Abstract
This report demonstrates that high levels of lameness can be induced by a limited bacterial challenge in drinking water for birds raised on litter flooring, comparable with lameness induced by the gold standard for inducing lameness, growth on suspended wire flooring. The bacterium used in the challenge was cultured from lesions in birds induced for bacterial chondronecrosis with osteomyelitis (BCO) in the wire-flooring model so the epidemiology appears similar. The litter-flooring model could better approximate broiler operations. Furthermore, the work demonstrates that 2 commercial probiotics (GalliProTect and GalliProMax) can reduce lameness in the bacterial challenge litter-flooring model. Lameness attributable to BCO is one of the most significant animal welfare issues for broiler production. The wire-flooring and litter-flooring models afford alternatives for understanding the etiology, and epidemiology of BCO, and development of management strategies to reduce lameness. Probiotics afford a promising management strategy. The results suggest that the probiotic protection may extend beyond just intestinal health and intestinal barrier function.
Key words: broiler, lameness, chondronecrosis, bacteria, Staphylococcus
Introduction
Lameness is one of the most significant animal welfare issues in the broiler industry, resulting in annual losses of millions of dollars. A wire-flooring model has been shown to induce high incidence of lameness in broilers (Wideman et al., 2012; Wideman and Prisby, 2013; Wideman et al., 2013; Wideman et al., 2014; Wideman, 2016). Bacterial chondronecrosis with osteomyelitis (BCO) of the proximal tibiae and femora is the most prevalent form of lameness in this model (Wideman et al., 2012; Wideman and Prisby, 2013; Wideman et al., 2013; Wideman, 2016). The wire-flooring system led to a model for BCO susceptibility based on the vasculature and growth plate dynamics (Wideman and Prisby, 2013; Wideman, 2016). The predominant isolates from BCO lesions using the wire floor model on our research farm is Staphylococcus agnetis and BCO lameness is sometimes associated with a significant bacteremia (Al-Rubaye et al., 2015). The isolates of S. agnetis appear to be from a clonal population, and administration of the type strain, isolate 908, in the drinking water increases the incidence of lameness (Al-Rubaye et al., 2015). Further investigations on the BCO lameness model demonstrated translocation of bacteria into the blood for birds on litter or wire flooring; and evidence for transmission of BCO-inducing pathogens within a flock (Al-Rubaye et al., 2017). We now report further investigations on, and expansion of, the BCO lameness model system. We now report that S. agnetis 908 can induce high levels of BCO lameness in birds raised on litter flooring. We also report that BCO lameness can be transmitted through the air to broilers in separate pens. Furthermore, broilers fed either of 2 commercial probiotics are protected against the airborne spread of the infection. These data provide a model system for studying treatments and management strategies for reducing BCO lameness in broiler operations.
Materials and methods
Lameness Trials
All animal experiments were approved by the University of Arkansas Institutional Animal Care and Use Committee under protocols 15043, 16073, 16073-1, and 17067. One-day-old chicks representing surplus males from a female broiler-breeder product were placed in 1.5 × 3 m pens on fresh wood shaving litter at 60 per pen, with a nipple water line supplied with city tap water on one 5 ft. side and 2 feeders on the other side.
Feed was standard starter through day 35 and finisher through day 56. For administration of probiotics, the commercial product (Table 1) was added by the University of Arkansas Poultry Research Feed mill to standard feed formula (Supplementary Table 1) before pelleting. Lines and mixers were flushed and cleaned between formulations. Samples of the pelleted feed were shipped to the supplier for verification of probiotic viability. For these experiments, we had up to 4 treatment groups as described in Table 1. In all experiments, there was a source population that was challenged with S. agnetis 908 in the drinking water, and a control population that was not challenged but was housed in the same room “down wind” of the source population. Other treatments were the same as the control but with administration of probiotics in the feed. Treatment pens were arrayed using a randomized block design.
Table 1.
Treatment designations and descriptions for treatments in experiments 1 and 2.
