was performed.
The first evaluation was made according to the Dowsland's method (2008) on a 3-point scale, where 0 (k1) means no lesions or very small ones, slight superficial discoloration, and mild keratosis of the epidermis; 1 (k2) means mild skin lesions, discoloration, superficial damage, and dark spots on the foot pads; and 2 (k3) means severe damage, ulcers, eschars, hemorrhages, and swelling of the paws.
As the second method, the Butterworth's method (2009) on a 5-point scale was used, where 0 (k1) means no skin discoloration, smooth epidermis, and small and invisible lesions; 1 (k2) means no discoloration and keratosis of the epidermis; 2 (k3) means discoloration of the epidermis and erosions; 3 (k4) means discoloration of the epidermis, erosions, and hyperkeratosis; 4 (k5) means discoloration, ulcers, and hyperkeratosis.
Slaughter and Meat Quality
Slaughter was carried out by electric stunning and cutting off the head between 1 and 2 cervical vertebras and rapid loss of blood. Fifteen minutes after the slaughter, a pH measurement of the carcass (pH15) was performed by introducing a 2.5-cm dagger electrode under the surface of the breast muscle. An Elmetron pH meter (Zabrze, Poland) was used. The carcasses were cooled in a cold storage for 24 h at 2°C. After 24 h, a measurement of carcass acidity at the same place in the breast muscle was performed (pH24). The carcasses were weighed using a Radwag weighing scale (Radwag, Radom, Poland). Ten chickens out of the selected ones were dissected (Ziołecki and Doruchowski, 1989) to determine the slaughter value and basic tissue composition (meat, fat, and bone content) of carcasses. The breast muscles, leg muscles, skin with subcutaneous fat, and depot fat, as well as offal (heart, stomach, liver), wings with skin, neck with skin, and the rest of the carcass or the trunk, femur, and lower leg bones were separated. All elements were weighed. The breast muscles and leg muscles (with no bones, but without removing tendons and intramuscular fat) were subjected to further analysis 24 h postmortem. Color measurements were made on the CIE Lab scale, determining the lightness (L∗), redness (a∗), and yellowness (b∗) of the breast and leg muscles, with the use of a colorimeter (CR400 model; Konica Minolta, Tokyo, Japan). Subsequently, the measurement of drip loss for the breast muscles, as well as of water-holding capacity for the breast muscles and leg muscles, was performed. Measurements of meat color, drip loss, and water-holding capacity were conducted according to the methodology described by Biesek et al. (2020). Samples of the breast muscles and leg muscles were ground separately for each group, and combined samples of 90 g were collected for testing for the chemical composition of meat, including protein, collagen, salt, fat, and water content. The tests were carried out in accordance with the PN-A-82019: 2010 standard. A FOSS FoodScan device (FOSS, Warsaw, Poland), with near-infrared transmission using a spectrometer (artificial neural network [ANN] calibration) was applied.
Intestinal Morphological Assessment
At the end of the experimental period, 10 chickens from each treatment were chosen to histological examinations. Samples for histological analyses (of approx. 3 cm) were collected immediately after the slaughter of chickens from the middle portion of the jejunum. Individual sections of the intestine were rinsed with 0.9% physiological saline and then fixed in a solution of 4% formalin buffered with CaCO3. The fixed samples were dehydrated, X-rayed, and impregnated with paraffin in a tissue processor (Thermo Shandon, Waltham, MA) and then embedded using a station for embedding biological material in paraffin blocks (Medite, Burgdorf, Germany). The blocks formed in this way were cut on a rotary microtome (Thermo Shandon) into 10-μm-thick sections, which were placed on microscope slides coated with chicken egg white with the addition of glycerine and placed in an incubator at 38°C for 24 h. Before staining, the slides were deparaffinized and hydrated. Subsequently, they were stained using the periodic acid-Schiff method with the Schiff reagent for morphometric analysis of the jejunum. The measurements of the height and width of intestinal villi and the depth of intestinal crypts were performed using a NIKON Ci-L microscope equipped with a 5.9 MPix NIKON DS-Fi3 camera and NIS ELEMENTS software, enabling linear and planimetric measurements and digital analysis of microscopic images. In order to perform measurements of the height of intestinal villi, 10 villi were randomly selected. The villus height was measured from its top to the base at the intestinal crypt orifice. The villus width was measured at its mid-length. Subsequently, the villi surface was calculated according to the formula proposed by Sakamoto et al. (2000): (2π) × (VW/2) × (VH), where VW = villus width and VH = villus height. The depth of intestinal crypts was measured between 10 villi.
