Abstract
Recent reports showed a positive correlation between frozen–thawed rooster sperm DNA integrity and the concentrations of valine in seminal plasma. The present study evaluated the effect of supplementing valine to semen extender for freezing sperm of 2 endangered local Spanish chicken breeds with different sperm cryoresistance: Red Villafranquina (VF) showing low sperm DNA integrity after cryopreservation and Quail Castellana that shows higher DNA integrity. One pool of semen per breed was obtained twice a week for 10 wk (n = 40, 20 per breed). Each pool was divided into 2 fractions. One of these fractions was frozen in presence of valine as additive in the extender (concentration 10 mmol), whereas the other was used as control. The evaluation of the samples before and after freezing-thawing included motility (CASA-Mot system), viability (propidium iodide and SYBR-14), DNA integrity (terminal deoxynucleotidyl transferase dUTP nick end labeling), and fertility rate (percentage of eggs with blastoderm development after artificial insemination). Supplementation of valine increased several motility variables of fresh semen. In VF breed, valine increased percentage of progressive motile sperm (P = 0.025), curvilinear velocity (P = 0.033), straight-line velocity (P = 0.040), and average path velocity (P = 0.033), whereas progressive motile sperm (P = 0.019), curvilinear velocity (P = 0.006), straight-line velocity (P = 0.003) and average path velocity (P = 0.004) were improved in the Quail Castellana breed. Valine addition increased the DNA integrity of cryopreserved semen (decreased post-thaw DNA fragmentation) in both breeds, with a significant effect (P = 0.002) in VF (36.3% VF-control vs 31%VF-valine). As expected, Quail Castellana cryopreserved sperm control showed higher fertility rate (34.4% ± 12.1) than VF cryopreserved sperm control (16.1% + 6.2). Supplementing valine to the freezing extender doubled (P = 0.026) the fertility rate of VF (32.6% ± 12.2) compared with the control (16.1% + 6.2). In conclusion, supplementation of valine to chicken freezing extenders shows a positive effect on DNA fragmentation and fertilizing ability of frozen–thawed sperm, with a better response in a breed considered as the lowest freezer in our conservatory.
Key words: valine, sperm cryoresistance, DNA integrity
Introduction
Ex situ conservation of avian reproductive cells in gene cryobanks results in a strategic tool to secure the genetic diversity and give complementary support to in situ poultry conservation programs (Blesbois et al., 2007). Different freezing protocols have been developed for cryopreserving rooster semen; however, the variable and usually low fertility rates currently obtained by artificial insemination with frozen–thawed sperm remain the main obstacle for commercial application or genetic preservation (Long, 2006). Compared with other species, the physiology of avian spermatozoa makes them more susceptible to cell cryodamage, thus dramatically decreasing their fertilizing capacity. For instance, they possess a spindle form with very low cytoplasmic content and a very long flagellum making them highly susceptible to cryoinjury during freeze-thawing (Long, 2006; Blesbois, 2012; Thananurak et al., 2019). Along with this, the cold shock, intracellular ice formation, and production of reactive oxygen species (ROS) result in diverse sperm injuries compromising the fluidity and permeability of membranes, sperm metabolism, motility, DNA integrity, and fertilizing ability (Partyka et al., 2010; Santiago-Moreno et al., 2019; Thananurak et al., 2019). For instance, the mitochondrion is the main sperm metabolic site and the main producer of ROS; the large number of mitochondria present in avian midpiece (in rooster, approximately 30; Etches, 1998) makes poultry spermatozoa prone to oxidative and other metabolic stress. For example, lipid peroxidation of sperm membranes induced by ROS leads to loss of flexibility and production of by-products, such as the mutagenic and genotoxic molecule malondialdehyde, causing indirect damage to DNA (Wagner et al., 2018). DNA damage and lipid peroxidation in mitochondrial membranes may lead to DNA fragmentation by a cytochrome C–mediated pathway (apoptosis) (Ansari et al., 2019).
DNA integrity in sperms is essential for embryo development, hence cryopreservation procedures must ensure the intact conservation of sperm DNA (Pérez-Cerezales et al., 2009). Although chicken spermatozoa do possess protective antioxidant systems of cytoplasmic origin (Surai et al., 1998), the lack of cytoplasm decreases the capacity to counteract the oxidative stress. Contrary to this, seminal plasma contains large amount of antioxidants (Henkel, 2011). Moreover, addition of antioxidants such as amino acids and amino acid–derived glutathione to extenders has demonstrated to reduce damage to spermatozoa in different mammalian species such as goat, bull, or buffalo (Kundu et al., 2001; Tuncer et al., 2010; Topraggaleh et al., 2014). In birds, studies are limited, but amino acids such as glutamine (Khiabani et al., 2017) or glycine (Lorenz and Tyler, 1951) have also been successfully used. A previous study in our laboratory with 12 Spanish breeds of rooster (Santiago-Moreno et al., 2019) suggested that the seminal plasma amino acid profile of each breed could be relevant regarding sperm cryoresistance. Results of this study showed that concentration of certain amino acids, such as valine, had a negative correlation with DNA fragmentation and a positive correlation with sperm viability in frozen–thawed sperm samples. Valine is an essential aliphatic and hydrophobic amino acid that is involved in multiple biological processes such as protein synthesis, energy production, lipolysis, glucose transportation, embryo development, and so on. (Zhang et al., 2017). Besides this, valine may be metabolized/oxidized in mitochondria. (Hutson and Hall, 1993). Considering all these elements, in the present study, we selected valine to be tested as a possible additive in chicken semen freezing extenders. Two different Spanish chicken breeds with different sperm cryoresistance were selected: Red Villafranquina (VF) showing low sperm DNA integrity after cryopreservation and Quail Castellana (QC) that shows higher DNA integrity (Santiago-Moreno et al., 2019).
