Abstract
Androgen hormones are important compounds related to body composition and exercise performance in athletes. The intake of Dioscorea esculenta, known as lesser yam, contains diosgenin and resistance training have been shown to normalize the secretion of androgen hormones. This study aimed to clarify the level of androgen hormone secretion and the effects of Dioscorea esculenta intake with resistance training on muscle hypertrophy and strength in athletes. First, in a cross-sectional study, we compared the serum androgen hormone [dehydroepiandrosterone (DHEA), testosterone, and 5α-dihydrotestosterone (DHT)] levels between sprint athletes (n = 15) and non-athletes (n = 15). Second, in an 8-week intervention study, sprint athletes were randomly divided into 2 groups: resistance training with placebo (n = 8) or with Dioscorea esculenta (2,000 mg/day) intake (n = 7). The serum DHEA, free testosterone, and DHT levels were lower in athletes than in non-athletes. Dioscorea esculenta intake combined with resistance training increased the arm fat-free mass, the 1 repetition maximum of deadlift and snatch, and the serum DHEA, free testosterone, and DHT levels, compared with resistance training and placebo intake. The results suggested that Dioscorea esculenta intake combined with resistance training has further effects on muscle hypertrophy and strength in athletes by restoring secretion of androgen hormones.
Keywords: 5α-dihydrotestosterone, Dioscorea esculenta, resistance training, muscle hypertrophy, sprint athletes
Introduction
Androgen hormones such as dehydroepiandrosterone (DHEA), testosterone, and 5α-dihydrotestosterone (DHT) have many important roles, including regulation of muscle protein synthesis,(1–3) energy metabolism,(4,5) bone turnover,(6) immune function,(7) motor skills,(8) and behavioral motivation and condition.(9) Therefore, androgen hormones are important compounds related to body composition and exercise performance in athletes. However, several studies to date have shown that the baseline serum testosterone levels are lower in some athletes, such as endurance-trained runners (high mileage running, training for more than 5 years) and resistance-trained weightlifters (training for more than 2 h/day at least 4 days/week for more than 1 year), than in non-athletes.(10–13) Furthermore, in our recent study, high-intensity exercise, but not low- to medium-intensity exercise, increased the circulating androgen hormone levels in endurance athletes.(14) If chronic decline not only in testosterone secretion but also in DHEA and DHT secretion occurs in athletes, this impairment will be a critical issue affecting body composition and exercise performance.
In our previous study using an obese rat model with impaired androgen hormone secretion, chronic supplementation of DHEA induced an acceleration in the secretion of testosterone and DHT, and DHT secretion was associated with increases in muscle mass.(15) Furthermore, in healthy men, treatment with supraphysiologic doses of testosterone led to an increase in muscle mass and strength.(16) Thus, for athletes with decreased androgen hormone secretion, it is necessary to search for treatments that can normalize the secretion of androgen hormones, especially DHT, within the physiological range.
Diosgenin, a steroid sapogenin, has a steroid molecular formula similar to that of DHEA.(17) Dioscorea esculenta, known as lesser yam, contains diosgenin. In vivo and vitro studies have shown that intake of Dioscorea esculenta causes no adverse effects or toxicities.(17) Additionally, our recent study demonstrated that chronic Dioscorea esculenta intake in type 2 diabetic rats did not cause adverse effects despite increasing the serum and muscle DHEA and DHT levels.(18) Therefore, intake of Dioscorea esculenta may restore the declining androgen hormone secretion in athletes, which will then lead to increases in muscle mass and strength. Additionally, our recent studies showed that resistance training enhanced the serum and muscle DHEA, free testosterone, and DHT levels, and that the elevation of muscle androgen hormone levels were associated with training-induced increases in muscle mass and strength in healthy older men(19) and type 2 diabetic rats.(1) Moreover, androgen hormone treatment augmented the resistance training-induced increases in muscle mass and strength in normal men(16) and older patients,(20) respectively. However, the effects of the combination of Dioscorea esculenta intake and resistance training on muscle hypertrophy and strength in athletes remain unclear.
Therefore, the aim of the present study was to clarify the status of androgen hormone (DHEA, testosterone, and DHT) secretion in athletes, and whether Dioscorea esculenta intake combined with resistance training would produce effects on muscle hypertrophy and strength in athletes by restoring the circulating androgen hormone levels. First, to confirm the decline not only in testosterone but also DHEA and DHT secretion in athletes through a cross-sectional study, we compared the serum androgen hormone levels between athletes and non-athletes. Second, by using a randomized controlled intervention trial, athletes were randomly divided into 2 groups (resistance training with placebo intake and resistance training with Dioscorea esculenta intake), and body composition, maximal muscle strength, blood parameters, and serum androgen hormone levels before and after 8 weeks of intervention were measured.
