Abstract
Our study is to investigate the preoperative prognostic value of the Controlling Nutritional Status score in intrahepatic cholangiocarcinoma patients after curative resection. One hundred and sixty-seven patients admitted to our hospital between January 2012 and December 2018 were included retrospectively. Time-dependent receiver operating characteristic (ROC) curve analysis was conducted to evaluate the ability of the Controlling Nutritional Status score to predict recurrence and survival. Patients with high Controlling Nutritional Status score (≥3) had significantly poorer RFS compared to those with low Controlling Nutritional Status score (low: <3) (p = 0.000) in Kaplan–Meier survival curve. Multivariate analyses identified Controlling Nutritional Status score, lymph node metastasis, tumor numbers and preoperative CEA as independent prognostic factors for RFS. Lymph node metastasis was the independent risk factor of OS. The Cox regression model with Controlling Nutritional Status score had better prognostic value for recurrence than the Cox regression model without Controlling Nutritional Status score in long-time alcohol consumption intrahepatic cholangiocarcinoma patients (AUC: 0.760 vs 0.706, p = 0.036). CONUT score may be a more powerful prognostic biomarker, which is tightly associated with other tumor characteristics, to predict recurrence but not survival, especially in long-time alcohol consumption intrahepatic cholangiocarcinoma patients after curative-intent surgery.
Keywords: intrahepatic cholangiocarcinoma, CONUT score, alcoholic, nutritional state
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary malignant tumor of the liver, and its incidence is increasing yearly.(1–3) Patients in different areas had different specific risk factors. In Thailand, liver flukes was the most common exposed factor; in China, hepatitis B and liver cirrhosis might be a common exposed factor; in other area, biliary disorders such as biliary stones and primary sclerosing cholangitis were seen as risk factors.(1) Radical resection is the primary treatment option for ICC,(4) but only 20–40% of patients are able to undergo this surgery. Even if radical resection is performed, the recurrence rate at 5 years after surgery can still be as high as 70%. Tumor recurrence and metastasis is the main cause of postoperative death. More than half of patients experience recurrence within 1 year, and the median recurrence-free survival time is only 11.2 months.(5) Some studies have reported that recurrence and metastasis mainly occur in the liver within 2 years after surgery.(6) Patients with tumor size >7.5 cm and multifocal tumor were assumed as high-risk population who had less disease free survival probability,(6) but in this study, researchers did not take preoperative nutritional status into consideration.
Nutritional and immunological status has been shown to be related to prognosis for some malignant tumors,(7) including ICC. For example, low preoperative serum prealbumin has been shown to be associated with poor long-term survival after hepatic resection in patients with HCC,(8) and prognostic nutritional index (PNI) can predict the prognosis of GIST.(9) Controlling Nutritional Status (CONUT) is a scoring tool to assess nutritional status calculated from the serum albumin concentration, total blood cholesterol level, and total peripheral lymphocyte count; it reflects nutritional and immune status. The prognostic significance of CONUT has been reported in patients undergoing curative surgery for colorectal cancer, gastric cancer and hepatocellular carcinoma.(10–12) However, the prognostic significance of CONUT score for ICC has not been reported.
No previous reports have evaluated the relationship between preoperative CONUT score and clinical outcomes after hepatectomy for ICC. This retrospective study evaluated the prognostic significance of CONUT score on clinical outcomes and verified whether CONUT can predict survival in patients undergoing hepatectomy for ICC.
Materials and Methods
Patients
This retrospective study included 167 ICC patients who underwent hepatectomy at our hospital between January 2012 and December 2018. The inclusion criteria were as follows: (1) Patients diagnosed with ICC by pathologists after curative resection; (2) Patients aged 18 and 80 years old; (3) Informed consent had been obtained before surgery. The exclusion criteria were: (1) Patients underwent palliative surgery, including gross-positive margin; (2) Perioperative mortality due to surgical complication; (3) Patients with other malignancy; (4) Loss of follow-up data. Patients were checked periodically for early recurrence by diagnostic imaging, such as ultrasonography, computed tomography (CT) and abdominal magnetic resonance imaging (MRI) every 3 months for 2 years after surgery then 6 months up to 5 years. Causes of death and patterns of recurrence were determined by reviewing medical records, including laboratory data, ultrasonography, CT and MRI or by direct contact with family members. Preoperative blood test results within one month before surgery, including serum albumin, total cholesterol (TC), gamma glutamyl transpeptidase and total peripheral blood lymphocyte count (TLC), were obtained from the patients’ records. Long-time alcohol consumption was defined as a daily consumption of more than 20 g of ethanol in women and more than 40 g of ethanol in men for more than 5 years.(13–15) This study was approved by the Ethics Committee of Chinese Academy of Medical Sciences Cancer Hospital. Informed consent had been obtained from all patients.
Postoperative outcome evaluation
Clinical findings and surgical outcomes were extracted from the medical records. The observation period was from the date of surgery until the date of death or loss to follow-up. Recurrence free survival (RFS) was calculated from the date of first hepatectomy to the date of first recurrence or metastasis. Overall survival (OS) was calculated from the date of first hepatectomy to the date of death from any cause or the date of censoring.
Statistical analysis
Categorical variables were compared using χ2 test or Fisher’s exact test, and continuous variables were compared using t test. Receiver operating characteristic (ROC) curves were generated, and the area under the curve (AUC) was determined for survival analysis and to identify the optimal cut-off value for the CONUT score. Survival curves were calculated according to the Kaplan–Meier method. Possible prognostic factors for RFS and OS were subjected to multivariate analysis using a Cox’s proportional hazards model and stepwise AIC analysis. The accepted level of significance was p<0.05. All statistical analyses were performed using the statistical packages R ver. 3.6.1 (http://www.r-project.org; The R Foundation) and Empower® (www. empowerstates.com; X&Y Solutions, Inc., Boston, MA).