| Treatment | Description |
|---|---|
| Source | Control diet challenge with Staphylococcus agnetis 908 105 CFU/mL in drinking water on days 20&21 |
| Control | Control diet |
| GalliProTect | GalliPro Tect diet 3.2 × 109 CFU Bacillus licheniformis DSM17236/g of product at 0.5 kg/metric ton of feed |
| GalliProMax | GalliPro Max diet 1.6 × 109 CFU Bacillus subtilis DSM17299/g of product at 0.5 kg/metric ton of feed |
Computer controllers regulated the temperature, photoperiod, and ventilation. Tunnel ventilation and evaporative cool pads were automatically activated when needed. The photoperiod was set for 23 h light:1 h dark for the duration of the experiment. Thermoneutral temperature targets were as follows: 90°F for day 1 to day 3, 88°F for day 4 to day 6, 85°F for day 7 to day 10, 80°F for day 11 to day 14, and 75°F thereafter. On day 19, all pens were culled to 50 birds. Pens challenged with S. agnetis were located “upwind” from all the other pens so that the ventilation flow would be from the challenged pens (source) to the downwind treatment pens (control or probiotic). For pens challenged with S. agnetis in the drinking water, at 7 AM on day 20, the hose to the nipple waterers was attached to an elevated 20L carboy. The bacteria (stationary overnight culture) were mixed into tap water in the carboy to 105 CFU/mL. At 7 PM on day 21, the nipple supply was restored to the tap water. All water lines were flushed with dilute bleach and fresh tap water before any experiment. Beginning with day 20, all birds were encouraged twice per day to move using standard kitchen brooms. Any bird that was reticent to move was marked with spray paint. Birds that were unwilling or unable to walk were diagnosed as “clinically lame” and euthanized. All birds that died or were diagnosed as clinical lame were recorded by date and pen number. Necropsy for BCO lameness was as described (Wideman, 2016) to assign as either N = normal proximal femur head or proximal tibia head; KB = kinky back (spondylolisthesis); FHS = proximal femoral head separation (epiphyseolysis); FHT = proximal femoral head transitional degeneration; FHN = proximal femoral head necrosis; THN = proximal tibial head necrosis; or other = symptoms other than BCO. Total BCO lame included all birds diagnosed as having FHS, FHT, FHN, THN, or KB. Some lame birds were also sampled for bacterial species in blood and BCO lesions as described (Al-Rubaye et al., 2015; Al-Rubaye et al., 2017). Air sampling used open petri dishes of ChromAgar Orientation (DRG International, Springfield, NJ). Species identification included subculture on ChromAgar Orientation, and ChromAgar Staphylococcus (DRG International) followed by PCR sequencing of 16S rRNA (Al-Rubaye et al., 2015; Al-Rubaye et al., 2017).
Transepithelial Electrical Resistance and Short-Circuit Current
Ileal transepithelial electrical resistance (TEER) and short-circuit current (SCC) was from 10 apparently healthy birds from each treatment on day 57. A 5-cm section of proximal ileum (1 cm distal to Meckel's diverticulum) was excised from freshly euthanized birds, rinsed with cold 1× phosphate-buffered saline, and were transported in fresh Kreb's buffer (Koltes et al., 2017) on ice to the main campus (about 5 min), and analyzed immediately. The TEER and SCC were measured in the Kreb's solution in an 8 chamber Navicyte Vertical EasyMount Ussing system (Warner Instruments, Hamden, CT) (Koltes et al., 2017). Data were captured in Acquire & Analyze revII (Physiologic Instruments, San Diego, CA) over a 20-min period. When all sample TEER and SCC were stable, an average of 5 min were used for the final data analyses.
Histopathology Analyses
Intestinal samples (3 cm section) for histopathology were the distal jejunum (1 cm proximal to Meckel's diverticulum) and the proximal ileum (1 cm distal to Meckel's diverticulum). Sections were collected on day 57 from euthanized apparently healthy birds. Samples were flushed with 1× phosphate-buffered saline and fixed for 1 to 2 d in phosphate-buffered 10% formalin (Cat# JTM518-3, J.T. Baker). The fixed samples were processed through the histology laboratory in the Department of Poultry Science at the University of Arkansas. Hematoxylin–Eosin stained sections were imaged on an Olympus inverted scope at 400× using a CCD camper to display on an LCD monitor. Villus length was measured on the monitor screen with a flexible ruler. Calibration was based on a stage micrometer. For villus length and pathology, at least 4 sections were examined for each tissue for each bird (8–10 birds per treatment). For each section, villus length was measured and gross pathology (villus tip integrity) was scored, on 4 sides (top, left, right, and bottom). For some sections, villus length and tip integrity could not be measured on all 4 sides owing to tissue damage in sectioning.
Statistical Analyses
Data were statistically evaluated using either the T-test function in Microsoft Excel (Office 365, Microsoft Corp., Redmond, WA) or a generalized linear model module in R.3.4.2 (https://cran.r-project.org/bin/windows/base/old/3.4.2/) to produce P-values between treatments. Significant difference was accepted at P ≤ 0.05.