Methods
Participants
For experiment 1, 15 male sprint athletes (mean age: 20 ± 1 years, athletic history: 7.1 ± 2.9 years) who were members of a collegiate track and field team (regular track training: 5 days/week, 2–3 h/day; resistance training: 2 times/week) and 15 male non-athletes [(sedentary control adults) mean age: 21 ± 1 years] who were sedentary or moderately active (not engaging in vigorous sports activity) participated in a cross-sectional study. Among the 15 sprint athletes, 8 of 400 m runners, 6 of 400 m hurdlers and 1 of 200 m runners were participated. All participants included in this study did not smoke or take any medications. For experiment 2, all athletes volunteered to participate in an intervention study. All participants were given an oral and written briefing about this study, and each of them provided written informed consent for participation. The study was approved by the ethics committee of Ritsumeikan University and was conducted in accordance with the Declaration of Helsinki. All athletes were instructed to continue their club activities and usual diets throughout the experimental period.
Experimental design
In experiment 1, the height, body weight, body mass index (BMI), and serum DHEA, free testosterone, and DHT levels of sprint athletes (n = 15) and non-athletes (sedentary control adults: n = 15) were measured. In experiment 2, the 15 sprint athletes were randomly divided into 2 groups: resistance training with placebo intake (n = 8) and resistance training with Dioscorea esculenta intake (n = 7). Body composition; maximal muscle strength; serum DHEA, free testosterone, and DHT levels; and triglyceride and total cholesterol levels were measured before and after 8 weeks of resistance training intervention. At the beginning and end of the intervention period, fasting blood tests were done with blood samples drawn in the morning (8:00–9:00 AM) at least 48 h after the last resistance training session to avoid the immediate acute effects of exercise and circadian rhythm. All participants were instructed not to eat or drink fluids other than water at least 12 h before blood sampling.(21) The serum sample was immediately centrifuged at 1,500 g and 4°C for 15 min. Blood samples were stored at –80°C until use. Room temperature was maintained at 22–24°C during the analysis.
Resistance training intervention
Resistance training sessions for athletes were carried out 3 days per week for 8 weeks.(19) Experienced trainers supervised all training sessions to ensure proper technique and progression in each exercise session. Each session included 12 exercises: bench press, high clean, swing-up arch, deadlift, snatch, sit-up, back extension, side raise, shoulder press, squatting, upright rowing, and dumbbell fly. The weight used during the resistance exercise in this study was 70% of each participant’s predetermined 1 repetition maximum (1RM) for 4 sets of 10 repetitions. The rest period between sets was 1 min.
Measurement of 1 repetition maximal strength
Bench press, high clean, deadlift, snatch, and squatting 1RM strength tests were performed before and after the resistance training intervention.(20) The participants lifted increasingly heavy weights, and the maximal amount of weight that they could lift was recorded as the 1RM within 5 sets for each exercise. The same investigator measured the 1RM strength before and after resistance training intervention with the same levels of vocal encouragement.
Measurement of body composition
The percent body fat, whole-body fat-free mass, and arm and leg fat-free masses were measured using dual-energy x-ray absorptiometry (Lunar iDXA; GE Healthcare UK, Buckinghamshire, UK).
Dioscorea esculenta or placebo intake
Dioscorea esculenta or placebo tablets were taken in a randomized, double-blinded, placebo-controlled study.(22) Dioscorea esculenta tablet was ingested at 2,000 mg/day once a day, within 30 min after every dinner. The placebo tablet contained dextrin in the same amount as the content of the Dioscorea esculenta tablet.(23)
Measurement of serum DHEA, free testosterone, and DHT levels
Serum DHEA (Enzo Life Sciences, Farmingdale, NY), free testosterone (IBL International, Hamburg, Germany), and DHT (IBL International) levels were determined using sandwich enzyme-linked immunosorbent assay kits. Immobilized polyclonal antibodies were raised against DHEA, free testosterone, and DHT, whereas the secondary horseradish peroxidase-coupled antibodies were monoclonal. Optical density at 405 or 450 nm was quantitated using a microplate reader (X-Mark microplate spectrophotometer; Bio-Rad Laboratories, Hercules, CA).
Measurement of total cholesterol and triglyceride levels
The fasting triglyceride and total cholesterol levels in serum were determined using standard enzymatic techniques, as previous described.(24)
Statistical analysis
All values are expressed as mean ± SE. For each parameter, the differences between groups and time points were assessed using a 2-way repeated-measures analysis of variance. Unpaired Student’s t tests were used to compare differences between athletes and non-athletes or the changes from baseline to 8 weeks between the resistance training with placebo intake group and the resistance training with Dioscorea esculenta intake group. The relationships between changes in serum DHEA, free testosterone, or DHT levels and changes in arm fat-free mass or the 1RMs of deadlift and snatch from baseline to 8 weeks in the Dioscorea esculenta intake with resistance training group were determined using Pearson correlation coefficients. P<0.05 was defined as statistically significant. All statistical analyses were performed using StatView (ver. 5.0; SAS Institute, Tokyo, Japan).