Statistical Analysis
The data were analysed in the Statistica statistical software 10.0 (Statsoft, Kraków, Poland) (2011). One-way ANOVA was used. The mean values for individual groups and standard deviation (±SD) were calculated. Statistically significant differences were identified using the Scheffé's test, with a significance level of P < 0.05. For in vivo performance as an experimental unit, each repetition for every group was taken. For meat quality and histological characteristics, each bird with a padlock stamp and number was considered as an experimental unit.
Results
Growth Performance
Production results are shown in Table 2. The body weight of one-day-old chicks in each group was similar (P > 0.05). Body weight was monitored on day 14, 28, and 42 of rearing. Body weight and growth rate index were not affected by the addition of each aluminosilicate. In group 3, the chickens showed the highest FI compared with the control group and group 2, where the addition of halloysite was applied, while, in group 2, the FCR was higher than that in the other groups. Nevertheless, these differences were not confirmed statistically (P > 0.05). The results obtained after the calculation of EPEF showed that each group was characterised by effective production. In group 3, EPEF was the highest, while in group 2, its value was the lowest. No statistically significant differences between the groups (P > 0.05) were revealed.
Table 2.
Least squares means and SD for production results of broiler chickens∗.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| Body weight of one-day-old chicks (g) | 44.5 ± 3.1 | 44.9 ± 3.3 | 46.0 ± 4.3 |
| Body weight (g) | |||
| Day 14 | 466.6 ± 41.1 | 479.9 ± 50.2 | 483.7 ± 42.8 |
| Day 28 | 1,567 ± 207 | 1,558 ± 199 | 1,614 ± 182 |
| Day 42 | 2,899 ± 421 | 2,802 ± 447 | 3,003 ± 490 |
| Growth rate index (%) | |||
| Day 14 | 165 ± 3.5 | 165 ± 4.3 | 165 ± 3.7 |
| Day 28 | 107 ± 14.9 | 105 ± 13.1 | 107 ± 9.2 |
| Day 42 | 61.0 ± 26.1 | 56.0 ± 18.9 | 58.0 ± 27.2 |
| FI (kg) | |||
| FI (kg) | 5.36 ± 0.68 | 5.39 ± 0.42 | 5.48 ± 0.24 |
| FCR (kg/kg) | 1.85 ± 0.34 | 1.92 ± 0.27 | 1.82 ± 0.21 |
| European production efficiency factor | 358.00 | 327.00 | 373.00 |
Abbreviations: FCR, feed conversion ratio; FI, feed intake.
No statistically significant difference; P > 0.05.
Footpad Dermatitis Evaluation
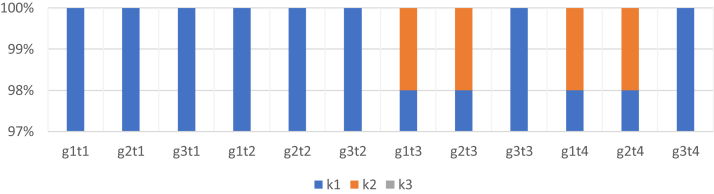
The evaluation of the health status of chicken foot pads was carried out in 4 terms. Evaluation using a 3-point Downsland's method (2008) showed that in terms 3 and 4, in the group of chickens reared without the addition of aluminosilicates, mild skin lesions, discoloration, superficial damage, and dark spots on the foot pads were demonstrated at the level of 2% of the entire group. The same results were found in the group of chickens where the addition of halloysite was used. In the group of birds where zeolite was used, no lesions or very small ones, slight superficial discoloration, or mild keratosis of the epidermis on the foot pads of chickens was demonstrated. In terms 1 and 2, in all groups, no skin lesions on the foot pads of chickens were found (Figure 1).
Figure 1.
Evaluation of the condition of foot pads in chickens according to the Downsland's method (2008). g, group; t, term, for example, g1t1, group 1, term 1; (k1) means no lesions or very small ones, slight superficial discoloration, mild keratosis of the epidermis; (k2) mild skin lesions, discoloration, superficial damage, dark spots on the foot pads; and (k3) severe damage, ulcers, eschars, hemorrhages, swelling of the paws.