Material and methods
Experimental Birds
The birds used in this study were 40 Spanish chicken roosters from endangered local breeds (VF and QC) and 30 randomly chosen hens. To avoid the breed factor in the fertility test, a third breed of hens, Black-Barred Andaluza was included in the study together with VF and QC. The birds, all of which were 1 yr of age at the beginning of the experiment, were housed under natural photoperiod and temperature conditions in 2 12 m2 sand-floor pens with partial roof cover at the El Encín Research Station (Madrid, Spain; 40° 31′ N). These birds were raised as part of the INIA's genetic resources conservation program (Campo and Orozco, 1982; Campo, 1998). All birds were fed a commercial feed containing 16% CP, 2,700 kcal of ME/kg, 3.5% Ca, and 0.5% available P over the entire experimental period. Animals were handled as per procedures approved by the INIA Ethics Committee and were performed in accordance with the Spanish Policy for Animal Protection (RD53/2,013), which conforms to European Union Directive 86/609 regarding the protection of animals used in scientific experiments.
Experimental Design
A pool of seminal plasma for each breed was obtained twice a week from February to June 2018 (n = 40 roosters; 20 per breed). To investigate the role of valine on cryoresistance of rooster sperm, the pooled semen samples were divided into 2 aliquots. One aliquot was frozen using extender (Lake-Ravie 84) with presence of valine (concentration 10 mmol) and the other was frozen using the same extender without valine. Sperm variables were analyzed prior and after freezing-thawing. Preliminary studies in our laboratory on the effect of supplementing different concentrations of valine (1.5, 10, and 20 mmol) to the chicken extender pointed out 10 mmol (5 mmol when mixed with semen 1:1) as the optimal concentration for reducing post-thaw DNA fragmentation without observing a negative effect on the motility variables (unpublished results). Thus, this was the concentration used to supplement the sperm samples of QC and VF roosters.
The fertilization capacity of the sperm cells from QC (better freezer) and VF (bad freezer) was estimated in artificial insemination of 30 hens (see details in the following sections).
Semen Collection, Management, and Freezing
Semen was collected twice weekly over the study period, in 15-mL graduated centrifuge tubes (Sterilin) using the massage technique described by Burrows and Quinn (1936). Pools of semen for each breed were made on each occasion. Each pool was immediately divided into 2 equal aliquots. The volume of the aliquots was dependent on the total volume of the pool collected per breed and ranged between 0.225 mL and 1.725 mL. One of them was diluted 1:1 (v/v) using a Lake-Ravie medium (Lake and Ravie, 1984) composed of sodium glutamate (1.92 g), glucose (0.8 g), magnesium acetate 4H2O (0.08 g), potassium acetate (0.5 g), polyvinylpyrrolidone (Mr 10,000; 0.3 g), and 100 mL H2O (final pH 7.08, final osmolality 343 mOsm/kg; hereinafter referred to as Lake-Ravie-84). The other aliquot was diluted 1:1 (v/v) using the Lake-Ravie-84 (LR) supplemented with 10 mmol L-valine (LR-Val). The extender was held in the hand (about 37°C) previous addition to sperm, and dilution was performed at field temperature (15°C–30°C throughout the study period). The diluted, pooled semen samples were then immediately cooled to 5°C, transported to the laboratory, and sperm concentration and sperm variables (sperm motility variables, plasma membrane integrity) examined (within 45 min of collection). Afterward, each pool was diluted as required with LR or LR-Val media to a concentration of 1,200 × 106 sperm/mL. Glycerol was then added to the diluted samples, to leave a final 8% concentration, and equilibrated for 10 min at 5°C. After equilibration, the samples were loaded into 0.25-mL French straws and then frozen using a computer freezer – IceCube 1,810 unit (Minitüb; Tiefenbach, Germany) – with the following cooling rate: from 5°C to −35°C at 7°C/min and then from −35°C to −140°C at 60°C/min (Santiago-Moreno et al., 2011). The frozen straws were then plunged into and maintained in liquid nitrogen (at −196°C) until thawing. For thawing, the straws were warmed for 3 min in LR or LR-Val bath (both in continuous stirring) at 5°C.
Assessment of Sperm Variables
Sperm concentration and motility were assayed using a computer-aided sperm analyses system coupled to a phase contrast microscope (Nikon Eclipse model 50i; Nikon Instruments Europe B.V., Izasa S.A.; negative contrast) and using Sperm Class Analyzer (SCA; Barcelona, Spain) v.4.0. Software (Microptic S.L., Barcelona, Spain). For motility analysis, sperm samples were diluted to a concentration of approximately 40 million sperm/mL and loaded onto warmed (38°C) 20-μm Leja 8-chamber slides (Leja Products B.V., Nieuw-Vennep, The Netherlands). The percentage of motile spermatozoa and the percentage showing progressive motility were recorded. Sperm movement characteristics – curvilinear velocity (VCL), straight-line velocity, average path velocity (VAP), amplitude of lateral head displacement were also recorded. A minimum of 3 fields and 200 sperm tracks were evaluated at a magnification of 100x for each sample (image acquisition rate 25 frames/s).
Propidium iodide and SYBR-14 were used as fluorochromes in the examination of membrane integrity (Chalah and Brillard, 1998); 200 cells were examined using an epifluorescence microscope at 400 × (wavelength: 450–490 nm).