Results
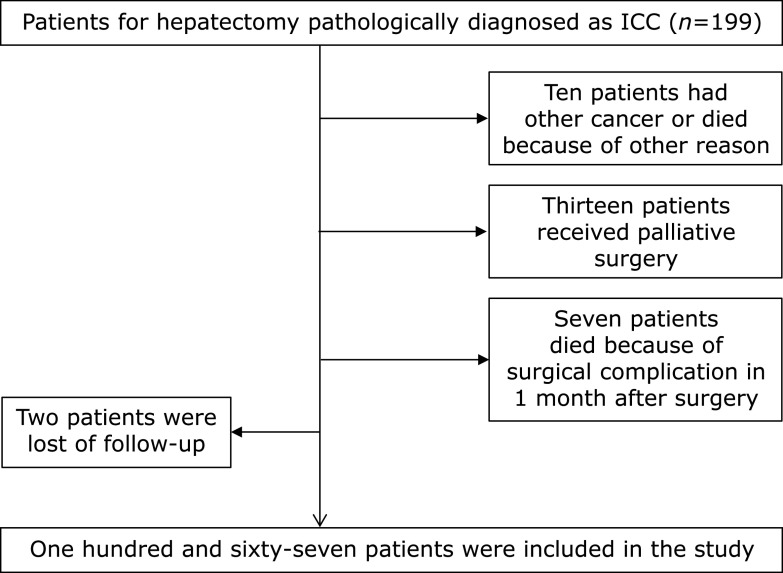
A flow diagram for the selection of ICC patients included in the final analysis is shown in Fig. 1. The overall median follow-up period was 14 (range 4–58) months, with the longest follow-up period being 84 months. During the follow-up period, 112 (64.7%) patients experienced recurrence, and the median RFS was 8 (range 1.2–45.6) months. As of 20th May, 2019, 79 (45.6%) patients had died, and 76 of those deaths were due to tumor recurrence or metastasis.
Fig. 1.
A flow diagram for the selection of ICC patients.
The CONUT score was calculated using serum albumin concentration, peripheral TLC and TC concentration, as described in Table 1.(16) The optimal cut-off value for the CONUT score was determined by ROC analysis and by using the maximum Youden Index. The AUC of the CONUT score based on the endpoint of recurrence was 0.679 (sensitivity = 43.1%, specificity = 83.7%, p = 0.000) (Fig. 2A). The CONUT score was evaluated as a dichotomized variable (low: <3 and high: ≥3). Then CONUT score was reclassified as low CONUT score group and high CONUT score group.
Table 1.
Controlling Nutritional Status (CONUT) scoring system
| Parameter | Normal | Light | Moderate | Severe |
|---|---|---|---|---|
| Serum albumin (g/dl) | 3.5–4.5 | 3.0–3.49 | 2.5–2.9 | <2.5 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocytes (/ml) | >1,600 | 1,200–1,599 | 800–1,199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Cholesterol (mg/dl) | >180 | 140–180 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Screening total score | 0–1 | 2–4 | 5–8 | 9–12 |
Fig. 2.
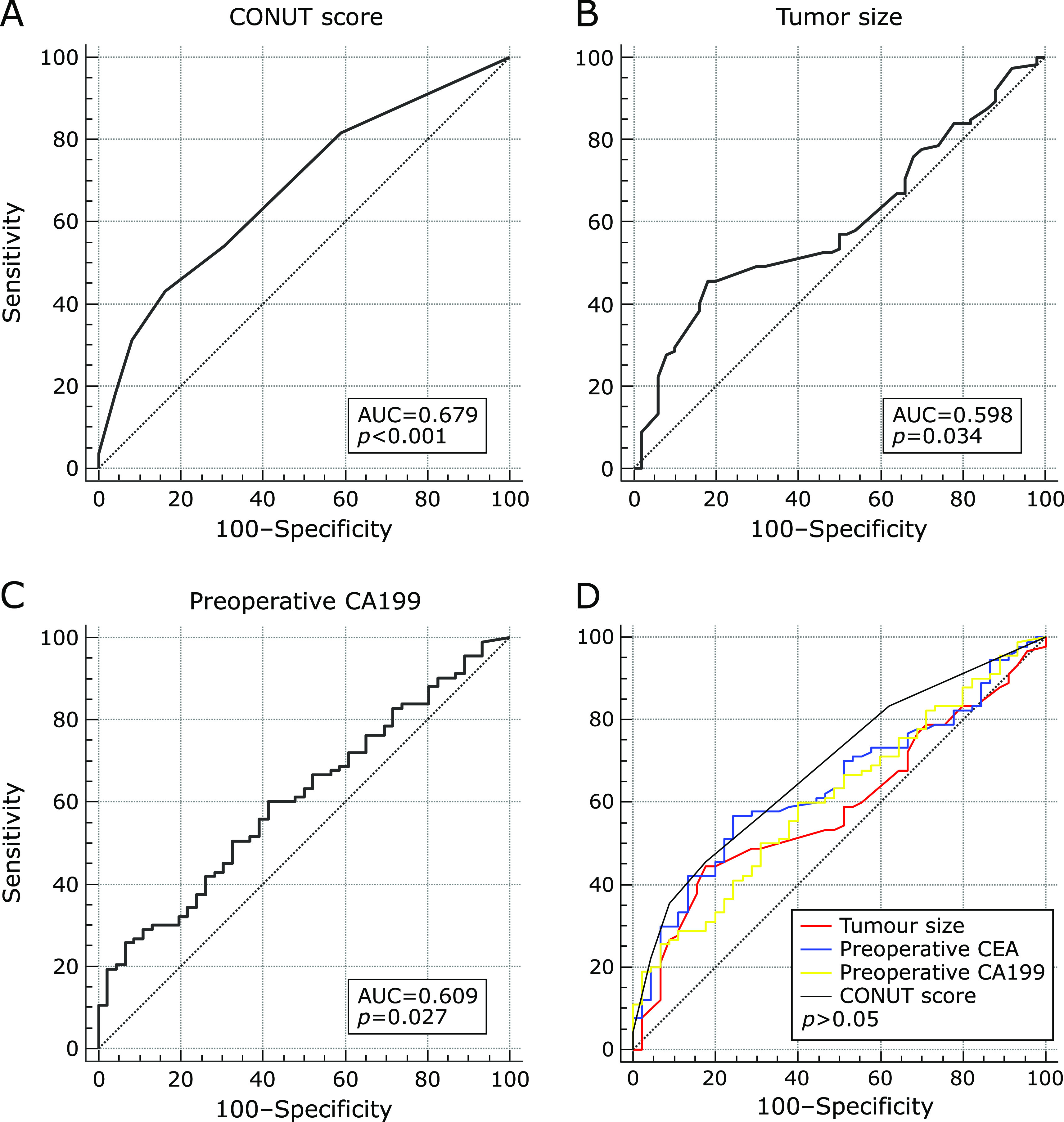
The receiver operating curve (ROC) for postoperative recurrence was plotted to verify the optimum cut-off value of CONUT score (A)/tumor size (B)/CA199 (C) for RFS, and comparison of the AUC between CONUT score/tumor size/CA199/CEA for RFS (D).