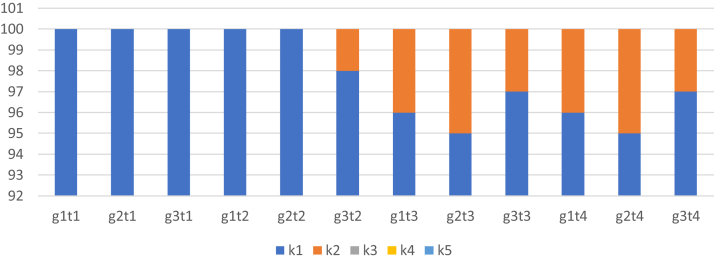
In turn, during evaluation with a more detailed (5-point) Butterworth's method (2009), in term 2 of the evaluation, no discoloration and keratosis of the epidermis were observed in group 3. In subsequent terms (3 and 4), keratosis of the epidermis affected 3% of the group. However, in groups 1 and 2, changes in terms 3 and 4 at the level of 4 and 5% were demonstrated. The aforementioned scale showed no discoloration and keratosis of the epidermis (Figure 2).
Figure 2.
Evaluation of the condition of foot pads in chickens according to the Butterworth's method (2009). g, group; t, term, for example, g1t1, group 1, term 1; where (k1) means no skin discoloration, smooth epidermis, small and invisible lesions; (k2) no discoloration, keratosis of the epidermis; (k3) discoloration of the epidermis, erosions; (k4) discoloration of the epidermis, erosions, hyperkeratosis; (k5) discoloration, ulcers, hyperkeratosis.
Meat Traits
After the dissection was performed, the carcass yield of chickens and the percentage of selected elements of the carcass were calculated (Table 3). The significantly higher carcass yield was found in the control group (1) compared with the group with the addition of halloysite (2) (P < 0.05), which is confirmed by the EPEF indicator, which was equally low among the study groups. In the group with the addition of halloysite, the percentage of neck with skin in carcass was significantly higher than that in the control group, P < 0.05. The results related to the percentage of wings and the rest of the carcass as well as the weight of offal were similar in all groups (P > 0.05).
Table 3.
Least squares means and SD for characteristics of chicken carcasses.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| Carcass yield | 74.7a ±2.8 | 71.8b ± 1.1 | 74.2a,b ± 0.9 |
| % in carcass of | |||
| Wings | 8.9 ± 0.8 | 10.6 ± 2.7 | 9.2 ± 1.2 |
| Neck with skin | 4.2b ± 0.5 | 5.1a ±0.8 | 4.6a,b ± 0.6 |
| The remaining parts | 25.0 ± 2.5 | 24.0 ± 2.3 | 24.1 ± 1.9 |
| Offal weight (g) | 122.8 ± 13.7 | 109.4 ± 7.9 | 114.2 ± 11.0 |
a,bMeans within a row lacking a common superscript differ, P < 0.05; lack of letters in some values means that no significant differences were found, P > 0.05.
Carcass muscle content was expressed as a percentage of the breast muscles and legs muscles and as a total in the carcass. Simultaneously, the percentage of skin with subcutaneous fat and abdominal fat in the carcass was calculated (Table 4). Statistically significant differences were demonstrated in the percentage of skin with subcutaneous fat in the carcass. A significantly higher percentage share of skin with subcutaneous fat in the carcass was found in group 2 than in group 3 (P < 0.05). No significant differences in breast, leg, and total muscle percentage share in carcass between groups were found (P > 0.05).
Table 4.
Least squares means and SD for muscle and fat content (%) of chicken carcasses.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| % of muscles in the carcass | |||
| Breast muscles | 31.2 ± 1.8 | 28.8 ± 2.3 | 31.1 ± 1.5 |
| Leg muscles | 21.2 ± 1.0 | 21.1 ± 1.5 | 21.6 ± 1.5 |
| Total | 52.4 ± 2.3 | 49.8 ± 3.2 | 52.7 ± 1.8 |
| % of skin with subcutaneous fat in the carcass | 8.9a,b ± 0.9 | 10.4a ±2.0 | 8.7b ± 0.8 |
| % of abdominal fat in the carcass | 1.2 ± 0.3 | 1.9 ± 0.8 | 1.2 ± 0.4 |
a,bMeans within a row lacking a common superscript differ, P < 0.05; lack of letters in some values means that no significant differences were found, P > 0.05.
