Abstract
The purpose of this study was to evaluate the effects of chitosan oligosaccharides (COS) on intestinal permeability, morphology, antioxidant status, and inflammatory response in heat-stressed broilers. A total of 108 thirty-five-day-old Chinese yellow-feather broilers (body weight 470.31 ± 13.15 g) were randomly allocated to 3 dietary treatments as follows: CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress. Each treatment had 6 replication pens and 6 broilers per pen. Compared with the CON group, heat stress decreased (P < 0.05) the relative weight of duodenum and jejunum; the relative length and villus height (VH) of duodenum, jejunum, and ileum; the ileum VH to crypt depth ratio; duodenum mucosal catalase (CAT) activity; and jejunum mucosal glutathione peroxidase (GSH-Px) and CAT activity, whereas it increased (P < 0.05) serum diamine oxidase (DAO) activity and D-lactate acid (D-LA) content, duodenum and jejunum mucosal malondialdehyde (MDA) and interleukin-1β (IL-1β) content, and ileum mucosal tumor necrosis factor-α content. Compared to the HS group, dietary COS supplementation increased (P < 0.05) the relative length of duodenum, jejunum, and ileum; the VH of jejunum and ileum; and duodenum and jejunum mucosal GSH-Px activity, whereas it decreased (P < 0.05) serum DAO activity and D-LA concentration and duodenum and jejunum mucosal MDA and IL-1β content. These results suggested that dietary COS supplementation had beneficial effects on intestinal morphology by increasing jejunum and ileum VH; permeability by decreasing serum DAO activity and D-LA content; antioxidant capacity by decreasing duodenum and jejunum mucosal MDA content and by increasing duodenum and jejunum GSH-Px activity; and inflammatory response by decreasing duodenum and jejunum mucosal IL-1β content.
Key words: chitosan oligosaccharide, heat stress, intestinal oxidative status, intestinal inflammation, yellow-feather broiler
Introduction
The gut tract played not only an important role in digestibility and absorption of nutrients but also a vital role as body barrier (Hao et al., 2012); therefore, gut health was important for animal health and performance. Pawar et al. (2016) reported that high temperature disturbed physiological homeostasis in broilers and impaired the function of digestive (Quinteiro-Filho et al., 2012) and immune system (Sugiharto et al., 2016), which lead to gut inflammation and dysfunction, including intestinal morphology, immune system, and barrier function (Song et al., 2017; Wang et al., 2018; Cheng et al., 2019). Heat stress impaired intestinal function by inducing overproduction reactive oxygen species (ROS) and proinflammatory cytokines, accompanied with increasing intestinal permeability (Song et al., 2017; Cheng et al., 2019). Therefore, alleviating intestinal inflammation and oxidative stress was an effective way to mitigating intestinal damage. Recent studies demonstrated that gut microbiota balance can alleviate inflammatory response by regulation of microbiota-gut-immunity axis (Brandsma et al., 2015), and there were growing evidences indicating that dietary oligosaccharides supplementation was an effective nutritional manipulation way to alleviate heat stress in broilers (Cheng et al., 2018; Tavaniello et al., 2020).
Chitosan oligosaccharides (COS), one of the functional oligosaccharides, was the degraded product of chitosan or chitin, the second most abundant polysaccharide in nature next to cellulose (Zou et al., 2016). Compared with chitosan, COS had lower molecular weight, better solubility, and less viscous (Naveed et al., 2019). Previous studies demonstrated that COS had various biological activities, including antibacterial (Li et al., 2017; Wan et al., 2017), antioxidant (Li et al., 2017), anti-inflammatory (Xiong et al., 2015; Hyung et al., 2016), immune-stimulating (Li et al., 2019), and free radical–scavenging capacity (Je et al., 2004). In addition, Qiao et al. (2011) reported that COS can ameliorate liver, lung, and kidney inflammation and oxidative stress in lipopolysaccharide-challenged mice. Lan et al. (2019) reported that COS can alleviate liver, spleen, and kidney inflammation and oxidative stress in heat-stressed rats. Moreover, former studies indicated that dietary COS supplementary had beneficial effects on growth performance (Li et al., 2007; Zhou et al., 2009), intestinal morphology (Liu et al., 2008; Li et al., 2019), barrier function (Yang et al., 2012; Li et al., 2019), immunity (Li et al., 2019), and antioxidant capacity (Li et al., 2017) of broilers and weaning pigs. However, limited research studies were available concerning the protective effects of COS on intestinal damage of heat-stressed broilers. However, dietary supplementation with mannan oligosaccharides, cello-oligosaccharides, and galacto-oligosaccharides was efficient to alleviate the detrimental effects of heat stress–induced intestinal damage in broilers (Song et al., 2013; Varasteh et al., 2015; Cheng et al., 2019). Intestinal damage was highly related to intestinal inflammation response and oxidative status. Therefore, we hypothesized that COS could alleviate intestinal damage of heat-stressed broilers by alleviating inflammatory response and improving antioxidant capacity. The purpose of this study was to evaluate the effects of COS on intestinal permeability, morphology, antioxidant status, and inflammatory response in heat-stressed broilers.
