Abstract
The negative effects of dietary antibiotics have become a widespread concern. It is imperative to search for a new type of green, safe, and efficient feed additive that can replace antibiotics. This study was to investigate the effects of glucose oxidase (GOD) on growth performance, immune function, and intestinal barrier in ducks infected with Escherichia coli O88. First, we established the E. coli challenge model of ducks through a preliminary experiment and then carried out the formal experiment by using 144 1-day-old male lean Peking ducklings (50 ± 2.75 g). All ducks were randomly assigned to 1 of 3 dietary treatment groups of basal diet (control), 30 mg/kg virginiamycin (antibiotic), and 200 U/kg GOD (1,000 U/g). Each group consisted of 6 replications with 8 birds per replicate. At day 7, all ducks were orally administered 0.2 mL E coli O88 (3 × 109 cfu/mL) twice, 8 h apart based on the preliminary experiment. The experiment lasted for 28 d. Dietary supplementation with GOD improved growth performance of ducks infected with E. coli. The GOD increased contents of Ig in plasma and secreted Ig A in jejunal mucosa. The GOD group had lower concentrations of inflammatory cytokines (tumor necrosis factor-α, IL-1β, and IL-6) and their upstream regulator Toll-like receptor 4 in the jejunum of ducks than the control group. Supplementation with GOD increased villus height and decreased crypt depth in the jejunum. The gene expression of tight junction proteins (zonula occludens-1, claudin-1 and claudin-2) was enhanced by adding GOD. The GOD decreased intestinal permeability by reducing the concentrations of diamine oxidase and D-lactic in plasma of ducks. There were no significant differences in almost all the indices tested between the GOD and the antibiotic groups. In conclusion, supplementation of GOD improved growth performance, immune function, and intestinal barrier of ducks infected with E. coli O88. Glucose oxidase may serve as a promising alternative therapy to antibiotics to relieve or prevent colibacillosis in ducks.
Key words: glucose oxidase, duck, Escherichia coli challenge, immune function, intestinal barrier
Introduction
The application of antibiotics in feed has greatly improved growth performance of animals and promoted the rapid development of animal husbandry. But, the longtime addition of antibiotics to animal feed may result in many negative effects, such as antimicrobial resistance, drug residue issues, and an imbalance of gut microbiota (Sorum and Sunde, 2001; Stanton, 2013; Cheng et al., 2014). There are also a number of publications that suggest a link between the use of antibiotics in animal production and the increase in human infections of antibiotic-resistant bacteria (Van et al., 2001; Laube et al., 2013). It is imperative to develop efficient, green, and safe feed additives that can stimulate animals’ production potential and maintain body health and replace antibiotics in feed.
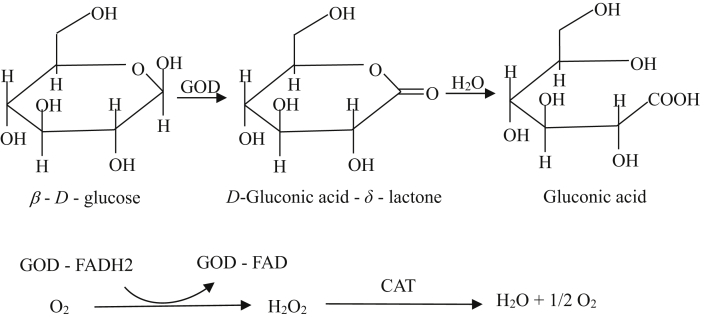
Glucose oxidase (GOD) is an aerobic dehydrogenase, which uses molecular oxygen to catalyze and oxidize β-D-glucose to gluconic acid, produce hydrogen peroxide and consume large amounts of oxygen at the same time (Bankar et al., 2009). The process of glucose oxidation catalyzed by GOD is shown in Figure 1. As a key metabolite of GOD, gluconic acid, also could improve animals’ growth performance (Biagi et al., 2006). Glucose oxidase plays the role in reducing the body's oxidative damage, maintaining health, and promoting growth by eliminating oxygen free radicals. This enzyme has been widely used in animal production by its characteristics of producing acid, deoxygenation, and sterilization (Kapat et al., 1998). Heenkenda et al. (2019) have been shown that 0.025% GOD could significantly improve the BW of broilers. Wu et al. (2019) also indicated that dietary supplement GOD could significantly influence the day 1 to 21 growth performance of broilers, even GOD could achieve to the effect similar with antibiotic group. Tang et al. (2016) and Mu et al. (2018) introduced that GOD significantly improve the ADG and decrease the feed-to-gain ratio (F:G) of weaned piglets.
Figure 1.
Process of glucose oxidation catalyzed by glucose oxidase. Abbreviations: CAT, catalase; FAD, flavin adenine dinucleotide; GOD, glucose oxidase; H2O2, hydrogen peroxide.
However, there is limited information regarding the effects of dietary supplementation with GOD on ducks. The objective of the current experiment was to evaluate the effects of GOD on growth performance, immune function, and intestinal barrier of ducks infected with Escherichia coli O88 and to explore feasibility of substituting antibiotics by GOD.
Materials and methods
Ethics Statement
All the experimental procedures were approved by the Institutional Animal Use and Care Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences and performed as per the guidelines set by the National Institute of Animal Health.
