Abstract
The effects of Lacto-Immuno-Vital synbiotic preparation on gene expression of IgA, MUC-2, and growth factor IGF-2 in the jejunum and on BW gain in broiler chickens were studied. A flock of 64,400 1-day-old Hybrid ROSS 308 chickens was inducted in the 42-day experiment. The chickens were divided into 2 equally size groups in separate halls. The chickens in the experimental (E) group received 500 g of Lacto-Immuno-Vital in 1,000 L of drinking water. The preparation was administered daily from the first day (day 1) to day 7 of the experiment. From day 7 to day 22, it was given in pulsed manner (every third day) at a dose of 300 g in 1,000 L of drinking water. The broiler chickens in the E group gained more weight (P < 0.001) compared with control from day 10 to day 42. Death of animals during feeding period was 1,078 chickens in the E group compared with 1,115 dead chickens in the control group. Feed conversion ratio was 1.61 kg of supplemented diet/kg of BW in the E group compare with 1.67 kg of nonsupplemented diet/kg of BW in control. The relative expression of IgA gene in the jejunum was upregulated on day 22 in the E group compared with control (P < 0.05), whereas relative expression of MUC-2 gene was upregulated in the E group compared with control on day 8 and day 22 (P < 0.05; P < 0.001). Similarly, relative expression of IGF-2 gene was upregulated in the E group compared with control on both samplings (P < 0.01). The composition of Lacto-Immuno-Vital synbiotic preparation showed beneficial effects on growth performance, feed conversion ratio, morbidity, mortality, and selected parameters of mucosal immunity in the chicken jejunum.
Key words: synbiotic preparation, growth factor, mucin, Iga, broiler
Introduction
Worldwide consumption of poultry meat has increased both in developed and in developing countries. Chicken meat is still popular because of its high-quality protein content and relatively low prices compared with other types of meat (Beski et al., 2015).
In today's consumer-oriented world, it is very important to produce healthy and safe animal products. In this context, the healthy and properly functional gastrointestinal tract of animals forms the basis for safe food production. Animal metabolism is a complex process, which is also regulated by the presence of both host and commensal intestinal microbiota. In the small intestine, the mucosal surface is particularly exposed to pathogens and is therefore covered with a loosely attached mucus layer. MUC-2 gene is a major component of the loose mucus layer secreted by goblet cells, limiting microbial adherence and regulating growth (Butler, 2015). IgA antibodies are among the most important humoral immune factors present on mucosal surfaces, where in addition to protecting against absorption of mucosal antigens, they play a strategic role in inhibiting inflammatory effects (Herich, 2017). Moreover, in the dynamic environment of the developing chicken intestine, growth factors represent important mediators of gastrointestinal repair, with key roles in cellular processes such as proliferation, differentiation, migration, and survival (Rowland et al., 2013). Similarly, intestinal development is also modified by insulin-like growth factors (IGF). It has been shown that IGF-2 is involved in mechanisms that control the differentiation of the intestinal epithelium (Georgiev et al., 2003). In addition, IGF-2 plays an essential role in the growth process of skeletal muscle and the growth plate of developing bone. Even in developing endochondral bones, chondrocyte proliferation is absolutely dependent on IGF signaling (Kawai and Rosen, 2012).
To improve the quality of chicken meat, alternative substances are increasingly used including probiotics and β-glucans. Specific probiotic strains can improve animal growth by modulating the intestinal microbiota as well as the secretion of IgA and mucin. Likewise, β-glucans modulate the intestinal morphology by increasing the number of mucin-producing goblet cells, as well as cells expressing secretory IgA (sIgA) with increased sIgA in the intestinal lumen. At the same time, they reduce bacterial translocation to various other organs (Anwar et al., 2017).
Several studies focusing on the relationship between the gut microbiota and immunology have emphasized the importance of using synbiotics to promote farm animal health. On the other hand, most studies investigating the effects of synbiotics concentrate on humans (Markowiak and Śliżewska, 2017). In general, a synbiotic is defined as a combination of prebiotics and probiotics, which synergically support gastrointestinal health by improving survival and adherence of live microbial dietary supplements (Yari and Hekmatdoost, 2019). Lacto-Immuno-Vital is a synbiotic preparation that improves conditions for the development of beneficial microbiota, thereby enhancing mucosal immunity in the intestine. Although the effects of synbiotics have been clarified, important information regarding their influence on chicken health is still incomplete. The aim of this study was therefore to evaluate the effects of Lacto-Immuno-Vital synbiotic preparation on selected parameters of mucosal immunity (IgA, MUC-2) and growth factor IGF-2 in the jejunum and on BW gain in broiler chickens.