Physicochemical Traits of Breast and Leg Muscles
Values of pH15 and pH24 (Table 5) were found to be similar among the groups (P > 0.05). In turn, significant differences in meat color were demonstrated by assessing it on the basis of L∗, a∗, and b∗ indicators. Color saturation, expressed as lightness (L∗), was highest (P < 0.05) in group 1 and 3 compared with group 2 (halloysite). On the other hand, the breast muscles of group 2 had a significantly higher redness than those in group 3. In turn, both experimental groups did not differ significantly from the control group 1. In case of the b∗ parameter (yellowness), a significantly lower value was found in group 2 than in group 1 and group 3 (P < 0.05). The breast muscles were tested for their water-holding capacity. The expressed values represent the percentage of water loss. After a 24-hour drip loss analysis, it was shown that the breast muscles in group 2, where halloysite was used, had significantly the largest water loss, compared to the other groups (P < 0.05). In turn, the analysis of water-holding capacity expressed as water absorption showed no significant differences between the groups (P > 0.05). The assessment of the chemical composition of the breast muscles did not show significant differences in terms of protein and collagen content (P > 0.05). The intramuscular fat and salt content in breast muscles from group 3 was significantly higher than that in group 1. Also, in group 2, significantly higher salt content was found than in group 1. Differences of water content in breast muscles were found between every group (P < 0.05).
Table 5.
Least squares means and SD for physicochemical traits of chicken breast muscles.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| pH15 | 5.9 ± 0.2 | 5.9 ± 0.2 | 6.0 ± 0.2 |
| pH24 | 5.9 ± 0.3 | 5.9 ± 0.1 | 6.0 ± 0.2 |
| Color | |||
| L∗ | 55.0a ±3.4 | 53.2b ± 2.7 | 55.4a ±3.1 |
| a∗ | 3.8a,b ± 1.1 | 4.1a ±1.8 | 3.5b ± 1.0 |
| b∗ | 5.0a ±2.3 | 3.7b ± 1.2 | 4.6a ±0.9 |
| Drip loss (%) | 1.7b ± 0.6 | 3.1a ±1.6 | 2.0b ± 0.7 |
| Water-holding capacity (%) | 34.5 ± 3.7 | 36.8 ± 4.7 | 37.6 ± 1.7 |
| Protein (%) | 23.1 ± 0.05 | 23.0 ± 0.04 | 23.0 ± 0.3 |
| Fat (%) | 1.7b ± 0.03 | 1.8b ± 0.02 | 2.3a ±0.05 |
| Water (%) | 75.4a ±0.02 | 75.1b ± 0.09 | 74.6c ±0.08 |
| Collagen (%) | 0.70 ± 0.1 | 0.80 ± 0.1 | 0.80 ± 0.3 |
| Salt (%) | 0.10b ± 0.03 | 0.22a ±0.04 | 0.22a ±0.01 |
a,bMeans within a row lacking a common superscript differ, P < 0.05; lack of letters in some values means that no significant differences were found, P > 0.05.
In addition, an analysis of the color, water-holding capacity, and basic chemical composition of chicken leg muscles was made (Table 6). A significantly higher red saturation was found in both experimental groups (2 and 3) than in group 1 (P < 0.05); however, in case of yellow colour (b∗), only group 2 was characterised by a significantly higher saturation than group 1. Leg muscles in group 3, where zeolite was used, were characterised by a larger water loss than in group 1 or 2. At the same time, in this group (3), leg muscles had significantly higher protein content, while less fat and collagen, than those in the control group (P < 0.05). In turn, group 2 (halloysite) was characterised by significantly lower content of fat than groups 1 and 3, lower collagen content than group 1, and a higher content of water and salt than groups 1 and 3 (P < 0.05).
Table 6.
Least squares means and SD for physicochemical traits of chicken leg muscles.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| Color | |||
| L∗ | 52.3 ± 2.8 | 52.6 ± 1.6 | 51.2 ± 2.5 |
| a∗ | 4.4b ± 0.9 | 5.4a ±1.7 | 5.0a ± 1.4 |
| b∗ | 3.5b ± 2.4 | 3.9a ±1.9 | 3.6b ± 1.3 |
| Water-holding capacity (%) | 34.6b ± 4.2 | 33.1b ± 2.7 | 39.2a ±8.7 |
| Protein (%) | 18.8b ± 0.08 | 18.9a,b ± 0.02 | 19.0a ±0.1 |
| Fat (%) | 8.0a ±0.04 | 7.2c ±0.03 | 7.7b ±0.05 |
| Water (%) | 72.5b ± 0.06 | 73.1a ±0.1 | 72.6b ± 0.05 |
| Collagen (%) | 1.5a ±0.07 | 1.4b ± 0.03 | 1.4b ± 0.1 |
| Salt (%) | 0.3b ± 0.04 | 1.2a ±0.8 | 0.3b ± 0.03 |
a,bMeans within a row lacking a common superscript differ, P < 0.05; lack of letters in some values means that no significant differences were found, P > 0.05.