Materials and methods
Experiment Design and Dietary Treatments
The experimental procedures were approved by the Animal Care and Use Committee of Guangdong Ocean University (SYXK-2018-0147). A total of 108 thirty-five-day-old Chinese indigenous yellow-feather broilers (Frizzled chicken, body weight 470.31 ± 13.15 g) were purchased from a local breeding company (Zhanjiang, Guangdong province, China), and randomly allocated to 3 treatments. Dietary treatments were CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS and raised under cycle heat stress. Broilers of each replication were assigned in battery pens (124 cm length × 64 cm width × 40 cm height). The basal diets (Table 1) were formulated to meet or exceed the nutrient requirement of the Feeding Standard of Chicken, China (NY/T 33-2004). COS was purchased from Jiangsu Xinrui Biotechnology Co., Ltd. (HPLC purity 95%, deacetylation degree over 95%, and average molecular weight below 32 kDa). The feed was provided in mash form, and the supplementation of COS to the basal diet at the expense of corn.
Table 1.
Basal diet composition (as-fed basis).
| Ingredients | Content, % |
|---|---|
| Corn | 69.95 |
| Soybean | 22.10 |
| Soybean oil | 2.70 |
| Calcium hydrogen phosphate | 1.70 |
| Shell power | 1.93 |
| Salt | 0.35 |
| Met | 0.10 |
| Lys | 0.05 |
| Zeolite powder | 0.80 |
| Vitamin premix1 | 0.16 |
| Mineral premix2 | 0.16 |
| Total | 100.00 |
| Nutrient level | |
| Metabolic energy, MJ/kg | 12.65 |
| Crude protein, % | 16.29 |
| Calcium, % | 1.18 |
| Total phosphorus, % | 0.62 |
| Available phosphorous, % | 0.41 |
| Met, % | 0.36 |
| Lys, % | 0.87 |
| Met + Cys, % | 0.64 |
Provided per kilogram of complete diet: 12,8000IU vitamin A, 1,600IU vitamin D3, 60IU vitamin E, 1.6 mg vitamin K3, 0.12 mg biotin, 50 mg choline, 1.2 mg folic acid, 32 mg nicotinic acid, 16 mg pantothenic acid, 4.8 mg riboflavin, 2.4 mg thiamine (VB1), 3.2 mg vitamin B6, and 0.03 mg vitamin B12.
Provided per kilogram of diet: Mg, 79 mg as manganese oxide; Zn, 60 mg as zinc oxide; Cu 100 mg as copper sulfate; Fe, 120 mg as iron sulfate; I, 0.96 mg as potassium iodine; Co, 0.16 mg as cobalt sulfate; and Se, 0.24 mg as sodium selenite.
Sample Preparation
At the end of the experiment, after 12-hour fast, 6 broilers per treatment (1 broiler from each replication pen) were randomly selected, and blood samples were collected from the brachial vein into nonheparinized tubes and centrifuged at 3,000 g for 10 min at 4°C to obtain serum. The serum samples were stored at −20°C until analysis. Then the broilers were individually weighed and euthanized by cervical dislocation. The length of duodenum (from the pyloric junction to the distal most point of insertion of the duodenum mesentery), jejunum (from the distal most point of insertion of the duodenum mesentery to the junction with Meckel's diverticulum), and ileum (from the junction with Meckel's diverticulum to ileo-caecal junction) was determined with a flexible tape on a glass surface to prevent inadvertent stretching. Then the empty weight of duodenum, jejunum, and ileum were weighted. The relative length and weight of duodenum, jejunum, and ileum were expressed as a percentage of live body weight (cm/kg and g/kg) (Mahdzvi and Torki., 2009). In addition, samples from the duodenum, jejunum, and ileum (2 cm at the midpoint) were fixed in 10% buffered formalin for morphology examination. Then the remaining duodenum, jejunum, and ileum were opened longitudinally and flushed with ice-cold phosphate-buffered saline. Mucosa of each segment sample was collected using a sterile glass microscope slide, rapidly stored in liquid nitrogen, and then frozen at −80°C until analysis.
