Abstract
This study was conducted to evaluate potential hormonal mechanisms associated with the stress response, thermoregulation, and metabolic changes of broiler chickens exposed to high environmental temperature. Nine hundred 1-day-old male broiler chicks (Ross 708) were placed in floor pens and raised to 24 d. At 24 d, chicks were randomly assigned to 1 of 2 treatments, heat stress (HS) or no HS, and allocated into battery cages in 8 batteries (10 birds per cage, 2 cages per battery). On day 31, blood was collected prior to HS and analyzed using an iSTAT analyzer. Half of the batteries were then moved into 2 rooms with an elevated ambient temperature (35°C) for 8 h. The remaining batteries stayed in the thermoneutral rooms with an ambient temperature of 22°C. Beginning at 5 h after the initiation of HS, blood was collected and analyzed using an iSTAT analyzer, birds were euthanized, and hypothalamus and pituitary samples were collected (16 birds per treatment), flash frozen, and stored at −80°C until RNA extraction. Reverse transcription-quantitative PCR was used to compare mRNA levels of key corticotropic and thyrotrophic genes in the hypothalamus and pituitary. Levels of mRNA for each target gene were normalized to PGK1 (pituitary) and GAPDH (hypothalamus) mRNA. Differences were determined using mixed model ANOVA. HS decreased (P < 0.05) feed intake, BW, bicarbonate, potassium, CO2, and triiodothyronine, while it increased mortality, glucose, pH, plasma thyroxine, and corticosterone. Expression of pituitary corticotropin-releasing hormone receptor 1 was downregulated (P < 0.001), while corticotropin-releasing hormone receptor 2 mRNA levels were higher (P = 0.001) in HS birds. HS increased expression of thyroid hormone receptor β (P = 0.01) (2.8-fold) and thyroid stimulating hormone β (P = 0.009) (1.4-fold). HS did not affect levels of mRNA of genes evaluated in the hypothalamus. Results showed that HS significantly affected both the thyrotropic and corticotropic axes. Understanding the role and regulation of these pathways during HS will allow researchers to better evaluate management strategies to combat HS.
Key words: broiler, heat stress, physiology, hypothalamus, pituitary
Introduction
As global temperatures continue to increase, poultry producers are challenged to find ways to alleviate the negative effects of heat stress (HS) on poultry raised for meat and eggs. When the environmental temperature and RH are high, a negative balance is created between the amount of heat that can flow from the animal's body to the environment (Sahin et al., 2009; Lara and Rostagno, 2013). To maintain their body temperature within a comfortable range, birds reduce their level of physical activity and feed intake, thereby reducing the amount of metabolic heat generated. Despite these adjustments in behavior, if the temperature rises to a point where the bird is unable to naturally cool down and an external method of heat dissipation is not used to reduce the ambient temperature, birds experience HS and often die from complications arising from this condition. As a result of HS, poultry producers in the United States and around the world have suffered reduced productivity, and increased mortality and economic losses (Abdelqader and Al-Fataftah, 2014; Habashy et al., 2017). In addition to the negative effects HS has on production, producers have gained more scrutiny from animal welfare groups, making this an even greater concern from an animal well-being point of view (Lara and Rostagno, 2013). Hence it is imperative that a suitable HS abatement strategy be developed. For an effective strategy to be identified, it is important that the underlying physiological mechanisms that control the response to HS be evaluated and understood.
Birds that are stressed respond with a myriad of mechanisms through the communication of various organs and tissues. These events are displayed by a change in behavior and performance, which are often the focus when evaluating animal health and well-being. However, as technology has improved, the underlying mechanisms that coordinate these responses can now be understood more thoroughly, allowing for the development of more specific and stringent mitigation strategies. The hypothalamus and pituitary are components of the neuroendocrine system that play critical roles in maintaining vertebrate homeostasis by regulating metabolism, growth, body composition, reproduction, and the stress response (Ellestad et al., 2011; Frigerio, 2012). If birds are exposed to a stressor for an extended period, a disruption in physiological processes can be harmful (Yahav, 2015) and result in death. Therefore, to counteract the negative consequences of a stressor, various organs under the action of the neuroendocrine system are activated to regain homeostasis.
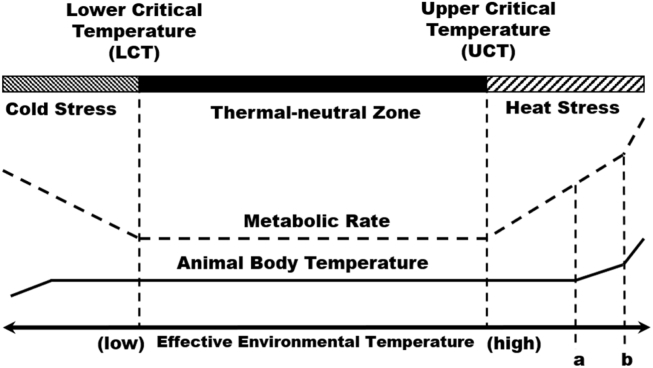
In birds, like other vertebrates, the hypothalamo–pituitary–adrenal axis regulates the response to a stressor (Kuenzel and Jurkevich, 2010). The hypothalamus functions as a central processor that responds to both external environmental stimuli (e.g., temperature) and internal physiological signals (e.g., hormones and metabolite levels), integrates these cues, and modulates physiological processes. Specifically, body temperature is monitored by thermoreceptors located in the aortic arch and carotid arteries that signal to control centers located in the hypothalamus (Hammel and Pierce, 1968). The effective environmental temperature (EET) is a combination of air temperature, RH, air velocity, and radiant heat from the surrounding surfaces (Sahin et al., 2009). When the EET drops below a lower critical temperature, the thermoreceptors signal to the central nervous system, initiate shivering to generate heat, and stimulate thyroid hormone secretion to increase the metabolic rate and heat production. On the other hand, when the EET rises above an upper critical temperature (UCT), the central nervous system initiates panting to cool the body through evaporation. The range of temperatures between the lower critical temperature and UCT is the thermal neutral zone, where no energy is expended specifically to maintain body temperature (Figure 1). When the temperature rises above the UCT and the bird's natural mechanisms are unable to efficiently cool it, an external source must be utilized to lower the EET.