The relationships between the CONUT score and clinicopathologic features are shown in Table 2. Patients with larger tumors and higher preoperative CA199 levels more commonly had a high CONUT score, and patients with positive lymph node metastasis and perineural invasion had higher CONUT score compared to negative patients. Other clinicopathologic features were not significantly related to the CONUT score.
Table 2.
Clinicopathologic features of patients with Intrahepatic cholangiocarcinoma
| Item | CONUT <3 (n = 112) | CONUT ≥3 (n = 57) | p |
|---|---|---|---|
| Age | 57.19 ± 9.36 | 59.47 ± 8.12 | 0.103 |
| Gender (male:female) | 67:44 | 28:28 | 0.251 |
| History of long-term alcohol consumption | 28 (25.0) | 11 (19.3) | 0.406 |
| History of biliary stones | 16 (14.3) | 12 (21.1) | 0.263 |
| HBsAg (+) | 28 (25.0) | 7 (12.3) | 0.069 |
| Liver cirrhosis | 49 (43.8) | 13 (23.2) | 0.009* |
| Preoperative therapy | 7 (6.3) | 4 (7.0) | 0.848 |
| Tumor margin (<1 mm) | 42 (37.5) | 30 (52.6) | 0.094 |
| Mass-forming tumor | 111 (99.1) | 54 (94.7) | 0.258 |
| Centre-located tumor | 60 (53.6) | 25 (43.9) | 0.233 |
| Poorly differentiated | 62 (55.4) | 33 (57.9) | 0.867 |
| Multiple tumors | 19 (17.0) | 14 (24.6) | 0.303 |
| Tumor size (cm) | 4.860 ± 2.1814 | 6.779 ± 2.6321 | 0.000* |
| TNM stage: T1 | 58 (51.8) | 21 (36.8) | 0.066 |
| TNM stage: T2 | 31 (27.7) | 16 (28.1) | 0.957 |
| Positive lymph node metastasis (TNM stage: N1) | 18 (16.1) | 26 (45.6) | 0.000* |
| Microvascular invasion | 39 (34.8) | 22 (38.6) | 0.734 |
| Capsular invasion | 80 (71.4) | 45 (78.9) | 0.244 |
| Perineural invasion | 29 (25.8) | 27 (47.3) | 0.006* |
| Postoperative therapy before recurrence | 41 (36.7) | 23 (40.4) | 0.489 |
| Preoperative CEA (ng/ml) | 6.60 ± 35.28 | 18.69 ± 50.15 | 0.109 |
| Preoperative CA199 (U/ml) | 501.79 ± 3,183.57 | 1,792.14 ± 2,874.04 | 0.015* |
| Preoperative GGT (U/L) | 101.17 ± 191.25 | 118.18 ± 173.92 | 0.574 |
| Preoperative ALP (U/L) | 98.13 ± 101.70 | 123.56 ± 80.12 | 0.102 |
| Preoperative TBIL (µmol/L) | 16.16 ± 20.61 | 19.06 ± 52.88 | 0.611 |
| Preoperative ALT (U/L) | 34.45 ± 52.44 | 34.53 ± 68.21 | 0.993 |
| Preoperative AST (U/L) | 29.07 ± 29.02 | 29.46 ± 30.91 | 0.938 |
Data are the mean with SD in parentheses for continuous variables, with p values from t tests; data are numbers with percentages in parentheses for categorical variables, with p values from chi-square test, *significant at p<0.05.
