Abstract
Although it has rapidly decreased since the early 2000s, fowl typhoid still occurs in commercial layer chickens, causing a significant economic loss in Korea. There is growing concern about the emergence of new pathogenic strains of the causative agent, Salmonella Gallinarum, which is able to overcome vaccine immunity. It has also been suspected that the poultry red mite, Dermanyssus gallinae, which is commonly found in layer chicken farms, may be an important cause of the recurrence of fowl typhoid in the farms. This study was conducted to examine changes in the virulence of recent isolates of S. Gallinarum obtained from layer farms and estimate the potential of the disease transmission of D. gallinae in the farms. Clinical and environmental samples and mites collected from layer farms affected by fowl typhoid between 2013 and 2018 were tested for S. Gallinarum. The isolates were characterized by genotypic analyses and in vitro virulence assays with chicken-derived cell lines. Vaccine protection against recent isolates was examined in the chickens. A total of 45 isolates of S. Gallinarum were collected and there was no evidence of changes in their virulence. It has also been demonstrated that the S. Gallinarum 9R vaccine strain widely used in Korea is still effective in controlling fowl typhoid if the susceptibility of birds to the disease is not increased by stress. Salmonella Gallinarum isolated from the outer and inner parts of D. gallinae, environmental dust, and dead birds of the same farm showed the same or closely related genotypes. Consequently, the present study indicated that the horizontal transmission and environmental persistence of S. Gallinarum and the increased disease susceptibility of chickens in layer farms could be mediated by D. gallinae, causing persistent outbreaks of fowl typhoid.
Key words: Dermanyssus gallinae, Salmonella, Gallinarum, fowl typhoid, layer
Introduction
Fowl typhoid is a systemic disease in avian species, primarily chickens and turkeys, caused by Salmonella Gallinarum. The disease is of considerable economic significance in many countries of Central and South America, Africa, and Asia including Korea (Shivaprasad, 2000; Jones et al., 2001). Fowl typhoid can be transmitted not only horizontally, but also through eggs by transovarial infections (Shivaprasad and Barrow, 2013). Most of all, infected birds (reactors and carriers) are the most important means of the persistence and spread of the organism (Shivaprasad and Barrow, 2013). Effective control measures for the disease are accompanied by periodic monitoring of flocks and the elimination of infected birds (Shivaprasad and Barrow, 2013). However, in many countries, vaccines and antimicrobial drugs are commonly used to prevent and treat commercial layer chickens (Barrow and Freitas Neto, 2011).
Live vaccines based on attenuated S. Gallinarum strains (i.e., SG 9R) were introduced in Korea to control fowl typhoid in commercial layer chickens in the early 2000s (Kwon et al., 2010). The use of live vaccines, together with a nationwide control policy for the eradication of the disease in breeder chicken flocks, has rapidly decreased the incidence of the disease in Korea (Kwon et al., 2010; Kang et al., 2012a). Nevertheless, the disease still occurs in commercial layers causing significant economic loss (Kang et al., 2012a). The high susceptibility of brown layers occupying most of the layer population in Korea may have made the disease difficult to control (Kwon et al., 2010). However, outbreaks of the disease in vaccinated chicken flocks raised concerns as to whether new pathogenic strains of S. Gallinarum have emerged to overcome the vaccine immunity.
In addition, the poultry red mite Dermanyssus gallinae is a serious problem in layer chickens worldwide (Sparagano et al., 2014; George et al., 2015). This blood-feeding ectoparasite has been considered to be a potential vector implicated in the transmission of several poultry diseases, including fowl typhoid (Valiente Moro et al., 2009; Sparagano et al., 2014; Pugliese et al., 2019). Recent reports showed that the rate of infested farms reached more than 80% in some countries, such as the Netherlands, Poland, China, and Japan in Europe and Asia (Sparagano et al., 2014; George et al., 2015). Dermanyssus gallinae is commonly found in layer farms in Korea, as well, and has been suspected to be an important cause of the recurrence of fowl typhoid in farms previously affected by the disease. Therefore, this study was conducted to examine any changes associated with virulence in the recent isolates of S. Gallinarum obtained from layer farms and estimate the potential of the disease transmission mediated by D. gallinae in the farms.
Materials and methods
Samples and Bacterial Isolation
Salmonella Gallinarum was isolated from chicken clinical samples submitted to the Animal and Plant Quarantine Agency (APQA) in South Korea for diagnosis between 2013 and 2018. The clinical (n = 8) and environmental (dust) (n = 10) samples and D. gallinae (n = 10) were also collected from 20 layer farms affected by fowl typhoid between 2017 and 2018 and were tested for S. Gallinarum. All flocks surveyed were housed in multitier cage systems and were infested with D. gallinae. Fowl typhoid was detected in the flocks within the last 3 yr and the current flocks from which samples were collected had been vaccinated with the SG 9R at least twice.