Results
Cross-sectional study (experiment 1)
Characteristics and serum androgen hormone levels in athletes and non-athletes. Male sprint athletes and non-athletes participated in a cross-sectional study. Height, body weight, and BMI were not significantly different between athletes and non-athletes (Table 1). The serum DHEA (p<0.001), free testosterone (p = 0.026), and DHT (p = 0.012) levels were significantly lower in athletes than in non-athletes (Fig. 1).
Table 1.
Subject characteristics between athletes and non-athletes
| Athletes (n = 15) | Non-athletes (n = 15) | Unpaired t test | |
|---|---|---|---|
| Height (cm) | 173.8 ± 1.8 | 174.8 ± 1.4 | 0.660 |
| Body weight (kg) | 67.3 ± 2.0 | 63.3 ± 1.1 | 0.138 |
| BMI (kg/m2) | 22.0 ± 0.6 | 21.1 ± 0.3 | 0.207 |
The values are expressed as mean ± SE. BMI, body mass index.
Fig. 1.
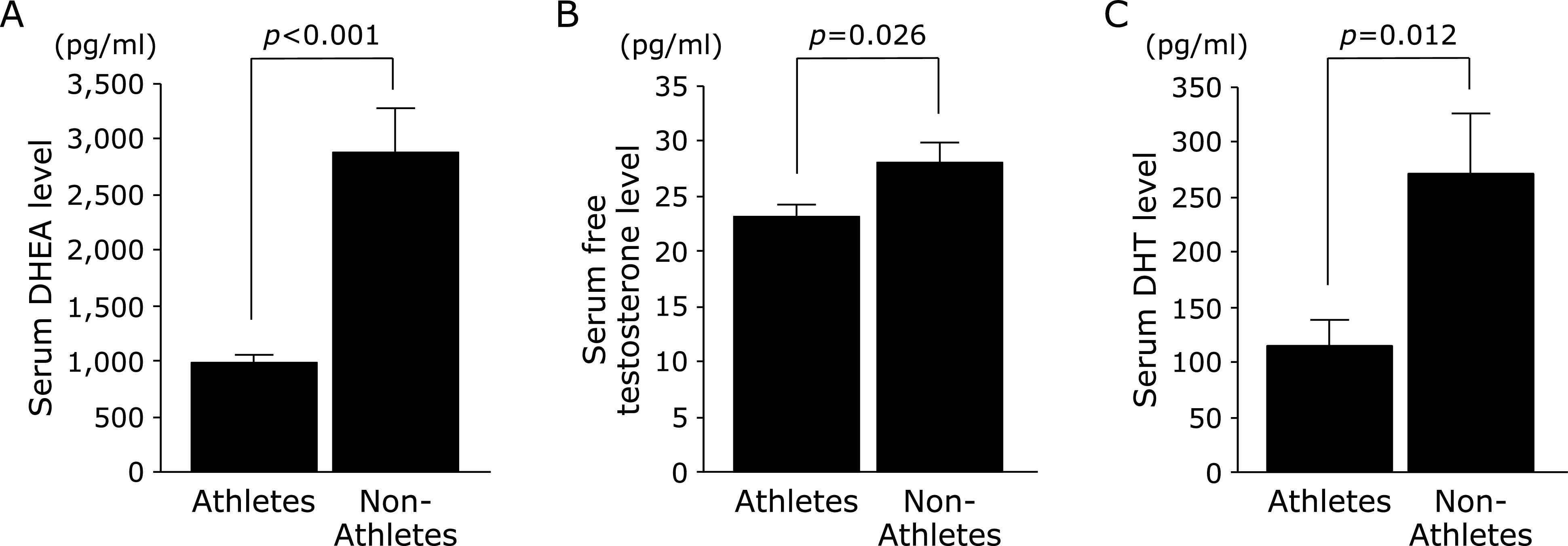
Serum (A) dehydroepiandrosterone (DHEA), (B) free testosterone, and (C) 5α-dihydrotestosterone (DHT) levels in athletes and non-athletes. The values are expressed as mean ± SE.
Intervention study (experiment 2)
Comparison of characteristics and maximal muscle strengths between resistance training with placebo intake and resistance training with Dioscorea esculenta intake. All sprint athletes were randomly divided into the resistance training with placebo intake group and the resistance training with Dioscorea esculenta intake group in the intervention study. No significant difference was observed in height, body weight, BMI, body fat percentage, whole-body fat-free mass, arm and leg fat-free masses, triglyceride level, and total cholesterol level between the resistance training with placebo intake group and the resistance training with Dioscorea esculenta intake group before and after the intervention (Table 2). The 1RMs of bench press, high clean, deadlift, snatch, and squatting were not significantly different between the 2 groups before and after the intervention (Table 2).
