Abstract
Increased demand in consumer choice has resulted in a wide variety of egg selection available in the retail market. Specialty and designer chicken eggs represent a portion of the table egg market that is increasing in size. Egg quality is known to be of great importance in all eggs as it relates to food safety, consumer preferences, and product value. In this study, egg quality characteristics were analyzed using a one-way ANOVA to evaluate 2 commercially available conventional egg brands (A and B) and 4 commercially available designer egg brands (C–F). Three hundred nine eggs were evaluated for shell and content weight, dimensional measurements, and breakage force. Calculations were completed to determine %yolk and albumen, yolk index, and Haugh units (HU), followed by an accelerated lipid oxidation study. No significant variation exists in breakage force. Brands A–E meet AA grade standard at a score of 72 HU or above, while brand F, a pasture-raised brand, meets the A grade standard, falling between 60 and 71 HU. Brand F has the highest yolk fan color value (10.41 ± 0.193, P < 0.001) and the lowest yolk index (0.523 ± 0.013, P < 0.05). In addition, brand F has the lowest albumen height (P < 0.001). As albumen height is an indication of freshness, and as all eggs were of equivalent age, it is possible that brand F exhibits overall lower quality than other brands. The conventionally raised white eggs of A experienced the greatest increase in % free fatty acids, which would likely result in off-flavors from hydrolytic rancidity. The organic cage-free D eggs have a significantly greater peroxide value (17.3 ± 2.9, P < 0.001), relative to all other brands, and is over the 10 mEg/kg threshold, which would be considered an unsuitable product for consumption. Ultimately, the measures of egg quality used in this study are essential for evaluating the delivery of the specialty market to the consumer and may indicate that improved measures of quality are needed to truly differentiate between the different egg types and their quality.
Key words: designer eggs, free fatty acids, oxidation
Introduction
Specialty raised and designer eggs meet production characteristics which include cage-free, enriched housing, certified organic, vegetarian or soy-free diets, or value-added nutrients to the hen's diet which results in a nutritionally altered egg (American Egg Board; Shallo, 2001). Conventional egg production serves the majority of US egg production, and capitol production costs within a conventional system are more than half that of eggs produced in enriched or aviary environments (Matthews and Sumner, 2015; USDA, 2018). Studies over the last decade have indicated consumers may prefer eggs raised beyond conventional practices (Heng et al., 2013; Lusk, 2019); however, consumers may not be willing to pay a premium for designer and specialty eggs (Chang et al., 2010). Despite the challenge an increase in price brings when consumers are forced to make purchasing decisions at a retailer, consumer preference for perceived increased welfare legitimizes specialty and designer production practices, and as a result, an increasing number of retailers are mandating production or dietary standards beyond national standards. As of February 2017, 217 food-providing companies have committed to cage-free eggs. Not only has there been an increase in organic eggs but also there has been an increase of about 5.7 million cage-free layers over the last 5 y (USDA, 2018). This exemplifies how the demand for designer eggs continues to increase with time. However, an increase of about 200 million cage-free hens would be necessary to satisfy these demands by 2026 (USDA, 2018).
Eggs of high quality are needed to sustain niche markets for specialty and designer eggs–seeking consumers. Egg quality defines those characteristics of an egg that affect consumer acceptability and preference. The parameters for egg grading include shell cleanliness, strength, texture, and shape; the relative viscosity of the albumen; and the shape and firmness of the yolk. While the consumer is concerned about broken or damaged eggshell during purchase, more importantly is the interior egg quality, which begins to wane after egg laying. Efficient egg gathering, cooling, and refrigeration at appropriate humidity can maintain this feature. As eggs age in cold storage, the water gradient and pH of the yolk and albumen continue to change (Heath, 1977). These changes result in an increase in free fatty acids and other lipid oxidation products that will then cause changes in yolk flavor and may also cause off-odors (Wang et al., 2017).