Results and discussion
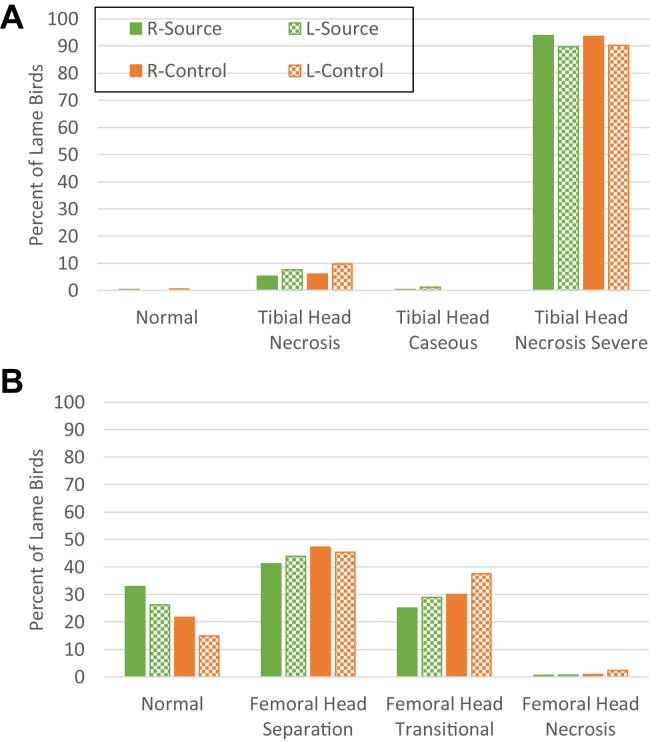
In our previous publications, we had reported that S. agnetis 908 can induce lameness at 80 to 90% for birds raised on wire flooring (Al-Rubaye et al., 2015; Al-Rubaye et al., 2017). This work also demonstrated that S. agnetis 908 can be transmitted from challenged birds to pen mates for birds raised on wire flooring. From that work, we hypothesized that contact or aerosols could spread the infection. Therefore, for experiment 1, we tested whether S. agnetis 908 administration could induce BCO lameness for broilers raised on standard litter flooring. We also tested whether the BCO infection could be transmitted to broilers in the same environment but not in direct contact with the challenged birds. For experiment 1, we had 14 pens of 50 birds in each of 2 treatment groups (n = 700 birds per treatment), source and control (Table 1). Source received a challenge with S. agnetis 908 in drinking water for day 20 and day 21 (Methods), whereas control received no challenge but was located within the same room. Cumulative lameness through day 56 shows that birds raised on litter and administered S. agnetis 908 at 105 CFU/mL in drinking water for day 20 and day 21 experienced cumulative lameness at 47% (Figure 1). The unchallenged control birds in the separated pens experienced a cumulative lameness of 31%, and the appearance of lameness in the control birds was delayed by about 5 d relative to the challenged source birds. The average counts per pen of lame birds in the source treatment was 23.4 ± 0.7 (SEM) and in the control pens lame bird counts was 15.4 ± 1.0 (T-test P-value = 1.5 × 10−6). Although we had no negative control population in a separate facility, typical lameness values for broiler breeders on litter flooring are usually less than 5% (Wideman et al., 2012; Wideman et al., 2015; Wideman, 2016), except in cases of lameness outbreaks (personal communications from industry sources). The severity of the BCO lesions in lame birds was very similar for the source birds and the control birds (Figure 2). Microbiological sampling of blood and BCO lesions from the control birds exclusively recovered S. agnetis. Therefore, the bacteria had spread from the source to the control birds, as we had found for birds raised on wire flooring (Al-Rubaye et al., 2015; Al-Rubaye et al., 2017). In addition, we sampled the air within the building using 10 open petri plates of CHROMAgar Orientation. We recovered thousands of pink colonies that and primarily recovered Staphylococcus saprophyticus but approximately 3% of the colonies were S. agnetis. Therefore, the bacterium in the source birds was being aerosolized within the facility.
Figure 1.
Cumulative percentage lameness for birds raised on litter when challenged with Staphylococcus agnetis 908, or unchallenged birds in the same room for experiment 1. All birds were raised on standard wood shavings. Source and control are as described in Table 1.
Figure 2.
(A) Proximal tibial and (B) femoral head lesion diagnoses for lame birds through the course of experiment 1. Treatments as described in Figure 1 and Table 1. Percentage of lame birds with the indicated BCO lesion is plotted for each treatment. Abbreviations: R, right; L, left; BCO, bacterial chondronecrosis with osteomyelitis.