Table 2.
Comparison of characteristics and maximal muscle strengths between resistance training with placebo intake and resistance training with Dioscorea esculenta intake and changes (Δ) in characteristics and maximal muscle strengths in athletes
| RT + PL (n = 8) |
RT + Dio (n = 7) |
Two-way ANOVA | ΔRT + PL | ΔRT + Dio | Unpaired t test | ||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | ||||||
| Height (cm) | 171.5 ± 2.0 | 172.5 ± 2.1 | 176.4 ± 2.9 | 176.3 ± 2.9 | 0.820 | ||||
| Body weight (kg) | 62.5 ± 1.3 | 64.4 ± 1.8 | 65.3 ± 1.6 | 66.3 ± 1.7 | 0.798 | 1.85 ± 0.68 | 1.01 ± 0.70 | 0.405 | |
| BMI (kg/m2) | 21.3 ± 0.3 | 21.6 ± 0.4 | 21.0 ± 0.5 | 21.3 ± 0.4 | 0.984 | 0.35 ± 0.23 | 0.33 ± 0.16 | 0.957 | |
| Body fat (%) | 7.5 ± 0.7 | 7.4 ± 0.6 | 6.1 ± 0.3 | 6.7 ± 0.2 | 0.493 | –0.15 ± 0.38 | 0.59 ± 0.31 | 0.160 | |
| Whole fat free mass (kg) | 55.8 ± 1.7 | 56.5 ± 1.5 | 58.8 ± 1.6 | 59.7 ± 1.7 | 0.934 | 0.65 ± 0.39 | 0.93 ± 0.28 | 0.580 | |
| Arm fat free mass (kg) | 6.3 ± 0.2 | 6.4 ± 0.2 | 6.3 ± 0.2 | 6.7 ± 0.2 | 0.548 | 0.10 ± 0.14 | 0.36 ± 0.05* | 0.049 | |
| Leg fat free mass (kg) | 19.3 ± 0.6 | 19.6 ± 0.6 | 20.3 ± 0.6 | 20.7 ± 0.6 | 0.949 | 0.33 ± 0.14 | 0.41 ± 0.15 | 0.697 | |
| Triglyceride (mg/dl) | 73.6 ± 7.3 | 85.0 ± 9.0 | 83.5 ± 10.8 | 85.5 ± 11.3 | 0.626 | 11.38 ± 6.10 | 2.00 ± 11.05 | 0.443 | |
| Total cholesterol (mg/dl) | 184.1 ± 7.6 | 197.3 ± 9.7 | 179.7 ± 10.3 | 186.0 ± 10.9 | 0.725 | 13.13 ± 5.13 | 6.29 ± 4.12 | 0.327 | |
| High clean 1RM (kg) | 76.3 ± 7.1 | 77.5 ± 5.8 | 80.4 ± 4.9 | 80.4 ± 3.7 | 0.914 | 1.25 ± 2.31 | 0.00 ± 1.44 | 0.666 | |
| Snach 1RM (kg) | 50.4 ± 4.0 | 52.1 ± 3.2 | 51.1 ± 2.6 | 59.3 ± 3.5 | 0.349 | 1.56 ± 1.24 | 8.21 ± 3.65 | 0.091 | |
| Bench press 1RM (kg) | 75.0 ± 4.7 | 82.2 ± 5.1 | 84.2 ± 6.9 | 89.6 ± 8.2 | 0.886 | 7.19 ± 1.92 | 5.42 ± 2.08 | 0.547 | |
| Squat 1RM (kg) | 126.3 ± 9.1 | 135.6 ± 5.0 | 124.3 ± 6.1 | 130.0 ± 3.1 | 0.777 | 9.38 ± 5.04 | 5.71 ± 4.29 | 0.595 | |
| Dead lift 1RM (kg) | 121.4 ± 8.0 | 121.4 ± 7.0 | 120.7 ± 7.6 | 133.6 ± 6.2 | 0.384 | 0.00 ± 3.27 | 12.86 ± 4.61* | 0.037 | |
The values are expressed as mean ± SE. BMI, body mass index; 1RM, 1 repetition maximum; RT + PL, resistance training with placebo intake group; RT + Dio, resistance training with Dioscorea esculenta intake group. *p<0.05 vs ΔRT + PL.
Comparison of serum androgen hormone levels between resistance training with placebo intake and resistance training with Dioscorea esculenta intake. The serum DHEA, free testosterone, and DHT levels between the resistance training with placebo intake group and the resistance training with Dioscorea esculenta intake group were not significantly different before and after the intervention.