Preliminary Test
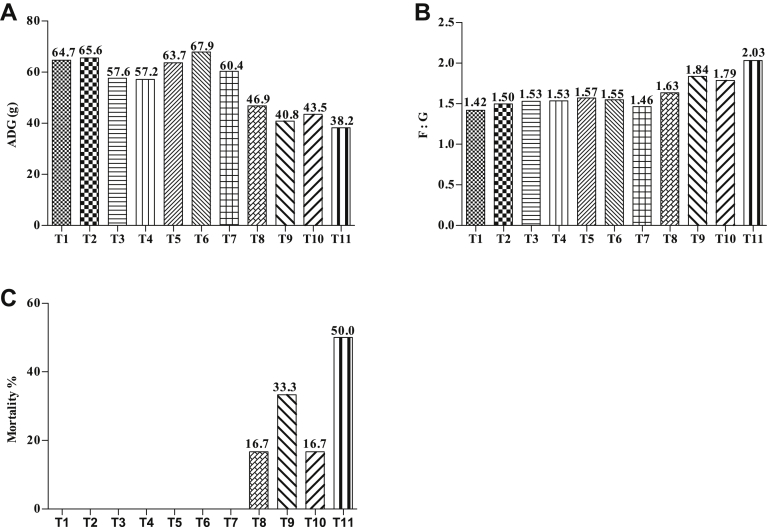
Sixty-six 1-day-old male lean Peking ducklings from 1 commercial hatchery (Institute of Animal Sciences, CAAS) were randomly divided into 11 groups with 6 each group. At day 7, ducks in the control group (treatment 1, T1) and 10 test groups (T2–T11) were orally administered with sterilized LB broth medium or E. coli O88 (1 × 109 cfu/mL) at different dose and times; the E. coli O88 strain was obtained from China Veterinary Culture Collection Center. The infection scheme is showed in Table 1. All the ducks were fed the same diet. The diet for the starter (day 1 to day 14) and the grower (day 15 to day 28) phases were formulated as per the nutrient requirements for meat-type ducks published by the Ministry of Agriculture of the People's Republic of China (2012). The results showed that the 14-day-old ducks in T4–T11 groups exhibited lethargy, drooping wings, gray white or green loose feces, and mucus in nasal cavity compared with the control group; however, no significant change was observed for ducks in T2 and T3 groups. Further dissection revealed typical pathologic changes of colibacillosis for the ducks in T9 and T11 groups, such as pericardial thickening, yellow or white fibrous exudates filled in the pericardium, enlarged liver, and white or yellowish fibrous exudates covered the liver. In addition, as shown in Figure 2, the ducks from day 1 to day 14 in T8–T11 groups had lower ADG and higher F:G than the control group. There was no death for ducks in T1–T7 groups, the mortality of ducks in T8–T11 groups were 17, 33, 17, and 50%, respectively. Finally, considering the results of clinical diagnosis, pathologic examination, growth performance, and mortality, we selected the infection scheme of orally administered E. coli O88 (6 × 108 cfu) twice, 8 h apart.
Table 1.
Pre-experimental grouping and infection methods.
| Group | Reagent | Dosage/mL | Inoculation quantity/cfu | Time |
|---|---|---|---|---|
| T1 | LB broth medium | 1 | - | 1 |
| T2 | 1E. coli O88 | 0.05 | 0.5 × 108 | 1 |
| T3 | E. coli O88 | 0.05 | 0.5 × 108 | 2 |
| T4 | E. coli O88 | 0.10 | 1 × 108 | 1 |
| T5 | E. coli O88 | 0.10 | 1 × 108 | 2 |
| T6 | E. coli O88 | 0.20 | 3 × 108 | 1 |
| T7 | E. coli O88 | 0.20 | 3 × 108 | 2 |
| T8 | E. coli O88 | 0.60 | 6 × 108 | 1 |
| T9 | E. coli O88 | 0.60 | 6 × 108 | 2 |
| T10 | E. coli O88 | 1.00 | 1 × 109 | 1 |
| T11 | E. coli O88 | 1.00 | 1 × 109 | 2 |
Abbreviations: E. coli, Escherichia coli; T, treatment.
The concentration of E. coli O88 was 1 × 109 cfu/mL.
Figure 2.
Effects of different infection methods on growth performance of 14-day-old ducks in preliminary test. (A) The ADG of 1- to 14-day-old ducks. (B) The F:G of 1- to 14-day-old ducks. (C) The mortality of ducks. Abbreviations: T, treatment; F:G, feed-to-gain ratio.
Animals, Diets, and Experimental Design
A total of 144 1-day-old male lean Peking ducklings (50 ± 2.75 g) from 1 commercial hatchery (Institute of Animal Sciences, CAAS) were randomly assigned to 3 groups with 6 replications of 8 birds per replicate. The test diets consisted of a corn–soybean basal diet (control) and 2 diets supplemented with 30 mg/kg virginiamycin (antibiotic) and 200 mg/kg GOD (1,000 U/g) on basal diet; virginiamycin and GOD were obtained from Beijing Challenge Biotechnology Co. Ltd. (Beijing, China). The basal diet of the experiment was the same as that of the preliminary test. The experiment lasted for 28 d. Ducks were reared in the 3-layer wire cages, allowed ad libitum access to diets and water, and subjected to a photoperiod of 24 h of light on day 1 to day 7 and 23 h of light and 1 h of dark thereafter. The temperature of the room was maintained at 32°C on first wk and gradually lowered to 25°C. At day 7, all ducks were orally administered with 0.2 mL E coli O88 (3 × 109 cfu/mL) twice, 8 h apart.
Sampling
At day 9, day 14, and day 28, after 6 h of fasting, all ducks were weighed, and feed intake was measured on a per cage basis. ADG, ADFI, and F:G were calculated.
One duck of average BW from each pen was randomly selected and slaughtered by bleeding the left jugular vein after fasting 6 h at day 9, day 14, and day 28. Blood was withdrawn by cardiac puncture into EDTA anticoagulated tubes, centrifuged at 1,300 × g for 10 min at 4°C, and stored at −20°C to determine immune indices in plasma. Weights of the liver, spleen, and bursa of Fabricius were recorded. Organ indices were the ratio of organ weight to BW. The middle segments (9 cm in length) of the jejunum were excised, removed the intestinal digesta, lightly rinsed with sterile PBS, and then divided into 3 parts, one frozen immediately in liquid nitrogen and then stored at −80°C in a freezer for testing the gene expression of tight junction proteins, another scraped off the intestinal mucosa with a cover slip in the clean bench to measure the content of secreted Ig A (sIgA), and the other fixed in 10% formalin (v/v) for morphologic examination.