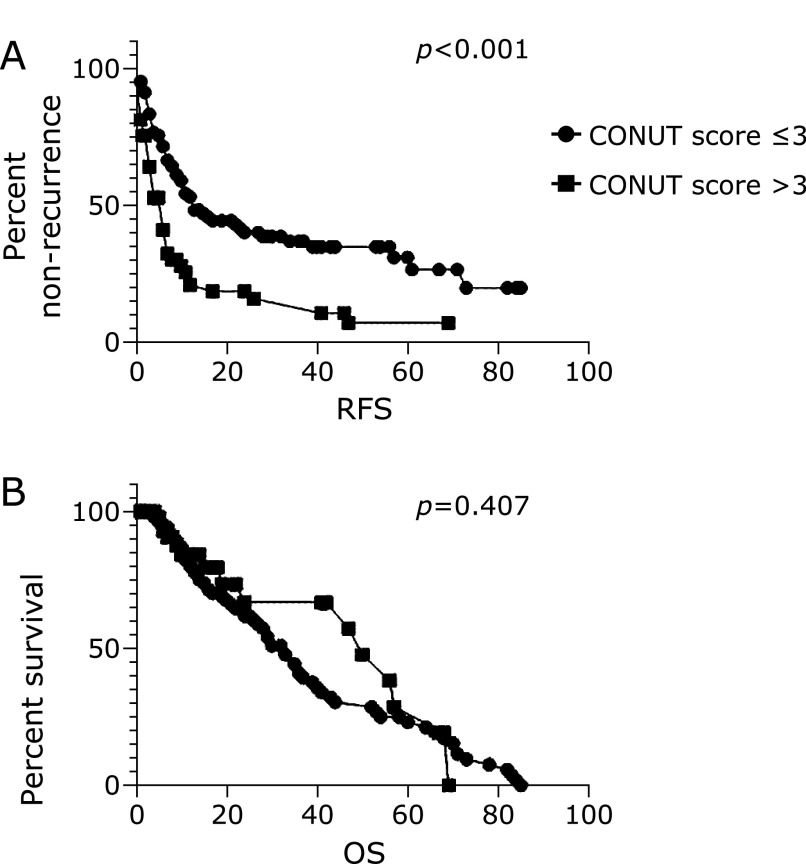
RFS was significantly higher [10 (range 2.4–55.2) months] in patients with a low CONUT score compared to those with a high CONUT score [5.5 (range 1.0–41.0) months] (p<0.0001), while OS was not different (p = 0.407) (Fig. 3A and B).
Fig. 3.
Kaplan–Meier analysis of CONUT groups (low: <3 and high: ≥3) for RFS (A) and OS (B).
As mentioned above, lymph node metastasis status, perineural invasion status, tumor size and preoperative CA199, the four factors were significantly different in low and high CONUT score groups. Low CONUT score group had better prognosis both in positive and negative lymph node metastasis group (Fig. 4A and B). Tumor size were dichotomized into two groups (<6.2 cm and ≥6.2 cm) according to ROC analysis (Fig. 2B). When tumor size was less than 6.2 cm, low CONUT score group had longer RFS, while when tumor size was more than 6.2 cm, there was no difference between low and high CONUT score groups (Fig. 4C and D). In the same way, preoperative CA199 were divided into two groups (<793.8 U/ml and ≥793.8 U/ml) (Fig. 2C). When CA199 was less than 793.8 U/ml, low CONUT score group had longer RFS, while when CA199 was more than 793.8 U/ml, there was no difference between low and high CONUT score groups (Fig. 4E and F). However, in positive perineural invasion group, low CONUT score group had better prognosis, while in negative perineural invasion group, there was no difference between low and high CONUT score groups (Fig. 4G and H).
Fig. 4.
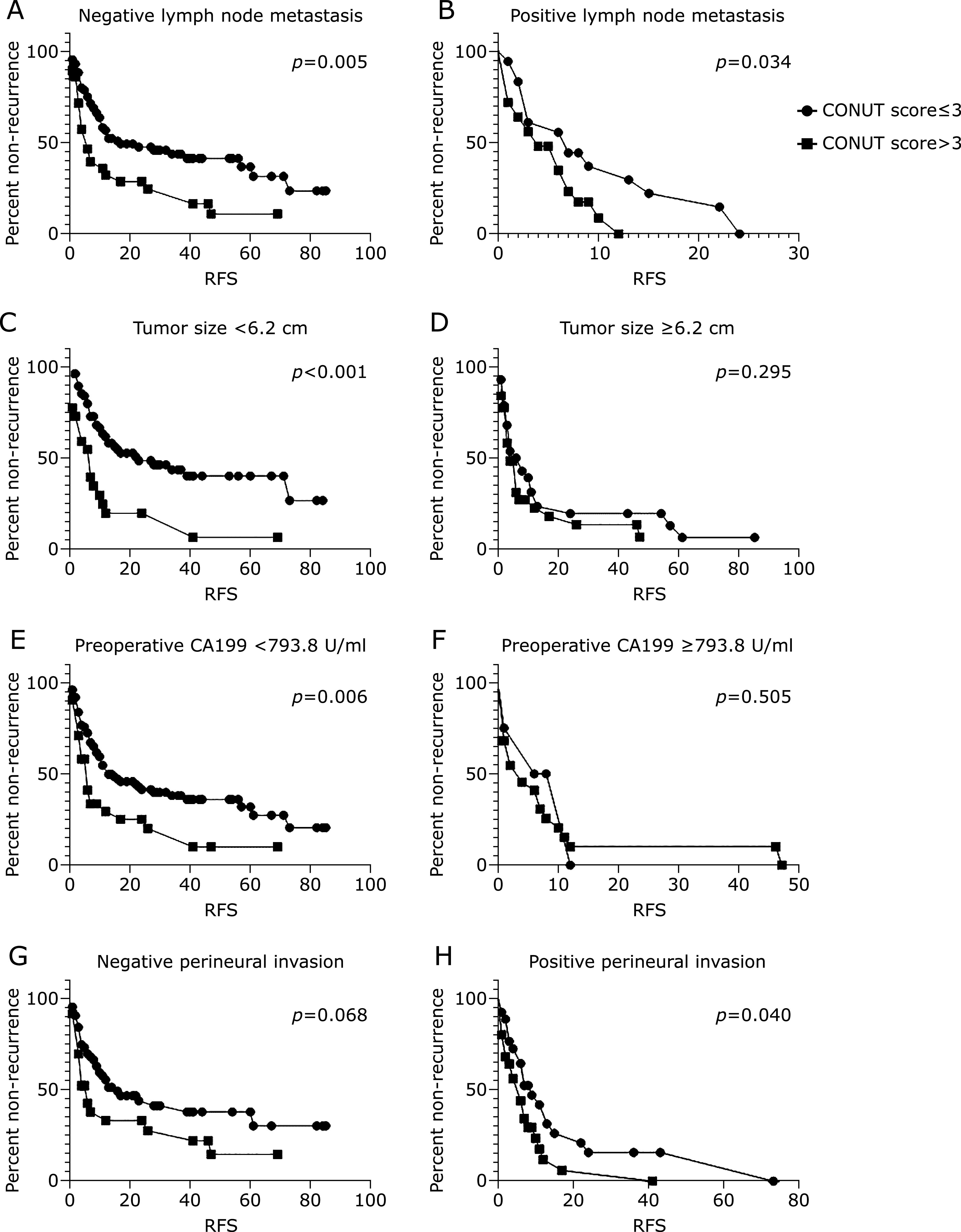
Kaplan–Meier analysis of CONUT groups (low: <3 and high: ≥3) for RFS in negative and positive lymph node metastasis group (A, B), in small and large tumor size group (small: <6.2 cm and large: ≥6.2 cm) (C, D), in low and high CA199 group (low: <793.8 U/ml and high ≥793.8 U/ml) (E, F), and in negative and positive perineural invasion group (G, H).