For bacterial isolation, liver or spleen samples were aseptically swabbed and cultured in tryptic soy broth (BD Biosciences, Sparks, MD) at 37°C for 24 h. The culture was streaked onto MacConkey agar (BD Biosciences) and Rambach agar (Merck, Darmstadt, Germany) and incubated at 37°C for 24 h. Dust samples were pre-enriched in buffered peptone water (BD Biosciences) (1:10 ratio) at 37°C for 18 h and transferred to tetrathionate broth (BD Biosciences) and Rappaport-Vassiliadis broth (Merck). They were incubated at 42°C for 24–48 h. Then, the cultures were streaked onto MacConkey agar and Rambach agar and incubated at 37°C for 24 h. D. gallinae was sampled by collecting the mite-containing dust from the cage environment in plastic containers, and approximately 100 specimens from each pooled sample were cleaned with sterile water, and disinfected with 4% formaldehyde solution for 7 min with agitation, and washed in sterile water as described previously (Zeman et al., 1982). Disinfected and nondisinfected specimens (approximately 100 bodies) from the same pooled sample were ground in sterile mortars and cultured in tetrathionate broth and Rappaport-Vassiliadis broth at 42°C for 24–48 h. The cultures were streaked onto MacConkey agar and Rambach agar and were incubated at 37°C for 24 h.
Subsequently, suspected colonies were selected from the plates and were subjected to conventional biochemical tests (Ewing, 1986) or VITEK 2 (bioMerieux Inc., Hazelwood, MO) analysis to identify Salmonella. The serotype and biotype of the isolates were determined by the APQA following conventional methods (Ewing, 1986; Grimont and Weill, 2007) and polymerase chain reaction using primers SG-L (5′-GATCTGCTGCCAGCTCAA-3′), SG-R (5′-GCGCCCTTT-TCAAAACATA-3′), SGP-L (5′-CGGTGTACTGCCCGCTAT-3′), SGP-R (5′-CTGGGCATTGACGCAAA-3′), 9R-L (5′-CTTTACGGGCAAACCACAGT-3′), and 9R-R (5′-TGAGATGGAAAAAGAGCAGCA-3′) as described previously (Kang et al., 2012b). Only one isolate per source per case was included in this study.
Genotypic Analysis
Pulsed-field gel electrophoresis (PFGE) was performed using the restriction endonuclease XbaI as described previously (Ribot et al., 2006). The conditions were as follows: separation in 1% SeaKem Gold agarose gel (FMC Bioproducts, Rockland, ME) in 0.5 × TBE buffer (Novex, Carlsbad, CA) at 14°C and 6 V/cm for 18 h, with switch times from 2.2 to 63.8 s on a CHEF-Mapper (Bio-Rad Laboratories, Hercules, CA). The gel images were analyzed using Bionumerics software (Applied Maths, Sint-Martens-Latem, Belgium). A dendrogram was generated from the Dice coefficients of similarity by the unweighted pair group method using average linkages.
Multilocus variable-number tandem-repeat analysis (MLVA) was performed with 4 loci (SGTR1 to SGTR4) as described previously (Kang et al., 2011). The MLVA profiles were determined using character data based on the number of repeated units and a minimum spanning tree was constructed using Bionumerics software.
In Vitro Virulence Assays
The virulence of the isolates was tested using infection experiments with the chicken hepatocellular carcinoma epithelial cell line (LMH) and the chicken macrophage cell line (HD11) as described previously with some modifications (Barrow and Lovell, 1989; Chadfield et al., 2003). For the host cell invasion assay, LMH cells were seeded in a 24-well tissue culture plate coated with 0.1% gelatin in Waymouth's MB 752/1 medium (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) at 105 cells/well and grown overnight at 37°C in a 5% CO2 atmosphere. The cells were inoculated with bacteria at a multiplicity of infection of 10. The plates, including the bacteria and cells, were incubated for 2 h at 37°C in a 5% CO2 atmosphere and then each well was washed three times with phosphate-buffered saline (PBS). Waymouth's medium with 10% FBS-containing amikacin (100 μg/mL) was added to the wells and the cells were incubated for another 1.5 h at 37°C with 5% CO2 to kill the extracellular bacteria. Then, each well was washed three times with PBS and the cells were lysed with 0.1% Triton X-100 in PBS. The released bacteria were quantified by plating on LB agar (Life Technologies) to determine the percentage invasion (% survival) relative to the inoculated bacteria. The data were analyzed based on 2 independent experiments.
For the survival assay in the HD11 cell line, HD11 cells were seeded in a 24-well tissue culture plate with Iscove's Modified Dulbecco's Medium (Sigma Aldrich, MO) containing 10% FBS and 150 mmol L-glutamine at 105 cells/well and grown overnight at 41°C in a 5% CO2 atmosphere. The log-phase bacterial culture was opsonized with 10% chicken serum at 37°C for 30 min. The opsonized culture was added to the HD11 cells at a multiplicity of infection of 10. The bacteria were incubated with cells for 1 h, and then the medium was replaced with Iscove's Modified Dulbecco's Medium with 10% FBS and 150 mmol L-glutamine containing amikacin (100 μg/mL) and incubated for 1 h to kill the extracellular bacteria. The cells were then washed three times with antibiotic-free medium. The initial intracellular bacterial count (1-h survival) was made by lysing the cells with 0.1% Triton X-100 in PBS and then plating the lysate on LB agar. To determine the persistence of bacteria, the cells were maintained in medium containing amikacin (10 μg/mL). At 4 and 24 h after infection, the monolayers were washed three times with PBS and the cells were lysed with 0.1% Triton X-100 in PBS. The released bacteria were quantified to determine the percentage recovery (% survival) relative to the inoculated bacteria. The data were analyzed based on three independent experiments.