Changes in characteristics and maximal muscle strengths. The changes in body weight, BMI, body fat percentage, whole-body and leg fat-free masses, triglyceride level, and total cholesterol level were not significantly different between the 2 groups (Table 2). Compared with the resistance training with placebo intake group, the resistance training with Dioscorea esculenta intake group showed significantly increased arm fat-free mass (p = 0.049, Table 2). Additionally, no significant differences in the changes in the 1RMs of bench press, high clean, and squatting were observed between the 2 groups (Table 2). The 1RM of deadlift in the resistance training with Dioscorea esculenta intake group was significantly increased compared with that in the resistance training with placebo intake group (p = 0.037, Table 2), whereas the 1RM of snatch tended to increase in the resistance training with Dioscorea esculenta intake group as compared with the resistance training with placebo intake group (p = 0.091, Table 2).
Changes in serum androgen hormone levels. The serum DHEA (p = 0.044) and DHT (p = 0.035) levels in the resistance training with Dioscorea esculenta intake group were significantly increased as compared with those in the resistance training with placebo intake group (Fig. 2A and C), whereas the serum free testosterone level tended to increase in the resistance training with Dioscorea esculenta intake group as compared with the resistance training with placebo intake group (p = 0.095, Fig. 2B).
Fig. 2.
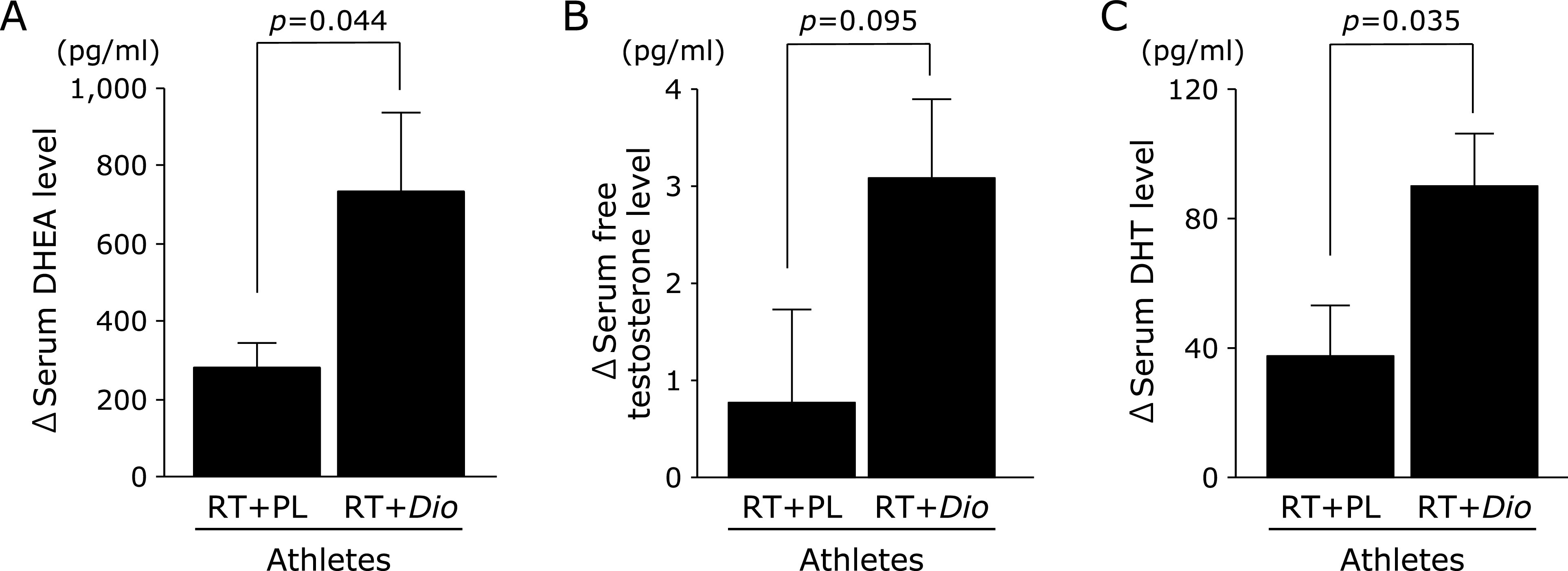
Changes in the levels of serum (A) dehydroepiandrosterone (DHEA), (B) free testosterone, and (C) 5α-dihydrotestosterone (DHT) between resistance training with placebo intake and resistance training with Dioscorea esculenta intake in athletes. RT + PL, resistance training with placebo intake group; RT + Dio, resistance training with Dioscorea esculenta intake group. The values are expressed as mean ± SE.