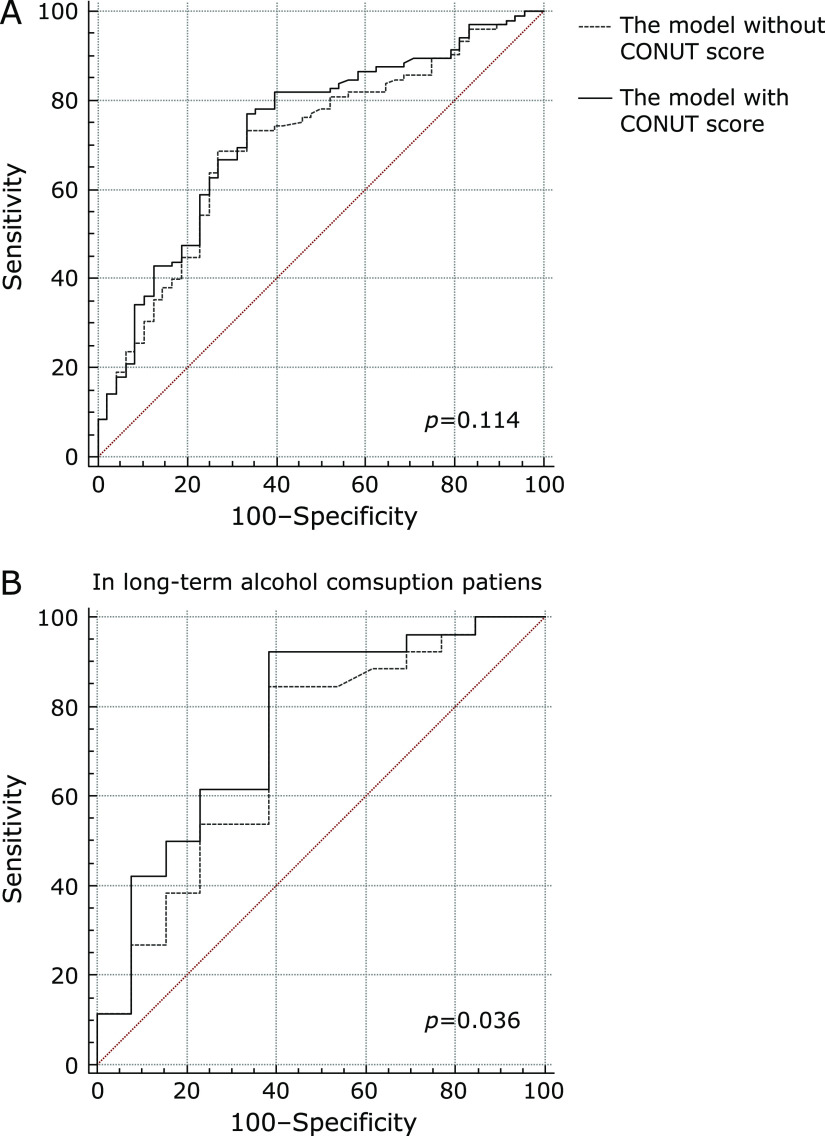
The results of the Cox regression hazard model for predictors of RFS are shown in Table 3. The covariates included in the analysis were age, gender, history of long-time alcohol consumption and biliary stones, pre- and postoperative therapy before recurrence, lymph node metastasis status, tumor characteristics as size, numbers, differentiation, microvascular invasion, capsular invasion, perineural invasion and tumor margin, preoperative CEA, preoperative CA199 and preoperative CONUT score. In univariate analysis, tumor size and numbers, lymph node metastasis status, microvascular invasion, perineural invasion, preoperative CEA, CA199 and CONUT score were associated with RFS. Multivariate analysis identified the CONUT score (HR = 1.658, 95% CI: 1.083–2.537, p = 0.001), lymph node metastasis status (HR = 2.337, 95% CI: 1.484–3.680, p<0.001), tumor numbers (HR = 2.096, 95% CI: 1.296–3.389, p = 0.003) and preoperative CEA (HR = 1.008, 95% CI: 1.003–1.012, p = 0.001) for RFS and showed that negative lymph node metastasis, single tumor, low CONUT score and low preoperative CEA were independently associated with better RFS (Table 4). The COX regression model (stepwiseAIC) is 0.00757 × (preoperative CEA) + 0.50540 × (high CONUT score group = 1) + 0.73987 × (multiple tumor = 1) + 0.84899 × (positive lymph node metastasis = 1). When the endpoint was recurrence in 12 months, the AUC of the model was 0.753 (sensitivity = 66.0%, specificity = 80.4%, p<0.001). Compared to the COX regression model without CONUT score, which was 0.00870 × (preoperative CEA) + 0.82011 × (multiple tumor = 1) + 1.00607 × (positive lymph node metastasis = 1), the model with CONUT score did not show significant advantages for predicting recurrence (Fig. 5A, p = 0.114).