Intestinal Morphological Assessment
Morphometric parameters of intestinal villi (height, width, and surface area) and depth of crypts in the jejunum of chickens after 6 wk of rearing are presented in Table 7. In the jejunum of chickens of group 2 (1% addition of halloysite), a greater height (P < 0.05) and surface area (P < 0.01) of intestinal villi were found, in comparison to group 3 (1% addition of zeolite). Intermediate values were found for the control group (P > 0.05). The width of intestinal villi was higher in group 2 and control group than that in group 3 (P < 0.05). Statistically significant deeper crypts were observed in the group of chickens whose feed and litter were supplemented with halloysite (2), compared to chickens in which the addition of zeolite was used (P < 0.01).
Table 7.
Least squares means and SD for morphology of the jejunum of chickens.
| Indicators | Group |
||
|---|---|---|---|
| 1, Control | 2, Halloysite | 3, Zeolite | |
| Villus height (μm) | 1,376.83a,b ± 64.22 | 1,481.60a ± 30.76 | 1,174.92b ± 111.18 |
| Villus width (μm) | 175.86a ± 16.57 | 180.19a ± 12.41 | 123.70b ± 11.26 |
| Villus surface area (μm2) | 765,406.12A,B ± 93,139.49 | 841,100.13A ± 73,222.67 | 464,094.60B ± 62,704.24 |
| Crypt depth (μm) | 162.73A,B ± 4.50 | 176.73A ± 15.81 | 127.69B ± 3.05 |
a,b,A,BThe values show a statistically significant difference; uppercase characters for P < 0.01; lowercase characters for P < 0.05.
Discussion
Natural aluminosilicates constitute not only potential means to increase production safety but also the impact of their addition to feed or litter on production results and the quality of obtained raw material is assumed. In the study of Schneider et al. (2016), the addition of zeolite to feed (5 g/kg) and litter (100 g/kg) was used in broiler rearing. No effect of zeolite addition on the production results and characteristics of carcasses of broiler chickens was demonstrated. The obtained results were consistent with our own research. In turn, Nikolakakis et al. (2013) found that 2 to 3% addition of natural zeolite to feed for Cobb-500 broiler chickens had a positive effect on the FCR, BWG, as well as litter quality. Karamanlis et al. (2008) drew similar conclusions (2008). Other studies (Incharoen et al., 2009) showed no significant effect of the addition of zeolite and plant extract in the diet of Cochin Sanuki chickens on production results. However, along with the increased level of the additive, higher values of BWG and FI indices were observed. Differences between individual studies and our own results may result from environmental conditions and the genotype of chickens. EPEF in our results showed effective production because the values were much higher than those in the other studies (Kryeziu et al., 2018). Mallek et al. (2012) used 0.5 and 1% of zeolite addition to feed and investigated its effect on intestinal flora and chicken rearing results. The authors concluded that good weight gain can be caused by a reduction in the presence of mycotoxins in feed. The same study showed an improvement in the organoleptic properties of meat (Mallek et al., 2012). No significant differences in the occurrence of symptoms of footpad dermatitis could have been caused by good environmental conditions in the hen house and proper hygiene of the litter.