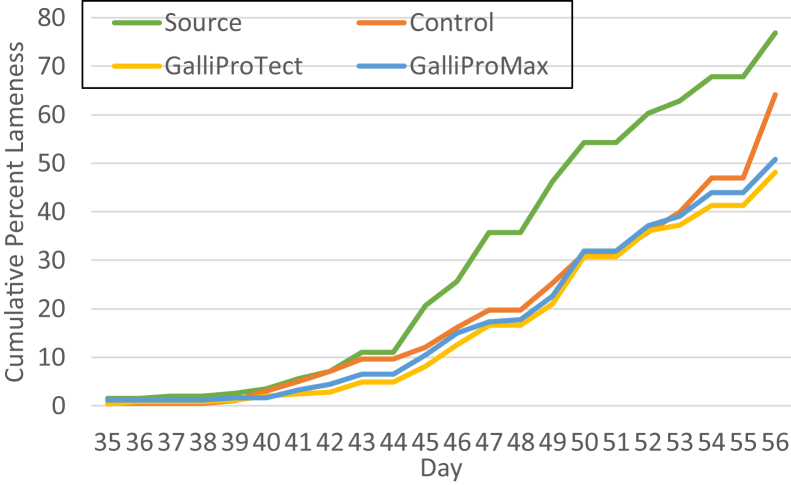
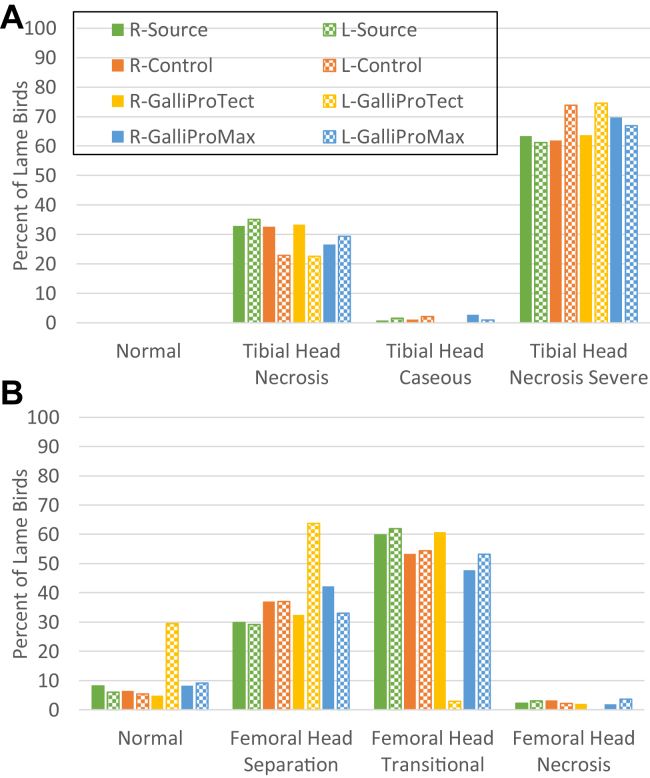
Previously, we had tested probiotics and gut health supplements for protection against BCO lameness for birds raised on wire flooring (Wideman et al., 2012; Wideman et al., 2015; Al-Rubaye et al., 2017). Specifically, we had reported that Biomin PoultryStar or Quality Technology International Incorporated BacPack 2X (combined prebiotic and probiotic) can reduce incidence of lameness by approximately 50% for birds raised on wire flooring (Wideman et al., 2012; Wideman et al., 2015). However, we later reported that when we challenged broilers with S. agnetis 908 in the drinking water at 20 d of age the protective effect of PoultryStar was overwhelmed (Al-Rubaye et al., 2017). Therefore, we designed experiment 2 to examine the activity of 2 commercial probiotics, GalliProTect and GalliProMax, for protective effect against the transmission of S. agnetis 908 induced BCO lameness from a challenged source treatment (Table 1). There were 4 pens of 50 birds each in the source (challenged with S. agnetis 908 on normal feed), and the control (normal feed). There were 5 pens each for the 2 probiotic products. The experiment was set up with the source pens “upwind” of the other pens relative to the exhaust fans. The control, GalliProTect, and GalliProMax pens were arrayed in a randomized, block design, and separated from the source pens by empty pens. Figure 3 shows the cumulative percentage lameness for the 4 treatments through day 56. Incidence of lameness in the source population was higher than in experiment 1 (77% vs. 56%). Experiment 1 was conducted from April 14 through June 8, 2016, whereas experiment 2 was March 8 through May 3, 2017, so outside temperatures (data from NOAA website) were milder during experiment 2 and there were no unusual heat stresses. The higher incidence of lameness in experiment 2 may reflect increasing contamination of the facility with S. agnetis 908 through repeated experiments similar to the increasing lameness with continued experiments on growth on wire flooring (Al-Rubaye et al., 2017). However, the facility is sprayed down with dilute bleach and power washed between experiments. The lameness incidence in the control and probiotic treatments were lower than the source pens and only during the last 4 d was there separation between the control treatment and the probiotic treatments, GalliProTect and GalliProMax, which were equivalent in their protective effect. The pen-to-pen variation in lame birds was similar within a treatment and nonlameness associated mortality was low (Table 2). P values for comparisons of the 4 treatments (Table 3) confirm that lameness in the source treatment was higher than the 3 other treatments, and that the 2 probiotic treatments were lower than the control treatment (P ≤ 0.02; Table 3). Lameness relative to the control was reduced by 25% by GalliProTect and 20% by GalliProMax, but the 2 probiotics were not significantly different from each other (P = 0.41; Table 3). The distribution of BCO lesion severity was not substantively different between the 4 treatments (Figure 4) except for reduced femoral lesion severity in the GalliprotTect for the left, but not the right, femoral head. We suspect this is an experimental aberration as we have reported left/right variability before (Wideman et al., 2012; Wideman et al., 2013; Gilley et al., 2014; Wideman, 2016; Al-Rubaye et al., 2017). We weighed 8 apparently healthy birds randomly selected from each of the treatments on day 56 and observed that the source birds were slightly heavier, at least relative to the control and GalliProMax (Table 4). Whether this difference was because the extreme disease pressure in the source treatment selected for highly robust birds, or was a statistical artifact, is not known without further investigation.