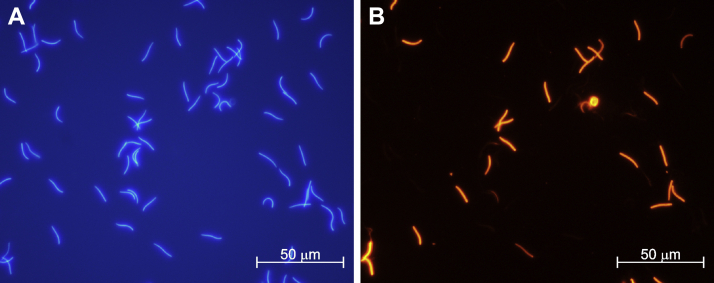
All sperm variables were measured again for each pool after their eventual thawing. In addition, DNA integrity was also assessed by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). For this, the kit “In Situ Cell Death Detection” (Roche, Basel, Switzerland) was used following manufacturer's instructions with minor changes to adapt the technique to the analyses of rooster sperm (Santiago-Moreno et al., 2019). Briefly, each sperm sample was diluted to 12 × 106 spermatozoa/mL in 4% formaldehyde. Subsequently, 10 μL of this dilution were placed on a glass slide and left to dry. Then, the spermatozoa were permeabilized with 0.1% of Triton X-100 in PBS. After a wash in PBS, fragmented DNA was nick end-labeled with tetramethylrhodamine-conjugated dUTP by adding 10 μL of the working solution provided by the kit – containing the substrates and the enzyme terminal transferase – on the sample. The reaction was conducted incubating the slides in a humid box for 1 h at 37°C. After a wash with PBS, the nucleus were counterstained with Hoechst at 0.1 mg/mL in PBS for 5 min in the dark. After an additional wash with PBS, the slides were mounted using Fluoromount (Sigma-Aldrich, MO) previous to observation under a fluorescent microscope (Eclipse E200; Nikon, Japan). Percentages of positive TUNEL spermatozoa (Figure 1) per sample were recorded by counting a minimum of 200 spermatozoa per microscopy preparation.
Figure 1.
DNA integrity assessment by TUNEL. DNA staining by Hoechst of all rooster sperm present in the microscope field (A) and the same field showing in red the sperm with DNA fragmentation, TUNEL (+) (B). Abbreviation: TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Cryoprotectant Removal and Artificial Insemination
Glycerol was removed before artificial insemination. Thawed samples were progressively diluted with Lake Centri medium at 5°C to a final dilution of 1:4 v/v via the following steps: 1:0.07, 1:0.18, 1:0.33, 1:0.6, 1:1.24, and 1:1.58 (v/v) (2-min intervals). These samples were then centrifuged at 600 × g for 10 min, the supernatant solution discarded, and the pellet resuspended (in the same volume as before centrifugation) in Lake 7.1. Briefly, the Lake Centri (method adapted from the study by Mocé et al., 2010) medium was composed of 1,000 mL H2O, 1.28 g potassium citrate tribasic monohydrate, 19.2 g sodium L-glutamate, 6.0 g D-fructose, 5.0 g TES, 5.1 g sodium acetate trihydrate, 0.8 g magnesium acetate tetrahydrate, and 5.2 mL of 1N sodium hydroxide (340–350 mOsm/kg, pH = 7.0–7.2). The Lake 7.1 medium (slightly modified from Lake and Ravie 1,979) was composed of 1,000 mL H2O, 0.8 g magnesium acetate tetrahydrate 4H2O, 1.28 g potassium citrate tribasic monohydrate, 15.2 g sodium L-glutamate, 6 g glucose, 30.5 g BES, and 58 mL of 1N sodium hydroxide (370 mOsm/kg, pH = 7.1).
Thirty randomly chosen hens were used for testing fertilizing ability of frozen–thawed semen of the VF and QC, that is 15 hens were inseminated with semen of good freezer and the remaining 15 with semen of the bad freezer. The AI of hens was performed with randomly chosen straws. Different frozen–thawed semen doses were used in each insemination session. The fertilization capacity was estimated from the percentage of fertilized eggs resulting from 6 consecutive intravaginal artificial inseminations (2 inseminations a week for 3 wk). All inseminations were performed between 15:00 h and 16:00 h. Artificial insemination procedures involved 300 million sperm/female at each insemination (250 μL). Eggs were collected from day 2 after the first insemination until 3 d after the last insemination. Fertility (% fertile/incubated eggs) was determined by blastoderm development after artificial insemination.
Statistical Analyses
Data are expressed as means ± SE. The sperm variables were not normally distributed as determined by Shapiro-Wilk test, regardless arcsin and log transformations. Thus, nonparametric analyses were used. Significant differences between the sperm variables of the control and the samples frozen with valine within each breed were determined by the Wilcoxon's test. Significant differences between breeds were determined by the Mann-Whitney U test. The association between fertility rates and the 2 kinds of diluent (LR or LR-Val) was assessed using the chi-square test. Correlations between TUNEL (+) and sperm motility ratios, between viability and motility ratios, and between TUNEL (+) and viability were determined by the Spearman test. All statistical calculations were made using TIBCO Statistica software v.13.3 (TIBCO Software Inc. Palo Alto, CA).