It has been shown that the bird's diet plays a crucial role in how quickly yolk lipids oxidize, as hens fed diet's with greater concentration (>5%) of omega-3 fatty acids tend to experience malodors and off-flavors more quickly, relative to hens fed a conventional corn- or soy-based diet (Aro et al., 2011). Previously published data on the effects of omega-3 enrichment on egg quality during storage report that increase in egg omega-3 does not decrease shell eggs' oxidative stability during storage (Marshall et al., 1994). However, this study only incorporated 1.5% menhaden oil and then measured thiobarbituric acid values, which only quantifies secondary lipid oxidation products, and is not an indicator of free fatty acid concentration or peroxide value (PV), which are primary oxidation products. Further studies of omega-3 eggs and other designer eggs with reference to shelf-stability and functionality are needed.
To evaluate quality, several parameters are used to determine the consistency in egg size and content, as well as contribute to grading of the egg. Furthermore, the structural integrity of an egg's shell and the yolk membrane integrity are directly related to food safety and possible economic loss. The yolk membrane integrity also influences the usability of the yolk and albumen in further processing (Kirunda and McKee, 2000). This is important in the egg breaker industry, as even minimal yolk contamination will decrease albumen foaming volume by 70% (John and Flor, 1931). Through using these quality parameters, this study aims to establish a better understanding of egg quality in commercially available designer egg brands, relative to conventional eggs.
Materials and methods
Five brands of brown eggs and one brand of white, all eggs labeled as A grading, were evaluated. Conventional, specialty, and designer designations are displayed in Table 1. A total of 309 eggs of the same age were evaluated from 2 sampling periods spanning 7 mo. Effort was taken to ensure egg-collection dates were consistent among brands. The sell by date on egg packaging in the United States does not exceed 30 d after the day the eggs were packed (USDA). To ensure consistency, all analyses were conducted on the 15th d before the sell by date printed on the packaging. Eggs were stored at 10°C refrigeration and used for analysis immediately after removal from refrigeration.
Table 1.
Brand-specific labeling parameters and cost per dozen.
| Brand | N | Color | Conv. raised | Certified organic | Enriched diet | Cage free | Free range | Pasture raised | Soy free | Cost/dozen1 |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 36 | White | x | $ | ||||||
| B | 26 | Brown | x | $ | ||||||
| C | 46 | Brown | x | x | $$$ | |||||
| D | 104 | Brown | x | x | x | $$ | ||||
| E | 82 | Brown | x | x | x | x | $$$ | |||
| F | 12 | Brown | x | x | $$$$ |
The lowest cost brand was given a $ designation. For every one US dollar cost over the lowest carton cost, an additional $ was assigned (i.e., a carton designated as $$$ costs 2 US dollars more than a carton designated as $).
Egg content weights and dimensions including total weight (shell, yolk, and albumen), yolk weight, albumen weight, yolk height, yolk diameter, and albumen height were evaluated. Total, shell, yolk, and albumen weights were determined for each egg using a digital scale (Mettler-Toledo, Columbus, Ohio). Traditional quality calculations were completed to determine percent yolk (%Y) and albumen (%A), calculated as (yolk weight)/(total weight) and (albumen weight)/(total weight), respectively. The yolk index (YI) was determined by measuring the width and height of the yolk with dial calipers. The measurements were taken with the yolk in the natural position when the egg was broken out: YI was calculated as (yolk height)/(yolk width). Hasugh units (HU) were calculated after albumen height was measured using a mounted digital Vernier Caliper (Marathon Watch Company Ltd., Richmond Hill, ON, Canada). HU was calculated as 100∗log((albumen height)-1.7((total weight)0.37+7.6)). Yolk color (YC) intensity was evaluated by visual comparison to a color yolk fan. A TA-XT Plus texture analyzer (Stable Micro Systems, Surrey, UK) was used to determine shell compression strength with a 45-mm probe, and yolk cohesiveness and stickiness were calculated using a TA-51 probe. Texture analyzer settings were as follows: pretest and posttest speed = 5 mm/s, and test speed = 10 mm/s. Shell breakage trigger force was set at 49 mN, and yolk trigger force testing was set at 9 mN. All tests were completed immediately after egg removal from 4°C. As this data collection was carried out for the purpose of evaluating commercial eggs available in the food retail setting, yolk cohesiveness and stickiness were determined to provide better sensory attribute descriptors to the act of at-home egg use and consumption. Cohesiveness is a term that is used specifically to describe semi-solid oral texture and is defined as the amount of sample that deforms without shearing or cutting (Meilgaard et al., 2016). Stickiness is a term that is used to describe the breakdown or manipulation of the food orally and is defined as the amount of mass that adheres to oral surfaces (Meilgaard et al., 2016).