Measurements
Plasma Indices
The concentration of Ig (IgA, IgG, and IgM), Toll-like receptor 4 (TLR4), cytokines (tumor necrosis factor α [TNF-α], IL 1β [IL-1β] and IL-6) were determined by the ELISA method using a Multiskan MK3 (Thermo Fisher Scientific, Waltham, MA). Diamine oxidase (DAO) content was measured by colorimetric method with spectrophotometry (Biomate 5; Thermo Electron Corporation, Rochester, NY). D-lactic acid (D-LA) in plasma was analyzed with the D-lactic Acid kit (Jiancheng Biological Engineering Research Institute, Nanjing, China). Because the presence of L-lactate dehydrogenase and L-lactate in plasma interferes with the estimation of D-LA, inactivation or removal of L-lactate dehydrogenase is necessary to measure the correct D-LA concentration, so we remove L-lactate dehydrogenase and L-lactate from plasma before measure D-LA as per the method reported by Nielsen et al. (2011).
The Content of sIgA in Intestinal Mucosa
The sIgA content of mucous membrane of the jejunum was measured by a Secretory Immunoglobulin A kit (Jiancheng Biological Engineering Research Institute, Nanjing, China).
RNA Extraction, cDNA Synthesis, and Real-time Reverse Transcription-Polymerase Chain Reaction Analysis
The gene expression of tight junction proteins (zonula occludens-1 [ZO-1], claudin-1 [CLDN-1], and CLDN-2) was determined by real-time fluorescence quantitative PCR. The total RNA was extracted using TRIzol reagents (Takara, Japan). cDNA was synthesized by 1 μg of total RNA using the Fast King RT Kit (Tiangen, China). Transcriptional changes were identified by quantitative PCR, which was performed using the Premix Ex Taq with SYBR Green (Tiangen, China) and the Bio-Rad Real-Time PCR system (Applied Biosystems, Carlsbad, CA). The thermocycle protocol lasted for 15 min at 95°C, followed by 40 cycles of 10s denaturation at 95°C, 34 s annealing/extension at 60°C, and then a final melting curve analysis to monitor purity of the PCR product. The β-actin was finally identified as the housekeeping gene because no variation in its expression was observed between treatments. Primer sequences were shown in Table 2. The 2−ΔΔCt method was used to estimate mRNA abundance. All the data were normalized to those of the housekeeping gene β-actin. The relative expression of the target gene mRNA in each group was calculated as follows: ΔCt = Ct (target gene) – Ct (β-actin) and ΔΔCt = ΔCt (treated group) – ΔCt (Tian et al., 2018). Relative gene expression levels were normalized to those of the eukaryotic reference gene β-actin.
Table 2.
Primers used for relative real-time PCR.1
| Gene name | Primers sequence (5′-3′) | GenBank accession |
|---|---|---|
| β-actin | F: TGAGAGTAGCCCCTGAGGAGCAC | NM_001310421.1 |
| R: TAACACCATCACCAGACTCCATCAC | ||
| ZO-1 | F: CACAGCAGCCCAGCAACGG | XM_027465580.1 |
| R: TCGCCTGCCACCTCTTCCATAG | ||
| CLDN-1 | F: AGCCTTCCTCTGCTGCTCCTG | XM_013108556.3 |
| R: AGTGCTGGTGGCTCCTCATCC | ||
| CLDN-2 | F: TCGCCTACTCCAACGCCTACC | XM_021271062.2 |
| R: GGGTTTGTGTGTCGGGCTGAC |
Abbreviations: CLDN-1, claudin-1; CLDN-2, claudin-2; F, forward; R, reverse; ZO-1, zonula occludens protein-1.
Primers designed by primer express software (Applied Biosystems, Foster City, CA).
Intestinal Morphology Analysis
The specimens of the jejunum were processed as per the hematoxylin-eosin staining staining method reported by Wu et al. (1996), and the histologic sections were examined using a light microscope (Olympus BX40; Olympus Optical Co., Hamburg, Germany) and computer-assisted image analysis (The ImageJ v 1.26; Wayne Rasband, National Institutes of Health, Bethesda, Maryland). Villus height (μm) was measured from the tip to the villus crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi. Ten fields were randomly selected to measure the villus height and the crypt depth and calculated the ratio of villus height to crypt depth (V:C).
Statistical Analysis
One-way ANOVA was performed using SPSS 19.0 software package for Windows (SPSS Inc., Chicago, IL). Mean values of treatment groups were compared using Duncan's multiple range test with P < 0.05 considered statistically significant. The results were expressed as mean ± SE.
Results
Growth Performance
Table 3 described the effects of GOD and antibiotic on growth performance for ducks. Supplementation of GOD and antibiotic increased (P < 0.05) ADG of ducks by 1.84 g and 1.75 g from day 1 to day 9 compared with the control group, but no differences were observed at other time points. Supplementation of GOD and antibiotic did not affect (P > 0.05) ADFI and F:G of ducks during all the periods.
Table 3.
Effects of glucose oxidase on growth performance of ducks infected by Escherichia coli.
| Items | Control | Antibiotic | Glucose oxidase | P-value |
|---|---|---|---|---|
| Day 1 to 9 | ||||
| ADG, g | 27.58 ± 1.24b | 29.42 ± 1.22a | 29.33 ± 1.31a | 0.039 |
| ADFI, g | 36.34 ± 2.05 | 38.29 ± 1.43 | 38.03 ± 1.68 | 0.142 |
| F:G | 1.32 ± 0.05 | 1.30 ± 0.02 | 1.30 ± 0.04 | 0.683 |
| Day 9 to 14 | ||||
| ADG, g | 62.75 ± 5.08 | 60.05 ± 4.35 | 64.10 ± 4.46 | 0.333 |
| ADFI, g | 79.79 ± 5.56 | 80.43 ± 6.09 | 82.77 ± 4.26 | 0.608 |
| F:G | 1.28 ± 0.09 | 1.34 ± 0.03 | 1.29 ± 0.08 | 0.328 |
| Day 14 to 28 | ||||
| ADG, g | 77.96 ± 4.77 | 80.11 ± 7.81 | 81.26 ± 7.24 | 0.697 |
| ADFI, g | 137.67 ± 6.78 | 140.62 ± 10.62 | 139.81 ± 6.68 | 0.817 |
| F:G | 1.77 ± 0.05 | 1.76 ± 0.05 | 1.73 ± 0.10 | 0.614 |
| Day 1 to 28 | ||||
| ADG, g | 58.94 ± 3.41 | 60.32 ± 4.39 | 61.43 ± 3.72 | 0.549 |
| ADFI, g | 92.20 ± 4.19 | 94.92 ± 5.29 | 94.36 ± 4.02 | 0.560 |
| F:G | 1.57 ± 0.05 | 1.58 ± 0.04 | 1.54 ± 0.06 | 0.404 |
a,bMeans within a row lacking a common superscripts differ (P < 0.05).