Serum Parameters Determination
The serum diamine oxidase (DAO) activity and D-lactate acid (D-LA) concentration were measured with commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, P. R. China) according to the manufacturers' instruction.
Intestinal Morphology Examination
The intestinal segments were fixed in 10% buffered formalin for 48 h at room temperature and subsequently dehydrated through a graded ethanol series, then cleared with xylene and finally embedded in paraffin for histological examination. For the histological analysis to measure villus height (VH) and crypt depth (CD), serial tissue sections of 4 μm were cut and mounted 4 sections per side. The sections were deparaffinized, rehydrated, and rinsed in distilled water. Finally, the section was stained with hematoxylin for 2 min and eosin for 40 s, then dehydrated and mounted. The gastrointestinal morphometric variables evaluated VH, CD, and the ratio of VH to CD (VH:CD). Morphological parameters were measured using the Image Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD). Each sample was subjected to 6 replicate measurements for each variable studied, then averaged to generate a mean value for each broiler. The VH was measured from the top of the villus to the top of the lamina propria. CD was measured from the base upward to the region of transition between the crypt and villus.
Determination of Intestinal Mucosal Oxidative Status and Cytokines
About 1 g of duodenum, jejunum, and ileum mucosa sample was homogenized at a ratio of 1:9 (weight/volume) with ice-cold phosphate-buffered saline. Homogenate was centrifuged at 3,000 g for 10 min at 4°C to obtain supernatant and immediately conduct the analysis. The protein concentration of the supernatant was determined by the Bradford method using bovine serum albumin as the standard. The activity of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) and the content of malondialdehyde (MDA), interleukin-1β (IL-1β), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) were measured with corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer's instruction.
Statistical Analysis
The pen was used as the experiment unit, and all data were analyzed with SAS 9.1 (SAS Institute Inc., Cary, NC). All data were analyzed using one-way ANOVA followed by Duncan's multiple range test to analyze differences among treatments. Differences were considered significant at P < 0.05.
Results
Relative Weight and Length of Small Intestine
Compared with the CON group, heat stress decreased (P < 0.05) the relative weight of duodenum and jejunum and the relative length of duodenum, jejunum, and ileum (Table 2). Compared with the HS group, dietary COS supplementation increased (P < 0.05) the relative length of duodenum, jejunum, and ileum.
Table 2.
Effects of chitosan oligosaccharides on the relative weight and length of small intestine in yellow-feather heat-stressed broilers.
| Item1 | CON | HS | HSC | SEM | P value |
|---|---|---|---|---|---|
| Relative weight (g/kg) | |||||
| Duodenum | 6.03a | 4.28b | 5.18ab | 0.47 | 0.2645 |
| Jejunum | 8.62a | 5.69b | 6.16b | 0.58 | 0.0884 |
| Ileum | 5.49 | 3.86 | 4.26 | 0.59 | 0.5713 |
| Relative length (cm/kg) | |||||
| Duodenum | 21.95a | 15.39b | 19.95a | 1.16 | 0.0823 |
| Jejunum | 41.72a | 31.94b | 40.29a | 1.99 | 0.0607 |
| Ileum | 44.01a | 27.61b | 34.79c | 2.23 | 0.0084 |
a-cDifferent superscript letters within the same row means significant difference (P < 0.05).
COS, chitosan oligosaccharides; CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Intestinal Morphology
Compared with the CON group, heat stress decreased (P < 0.05) VH of duodenum, jejunum, and ileum, as well as ileum VH:CD ratio (Table 3). Compared with the HS group, dietary COS supplementation increased (P < 0.05) VH of jejunum and ileum.
Table 3.