An accelerated lipid oxidation study was performed on egg yolks to determine the amount of free fatty acids and peroxides present in eggs day 1, and again 7 d later, at an incubation of 29°C following the study by Latimer (2012). Lipids were extracted from triplicate pooled samples for each of the egg brands following the study by Shinn and Proctor (2013). For lipid extraction, 2 yolks were homogenized in a single 50-mL centrifuge tube for a total of 3 tubes per bsrand. Two observations were made per tube and averaged.
The percent of free fatty acids and PVs were validated according to the Association of Official Analytical Chemists methodology (Latimer, 2012). Free fatty acid analysis is a measurement of fat acidity that reflects the amount of fatty acids hydrolyzed from triacylglycerol and may indicate improper storage of eggs. PV measures the transient products of lipid oxidation in the yolks. A low PV value may indicate the beginning of lipid oxidation, where peroxides are similar to products of oxidation being formed, or advanced oxidation, where peroxide have broken down into secondary oxidation products. Measuring PVs over time can help distinguish between these (Pike and O'Keefe, 2017).
All quality characteristics were analyzed using a one-way ANOVA and Tukey's honestly significant difference in JMP v.13.0.0 (Cary, NC). Significance was determined at P ≤ 0.05.
Results and discussion
Brand D cage-free, organic eggs had the largest average egg at 62.4 ± 0.323 g and were significantly larger than the enriched cage-free brand C eggs at 59.6 ± 0.344 g (Table 2). The largest yolk weight was observed in brand E, a free-range, organic, soy-free egg, at 21.4 ± 1.49 g. Brand E also had the smallest albumen weight at 30.3 ± 1.40 g.
Table 2.
Total egg weight including shell, yolk, and albumen weights (g) of brands A–F.
| Brand | Total weight (g) | Yolk weight (g) | Albumen weight (g) |
|---|---|---|---|
| A | 60.2 ± 0.600b,c | 16.5 ± 0.371b | 34.0 ± 0.501a |
| B | 61.8 ± 0.724a,b,c | 15.9 ± 0.228b | 33.7 ± 0.793a,b |
| C | 59.6 ± 0.344c | 14.9 ± 0.198b | 33.5 ± 0.652a,b |
| D | 62.4 ± 0.323a | 15.7 ± 0.142b | 35.5 ± 0.395a |
| E | 61.7 ± 0.341a,b | 21.4 ± 1.49a | 30.3 ± 1.40b |
| F | 61.5 ± 0.667a,b,c | 17.3 ± 0.611a,b | 34.6 ± 0.904a,b |
Different superscript letters in the same column represent values of significant differences (P < 0.05).
Conventional white (brand A) and brown (brand B) eggs had the largest yolk height at 24.3 ± 0.356 mm and 24.5 ± 0.452 mm, respectively (Table 3). Pasture-raised brand F had the smallest yolk height at 20.1 ± 0.477 mm. Interestingly, the yolks from brand E sustained a high number of breakages when eggs were cracked. Yet, this observation did not correlate to significant variations in the parameters measured for brand E. Albumen height is an indicator for the freshness and quality of an egg (Stadelman, 1995). As all eggs in this study are of equivalent age, meaning that brand F’s lower albumen height is an indicator of a lower quality. Strikingly, the albumen height of brand F was significantly lower than that of all other brands by at least 4.42 mm (P ≤ 0.05).
Table 3.