Relationships between changes in serum androgen hormone levels and changes in arm fat-free mass or maximal muscle strengths in the resistance training with Dioscorea esculenta intake group. In the resistance training with Dioscorea esculenta intake group, the changes in serum DHT level tended to positively correlate with the changes in arm fat-free mass (r = 0.677, p = 0.095), whereas the changes in serum DHEA and free testosterone levels did not correlate with the changes in arm fat-free mass. In addition, the changes in these serum androgen hormone levels did not correlate with the changes in the 1RMs of deadlift and snatch in the resistance training with Dioscorea esculenta intake group.
Discussion
In the cross-sectional study, we observed that athletes had decreased serum DHEA, free testosterone, and DHT levels. Furthermore, in the 8-week intervention study, the combination of Dioscorea esculenta intake and resistance training further increased the fat-free mass in the arms and the 1RMs of deadlift and snatch with augmentation of androgen hormone secretion in athletes, compared with the combination of resistance training and placebo intake. Additionally, the changes in serum DHT level tended to correlate with the changes in the arm fat-free mass in the resistance training with Dioscorea esculenta intake group, but the changes in serum DHEA and free testosterone levels did not show a correlation. Therefore, these findings suggest that Dioscorea esculenta intake combined with resistance training may have further effects of accelerating muscle hypertrophy and strength by restoring circulating DHT levels in athletes, who have reduced secretion of androgen hormones.
Treatment with androgen hormones is well known to increase muscle mass and strength in human and animal studies,(1–3,15,16,19) whereas treatment with supraphysiologic doses of androgen hormones may cause adverse effects such as increased risks of cardiac death(25,26) and cancer.(27,28) In our recent study using diabetic rats, chronic Dioscorea esculenta supplementation, which induced increases in the serum and muscle levels of DHEA and DHT, did not cause adverse effects or toxicities.(18) However, acute DHEA injection in healthy rats with normal androgen hormone secretion had little effects.(29) Therefore, the combination of Dioscorea esculenta intake and resistance training may have further effects on muscle hypertrophy and strength in athletes by restoring circulating androgen hormone levels within the physiological range. Although Dioscorea esculenta intake combined with resistance training enhanced the serum DHEA, free testosterone, and DHT levels, these increases only reached the same or lower level as in non-athletes. Thus, the restoration of circulating androgen hormone levels within the physiological range induced by Dioscorea esculenta intake combined with resistance training in athletes may be related to muscle hypertrophy and strength without adverse effects or toxicities.
In a previous study, resistance training-induced increases in muscle DHEA, free testosterone, and DHT levels were associated with muscle hypertrophy and strength in older men.(19) In this study, the combination of Dioscorea esculenta intake and resistance training further increased the DHEA, free testosterone, and DHT secretion in athletes. Moreover, the changes in DHT secretion tended to correlate with the changes in the arm fat-free mass in the Dioscorea esculenta intake with resistance training group, but the changes in DHEA and free testosterone secretion did not show a correlation. In our previous study, chronic administration of a DHT inhibitor suppressed the beneficial effects of resistance training-induced muscle hypertrophy.(1) DHT has more potent effects than DHEA and testosterone because it has greater affinity to the androgen receptor.(30,31) Additionally, chronic DHT injection increased the muscle androgen receptor mRNA expression and phosphorylation levels as well as the activation of mammalian target of rapamycin (m-TOR) and p70 ribosomal S6 kinase (p70S6K) signaling, the key regulator of muscle protein synthesis.(2,3) Therefore, the increase in circulating DHT levels induced by the combination of Dioscorea esculenta intake and resistance training may activate m-TOR/p70S6K signaling in skeletal muscle by enhancing binding to the androgen receptor, resulting in greater muscle hypertrophy.
Some athletes such as endurance-trained runners and resistance-trained weightlifters have lower baseline serum testosterone levels than non-athletes.(10–13) The present study revealed that sprint athletes had lower baseline serum levels of DHEA, free testosterone, and DHT than non-athletes. Additionally, our previous study showed that the baseline serum DHEA, free testosterone, and DHT levels tended to be lower in endurance athletes than in non-athletes.(14) Therefore, these results indicate that the baseline serum levels of androgen hormones are low in some athletes. As these androgen hormones are important in body composition and exercise performance,(1–9) it is highly beneficial to normalize the reduced androgen hormone secretion in various athletes by using a nutritional approach such as Dioscorea esculenta intake.
A reduction of circulating androgen levels in high mileage runners and endurance runners (training for more than 5 years) was observed, considering that high volume or long-term exercise training could affect androgen hormone secretion.(12,32) Therefore, as the sprint athletes in this study had been high volume training (average training: 5 days/week, 2–3 h/day) with long-term exercise (average athletic history: 7.1 ± 2.9 years), they may experience a reduction in circulating androgen levels. Additionally, other factors may be involved, including:(12) 1) endogenous steroid biosynthesis disorders of the testes; 2) changes in hepatic androgen clearance; 3) changes in muscle androgen metabolism; and 4) increased adrenal activity. However, the exact mechanisms contributing to the decrease in androgen hormone secretion in the sprint athletes involved in this study remains unclear.