Effects of chitosan oligosaccharides on intestinal mucosal morphology in yellow-feather heat-stressed broilers.
| Item1 | CON | HS | HSC | SEM | P value |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height (μm) | 552.08a | 469.81b | 510.76a,b | 21.67 | 0.2927 |
| Crypt depth (μm) | 124.59 | 108.80 | 115.69 | 10.26 | 0.8104 |
| Villus height:crypt depth | 4.64 | 4.47 | 4.45 | 0.45 | 0.8993 |
| Jejunum | |||||
| Villus height (μm) | 429.46a | 305.17b | 391.93a | 25.70 | 0.1104 |
| Crypt depth (μm) | 98.02 | 85.36 | 78.22 | 11.20 | 0.7463 |
| Villus height:crypt depth | 4.53 | 3.96 | 5.21 | 0.56 | 0.4395 |
| Ileum | |||||
| Villus height (μm) | 353.65a | 223.11b | 304.37a | 25.71 | 0.0055 |
| Crypt depth (μm) | 71.22 | 57.42 | 74.96 | 7.10 | 0.4106 |
| Villus height:crypt depth | 5.15a | 4.07b | 4.08b | 0.38 | 0.0669 |
a,bDifferent superscript letters within the same row means significant difference (P < 0.05).
COS, chitosan oligosaccharides; CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress.
Serum DAO Activity and D-LA Content
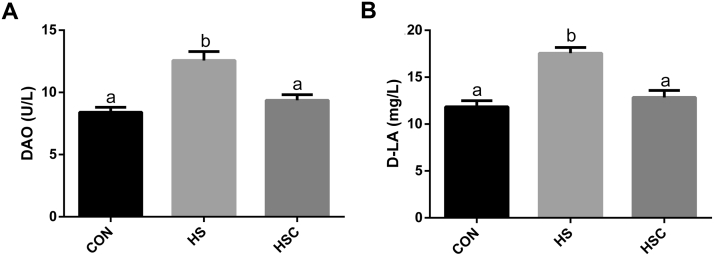
Compared with the CON group, heat stress increased (P < 0.05) serum DAO activity and D-LA content (Figure. 1). Compared with the HS group, dietary COS supplementation decreased (P < 0.05) serum DAO activity and D-LA content.
Figure 1.
Effects of chitosan oligosaccharides (COS) on (A) serum diamine oxidase (DAO) and (B) D-lactate acid (D-LA) in yellow-feather heat-stressed broilers. CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress. Values are mean ± SE (n = 6). The values with different superscript letters are different (P < 0.05).
Intestinal Antioxidant Status
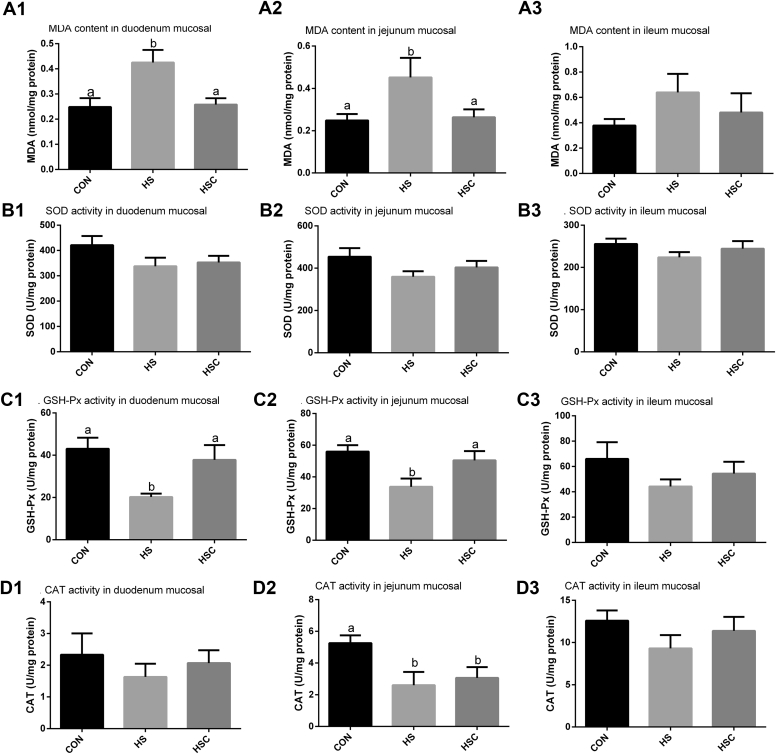
Compared with the CON group, heat stress increased (P < 0.05) duodenum and jejunum mucosal MDA content and decreased (P < 0.05) duodenum GSH-Px activity and jejunum mucosal GSH-Px and CAT activity (Figure 2). Compared with the HS group, dietary COS supplementation decreased (P < 0.05) duodenum and jejunum mucosal MDA content and increased (P < 0.05) duodenum and jejunum mucosal GSH-Px activity. No significant differences were observed in ileum mucosal MDA content, SOD, GSH-Px, or CAT activity among treatments (Figure. 2).