Yolk height, yolk diameter, and albumen height of brands A–F.
| Brand | Yolk height (mm) | Yolk diameter (mm) | Albumen height (mm) |
|---|---|---|---|
| A | 24.3 ± 0.356a | 37.9 ± 0.430a,b,c | 7.66 ± 0.129a,b |
| B | 24.5 ± 0.452a | 36.0 ± 0.388b,c | 7.50 ± 0.150a,b |
| C | 23.5 ± 0.412a | 37.1 ± 0.346a,b,c | 7.87 ± 0.125a,b |
| D | 23.4 ± 0.290a | 36.4 ± 0.183c | 7.85 ± 0.096a |
| E | 22.7 ± 0.553a,b | 37.6 ± 0.300a,b | 7.42 ± 0.118b |
| F | 20.1 ± 0.477b | 38.5 ± 0.749a,b,c | 3.00 ± 0.258c |
Different superscript letters in the same column represent values of significant differences (P < 0.05).
Brand E had a larger %Y and a lower %A than all other brands, by 6.9 and 6.7%, respectively (Table 4). Conversely, brands C and D had the lowest %Y and the largest %A.
Table 4.
Percent yolk and albumen of brands A–F.
| Brand | % Yolk2 | % Albumen1 |
|---|---|---|
| A | 27.3 ± 0.004b | 56.3 ± 0.005a |
| B | 26.5 ± 0.004b | 56.1 ± 0.004a,b |
| C | 25.3 ± 0.003b | 56.5 ± 0.005a,b |
| D | 25.2 ± 0.002b | 56.8 ± 0.005a |
| E | 34.5 ± 0.021a | 48.6 ± 0.026b |
| F | 27.6 ± 0.008a,b | 55.3 ± 0.010a,b |
Different superscript letters in the same column represent values of significant differences (P < 0.05).
Calculated as (albumen weight (g))/(total weight).
Calculated as (yolk weight (g))/(total weight).
The HU is an index that adjusts the height of the inner thick albumen according to the weight of the egg (Haugh, 1937) and relates well to the albumen quality of the egg. The higher the HU value, the better the albumen quality of eggs (Stadelman, 1995). HU can be used to measure quality greater than 72 to be considered AA grading (Jones, 2012). AA is the highest quality egg, followed by A and B. In this study, all the brands that were analyzed had been labeled as AA grade, with the exception of brand F (Table 5). Brand F had a HU lower than 72, which meets A quality grading.
Table 5.
Yolk index, Haugh units, and yolk fan color of brands A–F.
| Brand | Yolk index1 | Haugh unit2 | Yolk fan |
|---|---|---|---|
| A | 0.642 ± 0.011a,b | 98.6 ± 0.587a | 6.50 ± 0.197c |
| B | 0.680 ± 0.016a | 98.0 ± 0.606a | 7.13 ± 0.157b,c |
| C | 0.634 ± 0.012a,b | 99.6 ± 0.515a | 7.36 ± 0.125b |
| D | 0.643 ± 0.008a,b | 99.1 ± 0.416a | 6.84 ± 0.100c |
| E | 0.605 ± 0.016b,c | 97.3 ± 0.538a | 5.52 ± 0.119d |
| F | 0.523 ± 0.013c | 69.4 ± 2.20b | 10.4 ± 0.193a |
Different superscript letters in the same column represent values of significant differences (P < 0.05).
Calculated as (yolk height)/(yolk width).
Calculated as 100∗log((albumen height)-1.7((total weight)0.37 + 7.6)))).
Variability in HU has been noted in other studies between designer and conventional eggs (Jones et al., 2010). Specifically, conventional eggs were recorded as having higher HU than those of designer eggs. While Doležalová et al. (2010) indicated hens that raised in a litter system have shown to have a darker color yolk than those in a caged system, the finding was not fully consistent with our study. Brand F had a significantly darker colored yolk than the other brands at 10.4 ± 0.193. As a pasture-raised hen, variability in the diet due to foraging behavior is the likely cause of the darker coloration. Brand E was the lowest valued YC and was significantly lower than all other brands at 5.52 ± 0.119. The YC becomes significant in terms of a consumer perspective, as consumers prefer a darker colored yolk (Sass et al., 2018).