Figure 1.
Relationships between environmental temperature, animal body temperature, and metabolic rate. The EET is the combination of air temperature, humidity, air movement, conduction, and radiant heat. When the EET decreases below the LCT, the animal begins to shiver, which increases its metabolic rate to produce heat. When the EET increases above the UCT, the animal begins to pant, which also increases its metabolic rate. The range of EETs between the LCT and the UCT is the thermal neutral zone. Above the UCT, panting can maintain body temperature to a point when body temperature begins to increase along with EET (a). At some point, EET and body temperature increase too far, and spiraling hyperthermia results (b), which will result in death if the EET is not reduced. Abbreviations: EET, effective environmental temperature; LCT, lower critical temperature; UCT, upper critical temperature.
To ensure that the temperature in commercial poultry houses is maintained within a suitable range, houses in the United States and other developed countries are equipped with cooling and tunnel ventilation systems. However, even with proper cooling and ventilation systems, excessively high temperatures during a heat wave can easily raise the EET above the thermal neutral zone of chickens. In addition, farmers in some developing countries do not have access to state-of-the-art equipment, therefore making heat abatement in these countries an even greater challenge (Wang et al., 2018). Though there are several studies that highlight the negative consequences of HS on production (Niu et al., 2009; Quinteiro-Filho et al., 2010), few studies have focused on the physiological and molecular mechanisms that are affected by and are regulating the response to HS (Borges et al., 2004). A thorough understanding of the physiological and molecular mechanisms that regulate HS responses will enable researchers to better develop and evaluate management strategies to combat HS. Therefore, the objective of this study was to evaluate the effects of HS on the performance, physiology, and mRNA expression in the corticotropic and thyrotropic neuroendocrine axes of broiler chickens.
Materials and methods
Animal Husbandry and HS Challenge
Animal husbandry and experimental procedures were reviewed and approved by the institutional animal care and use committee of the University of Maryland. On day 1, nine hundred 1-day-old Ross 708 male broiler chicks were randomly placed into 1 of 8 floor pens in 2 rooms (4 pens per room). Birds had unrestricted access to a commercial corn-soybean starter diet (22.1% CP, 1.23% digestible Lys, 3,080 kcal/kg ME, 1% Ca, and 0.50% non-phytate phosphorus) until 18 d of age. On day 18, birds were switched to a commercial-type corn-soybean grower diet (20.7% CP, 1.12% digestible Lys, 3,150 kcal/kg ME, 0.8% Ca, and 0.35% non-phytate phosphorus). On day 24, chicks from each pen were allocated into battery cages in 8 Petersime grower batteries (Centrumstraat, Zulte, Belgium) (10 birds per cage, 2 cages per battery) in such a way that weight variation was minimized among cages and within the cage (n = 10 birds). Battery cages (199.4 cm long, 68.6 cm wide, and 29.2 cm high) were equipped with 4 nipple drinkers and 4 feeders (63.5 cm long, 8.9 cm wide, and 5.7 cm deep) and housed in 2 rooms under control conditions (18L:6D and at 22°C; 21°C–23°C). Feed intake and BW were monitored from day 28 until day 31 after HS and were adjusted for mortality and number of birds removed for sampling during HS. The HS challenge occurred on day 31 and simulated mid-day temperatures during a summer heat wave. For the HS challenge, half of the batteries (n = 4) from each room were placed in 2 rooms with an elevated ambient temperature (35°C; 34°C–36°C), while the remaining batteries stayed in the same rooms with an ambient temperature of 22°C (21°C–23°C). Birds were HS challenged for 8 h, and were monitored constantly for any sign of HS using our HS assessment score sheet (Table 1). When any bird reached category 4 (heavy panting, unresponsive to human approach or touch, glassy-eyed, and stupefied), they were removed from the battery cage, weighed, and moved to a control room at 22°C. Birds reaching category 4 were classified as a mortality, and were euthanized humanely.
Table 1.
Heat assessment score sheet.
| Score | Behavioral observations |
|---|---|
| 0 | Normal, no signs of heat stress |
| 1 | Mild panting |
| 2 | Heavy panting |
| 3 | Heavy panting, no movement away from human approach, but aware of their environment |
| 4 | Heavy panting, non-responsive to human approach or touch, glassy-eyed, stupefied |
Blood and Tissue Collection
Blood samples (1 mL) were taken from the brachial vein from 2 birds per cage from control and HS birds before (pre-stress) the HS heat challenge period. Starting at 5 h (5–8 h) after the application of HS, 2 birds per cage were weighed and blood samples (post-stress) were taken. Immediately after collection, blood samples were analyzed for pH, pO2, pCO2, HCO3−, glucose, Na+, and K+ using a VetScan i-Stat analyzer and EC8+ cartridges (Abaxis, Union City, CA). Subsequently, plasma was obtained by centrifugation (2,000 × g for 10 min at room temperature) and was stored at −20°C before analysis. Plasma samples were assayed for circulating levels of triiodothyronine (T3), thyroxine (T4), and corticosterone (CORT). Thyroid hormones were measured by radioimmunoassay (MP Biomedicals, Santa Ana, CA), while CORT was determined using an ELISA assay (Cayman, Ann Arbor, MI) according to the manufacturer's instructions. Immediately following blood collection, birds were euthanized, and hypothalamus and pituitary samples were collected from 16 birds per treatment (2 birds per cage per battery per room), flash frozen, and stored at −80°C until RNA extraction. Feed intake was recorded for each cage after the end of the HS challenge.