Material and methods
Experimental Design
The experiment was conducted in a commercial broiler chicken fattening farm, and the birds were handled and sacrificed in a humane manner. A flock of 64,400 1-day-old Hybrid ROSS 308 chickens were inducted in the 42-day experiment. The chickens were divided into 2 equal groups in separate halls. The chickens in the experimental (E) group received 500 g of Lacto-Immuno-Vital (Hajduvet Kft., Hungary) in 1,000 L of drinking water. Lacto-Immuno-Vital was administered daily from the first day (day 1) to day 7 of the experiment. From day 7 to day 22, it was given in a pulsed manner (every third day) at a dose of 300 g in 1,000 L of drinking water. The composition of Lacto-Immuno-Vital is shown in Table 1. The control (C) group received only the standard diet (see Table 2). Groups of 60 chickens randomly selected in each hall were weighed at 1, 5, 10, 16, 20, 26, 30, and 35 d of age (Table 3). For analyses, 16 chickens from each group (E, C) were taken from the halls. The sampling day were set at day 8 and day 22 of the experiment. The chickens were euthanized with an intra-abdominal injection of xylazine (Rometar 2%; SPOFA, Czech Republic) and ketamine (Narkamon 5%; SPOFA, Czech Republic) at doses of 0.7 mL/kg BW. Samples from the caudal part of the jejunum were collected during necropsy.
Table 1.
Composition of Lacto-Immuno-Vital.
| Probiotic strain | Cfu/g |
|---|---|
| Enterococcus faecium (CECT 4515) | 10 × 109 |
| Bacillus amyloliquefaciens (CECT 5940) | 10 × 109 |
| Mannan oligosaccharide | 12% |
| β-glucan (Saccharomyces cerevisiae) | 12% |
| Microbial protein | 10% |
Table 2.
Composition of feed mixtures.
| Components | Starter Day 1–Day 10 |
Grower I Day 11–Day 17 |
Grower II Day 18–Day 22 |
|---|---|---|---|
| Corn % | 42.77 | 43.31 | 46.14 |
| Soya extracted scrap % | 25.0 | 24.0 | 23.2 |
| Wheat % | 20.0 | 20.0 | 16.0 |
| Full-fat soya | 7.0 | 7.0 | 6.0 |
| Sunflower meal % | 0 | 0 | 1.5 |
| Rapeseed scrap % | 0 | 0 | 1.5 |
| Fodder lime % | 1.21 | 1.12 | 0.91 |
| Monocalcium phosphate % | 1.17 | 0.76 | 0.64 |
| Plant oil % | 0.6 | 1.7 | 2.1 |
| Premix % | 0.5 | 0.5 | 0.5 |
| Methionine % | 0.36 | 0.33 | 0.30 |
| Lysine % | 0.30 | 0.25 | 0.24 |
| Sodium bicarbonate % | 0.25 | 0.25 | 0.20 |
| Threonine % | 0.16 | 0.10 | 0.10 |
| Salt | 0.16 | 0.17 | 0.17 |
| Lupro-Cid nal % | 0.30 | 0.30 | 0.30 |
| FRA LeciMax dry % | 0.05 | 0.05 | 0.05 |
| l valine % | 0.05 | 0.07 | 0.01 |
| Anticoccidials | Maxiban G160 50 mg/kg |
Maxiban G160 50 mg/kg |
Sacox 70 mg/kg |
|---|---|---|---|
| Myco fix select | 0.08% | 0.08% | 0.08% |
| Declared values | |||
| Dry mass % | 87.83 | 87.91 | 87.95 |
| ns % | 20.33 | 19.80 | 19.47 |
| Fat % | 4.09 | 5.18 | 5.93 |
| Dietary fiber % | 2.65 | 2.62 | 3.08 |
| Ash % | 5.46 | 4.80 | 4.46 |
| MEn (mj.kg) | 12.53 | 12.90 | 13.04 |
| Lysine % | 1.27 | 1.20 | 1.19 |
| Methionine % | 0.64 | 0.61 | 0.59 |
| Met + lys % | 0.99 | 0.95 | 0.93 |
| Threonine % | 0.88 | 0.81 | 0.83 |
| Tryptophan % | 0.23 | 0.22 | 0.22 |
| Valine % | 0.95 | 0.94 | 0.87 |
| Ca % | 0.79 | 0.68 | 0.59 |
| P total % | 0.65 | 0.55 | 0.53 |
| Sodium % | 0.15 | 0.15 | 0.16 |
| Mg % | 0.14 | 0.14 | 0.14 |
| Zn (mg/kg) | 125.27 | 124.90 | 123.99 |
Table 3.
Effect of Lacto-Immuno-Vital on the weight of broiler chickens depending on age.
| Day of experiment | Control group (means + SD) | Experimental group (means + SD) |
|---|---|---|
| 1 d | 34.35 ± 0.16 | 34.44 ± 0.19 |
| 5 d | 118.60 ± 0.17 | 119.60 ± 0.36 |
| 10 d | 275.02 ± 0.25 | 285.42 ± 0.13∗ |
| 16 d | 589.56 ± 0.36 | 592.36 ± 0.30∗ |
| 20 d | 906.43 ± 0.15 | 910.17 ± 0.59∗ |
| 26 d | 1,443.09 ± 0.37 | 1,447.18 ± 0.28∗ |
| 30 d | 1,742.86 ± 1.97 | 1,791.35 ± 4.27∗ |
| 35 d | 1,980.38 ± 1.07 | 2,060.74 ± 1.46∗ |
| 42 d | 2,599.15 ± 2.94 | 2,709.93 ± 1.91∗ |
Means with superscripts are significantly different (P < 0.001).