Assessment of Vaccine Protection
Hy-Line Brown chickens were purchased from a farm known to be free from Salmonella. The chickens were maintained in wire cages and verified as Salmonella-free by bacterial culture and serology (serum plate agglutination and enzyme-linked immunosorbent assay). For examining the protective effect of vaccination against the recent isolates of S. Gallinarum, the chickens were divided into 10 groups of 10 birds each. All birds were vaccinated with S. Gallinarum live vaccine (Nobilis SG 9R; MSD Animal Health, Boxmeer, the Netherlands) at 4 wk old. Five groups were orally administered corticosterone (20 mg/L) every day starting at 4 wk old to establish a stress-induced depression model (Post et al., 2003). Then, each set of 4 groups with or without corticosterone administration was orally challenged with each of three recent isolates (N17SG007, N17SG014, and N18SG003), which were different in their genotypes and geographic origins, and one known virulent strain (SG06Q110) (Kang et al., 2012a) at a dose of 108 cfu/chicken at 7 wk old. All chickens of 8 challenged groups and 2 control groups were euthanized at 9 wk old. At the postmortem examination, the liver, spleen, and cecum, and cloacal swabs were collected from each dead bird and the other live birds euthanized 14 d after the challenge. Tissue samples of the liver and spleen were cultured as described above, and the cecal contents and cloacal swabs were cultured as described for environmental samples above.
Statistical Analysis
Differences in mortality and the bacterial isolation rates between the groups of chickens were assessed by the chi-square test. The percentage survival data were analyzed using the Student t test. The results with P < 0.05 were considered to be statistically significant.
Ethics Statement
All procedures involving animals were performed with the permission of the Animal Ethics Committee in the APQA (permission number 2018-972), Korea.
Results
Isolation of S. Gallinarum From Chickens and D. Gallinae
A total of 45 isolates of S. Gallinarum were obtained from the clinical and environmental samples and D. gallinae from chicken farms affected by fowl typhoid in this study. Twenty-seven of the isolates were obtained from the clinical samples (the liver and spleen) of the chicken flocks (14 layer, 11 broiler, and 2 broiler breeder flocks) diagnosed with fowl typhoid by the APQA between 2013 and 2018. In addition, 18 of the isolates were collected from 4 liver and three spleen samples, one house dust sample, and five D. gallinae samples (both outer and inner parts of 4 samples and the outer part of one sample) from 20 layer farms where fowl typhoid recurred between 2017 and 2018 in seven provinces (Gyeonggi, Chungbuk, Chungnam, Gyeongbuk, Gyeongnam, Jeonbuk, and Jeju) of Korea (Table 1).
Table 1.
Distribution of Salmonella Gallinarum in clinical and environmental samples and Dermanyssus gallinae collected from layer chicken farms (n = 20) affected by fowl typhoid between 2017 and 2018.
| Province | Farm | Sample | Treatment | Bacterial isolation1 |
|
|---|---|---|---|---|---|
| S. Gallinarum | Other Salmonella | ||||
| Gyeonggi | 17F03 | D. gallinae | External disinfection | − | − |
| None | − | − | |||
| 17F04 | Dust | None | − | − | |
| 17F08 | Dust | None | − | − | |
| 17F11 | D. gallinae | External disinfection | + | − | |
| None | + | − | |||
| Dust | None | − | + | ||
| Liver | None | + | − | ||
| Spleen | None | + | − | ||
| 17F14 | Spleen | None | + | − | |
| Jeonbuk | 17F05 | Dust | None | − | − |
| 17F06 | Dust | None | − | − | |
| 17F07 | Dust | None | − | − | |
| 18F04 | Dust | None | − | + | |
| 18F06 | D. gallinae | External disinfection | − | – | |
| None | − | − | |||
| Gyeongnam | 17F01 | D. gallinae | External disinfection | − | − |
| None | − | − | |||
| Liver | None | − | − | ||
| 17F09 | D. gallinae | External disinfection | + | − | |
| None | + | − | |||
| Dust | None | − | − | ||
| Chungnam | 17F02 | D. gallinae | External disinfection | − | − |
| None | − | − | |||
| Liver | None | + | − | ||
| 17F10 | D. gallinae | External disinfection | + | − | |
| None | + | − | |||
| Liver | None | + | − | ||
| Spleen | None | + | − | ||
| Dust | None | + | − | ||
| 18F03 | Liver | None | − | − | |
| Chungbuk | 17F13 | D. gallinae | External disinfection | + | − |
| None | + | − | |||
| Dust | None | − | − | ||
| 17F15 | Spleen | None | + | − | |
| 18F01 | D. gallinae | External disinfection | − | − | |
| None | + | − | |||
| Jeju | 17F12 | Liver | None | + | − |
| Spleen | None | + | − | ||
| Gyeongbuk | 18F02 | D. gallinae | External disinfection | − | − |
| None | − | − | |||
‘+’ = positive; ‘−’ = negative.