Reverse Transcription-Quantitative PCR (RT-qPCR) Assays
Total RNA was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. Before elution of RNA, an on-column deoxyribonuclease digestion was done to remove genomic DNA. Total RNA was quantified using Quant-iT RiboGreen RNA Quantification reagent (Invitrogen, Carlsbad, CA). After quantification, cDNA was synthesized from 1 μg of total RNA in 20 μL reactions using M-MLV RT kit (New England Biolabs, Ipswich, MA) following the manufacturer's instructions. To control for genomic DNA contamination, a sample was made by pooling total RNA from each sample and the reaction was conducted without reverse transcriptase (no RT). Reactions were diluted to 100 μL prior to use in PCR reactions. Primers (Table 2) for real time quantitative PCR for 4 genes were designed using Primer Express 2.0 (Applied Biosystems, Waltham, MA) and validated according to the procedures previously described (Ellestad et al., 2011). All other primers were previously designed, validated, and published (Ellestad et al., 2011). RT-qPCR was performed in duplicate 15 μL reactions that contained 2 μL cDNA template (20 ng), 0.6 μL (10 μm) of each forward and reverse primer, 7.5 μL 2X Master Mix (PCR buffer [50 mM KCl, 10 mM Tris–HCl, 0.1% triton-X-100], 0.12 U/μL Taq Polymerase, 0.2 μM dNTPs, 40 nM fluorescein [Invitrogen, Waltham, MA], and SYBR Green I Nucleic Acid Gel Stain diluted 1:10,000 [Invitrogen, Waltham, MA]), and 4.3 μL of water. PCR was performed for 40 cycles under the following conditions: 95°C for 15 s, 60°C for 30 s, and then 72°C for 30 s. The normalization genes used for relative expression calculations were PGK1 and GAPDH for the pituitary and hypothalamus, respectively. The data were transformed using the equation 2−ΔΔ Ct, where Ct represents the fractional cycle number when the amount of amplified product reached a fixed threshold for fluorescence. The data are presented as fold changes relative to the control.
Table 2.
Primer sequences for quantitative PCR.
| Gene | Forward | Reverse |
|---|---|---|
| Type II iodothyronine deiodinase | TGAAGCACATGGTGCTGTTTC | TTGCCCTTGGCTATGTGGAT |
| Thyroid hormone receptor β | AAATGGGGGTCTTGGCGTAG | ATGCCAGGAGGAAACCCTCT |
| Thyroid hormone receptor α | CATCTTCGACCTCGGCAAGT | GGTACGTCTCCTGGCACTTC |
| Glucocorticoid receptor | TTTGGAATTGCCATCGGTAT | GCCTGGAGACATTTCCGATA |
Statistical Analysis
In this study, the effect of 2 environments (treatments; HS vs. control) was studied using a completely randomized design. The experimental units were cages with 2 batteries in each room (10 birds per cage) (2 cages per battery). Data were analyzed for normality using the Shapiro–Wilk test and homogeneity of variance using SAS (version 9.4, SAS Institute Inc., Cary, NC) and statistical significance was set to P < 0.05. A mixed model ANOVA was used to compare blood metabolites and hormones before (pre-stress) and after HS (post-stress). All other data were analyzed using one-way ANOVA and Fisher's least significant difference post hoc comparisons for mean separation were used for each dataset. Levels of mRNA for each target gene were normalized to PGK1 and GAPDH mRNA levels in the pituitary and hypothalamus, respectively. Normalized RT-qPCR data were log2 transformed before statistical analysis. The percent mortality of each treatment was calculated, and the data were arc-sin transformed to adjust for normality.