Figure 2.
Effects of chitosan oligosaccharides (COS) on antioxidant status of the small intestine in yellow-feather heat-stressed broilers. CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress. Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase. Values are mean ± SE (n = 6). The values with different superscript letters are different (P < 0.05).
Intestinal Inflammatory Cytokines
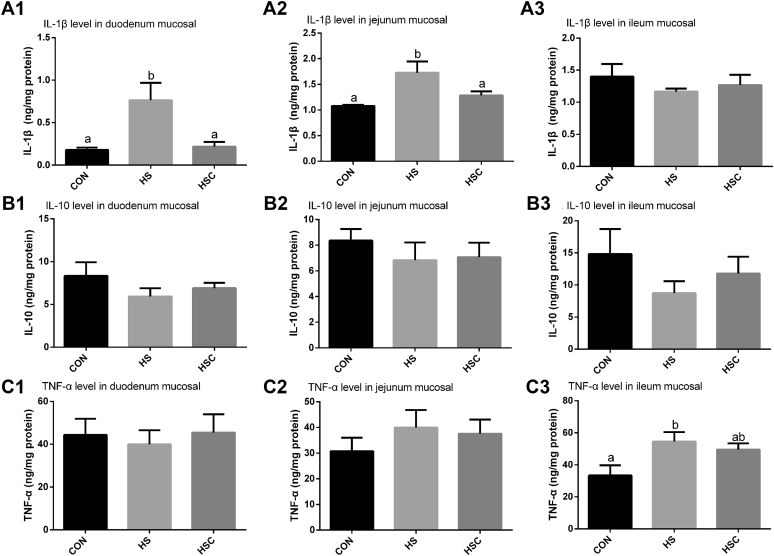
Compared with the CON group, heat stress increased (P < 0.05) duodenum and jejunum mucosal IL-1β level and ileum mucosal TNF-α level (Figure. 3). Compared with the HS group, dietary COS supplementation decreased (P < 0.05) duodenum and jejunum mucosal IL-1β level. No significant differences were observed in duodenum and jejunum mucosal IL-10 or TNF-α level and ileum mucosal IL-1β or IL-10 level among treatments.
Figure 3.
Effects of chitosan oligosaccharide (COS) on inflammatory cytokines of small intestine in yellow-feather heat-stressed broilers. CON group, basal diet and raised under normal temperature (24°C); HS group, basal diet and raised under cycle heat stress (34°C from 10:00–18:00 and 24°C for the rest time); HSC group, basal diet with 200 mg/kg COS supplementation and raised under cycle heat stress. Abbreviations: IL-1β, interleukin-1β; IL-10, interleukin-10; TNF-α, tumor necrosis factor-α. Values are mean ± SE (n = 6). The values with different superscript letters are different (P < 0.05).
Discussion
Heat stress induced multiple pathophysiological alterations and negative effects on growth performance of broilers (Quinterio-Filho et al., 2010). The gastrointestinal tract is considered as one of the main target organs primarily responsive to heat stress. Marchini et al. (2011) reported that heat stress decreased the length of the intestine in broilers. In this study, we also observed that heat stress decreased the relative length of duodenum, jejunum, and ileum. The results suggested that heat stress inhibited the development of the small intestine. However, dietary COS supplementation increased the relative length of duodenum, jejunum, and ileum in heat-stressed broilers, and these results suggested that COS may alleviate intestinal dysplasia induced by heat stress.
Maintaining the normal intestinal morphology was crucial for gut health and growth performance in broilers. The VH, CD, and VH:CD ratio were used as criteria that reflect nutrient digestion and absorption (Montagne et al., 2003). A shorter VH was associated with decreased nutrient absorption surface area (Xu et al., 2003). A deeper CD indicated fast tissue turnover and poor nutrient absorption (Yason and Schat et al., 1987). Previous studies indicated that heat stress induced detrimental effects on intestinal morphology of broilers, resulting in a shorter VH, deeper CD, and a lower VH:CD ratio (Song et al., 2013; Zhang et al., 2017). Similarly, in this study, heat stress decreased VH of duodenum, jejunum, and ileum and ileum VH:CD ratio. Li et al. (2019) reported that dietary COS supplementation increased duodenum VH and VH:CD ratio of duodenum and jejunum and decreased CD of duodenum and jejunum under normal conditions. Liu et al. (2010) reported that dietary COS supplementation can alleviate the damage on intestinal morphology of weanling pigs challenged with Escherichia coli by increasing ileum VH:CD ratio. The results of this study were consistent with aforementioned findings and show that dietary COS supplementation increased the VH of jejunum and ileum. These results suggested that dietary COS supplementation can alleviate heat stress–induced intestinal morphology damage in broilers.