Homogenization of Jejunum Samples and Isolation of Total RNA
Jejunum tissue samples were cut into 20-mg pieces, immediately placed in RNA Later solution (Qiagen, UK), and stored at −70°C before RNA purification, as described in the study by Karaffová et al. (2019).
Relative Expression of IgA, MUC-2, and IGF-2 Genes in Quantitative Real-Time PCR
The mRNA levels of IgA, MUC-2, and IGF-2 were determined. In addition, mRNA relative expression of the reference gene, coding glyceraldehyde-3-phosphate dehydrogenase, was determined based on stability of expression using BestKeeper software. The primer sequences used for quantitative real-time PCR are listed in Table 4. All primer sets allowed DNA amplification efficiencies between 94 and 100%.
Table 4.
List of primers used in qRT-PCR for target gene mRNA detection in chickens.
| Primer | Sequence 5′–3′ | Annealing temperature/time | References |
|---|---|---|---|
| IgA Fw | GTCACCGTCACCTGGACTACA | 59°C/30 s | Lammers et al., 2010 |
| IgA Rev | ACCGATGGTCTCCTTCACATC | ||
| MUC-2 Fw | GCTGATTGTCACTCACGCCTT | 54°C/1 min | Smirnov et al., 2006 |
| MUC-2 Rev | ATCTGCCTGAATCACAGGTGC | ||
| IGF-2 Fw | CTCTGCTGGAAACCTACTGT | 55°C/30 s | Mudroňová et al., 2018 |
| IGF-2 Rev | GAGTACTTGGCATGAGATGG | ||
| GAPDH Fw | CCTGCATCTGCCCATTT | 59°C/30 s | De Boever et al., 2008 |
| GAPDH Rev | GGCACGCCATCACTATC |
Abbreviation: qRT-PCR, quantitative real-time PCR.
Amplification and detection of specific products were performed using the CFX 96 RT system (Bio-Rad) and Maxima SYBR Green qPCR Master Mix (Thermo Scientific). Subsequent quantitative real-time PCR to detect relative expression of mRNA selected parameters was based on 36 cycles performed with initial denaturation at 94°C for 3 min, followed by denaturation at 93°C for 45 s. The optimal annealing temperature and time for each primer are shown in Table 4, and there was an elongation step at 72°C for 10 min. A melting curve from 50°C to 95°C with readings at every 0.5°C was produced for each individual quantitative real-time PCR plate. Analysis was performed after every run to ensure a single amplified product for each reaction. All real-time PCR reactions were performed in duplicate, and mean values of the duplicates were used for subsequent analysis. We also confirmed that the efficiency of amplification of each target gene was essentially 100% in the exponential phase of the reaction, where the quantification cycle (Cq) was calculated. The Cq values of the genes studied were normalized to the average Cq value of the reference gene (ΔCq), and the relative expression of each gene was calculated mathematically as 2−ΔCq.
Collection of Jejunum Samples for ELISA
During necropsy, jejunal segments were taken from the intestine at the same site in each chicken. Length of intestinal segments reached approximately 3 cm. Small pieces of intestinal loops were washed and prepared for determination of sIgA content as well MUC-2 production and secretion. Syringes were filled with an optimal volume (5 mL per each sample) of warm flushing solution (1 M tris/glycine buffer with 0.25% Tween 20, pH 7; Sigma-Aldrich). Then, a needle was inserted into one end of each intestinal loop, and by emptying the syringe in several pulses, the whole intestinal content was flushed out. The complete luminal brush-lined epithelial wall was flushed, and the content was emptied into 20-mL-volume test tubes. The jejunal flushes were centrifuged at 12,000 × g for 5 min (Hettich Rotina 75 420R Centrifuge DJB Labcare, UK), and the supernatants from each sample were used for ELISA (Husáková et al., 2015).
Detection of sIgA With Enzyme–Antibody Conjugate
To determine sIgA content in the jejunal flushes, we used a chicken IgA ELISA kit (Kamiya Biomedical Company). A 96-well microtiter plate was coated with affinity purified anti-chicken IgA antibody. Under laboratory conditions, the volume on each microtiter plate was incubated (22°C, 20 min), and subsequently, the content was aspirated and washed 3 times with solution, following the ELISA kit instructions. Determination of sIgA content was previously described by Karaffová et al. (2015).
Determination of Total MUC-2 by ELISA
For detection and determination of total MUC-2, we used a chicken MUC-2 ELISA kit (Kamiya Biomedical Company). For detection, 96-well microtiter plates were coated with affinity purified anti-chicken MUC-2 antibody. The plates were incubated, then washed and filled with 50 μL substrate solution in each well. The detected samples were diluted 1:5 in PBS with pH between 7.0 and 7.2 and added in 100-μL doses into predesignated wells in duplicates. Mixtures of balance solution in 10 μL and 50 μL of conjugate bound with horseradish peroxidase in stabilizing buffer were added into the plate wells, then incubated at 37°C for 1 h. Determination of total MUC-2 was previously described (Karaffová et al., 2019).