Abbreviations: Antibiotic, diets supplemented with virginiamycin at 30 mg/kg; Control, basal diets; F:G, feed-to-gain ratio; Glucose oxidase, diets supplemented with glucose oxidase(1,000 U/g) at 200 U/kg; mean values ± SE.
Immune Function
Organ Indices
Table 4 shows the effects of GOD and antibiotic supplementation on organ indices of ducks. Adding antibiotic significantly increased (P < 0.05) spleen index of 28-day-old ducks compared with the control and GOD groups, but no differences were observed at other time points. Supplementation of GOD and antibiotic did not affect (P > 0.05) liver and bursa indexes of ducks during all the periods.
Table 4.
Effects of glucose oxidase on organ indices of ducks infected by Escherichia coli O88, %.
| Items | Control | Antibiotic | Glucose oxidase | P-value |
|---|---|---|---|---|
| Day 9 | ||||
| Liver | 3.87 ± 0.58 | 4.26 ± 0.59 | 4.52 ± 0.33 | 0.115 |
| Spleen | 0.14 ± 0.03 | 0.15 ± 0.03 | 0.17 ± 0.05 | 0.308 |
| Bursa of Fabricius | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.21 ± 0.04 | 0.995 |
| Day 14 | ||||
| Liver | 5.20 ± 0.61 | 5.74 ± 0.70 | 5.43 ± 0.46 | 0.346 |
| Spleen | 0.28 ± 0.07 | 0.29 ± 0.07 | 0.34 ± 0.04 | 0.174 |
| Bursa of Fabricius | 0.36 ± 0.12 | 0.31 ± 0.05 | 0.40 ± 0.15 | 0.410 |
| Day 28 | ||||
| Liver | 2.28 ± 0.37 | 2.34 ± 0.33 | 2.29 ± 0.36 | 0.957 |
| Spleen | 0.11 ± 0.02b | 0.15 ± 0.04a | 0.11 ± 0.02b | 0.019 |
| Bursa of Fabricius | 0.09 ± 0.02 | 0.13 ± 0.05 | 0.12 ± 0.03 | 0.152 |
a,bMeans within a row lacking a common superscripts differ (P < 0.05).
Abbreviations: Antibiotic, diets supplemented with virginiamycin at 30 mg/kg; Bursa of Fabricius, bursa of Fabricius index; Control, basal diets; Glucose oxidase, diets supplemented with glucose oxidase(1,000 U/g) at 200 U/kg; Liver, liver index; Spleen, spleen index; mean values ± SE.
Ig Contents in Plasma
The effects of GOD and antibiotic on Ig contents in plasma of ducks are described in Table 5. An increase in plasma IgM content of 9-day-old (P = 0.079) and 14-day-old (P < 0.05) ducks were found in the GOD group. Adding antibiotic increased (P < 0.05) IgG content of ducks at day 9. There were no significant differences in all parameters mentioned previously between the GOD group and antibiotic group.
Table 5.
Effects of glucose oxidase on plasma immunoglobulins contents of ducks infected by Escherichia coli, g/L.
| Items | Control | Antibiotic | Glucose oxidase | P-value |
|---|---|---|---|---|
| Day 9 | ||||
| IgA | 0.82 ± 0.17 | 0.96 ± 0.16 | 0.98 ± 0.14 | 0.210 |
| IgG | 7.00 ± 0.74b | 8.60 ± 0.56a | 7.86 ± 1.16a,b | 0.028 |
| IgM | 0.61 ± 0.08 | 0.65 ± 0.09 | 0.75 ± 0.05 | 0.079 |
| Day 14 | ||||
| IgA | 0.74 ± 0.05 | 0.83 ± 0.10 | 0.86 ± 0.02 | 0.139 |
| IgG | 6.77 ± 0.11 | 7.57 ± 1.32 | 7.72 ± 1.53 | 0.589 |
| IgM | 0.56 ± 0.02b | 0.62 ± 0.08a,b | 0.68 ± 0.04a | 0.044 |
| Day 28 | ||||
| IgA | 0.79 ± 0.08 | 0.82 ± 0.08 | 0.88 ± 0.16 | 0.449 |
| IgG | 8.90 ± 0.83 | 8.22 ± 1.05 | 8.97 ± 1.25 | 0.523 |
| IgM | 0.62 ± 0.06 | 0.63 ± 0.03 | 0.73 ± 0.15 | 0.127 |
a,bMeans within a row lacking a common superscripts differ (P < 0.05).
Abbreviations: Antibiotic, diets supplemented with virginiamycin at 30 mg/kg; Control, basal diets; Glucose oxidase, diets supplemented with glucose oxidase (1,000 U/g) at 200 U/kg; mean values ± SE.
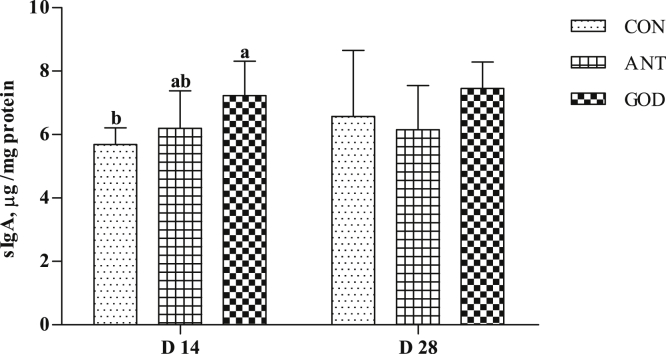
The Concentration of sIgA in the Jejunum Mucosa
Glucose oxidase significantly increased (P < 0.05, Figure 3) sIgA concentration in the jejunum mucosa of ducks at day 14 compared with the control group. There were no significant differences in sIgA concentration in the jejunum mucosa between the GOD group and antibiotic group.