Results
Breed Differences in Fresh Semen
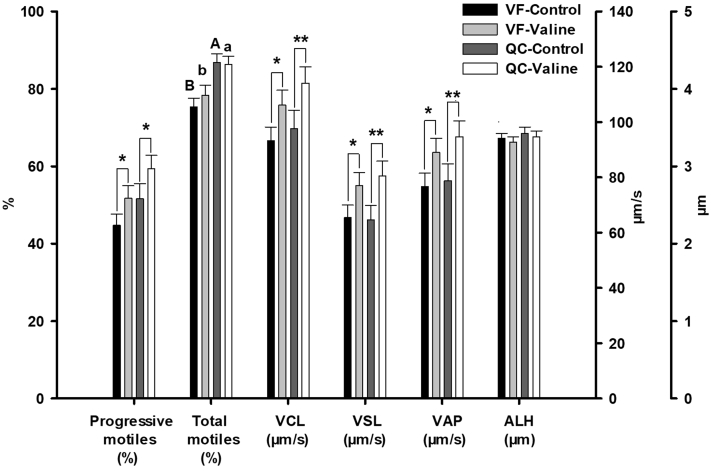
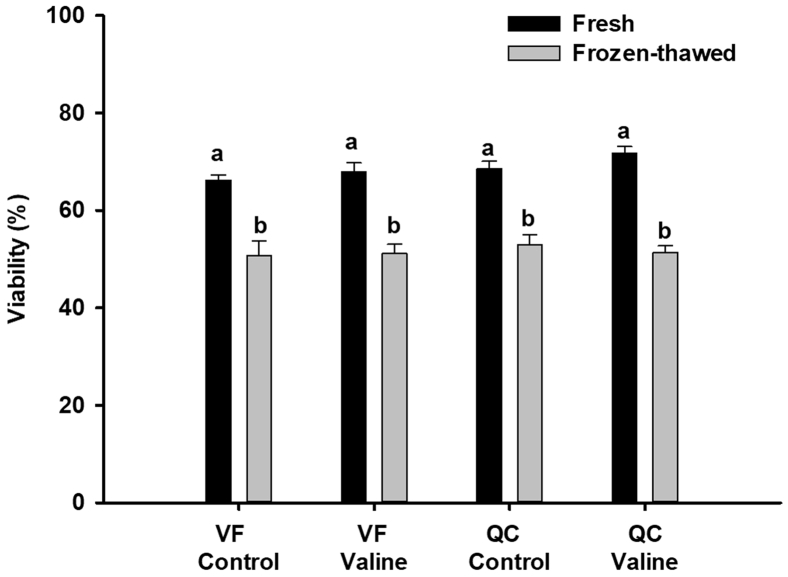
Comparison between fresh samples of VF control and QC control revealed significant effect of the breed on the percentage of total motile sperm (P < 0.001) (Figure 2), whereas no significant difference was found on viability (Figure 3).
Figure 2.
Motility sperm variables of fresh samples obtained from Red Villafranquina and Quail Castellana roosters, diluted in Lake Ravie 84 without (Control) or supplemented with 10 mmol valine. Different letters (a,b; A,B) indicate significant difference (P < 0.01; P < 0.001, respectively) between breeds. Appearance of ∗ or ∗∗ on means indicates significant difference (P < 0.05; P < 0.01, respectively) within each breed. n = 40 (20 samples per bread). Abbreviations: ALH amplitude of lateral head displacement; QC, Quail Castellana; VAP, average path velocity; VCL, curvilinear velocity; VF, Red Villafranquina; VSL, straight-line velocity.
Figure 3.
Viability values of fresh and frozen-thawed samples obtained from Red Villafranquina and Quail Castellana roosters, diluted with Lake Ravie 84 without (Control) or supplemented with 10 mmol valine. Different letters (a,b) indicate significant difference between treatments (control or valine) within the same breed (P < 0.001). n = 40 (20 samples per bread). Abbreviations: QC, Quail Castellana; VF, Red Villafranquina.
Breed Differences in Control Frozen–Thawed Semen
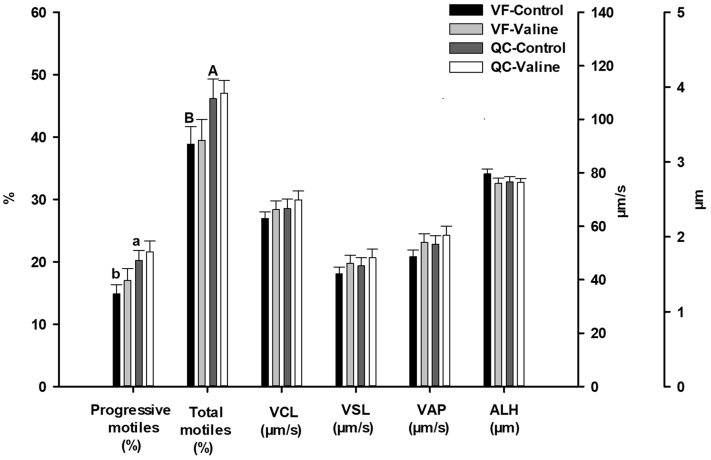
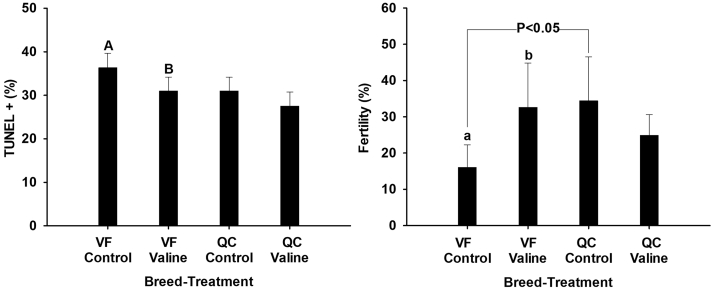
Concerning frozen–thawed semen, differences were found between VF control and QC control in percentage of progressive motile (P = 0.027) and total motile (P = 0.008) sperm (Figure 4), whereas no significant difference was found on viability (Figure 3). Concerning post-thawed DNA fragmentation (TUNEL +), differences between VF control and QC control were not significant, but the numerical differences (Figure 5) do suggest a higher susceptibility to DNA fragmentation in VF (36.3% ± 3.3) than in QC (30.9 ± 3.2).