Research on the effect of 1% zeolite addition to broiler chicken feed on meat quality was also conducted (Hashemi et al., 2014). The study showed no effect of the addition of zeolite on the color of the breast muscles; however, a higher level of water absorption in the muscles with the use of zeolite was found. A slightly higher level of L∗ and b∗ parameters in the leg muscles was also shown, without significant differences (P > 0.05). Differences between individual studies concerning the results of the physicochemical traits of chicken muscles may result from the oxidation of proteins in the muscles (Zhang et al., 2013), but the impact of material treatment after slaughter (or environmental conditions in the rearing house) is also important (Kuźniacka et al., 2020). Similar to our own research, the use of zeolite did not affect the protein content of chicken breast muscles in any way in the study conducted by Safaei et al. (2014). Nevertheless, the fat content was lower when using other aluminosilicates (bentonite). This is consistent with our own research on the chemical composition of the leg muscles; however, in breast muscles of chickens, a higher percentage of fat was found when zeolite was used. This may result from the use of various feeds where the fat levels are different. Nevertheless, this (higher intramuscular fat content) is not a negative feature because it is treated as a flavor carrier in meat (Zhao et al., 2019). The literature concerning the use of halloysite as an addition to feed and litter is more limited than in case of zeolite and its effect on production results and the quality of broiler chicken meat. Despite this, Kulok et al. (2005) concluded in their study that halloysite in the form of 1 or 2% addition to feed in pig fattening has a high effectiveness limiting the occurrence of bacteria, fungi, and aflatoxin B1.
In the present study, a positive effect of halloysite (layered hydrated aluminium hydrosilicate) on histomorphological parameters of the jejunum of chickens was demonstrated. In the group of broiler chickens, whose feed and litter was supplemented with halloysite, a larger surface area of the jejunum villi and deeper intestinal crypts were observed, which indicates both an increase in digestive and absorption intestinal functions as well as faster regeneration of villi cells in intestinal crypts (Porter et al., 2002; Xia et al., 2004; Wawrzyniak et al., 2017). Moreover, the crypt can be regarded as the villus factory, and a large crypt indicates fast tissue turnover and a high demand for a new tissue (Ma and Guo, 2008). Therefore, the present study has shown that halloysite can stimulate intestinal villi in the jejunum of chickens, as well as enhance regeneration of the intestinal epithelium by improving the condition and health of the intestine (Wu et al., 2013). In the study of Incharoen et al. (2009), in which dietary supplementation with the commercial ZEM product, which composed of zeolite (70%), enzymes extracted from plants such as pineapple and papaya (20%), and vermiculite (10%), in Sanuki Cochin chickens was performed, it has been shown that the intestinal villus height and villus area of all segments (duodenal, jejunum, and ileum) did not differ significantly. Considering only the use of zeolite, a similar effect was observed in our own research.
However, Wawrzyniak et al. (2017) observed an increase in the height and surface area of the villi, as well as the depth of the crypts in the duodenum and middle portion of the jejunum in broilers fed 2% zeolite. There is no available literature on the use of halloysite in the diet for broiler chickens and its effect on the morphology of the small intestine; therefore, further research in this area is indicated.
The study concerning the effect of aluminosilicates on production results and meat quality, as well as on the histomorphometric features of broiler chickens, showed no dependence in case of production results, while the quality of meat varied depending on the examined element (breast and leg muscles). In turn, histomorphometric examinations indicate the positive effect of the addition of halloysite on intestinal health. Currently, the addition of zeolite in the rearing of broiler chickens, based on the good carcass yield, could be proposed. However, it is equally important to continue this kind of research because of its scarcity in science.
Acknowledgments