Figure 3.
Cumulative percentage lameness for birds raised on litter when challenged with Staphylococcus agnetis 908, or unchallenged birds in the same room with and without probiotics in the feed, in experiment 2. Treatment groups are as described in Table 1.
Table 2.
Total lame, percent lame, and dead non-lame, birds for individual pens for each of the treatments through 56 d in experiment 2.
| Treatment |
Lame bird count/pen |
Percentage lame birds/pen |
Dead nonlame/pen |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pen | 1 | 2 | 3 | 4 | 5 | Avg1 | 1 | 2 | 3 | 4 | 5 | Avg | 1 | 2 | 3 | 4 | 5 |
| Source | 32 | 44 | 35 | 42 | 38.3 ± 2.5 | 64 | 88 | 71 | 84 | 77 | 0 | 0 | 1 | 0 | |||
| Control | 26 | 37 | 36 | 28 | 31.8 ± 2.4 | 52 | 74 | 73 | 56 | 64 | 0 | 0 | 1 | 0 | |||
| GalliProTect | 21 | 21 | 26 | 24 | 27 | 23.8 ± 1.1 | 43 | 42 | 54 | 48 | 54 | 48 | 1 | 0 | 2 | 0 | 0 |
| GalliProMax | 27 | 31 | 29 | 24 | 15 | 25.2 ± 2.5 | 54 | 65 | 60 | 48 | 30 | 51 | 0 | 2 | 2 | 0 | 0 |
Average (Avg) ± SEM calculated for total lame excluding dead nonlame.
Table 3.
Generalized linear model P values comparing different treatments in experiment 2.
| Treatment |
P values comparing treatment groups1 |
||
|---|---|---|---|
| Control | GalliProTect | GalliProMax | |
| Source | 0.003 | 0.000000001 | 0.00000008 |
| Control | 0.002 | 0.02 | |
| GalliProTect | 0.41 | ||
Experimental unit is individual bird. Birds that died of causes other than lameness were excluded from the calculations.
Figure 4.
(A) Proximal tibial and (B) femoral head lesion diagnoses for lame birds through the course of experiment 2. Treatments as described in Figure 3 and Table 1. Percentage of lame birds with the indicated BCO lesion is plotted for each treatment. Abbreviations: R, right; L, left; BCO, bacterial chondronecrosis with osteomyelitis.
Table 4.
Body weights for apparently healthy birds from each treatment on day 56 in experiment 2.
| Treatment | Average BW kg ± SEM1 |
P-value2 |
||
|---|---|---|---|---|
| Control | GalliProTect | GalliProMax | ||
| Source | 4.40 ± 0.05 | 0.021 | 0.17 | 0.047 |
| Control | 4.14 ± 0.07 | 0.17 | 0.34 | |
| GalliProTect | 4.27 ± 0.10 | 0.28 | ||
| GalliProMax | 4.19 ± 0.10 | |||
Average from 8 birds.
P-value computed by a T-test comparing the treatment in the first column to each of the other treatments.
We collected intestinal tissue from 10 apparently healthy birds on day 57 (held over from the end of the experiment) from the control, GalliProTect, and GalliProMax treatments. The proximal ileum was analyzed for permeability by TEER and SCC in Ussing chambers. The SCC was not different across treatments (P-value = 0.135). Average SCC for control was −16.08 ± 1.82; GalliProText was −11.35 ± 1.54; and GalliProMax was −14.43 ± 1.35. The TEER was significantly higher in GalliProTect and increased but not at the level of significance in GalliProMax (Figure 5). Histopathology was conducted on paired samples of the ileum and jejunum from the same birds for ileal TEER and SSC. Regions of the ileum and jejunum were also assessed for villus length and gross pathology (Materials and Methods). Villus length data are presented in Table 5. Ileal villus length was significantly higher (P-value < 0.001) in both probiotic treatments when compared with the control group. Villus length in the jejunum was lower than control for the GalliProTect but higher than control for GalliProMax. We also scored the jejunum for disrupted villus tips (Table 6). The control treatment had a higher number of birds with ruptured villus tips and 50% of the sections had evidence of ruptured villus tips. GalliProMax had the fewest birds with ruptured villus tips and the lowest percentage of sections with ruptured villus tips.