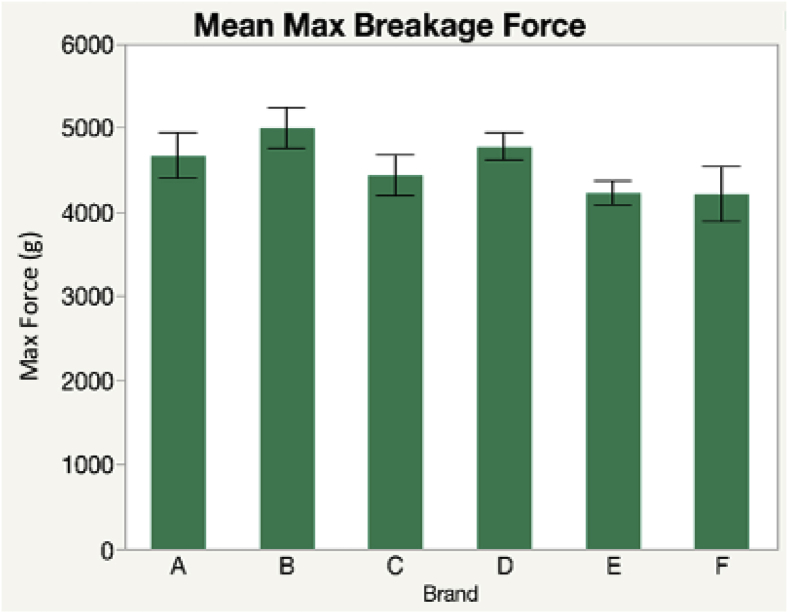
A greater breakage force signifies a stronger shell, which is ideal as the shell serves as a protection for the contents within the egg. Among all the brands that were analyzed, there was no significant variance in breakage force (Figure 1).
Figure 1.
Egg shell strength average among each brand measured as breakage force.
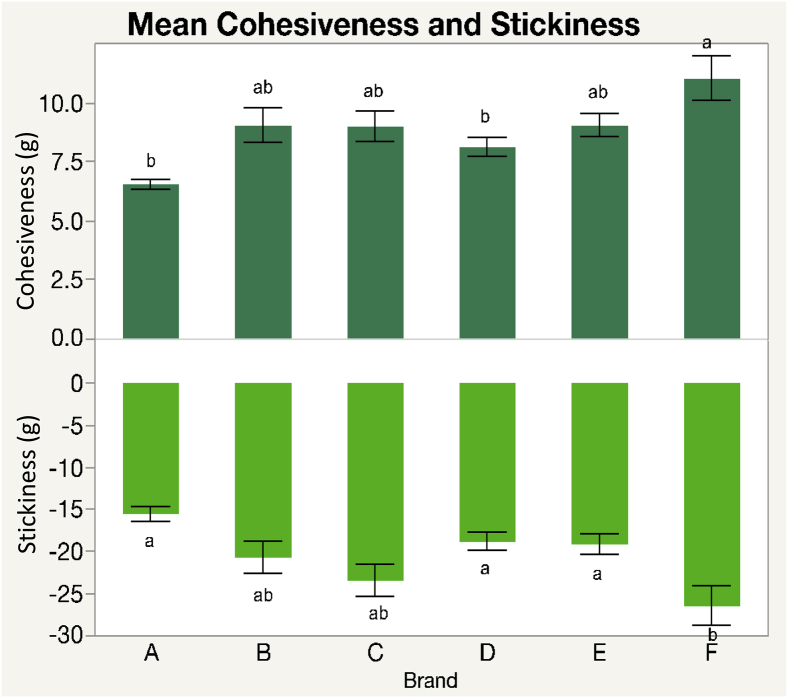
Brand F had the stickiest yolk among all brands (Figure 2), indicating further processing of brand F would result in a less clean separation of the yolk from the albumen than all other brands.
Figure 2.
Yolk cohesiveness and stickiness averaged among each brand.