Results
Effect of HS on Performance of Broilers
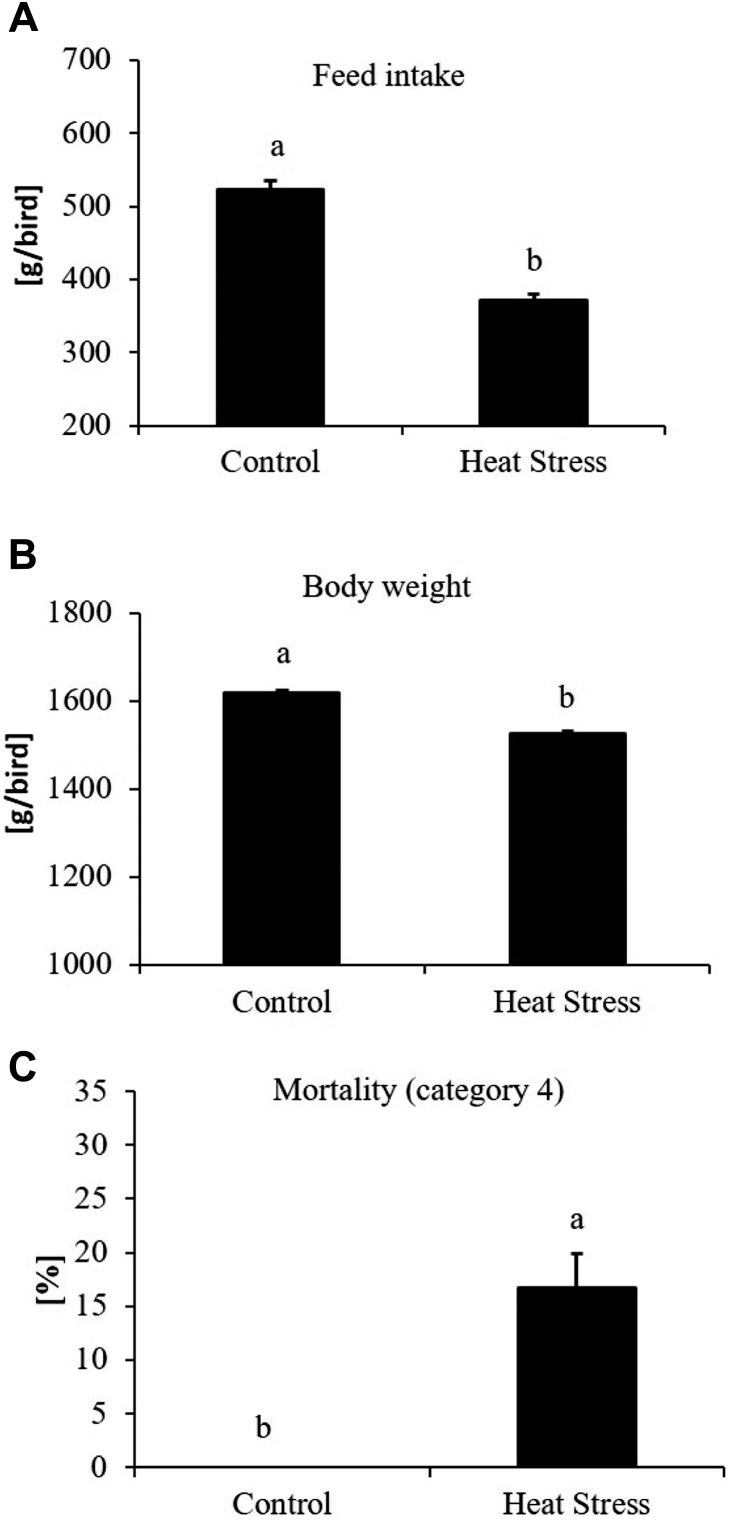
Broiler performance was determined by measuring feed intake and bird weight. Previous studies have shown that chickens decrease feed intake during periods of HS (Niu et al., 2009; Quinteiro-Filho et al., 2010), presumably to reduce the amount of metabolic heat generated. Results showed that birds that were exposed to 8 h of HS had a lower feed intake (P < 0.05) from day 28 to day 31 (Figure 2A) when compared to the control birds. The impact of HS was also evident in the differences between BW. Heat-stressed birds had a lower BW (P < 0.05) at the time of sampling on day 31 when compared to the control birds (Figure 2B). In this study, birds that died during HS or those that were classified as category 4 were recorded as mortality. HS resulted in a higher mortality rate, with 20% of the birds succumbing to the HS challenge while there were no deaths in the no HS group (Figure 2C).
Figure 2.
Average feed intake per bird from day 28 to day 31 (A), BW per bird from day 31 (B), and mortality (C) of broilers exposed to heat stress on day 31. Means with the same letter (a, b) do not differ significantly (P ≤ 0.05).
Effect of HS on Blood Gases, Circulating Metabolites, and Hormones in Broilers
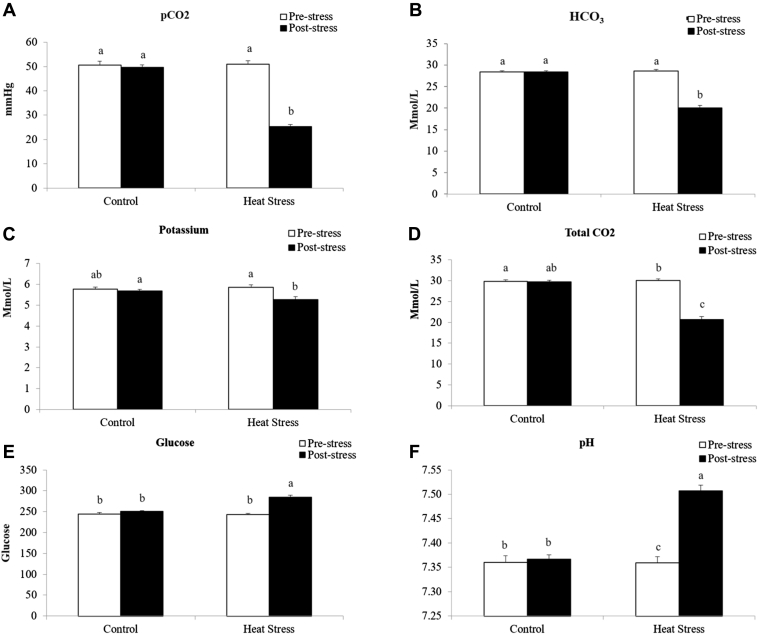
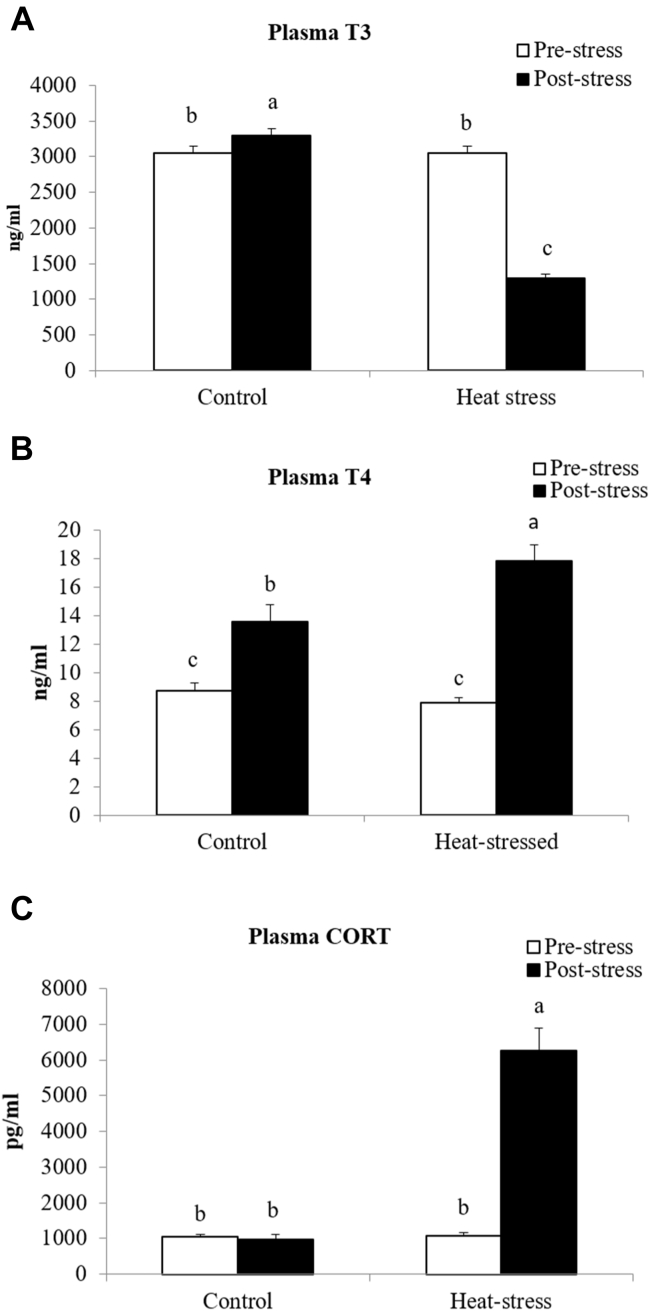