Genotypic Characteristics of S. Gallinarum Isolates
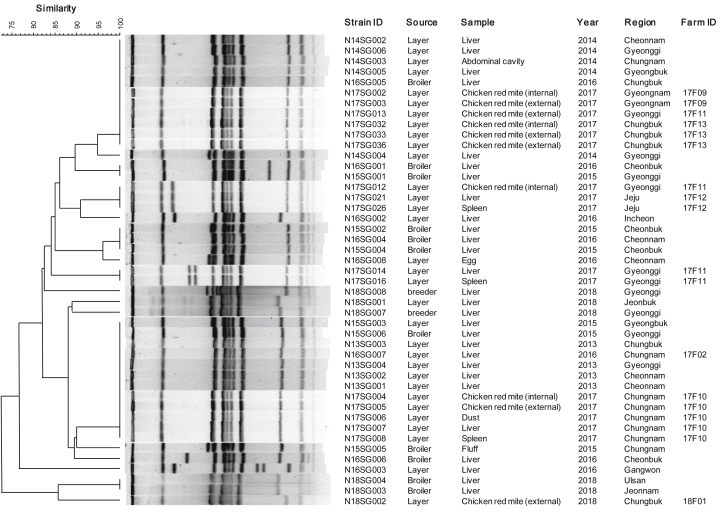
Forty-five isolates of S. Gallinarum were genotyped using PFGE and MLVA. Pulsed-field gel electrophoresis analysis showed a total of 17 patterns, which were grouped into six groups based on 85% similarity (Figure 1). In particular, isolates from the inside (internal) or surface (external) of D. gallinae (N17SG004 and N17SG005) based on external disinfection, dust (N17SG006), and the internal organs (liver and spleen) of dead chickens (N17SG007 and N17SG008) derived from the same farm were grouped together into a small group. In the MLVA analysis, a total of 21 profiles were identified (Figure 2). In addition, most isolates from D. gallinae with and without external disinfection, dust, and the internal organs of dead chickens derived from the same farm (17F10, 17F11) were the same type, although some isolates from the same farm were different but closely related types (Figure 2).
Figure 1.
Pulsed-field gel electrophoresis patterns of 45 Salmonella Gallinarum isolates from chickens, Dermanyssus gallinae, and environmental samples. Pulsed-field gel electrophoresis analysis shows a total of 17 patterns, with six groups based on 85% similarity. Isolates from the inside (internal) or outside (external) of D. gallinae (N17SG004 and N17SG005), dust (N17SG006), and the internal organs (liver and spleen) of dead chickens (N17SG007 and N17SG008) from the same farm belonged to a single group.
Figure 2.
Genotypic relationships between 45 Salmonella Gallinarum isolates from chickens, Dermanyssus gallinae, and environmental samples based on MLVA profiles. A minimum spanning tree was constructed using BioNumerics software (Applied Maths). A total of 21 allelic profiles were identified and their relationships are presented in the tree. Each circle denotes an MLVA type and its color presents the number of isolates with a particular MLVA type. The ovals include MLVA types of isolates from each farm indicated next to them. MLVA, multilocus variable-number tandem-repeat analysis.
In Vitro Virulence of S. Gallinarum Isolates
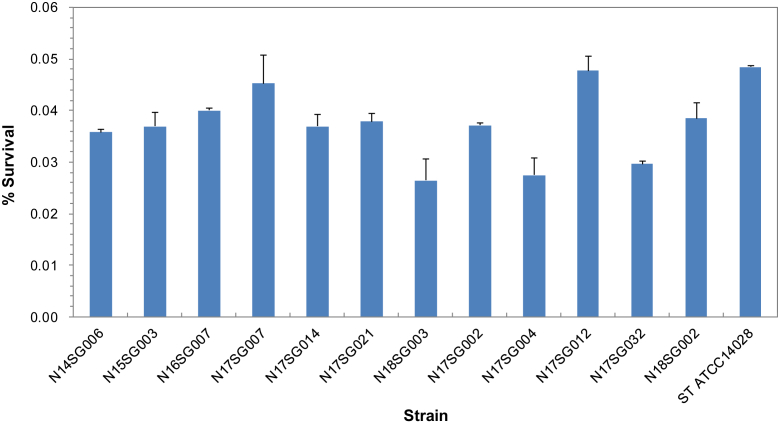
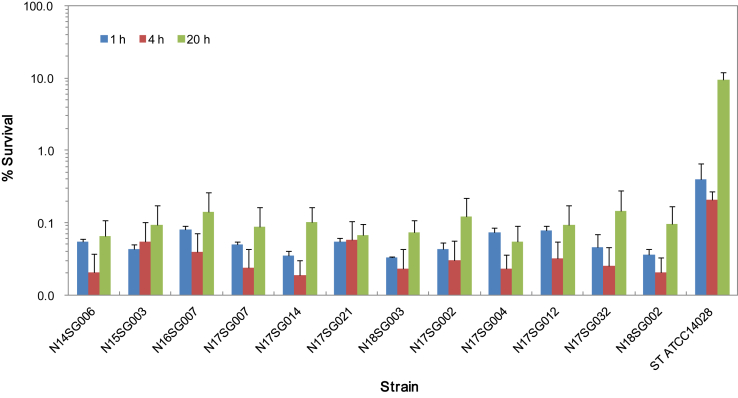
Fifteen representative isolates were selected based on their genotypes, sources, and temporal and geographic origins (Figure 1) and tested for their virulence using in vitro models with LMH and HD11 cells. The isolates were able to invade the LMH cells with variation, but there was no significant difference in invasion between the isolates from different sources and origins (Figure 3). In addition, the isolates inoculated into the HD11 cells were examined for their survival rate periodically. However, no significant difference was found between the isolates (Figure 4).