Figure 5.
Transepithelial electric resistance values for ileum tissue from apparently healthy birds on day 57 from 3 treatment groups in experiment 2. Values plotted are averages (error bars are SEM) from 10 birds from each treatment. Indicated P-values are relative to control. Where treatments share a letter there is no significant difference at P < 0.05.
Table 5.
Relative villus length for the ileum and jejunum for each of 3 treatment groups in experiment 2.
| Region | Treatment | N1 | Villus length (μmol/L) |
P-value2 |
|
|---|---|---|---|---|---|
| Average ± SEM | GalliProTect | GalliProMax | |||
| Ileum | Control | 137 | 1,085 ± 19 | 0.0006 | 0.000001 |
| Ileum | GalliProTect | 116 | 1,174 ± 19 | 0.12 | |
| Ileum | GalliProMax | 139 | 1,203 ± 15 | ||
| Jejunum | Control | 118 | 1,434 ± 27 | 0.04 | 0.17 |
| Jejunum | GalliProTect | 120 | 1,380 ± 14 | 0.0009 | |
| Jejunum | GalliProMax | 107 | 1,472 ± 25 | ||
Number of separate villus length measurements.
P-values based on unpaired student T-test.
Table 6.
Ruptured villus tip assessment for intestinal pathology for 3 treatment groups in experiment 2.
| Treatment | Number of birds | Birds showing ruptured villi1 | Percent of sections with ruptured villi1 |
|---|---|---|---|
| Control | 10 | 7 | 50 |
| GalliProTect | 9 | 5 | 44 |
| GalliProMax | 9 | 2 | 14 |
Based on 4 sections from jejunum from each bird.
These experiments were designed to extend our development of models for investigation of the epidemiology and etiology of BCO lameness in broilers. The wire-flooring model provides a method for inducing lameness and established that BCO was the primary form of lameness induced on wire flooring. Those investigations yielded a strain of S. agnetis that is the primary isolate from lame broilers in our facilities when birds are raised on wire flooring. We now extend the model using this S. agnetis isolate to demonstrate that this culture can induce significant levels of lameness in broilers raised on standard litter floors. This new litter floor model of BCO lameness recapitulates the lameness outbreaks that can occur in particular broiler houses where lameness is recorded in one area of the building and then spreads through the building. As was found with the wire-flooring model for lameness, some commercial probiotics can reduce the incidence or delay the onset of lameness. Probiotics are theorized to improve gut health and reduce pathogen colonization of the gut. However, even though we administer S. agnetis 908 in the drinking water to the source birds, the most likely transmission to the control, and probiotic treated birds is through aerosols. Therefore, the most likely route of the infection in those birds is the pulmonary route. An alternative is that the aerosolized bacteria contaminates feed or waterers in the other pens and is consumed rather than inhaled.
Conclusions
We were the first to report S. agnetis infections in poultry (Al-Rubaye et al., 2015), and there are few other reports of S. agnetis isolation from poultry (Poulsen et al., 2017). Before these reports, S. agnetis had been first identified in isolations from mastitis in cattle (Taponen et al., 2012; Calcutt et al., 2014; Adkins et al., 2017). Phylogenomic analysis of all known S. agnetis genomes has revealed that the poultry isolates may have radiated from a clade within the cattle isolates (Shwani et al., 2020). These analyses have failed to identify chicken-specific genetic determinants. In recent surveys of BCO lameness outbreaks in Arkansas broiler operations, we have isolated Staphylococcus aureus, Staphylococcus hyicus, Enterococcus cecorum, and Escherichia coli (Ekesi et al., unpublished data). Similar species of bacteria have been associated with BCO in other studies (Carnaghan, 1966; Devriese et al., 1972; Emslie and Nade, 1983; Kibenge et al., 1983; Mutalib et al., 1983; Griffiths et al., 1984; Julian, 1985; Daum et al., 1990; Duff, 1990; Hocking, 1992; Butterworth 1999; Butterworth et al., 2001; Ytrehus et al., 2007; Kense and Landman, 2011; Borst et al., 2015; Alstrup et al., 2016; Marek et al., 2016; Wideman, 2016). Whether these other BCO isolates are as virulent as S. agnetis 908 is not known, nor do we know whether they can be transmitted as readily as S. agnetis 908 under standard broiler rearing conditions. We have speculated that S. agnetis 908 evolved into a hypervirulent strain during the years of inducing BCO lameness in our research facilities. However, similar selection in broiler grower facilities can also be going on in each broiler operation. Each broiler operation could be evolving its own particular strain. This is consistent with anecdotal evidence from broiler operators that particular broiler houses have continuing issues with lameness incidence. The litter-flooring model for BCO lameness clearly demonstrates that the BCO epidemic can spread through the air for our S. agnetis model, and this may be likely for the other BCO-associated bacterial species. We have now demonstrated that that probiotics can reduce the spread of the infection through the air. If the primary spread of the infection is through the pulmonary route, then either the probiotics are also being introduced to the pulmonary system or the probiotic bacteria are eliciting an enhanced generalized innate immunity in major epithelial layers.