The primary way that birds dissipate heat is through gular flutter and then later through true panting or hyperventilation. Although this process is effective at dissipating heat, it results in the loss of CO2 and water. Changes in CO2 have a direct impact on blood pH, blood gases, and other circulating metabolites. Therefore, these parameters were measured during HS. Blood samples from HS birds had higher (P < 0.05) blood pH (Figure 3F) and glucose levels (Figure 3F) as well as decreased pCO2 (Figure 3A), HCO3 (Figure 3B), K+ (Figure 3C), and total CO2 (Figure 3D) levels when compared to the control birds. These results indicate that HS birds had an electrolyte and acid–base imbalance due to increased loss of CO2, also known as respiratory alkalosis. HS significantly decreased plasma T3 (Figure 4A) by 62%. Contrastingly, HS increased circulating levels of T4 (Figure 4B) by 38% when compared to the control birds. Birds subjected to the HS (6,000 pg/mL) challenge had a 6 X higher level of circulating CORT compared to the control birds (Figure 4C).
Figure 3.
Plasma partial pressure of CO2 (pCO2; A), bicarbonate (HCO3; B), potassium (C), CO2 (D), glucose (E), and pH (F) of broilers exposed to heat stress on day 31. Means with the same letter (a, b) do not differ significantly (P ≤ 0.05).
Figure 4.
Plasma triiodothyronine (T3; A), thyroxine (T4; B), and corticosterone (CORT; C) of broilers before and after exposure to heat stress on day 31. Blood samples were collected before and after heat stress. Means with the same letter (a, b) do not differ significantly (P ≤ 0.05).