Figure 3.
Invasion of chicken LMH cells by 15 recent isolates of Salmonella Gallinarum. The bars represent the percent survival (the ratio (%) of intracellular bacteria to inoculated bacteria) that was determined based on 2 independent experiments, and the error bars indicate the SEs. The isolates were able to invade the LMH cells with variation, but there was no significant difference in cell invasion between the isolates of different genotypes, sources, and temporal and geographic origins.
Figure 4.
Intracellular survival of 15 recent isolates of Salmonella Gallinarum in HD11 cells. The bars represent the percentage survival (the ratio (%) of intracellular bacteria to inoculated bacteria) that was determined based on three independent experiments, and the error bars indicate the SEs. The isolates inoculated into the HD11 cells were examined for their survival rate at 4 and 24 h after internalization. However, no significant difference was found between the isolates of different genotypes, sources, and temporal and geographic origins at the indicated time points.
Vaccine Protection Against Recent Isolates of S. Gallinarum
Of the vaccinated groups challenged with three recent isolates (N17SG007, N17SG014, and N18SG003), which belonged to different genotypic groups, and the known virulent strain (SG06Q110), the group challenged with SG06Q110 showed 10% (1/10) mortality until 14 d after the challenge (Table 2). No mortality was observed in the other vaccinated groups, indicating that no significant increase in virulence was observed in recent isolates compared with the existing pathogenic strain (Table 2). Of the vaccinated and stressed (corticosterone-treated) groups challenged with the same isolates as above, the mortalities were 20%, 40%, and 30% in the three groups challenged with N17SG007, N17SG014, and SG06Q110, respectively. Isolation of the challenge strains from the liver, spleen, cecum, and cloaca of the dead and live ducks was performed on the day of death and 14 d after the challenge. The isolation rates of the challenge strains in the liver, spleen, and cecal contents were not significantly different between the groups and between the vaccinated groups and the vaccinated and stressed groups (Table 2). The isolation rates of the challenge strains from the cloacal swabs were significantly lower in the vaccinated groups than in the vaccinated and stressed groups (P < 0.05) (Table 2).
Table 2.
Effect of immune stress on vaccine-induced protection against fowl typhoid in chickens.
| Group1 | Strain | Stress (Corticosterone)2 | Mortality (%) | Recovery of Salmonella Gallinarum (%) |
|||
|---|---|---|---|---|---|---|---|
| Liver | Spleen | Cecum | Cloaca3 | ||||
| 1 | N17SG007 | − | 0/10 (0) | 6/10 (60) | 9/10 (90) | 6/10 (60) | 0/10 (0) |
| 2 | N17SG007 | + | 2/10 (20) | 6/10 (60) | 8/10 (80) | 9/10 (90) | 2/10 (20) |
| 3 | N17SG014 | − | 0/10 (0) | 2/10 (20) | 8/10 (80) | 3/10 (30) | 0/10 (0) |
| 4 | N17SG014 | + | 4/10(40) | 8/10 (80) | 10/10 (100) | 8/10 (80) | 4/10 (40) |
| 5 | N18SG003 | − | 0/10 (0) | 5/10 (50) | 9/10 (90) | 6/10 (60) | 0/10 (0) |
| 6 | N18SG003 | + | 0/10 (0) | 4/10 (40) | 4/10 (40) | 6/10 (60) | 1/10 (10) |
| 7 | SG06Q110 | − | 1/10 (10) | 6/10 (60) | 9/10 (90) | 4/10 (40) | 1/10 (10) |
| 8 | SG06Q110 | + | 3/10 (30) | 7/10 (70) | 9/10 (90) | 7/10 (70) | 3/10 (30) |
| 9 | None | − | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) |
| 10 | None | + | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) | 0/10 (0) |
Vaccinated with SG9R (MSD) at 4 wk old and then inoculated at 7 wk old (108 cfu/chicken); euthanized at 9 wk old.
Corticosterone (20 mg/L): orally administered every day starting at 4 wk old.
P < 0.05 (normal vs. stressed).
Discussion
The present study characterized S. Gallinarum isolated from various samples collected from recent cases of fowl typhoid. The isolates showed a variety of genotypic groups based on PFGE patterns and MLVA profiles, indicating the occurrence of multiple clones in chicken flocks. The S. Gallinarum isolates originating from the same farm were the same or closely related genotypes, regardless of the sample types. In particular, it was found that all farms sampled between 2017 and 2018 were infested with D. gallinae.