Statistical Analysis
Statistical analysis of data was performed using t test in Minitab 16 software (SC & C Partner, Brno, Czech Republic). Differences between the mean values for the groups were considered statistically significant at P < 0.05, P < 0.01, and P < 0.001. Values are given as means ±SD.
Results
Measurements of average weight and relative expression of the IGF-2 gene in the jejunum were used to evaluate the effect of Lacto-Immuno-Vital on growth performance.
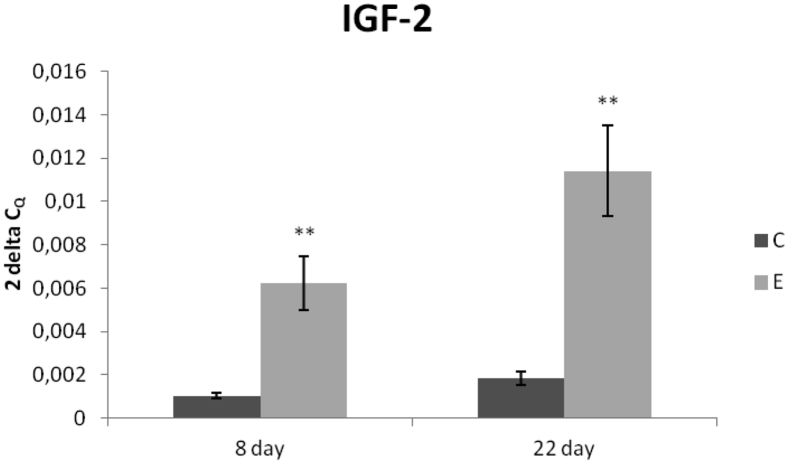
The E group of chickens in the hall fed the diet supplemented with Lacto-Immuno-Vital demonstrated higher average weight (P < 0.001) from day 10 to day 42 of the experiment (Table 3) compared to the C broilers in the other hall. As for the observed mortality, 1,078 chickens in the E group died during the feeding period compared with 1,115 chickens in the C group. Similarly, lower number of chickens because of crawling (dwarfism—428; locomotor system—202) was found in the E group compared with the C group (dwarfism—456; locomotor system—212). Feed conversion ratio was 1.61 kg of supplemented diet/kg of BW in the E group compared with 1.67 kg of nonsupplemented diet/kg of BW in the C group. Relative expression of IGF-2 gene was markedly upregulated in the E group (P < 0.01) compared with the C group, on both samplings (Figure 1).
Figure 1.
Relative expression of IGF-2 gene in the jejunum of chickens fed with Lacto-Immuno-Vital. Results at each time point are the median of 2−ΔCq. Superscripts indicate significant differences between the control and experimental groups. ∗∗P < 0.01. Abbreviations: C, control group; E, experimental group; IGF, insulin-like growth factor.
To evaluate the effect of Lacto-Immno-Vital on mucosal protection, measurements of the relative expression for MUC-2 and IgA genes in the jejunum and concentration of MUC-2 and sIgA in intestinal flush were performed.
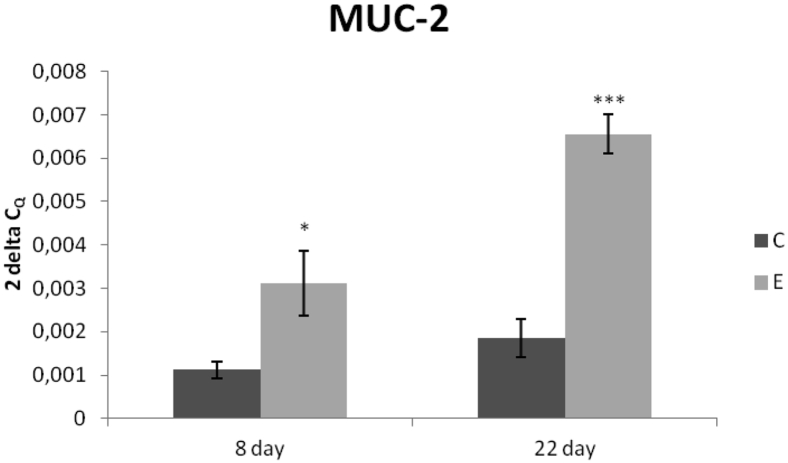
Relative expression of MUC-2 gene showed significant upregulation in the E group when compared with the C group in both samplings (P < 0.05; P < 0.001) (Figure 2).
Figure 2.
Relative expression of MUC-2 gene in the jejunum of chickens fed with Lacto-Immuno-Vital. Results at each time point are the median of 2−ΔCq. Superscripts indicate significant differences between the control and experimental groups. ∗P < 0.05; ∗∗∗P < 0.001. Abbreviations: C, control group; E, experimental group; MUC-2, mucin 2.