Authors thank the Kuyavian-Pomeranian Association of Poultry Breeders and Egg Producers (Poland) (www.bezpiecznaferma.pl) for the opportunity to conduct research and implement their results for further poultry practice.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Altınçekiç S.Ö., Sözcü A. Manure management and environmental pollution in animal production. Works Fac. Agric. Food Sci. Univ. Sarajevo, LXI. 2016;66:72–76. Sarajevo. [Google Scholar]
- Biesek J., Kuźniacka J., Banaszak M., Adamski M. The quality of carcass and meat from geese fed diets with or without Soybean meal. Animals. 2020;10:200. doi: 10.3390/ani10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgili S.F., Hess J.B., Blake J.P., Macklin K.S., Saenmahayak B., Sibley J.L. Influence of bedding material on footpad dermatitis in broiler chickens. J. Appl. Poult. Res. 2009;18:583–589. [Google Scholar]
- Butterworth A. Animal welfare indicators and their use in society. In: Smulders, Algers B., editors. Welfare of Production Animals: Assessment and Management of Risks. Food Safety Assurance and Veterinary Public Health Series 5. Wageningen Academic Publisher; Wageningen: 2009. [Google Scholar]
- Cabuk M., Alcicek A., Bozkurt M., Akkan S. Effect of Yucca schidigera and natural zeolite on broiler performance. Int. J. Poult. Sci. 2004;3:651–654. [Google Scholar]
- Cohuo-Colli J.M., Salinas-Ruiz J., Hernandez-Cazares A.S., Hidalgo-Contreras J.V., Brito-Damian V.H., Velasco-Velasco J. Effect of litter density and foot health program on ammonia emissions in broiler chickens. J. Appl. Poult. Res. 2018;27:198–205. [Google Scholar]
- Dowsland I. Aviagen; Newbridge: 2008. Broiler foot health – Controlling Foot Pad Dermatitis [Ross Tech Note] pp. 1–5. [Google Scholar]
- Hashemi S.R., Davoodi D., Dastar B., Bolandi N., Smaili M., Mastani R. Meat quality Attributes of broiler chickens fed diets supplemented with Silver Nanoparticles coated on zeolite. Poult. Sci. J. 2014;2:183–193. [Google Scholar]
- Incharoen T., Khambualai O., Yamauchi K. Performance and histological changes of the intestinal villi in chickens fed dietary natural zeolite including plant extract. Asian J. Poult. Sci. 2009;3:42–50. doi: 10.1080/00071660802662788. [DOI] [PubMed] [Google Scholar]
- Karamanlis X., Fortomaris P., Arsenos G., Dosis I., Papaioannou D., Batzios C., Kamarianos A. The effect of a natural zeolite (clinoptilolite) on the performance of broiler chickens and the quality of their litter. Asian-Australasian J. Anim. Sci. 2008;21:1642–1650. [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Lamot D.M., Stegeman J.A., Smidt H. Take care of the environment: housing conditions affect the interplay of nutritional interventions and intestinal microbiota in broiler chickens. Anim. Microbiome. 2019;1:10. doi: 10.1186/s42523-019-0009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korniewicz D., Kołacz R., Dobrzański Z., Korniewicz A., Kulok M. Effect of dietary halloysite on the quality of feed and Utilization of nutrients by Fatteners. Electron. J. Polish Agric. Universities. 2006;9:59. [Google Scholar]
- Kryeziu A.J., Mestani N., Berisha S.h., Kamberi M.A. The European performance indicators of broiler chicken as influenced by stocking density and sex. Agron. Res. 2018;16:483–491. [Google Scholar]
- Kulok M., Kołacz R., Dobrzanski Z., Wolska I. The influence of Halloysite on the content of bacteria, fungi and mycotoxins in feed mixtures. ISAH. 2005;2:354–357. [Google Scholar]
- Kuźniacka J., Adamski M., Czarnecki R., Banaszak M. Results of rearing broiler chickens under various systems. J. Agric. Sci. 2014;6:19–25. [Google Scholar]
- Kuźniacka J., Biesek J., Banaszak M., Rutkowski A., Kaczmarek S., Adamski M., Hejdysz M. Effect of dietary protein Sources Substituting Soybean meal on growth performance and meat quality in Ducks. Animals. 2020;10:133. doi: 10.3390/ani10010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.L., Guo T. Intestinal morphology, brush border and digesta enzyme activities of broilers fed on a diet containing Cu2+-loaded montmorillonite. Br. Poult. Sci. 2008;49:65–73. doi: 10.1080/00071660701816956. [DOI] [PubMed] [Google Scholar]
- Mahardhika B.P., Mutia R., Ridla M. Efforts to reduce ammonia gas in broiler chicken liter with the use of probiotics. IOP Conf. Series: Earth Environ. Sci. 2019;399:012012. [Google Scholar]