DAO, an intracellular enzyme, was mainly produced in the small intestine mucosa and mainly existed in cytoplasm (Thompson et al., 1987). D-LA was the end product of intraintestinal bacteria (Vella and Farrugia, 1998). When the intestinal barrier was damaged, the permeability of intestinal barrier increased, and a large amount of DAO and D-LA were released into blood (Cheng et al., 2019). Therefore, serum DAO activity and D-LA concentration can work as markers to monitoring intestinal permeability and barrier injury. Previous studies indicated that heat stress increased serum DAO activity and D-LA concentration in broilers (Wu et al., 2018; Cheng et al., 2019). In agreement with former results, we also observed higher serum DAO activity and D-LA concentration in heat-stressed broilers. These results, together with intestinal morphology damage, suggested that heat stress damages intestinal barrier integrity. Former studies indicated that dietary COS supplementation had lower serum DAO activity and D-LA concentration in animals under normal conditions (Yang et al., 2012; Zhao et al., 2017; Li et al., 2019). Similarly, in this study, we observed that dietary COS supplementation decreased serum DAO activity and D-LA concentration in heat-stressed broilers. These results suggested that dietary COS supplementation can alleviate intestinal barrier function damage by decreasing intestinal permeability and maintaining intestinal morphology in heat-stressed broilers.
Oxidative stress was another crucial factor in intestinal function disruption (Cheng et al., 2019). Oxidative stress was a result of the imbalance between the relative levels of ROS and the available antioxidants. Heat stress resulted in overproduction of ROS (Mujahid et al., 2005), lipid peroxidation (Huang et al., 2015), and disruption of the balance between oxidation and antioxidant system (Zhang et al., 2018). In this study, we also observed heat stress induced higher duodenum and jejunum mucosal MDA content, lower duodenum mucosal GSH-Px activity, and jejunum mucosal GSH-Px and CAT activity. Dietary COS supplementation improved the activity of dietary COS supplementation improved the activity of total anti-oxidant capacity, GSH-Px, and SOD and decreased the MDA content of intestine mucosa in broilers under normal conditions (Li et al., 2017, 2019). As expected, dietary COS supplementation decreased MDA content and increased duodenum and jejunum mucosa GSH-Px activity, suggesting that COS supplementation can alleviate heat stress–induced intestine mucosal oxidative stress. Dietary COS supplementation improved intestinal mucosal antioxidant capacity of heat-stressed broilers mainly because of the antioxidant capacity of COS (Naveed et al., 2019).
The production of proinflammatory cytokines had detrimental effects on intestinal integrity. Cytokines played vital roles in inflammatory and immune response; the intestinal barrier disfunction always accompanied increased intestinal mucosal IL-1β, IL-6, and IL-8 levels (Dann et al., 2008; Akbari et al., 2015; Song et al., 2017). Therefore, suppressing the overproduction of proinflammatory cytokines was an effective way to alleviate the intestinal disfunction. In this study, heat stress increased duodenum and jejunum mucosal IL-1β content and ileum mucosal TNF-α content. As expected, dietary COS supplementation decreased duodenum and jejunum mucosal IL-1β content, which was consistent with the results that dietary COS supplementation can modulate the production of inflammatory cytokines and immunoglobulin (Xiong et al., 2015; Wan et al., 2017), indicating that COS had beneficial effects on alleviating intestinal inflammatory response in heat-stressed broilers.
Conclusion
In conclusion, heat stress impaired intestinal morphology (decreased duodenum, jejunum, and ileum VH, and ileum VH:CD; 552.08 μm vs. 469.81 μm; 429.46 μm vs. 305.17 μm; 353.65 μm vs. 223.11 μm; 5.15 vs. 4.07, respectively), permeability (increased serum DAO activity and D-LA content), antioxidant status (increased duodenum and jejunum mucosal MDA content, decreased duodenum and jejunum GSH-Px activity, and jejunum mucosal CAT activity), and enhanced inflammatory response (increased duodenum and jejunum mucosal IL-1β content and ileum mucosal TNF-α content) in heat-stressed broilers. Dietary COS supplementation had beneficial effects on intestinal morphology (increased jejunum and ileum VH; 391.93 μm vs. 305.17 μm; 304.37 μm vs. 223.11 μm, respectively), permeability (decreased serum DAO activity and D-LA content), antioxidant capacity (decreased duodenum and jejunum mucosal MDA content, increased duodenum and jejunum GSH-Px activity), and inflammatory response (decreased duodenum and jejunum mucosal IL-1β content). These results suggested that dietary COS can alleviate heat stress–induced intestinal oxidative stress and inflammatory response in broilers.