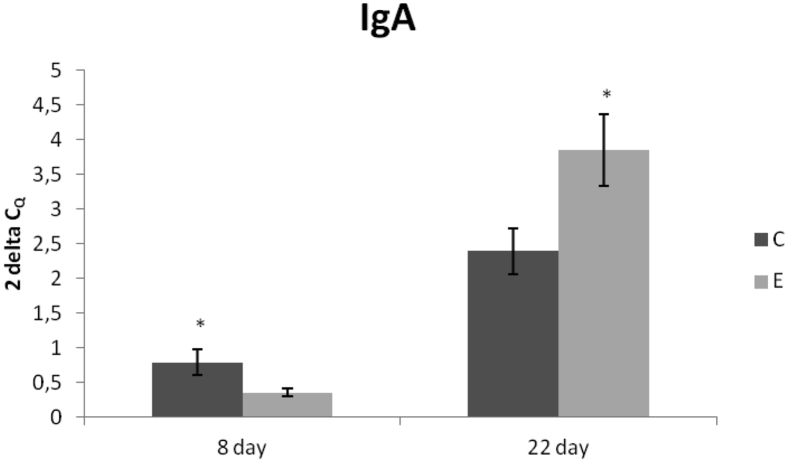
However, the relative expression of IgA gene in the jejunum was upregulated in the C group compared with the E group on day 8 of the experiment (P < 0.05). The opposite result was recorded on day 22, when gene expression was upregulated in the E group (P < 0.05) compared with the C group(Figure 3).
Figure 3.
Relative expression of IgA gene in the jejunum of chickens fed with Lacto-Immuno-Vital. Results at each time point are the median of 2−ΔCq. Superscripts indicate significant differences between the control and experimental groups. ∗P < 0.05. Abbreviations: C, control group; E, experimental group.
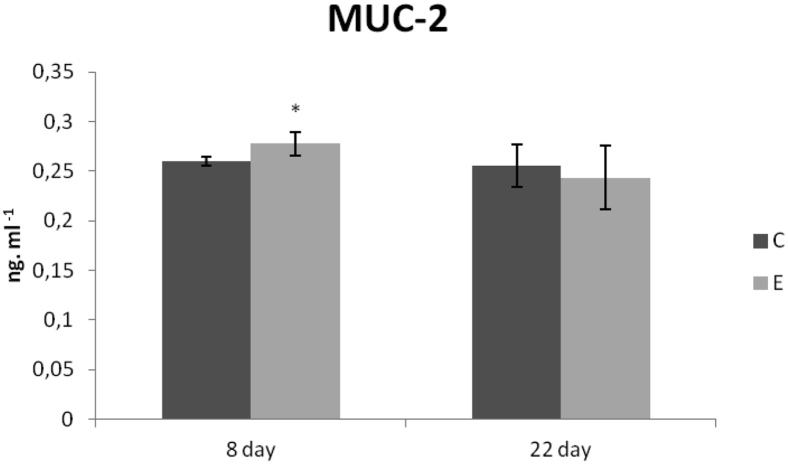
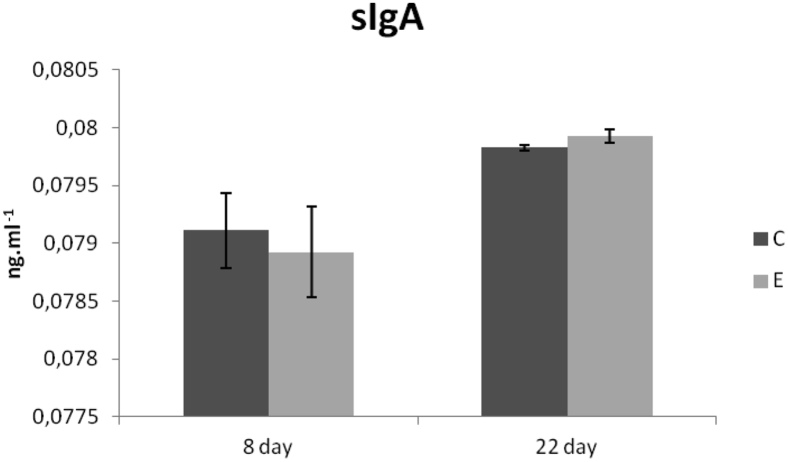
Concentration of MUC-2 (ng/mL) in the intestinal flush from the jejunum was increased in the E group compared with the C group (P < 0.05) on day 8. Interestingly, MUC-2 concentration was almost the same in both groups on day 22 of the experiment (Figure 4). Similarly, concentration of sIgA (ng/mL) in the intestinal flush from the jejunum was very resembled in both groups on both samplings (Figure 5).
Figure 4.
Mucin 2 concentrations (ng/mL) in the jejunum of chickens fed with Lacto-Immuno-Vital. Superscripts indicate significant differences between the control and experimental groups. ∗P < 0.05. Abbreviations: C, control group; E, experimental group; MUC-2, mucin 2.
Figure 5.
sIgA concentrations (ng/mL) in jejunum of chickens fed with Lacto-Immuno-Vital. Abbreviations: C, control group; E, experimental group; sIgA, secretory Ig A.