- Mallek Z., Fendri I., Khannous L., Hassena A.B., Traore A.I., Ayadi M.A., Gdoura R. Effect of zeolite (clinoptilolite) as feed additive in TUnisian broilers on the total flora, meat texture and the production of omega 3 polyunsaturated fatty acid. Lipids Health Dis. 2012;11:35. doi: 10.1186/1476-511X-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolakakis I., Dotas V., Lampros A. Kargopoulos H., Dotas D., Ampas Z. Effect of natural zeolite (clinoptilolite) on the performance and litter quality of broiler chickens. Turkish J. Vet. Anim. Sci. 2013;37:682–686. [Google Scholar]
- Özlem V.A., Ebru Y. Suzan I.E., Shazaib R.M. Comparison of slaughter yields and some meat quality parameters in broilers reared on sepiolite-supplemented wood shavings and rice hulls. Poult. Sci. 2019;98:1678–1683. doi: 10.3382/ps/pey536. [DOI] [PubMed] [Google Scholar]
- Pilarska A., Dach J., Pilarski K., Boniecki P. Produkcja i wykorzystanie pasz w Polsce: Stan aktualny i tendencje. Technika Rolnicza Ogrodnicza Leśna. 2013;6:24–27. (in Polish) [Google Scholar]
- Porter E.M., Bevins C.L., Ghosh D., Ganz T. The multifaceted Paneth cell. Cell Mol. Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proch A., Malik D.S., Singh Y., Sandhu K.S., Sharma A., Sethi A.P.S. Effect of litter and dietary amendments on ammonia concentration, broiler performance and liter quality in winter. Indian J. Anim. Res. 2019;53:973–978. [Google Scholar]
- Safaei M., Boldaji F., Daster B., Hassani S., Mutalib M.S.A., Rezaei R. Effect of Inclusion Kaolin, Bentonite and zeolite in dietary on chemical composition of broiler chickens meat. Asian J. Anim. Vet. Adv. 2014;9:56–63. [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Schneider A.F., Almeida D.S.D., Yuri F.M., Zimmermann O.F., Gerber M.W., Gewehr C.E. Natural zeolites in diet or litter of broilers. Br. Poult. Sci. 2016;57:257–263. doi: 10.1080/00071668.2016.1150962. [DOI] [PubMed] [Google Scholar]
- Smulikowska S., Rutkowski A. Poultry Feeding Standards. 5th ed. The Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences; Jabłonna, Poland: 2018. Recommended Allowances and Nutritive value of Feedstuffs; pp. 58–65. (In Polish) [Google Scholar]
- Vissers L.S.M., de Jong I.C., van Horne P.L.M., Saatkamp H.W. Global Prospects of the Cost-efficiency of broiler welfare in middle-Segment production systems. Animals. 2019;9:473. doi: 10.3390/ani9070473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrzyniak A., Kapica M., Stępień-Pyśniak D., Szewerniak R., Olejarska A., Jarosz Ł. Effect of feeding transcarpathian zeolite on gastrointestinal morphology and function in broiler chickens. Braz. J. Poult. Sci. 2017;19:737–746. [Google Scholar]
- Wu Q.J., Wang L.C., Zhou Y.M., Zhang J.F., Wang T. Effects of clinoptilolite and modified clinoptilolite on the growth performance, intestinal microflora, and gut parameters of broilers. Poult. Sci. 2013;92:684–692. doi: 10.3382/ps.2012-02308. [DOI] [PubMed] [Google Scholar]
- Xia M.S., Hu C.H., Xu Z.R. Effects of copper-bearing montmorillonite on growth performance, digestive enzyme activities, and intestinal microflora and morphology of male broilers. Poult. Sci. 2004;83:1868–1875. doi: 10.1093/ps/83.11.1868. [DOI] [PubMed] [Google Scholar]
- Zarnab S., Chaudhary M.S., Javed M.T., Khatoon A., Saleemi M.K., Ahmed T., Tariq N., Manzoor F., Javed I., Zhang H., Zhenhua X., Peng Y. Effects of Induced high ammonia concentration in Air on Gross and Histopathology of different body Organs in experimental broiler birds and its Amelioration by different Modifiers. Pakistan Vet. J. 2019;39:371–376. [Google Scholar]
- Zhang W., Marwan A.H., Samaraweera H., Lee E.J., Ahn D.U. Breast meat quality of broiler chickens can be affected by managing the level of nitric oxide. Poult. Sci. 2013;92:3044–3049. doi: 10.3382/ps.2013-03313. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang T., Xie J., Xiao Q., Cheng J., Chen F., Wang S., Sun B. Formation mechanism of aroma compound in a glutathione-glucose reaction with fat or oxidized fat. Food Chem. 2019;270:436–444. doi: 10.1016/j.foodchem.2018.07.106. [DOI] [PubMed] [Google Scholar]
- Zhou P., Tan Y.Q., Zhang L., Zhou Y.M., Gao F., Zhou G.H. Effects of dietary supplementation with the Combination of zeolite and attapulgite on growth performance, nutrient Digestibility, Secretion of digestive enzymes and intestinal health in broiler chicken. Asian-Australasian J. Anim. Sci. 2014;27:1311–1318. doi: 10.5713/ajas.2014.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziołecki J., Doruchowski W. COBRD Poznań; Poland: 1989. Methods for the Evaluation of Meat Quality and Yield; pp. 1–22. (in Polish) [Google Scholar]