Figure 4.
Motility sperm variables of frozen-thawed semen from Red Villafranquina and Quail Castellana roosters, frozen in Lake Ravie 84 without (Control) or with 10 mmol valine. Different letters (a,b; A,B) indicate significant differences between breeds (P < 0.05 or P < 0.01, respectively). n = 40 (20 samples per bread). Abbreviations: ALH amplitude of lateral head displacement; QC, Quail Castellana; VAP, average path velocity; VCL, curvilinear velocity; VF, Red Villafranquina; VSL, straight-line velocity.
Figure 5.
TUNEL (+) and fertility rates obtained from thawed samples of Red Villafranquina and Quail Castellana roosters, frozen without (Control) or with valine 10 mmol. Different letters (a,b; A,B) indicate significant differences between treatments (control or valine) within the same breed (P < 0.05; P < 0.01, respectively). Line over bars indicates significant differences between breeds. n = 40 (20 samples per bird). Abbreviations: TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VF, Red Villafranquina; QC, Quail Castellana.
A number of correlations were found between the viability values and some of the motility rates of both breeds. Regarding VF control, viability showed positive correlation with progressive motility (P < 0.01) and total motility (P < 0.001) (Table 1). About QC control, 1 correlation was found between viability and progressive motile sperm (P < 0.05) (Table 1)
Table 1.
Spearman correlations within each breed between different sperm assessment variables in frozen–thawed samples of Quail Castellana and Red Villafranquina.
| Sperm variables | TUNEL (+) (%) | Progressive motile sperm (%) | Total motile sperm (%) | VCL (μm/s) | VAP (μm/s) |
|---|---|---|---|---|---|
| VF control | |||||
| Viability | - | 0.64∗∗ | 0.73∗∗∗ | - | - |
| QC control | |||||
| Viability | - | 0.54∗ | - | - | - |
| VF valine | |||||
| Viability | −0.64∗∗ | - | - | - | - |
| TUNEL (+) | - | −0.58∗∗ | −0.57∗∗ | −0.49∗ | −0.50∗ |
∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Abbreviations: QC, Quail Castellana; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; VAP, average path velocity; VCL, curvilinear velocity; VF, Red Villafranquina.
With regard to fertility, comparison between the thawed samples of VF control and QC control revealed an effect of the breed (P = 0.037) on the post-thawed fertilizing capacity of the spermatozoa. QC control showed 2-fold higher fertility rate (34.4% ± 12.1) than VF control (16.1% ± 6.2) (Figure 5).
Valine Effect on Motility and Viability of Fresh Semen
The addition of valine to the freezing extender improved several motility variables of fresh semen of both breeds (Figure 2). With respect to VF, the variables improved were progressive motile sperm (P = 0.025), VCL (P = 0.033), straight-line velocity (P = 0.040), and VAP (P = 0.033). In QC roosters, the variables improved were progressive motile sperm (P = 0.019), VCL (P = 0.006), straight-line velocity (P = 0.003), and VAP (P = 0.004) (Figure 2). The addition of valine did not improve the sperm viability values in any of the breeds (Figure 3).
Valine Effect on Motility and Viability of Frozen–Thawed Semen
Regarding frozen–thawed semen the supplementation of valine did not improve any motility variable of any of the breeds (Figure 4) or did it for viability (Figure 3).
Valine Effect on DNA Fragmentation of Frozen–Thawed Semen
The post-thawed results of VF sperm supplemented with valine (VF-Val) showed 14.8% lower DNA fragmentation rate than VF control (31% vs. 36.3%, P = 0.002) (Figure 5).
Negative correlations were found in VF-Val between TUNEL (+) and progressive motile sperm (P < 0.01), total motile sperm (P < 0.01), VCL (P < 0.05), and VAP (P < 0.05) (Table 1). Moreover VF frozen–thawed sperm, but not QC, showed a negative correlation between viability and DNA fragmentation (TUNEL +) (P < 0.01) (Table 1).
Valine Effect on Fertilizing Ability of Frozen–Thawed Semen
Supplementing valine to the freezing extender doubled (P = 0.026) the fertility rate of frozen–thawed semen of VF (32.6% ± 12.2) compared with the control (16.1% + 6.2). In contrast, in QC, the difference between the fertility in QC control and QC valine was not significant, whereas the numerical results would suggest a decrease in the fertility rate by almost a third (24.8% ± 5.7) (Figure 5).
Discussion
A previous work of our group with 12 different breeds of rooster (Santiago-Moreno et al., 2019) demonstrated that concentration of endogenous valine in rooster seminal plasma was negatively correlated with post-thawed DNA fragmentation. In the present study, we demonstrated that supplementing valine to a chicken freezing extender can, depending on the breed, improve motility variables in fresh sperm as well as increase DNA cryoresistance and fertilization capacity of post-thawed spermatozoa, mainly in the lowest fertility breed.