In this study, intake of Dioscorea esculenta combined with resistance training enhanced the androgen hormone levels in athletes with deteriorated androgen hormone secretion. However, the mechanism of the restoration of androgen hormone secretion remains unclear. Dioscorea esculenta contains diosgenin, a steroid molecular formula similar to DHEA.(17) In a previous study, diosgenin treatment enhanced the serum and muscle DHEA and DHT levels in type 1 diabetic rats.(33) Therefore, Dioscorea esculenta may restore the serum DHEA, free testosterone, and DHT levels in athletes through its diosgenin content. In this study, the ingested Dioscorea esculenta contained approximately 20 mg/day of diosgenin because 1,000 mg dry weight of Dioscorea esculenta contains about 9.5 mg of diosgenin (data not shown) and the participants ingested 2,000 mg/day of Dioscorea esculenta. Thus, athletes may be able to restore their circulating androgen hormone levels by taking approximately 20 mg/day of diosgenin. However, it is still unclear how diosgenin is absorbed into the body and whether diosgenin is mimicked or complemented to DHEA in the blood. Therefore, further study is required to understand the absorption and metabolism of diosgenin. Additionally, although diosgenin may be contained in other foods, this study did not examine dietary records during the intervention.
In conclusion, the findings of this study indicated that Dioscorea esculenta intake combined with resistance training has further effects on muscle hypertrophy and strength in sprint athletes by restoring circulating DHT levels within the physiological range.
Author Contributions
MI conceived and designed research. NHorii, NHasegawa, SF, KI, MU, and TH performed formal analysis. NHorii, TH and MI wrote the manuscript and all authors reviewed and approved the manuscript.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (KAKENHI No. 17H02182 and 19K22828 to MI).
Abbreviations
- BMI
body mass index
- DHEA
dehydroepiandrosterone
- DHT
5α-dihydrotestosterone
- m-TOR
mammalian target of rapamycin
- p70S6K
p70 ribosomal S6 kinase
- 1RM
one repetition maximum
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Horii N, Sato K, Mesaki N, Iemitsu M. Increased muscular 5α-dihydrotestosterone in response to resistance training relates to skeletal muscle mass and glucose metabolism in type 2 diabetic rats. PLoS One 2016; 11: e0165689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu T, Shen Y, Pink H, et al. Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J Steroid Biochem Mol Biol 2004; 92: 447–454. [DOI] [PubMed] [Google Scholar]
- 3.Zeng F, Zhao H, Liao J. Androgen interacts with exercise through the mTOR pathway to induce skeletal muscle hypertrophy. Biol Sport 2017; 34: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horii N, Sato K, Mesaki N, Iemitsu M. DHEA administration activates transcription of muscular lipid metabolic enzymes via PPARα and PPARδ in obese rats. Horm Metab Res 2016; 48: 207–212. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Iemitsu M, Aizawa K, Ajisaka R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab 2008; 294: E961–E968. [DOI] [PubMed] [Google Scholar]
- 6.Liang X, Glowacki J, Hahne J, Xie L, LeBoff MS, Zhou S. Dehydroepiandrosterone stimulation of osteoblastogenesis in human MSCs requires IGF-I signaling. J Cell Biochem 2016; 117: 1769–1774. [DOI] [PubMed] [Google Scholar]
- 7.Du C, Khalil MW, Sriram S. Administration of dehydroepiandrosterone suppresses experimental allergic encephalomyelitis in SJL/J mice. J Immunol 2001; 167: 7094–7101. [DOI] [PubMed] [Google Scholar]
- 8.Wegner M, Koedijker JM, Budde H. The effect of acute exercise and psychosocial stress on fine motor skills and testosterone concentration in the saliva of high school students. PLoS One 2014; 9: e92953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR. Neurobiology of DHEA and effects on sexuality, mood and cognition. J Steroid Biochem Mol Biol 2015; 145: 273–280. [DOI] [PubMed] [Google Scholar]
- 10.Arce JC, De Souza MJ, Pescatello LS, Luciano AA. Subclinical alterations in hormone and semen profile in athletes. Fertil Steril 1993; 59: 398–404. [PubMed] [Google Scholar]
- 11.Ayers JW, Komesu Y, Romani T, Ansbacher R. Anthropomorphic, hormonal, and psychologic correlates of semen quality in endurance-trained male athletes. Fertil Steril 1985; 43: 917–921. [DOI] [PubMed] [Google Scholar]