Figure 3.
Effects of glucose oxidase on jejunal mucosa sIgA of ducks infected by Escherichia coli. Means with different letters are different (P < 0.05). Abbreviations: ANT, antibiotic group, diet supplemented with virginiamycin at 30 mg/kg; CON, control group, basal diet; GOD, glucose oxidase, diet supplemented with glucose oxidase (1,000 U/g) at 200 U/kg; sIgA, secreted Ig A.
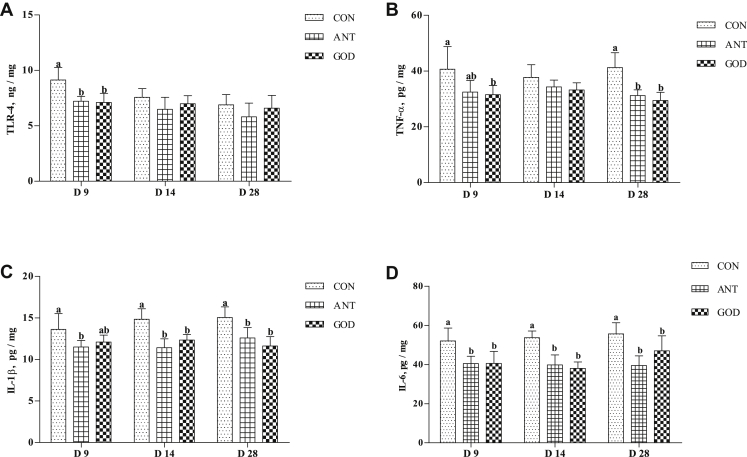
Proinflammatory Cytokine Concentrations in the Jejunum
Adding GOD and antibiotic dramatically reduced concentration of proinflammatory cytokine in different time point. The ducks in the GOD and antibiotic groups had lower (P < 0.05, Figure 4) TNF-α concentration at day 9 and day 28 but not on day 14. Glucose oxidase and antibiotic decreased (P < 0.05, Figure 4) the jejunal IL-1β concentration at day 14 and day 28 but not on day 9; the jejunal IL-6 concentration of ducks in the GOD and antibiotic groups were decreased (P < 0.05, Figure 4) at all the time point. There were no significant differences in proinflammatory cytokine concentration in the jejunum between the GOD group and antibiotic group. Adding GOD and antibiotic can also reduce (P < 0.05, Figure 4) upstream regulator TLR4 concentration at day 9 in the jejunum of ducks.
Figure 4.
Effects of glucose oxidase on jejunal proinflammatory cytokine of ducks infected by Escherichia coli. (A) The concentration of TLR4. (B) The concentration of TNF-α; (C) The concentration of IL-1β; (D) The concentration of IL-6. Means with different letters are different (P < 0.05). Abbreviations: ANT, antibiotic group, diet supplemented with virginiamycin at 30 mg/kg; CON, control group, basal diet; GOD, glucose oxidase, diet supplemented with glucose oxidase (1,000 U/g) at 200 U/kg; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor α.
Intestinal Barrier
Intestinal Morphology
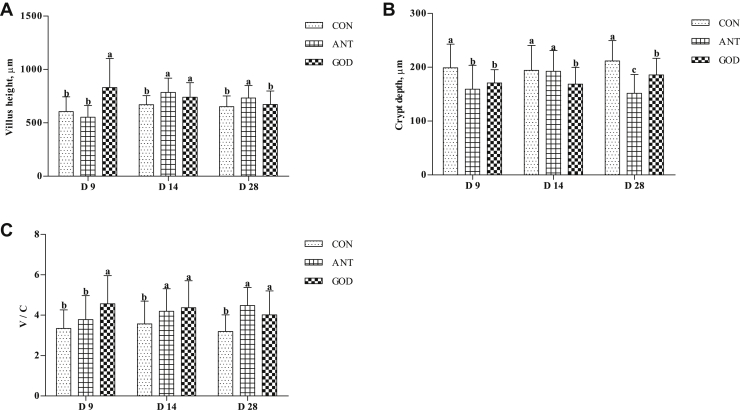
In comparison with the control group, both the antibiotic group and GOD group better maintained the villus integrity of the jejunum (Figure 5). Adding GOD enhanced (P < 0.05) the jejunal villus height of ducks at day 9 and day 14 but not on day 28, decreased (P < 0.05) crypt depth, and improved the V:C at day 9, day 14, and day 28. The ducks in the antibiotic group had higher (P < 0.05) jejunal villus height and V:C at day 14 and day 28 and lower (P < 0.05) crypt depth at day 9 and day 28 compared with the control group.
Figure 5.
Effects of glucose oxidase on intestinal morphology of ducks infected by Escherichia coli. (A) The villus height in jejunal of ducks. (B) The crypt depth in jejunal of ducks. (C) The ratio of villus height to crypt depth in jejunal of ducks. Means with different letters are different (P < 0.05). Abbreviations: ANT, antibiotic group, diet supplemented with virginiamycin at 30 mg/kg; CON, control group, basal diet; GOD, glucose oxidase, diet supplemented with glucose oxidase (1,000 U/g) at 200 U/kg; V:C, the ratio of villus height to crypt depth.