Discussion
Probiotics and combinations of probiotics and prebiotics (synbiotics) have been introduced as an alternative to antibiotics and growth promoters in poultry production. The use of synbiotics could be a promising option.
Data on the average BW of the broiler chickens showed improved growth performance. In our experiment, Lacto-Immuno-Vital was administered from the first day. The first wk after hatching is crucial for broilers' pectoralis major muscle development (Halevy et al., 2000; Žitňan et al., 2019). Malnutrition or enteral infection during this period can have irreversible negative effects on growth performance (Dina and Hams, 2016). Moreover, preventive early application of Enterococcus faecium has been shown to decrease cecal pathogenic microorganisms, promoting the development of the small intestine and its protective barrier (Herich et al., 2010; Ševčíková et al., 2016) and stimulating innate and acquired immune responses (Levkut et al., 2012; Dina and Hams, 2016). After 7 d of our experiment, the Lacto-Immuno-Vital dosing frequency was reduced. Levkut et al. (2009) demonstrated antimicrobial effects of E. faecium against pathogens on day 7 after continuous administration of the probiotic bacteria. Similarly, our previous results showed that 21 d of feeding with E. faecium had protective effect on the immune response in chickens (Levkut et al., 2012). However, the economic cost of long-term synbiotic administration played an important role in our experiment. This prompted us to stop the diet supplementation with Lacto-Immuno-Vital on day 23 of the present experiment and then to check for permanent improvement in the chickens' growth performance and health status. In our trial, the weight gain increased by 110.78 g for chickens in the experimental group on day 42 of the experiment. Beneficial effect of Lacto-Immuno-Vital was demonstrated also on feed conversion ratio (increased 3.6%), morbidity (decreased 6%), and mortality (decreased 3.4%).
Several studies have observed the stimulating effect of Bacillus amyloliquefaciens alone on the average daily weight gain in chickens (Ahmed et al., 2014; Lei et al., 2015). E. faecium has been shown to support gut villi development and thereby affect the capacity for digestion and absorption in a positive way (Herich et al., 2010; Ševčíková et al., 2016). Similarly, Mallo et al. (2010) reported that addition of E. faecium CECT4515 (106 cfu/g) improved intestinal microbiota balance by increasing the number of Lactobacillus and reducing the number of coliforms in the ileum, cecum, and faeces, thus promoting the growth of weaned piglets. However, several studies have shown no significant effect on feed conversion and thus on the growth of broiler chickens when fed a diet supplemented with B. amyloliquefaciens alone (Wizna et al., 2009; Jerzsele et al., 2012). Moreover, the effect of the combination of B. amyloliquefaciens CECT5940 and E. faecium CECT4515 on broiler chickens has not been fully clarified so far.
Supplementation of Lacto-Immuno-Vital in the broiler diet in the present experiment increased relative expression of IGF-2 in the chicken jejunum on day 8 and day 22 (sampling day). It is known that IGF are essential for the growth and development of muscle (Fu et al., 2015). Furthermore, IGF contribute to maintaining the satellite cell niche by reducing depletion (Chakravarthy et al., 2000) and inhibiting the degradation of myofibers derived from chick embryonic myoblasts (Janeczko and Etlinger, 1984).
The basic protection of the mucous membranes is mediated by mucin produced by goblet cells, which is either localized on the cell membrane or secreted into the lumen to form a mucosal layer. Mucus is necessary for ensuring of hydration and physical protection and also serves as a reservoir for antimicrobial molecules (Robbe-Masselot et al., 2008). The gel forming MUC-2 provides not only nutrients but also attachment sites for host bacteria, and it can contribute to the selection of species-specific intestinal microbiota (Johansson et al., 2011).
The results of the present study demonstrate that a broiler diet supplemented with Lacto-Immuno-Vital stimulates the gene expression of MUC-2, total IgA, as well as secretion of MUC-2 in the jejunum of broiler chickens even on the eighth day of their age. The influence of E. faecium EF55 on the dynamics of intestinal mucin production in birds infected with Salmonella Enteritidis was previously demonstrated by Levkut et al. (2012). Similarly, in a recent study, Luan et al. (2019) reported that treatment with B. amyloliquefaciens CECT5940 upregulated gene expression of MUC-2 on the mucosal surface of the respiratory tract in broilers.
Two of the main components of Lacto-immuno-vital are the gram-positive strains B. amyloliquefaciens CECT5940, which has been shown to increase modified IgG and IgA levels in the serum of broilers (Ahmed et al., 2014), as well as E. faecium CECT4515, which has a positive effect on growth and feed intake of broiler chickens (Sanchez et al., 2007). In addition, the preparation includes an extract from the yeast strain Saccharomyces cerevisiae, which contains a large amount of peptides, a mannan oligosaccharide and a β-glucan, which binds pathogenic microorganisms, inhibits their attachment to cells,and increases the length of intestinal villi. Moreover, β-glucans increase the gene expression of tight junction proteins, thereby ensuring integrity of the intestinal wall in chickens (Anwar et al., 2017). In mice, dendritic cell uptake of B. amyloliquefaciens SQR9 alone induced the expression of cytokines and secretion of sIgA (Huang et al., 2016). Secretory IgA produced by IgA+ plasma cells is transported to the lumen of the mucosal layer by epithelial cells, where it protects the epithelium against colonization by pathogens (Macpherson et al., 2008).