The wire-flooring model for inducing lameness and the litter-flooring model for BCO lameness epidemics, provide avenues to develop strategies and evaluate management programs to reduce a continuing animal welfare concern.
Acknowledgments
AH was supported on a fellowship from the Iraqi government. Chr Hansen provided the funding for some of this work and provided input on the design of some experiments.
Conflict of Interest Statement: The authors declare that they have no competing interests or conflict of interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.08.067.
Supplementary data
References
- Adkins P.R.F., Middleton J.R., Calcutt M.J., Stewart G.C., Fox L.K. Species identification and strain typing of Staphylococcus agnetis and Staphylococcus hyicus isolates from bovine milk by use of a novel multiplex PCR assay and pulsed-field gel electrophoresis. J. Clin. Microbiol. 2017;55:1778–1788. doi: 10.1128/JCM.02239-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rubaye A.A.K., Couger M.B., Ojha S., Pummill J.F., Koon J.A., II, Wideman R.F., Jr., Rhoads D.D. Genome analysis of Staphylococcus agnetis, an agent of lameness in broiler chickens. PLoS One. 2015;10:e0143336. doi: 10.1371/journal.pone.0143336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rubaye A.A.K., Ekesi N.S., Zaki S., Emami N.K., Wideman R.F., Rhoads D.D. Chondronecrosis with osteomyelitis in broilers: further defining a bacterial challenge model using the wire flooring model. Poult. Sci. 2017;96:332–340. doi: 10.3382/ps/pew299. [DOI] [PubMed] [Google Scholar]
- Alstrup A.K.O., Nielsen K.M., Schønheyder H.C., Jensen S.B., Afzelius P., Leifsson P.S., Nielsen O.L. Refinement of a hematogenous localized osteomyelitis model in pigs. Scand. J. Lab. Anim. Sci. 2016;42 [Google Scholar]
- Borst L.B., Suyemoto M.M., Scholl E.H., Fuller F.J., Barnes H.J. Comparative genomic analysis identifies divergent genomic features of pathogenic Enterococcus cecorum including a type IC CRISPR-Cas system, a capsule locus, an epa-like locus, and putative host tissue binding proteins. PLoS One. 2015;10:e0121294. doi: 10.1371/journal.pone.0121294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. Infectious components of broiler lameness: a review. Worlds Poult. Sci. J. 1999;55:327–352. [Google Scholar]
- Butterworth A., Reeves N.A., Harbour D., Werrett G., Kestin S.C. Molecular typing of strains of Staphylococcus aureus isolated from bone and joint lesions in lame broilers by random amplification of polymorphic DNA. Poult. Sci. 2001;80:1339–1343. doi: 10.1093/ps/80.9.1339. [DOI] [PubMed] [Google Scholar]
- Calcutt M.J., Foecking M.F., Fry P.R., Hsieh H.-Y., Perry J., Stewart G.C., Scholl D.T., Messier S., Middleton J.R. Draft genome sequence of bovine mastitis isolate Staphylococcus agnetis CBMRN 20813338. Genome Announc. 2014;2 doi: 10.1128/genomeA.00883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaghan R.B.A. Spinal cord compression due to spondylitis caused by Staphylococcus pyogenes. J. Comp. Pathol. 1966;76:9–14. doi: 10.1016/0021-9975(66)90042-9. [DOI] [PubMed] [Google Scholar]
- Daum R.S., Davis W.H., Farris K.B., Campeau R.J., Mulvihill D.M., Shane S.M. A model of Staphylococcus aureus bacteremia, septic arthritis, and osteomyelitis in chickens. J. Orthop. Res. 1990;8:804–813. doi: 10.1002/jor.1100080605. [DOI] [PubMed] [Google Scholar]
- Devriese L.A., Devos A.H., Beumer J. Staphylococcus aureus colonization on poultry after experimental spray inoculations. Avian Dis. 1972;16:656–665. [PubMed] [Google Scholar]
- Duff S.R.I. Diseases of the musculoskeletal system. In: Jordan F., editor. Poultry Diseases. Bailliere; Tindall, UK: 1990. [Google Scholar]
- Emslie K., Nade S. Acute hematogenous Staphylococcal osteomyelitis. A description of the natural history in an avian model. Am. J. Pathol. 1983;110:333. [PMC free article] [PubMed] [Google Scholar]
- Gilley A.D., Lester H., Pevzner I.Y., Anthony N.B., Wideman R.F. Evaluating portable wire-flooring models for inducing bacterial chondronecrosis with osteomyelitis in broilers. Poult. Sci. 2014;93:1354–1367. doi: 10.3382/ps.2013-03781. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Hopkinson W., Lloyd J. Staphylococcal necrosis of the head of the femur in broiler chickens. Aust. Vet. J. 1984;61:293. doi: 10.1111/j.1751-0813.1984.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. Musculo-skeletal disease in heavy breeding birds. In: Whitehead C.C., editor. Bone Biology and Skeletal Disorders in Poultry. Carfax Publishing Company; Abingdon, United Kingdom: 1992. pp. 297–309. [Google Scholar]