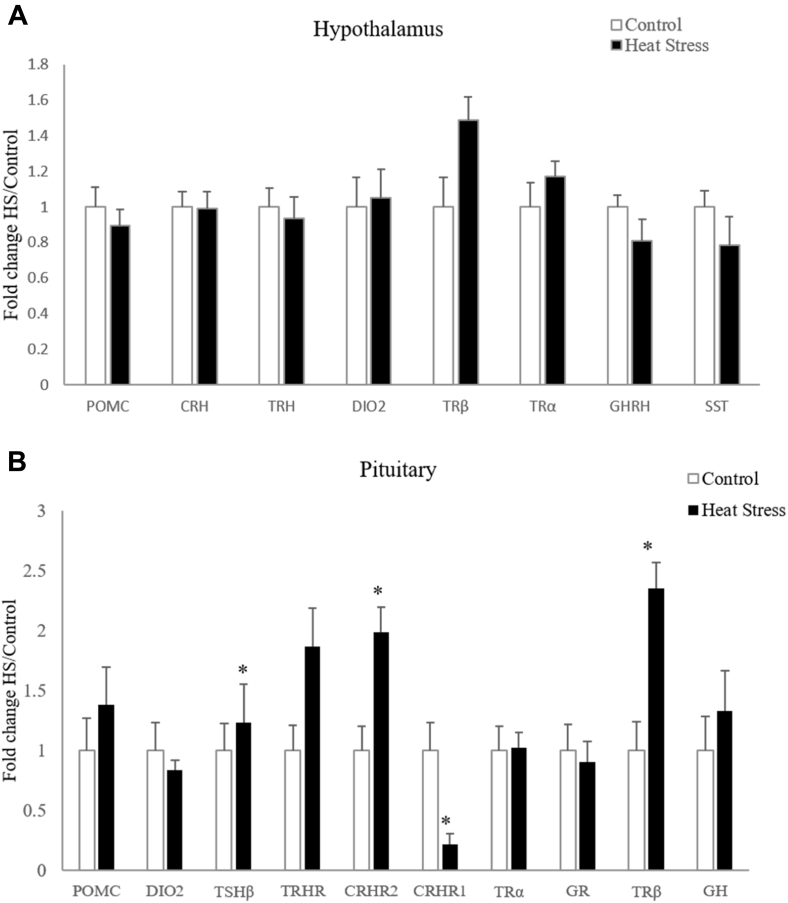
Effect of Heat Stress on Gene Expression in the Pituitary and Hypothalamus
To further define the effects of HS, differences in the expression of genes in the corticotropic and thyrotropic neuroendocrine axes were determined using RT-qPCR. There were no significant changes in mRNA levels for pro-opiomelanocortin (POMC), corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), type II iodothyronine deiodinase, thyroid hormone receptor β (TRβ), thyroid hormone receptor α, growth hormone-releasing hormone, and somatostatin in the hypothalamus (Figure 5A). HS increased the activity of the corticotropic axis. In addition to the increased circulating CORT levels, gene expression results showed that the pituitary expression of corticotropin-releasing hormone receptor 1 (CRHR1) was decreased (P < 0.001) due to HS (Figure 5B). HS also affected levels of mRNA for genes in the thyrotropic axis. In the pituitary, mRNA abundance for thyroid stimulating hormone β (TSHβ), corticotropin-releasing hormone receptor 2 (CRHR2) (P = 0.001), and TRβ were increased (P = 0.01) due to HS. In contrast, levels of mRNA for POMC, type II iodothyronine deiodinase, TRH receptor, thyroid hormone receptor α, glucocorticoid receptor, and growth hormone were not affected by HS.
Figure 5.
Levels of mRNA for POMC, CRH, TRH, DIO2, TRβ, TRα, GHRH, and SST in the hypothalamus (A) and POMC, DIO2, TSHβ, TRHR, CRHR2, CRHR1, TRα, GR, TRβ, and GH in the pituitary (B) of broilers exposed to HS on day 31. Normalized data are presented relative to the control for each gene. Means with an asterisk (∗) differ significantly from the control (P ≤ 0.05). Abbreviations: CRH, corticotropin-releasing hormone; CRHR1, corticotropin-releasing hormone receptor 1; CRHR2, corticotropin-releasing hormone receptor 2; DIO2, type II iodothyronine deiodinase; GH, growth hormone; GHRH, growth hormone-releasing hormone; GR, glucocorticoid receptor; HS, heat stress; POMC, pro-opiomelanocortin; SST, somatostatin; TRα, thyroid hormone receptor α; TRβ, thyroid hormone receptor β; TRH, thyrotropin-releasing hormone; TRHR, thyrotropin-releasing hormone receptor; TSHβ, thyroid stimulating hormone β.
Discussion
HS continues to pose a problem for poultry production around the world. Birds exposed to HS experience great discomfort and in some cases end up dying resulting in high mortality rates. Consequently poultry producers suffer significant economic losses (Habashy et al., 2017). Although some houses are equipped with state-of-the-art cooling equipment, high temperatures during the summer months can result in heat waves. During heat waves, cooling equipment is often not efficient enough to reduce the EET in a timely manner. Additionally, some of the cooling strategies utilize cooling pads or foggers that rely on the use of water to reduce the temperature. Consequently this may result in an increase in RH leading to a decrease in EET. In addition, in some countries, poultry producers do not have access to this equipment, making cooling an even greater challenge. Birds exposed to unabated heat for extended periods utilize evaporative cooling, an energetically expensive process which also results in loss of water (Molokwu and Olsson, 2016). Consequently, birds also alter their behavior and metabolism through the concerted actions of various tissues and organs to reduce the amount of heat they generate (Jastrebski et al., 2017). Changes in metabolism and behavior are often reflected in bird performance. In the present study, feed intake and BW were significantly decreased by HS. Similar results were obtained by Quinteiro-Filho et al. (2010), who also reported decreased performance in broilers that were heat stressed. Although some birds can cope with HS by reducing feed intake and their level of physical activity, if the temperature is not reduced within a certain time some birds succumb to high temperatures and those that survive are sometimes unable to fully recover. According to the National Chicken Council, broiler producers currently have a mortality rate lower than 5% (www.nationalchickencouncil.org) with most of the mortality occurring in the first week (Yassin et al., 2009). A high mortality rate results in a significant decrease in the amount of marketable products and can lead to economic loss to producers. In the present study, 20% of the birds exposed to the HS challenge were unable to recover and either died or were humanely euthanized.
One of the negative consequences of HS is respiratory alkalosis. In addition to reducing their level of physical activity, birds also utilize gular flutter and panting to dissipate excess heat. However, as they increase panting, they exhale CO2 and decrease the amount of bicarbonate in the blood, consequently decreasing the concentration of hydrogen ions that increases the pH of plasma (Zaboli et al., 2017). In this study, HS decreased CO2 and bicarbonate and increased pH. In addition, birds that were heat stressed also had a significantly lower level of circulating K+. Respiratory alkalosis promotes the loss of K+ in the urine and can also result in hemodilution due to increased water intake (Park and Kim, 2016). Combined, these results indicate that heat-stressed birds experienced respiratory alkalosis and other perturbations in blood chemistry. These changes in blood chemistry likely contributed to the mortality observed.