The impact of D. gallinae has increased in Korea over the past decades because the use of synthetic acaricides in farms has been restricted due to the increase in ecofriendly farms certified by the government since the 2000s. In addition, climate warming may have facilitated the proliferation of D. gallinae in farms as described in many other countries (Skuce et al., 2013; Sigognault Flochlay et al., 2017). It has been shown that D. gallinae can act as a mechanical or biological vector by carrying and transmitting a variety of pathogens on poultry premises (Valiente Moro et al., 2007, 2009; Huong et al., 2014). Dermanyssus gallinae was also reported to act as a reservoir of S. Gallinarum and was implicated in the persistence of the pathogen in farms (Zeman et al., 1982; Pugliese et al., 2019). In the present study, S. Gallinarum was isolated from the outside and inside of D. gallinae, as well as from environmental dust, and shared common genotypes with clinical isolates from dead birds of a farm. This finding indicates that D. gallinae is involved in the transmission of S. Gallinarum within farms mechanically and potentially biologically, in addition to its role as a reservoir of S. Gallinarum. This is consistent with previous reports and strongly supports that D. gallinae infestation can be an important factor resulting in persistent outbreaks of fowl typhoid in layer chicken flocks.
Recent isolates of S. Gallinarum representing genotypes, sources, and temporal and geographic origins were tested for their virulence in in vitro models using chicken hepatocytes and macrophages in this study. The invasion of host cells and survival in macrophages are essential features in the virulence of Salmonella in the host (Chadfield et al., 2003; He et al., 2012). The in vitro virulence assays with chicken cell lines in this study did not show any increase in virulence distinguishing recent isolates of S. Gallinarum regardless of sources and temporal and geographic origins.
Dermanyssus gallinae sucks the blood of chickens and causes stress, such as skin irritations and restlessness, anemia, and death in the affected birds, as well as facilitating the potential spread of diseases (Shivaprasad and Barrow, 2013; George et al., 2015). Consequently, infestation with D. gallinae can cause significant economic losses in the chicken industry because of a poor feed conversion ratio, a drop in egg production, a higher susceptibility to diseases, and an increased mortality rate (Sigognault Flochlay et al., 2017; Sleeckx et al., 2019). All layer chicken flocks, which were recently confirmed to be affected by fowl typhoid in this study, were infested with D. gallinae. Therefore, the constant stress posed by these infestations may have increased the susceptibility of the flocks to fowl typhoid. In the present study, we tried to examine the effect of stress on the infection with S. Gallinarum in vaccinated chickens using a chicken stress model produced by the administration of corticosterone as described previously (Post et al., 2003). The chickens were vaccinated with a live S. Gallinarum vaccine (SG 9R), which was most commonly used in Korea, and experimentally challenged by recent isolates of S. Gallinarum. In addition, a duplicate vaccinated group was continuously stressed by the stress-related hormone corticosterone and was challenged in the same way. As expected, the stressed groups showed higher mortality and bacterial isolation rates compared with the unstressed groups, indicating the increased susceptibility to S. Gallinarum infection in stressed chickens. In this stressed chicken model, there were no significant differences in virulence between the recent isolates and an old control isolate of S. Gallinarum within the vaccinated groups and the vaccinated and stressed groups. This finding also supports the lack of evidence on the emergence of new pathogenic S. Gallinarum strains with increased virulence. Instead, chickens can be more susceptible to fowl typhoid by stress induced by D. gallinae infestations, although they were appropriately vaccinated against the disease.
Concern about the impact of D. gallinae infestations has been growing in many countries, including Korea. Conventional cage housing systems for layer chicken flocks favor the proliferation and infestation of D. gallinae (Hamidi et al., 2011; Sigognault Flochlay et al., 2017). In addition, the climate is changing rapidly as a result of global warming, providing an environment more favorable for the proliferation of D. gallinae (Skuce et al., 2013). Restriction on the use of synthetic acaricides has led to the increased application of natural products, such as plant-derived extracts and essential oils (Maurer et al., 2009; Lee et al., 2019). A variety of other control strategies, including vaccination and biological and physical control measures, have also been described (Maurer et al., 2009; Sparagano et al., 2014). However, it is known that no single treatment method is sufficient for controlling D. gallinae. The use of integrated pest management, which is a combined process including the prevention, monitoring, and control of D. gallinae, and a high level of biosecurity are considered to be the best approach to solve the pest problem (Sparagano et al., 2014; Sylejmani et al., 2016). Such successful control and management measures for D. gallinae are necessary to prevent diseases including fowl typhoid on infested farms.
As mentioned above, the findings of the present study strongly support that the recurrent and persistent outbreaks of fowl typhoid can be mediated by D. gallinae in the infested farms. Nevertheless, a previous study showed that the vaccination of chicks prevented the recurrence of fowl typhoid although the D. gallinae infestation persisted (Pugliese et al., 2019). They suspected that the high level of antibody to S. Gallinarum in vaccinated chickens might have impaired the circulation of the pathogen and reduced its contamination level in D. gallinae (Pugliese et al., 2019). Indeed, there are some differences in the properties of live S. Gallinarum vaccines available worldwide. In Korea, the live vaccine strain, SG 9R, has been most commonly used to control fowl typhoid in commercial layer chickens, although the S. Gallinarum SR2-N6 vaccine strain has also been used (Lee et al., 2007; Kang et al., 2012b). All layer chicken farms surveyed in this study were also vaccinated with SG 9R. The 2 vaccine strains have altered lipopolysaccharide, resulting in semi-rough colony morphology and a low-level serological response (Silva et al., 1981; Cho et al., 2015). The low level of antibody in the chickens vaccinated with lipopolysaccharide-defective vaccine strains may have some limitations in preventing the circulation of S. Gallinarum in D. gallinae and the exposure of birds to the mite-harboring pathogen, although this needs further confirmation.