Comparison between VF control (bad freezer) and QC control (better freezer) revealed superiority of QC in fresh and frozen–thawed sperm regarding different semen parameters including, total motility ratios, DNA integrity, and finally fertility. Addition of valine to semen extender to dilute fresh samples of VF and QC improved several motility values of both breeds. This is an important issue considering that once the chicken sperm has been inseminated in the vagina, only 1% of the initial sperm population reaches the sperm storage tubules from where only 1% reaches the fertilization site in the infundibulum (Blesbois, 2018). Thus, high motility sperm is essential to obtain high fertility rates (Blesbois, 2018). The beneficial effect of valine on motility was previously reported in a study about the composition of free amino acids in sperm of several fish species, where motility of perch semen was positively influenced by the presence of valine (Lahnsteiner, 2010). Regarding human, the exploration on seminal plasma amino acid disorder in patients with asthenozoospermia revealed significantly lower values of this amino acid than in healthy controls, pointing out valine as a potential biomarker closely related to the clinical parameters of this pathology (Li et al., 2019). The mechanism by which valine exerts a beneficial effect on sperm motility is still unclear (Lahnsteiner, 2010; Li et al., 2019); however, it has been observed that there is a direct effect of dietary supplemented valine on the antioxidant enzymes superoxide dismutase and glutathione peroxidase of hypercholesterolemic rats (Cojocaru et al., 2014).
Although valine significantly increased several motility values in fresh rooster samples of both breeds, the corresponding differences were not significant in frozen–thawed samples of either breed. However, a significant beneficial effect of valine was observed regarding DNA cryoresistance and fertility rate of VF. Supplementation of valine significantly decreased the post-thawed DNA fragmentation rate and doubled the fertility rate of VF-Val, a rate comparable with that of QC control. This achievement is of special importance considering that fertility rate of VF control was significantly lower than QC control. It is noteworthy that, despite the significantly higher post-thawed motility values of QC-Val than those of VF-Val, this superiority was not reflected in the fertility rate in the present experiment. As discussed afterward, the impact of modifying the endogenous valine concentration may be reflected in the sperm oxidative status, affecting another sperm functions such as the acrosome reaction or fertilizing capacity. Therefore, further study is required to confirm the effect of valine on VF and QC fertility rate, considering assessment of features as acrosome integrity or ROS generation.
Because mitochondria play a role in both apoptotic DNA fragmentation and in oxidative energy metabolism for motility, the improved DNA cryoresistance observed in VF could also suggest an increase in post-thawed mitochondrial integrity. At this respect, Bollwein et al. (2008) communicated the existence of some interdependency between the DNA fragmentation index and the loss of mitochondrial function when frozen bovine sperm was thawed and maintained for 3 h at 38°C, a relationship that is expected to have an impact on motility parameters. A study on human sperm communicated a negative correlation between DNA fragmentation and progressive motility (Marchetti, 2002). It is not surprising then that TUNEL (+) values of VF would be negatively correlated with some post-thawed motility variables.
Reactive oxygen species can exert a negative effect on sperm DNA, inducing the production of oxidative products as the 8-oxo-7,8-dihydroxyguanosine that causes DNA fragmentation and mutagenic modifications (Ménézo et al., 2007). This can result in accumulation of the protein p53, inducing mitochondria to release cytochrome C and start the apoptosis program. Knowing that redox status can either trigger or inhibit cytochrome C–induced apoptosis (Hampton and Orrenius, 1998), the decrease in the DNA fragmentation rate of VF-Val, possibly indicating lower fraction of apoptotic sperm cells, could suggest the involvement of valine in maintaining the optimal oxidative status of spermatozoa and mitochondria. At this respect, it is noteworthy that different species/breed/individual oxidative status might shift the kind of response to valine supplementation going from a positive effect on one species/breed/individual to a less positive or absence of effect on another. This could explain the different response of valine supplementation on DNA fragmentation and fertility rate of VF and QC. Whether or not that is the case, the negative correlations observed between DNA fragmentation and several motility ratios in VF-Val would further corroborate that valine, in some way, assists in maintaining mitochondrial integrity.
Together with leucine and isoleucine, valine forms part of the 3 aliphatic branched-chain amino acids. Branched-chain amino acids are some of the most hydrophobic amino acids which make them important in determining the structure of globular proteins as well as in the interaction of the transmembrane domains of membranous proteins with phospholipid bilayers (Brosnan and Brosnan, 2006). It is known that human catabolism of branched-chain amino acids starts with their transamination to branched-chain α-ketoacids. At the same time, the α–branched-chain ketoacid (α-ketoisovalerate, for the case of valine) (Adeva-Andany et al., 2017) is converted into its respective amino acid. α-ketoacids have demonstrated to be implicated in decreasing oxidative stress thanks to their capacity to reduce H2O2 to H2O (Holleman, 1904). Thus, valine could potentially act as an antioxidant agent decreasing or avoiding the production of harmful ROS involved directly or indirectly in low motility, DNA fragmentation, and low fertility. The different effect of valine on the fertility rate of VF and QC could be explained by different needs to strengthen their antioxidant response capacity. While in VF, supplementation of valine could be necessary to reach enough antioxidant capacity to decrease ROS damages involved in DNA fragmentation, in QC, it could result irrelevant or even produce an harmful effect, decreasing the controlled and localized ROS that, as observed in mammals, are needed for capacitation, hyperactivation, acrosome reaction, and fertilizing capacity (Aitken, 1995; O'Flaherty et al., 2006; Wagner et al., 2018). Other possible mechanisms suggested to date by which supplementing amino acids to extenders can exert a protective effect are the existence of some unique targets on the plasma membrane that are protected by selected amino acids (Kruuv and Glofcheski, 1992) or intracellular protection against denaturing effects of hyperosmolality in the unfrozen fraction and within the cells during slow freezing (Withers and King, 1979). Thus valine supplementation can display, depending on the breed, a positive impact on sperm motility, DNA integrity, and fertility rate, thanks to its potential role as an antioxidant agent or a protector agent.