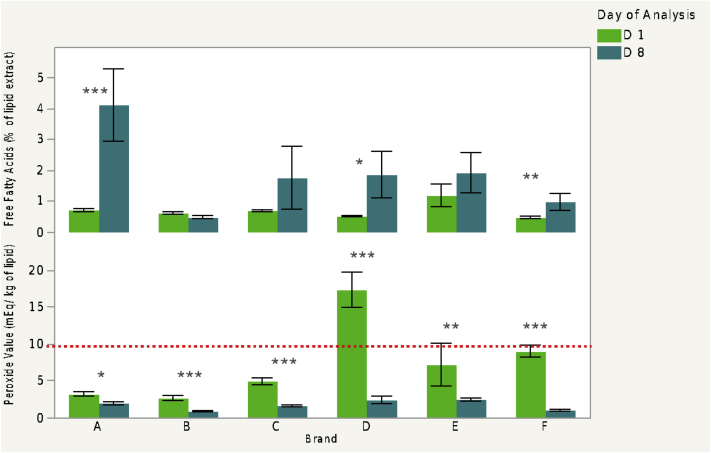
When evaluating oxidation indicators, brands A, D, and F experienced a significant increase in percent free fatty acids, with brand A experiencing the greatest increase (Figure 3). An increase in percent free fatty acids may result in the production of off-flavors from hydrolytic rancidity. Furthermore, the significant increase in free fatty acids for conventional brand A may be an indicator that the egg was handled or stored improperly and some point during the supply chain.
Figure 3.
Fatty acid and peroxide value comparison between brands at day 1 and 8. The dashed line represents the 10-mEg/kg threshold for peroxide values.a
Brand D had a significantly greater PV that was also above the 10 mEg/kg threshold, which is considered unsuitable for consumption. At day 8, all PVs were significantly lower, relative to day 1. It is likely that all the brands experienced lipid oxidation that progressed into secondary oxidation products such as aldehydes or ketones, which would result in the PV to decrease overtime as autooxidation continued (Pike and O'Keefe, 2017).
In this study, egg brands of various conventional and specialty designations were shown to produce high-quality products. Overall, as an A-graded egg, brand F exhibits lower quality measurements than other brands, which contributes to the downgrading of the brand. As a dark yolk, pasture-raised egg, this brand does meet a specialized consumer preference, despite a cost 4 times that of the conventional egg; however, the yolk and albumen content, and the yolk stickiness, does not meet the quality standards demonstrated from the other brands examined. Similar to other studies on designer egg quality, these results indicate that current US egg quality standards should effectively define quality for commercially produced conventional cage, enriched colony cage, and cage-free aviary eggs, and those quality standards should be more indicative of the egg's behavior in response to moving through the supply chain, such as possible oxidation or other physiochemical changes that may occur as a result of improper storage (Jones et al., 2014).
Ideally, product quality should not be compromised when paying for a premium product. Focusing on the designer eggs evaluated, the main issues facing optimizing egg quality revolve around off flavors developing over time and meeting USDA-quality grading standards. Ensuring a consistent market for high-quality products is essential to ensuring the sustainability of the specialty and designer egg market.
Acknowledgments
This material is bassed on the work supported by the CSU-LSAMP program funded by NSF the under grant #HRD-1826490, CSU Office of the Chancellor, and Fresno State.
Disclosures
The authors declare no conflicts of interest.