Stress responses in poultry are mediated mainly by activation of the hypothalamo–pituitary–adrenal axis of the sympathetic nervous system (Lin et al., 2006). The primary glucocorticoid or stress hormone in chickens is CORT. Results in this study showed that heat-stressed birds had a significantly higher level of CORT. This also coincided with an increase in the level of circulating glucose, a known effect of elevated CORT. Combined, these results serve as an indication that HS resulted in a cascade of downstream stress-related events. The secretion of CORT has been shown to induce the breakdown of muscle protein and adipose tissue, promoting gluconeogenesis and improving resistance to stress (Xu et al., 2018). Hence the increase in glucose could be a result of increased hepatic gluconeogenesis, in which lactate is partly converted to glucose (Willemsen et al., 2011). According to Xie et al. (2015), an increase in blood glucose may be part of the fight-or-flight response that aids in the survivability of chickens, with birds that survive a stressor having higher levels of circulating glucose. They found that broiler breeders exposed to HS above 33°C had significant changes in their blood metabolites including circulating glucose. In addition to CORT, the thyroid hormones have been shown to be impacted during HS. Thyroid hormones have various effects on development and energy metabolism in vertebrates (Decuypere et al., 2005), as well as increasing respiratory rate (Souza et al., 2016). The thyroid gland secretes T4, which is converted into T3 by the liver and other tissues. T3 is the primary regulator of metabolic rate and heat production in vertebrates. Our results showed that while HS increased the T4 concentration, the T3 concentration was significantly decreased. Thyroid hormone secretion is negatively correlated with ambient temperature, and at high ambient temperatures the activity of thyroid hormones is reduced thereby depressing bird performance (Abhay et al., 2015). This could be considered a protective effect developed by the birds to reduce their level of activity and decrease the amount of heat generated. The decrease in plasma T3 and increase in circulating T4 levels suggest that decreased hepatic conversion of T4 to T3 by type I iodothyronine deiodinase in the liver might be part of the mechanism involved. Alternatively, a reduction in negative feedback on the thyrotropic axis due to lower T3 levels might have resulted in increased secretion of T4 by the thyroid gland.
In the present study, corticotropic, thyrotropic, and somatotropic genes expressed in the hypothalamus and pituitary were profiled to determine the effect of HS on their expression. Our results confirmed that HS influenced the corticotropic axis. In addition to the increased levels of CORT in circulation, CRHR1 mRNA levels in the pituitary were significantly decreased by HS. Corticotropin-releasing hormone receptor 1 is the primary CRH receptor mediating CRH regulation of adrenocorticotropic hormone (ACTH) secretion (Mo et al., 2015). The decrease in pituitary mRNA levels for CRHR1 in response to HS might be in response to the elevated levels of plasma CORT observed through negative feedback regulation of the corticotropic axis. In contrast, pituitary mRNA levels for CRHR2 were increased in response to HS. Previous reports suggest that CRHR2 is involved in CRH regulation of thyroid stimulating hormone secretion, rather than ACTH secretion, from the anterior pituitary gland (De Groef et al., 2004). Our observation of increased levels of pituitary CRHR2 mRNA and decreased plasma levels of T3 during HS is consistent with reduced negative feedback regulation by T3 of CRHR2 expression in pituitary thyrotrophs. Consistent with reduced negative feedback in the thyrotropic axis, we observed a large increase in mRNA levels for TRβ and a modest increase in mRNA levels for TSHβ in the pituitary gland in response to HS. Levels of TRβ mRNA in the hypothalamus were numerically but not statistically greater in response to HS. Analysis of mRNA levels for growth hormone in the anterior pituitary gland and growth hormone-releasing hormone and somatostatin in the hypothalamus revealed no effects of HS. However, we cannot exclude the possibility that prolonged or chronic HS could affect the somatotropic axis. Taken together, our results indicate that the effects of HS on corticotropic and thyrotropic gene expression occurred primarily at the level of the anterior pituitary, rather than the hypothalamus, and that these effects were consistent with alterations in negative feedback from circulating levels of CORT and T3, respectively. This might suggest that hypothalamic or pituitary responses to HS are not driving but rather responding to alterations in CORT and T3 during HS. However, it must be noted that only mRNA levels were measured. Altered release of CRH and TRH peptides from the hypothalamus into the portal vessels and ACTH and thyroid stimulating hormone from the anterior pituitary gland into the systemic circulation could be driving changes in circulating levels of CORT and T3 without any underlying changes in CRH, TRH, POMC, or TSHβ mRNA levels.
In conclusion, acute HS in broiler chickens resulted in alterations in blood gasses and blood chemistry, consistent with respiratory alkalosis, increased plasma CORT and decreased T3, consistent with stress responses and reduced metabolism, and adaptations in pituitary expression of corticotropic and thyrotropic axis genes, consistent with responses to negative feedback loops. However, repeated or chronic increases in circulating CORT would likely increase catabolism of skeletal muscle, and similar prolonged decreases in circulating T3 would likely contribute to reduced body growth. Mechanisms linking the observed changes in blood chemistry and hormone levels with bird performance during HS conditions remain to be established, and effects of chronic HS on the parameters measured and any possible adaptations of these systems during chronic HS remain unstudied.
Disclosures
All authors declare that no conflicts of interest exist with the research presented in this manuscript.