- Julian R.J. Osteochondrosis, dyschondroplasia, and osteomyelitis causing femoral head necrosis in turkeys. Avian Dis. 1985;29:854–866. [PubMed] [Google Scholar]
- Kense M.J., Landman W.J.M. Enterococcus cecorum infections in broiler breeders and their offspring: molecular epidemiology. Avian Pathol. 2011;40:603–612. doi: 10.1080/03079457.2011.619165. [DOI] [PubMed] [Google Scholar]
- Kibenge F.S., Wilcox G., Pass D. Pathogenicity of four strains of Staphylococci isolated from chickens with clinical tenosynovitis. Avian Pathol. 1983;12:213–220. doi: 10.1080/03079458308436164. [DOI] [PubMed] [Google Scholar]
- Koltes D.A., Lester H.D., Frost M., Aldridge D., Christensen K.D., Scanes C.G. Effects of bacitracin methylene disalicylate and diet change on gastrointestinal integrity and endotoxin permeability in the duodenum of broiler chicken. BMC Res. Notes. 2017;10:470. doi: 10.1186/s13104-017-2781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek A., Stepień-Pyśniak D., Pyzik E., Adaszek Ł., Wilczyński J., Winiarczyk S. Occurrence and characterization of Staphylococcus bacteria isolated from poultry in Western Poland. Berl. Munch. Tierarztl. Wochenschr. 2016;129:147–152. [PubMed] [Google Scholar]
- Mutalib A., Riddell C., Osborne A.D. Studies on the pathogenesis of Staphylococcal osteomyelitis in chickens. II. Role of the respiratory tract as a route of infection. Avian Dis. 1983;27:157–160. [PubMed] [Google Scholar]
- Poulsen L.L., Thøfner I., Bisgaard M., Olsen R.H., Christensen J.P., Christensen H. Staphylococcus agnetis, a potential pathogen in broiler breeders. Vet. Microbiol. 2017;212:1–6. doi: 10.1016/j.vetmic.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Shwani A., Adkins P.R.F., Ekesi N.S., Alrubaye A., Calcutt M.J., Middleton J.R., Rhoads D.D. Whole genome comparisons of Staphylococcus agnetis isolates from cattle and chickens. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.00484-20. e00484-00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taponen S., Supré K., Piessens V., Van Coillie E., De Vliegher S., Koort J.M.K. Staphylococcus agnetis sp. nov., a coagulase-variable species from bovine subclinical and mild clinical mastitis. Int. J. Syst. Evol. Microbiol. 2012;62:61–65. doi: 10.1099/ijs.0.028365-0. [DOI] [PubMed] [Google Scholar]
- Wideman R.F. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult. Sci. 2016;95:325–344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Gilley A., Reynolds D., Lester H., Yoho D., Hughes J.M., Pevzner I. Susceptibility of 4 commercial broiler crosses to lameness attributable to bacterial chondronecrosis with osteomyelitis. Poult. Sci. 2013;92:2311–2325. doi: 10.3382/ps.2013-03150. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Kwon Y.M., Blankenship J., Lester H., Mitchell K.N., Pevzner I.Y., Lohrmann T., Schleifer J. Prophylactic administration of a combined prebiotic and probiotic, or therapeutic administration of enrofloxacin, to reduce the incidence of bacterial chondronecrosis with osteomyelitis in broilers. Poult. Sci. 2015;94:25–36. doi: 10.3382/ps/peu025. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Al-Rubaye A., Reynolds D., Yoho D., Lester H., Spencer C., Hughes J.D., Pevzner I.Y. Bacterial chondronecrosis with osteomyelitis in broilers: influence of sires and straight-run versus sex-separate rearing. Poult. Sci. 2014;93:1675–1687. doi: 10.3382/ps.2014-03912. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Hamal K.R., Stark J.M., Blankenship J., Lester H., Mitchell K.N., Lorenzoni G., Pevzner I. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult. Sci. 2012;91:870–883. doi: 10.3382/ps.2011-01907. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Prisby R.D. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front. Endocrinol. (Lausanne) 2013;3:183. doi: 10.3389/fendo.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ytrehus B., Carlson C., Ekman S. Etiology and pathogenesis of osteochondrosis. Vet. Pathol. 2007;44:429–448. doi: 10.1354/vp.44-4-429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.