- 12.De Souza MJ, Miller BE. The effect of endurance training on reproductive function in male runners. A ‘volume threshold’ hypothesis. Sports Med 1997; 23: 357–374. [DOI] [PubMed] [Google Scholar]
- 13.Hackney AC, Sinning WE, Bruot BC. Reproductive hormonal profiles of endurance-trained and untrained males. Med Sci Sports Exerc 1988; 20: 60–65. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Iemitsu M, Katayama K, Ishida K, Kanao Y, Saito M. Responses of sex steroid hormones to different intensities of exercise in endurance athletes. Exp Physiol 2016; 101: 168–175. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Iemitsu M, Aizawa K, Mesaki N, Fujita S. Increased muscular dehydroepiandrosterone levels are associated with improved hyperglycemia in obese rats. Am J Physiol Endocrinol Metab 2011; 301: E274–E280. [DOI] [PubMed] [Google Scholar]
- 16.Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996; 335: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Raju J, Patlolla JM, Swamy MV, Rao CV. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol Biomarkers Prev 2004; 13: 1392–1398. [PubMed] [Google Scholar]
- 18.Sato K, Fujita S, Iemitsu M. Dioscorea esculenta-induced increase in muscle sex steroid hormones is associated with enhanced insulin sensitivity in a type 2 diabetes rat model. FASEB J 2017; 31: 793–801. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Iemitsu M, Matsutani K, Kurihara T, Hamaoka T, Fujita S. Resistance training restores muscle sex steroid hormone steroidogenesis in older men. FASEB J 2014; 28: 1891–1897. [DOI] [PubMed] [Google Scholar]
- 20.Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab 2006; 291: E1003–E1008. [DOI] [PubMed] [Google Scholar]
- 21.Takami M, Aoi W, Terajima H, Tanimura Y, Wada S, Higashi A. Effect of dietary antioxidant-rich foods combined with aerobic training on energy metabolism in healthy young men. J Clin Biochem Nutr 2019; 64: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai N, Yagyu S, Hata A, et al. Maslinic acid derived from olive fruit in combination with resistance training improves muscle mass and mobility functions in the elderly. J Clin Biochem Nutr 2019; 64: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura Y, Inaguma A, Watanabe S, et al. Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness. Int J Sport Nutr Exerc Metab 2010; 20: 236–244. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa N, Fujie S, Horii N, et al. Aerobic exercise training-induced changes in serum C1q/TNF-related protein levels are associated with reduced arterial stiffness in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 2018; 314: R94–R101. [DOI] [PubMed] [Google Scholar]
- 25.Montisci M, EI Mazloum R, Cecchetto G, et al. Anabolic androgenic steroids abuse and cardiac death in athletes: morphological and toxicological findings in four fatal cases. Forensic Sci Int 2012; 217: e13–e18. [DOI] [PubMed] [Google Scholar]
- 26.Thiblin I, Lindquist O, Rajs J. Cause and manner of death among users of anabolic androgenic steroids. J Forensic Sci 2000; 45: 16–23. [PubMed] [Google Scholar]
- 27.Calhoun KE, Pommier RF, Muller P, Fletcher WS, Toth-Fejel S. Dehydroepiandrosterone sulfate causes proliferation of estrogen receptor-positive breast cancer cells despite treatment with fulvestrant. Arch Surg 2003; 138: 879–883. [DOI] [PubMed] [Google Scholar]
- 28.Ahonen MH, Zhuang YH, Aine R, Ylikomi T, Tuohimaa P. Androgen receptor and vitamin D receptor in human ovarian cancer: growth stimulation and inhibition by ligands. Int J Cancer 2000; 86: 40–46. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, Iemitsu M, Aizawa K, Ajisaka R. DHEA improves impaired activation of Akt and PKC zeta/lambda-GLUT4 pathway in skeletal muscle and improves hyperglycaemia in streptozotocin-induced diabetes rats. Acta Physiol (Oxf) 2009; 197: 217–225. [DOI] [PubMed] [Google Scholar]
- 30.Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS 2000; 108: 838–846. [DOI] [PubMed] [Google Scholar]
- 31.Labrie F, Luu-The V, Bélanger A, et al. Is dehydroepiandrosterone a hormone? J Endocrinol 2005; 187: 169–196. [DOI] [PubMed] [Google Scholar]
- 32.Hackney AC, Lane AR. Low testosterone in male endurance-trained distance runners: impact of years in training. Hormones (Athens) 2018; 17: 137–139. [DOI] [PubMed] [Google Scholar]
- 33.Sato K, Fujita S, Iemitsu M. Acute administration of diosgenin or dioscorea improves hyperglycemia with increases muscular steroidogenesis in STZ-induced type 1 diabetic rats. J Steroid Biochem Mol Biol 2014; 143: 152–159. [DOI] [PubMed] [Google Scholar]