Acknowledgments
Funding was provided by Agriculture and Food Research Initiative Competitive Grant # 2017-67015-26572 from the USDA National Institute of Food and Agriculture to T. E. Porter. The authors would like to thank Mason Trappio, Joy Penaso, and the staff at the animal care facility for their assistance with conducting this study.
References
- Abdelqader A., Al-Fataftah A.-R. Thermal acclimation of broiler birds by intermittent heat exposure. J. Therm. Biol. 2014;39:1–5. [Google Scholar]
- Abhay K., Tapan K., David C.C., Sudipto H. Effects of supplementation of betaine hydrochloride on physiological performances of broilers exposed to thermal stress. Open Access Anim. Physiol. 2015;7:111–120. [Google Scholar]
- Borges S.A., Fischer da Silva A.V., Majorka A., Hooge D.M., Cummings K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram) Poult. Sci. 2004;83:1551–1558. doi: 10.1093/ps/83.9.1551. [DOI] [PubMed] [Google Scholar]
- Decuypere E., Van As P., Van der Geyten S., Darras V.M. Thyroid hormone availability and activity in avian species: a review. Domest. Anim. Endocrinol. 2005;29:63–77. doi: 10.1016/j.domaniend.2005.02.028. [DOI] [PubMed] [Google Scholar]
- De Groef B., Grommen S.V.H., Mertens I., Schoofs L., Kühn E.R., Darras V.M. Cloning and tissue distribution of the chicken type 2 corticotropin-releasing hormone receptor. Gen. Comp. Endocrinol. 2004;138:89–95. doi: 10.1016/j.ygcen.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ellestad L.E., Saliba J., Porter T.E. Ontogenic characterization of gene expression in the developing neuroendocrine system of the chick. Gen. Comp. Endocrinol. 2011;171:82–93. doi: 10.1016/j.ygcen.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Frigerio F. The HPA axis and the regulation of energy balance. In: Luzi L., editor. Cellular Physiology and Metabolism of Physical Exercise. Springer; Milano: 2012. pp. 109–121. [Google Scholar]
- Habashy W.S., Milfort M.C., Fuller A.L., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017;61:2111–2118. doi: 10.1007/s00484-017-1414-1. [DOI] [PubMed] [Google Scholar]
- Hammel H.T., Pierce J.B. Regulation of internal body temperature. Annu. Rev. Physiol. 1968;30:641–710. doi: 10.1146/annurev.ph.30.030168.003233. [DOI] [PubMed] [Google Scholar]
- Jastrebski S.F., Lamont S.J., Schmidt C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis. PLoS One. 2017;12:1–15. doi: 10.1371/journal.pone.0181900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzel W.J., Jurkevich A. Molecular neuroendocrine events during stress in poultry. Poult. Sci. 2010;89:832–840. doi: 10.3382/ps.2009-00376. [DOI] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Mo C., Cai G., Huang L., Deng Q., Lin D., Cui L., Wang Y., Li J. Corticotropin-releasing hormone (CRH) stimulates cocaine- and amphetamine-regulated transcript gene (CART1) expression through CRH type 1 receptor (CRHR1) in chicken anterior pituitary. Mol. Cell. Endocrinol. 2015;417:166–177. doi: 10.1016/j.mce.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Molokwu M.N., Olsson O. Body temperature regulation in Hot environments. PLoS One. 2016;11:1–9. doi: 10.1371/journal.pone.0161481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Park S.O., Kim W.K. Effects of betaine on biological functions in meat-type ducks exposed to heat stress. Poult. Sci. 2016;96:1515. doi: 10.3382/ps/pew416. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Kucuk O., Hayirli A., Prasad A.S. Role of dietary zinc in heat-stressed poultry: a review. Poult. Sci. 2009;88:2176–2183. doi: 10.3382/ps.2008-00560. [DOI] [PubMed] [Google Scholar]
- Souza L. F. A. de, Espinha L.P., de Almeida E.A., Lunedo R., Furlan R.L., Macari M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest. Sci. 2016;192:39–43. [Google Scholar]
- Wang Y., Saelao P., Chanthavixay K., Gallardo R., Bunn D., Lamont S.J., Dekkers J.M., Kelly T., Zhou H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 2018;97:770–780. doi: 10.3382/ps/pex363. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Li Y., Willems E., Franssens L., Wang Y., Decuypere E., Everaert N. Intermittent thermal manipulations of broiler embryos during late incubation and their immediate effect on the embryonic development and hatching process. Poult. Sci. 2011;90:1302–1312. doi: 10.3382/ps.2011-01390. [DOI] [PubMed] [Google Scholar]
- Xie J., Tang L., Lu L., Zhang L., Lin X., Liu H.-C., Odle J., Luo X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lai X., Li Z., Zhang X., Luo Q. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 2018;97:4073–4082. doi: 10.3382/ps/pey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S. Academic Press; London, UK: 2015. Regulation of body temperature: strategies and mechanisms. Pages 869–905 in Sturkie's Avian Physiology. Colin G. Scanes, ed. [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., van Riel J. Field study on broilers’ first-week mortality. Poult. Sci. 2009;88:798–804. doi: 10.3382/ps.2008-00292. [DOI] [PubMed] [Google Scholar]
- Zaboli G.-R., Rahimi S., Shariatmadari F., Torshizi M.A.K., Baghbanzadeh A., Mehri M. Thermal manipulation during Pre and Post-Hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult. Sci. 2017;96:478–485. doi: 10.3382/ps/pew344. [DOI] [PubMed] [Google Scholar]