References
- Aro H., Rokka T., Valaja J., Hiidenhovi J., Huopalahti R., Ryhanen E.L. Functional and sensory properties of hen eggs with modified fatty acid compositions. Food Func. 2011;2:671–677. doi: 10.1039/c1fo10132c. [DOI] [PubMed] [Google Scholar]
- Chang J.B., Lusk J.L., Norwood F.B. The price of happy hens: a hedonic analysis of retail egg prices. J. Agri. Resour. Econ. 2010;35:406–423. [Google Scholar]
- Doležalová J., Dvořák P., Straková E., Suchý P. Variation in egg yolk colour in different systems of rearing laying hens. Acta Vet. Brno. 2010;79:13–19. [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. Food Tech. 1937;5:356–367. [Google Scholar]
- Heath J.L. Chemical and related osmotic changes in egg albumen during storage. Poult. Sci. 1977;56:822–828. [Google Scholar]
- Heng Y., Peterson H.H., Li X.H. Consumer attitudes toward farm-animal welfare: the case of laying hens. Agri. Resour. Econ. 2013;38:418–434. [Google Scholar]
- Jones D. Haugh unit: Gold standard of egg quality. Natl. Egg Quailty Sch. Proc. 2012;7:47–51. [Google Scholar]
- Jones D.R., Musgrove M.T., Anderson K.E., Thesmar H.S. Physical quality and composition of retail shell eggs. Poult. Sci. 2010;89:582–587. doi: 10.3382/ps.2009-00315. [DOI] [PubMed] [Google Scholar]
- Jones D.R., Karcher D.M., Abdo Z. Effect of a commercial housing system on egg quality during extended storage. Poult. Sci. 2014;93:1282–1288. doi: 10.3382/ps.2013-03631. [DOI] [PubMed] [Google Scholar]
- Kirunda D.F.K., McKee S.R. Relating Quality characteristics of aged eggs and fresh eggs to vitelline membrane strength as determined by a texture analyzer. Poult. Sci. 2000;79:1189–1193. doi: 10.1093/ps/79.8.1189. [DOI] [PubMed] [Google Scholar]
- Latimer G.W. 19th ed. AOAC International; Gaithersburg, MD: 2012. Official Methods of Analysis of AOAC International. [Google Scholar]
- Lusk J.L. Consumer preferences for cage-free eggs and impacts of retailer pledges. Agribusiness. 2019;35:129–148. [Google Scholar]
- Marshall A.C., Sams A.S., Elswyk M.E. Oxidative stability and sensory uailty of stored eggs from hens fed 1.5 percent menhaden oil. J. Food Sci. 1994;59:561–563. [Google Scholar]
- Matthews W.A., Sumner D.A. Effects of housing system on the costs of commercial egg production. Poult. Sci. 2015;94:552–557. doi: 10.3382/ps/peu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilgaard M.C., Civille G.V., Carr B.T. 5th ed. CRC Press; Boca Raton, FL: 2016. Sensory Evaluation Techniques. [Google Scholar]
- Pike O.A., O’Keefe S. Food Analysis. Springer; Cham: 2017. Fat characterization; pp. 407–429. [Google Scholar]
- Sass C.A.B., Kuriya S., da Silva G.V., Silva H.L.A., da Cruz A.G., Esmerino E.A., Freitas M.Q. Completion task to uncover consumer's perception: a case study using distinct types of hen's eggs. Poult. Sci. 2018;97:2591–2599. doi: 10.3382/ps/pey103. [DOI] [PubMed] [Google Scholar]
- Shallo H.S. Proceedings of the 54th Reciprocal Meat Conference. 2001. Designer foods: egg products; pp. 169–171. American Meat Science Association, Savory, IL. [Google Scholar]
- Shinn S.E., Proctor A. Rapid lipid extraction from egg yolks. J. Am. Oil. Chem. Soc. 2013;90:315–316. [Google Scholar]
- Stadelman W.J. Quality Identification of shell eggs. In: Stadelman W.J., Newkirk D., Newby L., editors. Egg Science and Technology. 4nd ed. CR Press; Boca Raton, FL: 1995. [Google Scholar]
- John J.L., St., Flor I.H. A study of whipping and coagulation of eggs of varying quality. Poult. Sci. 1931;10:71–74. [Google Scholar]
- USDA AMS Livestock, Poultry, and Seed Program, Agricultural Analytics Division . 2018. Egg Markets Overview. [Google Scholar]
- Wang Q., Jin G., Wang N., Guo X., Jin Y., Ma M. Lipolysis and oxidation of lipids during egg storage at different temperatures. Czech J. Food Sci. 2017;35:229–235. [Google Scholar]