Table 3.
Multivariate cox regression analyses for factors predicting RFS
| Item | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age | 1.003 (0.983–1.022) | 0.794 | |||
| Female | 1.190 (0.815–1.737) | 0.368 | |||
| History of long-term alcohol consumption | 1.039 (0.664–1.628) | 0.866 | |||
| History of biliary stones | 1.150 (0.701–1.887) | 0.581 | |||
| HBsAg (+) | 1.188 (0.764–1.848) | 0.444 | |||
| Liver cirrhosis | 1.170 (0.794–1.725) | 0.427 | |||
| Preoperative therapy | 1.203 (0.585–2.473) | 0.616 | |||
| Tumor margin (<1 mm) | 0.883 (0.602–1.293) | 0.521 | |||
| Mass-forming tumor | 0.405 (0.127–1.290) | 0.126 | |||
| Centre-located tumor | 1.338 (0.920–1.946) | 0.127 | |||
| Poorly differentiated | 1.409 (0.949–2.091) | 0.089 | |||
| Multiple tumors | 1.757 (1.120–2.757) | 0.014* | 2.096 (1.296–3.389) | 0.003* | |
| Tumor size (cm) | 1.125 (1.037–1.221) | 0.005* | |||
| Positive lymph node positive | 2.549 (1.678–3.872) | 0.000* | 2.337 (1.484–3.680) | <0.001* | |
| Microvascular invasion | 1.513 (1.022–2.239) | 0.038* | |||
| Capsular invasion | 1.423 (0.883–2.292) | 0.147 | |||
| Perineural invasion | 1.835 (1.227–2.745) | 0.003* | |||
| Postoperative therapy before recurrence | 0.946 (0.636–1.408) | 0.785 | |||
| Preoperative CEA (ng/ml) | 1.007 (1.004–1.011) | 0.000* | 1.008 (1.003–1.012) | 0.001* | |
| Preoperative CA199 (U/ml) | 1.000 (1.000–1.000) | 0.000* | |||
| CONUT score | 1.315 (1.182–1.462) | 0.000* | 1.658 (1.083–2.537) | 0.020* | |
*Significance at p<0.05.
Table 4.
Comparison the AUC of two models for predicting recurrence in stratified analysis
| Item | The model with CONUT score (95% CI) | The model without CONUT score (95% CI) | p |
|---|---|---|---|
| Female | 0.715 (0.608–0.806) | 0.691 (0.584–0.785) | 0.081 |
| History of long-time alcohol consumption | 0.760 (0.597–0.882) | 0.706 (0.538–0.840) | 0.036* |
| History of biliary stones | 0.689 (0.489–0.860) | 0.655 (0.436–0.835) | 0.199 |
| Live cirrhosis | 0.774 (0.643–0.874) | 0.738 (0.605–0.846) | 0.074 |
| HBsAg (+) | 0.590 (0.406–0.757) | 0.570 (0.387–0.740) | 0.505 |
*Significance at p<0.05.
Fig. 5.
Comparison of the AUC between the Cox regression model with CONUT score and without CONUT score for RFS (A), and in patients who had long-time alcohol consumption history for RFS (B).
However, in long-time alcohol consumption patients, the model with CONUT score had better prognostic value for recurrence (p = 0.036, Fig. 5B, Table 4). In stratified analysis of other exposed factors, such as gender, history of biliary stones, hepatitis and liver cirrhosis, the model with CONUT score was not different with the model without CONUT score (Table 4).
The results of the Cox regression hazard model for predictors of OS are shown in Table 5. In univariate analysis, age, gender, history of long-time alcohol consumption, tumor size, lymph node metastasis status, microvascular invasion, perineural invasion, preoperative CEA and CA199 were associated with OS. In multivariate analysis, lymph node metastasis was independently risk factors of survival (Table 5).
Table 5.
Multivariate cox regression analyses for factors predicting OS
| Factor | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Age | 1.024 (1.002–1.047) | 0.031* | |||
| Female | 1.467 (0.949–2.268) | 0.085 | |||
| History of long-term alcohol consumption | 1.720 (1.021–2.899) | 0.042* | |||
| History of biliary stones | 1.195 (0.659–2.167) | 0.558 | |||
| HBsAg (+) | 1.372 (0.769–2.451) | 0.285 | |||
| Liver cirrhosis | 1.246 (0.796–1.953) | 0.402 | |||
| Preoperative therapy | 1.330 (0.603–2.933) | 0.479 | |||
| Tumor margin (<1 mm) | 0.886 (0.564–1.391) | 0.599 | |||
| Mass-forming tumor | 0.735 (0.180–3.006) | 0.668 | |||
| Centre-located tumor | 1.203 (0.781–1.851) | 0.402 | |||
| Poorly differentiated | 1.542 (0.961–2.474) | 0.073 | |||
| Multiple tumors | 1.633 (0.958–2.785) | 0.072 | |||
| Tumor size (cm) | 1.129 (1.034–1.232) | 0.007* | |||
| Positive lymph node metastasis | 3.157 (1.965–5.071) | 0.000* | 2.349 (1.224–4.508) | 0.010* | |
| Microvascular invasion | 1.592 (1.007–2.515) | 0.046* | |||
| Capsular invasion | 0.944 (0.563–1.583) | 0.828 | |||
| Perineural invasion | 2.197 (1.370–3.521) | 0.001* | |||
| Postoperative therapy before recurrence | 1.538 (0.942–2.509) | 0.085 | |||
| Preoperative CEA (ng/ml) | 1.006 (1.003–1.010) | 0.001* | |||
| Preoperative CA199 (U/ml) | 1.000 (1.000–1.000) | 0.000* | |||
| CONUT score | 1.014 (0.871–1.180) | 0.857 | |||
*Significance at p<0.05.