Acknowledgements
Financial support provided by program for scientific research start-up funds of Guangdong Ocean University (101402/R18005), College Students' Innovative Entrepreneurial Training Plan Program (S202010566030), and Key Platform Project of Innovation strong school Engineering by Department of Education of Guangdong Province (2018302) is gratefully acknowledged.
Disclosures
The authors declare no conflicts of interest.
References
- Akbari P., Braber S., Alizadeh A., Verheijden K.A., Schoterman M.H., Kraneveld A.D., Garssen J., Fink-Gremmels J. Galacto-oligosaccharides protect the intestinal barrier by maintaining the tight junction network and modulating the inflammatory responses after a challenge with the mycotoxin deoxynivalenol in human Caco-2 cell monolayers and B6C3F1 mice. J. Nutr. 2015;145:1604–1613. doi: 10.3945/jn.114.209486. [DOI] [PubMed] [Google Scholar]
- Brandsma E., Houben T., Fu J., Shiri-Sverdlov R., Hofker M.H. The immunity–diet–microbiota axis in the development of metabolic syndrome. Curr. Opin. Lipidol. 2015;26:73–81. doi: 10.1097/MOL.0000000000000154. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Chen Y., Chen R., Su Y., Zhang R., He Q., Wang K., Wen C., Zhou Y. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019;98:4767–4776. doi: 10.3382/ps/pez192. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Du M., Xu Q., Chen Y., Wen C., Zhou Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018;75:106–111. doi: 10.1016/j.jtherbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Dann S.M., Spehlmann M.E., Hammond D.C., Iimura M., Hase K., Choi L.J., Hanson E., Eckmann L. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 2008;180:6816–6826. doi: 10.4049/jimmunol.180.10.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Gu X., Wang X. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012;91:781–789. doi: 10.3382/ps.2011-01627. [DOI] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Hyung J.H., Ahn C.B., Kim B.I., Kim K., Je J.Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016;793:43–48. doi: 10.1016/j.ejphar.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Je J.Y., Park P.J., Kim S.K. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem. Toxicol. 2004;42:381–387. doi: 10.1016/j.fct.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lan R., Li S., Chang Q., Zhao Z. Chitosan oligosaccharides protect Sprague Dawley rats from cyclic heat stress by attenuation of oxidative and inflammation stress. Animals. 2019;9:1074–1084. doi: 10.3390/ani9121074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cheng Y., Chen Y., Qu H., Zhao Y., Wen C., Zhou Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals. 2019;9:493–505. doi: 10.3390/ani9080493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X., Ding X., Peng X., Chi X., Cui H., Zuo Z., Fang J. Effect of chitosan oligosaccharides on antioxidant function, lymphocyte cycle and apoptosis in ileum mucosa of broiler. Kafkas. Univ. Vet. Fak. Derg. 2017;23:571–577. [Google Scholar]
- Li X., Piao X., Kim S., Liu P., Wang L., Shen Y., Jung S., Lee H. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult. Sci. 2007;86:1107–1114. doi: 10.1093/ps/86.6.1107. [DOI] [PubMed] [Google Scholar]
- Liu P., Piao X., Kim S., Wang L., Shen Y., Lee H., Li S. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008;86:2609–2618. doi: 10.2527/jas.2007-0668. [DOI] [PubMed] [Google Scholar]
- Liu P., Piao X., Thacker P., Zeng Z., Li P., Wang D., Kim S. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010;88:3871–3879. doi: 10.2527/jas.2009-2771. [DOI] [PubMed] [Google Scholar]
- Mahdzvi R., Torki M. Study on usage period of dietary protected butyic acid on performance carcass characteristics, serum metabolite levels and humoral immune response of broiler chickens. J. Anim. Vet. Adv. 2009;8:1702–1709. [Google Scholar]
- Marchini C., Silva P., Nascimento M., Beletti M., Silva N., Guimarães E. Body weight, intestinal morphometry and cell proliferation of broiler chickens submitted to cyclic heat stress. Int. J. Poult. Sci. 2011;10:455–460. [Google Scholar]