The Gene Expressions of Tight Junction Proteins in the Jejunum
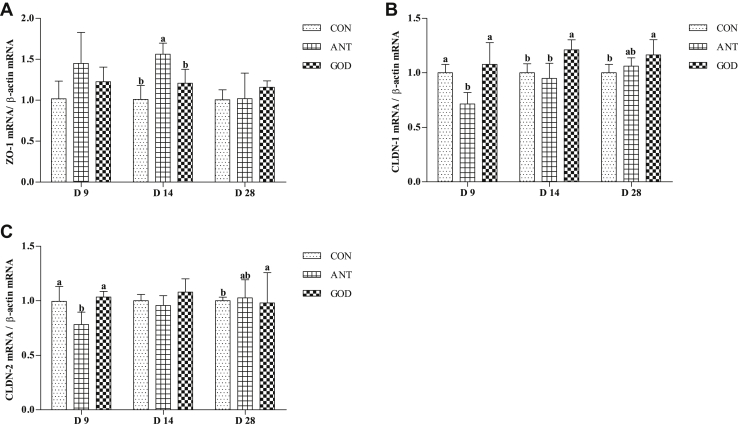
As shown in Figure 6, the antibiotic group had higher (P < 0.05) ZO-1 expression in 9-day-old ducks than the control group. The 14 28-day-old ducks in the GOD group had higher (P < 0.05) expression of CLDN-1. A higher (P < 0.05) gene expression of CLDN-2 was observed in 28-day-old ducks in the GOD group in contrast to the control group.
Figure 6.
Effects of glucose oxidase on jejunal tight junction proteins gene expression ducks infected by Escherichia coli. (A) The mRNA level of ZO-1. (B) The mRNA level of CLDN-1. (C) The mRNA level of CLDN-2. Means with different letters are different (P < 0.05). Abbreviations: ANT, antibiotic group, diet supplemented with virginiamycin at 30 mg/kg; CON, control group, basal diet; CLDN-1, claudin-1; CLDN-2, claudin-2; GOD, glucose oxidase, diet supplemented with glucose oxidase (1,000 U/g) at 200 U/kg; ZO-1, zonula occludens-1.
Intestinal Permeability
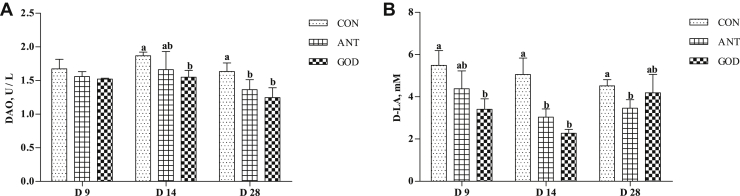
The plasma DAO and D-LA concentration were considered as an important symbol of intestinal permeability (Figure 7). The DAO level at day 14 and day 28 and D-LA level at day 9 and day 14 of ducks were lower (P < 0.05) in the GOD group than that in the control group. Adding antibiotic decreased (P < 0.05) the DAO level of ducks at day 28 and Day-LA level at day 14 and day 28 compared with control. There were no significant differences in the plasma DAO and D-LA content between the GOD group and antibiotic group.
Figure 7.
Effects of glucose oxidase on intestinal permeability of ducks infected by Escherichia coli. (A) The concentrations of DAO in plasma of ducks. (B) The concentrations of D-LA in plasma of ducks. Means with different letters are different (P < 0.05). Abbreviations: ANT, antibiotic group, diet supplemented with virginiamycin at 30 mg/kg; CON, control group, basal diet; DAO, diamine oxidase; D-LA, D-lactic acid; GOD, glucose oxidase, diet supplemented with glucose oxidase (1,000 U/g) at 200 U/kg.
Discussion
Our results showed that supplement of GOD improved the growth performance, especially ADG of 9-day-old ducks, which was in agreement with the previous studies in broilers (Heenkenda et al., 2019; Wu et al., 2019) and pigs (Tang et al., 2016). Similar result was also reported that 100 and 200 g/ton GOD significantly improved ADG and decreased F:G of ducks (Tang et al., 2015). Liu. (2017) indicated that adding 150 and 200 g/ton GOD in feed can significantly enhance the BW of 21-day-old and 39-day-old ducks, improve ADG, and decrease the mortality of ducks in various periods. The result in present study was derived from producing acid, deoxygenation, and sterilization properties of GOD.
E. coli infection can cause atrophy of the bursa of Fabricius, congestion and enlargement of the spleen, and exudative inflammation of the liver (Wani et al., 2017). Wang et al. (2018) indicated that the adding GOD in feed had no significant effect on the immune organ development of Chinese yellow-feathered broilers. The finding was consistent with our results.
Ig are a class of specific active proteins that can be transformed into antibody by antigen induction, such as IgM, IgG, IgA, and so on. IgM can regulate, sterilize, agglutinate, and activate the traditional way of complement in the early stage of pathogen infection; IgG participates in humoral immunity and plays the roles of phagocytosis, agglutination, precipitation of antigens, neutralization of viruses and toxins, and activation of complement. IgA can bind to and clear antigens without causing inflammation, thus maintain the internal environment (Kerr, 1990; Galzie, 1991; Heyman and Shulman, 2016). Our results found that the GOD and antibiotic can increase the contents of IgM in plasma of ducks in the starter phases; the antibiotic can also increase plasma IgG content of ducks on day 9. Similar result was also reported that the addition of 400 g/ton GOD promoted serum Ig levels so as to enhance the piglet's resistance to pathogenic microorganisms (Mu et al., 2018). Dimeric IgA, known as sIgA, has immunoprotective function at the mucosal level (Curtis, 2017). The present study also found that GOD increased the sIgA content in jejunum mucosa of 14-day-old ducks infected by E. coli. The aforementioned results indicated that GOD enhanced immunity by enhancing humoral immunity, reducing inflammation, and protecting intestinal mucosa.
Inflammation is a major immune response of organism, but excessive immune response will lead to a sharp rise of cytokines, form inflammatory cytokines storm, result in disorder of immune system, and cause irreversible damage to the host organs. In the present study, the concentration of immune pathogen pattern recognition receptor (TLR4),proinflammatory cytokine (TNF-α), and chemokine (IL-1β, IL-6) were measured, which are key mediators in the regulation of immune response. It was reported that the infection of E. coli can activate TLR4 signal pathway and produce inflammatory reaction, which can increase the expression of TNF-α, IL-1β, and IL-6, affect the normal function of immune system, and threaten the health of animals (Sandford et al., 2011; Nie et al., 2012). We found that adding GOD attenuated the concentration of TNF-α, IL-1β, and IL-6 in response to E. coli stimulation, simultaneously, reduced the concentration of upstream regulator TLR4 of ducks on day 9. This was consistent with previous results from the study by Wang et al. (2018) which reported that feeding GOD helped to reduce the concentrations of proinflammatory cytokines and chemokines in the intestine of yellow-feathered broilers. It indicated that GOD can inhibit the activation of proinflammatory signaling pathway, alleviate the adverse effect of inflammatory on organs, and maintain animal health.