Discussion
This retrospective study demonstrated that a preoperative CONUT score is an independent prognostic factor of survival in ICC patients. This study is the first to identify the prognostic significance of CONUT score in patients with ICC after hepatectomy.
In our study, the CONUT score alone was predictive of recurrence: there were significant differences in RFS (p = 0.000) between the high CONUT score group and the low CONUT score group. Many studies have shown an association between the CONUT score and malignant tumor prognosis:(17–19) the CONUT score was shown to be an independent prognostic factor for patients with oligometastatic prostate cancer,(20) gastric cancer, renal cell carcinoma,(21) and other cancers. Thus, our findings are consistent with currently reported research. Malnutrition may result in an unfavourable prognosis(22) and impaired nutrition status represents poorer physical status, which slows down recovery after surgery and can lead to tumor progression. Furthermore, tumors that are more aggressive may cause the patient to have a worse nutritional status.
Lymph node metastasis (LNM) is an important prognostic indicator for patients with ICC.(23,24) In this study, 44 (26.0%) patients had positive lymph node metastasis and the multivariate analysis indicated that lymph node metastasis status was significantly related to prognosis. CONUT score was associated with lymph node metastasis status in our centre, and high CONUT score group had more positive LNM patients (Table 2). However, CONUT score was predictive for recurrence both in positive and negative LNM group, which indicated that CONUT score was the independent risk factor apart from LNM.
Some studies have reported tumor size and CA199 as predictive factors for recurrence in ICC.(25,26) In our study, tumor size and CA199 were risk factors in univariate COX regression analysis. CONUT score was associated with the two factors as well, high CONUT score group had larger tumor size and preoperative CA199. Interestingly, only in small tumor size and low CA199 group, high CONUT score group had poorer prognosis. In large tumor size and high CA199 group, CONUT score was not related with RFS. Hence, if tumor size was more than 6.2 cm or CA199 was more than 793.8 U/ml, CONUT score lost its meaning of prediction for recurrence. In other words, tumor size and CA199 were more predictive compared to CONUT score in more severe situations. Though tumor size and CA199 were not identified in multivariate COX regression analysis for recurrence due to statistical interaction with CONUT score, the two factors were still important risk factors for prognosis of ICC.
Perineural invasion is highly correlated with postoperative recurrence and poor prognosis in head and neck squamous cell carcinoma.(27) Only a few studies indicated(28,29) indicated that perineural invasion was a prognostic factor in cholangiocarcinoma. In our study, total 56 (33.5%) patients had perineural invasion, and in univariate COX regression analysis, perineural invasion was a risk factor for both RFS and OS. High CONUT group had more proportional patients with perineural invasion, which indicated that CONUT score was associated with perineural invasion. However, only in positive perineural invasion, CONUT score was predictive for recurrence while in negative perineural invasion, CONUT score was not related to prognosis. Therefore, in patients with positive perineural invasion, malnutritional status was associated with poor prognosis.
Above all, the nutritional status such as CONUT score might be an indicator of the degree of malignancy. In our study, preoperative CONUT score was associated with tumor size, perineural invasion, lymph node metastasis and CA199, which were tumor features of aggressiveness. That might explain why CONUT score was an independent predictive risk factor for recurrence of ICC patients. Compared with tumor size, preoperative CA199 and CEA, CONUT score did not show special advantages in predicting recurrence (Fig. 2D). The AUC of CONUT score, tumor size, preoperative CEA and CA199 was 0.687, 0.588, 0.641 and 0.611, respectively. P value between every two of the four risk factors was more than 0.05. Therefore, CONUT score was not inferior to the four risk factors for prognosis.
However, the prognostic Cox model with CONUT score for recurrence did show advantages in ICC patients with history of long-time alcohol consumption, even though there was no significant difference of CONUT score before operation between patients with or without history of long-time alcohol consumption (1.7 vs 2.0, p = 0.378). It indicated that for patients who might be potentially malnourished, the evaluation of nutritional status like CONUT score would be helpful to predict prognosis of the malignant tumors.
CONUT score was not a risk factor of survival. There was no difference on survival between low and high CONUT score group. Despite some studies indicated that CONUT was an effective independent predictor of OS,(12,30) in our study, we could not approve its influence on survival. Lymph node metastasis was the independent risk factor for survival, and even though CONUT score was associated with lymph node metastasis status, it was not identified in survival analysis.
There are some limitations to our study. First, it is a retrospective study collected data from 2012 to 2018. The pathological definition of AJCC TMN stage has changed in 6 years, though we reclassify patients according to AJCC 8th edition of TMN stage, there still could be some inconsistent description in medical data recording over the 6 years. Second, the number of patients included was small so it hardly set the training cohort and validation cohort. The conclusion would have bias as well, especially the cut-off values of tumor size and CA199 would be different with other studies. Third, the median follow-up period was only 14 months, which may be too short to form valid conclusions about long-term survival. That would be the reason why the CONUT score was not predictive for survival. Further large-scale, prospective and randomized trials are needed to confirm the results of this study.