The persistent outbreaks of fowl typhoid, even in vaccinated chicken flocks, are a significant challenge and pose a substantial economic burden in the chicken industry. The present study indicated that the horizontal transmission and environmental persistence of S. Gallinarum in layer farms could be mediated by D. gallinae. This study also demonstrated that the stress induced by D. gallinae infestation might negatively affect the protective efficacy of S. Gallinarum vaccines. Overall the findings suggest that widespread infestations of D. gallinae in layer farms can cause persistent outbreaks of fowl typhoid, even in vaccinated birds. Effective monitoring and control measures for D. gallinae should be established to prevent and eliminate the contamination on farms, thus preventing fowl typhoid. In addition, specific studies are needed to confirm the antibody-mediated reduction of S. Gallinarum contamination in D. gallinae and estimate the protective efficacy of different types or combinations of S. Gallinarum vaccines in chicken flocks infested with D. gallinae.
Acknowledgments
The authors thank Su-Jeong Kim for technical assistance. This work was supported by grants funded by the Animal and Plant Quarantine Agency, Republic of Korea [grant numbers N-1549085-2017-36-01 and Z-1543084-2017-18-01].
Disclosures
The authors declare no conflicts of interest.
References
- Barrow P.A., Freitas Neto O.C. Pullorum disease and fowl typhoid--new thoughts on old diseases: a review. Avian Pathol. 2011;40:1–13. doi: 10.1080/03079457.2010.542575. [DOI] [PubMed] [Google Scholar]
- Barrow P.A., Lovell M.A. Invasion of Vero cells by Salmonella species. J. Med. Microbiol. 1989;28:59–67. doi: 10.1099/00222615-28-1-59. [DOI] [PubMed] [Google Scholar]
- Chadfield M.S., Brown D.J., Aabo S., Christensen J.P., Olsen J.E. Comparison of intestinal invasion and macrophage response of Salmonella Gallinarum and other host-adapted Salmonella enterica serovars in the avian host. Vet. Microbiol. 2003;92:49–64. doi: 10.1016/s0378-1135(02)00290-0. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Ahn Y.J., Kim T.E., Kim S.J., Huh W., Moon Y.S., Lee B.H., Kim J.H., Kwon H.J. Establishment of a live vaccine strain against fowl typhoid and paratyphoid. Korean J. Vet. Res. 2015;55:241–246. [Google Scholar]
- Ewing W.H. 4th ed. Elsevier Science Publishing Co. Inc.; New York, NY: 1986. Edwards and Ewing's Identification of Enterobacteriaceae. [Google Scholar]
- George D.R., Finn R.D., Graham K.M., Mul M.F., Maurer V., Moro C.V., Sparagano O.A. Should the poultry red mite Dermanyssus gallinae be of wider concern for veterinary and medical science? Parasit. Vectors. 2015;8:178. doi: 10.1186/s13071-015-0768-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont P.A.D., Weill F.-X. 9th ed. W. H. O. Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur; Paris, France: 2007. Antigenic Formulae of the Salmonella Serovars. [Google Scholar]
- Hamidi A., Sherifi K., Muji S., Behluli B., Latifi F., Robaj A., Postoli R., Hess C., Hess M., Sparagano O. Dermanyssus gallinae in layer farms in Kosovo: a high risk for Salmonella prevalence. Parasit. Vectors. 2011;4:136. doi: 10.1186/1756-3305-4-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Genovese K.J., Swaggerty C.L., Nisbet D.J., Kogut M.H. A comparative study on invasion, survival, modulation of oxidative burst, and nitric oxide responses of macrophages (HD11), and systemic infection in chickens by prevalent poultry Salmonella serovars. Foodborne Pathog. Dis. 2012;9:1104–1110. doi: 10.1089/fpd.2012.1233. [DOI] [PubMed] [Google Scholar]
- Huong C.T., Murano T., Uno Y., Usui T., Yamaguchi T. Molecular detection of avian pathogens in poultry red mite (Dermanyssus gallinae) collected in chicken farms. J. Vet. Med. Sci. 2014;76:1583–1587. doi: 10.1292/jvms.14-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A., Wigley P., Page K.L., Hulme S.D., Barrow P.A. Salmonella enterica serovar Gallinarum requires the Salmonella pathogenicity island 2 type III secretion system but not the Salmonella pathogenicity island 1 type III secretion system for virulence in chickens. Infect. Immun. 2001;69:5471–5476. doi: 10.1128/IAI.69.9.5471-5476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.S., Kwon Y.K., Kim H.R., Oh J.Y., Kim M.J., An B.K., Shin E.G., Kwon J.H., Park C.K. Comparative proteome and transcriptome analyses of wild-type and live vaccine strains of Salmonella enterica serovar Gallinarum. Vaccine. 2012;30:6368–6375. doi: 10.1016/j.vaccine.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Kang M.S., Kwon Y.K., Kim H.R., Oh J.Y., Kim M.J., An B.K., Shin E.G., Kwon J.H., Park C.K. Differential identification of Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum and the biovar Gallinarum live vaccine strain 9R. Vet. Microbiol. 2012;160:491–495. doi: 10.1016/j.vetmic.2012.05.041. [DOI] [PubMed] [Google Scholar]