Absorption of nutrients is mainly in the small intestine, especially in the villus of the intestinal wall. The height of the villus determines the area of intestinal absorption and affects the growth and development of animals (Wilson et al., 2018). The depth of the crypt represents production speed and maturation rate of intestinal epithelial cells (Xing et al., 2015). The V:C reflects the functional state of the intestine; the decrease of V:C indicates the decrease of absorption function (He et al., 2018). Our study showed that the addition of GOD increased the height of the villus, decreased the depth of the crypt, and enhanced the V:C compared with the control group and similarly with the antibiotic group. It indicated that GOD can repair the intestinal damage caused by E. coli infection, enhance the absorption of nutrients, and promote the growth performance of animals.
A good mechanical barrier can effectively prevent bacteria, endotoxins, and other harmful substances from penetrating the intestinal mucosa (Monaco et al., 2011; Tian et al., 2018). Tight junction proteins, such as ZO-1, CLDN-1, and CLDN-2, are critical in the maintenance of intestinal integrity and barrier function. Studies have suggested that the expression of intestinal tight junction proteins were influenced by the expression of individual protein or the cause of intestinal insult and also oppositely correlated with gut permeability (Bruewer et al., 2003; Kim et al., 2019). Diamine oxidase is located mainly in the small intestinal mucosa, particularly near the tips of villi, and reflects small intestinal integrity and maturity (Hesterberg et al., 1984; Dagostino et al., 1988; Wolvekamp and Bruin, 1994), as they are normally presented in very small amounts in blood circulation. Increased serum D-LA and DAO levels reflect changes in intestinal permeability, suggesting that the intestinal barrier function has been damaged (Nielsen et al., 2011; Fukudome et al., 2014). In this study, relative abundances of jejunal transcripts of ZO-1, CLDN-1, and CLDN-2 increased in the GOD group than that in the control group. Meanwhile, dietary supplementation of GOD decreased the levels of plasma DAO and D-LA compared with the control group, even lower than the antibiotic group in later periods. It indicated that GOD can improve intestinal physical barrier and relieve intestinal damage of ducks infected by E. coli.
In conclusion, GOD improved growth performance, immune function, and intestinal barrier of ducks infected by E. coli. Glucose oxidase affected immune function of ducks in 3 ways that include reducing the invasion of pathogens by enhancing intestinal barrier function, inhibiting inflammatory reaction by blocking the expression of inflammatory factors, and enhancing humoral immunity by improving the contents of Ig. In addition, GOD can also promote the absorption of nutrients by improving the intestinal morphology. There were no obvious differences in almost all indices between GOD and antibiotic treatments. Therefore, GOD may serve as a promising alternative therapy to antibiotics to relieve or prevent colibacillosis in ducks.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2017YFD0601001) and the Collaborative Innovation Task in Agricultural Science and Technology Innovation Program (CAAS-XTCX2016011-01).
Disclosures
The authors declare no conflicts of interest.
References
- Bruewer M., Luegering A., Kucharzik T., Parkos C., Madara J., Hopkins A.M., Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Biagi G., Piva A., Moschini M., Vezzali E., Roth F.X. Effect of gluconic acid on piglet growth performance, intestinal microflora, and intestinal wall morphology. J. Anim. Sci. 2006;84:370–378. doi: 10.2527/2006.842370x. [DOI] [PubMed] [Google Scholar]
- Bankar S.B., Bule M.V., Singhal R.S., Ananthanarayan L. Glucose oxidase--an overview. Biotechnol. Adv. 2009;27:489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Cheng G.Y., Hao H.H., Xie S.Y., Wang X., Dai M.H., Huang L.L., Yuan Z.H. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front. Microbiol. 2014;5:217. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis J.L. A hairline crack in the levee: Focal secretory IgA deficiency as a first step toward emphysema. Am. J. Respir. Crit. Care Med. 2017;195:970–973. doi: 10.1164/rccm.201612-2509ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagostino L., Daniele B., Pignata S., Greco L., Mazzacca G. Postheparin plasma diamine oxidase in subjects with small bowel disease, diagnostic efficiency of a simplified test. Digestion. 1988;41:46–54. doi: 10.1159/000199731. [DOI] [PubMed] [Google Scholar]
- Fukudome I., Kobayashi M., Dabanaka K., Maeda H., Okamoto K., Okabayashi T., Baba R., Kumagai N., Oba K., Fujita M., Hanazaki K. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2014;47:100–107. doi: 10.1007/s00795-013-0055-7. [DOI] [PubMed] [Google Scholar]
- Galzie Z. Structure and function of human and murine receptors for IgG. Indian J. Biochem. Bio. 1991;28:77–82. [PubMed] [Google Scholar]
- Hesterberg R., Sattleb J., Lorenz W., Stahlknecht C.D., Barth H., Crombach M. Histamine content, diamine oxidase activity and histamine methyl transferase activity in human tissues: Fact or fictions? Agents. Actions. 1984;14:324–334. doi: 10.1007/BF01973821. [DOI] [PubMed] [Google Scholar]
- Heyman B., Shulman M.J. Structure, function, and production of immunoglobulin M (IgM) In: Michael J.H.R., editor. Encyclopedia of Immunobiology. 2016. pp. 1–14. Academic Press, Toronto, Canada. [Google Scholar]
- He X., Lu Z., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agr. 2018;98:4471–4478. doi: 10.1002/jsfa.8971. [DOI] [PubMed] [Google Scholar]