In conclusion, the CONUT score may be a useful prognostic indicator in patients with ICC. Given that serum markers can be measured quickly, easily and noninvasively, the CONUT score may represent a useful biological marker in routine clinical settings especially in patients with history of long-time alcohol consumption or who would be potentially malnourished.
Author Contributions
Study Concept and design: LW and JW; Acquisition of data: FW and WR; Analysis and interpretation of data: YZ, YL and ST; Drafting of the Manuscript: All authors; Final approval of the Manuscript: All authors.
Acknowledgments
It was supported by Beijing Hope Run Special Fund of Cancer Foundation of China (LC2018A15).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol 2015; 29: 221–233. [DOI] [PubMed] [Google Scholar]
- 2.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology 2017; 15: 95–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massarweh NN, El-Serag HB. Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control 2017; 24: 1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep 2017; 19: 2. [DOI] [PubMed] [Google Scholar]
- 5.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013; 153: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doussot A, Gonen M, Wiggers JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg 2016; 223: 493–505. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Xiao G. Prognostic significance of the ratio of fibrinogen and albumin in human malignancies: a meta-analysis. Cancer Manag Res 2019; 11: 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao YY, Teng CL, Peng NF, et al. Serum prealbumin is negatively associated with survival in hepatocellular carcinoma patients after hepatic resection. J Cancer 2019; 10: 3006–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, Mei Y, Zhu Q, et al. Relationship of prognostic nutritional index with prognosis of gastrointestinal stromal tumors. J Cancer 2019; 10: 2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi K, Yagi T, Umeda Y, et al. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg 2017; 41: 2353–2360. [DOI] [PubMed] [Google Scholar]
- 11.Hirahara N, Tajima Y, Fujii Y, et al. Controlling Nutritional Status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: a propensity score-matched analysis. Surg Endosc 2019; 33: 4143–4152. [DOI] [PubMed] [Google Scholar]
- 12.Lin ZX, Ruan DY, Jia CC, et al. Controlling nutritional status (CONUT) score‐based nomogram to predict overall survival of patients with HBV‐associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol 2020; 22: 370–380. [DOI] [PubMed] [Google Scholar]
- 13.Roerecke M, Vafaei A, Hasan OSM, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol 2019; 114: 1574–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver 2017; 11: 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlov CS, Casazza G, Semenistaia M, et al. Ultrasonography for diagnosis of alcoholic cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev 2016; 3: CD011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005; 20: 38–45. [PubMed] [Google Scholar]
- 17.Okamoto S, Ureshino H, Kidoguchi K, et al. Clinical impact of the CONUT score in patients with multiple myeloma. Ann Hematol 2020; 99: 113–119. [DOI] [PubMed] [Google Scholar]
- 18.Ahiko Y, Shida D, Horie T, et al. Controlling nutritional status (CONUT) score as a preoperative risk assessment index for older patients with colorectal cancer. BMC Cancer 2019; 19: 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Huang Y, Lu M, et al. Controlling Nutritional Status (CONUT) score is a predictor of post-operative outcomes in elderly gastric cancer patients undergoing curative gastrectomy: a prospective study. Cancer Manag Res 2019; 11: 9793–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Wu Y, Zhang Z, et al. Controlling Nutritional Status score: a new prognostic indicator for patients with oligometastatic prostate cancer. Curr Probl Cancer 2019; 43: 461–470. [DOI] [PubMed] [Google Scholar]
- 21.Elghiaty A, Kim J, Jang WS, et al. Preoperative controlling nutritional status (CONUT) score as a novel immune-nutritional predictor of survival in non-metastatic clear cell renal cell carcinoma of ≤ 7 cm on preoperative imaging. J Cancer Res Clin Oncol 2019; 145: 957–965. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Kanaji S, Yamamoto M, Oshikiri T, Nakamura T, Kakeji Y. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg 2019; 43: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 23.Bagante F, Gani F, Spolverato G, et al. Intrahepatic cholangiocarcinoma: prognosis of patients who did not undergo lymphadenectomy. J Am Coll Surg 2015; 221: 1031–1040.e1–4. [DOI] [PubMed] [Google Scholar]
- 24.Sahara K, Tsilimigras DI, Merath K, et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: which patients benefit the most from nodal evaluation? Ann Surg Oncol 2019; 26: 2959–2968. [DOI] [PubMed] [Google Scholar]
- 25.Zheng BH, Yang LX, Sun QM, et al. A new preoperative prognostic system combining CRP and CA199 for patients with intrahepatic cholangiocarcinoma. Clin Transl Gastroenterol 2017; 8: e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliante F, Ardito F, Mattiucci GC, et al. Patterns of recurrence following liver resection for intrahepatic cholangiocarcinoma (IHC) and recurrence location risk score (RLRS) to identify patients suitable for loco-regional treatment and volumes of interest. Int J Radiat Oncol Biol Phys 2010; 78: S196. [Google Scholar]
- 27.Saidak Z, Lailler C, Clatot F, Galmiche A. Perineural invasion in head and neck squamous cell carcinoma: background, mechanisms, and prognostic implications. Curr Opin Otolaryngol Head Neck Surg 2020; 28: 90–95. [DOI] [PubMed] [Google Scholar]
- 28.Murakami Y, Uemura K, Sudo T, et al. Perineural invasion in extrahepatic cholangiocarcinoma: prognostic impact and treatment strategies. J Gastrointest Surg 2013; 17: 1429–1439. [DOI] [PubMed] [Google Scholar]
- 29.Shen FZ, Zhang BY, Feng YJ, et al. Current research in perineural invasion of cholangiocarcinoma. J Exp Clin Cancer Res 2010; 29: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirahara N, Matsubara T, Hayashi H, Takai K, Nakada S, Tajima Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther 2018; 25: e524–e532. [DOI] [PMC free article] [PubMed] [Google Scholar]