In conclusion, the results of this study show that supplementation with valine to chicken extenders can have a positive effect on DNA fragmentation and fertility rates of frozen–thawed sperm, improving the current cryopreservation protocols used for endangered Spanish native breeds. However, as seen in this study, this effect can be breed-specific: valine had a positive effect on VF sperm, reducing DNA fragmentation and doubling the fertility rate (from 16.1 to 32.6%), whereas for the case of QC, it did not affect DNA cryoresistance or fertility rate. This fact could be attributable to breed differences in the seminal free amino acid profile or other seminal components, which may also affect the antioxidant capacity. Thus, supplementation of valine to chicken extender is recommended in these native breeds with low fertility rates after AI using frozen–thawed sperm.
Disclosures
The authors declare no conflicts of interest. 2015-00002-00-00.
Acknowledgments
This paper is dedicated to the memory of our dear co-worker, Dr. Serafín Pérez Cerezales, who recently passed-away. Serafín took part in the DNA analyses, and in the critical revision of the original manuscript. This research is part of a project that received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement Nº 677353 IMAGE. Part of this research was funded by Zoitechlab S.L.-INIA contract CON 18-141, and INIA project RZP2015-0002-00-00.
References
- Adeva-Andany M.M., López-Maside L., Donapetry-García C., Fernández-Fernández C., Sixto-Leal C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids. 2017;49:1005–1028. doi: 10.1007/s00726-017-2412-7. [DOI] [PubMed] [Google Scholar]
- Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- Ansari M.S., Rakha B.A., Akhter S., Blesbois E., Santiago-Moreno J. Effect of cryopreservation on lipid peroxidation, antioxidant potential, chromatin integrity, and mitochondrial activity of Indian Red Jungle fowl ( Gallus gallus murghi ) semen. Biopreserv Biobank. 2019;17:288–295. doi: 10.1089/bio.2018.0111. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Seigneurin F., Grasseau I., Limouzin C., Besnard J., Gourichon D., Coquerelle G., Rault P., Tixier-Boichard M. Semen cryopreservation for ex situ management of genetic diversity in chicken: creation of the French avian cryobank. Poult. Sci. 2007;86:555–564. doi: 10.1093/ps/86.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesbois E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 2012;49:141–149. [Google Scholar]
- Blesbois E. Encyclopedia of Reproduction. Elsevier.Science BV; Amsterdam, The Netherlands: 2018. Bird reproduction overview; pp. 579–585. [Google Scholar]
- Bollwein H., Fuchs I., Koess C. Interrelationship between plasma membrane integrity, mitochondrial membrane potential and dna fragmentation in cryopreserved bovine spermatozoa. Reprod. Domest. Anim. 2008;43:189–195. doi: 10.1111/j.1439-0531.2007.00876.x. [DOI] [PubMed] [Google Scholar]
- Brosnan J.T., Brosnan M.E. Branched-chain amino acids : enzyme and substrate regulation. J. Nutr. 2006;136:207S–211S. doi: 10.1093/jn/136.1.207S. [DOI] [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1936;16:19–24. [Google Scholar]
- Campo J.L. Proceedings of the 6th World Congress on Genetic Applied to Livestock Production. 1998. Conservation and genetical study of Spanish chicken breeds; pp. 155–158. University of New England, Armidale. [Google Scholar]
- Campo J.L., Orozco F. Proceedings of the Second World Congress on Genetics Applied to Livestock Production. 1982. Conservation and genetical study of Spanish chicken breeds; pp. 88–93. Editorial Garsi, Madrid. [Google Scholar]
- Chalah T., Brillard J.P. Comparison of assessment of fowl sperm viability by eosin-nigrosin and dual fluorescence (SYBR-14/PI) Theriogenology. 1998;50:487–493. doi: 10.1016/s0093-691x(98)00155-1. [DOI] [PubMed] [Google Scholar]
- Cojocaru E., Filip N., Ungureanu C., Filip C., Danciu M. Effects of valine and leucine on some antioxidant enzymes in hypercholesterolemic rats. Health. 2014;06:2313–2321. [Google Scholar]
- Etches R.J. Acribia; Zaragoza: 1998. Reproducción Aviar. Page 236 in. [Google Scholar]
- Hampton M.B., Orrenius S. Redox Regulation of apoptotic cell death in the immune system. Toxicol. Lett. 1998;102–103:355–358. doi: 10.1016/s0378-4274(98)00333-6. [DOI] [PubMed] [Google Scholar]
- Henkel R.R. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J. Androl. 2011;13:43–52. doi: 10.1038/aja.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleman M.A.F. Notice Sur l’action de l’eau Oxygénée Sur Les Acides Alpha-Cétoniques at Sur Les Dicétones 1.2. Recl. Trav. Chim. 1904;23:169–171. [Google Scholar]
- Hutson S.M., Hall T.R. Identification of the mitochondrial branched chain aminotransferase as a branched chain α-keto acid transport protein. J. Biol.Chem. 1993;268:3084–3091. [PubMed] [Google Scholar]
- Khiabani A.B., Moghaddam G., Kia H.D. Effects of adding different levels of glutamine to modified beltsville extender on the survival of frozen rooster semen. Anim. Reprod. Sci. 2017;184:172–177. doi: 10.1016/j.anireprosci.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Kruuv J., Glofcheski D.J. Protective effects of amino acids against freeze-thaw damage in mammalian cells. Cryobiology. 1992;29:291–295. doi: 10.1016/0011-2240(92)90028-z. [DOI] [PubMed] [Google Scholar]