- Heenkenda H.M.D.P.B., N K Illippangama I.P.A.U., Arsecularatne M.D.N.A.F., Palliyeguru M.W.C.D., Jayasena D.D. Effect of glucose oxidase on growth performance and meat quality of broiler chicken. Int. Res. Conf. UWU-2019. 2019:594. (Abstr.) [Google Scholar]
- Kerr M.A. The structure and function of human IgA. Biochem. J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapat A., Jung J.K., Park Y.H. Improvement of extracellular recombinant glucose oxidase production in fed-batch culture of Saccharomyces cerevisiae: effect of different feeding strategies. Biotechnol. Lett. 1998;20:319–323. [Google Scholar]
- Kim K., He Y.J., Xiong X., Ehrlich A., Li X.D., Raybould H., Atwill E.R., Maga E.A., Jørgensen J., Liu Y.H. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019;10:52. [Google Scholar]
- Laube H., Friese A., Salviati C.V., Guerran B., Käsbohrer A., Kreienbrock L., Roesler U. Longitudinal monitoring of extended-spectrum-beta-lactamase/AmpC-producing Escherichia coli at German broiler chicken fattening farms. Appl. Environ. Microbiol. 2013;79:4815–4820. doi: 10.1128/AEM.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.Q. Univ. Shandong Agriculture; China: 2017. Effects of Glucose Oxidase on Growth Promoting and Immune Protection in Meat Ducks. MD Diss. [Google Scholar]
- Monaco M.H., Kashtanov D.O., Wang M., Walker D.C., Rai D., Jouni Z.E., Miller M.J., Donovan S.M. Addition of polydextrose and galactooligosaccharide to formula does not affect bacterial translocation in the neonatal piglet. J. Pediatr. Gastr. Nutr. 2011;52:210–216. doi: 10.1097/MPG.0b013e3181ffcaee. [DOI] [PubMed] [Google Scholar]
- Mu S.Q., Li N., Yan J., Zheng X., Ma Y., Li Q.J., Zhang C.H. Effect of glucose oxidase on the growth performance and serum biochemical indexes of piglets. Chin. J. Anim. Husbandry. Vet. Med. 2018;45:2212–2218. [Google Scholar]
- Nielsen C., Lindholt J.S., Erlandsen E.J., Mortensen F.V. D-lactate as a marker of venous-induced intestinal ischemia: an experimental study in pigs. Int. J. Surg. 2011;9:428–432. doi: 10.1016/j.ijsu.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Nie Q.H., Sandford E.E., Zhang X.Q., Nolan L.K., Lamont S.J. Deep sequencing-based transcriptome analysis of chicken spleen in response to avian pathogenic Escherichia coli (APEC) infection. PLoS One. 2012;7:e41645. doi: 10.1371/journal.pone.0041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorum H., Sunde M. Resistance to antibiotics in the normal flora of animals. Vet. Res. 2001;32:227–241. doi: 10.1051/vetres:2001121. [DOI] [PubMed] [Google Scholar]
- Sandford E.E., Orr M., Balfanz E., Bowerman N., Li X.Y., Zhou H.J., Johnson T.J., Kariyawasam S., Liu P., Nolan L.K., Lamont S.J. Spleen transcriptome response to infection with avian pathogenic Escherichia coli in broiler chicken. BMC Genomics. 2011;12:469. doi: 10.1186/1471-2164-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T.B. A call for antibiotic alternatives research. Trends. Microbiol. 2013;21:111–113. doi: 10.1016/j.tim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Tang H.O., Gao X.H., Yao B., Zhang G.M., Wang Z.X., Li X.C. Effects of glucose oxidase on growth performance, serum parameters and slaughter performance of meat ducks and its antidotal effect on aflatoxin B1. Anim. Nutr. 2015;27:2361–2367. [Google Scholar]
- Tang H.O., Yao B., Gao X., Yang P., Wang Z., Zhang G. Effects of glucose oxidase on the growth performance, serum parameters and faecal microflora of piglets. S. Afr. J. Anim. Sci. 2016;46:14–20. [Google Scholar]
- Tian S.Y., Wang J., Yu H., Wang J., Zhu W.Y. Effects of galacto-oligosaccharides on growth and gut function of newborn suckling piglets. J. Anim. Sci. Biotechno. 2018;9:75. doi: 10.1186/s40104-018-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van d.B.A. E., London N., Driessen C., Stobberingh E.E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 2001;47:763–771. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]
- Wolvekamp M.C.J., Bruin R.W.F.D. Diamine oxidase: an overview of historical, biochemical and functional aspects. Dig. Dis. 1994;12:2–14. doi: 10.1159/000171432. [DOI] [PubMed] [Google Scholar]
- Wu G., Meier S.A., Knabe D.A. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J. Nutr. 1996;126:2578–2584. doi: 10.1093/jn/126.10.2578. [DOI] [PubMed] [Google Scholar]
- Wani B.M., Darzi M.M., Mir M.S., Adil S., Shakeel I. Pathological and pharmacochemical evaluation of broiler chicken affected naturally with colibacillosis in Kashmir Valley. Int. J. Pharmacol. 2017;13:388–395. [Google Scholar]
- Wilson F.D., Cummings T.S., Barbosa T.M., Williams C.J., Gerard P.D., Peebles E.D. Comparison of two methods for determination of intestinal villus to crypt ratios and documentation of early age-associated ratio changes in broiler chickens. Poult. Sci. 2018;97:1757–1761. doi: 10.3382/ps/pex349. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Wang Y.B., Xu H., Mei X.Q., Gong L., Wang B.K., Li W.F., Jiang S.Q. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poult. Sci. 2018;97:3540–3549. doi: 10.3382/ps/pey216. [DOI] [PubMed] [Google Scholar]
- Wu S.R., Li T.H., Niu H.F., Zhu Y.F., Liu Y.L., Duan Y.L., Sun Q.Z., Yang X.J. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult. Sci. 2019;98:828–841. doi: 10.3382/ps/pey393. [DOI] [PubMed] [Google Scholar]
- Xing X.X., Jiang R.L., Wang L.C., Lei S., Zhi Y.H., Wu Y.C., Zhu M.F., Huang L.Q., Xia G.L., Chen Z.Q. Shenfu injection alleviates intestine epithelial damage in septic rats. Am. J. Emerg. Med. 2015;33:1665–1670. doi: 10.1016/j.ajem.2015.08.001. [DOI] [PubMed] [Google Scholar]