- Montagne L., Pluske J., Hampson D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed. Sci. Tech. 2003;108:95–117. [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Naveed M., Phil L., Sohail M., Hasnat M., Baig M.M.F.A., Ihsan A.U., Shumzaid M., Kakar M.U., Husain T., Akabar M. Chitosan oligosaccharide (COS): an overview. Int. J. Biol. Macromol. 2019;129:827–843. doi: 10.1016/j.ijbiomac.2019.01.192. [DOI] [PubMed] [Google Scholar]
- Pawar S., Sajjanar B., Lonkar V., Kurade N., Kadam A., Nirmal A., Brahmane M., Bal S. Assessing and mitigating the impact of heat stress on poultry. Adv. Anim. Vet. Sci. 2016;4:332–341. [Google Scholar]
- Qiao Y., Bai X.F., Du Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011;11:121–127. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L.R.M.D., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Rodrigues M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sá L.R.M.D., Ferreira A.J.P., Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Song Z., Cheng K., Zhang L., Wang T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017;69:184–190. doi: 10.1016/j.jtherbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Song J., Jiao L., Xiao K., Luan Z., Hu C., Shi B., Zhan X. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed. Sci. Tech. 2013;185:175–181. [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress–a review. Ann. Anim. Sci. 2017;17:591–604. [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.S., Vaughan W., Forst C., Jacobs D., Weekly J., Rikkers L. The effect of the route of nutrient delivery on gut structure and diamine oxidase levels. Jpen- Parenter. Enter. 1987;11:28–32. doi: 10.1177/014860718701100128. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10:e0138975. doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella A., Farrugia G. D-lactic acidosis: pathologic consequence of saprophytism. Mayo. Clinic. Proc. 1998;73:451–456. doi: 10.1016/S0025-6196(11)63729-4. [DOI] [PubMed] [Google Scholar]
- Wan J., Jiang F., Xu Q., Chen D., Yu B., Huang Z., Mao X., Yu J., He J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Advances. 2017;7:9669–9679. [Google Scholar]
- Wang W., Yan F., Hu J., Amen O., Cheng H. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Liu N., Wu X., Wang G., Lin L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018;97:2675–2683. doi: 10.3382/ps/pey123. [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H., Wang X., Hu Q., Liu C., Wu X., Deng D., Hou Y., Nyachoti C., Xiao D. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015;93:1089–1097. doi: 10.2527/jas.2014-7851. [DOI] [PubMed] [Google Scholar]
- Xu Z., Hu C., Xia M., Zhan X., Wang M. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang C., Ferket P., Hong Q., Zhou J., Cao G., Zhou L., Chen A. Effect of chito-oligosaccharide on growth performance, intestinal barrier function, intestinal morphology and cecal microflora in weaned pigs. J. Anim. Sci. 2012;90:2671–2676. doi: 10.2527/jas.2011-4699. [DOI] [PubMed] [Google Scholar]
- Yason C.V., Schat K. Pathogenesis of rotavirus infection in various age groups of chickens and turkeys: clinical signs and virology. Am. J. Vet. Res. 1987;48:977–983. [PubMed] [Google Scholar]
- Zhang J., Bai K., Su W., Wang A., Zhang L., Huang K., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X., Yang L., Chen X., Jiang R., Jin S., Geng Z. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhao P., Piao X., Zeng Z., Li P., Xu X., Wang H. Effect of Forsythia suspensa extract and chito-oligosaccharide alone or in combination on performance, intestinal barrier function, antioxidant capacity and immune characteristics of weaned piglets. Anim. Sci. J. 2017;88:854–862. doi: 10.1111/asj.12656. [DOI] [PubMed] [Google Scholar]
- Zhou T., Chen Y., Yoo J., Huang Y., Lee J., Jang H., Shin S., Kim H., Cho J., Kim I. Effects of chitooligosaccharide supplementation on performance, blood characteristics, relative organ weight, and meat quality in broiler chickens. Poult. Sci. 2009;88:593–600. doi: 10.3382/ps.2008-00285. [DOI] [PubMed] [Google Scholar]
- Zou P., Yang X., Wang J., Li Y., Yu H., Zhang Y., Liu G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181. doi: 10.1016/j.foodchem.2015.06.076. [DOI] [PubMed] [Google Scholar]