Despite these findings, diet supplementation with synbiotic preparation in our case had no significant effect on the concentration of sIgA in the jejunum of the E group. On the other hand, there was no infection, and the broilers remained in good condition. An alternative explanation could involve the combination of different strains, the concentration, or interactions between the strains used. In any case, there are only a few studies about the influence of B. amyloliquefaciens or E. faecium alone on the parameters of mucosal immunity in chickens.
Conclusion
Based on our results, it can be said that Lacto-Immuno-Vital improved growth performance of broilers during the experiment and decreased morbidity and mortality of chickens. Similarly, Lacto-Immuno-Vital increased feed conversion ratio. Moreover, gene expression of IgA, MUC-2, and IGF-2 gene and secretion of MUC-2 in the jejunum were increased in a nonchallenging model.
Disclosures
No potential conflict of interest was reported by the authors.
Acknowledgment
This work was supported by the Grant Agency for Science of Slovak Republic VEGA 1/0355/19 and Slovak Research and Developmental Agency APVV-15-0165.
References
- Ahmed S.T., Islam M., Mun H.S., Sim H.J., Kim Y.J., Yang C.J. Effects of Bacillus amyloliquefaciensas a probiotic strain on growth performance, cecal microflora, and fecal noxious gas emissions of broiler chickens. Poult. Sci. 2014;93:1963–1971. doi: 10.3382/ps.2013-03718. [DOI] [PubMed] [Google Scholar]
- Anwar M.I., Muhammad F., Awais M.M., Akhtar M. A review of β-glucans as a growth promoter and antibiotic alternative against enteric pathogens in poultry. Worlds Poult. Sci. J. 2017;73:651–661. [Google Scholar]
- Beski S., Swick R.A., Iji P.A. Specialized protein products in broiler chicken nutrition: a review. Anim. Nutr. 2015;1:47–53. doi: 10.1016/j.aninu.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.E. Collection, handling, and analysis of specimens for studies of mucosal immunity in animals of veterinary importance. In: Mestecky J., editor. Mucosal Immunology. 4th ed. Academic Press; Cambridge, MA: 2015. pp. 2369–2391. [Google Scholar]
- De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Dina M.W., Hams A.M. Effect of probiotic on Salmonella Enteritidis infection on broiler chickens. Egypt. J. Chem. Environ. Health. 2016;2:298–314. [Google Scholar]
- Fu X., Wang H., Hu P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015;72:1663–1677. doi: 10.1007/s00018-014-1819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev I.P., Georgieva T.M., Pfaffl M., Hammon H.M., Blum J.W. Insulin-like growth factor and insulin receptors in intestinal mucosa of neonatal calves. J. Endocrinol. 2003;176:121–132. doi: 10.1677/joe.0.1760121. [DOI] [PubMed] [Google Scholar]
- Halevy O., Geyra A., Barak M., Uni Z., Sklan D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000;130:858–864. doi: 10.1093/jn/130.4.858. [DOI] [PubMed] [Google Scholar]
- Herich R. Is the role of IgA in local immunity completely known? Food Agr. Immunol. 2017;28:223–237. [Google Scholar]
- Herich R., Kokinčáková T., Lauková A., Levkutová M. Effect of preventive application of Enterococcus faecium EF 55 on intestinal mucosa during salmonellosis in chickens. Czech J. Anim. Sci. 2010;55:42–47. [Google Scholar]
- Huang L., Qin T., Yin Y., Gao X., Lin J., Yang Q., Yu Q. Bacillus amyloliquefaciens SQR9 induces dendritic cell maturation and enhances the immune response against inactivated avian influenza virus. Sci. Rep. 2016;6:21363. doi: 10.1038/srep21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husáková E., Spišáková V., Herich R., Kolesárová M., Stašová D., Levkutová M., Levkut M. Expression of cytokines in chicken peripheral mononuclear blood cells (PMBCs) exposed to probiotic strains and Salmonella Enteritidis. Acta Vet. Brno. 2015;84:29–35. [Google Scholar]
- Chakravarthy M.V., Davis B.S., Booth F.W. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J. Appl. Physiol. 2000;89:1365–1379. doi: 10.1152/jappl.2000.89.4.1365. [DOI] [PubMed] [Google Scholar]
- Janeczko R.A., Etlinger J.D. Inhibition of intracellular proteolysis in muscle cultures by multiplication-stimulating activity. Comparison of effects of multiplication-stimulating activity and insulin on proteolysis, protein synthesis, amino acid uptake, and sugar transport. J. Biol. Chem. 1984;259:6292–6297. [PubMed] [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J., Galfi P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Johansson M.E., Larsson J.M., Hansson G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. USA. 2011;1:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaffová V., Bobíková K., Husáková E., Bobíková K., Levkut M., Herich R., Revajová V., Levkutová M., Levkut M. Interaction of TGF-β4 and IL-17 with IgA secretion in the intestine of chickens fed with E. faecium AL41 and challenged with S. Enteritidis. Res. Vet. Sci. 2015;100:75–79. doi: 10.1016/j.rvsc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Karaffová V., Bobíková K., Levkut M., Revajová V., Ševčíková Z., Levkut M. The influence of Farmatan® and Flimabend® on the mucosal immunity of broiler chicken1. Poult. Sci. 2019;98:1161–1166. doi: 10.3382/ps/pey517. [DOI] [PubMed] [Google Scholar]