- Kundu C.N., Das K., Majumder G.C. Effect of amino acids on goat cauda epididymal sperm cryopreservation using a chemically defined model system. Cryobiology. 2001;42:21–27. doi: 10.1006/cryo.2001.2296. [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F. A Comparative study on the composition and importance of free amino acids in semen of gilthead sea bream, sparus aurata, and perch, perca fluviatilis. Fish Physiol. Biochem. 2010;36:1297–1305. doi: 10.1007/s10695-010-9442-3. [DOI] [PubMed] [Google Scholar]
- Lake P.E., Ravie O. Effect of age and sex on the chemical composition of edible offal and blood from broilers. Br. Poult. Sci. 1984;25:145–150. doi: 10.1080/13632758408454852. [DOI] [PubMed] [Google Scholar]
- Li M.J., Zhang Z.M., Fan F., Ma P., Wang Y., Lu H.M. Exploring asthenozoospermia seminal plasma amino acid disorder based on GC-SIM-MS combined with chemometrics methods. Anal. Methods. 2019;11:2895–2902. [Google Scholar]
- Long J.A. Avian semen cryopreservation: what are the biological challenges? Poult. Sci. 2006;85:232–236. doi: 10.1093/ps/85.2.232. [DOI] [PubMed] [Google Scholar]
- Lorenz F.W., Tyler A. Extension of motile life span of spermatozoa on the domestic fowl by amino acids and proteins. Proc. Soc. Exp. Biol. Med. 1951;78:57–62. doi: 10.3181/00379727-78-18973. [DOI] [PubMed] [Google Scholar]
- Marchetti C. G Obert, Deffosez A., Formstecher P., Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum. Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- Ménézo Y.J.R., Hazout A., Panteix G., Robert F., Rollet J., Cohen-Bacrie P., Chapuis F., Clément P., Benkhalifa M. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod. Biomed. Online. 2007;14:418–421. doi: 10.1016/s1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- Mocé E., Grasseau I., Blesbois E. Cryoprotectant and freezing-process alter the ability of chicken sperm to acrosome react. Anim. Reprod. Sci. 2010;122:359–366. doi: 10.1016/j.anireprosci.2010.10.010. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C., de Lamirande E., Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006;41:528–540. doi: 10.1016/j.freeradbiomed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Partyka A., Nizański W., Łukaszewicz E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology. 2010;74:1019–1027. doi: 10.1016/j.theriogenology.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Pérez-Cerezales S., Martínez-Páramo S., Cabrita E., Martínez-Pastor F., de Paz P., Herráez M.P. Evaluation of oxidative dna damage promoted by storage in sperm from sex-reversed rainbow trout. Theriogenology. 2009;71:605–613. doi: 10.1016/j.theriogenology.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Santiago-Moreno J., Bernal B., Pérez-Cerezales S., Castaño C., Toledano-Diaz A., Esteso M.C., Gutiérrez-Adán A., López-Sebastián A., Gil M.G., Woelders H., Blesbois E. Seminal plasma amino acid profile in different breeds of chicken: role of seminal plasma on sperm cryoresistance. PLoS One. 2019;14:1–19. doi: 10.1371/journal.pone.0209910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Moreno J., Castaño C., Toledano-Díaz A., Coloma M.A., López-Sebastián A., Prieto M.T., Campo J.L. Semen cryopreservation for the creation of a Spanish poultry breeds cryobank: Optimization of freezing rate and equilibration time. Poult. Sci. 2011;90:2047–2053. doi: 10.3382/ps.2011-01355. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Cerolini S., Wishart G.J., Speake B.K., Noble R.C., Sparks N.H.C. Lipid and antioxidant composition of chicken semen and its susceptibility to peroxidation. Avian Pout. Biol. Rev. 1998;9:11–23. [Google Scholar]
- Thananurak P., Chuaychu-noo N., Thélie A., Phasuk Y., Vongpralub T., Blesbois E. Sucrose Increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019;98:4161–4171. doi: 10.3382/ps/pez196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topraggaleh T.R., Shahverdi A., Rastegarnia A., Ebrahimi B., Shafiepour V., Sharbatoghli M., Esmaeili V., Janzamin E. Effect of cysteine and glutamine added to extender on post-thaw sperm functional parameters of buffalo bull. Andrologia. 2014;46:777–783. doi: 10.1111/and.12148. [DOI] [PubMed] [Google Scholar]
- Tuncer P.B., Bucak M.N., Büyükleblebici S., Sariözkan S., Yeni D., Eken A., Akalin P.P., Kinet H., Avdatek F., Fidan A.F., Gündogăn M. The effect of cysteine and glutathione on sperm and oxidative stress parameters of post-thawed bull semen. Cryobiology. 2010;61:303–307. doi: 10.1016/j.cryobiol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Wagner H., Cheng J.W., Ko E.Y. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J. Urol. 2018;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers L.A., King P.J. Proline: a novel cryoprotectant for the freeze preservation of cultured cells of Zea mays L. Plant Physiol. 1979;64:675–678. doi: 10.1104/pp.64.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Zeng X., Ren M., Mao X., Qiao S. Novel metabolic and physiological functions of branched chain amino acids: a review. J. Anim. Sci. Biotechno. 2017;8:4–15. doi: 10.1186/s40104-016-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]