- Kang M.S., Kwon Y.K., Oh J.Y., Call D.R., An B.K., Song E.A., Kim J.Y., Shin E.G., Kim M.J., Kwon J.H., Chung G.S. Multilocus variable-number tandem-repeat analysis for subtyping Salmonella enterica serovar Gallinarum. Avian Pathol. 2011;40:559–564. doi: 10.1080/03079457.2011.613915. [DOI] [PubMed] [Google Scholar]
- Kwon Y.K., Kim A., Kang M.S., Her M., Jung B.Y., Lee K.M., Jeong W., An B.K., Kwon J.H. Prevalence and characterization of Salmonella gallinarum in the chicken in Korea during 2000 to 2008. Poult. Sci. 2010;89:236–242. doi: 10.3382/ps.2009-00420. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim H.K., Kim G.H. Toxicity and effects of essential oils and their components on Dermanyssus gallinae (Acari: Dermanyssidae) Exp. Appl. Acarol. 2019;78:65–78. doi: 10.1007/s10493-019-00363-7. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Mo I.P., Kang M.S. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 2007;36:495–498. doi: 10.1080/03079450701691278. [DOI] [PubMed] [Google Scholar]
- Maurer V., Perler E., Heckendorn F. In vitro efficacies of oils, silicas and plant preparations against the poultry red mite Dermanyssus gallinae. Exp. Appl. Acarol. 2009;48:31–41. doi: 10.1007/s10493-009-9254-2. [DOI] [PubMed] [Google Scholar]
- Post J., Rebel J.M., ter Huurne A.A. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult. Sci. 2003;82:1313–1318. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- Pugliese N., Circella E., Marino M., De Virgilio C., Cocciolo G., Lozito P., Cafiero M.A., Camarda A. Circulation dynamics of Salmonella enterica subsp. enterica ser. Gallinarum biovar Gallinarum in a poultry farm infested by Dermanyssus gallinae. Med. Vet. Entomol. 2019;33:162–170. doi: 10.1111/mve.12333. [DOI] [PubMed] [Google Scholar]
- Ribot E.M., Fair M.A., Gautom R., Cameron D.N., Hunter S.B., Swaminathan B., Barrett T.J. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 2006;3:59–67. doi: 10.1089/fpd.2006.3.59. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Shivaprasad H.L., Barrow P.A. Pullorum disease and fowl typhoid. In: Swayne D.E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V., editors. Diseases of Poultry. John Wiley & Sons, Inc.; Ames, IA: 2013. pp. 678–693. [Google Scholar]
- Sigognault Flochlay A., Thomas E., Sparagano O. Poultry red mite (Dermanyssus gallinae) infestation: a broad impact parasitological disease that still remains a significant challenge for the egg-laying industry in Europe. Parasit. Vectors. 2017;10:357. doi: 10.1186/s13071-017-2292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E.N., Snoeyenbos G.H., Weinack O.M., Smyser C.F. Studies on the use of 9R strain of Salmonella gallinarum as a vaccine in chickens. Avian Dis. 1981;25:38–52. [PubMed] [Google Scholar]
- Skuce P.J., Morgan E.R., van Dijk J., Mitchell M. Animal health aspects of adaptation to climate change: beating the heat and parasites in a warming Europe. Animal. 2013;7(Suppl 2):333–345. doi: 10.1017/S175173111300075X. [DOI] [PubMed] [Google Scholar]
- Sleeckx N., Van Gorp S., Koopman R., Kempen I., Van Hoye K., De Baere K., Zoons J., De Herdt P. Production losses in laying hens during infestation with the poultry red mite Dermanyssus gallinae. Avian Pathol. 2019;48:S17–S21. doi: 10.1080/03079457.2019.1641179. [DOI] [PubMed] [Google Scholar]
- Sparagano O.A., George D.R., Harrington D.W., Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- Sylejmani D., Musliu A., Ramadani N., Sparagano O., Hamidi A. Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. 2016;60:454–459. doi: 10.1637/11327-111415-Reg. [DOI] [PubMed] [Google Scholar]
- Valiente Moro C., Chauve C., Zenner L. Experimental infection of Salmonella Enteritidis by the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2007;146:329–336. doi: 10.1016/j.vetpar.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Valiente Moro C., De Luna C.J., Tod A., Guy J.H., Sparagano O.A., Zenner L. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp. Appl. Acarol. 2009;48:93–104. doi: 10.1007/s10493-009-9248-0. [DOI] [PubMed] [Google Scholar]
- Zeman P., Stika V., Skalka B., Bartik M., Dusbabek F., Lavickova M. Potential role of Dermanyssus gallinae De Geer, 1778 in the circulation of the agent of pullurosis-typhus in hens. Folia Parasitol. (Praha) 1982;29:371–374. [PubMed] [Google Scholar]