- Kawai M., Rosen C.J. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol. Metab. Clin. North. Am. 2012;41:323–333. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers A., Wieland W.H., Kruijt L., Jansma A., Straetemans T., Schots A., den Hartog G., Parmentier H.K. Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev. Comp. Immunol. 2010;34:1254–1262. doi: 10.1016/j.dci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Lei X., Piao X., Ru Y., Zhang H., Péron A., Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Australas J. Anim. Sci. 2015;28:239–246. doi: 10.5713/ajas.14.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkut M., Pistl J., Lauková A., Revajová V., Herich R., Ševčíková Z., Strompfová V., Szabóová R., Kokinčáková T. Antimicrobial activity of Enterococcus faecium EF 55 against Salmonela Enteritidis in chicks. Acta Vet. Hung. 2009;57:13–24. doi: 10.1556/AVet.57.2009.1.2. [DOI] [PubMed] [Google Scholar]
- Levkut M., Revajová V., Lauková A., Ševčíková Z., Spišáková V., Faixová Z., Levkutová M., Strompfová V., Pistl J., Levkut M. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF 55 and challenged with Salmonella Enteritidis. Res. Vet. Sci. 2012;93:195–201. doi: 10.1016/j.rvsc.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Luan S.J., Sun Y.B., Wang Y., Sa R.N., Zhang H.F. Bacillus amyloliquefaciens spray improves the growth performance, immune status, and respiratory mucosal barrier in broiler chickens. Poult. Sci. 2019;98:1403–1409. doi: 10.3382/ps/pey478. [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., McCoy K.D., Johansen F.E., Brandtzaeg P. The immune geography of IgA induction and function. Mucosal. Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Mallo J.J., Rioperez J., Honrubia P. The addition of Enterococcus faecium to diet improves piglet's intestinal microbiota and performance. Livest. Sci. 2010;133:176–178. [Google Scholar]
- Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudronová D., Karaffová V., Košcová J., Bartkovský M., Marcincáková D., Popelka P., Klempová T., Certík M., Macanga J., Marcincák S. Effect of fungal gamma-linolenic acid and beta-carotene containing prefermented feed on immunity and gut of broiler chicken. Poult. Sci. 2018;97:4211–4218. doi: 10.3382/ps/pey306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe-Masselot C., Herrmann A., Carlstedt I., Michalski J.C., Capon C. Glycosylation of the two O-glycosylated domains of human MUC2 mucin in patients transposed with artificial urinary bladders constructed from proximal colonic tissue. Glycoconj. J. 2008;25:213–224. doi: 10.1007/s10719-007-9079-3. [DOI] [PubMed] [Google Scholar]
- Rowland K.J., Choi P.M., Warner B.W. The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Semin. Pediatr. Surg. 2013;22:101–111. doi: 10.1053/j.sempedsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez J., Dzung B.D., Herranz C., Nes I.F., Cintas L.M., Hernández P.E. Amino acid and nucleotide sequence, adjacent genes, and heterologous expression of hiracin JM79, a sec-dependent bacteriocin produced by Enterococcus hirae DCH5, isolated from Mallard ducks (Anas platyrhynchos) FEMS Microbiol. Lett. 2007;270:227–236. doi: 10.1111/j.1574-6968.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P.R., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Ševčíková Z., Blanár J., Lauková A., Revajová V., Strompfová V., Levkut M. Effect of Enterococcus faecium EF 55 on morphpmetry and proloferative activity of intestinal mucosa in broilers infected with Salmonella Enteritidis. J. Vet. Res. 2016;60:261–265. [Google Scholar]
- Wizna Y.R., Abbas H., Dharma A., Kompiang I.P. Influence of dietary fermented tapioca by-products on the performance of broilers and ducklings. Int. J. Poult. Sci. 2009;8:902–904. [Google Scholar]
- Yari Z., Hekmatdoost A. Dietary interventions in fatty liver. In: Watson R.R., Preedy V.R., editors. Foods, Nutrients, and Dietary Supplements. Academic Press; Cambridge, MA: 2019. pp. 245–255. [Google Scholar]
- Žitňan R., Albrecht E., Kalbe C., Miersch C., Revajová V., Levkut M., Jr., Röntgen M. Muscle characteristics in chicks challenged with Salmonella Enteritidis and the effect of preventive application of probiotic Enterococcus faecium. Poult. Sci. 2019;98:2014–2025. doi: 10.3382/ps/pey561. [DOI] [PMC free article] [PubMed] [Google Scholar]